Abstract
BACKGROUND:
This study aimed to determine the effects of sepsis on brain integrity, memory, and executive function.
METHODS:
Twenty sepsis patients who were not diagnosed with sepsis-associated encephalopathy (SAE) but had abnormal electroencephalograms (EEGs) were included. The control group included twenty healthy persons. A neuropsychological test of memory and executive function and a brain magnetic resonance imaging scan were performed. The volumes of cortex and subcortex were measured using the FreeSurfer software. Acute Physiology and Chronic Health Evaluation II (APACHE II) score was used to determine the disease severity.
RESULTS:
In the sepsis group, the levels of immediate free recall, immediate cued recall, and delayed cued recall in the California Verbal Learning Test-II (CVLT-II) were significantly lower; the explicit memory (recollection process) in the process dissociation procedure test was lower; and the volumes of the left and right hippocampi were significantly lower compared with the control group. The volume of the presubiculum in the hippocampus of sepsis patients showed statistically significant decrease. In the sepsis group, the volumes of the left and right hippocampi were negatively correlated with the APACHE II score and positively with immediate free recall, immediate cued recall, and delayed cued recall in the CVLT-II; moreover, the hippocampal volume was significantly correlated with recollection but not with familiarity.
CONCLUSIONS:
Patients with abnormal EEGs during hospitalization but with no SAE still have reduced hippocampal volume and memory deficits. This finding indicates that sepsis leads to damage to specific parts of the hippocampus.
KEYWORDS: Hippocampus, Magnetic resonance imaging, Memory, Sepsis-associated encephalopathy
INTRODUCTION
Sepsis is a life-threatening, multiple-organ dysfunction condition caused by an infection that induces immune dysfunction in the host.[1] It is an important medical and health problem that yearly affects thousands of patients worldwide. Sepsis-associated encephalopathy (SAE) is a common complication of sepsis. SAE refers to diffuse brain dysfunction. The incidence of SAE is 8%–70%, and it is also one of the most common brain disorders in the intensive care unit.[2,3] So far, there are no clear diagnostic criteria for SAE. SAE diagnosis excludes direct infection of the central nervous system, head trauma, fat embolism, adverse drug reactions, other factors that affect consciousness, and diffuse cerebral dysfunction caused by the systemic inflammatory response.[4,5] A multicenter study found that 307 (23.0%) of 1,333 patients with severe sepsis had severely altered mental states.[2] However, sensitive diagnostic tools, such as electrophysiological tests for brain function, revealed that almost all sepsis patients possessed mental abnormalities.[6-8] Brain dysfunction is often overlooked because sepsis patients are not diagnosed with SAE during hospitalization.
SAE is a disorder with complex and unclear pathogenesis.[9] Cerebral microcirculation is one important pathophysiological symptom of SAE.[10] In sepsis patients with impaired brain autoregulation, the cerebral vessel responses to carbon dioxide concentration and extracellular pH become slow.[11] When systemic blood pressure drops sharply, inadequate cerebral perfusion will likely occur, leading to neurological dysfunction. Dysfunction of brain autoregulation leads to an inability to respond to changes in mean arterial pressure.[6,10,12,13] Related studies have shown that neuronal damage after cerebral ischemia is region-selective, and the vulnerabilities of neurons in sensitive areas are also different.[14] The hippocampus and neocortex are prone to damage after cerebral ischemia. Neurons in the CA1 area of the hippocampus are especially sensitive to transient ischemia, whereas apoptosis in the CA3 area is known as selective vulnerability. Sepsis patients have a higher risk of non-Alzheimer’s dementia compared with the general population.[15,16] At present, most clinical diagnoses of SAE are based only on clinical manifestations without electrophysiological or biochemical tests. As a result, many sepsis patients with hidden neurological dysfunction do not undergo comprehensive neuropsychological tests because they do not have significant psychiatric disorders in hospitals. The nervous system function in such patients is not clear; thus, most sepsis patients don’t receive corresponding rehabilitation treatment.
This study aimed to investigate whether sepsis causes loss of brain volume and cognitive function. We used magnetic resonance imaging (MRI) scans to evaluate the nervous system integrity of sepsis patients and healthy controls. We focused on the hippocampus, thalamus, caudate, putamen, pallidum, amygdala, and ventral diencephalon because these areas are considered as the most appropriate regions for analysis throughout the brain. The CA1 area of the hippocampus was also analyzed because CA1 is particularly sensitive to ischemia and hypoxia.[14] Neuropsychological tests were used to assess the neurological function of patients. Particular emphasis was placed on the cognitive and executive functions, which are susceptible to be influenced by ischemia and hypoxia.[17]
METHODS
Study population
This study included 20 sepsis patients who were not diagnosed clinically with SAE but had abnormal electroencephalograms (EEGs). These patients were admitted to the emergency department of the Second Affiliated Hospital of Xi’an Jiaotong University from June 2015 to November 2018, and the clinical data were collected and analyzed.
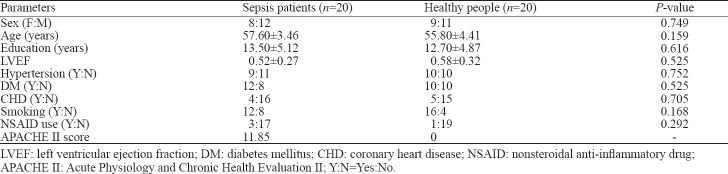
The patients were diagnosed with sepsis, which was defined in accordance with the criteria.[1] All patients had no change of mental state during hospitalization, but their EEGs revealed abnormalities, including background abnormalities, changes in cycles and rhythms, and epileptiform activity. Background abnormality was characterized by slow background activity, including diffuse slow waves, persistent θ waves, and slow delta waves or burst suppression, with or without autonomous background variation. The periodic and rhythmic changes were manifested as three-phase waves, periodic epileptic rhythms, intermittent rhythmic δ activities in the frontal region, pseudoperiodic epileptic discharges on one side or epileptic discharges on both sides, and abnormal seizure periods. All sepsis patients completed Acute Physiology and Chronic Health Evaluation II (APACHE II)[18] scoring (score 0 to 71) on admission. After admission, patients received empirical anti-infection treatment. For patients with positive blood cultures in the later stage, the anti-infection regimen was adjusted according to the blood culture results. All patients with hemodynamic abnormalities received active fluid resuscitation, and crystalloid fluid was preferred. All patients underwent an MRI examination within one month after hospital discharge. The control group was composed of 20 healthy people (from the Physical Examination Center of the Second Affiliated Hospital of Xi’an Jiaotong University); their sex, age, education level, hypertension, coronary heart disease (CHD), diabetes mellitus (DM), and other basic information were matched with those of the sepsis group. All participants were required to have no previous history of neurological diseases so as not to affect the results of this study. All participants underwent neuropsychological tests within one month after enrolling in the study. Table 1 summarizes the basic demographic information and clinical characteristics of the two groups.
Table 1.
Demographic and clinical characteristics of patients with sepsis and healthy controls
Neuropsychological assessments
Neuropsychological assessments were performed in all sepsis patients and healthy controls. All sepsis patients completed the test within one month after discharge, and participants in the control group completed the test within one month after joining the study. The assessments included the following cognitive functions: (1) general intelligence test using the Wechsler Adult Intelligence Scale-III (Chinese translation version);[19] (2) visual evaluation using the Hooper Visual Organization Test;[20] (3) memory function using the California Verbal Learning Test-II (CVLT-II);[21] (4) attention using the Stroop Test (ST);[22] (5) executive function using the Wisconsin Card Sorting Test;[23] (6) working memory using the Paced Auditory Serial Addition Test (PASAT 2 and 3);[24] and (7) changes in the familiarity and recognition functions in memory using the process dissociation procedure (PDP) test.[25]
Image acquisition
The MRI data were gathered from all 40 people. All images were acquired using a GE Signa HDxt 3.0T TX dual-gradient dual-RF source MRI scanner and a head orthogonal eight-channel phased-array coil. High-resolution axial 3D T1-weighted images of the entire brain were obtained using a 3D fast spoiled gradient-recalled sequence. The scanning parameters were as follows: time of repetition 8.0 ms; time of echo 3.1 ms; inversion time 400 ms; thickness 1 mm; flip angle 12°; matrix 240×240; number of excitation 1; and field of view 24 cm×18 cm. Moreover, 158 layers were scanned without interval.
Image analyses
FreeSurfer v6.0 (http://surfer.nmr.mgh.harvard.edu/) was used for image analysis. FreeSurfer is a powerful suite of tools that performs highly reliable and automated analysis of the human brain.[26] The analysis included volumetric segmentation of the brain structure and the segmentation of the hippocampus. FreeSurfer can accurately identify and segment cerebral cortex and subcortical structures in MRI imaging data and measure the changes of subpixel accuracy of cerebral cortex thickness with high sensitivity. FreeSurfer was used to segment the hippocampus and to obtain the volumes of different brain regions. The results of all scans and all automatic segmentation were visually examined to ensure proper skull dissection and correct segmentation.
Ethics statement
The Research Ethics Board of the Medical Center and Psychology Department of the Xi’an Jiaotong University approved this study.
RESULTS
Demographic information
There were no significant differences between the sepsis group and the healthy control group in general demographic data that included sex ratio, age, education level, hypertension, CHD, DM, tobacco use, and nonsteroidal anti-inflammatory drug (NSAID) use (Table 1).
Neuropsychological assessment
Figure 1 shows the results of clinical neuropsychological tests of different cognitive region functions. The results were tested by a normal distribution (Kolmogorov-Smirnov test). Data of the two groups were fitted to a normal distribution so that they could be compared using the t-test between two pairs of samples. According to the Wechsler Adult Intelligence Scale-III, no difference in the overall intelligence quotient (IQ) was found between the two groups (P>0.05), a condition that could be used as a background to explain other areas of performance. In addition, we didn’t find any significant differences in visual assessment, executive function, or attention tests between the two groups (P>0.05). Although the sepsis group showed a cognitive decline clinically, no significant difference was found compared with the healthy control group (P>0.05). The levels of immediate free recall, immediate cued recall, and delayed cued recall in the CVLT-II were significantly lower in sepsis patients (P<0.05). No significant difference in PASAT 2 or 3 was found between the two groups (P>0.05). The explicit memory (recollection process) of the PDP test was lower in the sepsis group compared with the control group, but no significant difference was found in the implicit memory familiarity.
Figure 1.
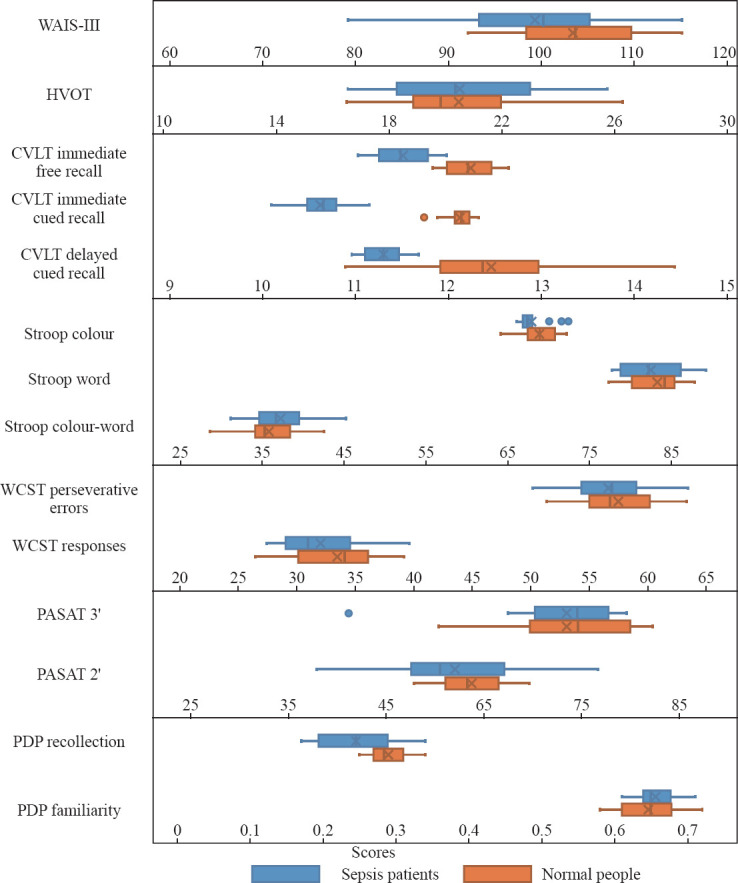
Results of clinical neuropsychological tests of different cognitive region functions between the two groups. WAIS-III: Wechsler Adult Intelligence Scale-III; HVOT: Hooper Visual Organization Test; CVLT: California Verbal Learning Test; WCST: Wisconsin Card Sorting Test; PASAT: Paced Auditory Serial Addition Test; PDP: process dissociation procedure.
Structural neuroimaging
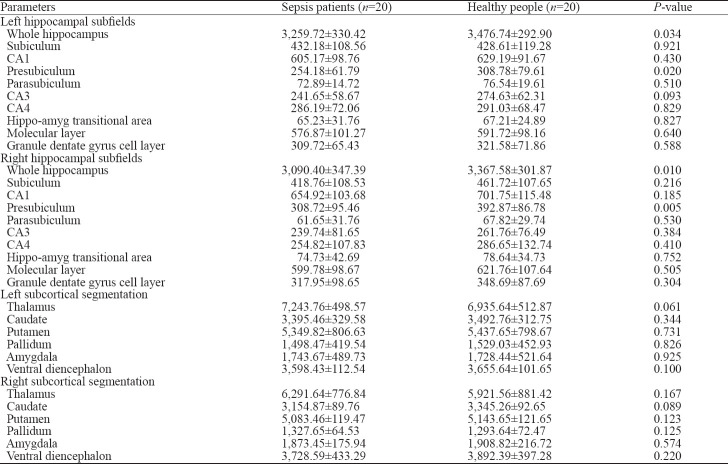
We used FreeSurfer v6.0 to automatically analyze and measure the volumes of different brain regions and hippocampal formation in the sepsis and control groups. The results indicated that the volumes of the left and right hippocampi in sepsis patients were significantly lower than those in the control group (P<0.05). Compared with the control group, the volume of the presubiculum in the hippocampus of sepsis patients decreased, and the difference was statistically significant (P<0.05). The volumes of CA1, CA3, CA4, molecular layer, granular dentate gyrus cell layer, and the hippo-amyg transitional area between the hippocampus and the amygdala were smaller in the sepsis group than in the healthy control group, but the differences were not statistically significant (P>0.05). There was no significant difference in subcortical volume between the two groups, including the thalamus, caudate, putamen, pallidum, amygdala, and ventral diencephalon (P>0.05), but we found that the subcortical volume in the sepsis group was smaller than that in the control group (Table 2).
Table 2.
The volume of different brain regions (mm3)
Correlations between hippocampal volumes and neuropsychological tests
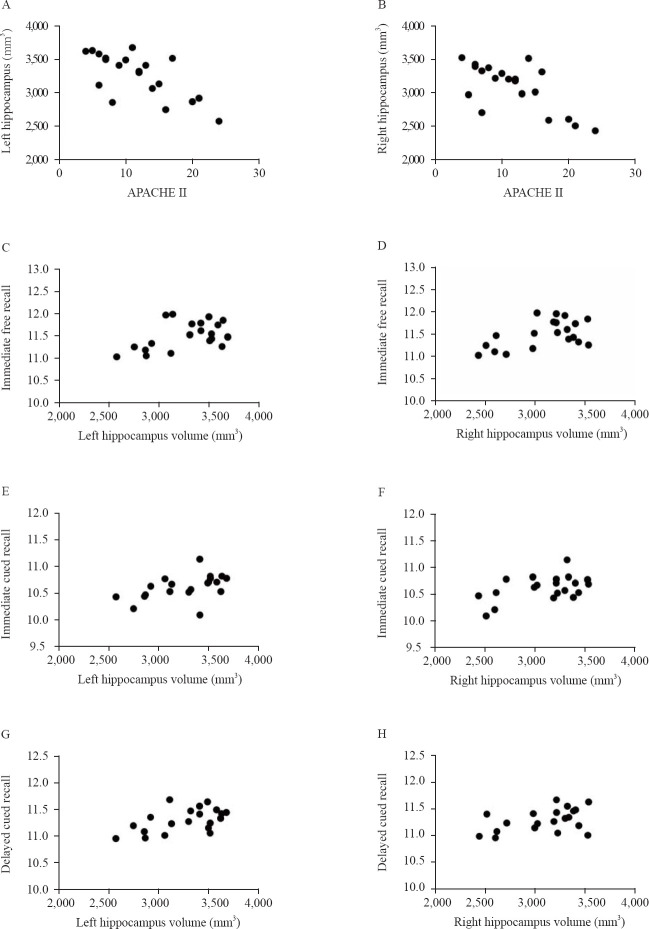
The APACHE II score of the sepsis group was negatively correlated with the volumes of left and right hippocampi (Figure 2A, r= –0.690, P<0.05; Figure 2B, r= –0.694, P<0.05), and the volumes of left and right hippocampi were positively correlated with immediate free recall (Figure 2C, r=0.496, P<0.05; Figure 2D, r=0.526, P<0.05), immediate cued recall (Figure 2E, r=0.484, P<0.05; Figure 2F, r=0.497, P<0.05), and delayed cued recall (Figure 2G, r=0.571, P<0.05; Figure 2H, r=0.448, P<0.05) of the CVLT-II (Figure 2). In the sepsis group, the hippocampal volume was significantly positively correlated with recollection but wasn’t correlated with familiarity (P<0.05). No correlation was found between the hippocampal volume and CVLT-II in the healthy control group (P>0.05), and between other cognitive tests and hippocampal volume in the two groups (P>0.05).
Figure 2.
Correlations between APACHE II scores and hippocampal volume (A and B) and between estimates of neuropsychological test results and hippocampal volumes (C–H) in the sepsis group (n=20).
DISCUSSION
Severe infection often causes neurological impairment. In a prospective cohort study, Yaffe et al[27] first reported a threefold increase in the risk of moderate-to-severe cognitive impairment after recovery from severe sepsis. A considerable number of patients develop abnormal neuropsychiatric function during hospitalization. After excluding direct infection of the central nervous system, head trauma, fat embolism, and adverse drug reactions that affect the state of consciousness, the diffuse brain dysfunction caused by the systemic inflammatory response can be diagnosed as SAE.[4,5] Many sepsis patients don’t receive comprehensive neuropsychological tests after discharge because they do not have obvious mental disorders during their hospital stay. Clinicians do not obtain a clear understanding of the nervous system function in sepsis patients; thus, many patients do not receive the corresponding rehabilitation treatment. In the present study, the volume of the hippocampus in sepsis patients without neurological and psychiatric abnormalities was found to be reduced compared with that in the control group.
Azabou et al[28] found that an abnormal EEG and mental disorders could occur in the early stage of sepsis. The investigators further analyzed the data of sepsis patients in the recovery stage or discharged from the hospital about one month after treatment. Thus, in our study, we performed neuropsychological tests in participants within one month after enrollment.
Several neuropsychological tests on cognitive functions were performed, including visual, attention, memory, executive, and working memory. Although no differences in IQ, visual assessment, executive function, or attention test were found between sepsis patients and controls, sepsis patients exhibited a lower relative performance in immediate free recall, immediate cued recall, and delayed cued recall. Sepsis increases the risk of dementia[29-31] that severely affects the integrity of cognition and function[32] and reduces the quality of life.[30] In this study, no significant decline in the executive and attention functions was found in the early stage after sepsis patients were discharged from the hospital. This finding indicated that the impairment of executive and attention functions might not be an acute process, and rehabilitation training might delay the decline in executive and attention functions, thereby improving the life quality of sepsis patients.
The memory function of sepsis patients, immediate free recall, immediate cued recall, or delayed cued recall declined significantly. The CA1 area and presubiculum of the hippocampus were related to the learning and memory functions.[31] The integrity of hippocampal neuronal structure and function is the premise of maintaining learning and memory; the decline in memory function in sepsis patients may be related to the decrease in the hippocampal volume.
In this study, the APACHE II scores on admission were negatively correlated with the hippocampal volume in the sepsis group. The results were similar to those of previous studies, indicating that the APACHE II score had a particular significance in the prognosis of patients with severe nervous system disorders.[33] The APACHE II scores of sepsis patients can be used as an index to evaluate the integrity of the nervous system.
The hippocampal volume was significantly correlated with recollection, but not with familiarity. The memory function of the hippocampus was controversial.[34,35] Some differences existed between familiarity and recollection as two different components of memory. The findings of this study supported the idea that the hippocampus had a function in recollection. The volume of the presubiculum in the hippocampus decreased in sepsis patients, indicating that recollection was related to this area.
Because of the effects of various inflammatory factors, sepsis patients often develop hypotension leading to insufficient cerebral perfusion and changes in brain microcirculation.[4,5] The neuronal damage after cerebral ischemia is region-selective, and the vulnerability of neurons in sensitive areas is also different during cerebral ischemia and hypoxia.[14] In this study, the volume of the presubiculum decreased in the sepsis patients, and this was consistent with the results of previous studies. The main subjects of previous studies were patients with whole brain chronic hypoxia or acute hypoxia. The findings of our study indicated that the whole brain hypoxic condition may exist in sepsis instead of a localized injury caused by local exposure to inflammatory factors alone.
These three results indicate that the factors that cause intracranial injury in sepsis patients may be more complicated than simple ischemia and hypoxia.
The volume of the presubiculum, which was not a vulnerable area of patients, also decreased in the areas considered to be vulnerable to ischemia. These results indicated that the factors, which caused intracranial injury in sepsis patients, might be more complicated. Of course, we cannot rule out inaccuracies due to the small sample size, the clarity of imaging, and the FreesSufer software division of the hippocampal formation.
The present study mainly focused on sepsis patients who didn’t have an obvious nervous system dysfunction during hospitalization but had abnormal EEGs. Because SAE is often diagnosed by clinical manifestations, some sepsis patients with abnormal EEGs are not timely recognized with SAE. Although most patients have early memory impairment, they are often discharged without evaluated with neuropsychological measures, and they are not considered in need of rehabilitation training. The impairment gradually affects the executive and attention functions, which can lead to challenges in life after discharge. Therefore, more advanced tests are needed to identify neurological abnormalities in sepsis patients during hospitalization, and early rehabilitation of sepsis patients who are not diagnosed with SAE should not be neglected. This approach may contribute to an improvement in the life quality of patients. We also found that executive and attention dysfunctions did not exist in sepsis patients at an early stage, and thus, by taking some steps early, we may be able to prevent or slow the decline in executive and attention functions.
There were some limitations in our study. The control group in our study was composed of healthy people. Although they were matched with the sepsis group for basic features such as sex, age, educational level, hypertension, CHD, diabetes, smoking, and NSAID use, sepsis patients may also have some unknown high risk of infection. Meanwhile, the study was a single-center, limited-sample study, which might affect the reliability of the results.
CONCLUSIONS
In general, sepsis can lead to a decrease in the size of the hippocampus, accompanied by early memory deficits. More advanced tests are needed to identify neurological abnormalities in sepsis patients during hospitalization. Even if they do not develop neurological dysfunction during hospitalization, sepsis patients should be assessed neurologically and, if necessary, treated with appropriate rehabilitation after hospital discharge. This strategy may improve long-term nervous system prognosis and quality of life, and reduce the incidence of non-Alzheimer’s dementia.
Footnotes
Funding: This work was supported by the Shanxi Province Key Scientific and Technological Project (2016YFJH2-05), and the Youth Project of the Second Affiliated Hospital of Xi’an Jiaotong University (YJ[QN]201523).
Ethical approval: The Research Ethics Board of the Medical Center and Psychology Department of the Xi’an Jiaotong University approved this study.
Conflicts of interest: The authors have no competing interests relevant to the present study.
Contributors: All authors read and approved the final version of the manuscript.
REFERENCES
- 1.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving Sepsis Campaign:International Guidelines for Management of Sepsis and Septic Shock 2016. Intensive Care Med. 2017;43(3):1–74. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 2.Sprung CL, Peduzzi PN, Shatney CH, Schein RM, Wilson MF, Sheagren JN, et al. Impact of encephalopathy on mortality in the sepsis syndrome. The Veterans Administration Systemic Sepsis Cooperative Study Group. Crit Care Med. 1990;18(8):801–6. doi: 10.1097/00003246-199008000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Young GB, Bolton CF, Austin TW, Archibald YM, Gonder J, Wells GA. The encephalopathy associated with septic illness. Clin Invest Med. 1990;13(6):297–304. [PubMed] [Google Scholar]
- 4.Chaudhry N, Duggal AK. Sepsis associated encephalopathy. Adv Med. 2014;2014(3):195–204. doi: 10.1155/2014/762320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papadopoulos MC, Davies DC, Moss RF, Tighe D, Bennett ED. Pathophysiology of septic encephalopathy:a review. Crit Care Med. 2000;28(8):3019–24. doi: 10.1097/00003246-200008000-00057. [DOI] [PubMed] [Google Scholar]
- 6.Schramm P, Klein KU, Falkenberg L, Berres M, Closhen D, Werhahn KJ, et al. Impaired cerebrovascular autoregulation in patients with severe sepsis and sepsis-associated delirium. Crit Care. 2012;16(5):R181. doi: 10.1186/cc11665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polito A, Eischwald F, Maho AL, Polito A, Azabou E, Annane D, et al. Pattern of brain injury in the acute setting of human septic shock. Crit Care. 2013;17(5):R204. doi: 10.1186/cc12899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sutter R, Kaplan PW. Clinical and imaging correlates of EEG patterns in hospitalized patients with encephalopathy. J Neurol. 2013;260(4):1087–98. doi: 10.1007/s00415-012-6766-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pandharipande PP, Sanders RD, Girard TD, McGrane S, Thompson JL, Shintani AK, et al. Effect of dexmedetomidine versus lorazepam on outcome in sepsis patients:an a priori-designed analysis of the MENDS randomized controlled trial. Crit Care. 2010;14(2):R38. doi: 10.1186/cc8916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Backer D, Donadello K, Sakr Y, Ospina-Tascon G, Salgado D, Scolletta S, et al. Microcirculatory alterations in patients with severe sepsis:impact of time of assessment and relationship with outcome. Crit Care Med. 2013;41(3):791–9. doi: 10.1097/CCM.0b013e3182742e8b. [DOI] [PubMed] [Google Scholar]
- 11.Taccone FS, Castanares-Zapatero D, Peres-Bota D, Vincent JL, Berre J, Melot C. Cerebral autoregulation is influenced by carbon dioxide levels in patients with septic shock. Neurocrit Care. 2010;12(1):35–42. doi: 10.1007/s12028-009-9289-6. [DOI] [PubMed] [Google Scholar]
- 12.Terborg C, Schummer W, Albrecht M, Reinhart K, Weiller C, Röther J. Dysfunction of vasomotor reactivity in severe sepsis and septic shock. Intensive Care Med. 2001;27(7):1231–4. doi: 10.1007/s001340101005. [DOI] [PubMed] [Google Scholar]
- 13.Pfister D, Siegemund M, Dell-Kuster S, Smielewski P, Rüegg S, Strebel SP, et al. Cerebral perfusion in sepsis-associated delirium. Crit Care. 2008;12(3):R63. doi: 10.1186/cc6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt-Kastner R, Freund TF. Selective vulnerability of the hippocampus in brain ischemia. Neuroscience. 2015;309(3):259–79. doi: 10.1016/j.neuroscience.2015.08.034. [DOI] [PubMed] [Google Scholar]
- 15.Girard TD, Jackson JC, Pandharipande PP, Pun BT, Thompson JL, Shintani AK, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2016;38(7):1513–20. doi: 10.1097/CCM.0b013e3181e47be1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chou CH, Lee JT, Lin CC, Sung YF, Lin CC, Muo CH, et al. Septicemia is associated with increased risk for dementia:a population-based longitudinal study. Oncotarget. 2017;8(48):84300–8. doi: 10.18632/oncotarget.20899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II:a severity of disease classification system. Crit Care Med. 1985;13(10):818–29. [PubMed] [Google Scholar]
- 19.Keilp JG, Corbera K, Slavov I, Taylor MJ, Sackeim HA, Fallon BA. WAIS-III and WMS-III performance in chronic Lyme disease. J Int Neuropsychol Soc. 2006;12(1):119–29. doi: 10.1017/S1355617706060231. [DOI] [PubMed] [Google Scholar]
- 20.Su CY, Lin YH, Wu YY, Wuang YP. Development of the Chinese version of the Hooper Visual Organization Test:normative data. Int J Rehabil Res. 2013;36(1):56–67. doi: 10.1097/MRR.0b013e3283588b95. [DOI] [PubMed] [Google Scholar]
- 21.Woods SP, Delis DC, Scott JC, Kramer JH, Holdnack JA. The California Verbal Learning Test–second edition:test-retest reliability, practice effects, and reliable change indices for the standard and alternate forms. Arch Clin Neuropsychol. 2006;21(5):413–20. doi: 10.1016/j.acn.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18(6):643. [Google Scholar]
- 23.MacAllister WS, Maiman M, Marsh M, Whitman L, Vasserman M, Cohen RJ, et al. Sensitivity of the Wisconsin Card Sorting Test (64-Card Version) versus the Tower of London (Drexel Version) for detecting executive dysfunction in children with epilepsy. Child Neuropsychol. 2018;24(3):354–69. doi: 10.1080/09297049.2016.1265101. [DOI] [PubMed] [Google Scholar]
- 24.Diehr MC, Cherner M, Wolfson TJ, Miller SW, Grant I, Heaton RK, et al. The 50- and 100-item short forms of the Paced Auditory Serial Addition Task (PASAT):demographically corrected norms and comparisons with the full PASAT in normal and clinical samples. J Clin Exp Neuropsychol. 2003;25(4):571–85. doi: 10.1076/jcen.25.4.571.13876. [DOI] [PubMed] [Google Scholar]
- 25.Jacoby LL. A process dissociation framework:separating automatic from intentional uses of memory. J Mem Lang. 1991;30(5):513–41. [Google Scholar]
- 26.Fischl B. FreeSurfer. Neuroimage. 2012;62(2):774–81. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yaffe K, Fox P, Newcomer R, Sands L, Lindquist K, Dane K, et al. Patient and caregiver characteristics and nursing home placement in patients with dementia. JAMA. 2002;287(16):2090. doi: 10.1001/jama.287.16.2090. [DOI] [PubMed] [Google Scholar]
- 28.Azabou E, Magalhaes E, Braconnier A, Yahiaoui L, Moneger G, Heming N, et al. Early standard electroencephalogram abnormalities predict mortality in septic intensive care unit patients. PLoS One. 2015;10(10):e0139969. doi: 10.1371/journal.pone.0139969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dunn N, Mullee M, Perry VH, Holmes C. Association between dementia and infectious disease:evidence from a case-control study. Alzheimer Dis Assoc Disord. 2005;19(2):91. doi: 10.1097/01.wad.0000165511.52746.1f. [DOI] [PubMed] [Google Scholar]
- 30.Winters BD, Eberlein M, Leung J, Needham DM, Pronovost PJ, Sevransky JE. Long-term mortality and quality of life in sepsis:a systematic review. Crit Care Med. 2010;38(5):1276. doi: 10.1097/CCM.0b013e3181d8cc1d. [DOI] [PubMed] [Google Scholar]
- 31.Duncan K, Ketz N, Inati SJ, Davachi L. Evidence for area CA1 as a match/mismatch detector:a high-resolution fMRI study of the human hippocampus. Hippocampus. 2012;22(3):389–98. doi: 10.1002/hipo.20933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yende S, Angus DC. Long-term outcomes from sepsis. Curr Infect Dis Rep. 2007;9(5):382. doi: 10.1007/s11908-007-0059-3. [DOI] [PubMed] [Google Scholar]
- 33.Huang Y, Chen J, Zhong S, Yuan J. Role of APACHE II scoring system in the prediction of severity and outcome of acute intracerebral hemorrhage. Int J Neurosci. 2016;126(11):1020–4. doi: 10.3109/00207454.2015.1099099. [DOI] [PubMed] [Google Scholar]
- 34.Wixted JT, Squire LR. The role of the human hippocampus in familiarity-based and recollection-based recognition memory. Behav Brain Res. 2010;215(2):197–208. doi: 10.1016/j.bbr.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kafkas A, Montaldi D. Familiarity and recollection produce distinct eye movement, pupil and medial temporal lobe responses when memory strength is matched. Neuropsychologia. 2012;50(13):3080–93. doi: 10.1016/j.neuropsychologia.2012.08.001. [DOI] [PubMed] [Google Scholar]