Abstract
BACKGROUND:
Fluid management is crucial to acute respiratory distress syndrome (ARDS) secondary to sepsis. However, choices of fluid resuscitation strategies and fluid input volumes remain a thorny problem. Our study aimed to elucidate the relationship between fluid balance and prognosis of ARDS patients secondary to sepsis.
METHODS:
Our study included 322 sepsis patients from Ruijin Hospital between 2014 and 2018, and 84 patients were diagnosed as ARDS within 72 hours after onset of sepsis according to Berlin ARDS Definition.
RESULTS:
Among the 322 sepsis patients, 84 (26.1%) were complicated with ARDS within 72 hours. ARDS patients had a lower oxygenation index (PaO2/FiO2 166.4±71.0 vs. 255.0±91.2, P<0.05), longer duration of mechanical ventilation (11 [6–24] days vs. 0 [0–0] days, P<0.05) than those without ARDS. Sepsis patients with ARDS showed daily positive net fluid balance during seven days compared with those without ARDS who showed daily negative net fluid balance since the second day with significant statistical differences. Among the 84 sepsis patients with ARDS, 58 (69.0%) died. Mean daily fluid input volumes were much lower in survivors than in non-survivors (43.2±16.7 mL/kg vs. 51.0±25.2 mL/kg, P<0.05) while output volumes were much higher in survivors (45.2±19.8 mL/kg vs. 40.2±22.7 mL/kg, P<0.05). Using binary logistic regression analysis, we found that the mean daily fluid balance was independently associated with mortality of sepsis patients complicating with ARDS (P<0.05).
CONCLUSIONS:
Early negative fluid balance is independently associated with a better prognosis of sepsis patients complicated with ARDS.
KEYWORDS: Sepsis, Acute respiratory distress syndrome, Fluid balance, Prognosis
INTRODUCTION
Sepsis is a severe critical care syndrome and a life-threatening condition with high morbidity and poor prognosis.[1,2] Acute respiratory distress syndrome (ARDS) is a type of acute diffuse, inflammatory lung injury caused by various factors inside and outside the lungs, leading increased pulmonary vascular permeability, increased lung weight, and loss of aerated lung tissue.[3] Many studies have reported that sepsis is one of the most common and lethal causes of ARDS,[4] and nearly 40% ARDS incidence results from sepsis.[5-7] ARDS, as a main cause of acute respiratory failure, is mainly characterized by increased pulmonary endothelial and epithelial permeability, diffuse alveolar injury, and severe pulmonary inflammation.[8] Proteinaceous fluid accumulation in the pulmonary interstitial area or alveoli reduces pulmonary compliance and volume for patients with ARDS.[9] Therefore, fluid management is crucial to ARDS. However, choices of fluid resuscitation strategies and fluid input volumes remain a thorny problem. Several relevant studies[10-14] have been conducted to explore this problem but showed contradictory results. Fluid resuscitation is also the mainstay of therapy for sepsis.[15] Restricted fluid resuscitation and negative fluid balance are shown to be beneficial to the prognosis of sepsis patients.[16-18] However, for ARDS patients secondary to sepsis, only a few studies have been conducted to illuminate the relationship between fluid resuscitation and prognosis. The study aimed to explore this problem.
METHODS
We conducted a single-center and retrospective study in Ruijin Hospital (2,100 beds) Affiliated to Shanghai Jiao Tong University School of Medicine in Shanghai, China. It was approved by the Ethics Committee of Ruijin Hospital (Reference number: 2017119). Because our study was retrospective, the patient’s consent was not necessary.
Inclusion criteria
Patients were identified for the study if they met all of the following inclusion criteria: (1) age ≥18 years; (2) diagnosis of sepsis;[19] (3) meeting the Berlin Definition for ARDS within 72 hours of sepsis onset;[20] (4) duration of emergency intensive care unit (EICU) stay more than 72 hours.
Exclusion criteria
Patients were excluded if they: (1) participated in other studies; (2) had inadequate medical records; (3) had concomitant pulmonary diseases; (4) died; (5) were discharged within 24 hours; or (6) needed emergency operations after EICU admission.
Data collection
Baseline data included demographics, sepsis category, source of sepsis, the origin of patients, comorbidities, Acute Physiologic and Chronic Health Evaluation II (APACHE II) scores, and Sequential Organ Failure Assessment (SOFA) scores during the first 24 hours after EICU admission. We also collected data of tidal volumes, PaO2/FiO2, positive end-expiratory pressure (PEEP), duration of hospital and EICU stay as well as mechanical ventilation. The primary end point was in-hospital mortality, and the secondary end points were duration of hospital and EICU stay as well as mechanical ventilation.
Daily fluid input included oral, enteral, and intravenous fluids. Daily fluid output included urine volume, ultrafiltration, and fluid loss from drains and tubes. We didn’t consider insensible water loss. Daily net fluid balance for seven days was calculated by subtracting daily fluid output from daily fluid input.
We divided all patients into the ARDS group and the non-ARDS group. Furthermore, patients of the ARDS group were divided into survivors and non-survivors.
Statistical analysis
Continuous demographic and clinical variables were described as mean±standard deviation (SD) or median (interquartile range [IQR]), depending on the normality measured by the Kolmogorov-Smirnov method. Categorical variables were described as numbers and percentages. We used Student’s t-test or Mann-Whitney U-test to compare data of continuous variables, and Pearson’s Chi-square, continuity correction, Fisher’s exact, or likelihood ratio tests for categorical variables. Binary logistic regression analysis was applied to analyze the relationship between indicators with statistical significance (P<0.05) and mortality. If the two-sided P-value was <0.05, comparisons were considered significant. Analyses of data were performed using SPSS Version 18.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
We assessed 405 patients in total, and 83 were excluded for various reasons which were listed in Figure 1. A total of 322 patients were enrolled finally, and 84 (26.1%) had ARDS within 72 hours after sepsis onset. Among patients with ARDS secondary to sepsis, 49 (58.3%) survived during the EICU stay (Figure 1).
Figure 1.
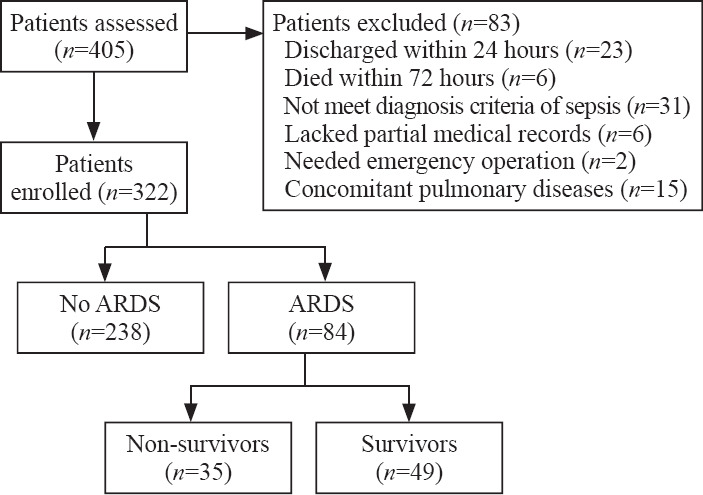
Study flow chart.
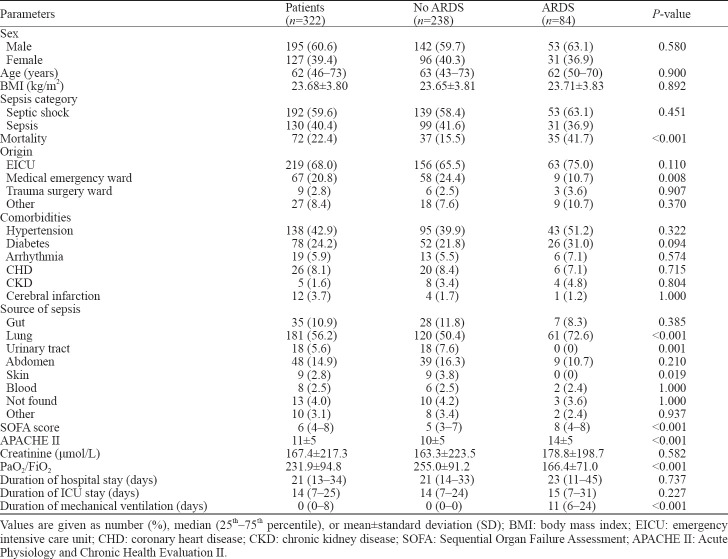
First, we divided sepsis patients into the ARDS group and the non-ARDS group. Epidemiologic statistics compared between the two groups were counted as follows: men accounted for 60.6%, the median age was 62 (IQR 46–73) years, the mean body mass index (BMI) was 23.68±3.80 kg/m2, and patients with sepsis (59.6%) occupied a higher proportion (Table 1).
Table 1.
Characteristics of sepsis patients with or without ARDS
Most patients were from EICU (68.0%) and medical emergency ward (20.8%). The most frequent comorbidities were hypertension (42.9%) and diabetes (24.2%). Lung (56.2%) and abdomen (14.9%) were the most common sources of sepsis (Table 1).
Sepsis patients had a lower PaO2/FiO2 ratio in the ARDS group than in the non-ARDS group (166.4±71.0 vs. 255.0±91.2, P<0.05). Besides, patients from the ARDS group had worse physical conditions, including higher illness severity scores and longer duration of mechanical ventilation (11 [6–24] days vs. 0 [0–0] days, P<0.05). Mortality rates were much higher in the ARDS group than in the non-ARDS group (41.7% vs. 15.5%, P<0.05). The duration of hospital and EICU stay didn’t show the statistical difference (Table 1).
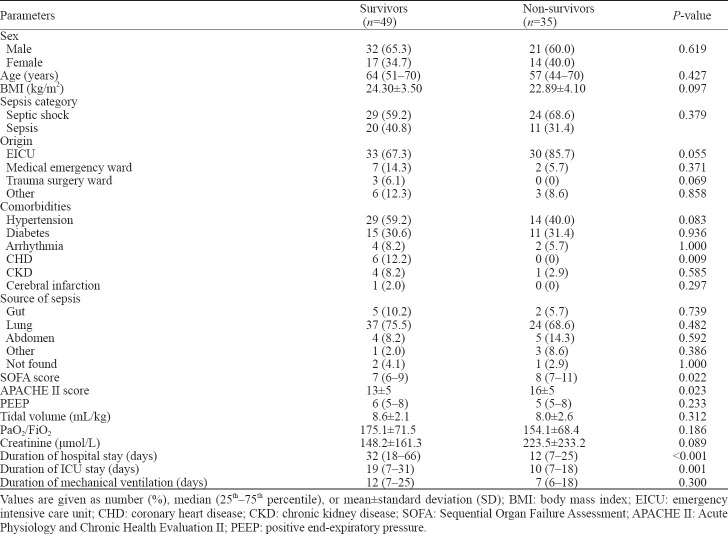
Non-survivors indeed had higher SOFA scores and APACHE II scores, as well as shorter duration of hospital and EICU stay than survivors. However, we didn’t find significant differences among tidal volumes, PaO2/FiO2 ratio, and duration of mechanical ventilation between the two groups (Table 2).
Table 2.
Characteristics of survivors and non-survivors
In the first 24 hours, mean fluid input volumes in the ARDS group were more than those in the non-ARDS group (50.1±22.1 mL/kg vs. 41.0±21.6 mL/kg, P<0.05). During the 7-day period, patients with ARDS had more mean daily fluid input volumes (46.3±20.8 mL/kg vs. 35.5±21.7 mL/kg, P<0.05) than those without ARDS, while mean daily output volumes between the two groups were similar (43.3±21.1 mL/kg vs. 40.7±21.3 mL/kg, P>0.05). Besides, we compared fluid input and output volumes between the two groups every day in seven days and found daily fluid input volumes were more in the ARDS group with significant differences while daily output volumes between the two groups didn’t show statistical differences except results on the 5th day.
Patients with ARDS showed daily positive net fluid balance for seven days, while those without ARDS showed daily negative net fluid balance since the second day with statistically significant differences (P<0.05) (Figure 2). We compared net fluid balance every day for seven days between the ARDS group and the non-ARDS group and found all results had significant differences (P<0.05).
Figure 2.
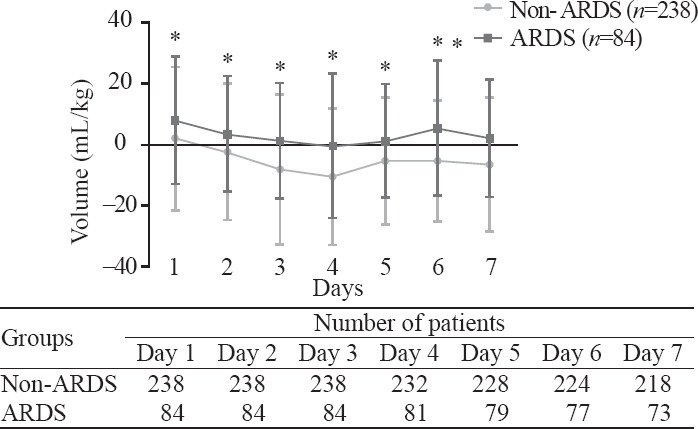
Mean fluid balance (mL/kg, mean±standard deviation) in sepsis patients with or without ARDS during seven continuous days after onset of sepsis. *Statistically significant difference at the P<0.05 level between two groups.
Our results showed that mean daily fluid input volumes were much lower in survivors than in non-survivors (43.2±16.7 mL/kg vs. 51.0±25.2 mL/kg, P<0.01) while output volumes were much higher in survivors (45.2±19.8 mL/kg vs. 40.2±22.7 mL/kg, P<0.05). We also compared daily fluid input and output for seven days between the two groups. Results of fluid input showed statistical differences only on the 4th and 6th day, and fluid output consequences didn’t show statistical differences except for the 7th day.
Survivors showed daily negative net fluid balance for seven days except for the first day, while non-survivors showed daily positive net fluid balance every day during the 7-day period with statistically significant differences (P<0.01) (Figure 3). We compared net fluid balance every day for seven days between survivors and non-survivors and found all results except the first day had statistically significant differences (P<0.05).
Figure 3.
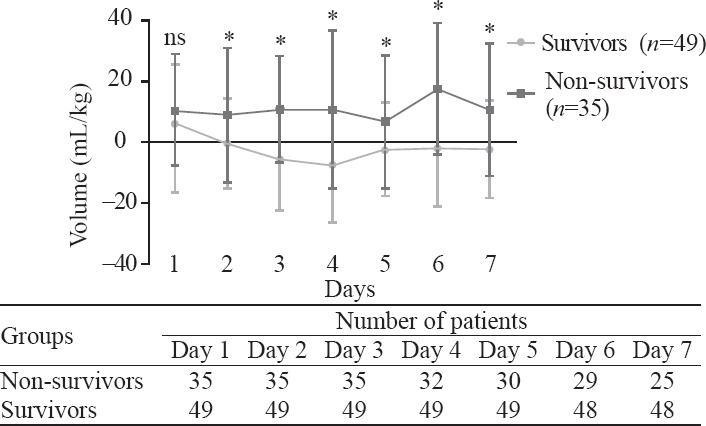
Mean fluid balance (mL/kg, mean±standard deviation) in survivors and non-survivors during seven continuous days in sepsis patients complicating with ARDS. *Statistically significant difference at the P<0.05 level between two groups. ns: not significant.
Finally, we used binary logistic regression analysis to measure which of the following indicators, including SOFA score, APACHE II score, and mean daily fluid balance, were independent prognostic factors. Results demonstrated that only mean fluid balance (P<0.01) was independently associated with the prognosis of sepsis patients with ARDS.
DISCUSSION
ARDS is a clinical syndrome characterized by refractory hypoxemia with high mortalities. A study conducted in 15 adult ICUs of Shanghai between 2001 and 2002 reported that ARDS incidence accounted for approximated 2% of all ICU admissions with a 90-day mortality rate of more than 70%.[21] The main treatment method of ARDS was etiology treatment, and fluid resuscitation was considered as an effective supportive treatment method of ARDS patients for increased capillary permeability and protein-rich fluid accumulation as the most important pathophysiological changes.[8] Many studies reported the relationship between fluid resuscitation and prognosis of ARDS, but results were contradictory. A large randomized study conducted by Wiedemann et al[10] compared a conservative and a liberal strategy of fluid management in 1,000 patients with acute lung injury, and concluded that patients from conservative fluid administration group had a shorter duration of mechanical ventilation and ICU stay while there was no significant difference in the primary outcome of the 60-day mortality. Another post-hoc analysis of a cohort of 313 children with ARDS revealed that positive fluid balance (in increments of 10 mL/[kg·day]) was associated with a significant increase in both mortality and prolonged duration of mechanical ventilation, independent of the presence of multiple organ system failure and the extent of oxygenation.[12] Rosenberg et al[13] assessed the fluid balance of 844 patients from 24 hospitals and 75 intensive care units and found that negative cumulative fluid balance at day 4 of acute lung injury was associated with significantly lower mortality. However, some investigators[11] found fluid resuscitation strategy might be a potential risk factor for long-term cognitive impairment. Sepsis, as the most frequent cause of ARDS, has a close relation with ARDS in critically ill patients. In 2002, an epidemiological study reported that sepsis was the most common cause of acute respiratory failure.[22] Another study conducted by the Korean Study Group on Respiratory Failure declared the incidence rate of sepsis-induced ARDS was 6.8% in Korea.[23] Fluid resuscitation is a lifesaving therapy for both sepsis and ARDS patients but only a few studies have been conducted to explore the relationship between fluid balance and prognosis of ARDS secondary to sepsis. Murphy et al[24] carried out a study containing 212 patients with septic shock complicated with ALI and found both early fluid management and late fluid management of septic shock complicated with ALI can influence patient outcomes. However, the term “acute lung injury (ALI)” was eliminated by the Berlin definition of ARDS in 2012.[20] Our study used the newest criteria of ARDS and mainly focused on ARDS patients secondary to sepsis. We included 322 patients, and ARDS patients accounted for 26.1%. In the group of sepsis complicated with ARDS, survivors had much lower input volumes and higher output volumes than non-survivors. Survivors showed daily negative net fluid balance since the second day, while non-survivors showed daily positive net fluid balance every day during the 7-day period with statistically significant differences except the first day. These results indicated that sufficient volume was necessary to resuscitate patients during the early time and excess fluid infusion should be avoided for fear of aggravation of pulmonary edema or injury of other organs. Negative fluid balance since the second day might be a prognostic factor for ARDS patients secondary to sepsis.
Besides fluid resuscitation, lung-protective ventilation was also an important strategy for ARDS patients, which could reduce mortality. Several clinical trials have demonstrated that lung-protective ventilation with low airway pressure and tidal volumes could prolong the survival time of patients with ARDS.[25-27] For patients with ARDS, the use of lower tidal volumes during ventilation was also an effective treatment that could reduce the release of inflammatory cytokines and detrimental lung stretch.[28-30] An early clinical trial reported the lower tidal volume was approximately 8.1 mL/kg compared with traditional tidal volume, which was approximately 12.2 mL/kg.[31] We didn’t find significant differences in tidal volumes between survivors and non-survivors because we employed low tidal volumes as a routine treatment for all ARDS patients. As for PEEP, there was a lack of an accurate method to define it. Some investigators thought higher PEEP values might improve the prognosis of ARDS patients.[32] We compared PEEP values between survivors and non-survivors and found no statistical difference. Acute kidney injury (AKI) was also reported to be an independent prognostic factor of patients with sepsis.[33,34] We wanted to clarify whether the positive fluid balance was due to AKI. Results showed that there was no statistical difference in creatinine on sepsis patients with or without ARDS (178.8±198.7 μmol/L vs. 163.3±223.5 μmol/L, P>0.05). We also compared the creatinine level between survivors and non-survivors of sepsis combined with ARDS. Although the creatinine level was higher in non-survivors than in survivors (223.5±233.2 μmol/L vs. 148.2±161.3 μmol/L), there was no statistical difference between the two groups (P>0.05). This indicated that positive fluid balance was not due to AKI and AKI was not an independent prognostic factor in our study.
Compared with sepsis patients without ARDS, the ARDS group did have higher SOFA and APACHE II scores, indicating a higher severity of the disease. However, we also knew that sepsis was a complex pathophysiological condition that might involve multiple organs. Due to the limited number of patients, we couldn’t make more detailed stratification to determine which factor had the most significant impact on prognosis. But our results showed that there was no difference in creatinine levels between the two groups, indicating AKI was not an important factor affecting the severity of the disease. However, the PaO2/FiO2 ratio was significantly lower, and the duration of mechanical ventilation was significantly longer in sepsis patients with ARDS, suggesting that the severity of ARDS might be an important factor affecting the severity of the entire disease, but not the only factor.
There were some limitations in our study. This was a monocentric and retrospective study, and enrolled patients were not enough. Besides, clinical data during the first several hours were not recorded which might be more important. And some clinical and laboratory parameters we omitted could influence our results in some degree.
CONCLUSIONS
We conclude that early negative fluid balance since the second day is independently associated with a better prognosis of ARDS patients secondary to sepsis. Early enough fluid resuscitation and late restricted fluid resuscitation might be beneficial to the prognosis of ARDS patients secondary to sepsis.
Footnotes
Funding: This work was supported by Shanghai Shenkang Hospital Development Center of China (SHDC12017116); Program for Outstanding Medical Academic, Shanghai Municipal Committee of Science and Technology (184119500900); Shanghai Municipal Commission of Health and Family Planning (2016ZB0206, ZHYY-ZXYJHZX-1-201702) and Shanghai Jiao Tong University School of Medicine (DLY201803) to Er-zhen Chen; Shanghai Municipal Commission of Health and Family Planning (201640089) to Zhi-tao Yang.
Ethical approval: The study was approved by the Ethics Committee of Ruijin Hospital (Reference number: 2017119).
Conflicts of interest: There are no competing interests involving this work.
Contributors: YMW wrote the first draft of this paper. All authors approved the final version.
REFERENCES
- 1.Vincent J-L, Marshall JC, Ñamendys-Silva SA, François B, Martin-Loeches I, Lipman J, et al. Assessment of the worldwide burden of critical illness:the Intensive Care Over Nations (ICON) audit. Lancet Respir Med. 2014;2(5):380–6. doi: 10.1016/S2213-2600(14)70061-X. [DOI] [PubMed] [Google Scholar]
- 2.Cohen J, Vincent JL, Adhikari NKJ, Machado FR, Angus DC, Calandra T, et al. Sepsis:a roadmap for future research. Lancet Infect Dis. 2015;15(5):581–614. doi: 10.1016/S1473-3099(15)70112-X. [DOI] [PubMed] [Google Scholar]
- 3.Rezoagli E, Fumagalli R, Bellani G. Definition and epidemiology of acute respiratory distress syndrome. Ann Transl Med. 2017;5(14):282. doi: 10.21037/atm.2017.06.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferguson ND, Kacmarek RM, Chiche JD, Singh JM, Hallett DC, Mehta S, et al. Screening of ARDS patients using standardized ventilator settings:influence on enrollment in a clinical trial. Intensive Care Med. 2004;30(6):1111–6. doi: 10.1007/s00134-004-2163-2. [DOI] [PubMed] [Google Scholar]
- 5.Fein AM, Calalang-Colucci MG. Acute lung injury and acute respiratory distress syndrome in sepsis and septic shock. Crit Care Clin. 2000;16(2):289–317. doi: 10.1016/s0749-0704(05)70111-1. [DOI] [PubMed] [Google Scholar]
- 6.Iscimen R, Cartin-Ceba R, Yilmaz M, Khan H, Hubmayr RD, Afessa B, et al. Risk factors for the development of acute lung injury in patients with septic shock:an observational cohort study. Crit Care Med. 2008;36(5):1518–22. doi: 10.1097/CCM.0b013e31816fc2c0. [DOI] [PubMed] [Google Scholar]
- 7.Stapleton RD, Wang BM, Hudson LD, Rubenfeld GD, Caldwell ES, Steinberg KP. Causes and timing of death in patients with ARDS. Chest. 2005;128(2):525–32. doi: 10.1378/chest.128.2.525. [DOI] [PubMed] [Google Scholar]
- 8.Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med. 2017;377(6):562–72. doi: 10.1056/NEJMra1608077. [DOI] [PubMed] [Google Scholar]
- 9.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 Pt 1):818–24. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 10.National Heart Lung and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–75. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 11.Mikkelsen ME, Christie JD, Lanken PN, Biester RC, Thompson BT, Bellamy SL, et al. The adult respiratory distress syndrome cognitive outcomes study:long-term neuropsychological function in survivors of acute lung injury. Am J Respir Crit Care Med. 2012;185(12):1307–15. doi: 10.1164/rccm.201111-2025OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flori HR, Church G, Liu KD, Gildengorin G, Matthay MA. Positive fluid balance is associated with higher mortality and prolonged mechanical ventilation in pediatric patients with acute lung injury. Crit Care Res Pract. 2011;2011:8541–42. doi: 10.1155/2011/854142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenberg AL, Dechert RE, Park PK, Bartlett RH. NIH NHLBI ARDS Network. Review of a large clinical series:association of cumulative fluid balance on outcome in acute lung injury:a retrospective review of the ARDSnet tidal volume study cohort. J Intensive Care Med. 2009;24(1):35–46. doi: 10.1177/0885066608329850. [DOI] [PubMed] [Google Scholar]
- 14.Cooke CR, Shah CV, Gallop R, Bellamy S, Ancukiewicz M, Eisner MD, et al. A simple clinical predictive index for objective estimates of mortality in acute lung injury. Crit Care Med. 2009;37(6):1913–20. doi: 10.1097/CCM.0b013e3181a009b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369(9):840–51. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 16.Silversides JA, Major E, Ferguson AJ, Mann EE, McAuley DF, Marshall JC, et al. Conservative fluid management or deresuscitation for patients with sepsis or acute respiratory distress syndrome following the resuscitation phase of critical illness:a systematic review and meta-analysis. Intensive Care Med. 2017;43(2):155–70. doi: 10.1007/s00134-016-4573-3. [DOI] [PubMed] [Google Scholar]
- 17.Sirvent JM, Ferri C, Baro A, Murcia C, Lorencio C. Fluid balance in sepsis and septic shock as a determining factor of mortality. Am J Emerg Med. 2015;33(2):186–9. doi: 10.1016/j.ajem.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 18.Alsous F, Khamiees M, DeGirolamo A, Amoateng-Adjepong Y, Manthous CA. Negative fluid balance predicts survival in patients with septic shock:a retrospective pilot study. Chest. 2000;117(6):1749–54. doi: 10.1378/chest.117.6.1749. [DOI] [PubMed] [Google Scholar]
- 19.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315(8):801–10. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.ARDS Definition Task Force. Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome:the Berlin Definition. JAMA. 2012;307(23):2526–33. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 21.Lu Y, Song Z, Zhou X, Huang S, Zhu D, Yang CBX, et al. A 12-month clinical survey of incidence and outcome of acute respiratory distress syndrome in Shanghai intensive care units. Intensive Care Med. 2004;30(12):2197–203. doi: 10.1007/s00134-004-2479-y. [DOI] [PubMed] [Google Scholar]
- 22.Esteban A, Anzueto A, Frutos F, Alía I, Brochard L, Stewart TE, et al. Characteristics and outcomes in adult patients receiving mechanical ventilation:a 28-day international study. JAMA. 2002;287(3):345–55. doi: 10.1001/jama.287.3.345. [DOI] [PubMed] [Google Scholar]
- 23.Kim WY, Hong SB. Sepsis and acute respiratory distress syndrome:recent update. Tuberc Respir Dis (Seoul) 2016;79(2):53–7. doi: 10.4046/trd.2016.79.2.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy CV, Schramm GE, Doherty JA, Reichley RM, Gajic O, Afessa B, et al. The importance of fluid management in acute lung injury secondary to septic shock. Chest. 2009;136(1):102–9. doi: 10.1378/chest.08-2706. [DOI] [PubMed] [Google Scholar]
- 25.Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338(6):347–54. doi: 10.1056/NEJM199802053380602. [DOI] [PubMed] [Google Scholar]
- 26.Acute Respiratory Distress Syndrome Network. Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–8. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 27.Villar J, Kacmarek RM, Perez-Mendez L, Aguirre-Jaime A. A high positive end-expiratory pressure, low tidal volume ventilatory strategy improves outcome in persistent acute respiratory distress syndrome:a randomized, controlled trial. Crit Care Med. 2006;34(5):1311–8. doi: 10.1097/01.CCM.0000215598.84885.01. [DOI] [PubMed] [Google Scholar]
- 28.Hickling KG, Walsh J, Henderson S, Jackson R. Low mortality rate in adult respiratory distress syndrome using low-volume, pressure-limited ventilation with permissive hypercapnia:a prospective study. Crit Care Med. 1994;22(10):1568–78. doi: 10.1097/00003246-199422100-00011. [DOI] [PubMed] [Google Scholar]
- 29.Hickling KG, Henderson SJ, Jackson R. Low mortality associated with low volume pressure limited ventilation with permissive hypercapnia in severe adult respiratory distress syndrome. Intensive Care Med. 1990;16(6):372–7. doi: 10.1007/BF01735174. [DOI] [PubMed] [Google Scholar]
- 30.Slutsky AS. Mechanical ventilation. Chest. 1993;104(6):1833–59. doi: 10.1378/chest.104.6.1833. [DOI] [PubMed] [Google Scholar]
- 31.Stewart TE, Meade MO, Cook DJ, Granton JT, Hodder RV, Lapinsky SE, et al. Evaluation of a ventilation strategy to prevent barotrauma in patients at high risk for acute respiratory distress syndrome. N Engl J Med. 1998;338(6):355–61. doi: 10.1056/NEJM199802053380603. [DOI] [PubMed] [Google Scholar]
- 32.Briel M, Meade M, Mercat A, Brower RG, Talmor D, Walter SD, et al. Higher vs. lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome:systematic review and meta-analysis. JAMA. 2010;303(9):865–73. doi: 10.1001/jama.2010.218. [DOI] [PubMed] [Google Scholar]
- 33.Bagshaw SM, George C, Bellomo R. Early acute kidney injury and sepsis:a multicentre evaluation. Crit Care. 2008;12(2):R47. doi: 10.1186/cc6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prowle JR. Sepsis-associated AKI. Clin J Am Soc Nephrol. 2018;13(2):339–42. doi: 10.2215/CJN.07310717. [DOI] [PMC free article] [PubMed] [Google Scholar]