Dear editor,
A new infectious disease outbreak, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is now spreading all around the world.[1] One of the most critical understandings of the current pandemic of the coronavirus disease 2019 (COVID-19) focuses on an imbalanced coagulation status. Based on the current evidence, we can divide the COVID-19-related hypercoagulable state into two categories[2]: patients without other pre-existing indications for anticoagulant therapy who develop a state of hypercoagulability linked to SARS-CoV-2 infection especially during the cytokine storm phase, and patients already on anticoagulation in whom we have to reevaluate their drug choices, dosages, and pharmacokinetics. At the same time, recent literature recommends the use of heparin in severe SARS-CoV-2 infection, both as thromboembolic prophylaxis and as an anticoagulant therapy, in light of possible anti-inflammatory and antiviral mechanisms and less pharmacological interferences.[3] This indication should be adopted in all patients except for those with prosthetic heart valves, in whom vitamin K antagonists continue to remain the drug of choice.[4] In this subgroup of patients, strict monitoring of PT-INR is required to maintain the therapeutic range. The purpose of this article is to underline the need for good quality evidence regarding the management of this high-risk category of the patient population.
CASE
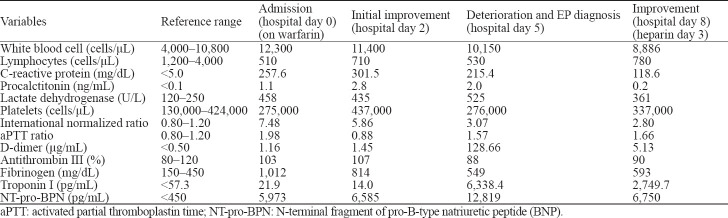
We reported a case of a 67-year-old Caucasian male who presented to our emergency department (ED) with complaints of fever and dyspnea for the last ten days. On the initial medical evaluation, the patient appeared hemodynamically stable, but with a respiratory rate of 36 breaths/minute and an oxygen saturation of 86% on room air. Blood gas analysis showed hypoxemic respiratory failure with a pressure of arterial oxygen/fraction of inspired oxygen (P/F) ratio of 229. Chest X-ray and lung ultrasound showed bilateral parenchymal thickening, compatible with changes of interstitial pneumonia. Blood tests showed lymphopenia and elevated C-reactive protein (CRP) and lactate dehydrogenase (LDH) (Table 1). Nasopharyngeal swab with polymerase chain reaction (PCR) confirmed positive for SARS-CoV-2 RNA-confirmed COVID-19-related pneumonia. He was therefore hospitalized in our High Dependency Unit (HDU) and treated with non-invasive continuous positive airway pressure (CPAP) ventilation, hydroxychloroquine, and antibiotics. His past medical history was unremarkable with the exception of aortic valve replacement with a mechanical prosthesis on the anticoagulant regimen of warfarin. Considering the prolonged baseline international normalized ratio (INR) and activated partial thromboplastin time (aPTT), we held warfarin until returning of the therapeutic range of INR, and we resumed warfarin with a target of INR at 2.5–3.5.
Table 1.
Laboratory data
After an initial improvement, we observed an exacerbation with a recurrence of fever and worsening respiratory exchanges, with a rise of inflammation markers and a dramatic increase of D-dimer levels. Computed tomographic pulmonary angiography demonstrated multiple pulmonary embolisms affecting segmental and sub-segmental branches of the pulmonary arteries. Subsequent treatment with intravenous unfractionated heparin (UFH) at an anti-coagulant dose obtained a marked improvement of both oxygenation and inflammation. No abnormalities were achieved after baseline screenings for cancer, liver failure, and congenital or acquired forms of thrombophilia. Moreover, laboratory findings were not consistent with disseminated intravascular coagulation (DIC).
DISCUSSION
We reported a case of pulmonary thromboembolism occurred despite a high INR-prothrombin time (PT) value during anticoagulant therapy with warfarin in a patient with SARS-CoV-2 pneumonia. Critically ill patients hospitalized in HDU exhibit high risk of venous thromboembolism (VTE) because of both individual patient-related risk factors and HDU-specific risk factors (immobilization, sedation, vasopressors, or use of central venous catheters). SARS-CoV-2 infection, especially in the case of a cytokine storm, probably causes a further imbalance of coagulation cascades without a clear increased incidence of disseminated intravascular coagulation.[5,6] Herein VTE seems to be connected with inflammation[7]: the clinical and laboratory improvements observed in our patient following intravenous heparin administration seem to support this hypothesis.[8]
Among SARS-CoV-2 pneumonia, patients with valve prostheses constitute an even higher risk category of hypercoagulation with little evidence regarding the optimal therapy for their management in this specific situation. We thus hypothesize that the monitoring of D-dimer levels associated with the inflammation parameters (e.g., CRP and IL-6) might be used for early recognition of SARS-CoV-2-associated prothrombotic pattern, despite therapeutic values of INR during warfarin treatment. Moreover, it is conceivable that viscoelastic testing (thromboelastography) may be useful in reflecting the actual coagulability status in patients with pre-existing coagulation disorders.[9]
In patients with prosthetic heart valves, low-molecular-weight heparin and UFH can be considered as a practical alternative, maintaining strict control of anti-FXa and aPTT, respectively, at the upper limit of the therapeutic range.[10]
Footnotes
Funding: None.
Ethical approval: This study was approved by the Ethical Committee of the hospital.
Conflicts of interest: There is no conflict of interest in this study.
Contributors: AA proposed the study, and wrote the first draft. All authors read and approved the final version.
REFERENCES
- 1.World Health Organization (WHO) Coronavirus disease (COVID-2019) situation reports. [Accessed on April 2 2020]. Available at https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports .
- 2.Testa S, Paoletti O, Giorgi-Pierfranceschi M, Pan A. Switch from oral anticoagulants to parenteral heparin in SARS-CoV-2 hospitalized patients. Intern Emerg Med. 2020;15(5):751–3. doi: 10.1007/s11739-020-02331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marietta M, Ageno W, Artoni A, de Candia E, Gresele P, Marchetti M, et al. COVID-19 and haemostasis:a position paper from Italian Society on Thrombosis and Haemostasis (SISET) Blood Transfus. 2020;18(3):167–9. doi: 10.2450/2020.0083-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitlock RP, Sun JC, Fremes SE, Rubens FD, Teoh KH. Antithrombotic and thrombolytic therapy for valvular disease:Antithrombotic Therapy and Prevention of Thrombosis, 9th ed:American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e576S–e600S. doi: 10.1378/chest.11-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han H, Yang L, Liu R, Liu F, Wu KL, Li J, et al. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020;58(7):1116–20. doi: 10.1515/cclm-2020-0188. [DOI] [PubMed] [Google Scholar]
- 6.Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–7. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, et al. COVID-19:consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–4. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–9. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spiezia L, Boscolo A, Poletto F, Cerruti L, Tiberio I, Campello E, et al. COVID-19-related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb Haemost. 2020;120(6):998–1000. doi: 10.1055/s-0040-1710018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fanikos J, Tsilimingras K, Kucher N, Rosen AB, Hieblinger MD, Goldhaber SZ. Comparison of efficacy, safety, and cost of low-molecular-weight heparin with continuous-infusion unfractionated heparin for initiation of anticoagulation after mechanical prosthetic valve implantation. Am J Cardiol. 2004;93(2):247–50. doi: 10.1016/j.amjcard.2003.09.054. [DOI] [PubMed] [Google Scholar]