Abstract
BACKGROUND:
Disturbance of mitochondrial fission and fusion (termed mitochondrial dynamics) is one of the leading causes of ischemia/reperfusion (I/R)-induced myocardial injury. Previous studies showed that mitochondrial aldehyde dehydrogenase 2 (ALDH2) conferred cardioprotective effect against myocardial I/R injury and suppressed I/R-induced excessive mitophagy in cardiomyocytes. However, whether ALDH2 participates in the regulation of mitochondrial dynamics during myocardial I/R injury remains unknown.
METHODS:
In the present study, we investigated the effect of ALDH2 on mitochondrial dynamics and the underlying mechanisms using the H9c2 cells exposed to hypoxia/reoxygenation (H/R) as an in vitro model of myocardial I/R injury.
RESULTS:
Cardiomyocyte apoptosis was significantly increased after oxygen-glucose deprivation and reoxygenation (OGD/R), and ALDH2 activation largely decreased the cardiomyocyte apoptosis. Additionally, we found that both ALDH2 activation and overexpression significantly inhibited the increased mitochondrial fission after OGD/R. Furthermore, we found that ALDH2 dominantly suppressed dynamin-related protein 1 (Drp1) phosphorylation (Ser616) and adenosine monophosphate-activated protein kinase (AMPK) phosphorylation (Thr172) but not interfered with the expression levels of mitochondrial shaping proteins.
CONCLUSIONS:
We demonstrate the protective effect of ALDH2 against cardiomyocyte H/R injury with a novel mechanism on mitochondrial fission/fusion.
KEYWORDS: Myocardial hypoxia/reoxygenation injury, Aldehyde dehydrogenase 2, Mitochondrial fission/fusion, Mitochondrial dynamics, Dynamin-related protein 1
INTRODUCTION
Acute myocardial infarction (AMI) is a leading cause of morbidity and mortality worldwide. Immediate reperfusion to the ischemic myocardium is the most effective way to limit infarct size and thereby reduce the risks of post-myocardial infarction (post-MI) complications and heart failure.[1] Paradoxically, however, reperfusion can result in independent myocardial damage, which has been known as ischemia/reperfusion (I/R) injury.[2,3] Therefore, innovative treatments targeting which provide cardioprotection against myocardial I/R injury will pave the way for the improvement of post-MI outcomes.
Accumulated evidence has shown that myocardial I/R injury could be attributed to the imbalance between mitochondrial fission and fusion.[4-6] Mitochondria, the center of energy supply, are vital to maintaining cellular homeostasis in the cardiomyocytes, the high energy-dependent organ. A paramount characteristic nature of mitochondria is that they undergo dynamic fission and fusion (termed mitochondrial dynamics) during the physiological processes, which are extremely important for the maintenance of a healthy mitochondrial population and cardiomyocyte survival.[7] However, the imbalance between mitochondrial fission and fusion always occurs in response to I/R stimuli, resulting in mitochondrial dysfunction and eventual apoptosis. The mitochondrial dynamics are reversible processes that depend on the coordination of mitochondrial shaping proteins including mitofusin 1 (Mfn1), mitofusin 2 (Mfn2) and optic atrophy 1 (Opa1) which are essential to fusion, as well as dynamin-related protein 1 (Drp1) and fission protein 1 (Fis1) which are the effectors of fission. Differential regulation of the expression or post-translational modification constitutes the core mechanisms for modulating mitochondrial morphology.[8] It has been known that Drp1 phosphorylation at Ser616 activated Drp1 and promoted its translocation to the mitochondria, thus contributing to mitochondrial fission; however, Drp1 phosphorylation at Ser637 led to the opposite outcome and preserved the elongated organelles.[8] In spite of the rapid development in the mechanistic insights into the mitochondrial dynamics in terms of the regulation of shaping proteins, other important regulatory nodes and their impact on the mitochondrial shaping proteins are not well understood.
Aldehyde dehydrogenase 2 (ALDH2), located at the mitochondrial matrix, has emerged as a key enzyme in protecting multiple organs against I/R injury, including heart, brain, and lung.[9-11] Accumulated evidence demonstrated that enhancement of ALDH2 activity or expression attenuated I/R-induced cardiomyocyte death while inhibition of ALDH2 activity or silencing its expression exacerbated myocardial I/R injury.[9,12,13] Additionally, our previous research showed that ALDH2 suppressed I/R-induced excessive mitophagy in cardiomyocytes, which is the downstream process of mitochondrial fission and is responsible for the elimination of shortened and damaged mitochondria.[13,14] ALDH2-mediated detoxification of cytotoxic aldehydes, including 4-hydroxy-2-nonenal, acrolein, and malondialdehyde, represents an established cardioprotective mechanism of ALDH2 during I/R injury. Despite this knowledge, it is yet unclear whether ALDH2 participates in the regulation of mitochondrial dynamics during myocardial I/R injury.
Here, the objectives of the study are: (1) to evaluate the effect of ALDH2 on cardiomyocyte apoptosis after I/R injury; (2) to investigate whether ALDH2 can inhibit I/R-induced excessive mitochondrial fission and the related connection with mitochondrial shaping proteins in an in vitro model of hypoxia/reoxygenation (H/R) in H9c2 cells.
METHODS
Cell culture
The H9c2 cells were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). H9c2 cells were cultured in Dulbecco’s modified Eagle’s media (DMEM, the glucose level of 4.5 g/L) (Gibco, Waltham, Massachusetts) supplemented with 10% (v/v) fetal bovine serum and 1% (v/v) penicillin/streptomycin (the complete media). Cells were maintained in a humidified incubator with 95% air/5% CO2 at 37 °C (normal oxygen concentration). H9c2 cells from passage 5 to passage 20 were used in this study.
Oxygen glucose deprivation/reoxygenation (OGD/R) model
H/R was induced by OGD/R. OGD was obtained by exposing cells in a hypoxic workstation (Don Whitley Scientific, Bradford, UK) containing 94% N2, 5% CO2, and 1% O2 at 37 °C with glucose concentration (1 g/L) and serum-deprived media. Cells were cultured in OGD media for 0.5 hours, 4 hours, 10 hours, 12 hours, or 14 hours, respectively; then cells were cultured under normal oxygen concentration with complete media for 1 hour. ALDH2 activator Alda-1 (Sigma-Aldrich, St. Louis, Missouri) with different concentrations (20 μM, 40 μM, or 80 μM) was added to the media 30 minutes before OGD according to previous studies.[13,15]
Adenovirus-mediated overexpression of ALDH2
The recombinant adenovirus with ALDH2 cDNA (Ad-ALDH2) and green fluorescent protein (Ad-GFP) as control were constructed by Hanbio Biotechnology (Shanghai, China). H9c2 cells were cultured with transfection reagent and infected with adenovirus at different multiplicities of infection (MOIs) for 12 hours, then cultured in complete media for an additional 48 hours before OGD/R procedure. Enhanced infection solution (Shanghai Genechem Co., Ltd, Shanghai, China) and 5 μg/mL polybrene (Shanghai Genechem) were used as transfection reagents. The MOI was determined by transfection efficiency evaluated by GFP expression under a fluorescence microscope (Olympus Corporation, Tokyo, Japan).
Evaluation of mitochondrial morphology
Mitochondria were stained with the MitoTracker Red CMXROS (Thermo Fisher Scientific, Waltham, Massachusetts), which is a red-fluorescent dye that could selectively label mitochondria in live cells. Briefly, H9c2 cells were incubated with 80 nM MitoTracker Red CMXROS in serum-free media at 37 °C for 30 minutes in the dark. Then the staining solution was replaced with fresh media, and cells were observed under a fluorescence microscope (Olympus Corporation). A hundred randomly chosen cells per group were designated as containing either predominantly (>50%) elongated or predominantly (>50%) fragmented mitochondria by three investigators blinded to the treatment. The percentage of cells with mitochondrial fragmentation was calculated by the ratio of cells with fragmented mitochondria to the total number of cells.[6,16,17]
Determination of cellular apoptosis
Apoptosis was measured by the terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) method and fluorescein isothiocyanate (FITC)-Annexin V/propidium iodide (PI) (FITC-Annexin V/PI) staining with flow cytometry assay.
For the TUNEL method, the In Situ Cell Death Detection Kit (Roche, Mannheim, Germany) was used according to the manufacturer’s instructions. Briefly, H9c2 cells grown on cover slip were fixed by 4% formaldehyde for 1 hour and permeated with 0.1% Triton X-100 for 2 minutes. Then, cells were incubated with TUNEL reaction mixture for 1 hour at 37 °C in the dark and incubated with 4’,6-diamidino-2-phenylindole (DAPI) (Boster, Wuhan, China) for 2 minutes. After observed under a fluorescence microscope (Olympus Corporation) and analyzed by Image-Pro Plus 6.0 (Media Cybernetics, Rockville, Maryland), the percentage of apoptotic cells was calculated by the ratio of cells with TUNEL-positive nuclei to the total nuclei.
For the FITC-Annexin V/PI staining assay, 105 cells were harvested and washed three times with phosphate-buffered saline. After incubated with FITC-Annexin V/PI (BD Biosciences, Franklin Lakes, New Jersey) for 15 minutes at room temperature in the dark, 1× binding buffer was added, and the cells were analyzed by flow cytometry (BD FACSCalibur Flow Cytometer, Franklin Lakes, New Jersey) within 1 hour. The percentage of apoptotic cells was calculated by the ratio of cells with FITC-Annexin V+PI– and FITC-Annexin V+PI+ to the total number of cells using FlowJo software (Ashland, Oregon).
Western blot analysis
Protein samples were separated on sodium dodecyl sulfate (SDS)-polyacrylamide gels and then transferred to polyvinylidene difluoride membranes (Merck Millipore, Darmstadt, Germany). After blocked with 5% nonfat dried milk, the membranes were incubated with primary antibodies overnight at 4 °C. The membranes were washed three times and then incubated with horseradish peroxidase-coupled secondary antibodies (1:10,000) for 1 hour at room temperature. The membranes were scanned by the Bio-Rad calibrated densitometer (Bio-Rad, Hercules, California) and analyzed by ImageJ software (U.S. National Institutes of Health, Bethesda, Marland). The primary antibodies used were ALDH2 (1:1,000, Abcam, Cambridge, UK), Drp1 (1:1,000, Abcam), Fis1 (1:1,000, Abcam), Mfn1 (1:1,000, Abcam), Mfn2 (1:1,000, Abcam), Opa1 (1:1,000, Abcam), phospho-Drp1 (Ser616) (1:1,000, Cell Signaling Technology, Danvers, Massachusetts), phospho-adenosine monophosphate-activated protein kinase α (AMPKα) (Thr172) (1:1,000, Cell Signaling Technology) and AMPKα (1:1,000, Cell Signaling Technology). All data were normalized by the internal control of β-actin.
Statistical analysis
Data were presented as means±standard error of the mean (SEM) from at least three independent experiments. An unpaired Student’s t-test was used for comparison between two groups. The one-way analysis of variances (ANOVA) followed by Tukey’s post hoc test was used for comparison among more than two groups. Two-sided P<0.05 was considered statistically significant. All analyses were performed with GraphPad Prism 7 (GraphPad Software, La Jolla, California).
RESULTS
ALDH2 activation inhibited H/R-induced apoptosis
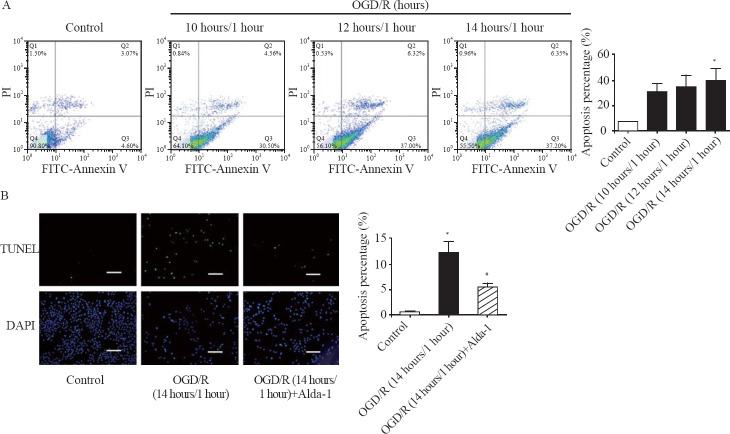
The percentage of apoptotic cells was increased in a time-dependent manner, with 7.6%, 31.1%, 34.6% and 39.6% in the control group, the OGD/R (10 hours/1 hour) group, the OGD/R (12 hours/1 hour) group, and the OGD/R (14 hours/1 hour) group, respectively, detected by flow cytometry analysis (Figure 1A). Notably, the apoptosis percentage was significantly increased by 4.2-fold in the OGD/R (14 hours/1 hour) group compared with that in the control group (Figure 1A). Consistently, the percentage of TUNEL-positive cardiomyocytes was markedly increased from 0.6% in the control group to 12.3% in the OGD/R (14 hours/1 hour) group (Figure 1B). However, ALDH2 activator Alda-1 significantly reduced the percentage of apoptotic cells by 55.9% compared with that in the OGD/R group (Figure 1B). Collectively, these results demonstrated that ALDH2 activation dramatically inhibited cellular apoptosis induced by H/R in H9c2 cells.
Figure 1.
ALDH2 activation inhibited OGD/R-induced cell apoptosis. A: representative photographs and quantitative data of apoptotic cardiomyocytes in the control group, the OGD/R (10 hours/1 hour) group, the OGD/R (12 hours/1 hour) group, and the OGD/R (14 hours/1 hour) group by FITC-Annexin V/PI staining using flow cytometry; B: representative photographs and quantitative data of apoptotic cardiomyocytes in the control group, the OGD/R (14 hours/1 hour) group, and the OGD/R (14 hours/1 hour) group+Alda-1 group by TUNEL staining; scale bar: 50 μM; data are means±standard error of mean (SEM); FITC-Annexin V/PI: fluorescein isothiocyanate (FITC)-Annexin V/propidium iodide (PI); TUNEL: terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling; DAPI: 4’,6-diamidino-2-phenylindole; *P<0.05 vs. control group; #P<0.05 vs. OGD/R (14 hours/1 hour) group.
Both ALDH2 activation and overexpression effectively inhibited H/R-induced mitochondrial fission
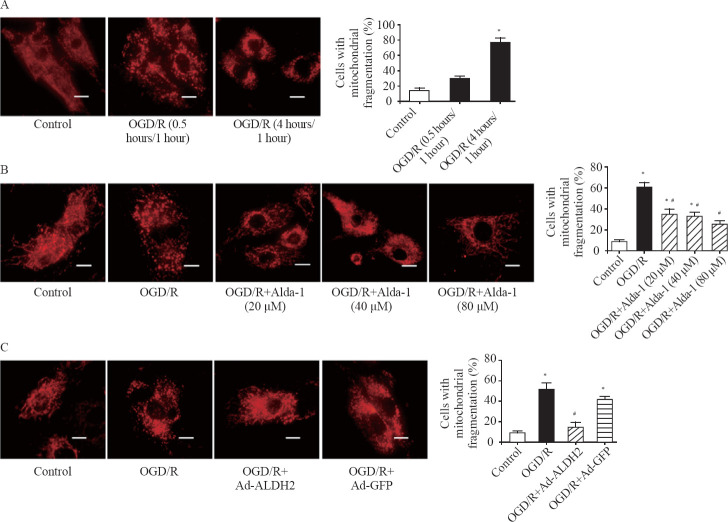
We found that mitochondria showed a long filamentous morphology in the control group while most mitochondria became smaller and punctate with significantly short in length in the OGD/R group (Figure 2A). The percentage of cells with mitochondrial fragmentation was increased in a time-dependent manner with 14.1%, 28.3%, and 73.4% in the control group, the OGD/R (0.5 hours/1 hour) group, and the OGD/R (4 hours/1 hour) group, respectively (Figure 2A). It was interesting to find that Alda-1 significantly reduced OGD/R-induced mitochondrial fission in a dose-dependent manner within the range of 20–80 μM (Figure 2B).
Figure 2.
Both ALDH2 activation and overexpression inhibited OGD/R-induced mitochondrial fission. A: representative photographs and the percentage of cells with mitochondrial fragmentation in the control group, the OGD/R (0.5 hours/1 hour) group, and the OGD/R (4 hours/1 hour) group by mitochondrial staining; scale bar: 25 μM; B: representative photographs and the percentage of cells with mitochondrial fragmentation in the control group, the OGD/R (4 hours/1 hour) group, and the OGD/R (4 hours/1 hour)+Alda-1 group in the concentration range of 20–80 μM by mitochondrial staining; scale bar: 25 μM; C: representative photographs and the percentage of cells with mitochondrial fragmentation in the control group, the OGD/R group, the OGD/R+Ad-ALDH2 group, and the OGD/R+Ad-GFP group; scale bar: 25 μM; Data are means±standard error of mean (SEM). *P<0.05 vs. control group; #P<0.05 vs. OGD/R group.
To further validate the effect of ALDH2 on mitochondrial morphology, we used the recombinant adenovirus with ALDH2 cDNA to enhance the expression of ALDH2. The MOI of 40 was determined by transfection efficiency evaluated by GFP expression, and the overexpression of ALDH2 was confirmed by Western blot analysis. We found that ALDH2 overexpression significantly reduced the percentage of cells with mitochondrial fragmentation by 72.3% compared with that in the OGD/R group (Figure 2C).
These results indicated that the enhancement of ALDH2 through both enzymatic activation and protein overexpression effectively protected mitochondria against excessive fission induced by H/R in H9c2 cells.
Phosphorylation levels of Drp1 (Ser616) and AMPK (Thr172) significantly reversed by ALDH2 under H/R
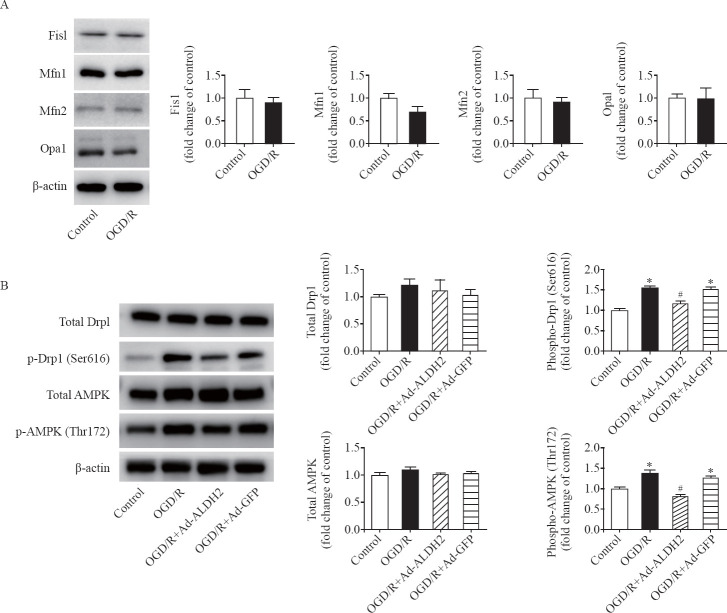
To gain insight into the mechanisms by which ALDH2 inhibited mitochondrial fission during OGD/R, we first detected the expression of each mitochondrial shaping proteins (Fis1, Mfn1, Mfn2, Opa1, and Drp1) during OGD/R. However, no changes in them were observed after OGD/R compared with those in the control group (Figures 3A and 3B). We then specifically detected the activated status of Drp1 after OGD/R and the effect of ALDH2 on it. The phosphorylation levels of Drp1 (Ser616) were increased by 56.0% after OGD/R compared with those in the control group. ALDH2 overexpression significantly decreased the phosphorylation levels of Drp1 to the levels in the control group (Figure 3B), suggesting ALDH2 dominantly restore the phosphorylation levels of Drp1 to the normal condition under H/R.
Figure 3.
ALDH2 suppressed Drp1 phosphorylation at Ser616 but not affected the expression levels of mitochondrial dynamics-regulating proteins. A: representative immunoblots and quantification of the expression levels of Fis1, Mfn1, Mfn2, and Opa1 in the control group and the OGD/R group; B: representative immunoblots and quantification of levels of total Drp1, phosphorylated Drp1 (p-Drp1) at Ser616, total AMPK, and phosphorylated AMPK (p-AMPK) at Thr172 in the control group, the OGD/R group, the OGD/R+Ad-ALDH2 group, and the OGD/R+Ad-GFP group; data are mean±standard error of mean (SEM); *P<0.05 vs. control group; #P<0.05 vs. OGD/R group.
Recently, the energy-sensing AMPK was shown to be required for cells to initiate rapid mitochondrial fragmentation under mitochondrial stress, which could recruit Drp1 from cytosol to mitochondrial surface to catalyze the fission reaction.[14] To examine whether ALDH2 has an effect on AMPK during OGD/R, we detected the expression of total AMPK and phosphorylation of AMPK. We found that the phosphorylation levels of AMPK (Thr172) were significantly increased by 37.7% after OGD/R (4 hours/1 hour) compared with those in the control group, whereas the expression levels of total AMPK were not affected under OGD/R. Moreover, ALDH2 overexpression markedly reduced the phosphorylation levels of AMPK compared with those in the OGD/R group (Figure 3B). Taken together, the findings suggested that ALDH2 may suppress excessive mitochondrial fission at least via reversing AMPK/Drp1 pathway under H/R (Figure 4).
Figure 4.
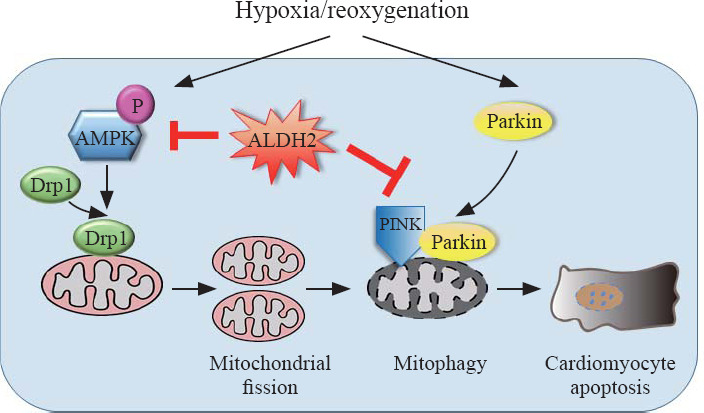
Schematic illustration of the protective effects of ALDH2 on H/R-induced cardiomyocyte damage. Under H/R, on the one hand, AMPK and Drp1 are activated by phosphorylation which triggers excessive mitochondrial fission, leading to apoptosis; on the other hand, demonstrated by our previous study,[13] PINK/Parkin-dependent mitophagy is activated, contributing to apoptosis; through suppressing either AMPK/Drp1 pathway or PINK/Parkin pathway, ALDH2 could exert cardioprotection effects.
DISCUSSION
In the present study, we demonstrate that the enhancement of ALDH2 reduces excessive mitochondrial fission and cardiomyocyte apoptosis under H/R in H9c2 cells. Moreover, we support a new mechanism by which ALDH2 inhibits mitochondrial fission at least via reversing AMPK/Drp1 pathway. To our knowledge, this is the first report to identify ALDH2 as an important mitochondrial shape-stabilizing factor in cardiomyocytes under H/R.
We observed increased mitochondrial fission after H/R in H9c2 cells, consistent with previous studies in which mitochondrial fission was increased in response to I/R injury both in vitro and in vivo.[6,8] The normal mitochondrial dynamics is necessary for maintaining cardiomyocyte survival, while excessive mitochondrial fission can contribute to I/R injury. It was reported that excessive mitochondrial fission resulted in the loss of ATP synthesis and increased reactive oxygen species production that led to cellular damage. Conversely, inhibiting excessive mitochondrial fission reduced reactive oxygen species, increased ATP production and O2 consumption in cardiomyocytes, and decreased infarct size after I/R.[18] Additionally, the imbalance between mitochondrial fission and fusion could result in a higher susceptibility to mitochondrial permeability transition pore (mPTP) opening, leading to an activation of the apoptotic pathway by the release of caspase family proteins and cell death at the time of myocardial reperfusion.[19] In reverse, the elongated mitochondria may accommodate a greater burden of mitochondrial calcium load and oxidative stress before undergoing mPTP opening compared with fragmented mitochondria.[6]
We found that ALDH2 effectively inhibited mitochondrial fission under H/R. Although it has been recognized that ALDH2 is located at the mitochondrial matrix for more than three decades,[20] few studies investigate the role of ALDH2 in the physiological process of mitochondrial damage or dysfunction. This pilot in vitro study revealed the encouraging findings that ALDH2 could preserve mitochondrial morphology and attenuate cardiomyocyte apoptosis. In our previous study, we found that ALDH2 suppressed H/R-induced phosphatase and tensin homolog (PTEN)-induced putative kinase 1 (PINK1)/Parkin-dependent mitophagy, further inhibiting myocardial apoptosis.[13] Combined with the present study, our results indicate that ALDH2 has influences on at least two facets of mitochondrial dynamics, mitochondrial fission and mitophagy, therefore exerting cardioprotection effect under H/R (Figure 4).
It is interesting to find that the phosphorylation levels of Drp1 (Ser616) were increased after H/R, which were dramatically decreased by ALDH2. This could explain why ALDH2 inhibited mitochondrial fission. Drp1 is mainly a cytosolic protein and is dynamically recruited to mitochondrial membranes under mitochondrial stress where it oligomerizes and drives membrane constriction in a GTP-dependent manner, inducing mitochondrial fission.[21,22] Drp1 activation during I/R injury can be post-translationally regulated by sumoylation/desumoylation and phosphorylation/dephosphorylation of serine residues.[8,23] I/R could up-regulate the expression of Drp1 and other important proteins regulating mitochondrial fission such as mitochondrial fission factor (MFF), mitochondrial fission regulator 1 (Mtfr1), and mitochondrial protein 18 kDa (MTP18).[24-27] Studies showed that I/R could down-regulate the expression of mitochondrial fusion proteins, including Mfn1, Mfn2, and Opa1.[6,28] However, we did not find the expression changes of these mitochondrial shaping proteins, including Drp1, Fis1, Mfn1, Mfn2, or Opa1. The inconsistency can be explained by the difference in the severity of ischemia or the cell types.
Drp1 was reported to have five phosphorylation sites at serine 585, 616, 637, 656, and 693, which were phosphorylated by different kinases. The phosphorylation status of Ser616, Ser637, and Ser656 was showed to participate in the regulation of Drp1 activation and mitochondrial fission in cardiomyocytes upon I/R.[8] The phosphorylation of Drp1 at Ser616 by cyclin-dependent kinase (CDK) 1/Cyclin B or CDK5 promoted mitochondrial fission, whereas Drp1 phosphorylation at serine 637 by protein kinase A (PKA) induced the detachment of Drp1 from mitochondria and inhibited mitochondrial fission.[21,29] Drp1 has become the target of regulating mitochondrial dynamics for the treatment of I/R-induced cardiomyocyte injury.[8] Studies showed that mitochondrial division inhibitor 1 (mdivi-1), a pharmacological inhibitor of Drp1, decreased the cells with mitochondrial fragmentation, inhibited mitochondrial permeability transition pore opening, reduced cell death, and attenuated myocardial infarct size induced by I/R injury.[6,29,30] Additionally, studies found that inhibiting the expression of Drp1 by siRNA could reduce cardiomyocyte apoptosis and infarct size in response to I/R.[31,32] In our study, we found that ALDH2 could restore the levels of phospho-Drp1 (Ser616) to normal conditions, providing convincing evidence supporting the role of ALDH2 in mitochondrial protection.
We also found that ALDH2 decreased the elevated expression of phospho-AMPK (Thr172) after H/R. AMPK is a central metabolic sensor and could be activated during myocardial I/R injury, which modulates glucose and fatty acid metabolism, mitochondrial function, endoplasmic reticulum stress, autophagy, and apoptosis.[33] For mitochondria, the activation of AMPK could rapidly trigger mitochondrial fission through recruiting Drp1 to the outer membrane of mitochondria.[14] Additionally, the activation of AMPK was shown to promote mitophagy of those mitochondrial fragments through its direct phosphorylation and activation of the highly conserved autophagy kinase ULK1 (Atg1 in yeast).[34,35] Although the AMPK activation has been reported to be an adaptive response following cardiac I/R injury, some studies[36,37] found that the AMPK-dependent acceleration of fatty acid oxidation occurred at the expense of glucose oxidation, and had the potential to be detrimental in the setting of I/R. Jaswal et al[38] found that inhibition of p38 MAPK and AMPK restored adenosine-induced cardioprotection in hearts stressed by antecedent ischemia by altering glucose utilization. Additionally, the role of AMPK in apoptosis is not clear, with both anti-apoptotic and pro-apoptotic actions being reported.[39,40] In this study, we didn’t investigate the direct effect of AMPK under cardiac H/R injury but our findings suggest that ALDH2 may suppress excessive mitochondrial fission at least via reversing AMPK/Drp1 pathway under H/R. ALDH2 was also reported to exert protective effects against ethanol cardiotoxicity through inhibition of AMPK phosphorylation-dependent autophagy.[41] Although the exact mechanism of how ALDH2 inhibits AMPK phosphorylation is still elusive, it is likely that ALDH2 affects the phosphorylated AMPK through detoxification of aldehydes, which is widely accepted as initiators to various changes of signaling pathways in myocardial I/R injury. For example, ALDH2 offered beneficial effects against myocardial I/R injury by restoring interrupted AMPK and Akt signaling induced by cytotoxic aldehyde.[12] Li et al[42] found that ALDH2 suppressed chronic pain-related myocardial I/R injury through inhibiting SIRT1 carbonylative inactivation and impairment of liver kinase B1 (LKB1)-AMPK interaction.
CONCLUSIONS
In conclusion, our data reveal a novel mechanism of the cardioprotective effect of ALDH2 on H/R injury, highlighting the role of ALDH2 in inhibiting mitochondrial fission. However, our study results are limited to the cardiomyocytes in vitro, and the present findings need to be verified in animal experiments for further investigation.
Footnotes
Funding: This study was supported by the National Key R&D Program of China (2017YFC0908700, 2017YFC0908703), National Natural Science Foundation of China (81772036, 81671952, 81873950, 81873953, 81570401, 81571934), National S&T Fundamental Resources Investigation Project (2018FY100600, 2018FY100602), Taishan Pandeng Scholar Program of Shandong Province (tspd20181220), Taishan Young Scholar Program of Shandong Province (tsqn20161065, tsqn201812129), Key R&D Program of Shandong Province (2018GSF118003) and the Fundamental Research Funds of Shandong University (2018JC011).
Ethical approval: This study was approved by the Ethics Committee of Shandong University.
Conflicts of interest: The authors declare that there is no conflict of interest.
Contributors: RZ and MYX contributed equally to this work. YGC, JLW, and RZ contributed to the conception and design. RZ, MYX, BSL, WJW, XHF, and BYZ contributed to the acquisition, analysis, and interpretation of data. All authors reviewed and approved the final version.
REFERENCES
- 1.Vogel B, Claessen BE, Arnold SV, Chan D, Cohen DJ, Giannitsis E, et al. ST-segment elevation myocardial infarction. Nat Rev Dis Primers. 2019;5(1):39. doi: 10.1038/s41572-019-0090-3. [DOI] [PubMed] [Google Scholar]
- 2.Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury:a neglected therapeutic target. J Clin Invest. 2013;123(1):92–100. doi: 10.1172/JCI62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357(11):1121–35. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 4.Dorn GW., 2nd Mitochondrial dynamics in heart disease. Biochim Biophys Acta. 2013;1833(1):233–41. doi: 10.1016/j.bbamcr.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Archer SL. Mitochondrial dynamics—mitochondrial fission and fusion in human diseases. N Engl J Med. 2013;369(23):2236–51. doi: 10.1056/NEJMra1215233. [DOI] [PubMed] [Google Scholar]
- 6.Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 2010;121(18):2012–22. doi: 10.1161/CIRCULATIONAHA.109.906610. [DOI] [PubMed] [Google Scholar]
- 7.Dorn GW., 2nd Evolving concepts of mitochondrial dynamics. Annu Rev Physiol. 2019;81:1–17. doi: 10.1146/annurev-physiol-020518-114358. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Liu X. Novel insights into the role of mitochondrial fusion and fission in cardiomyocyte apoptosis induced by ischemia/reperfusion. J Cell Physiol. 2018;233(8):5589–97. doi: 10.1002/jcp.26522. [DOI] [PubMed] [Google Scholar]
- 9.Chen CH, Budas GR, Churchill EN, Disatnik MH, Hurley TD, Mochly-Rosen D. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science. 2008;321(5895):1493–5. doi: 10.1126/science.1158554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo JM, Liu AJ, Zang P, Dong WZ, Ying L, Wang W, et al. ALDH2 protects against stroke by clearing 4-HNE. Cell Res. 2013;23(7):915–30. doi: 10.1038/cr.2013.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding J, Zhang Q, Luo Q, Ying Y, Liu Y, Li Y, et al. Alda-1 attenuates lung ischemia-reperfusion injury by reducing 4-hydroxy-2-nonenal in alveolar epithelial cells. Crit Care Med. 2016;44(7):e544–52. doi: 10.1097/CCM.0000000000001563. [DOI] [PubMed] [Google Scholar]
- 12.Ma H, Guo R, Yu L, Zhang Y, Ren J. Aldehyde dehydrogenase 2 (ALDH2) rescues myocardial ischaemia/reperfusion injury:role of autophagy paradox and toxic aldehyde. Eur Heart J. 2011;32(8):1025–38. doi: 10.1093/eurheartj/ehq253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ji W, Wei S, Hao P, Xing J, Yuan Q, Wang J, et al. Aldehyde dehydrogenase 2 has cardioprotective effects on myocardial ischaemia/reperfusion injury via suppressing mitophagy. Front Pharmacol. 2016;7:101. doi: 10.3389/fphar.2016.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toyama EQ, Herzig S, Courchet J, Lewis TL, Loson OC, Hellberg K, et al. Metabolism. AMP-activated protein kinase mediates mitochondrial fission in response to energy stress. Science. 2016;351(6270):275–81. doi: 10.1126/science.aab4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu B, Zhang R, Wei S, Yuan Q, Xue M, Hao P, et al. ALDH2 protects against alcoholic cardiomyopathy through a mechanism involving the p38 MAPK/CREB pathway and local renin-angiotensin system inhibition in cardiomyocytes. Int J Cardiol. 2018;257:150–59. doi: 10.1016/j.ijcard.2017.11.094. [DOI] [PubMed] [Google Scholar]
- 16.Brooks C, Wei Q, Cho SG, Dong Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest. 2009;119(5):1275–85. doi: 10.1172/JCI37829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang K, Long B, Zhou LY, Liu F, Zhou QY, Liu CY, et al. CARL lncRNA inhibits anoxia-induced mitochondrial fission and apoptosis in cardiomyocytes by impairing miR-539-dependent PHB2 downregulation. Nat Commun. 2014;5:3596. doi: 10.1038/ncomms4596. [DOI] [PubMed] [Google Scholar]
- 18.Disatnik MH, Ferreira JC, Campos JC, Gomes KS, Dourado PM, Qi X, et al. Acute inhibition of excessive mitochondrial fission after myocardial infarction prevents long-term cardiac dysfunction. J Am Heart Assoc. 2013;2(5):e000461. doi: 10.1161/JAHA.113.000461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maneechote C, Palee S, Chattipakorn SC, Chattipakorn N. Roles of mitochondrial dynamics modulators in cardiac ischaemia/reperfusion injury. J Cell Mol Med. 2017;21(11):2643–53. doi: 10.1111/jcmm.13330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braun T, Bober E, Singh S, Agarwal DP, Goedde HW. Evidence for a signal peptide at the amino-terminal end of human mitochondrial aldehyde dehydrogenase. FEBS Lett. 1987;215(2):233–6. doi: 10.1016/0014-5793(87)80152-7. [DOI] [PubMed] [Google Scholar]
- 21.Tilokani L, Nagashima S, Paupe V, Prudent J. Mitochondrial dynamics:overview of molecular mechanisms. Essays Biochem. 2018;62(3):341–60. doi: 10.1042/EBC20170104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kraus F, Ryan MT. The constriction and scission machineries involved in mitochondrial fission. J Cell Sci. 2017;130(18):2953–60. doi: 10.1242/jcs.199562. [DOI] [PubMed] [Google Scholar]
- 23.Ong SB, Hausenloy DJ. Mitochondrial morphology and cardiovascular disease. Cardiovasc Res. 2010;88(1):16–29. doi: 10.1093/cvr/cvq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Long B, Wang K, Li N, Murtaza I, Xiao JY, Fan YY, et al. miR-761 regulates the mitochondrial network by targeting mitochondrial fission factor. Free Radic Biol Med. 2013;65:371–79. doi: 10.1016/j.freeradbiomed.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang K, Zhang DL, Long B, An T, Zhang J, Zhou LY, et al. NFAT4-dependent miR-324-5p regulates mitochondrial morphology and cardiomyocyte cell death by targeting Mtfr1. Cell Death Dis. 2015;6:e2007. doi: 10.1038/cddis.2015.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang K, Gan TY, Li N, Liu CY, Zhou LY, Gao JN, et al. Circular RNA mediates cardiomyocyte death via miRNA-dependent upregulation of MTP18 expression. Cell Death Differ. 2017;24(6):1111–20. doi: 10.1038/cdd.2017.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao L, Zhuang J, Wang Y, Zhou D, Zhao D, Zhu S, et al. Propofol ameliorates H9c2 cells apoptosis induced by oxygen-glucose deprivation and reperfusion injury via inhibiting high levels of mitochondrial fusion and fission. Front Pharmacol. 2019;10:61. doi: 10.3389/fphar.2019.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cellier L, Tamareille S, Kalakech H, Guillou S, Lenaers G, Prunier F, et al. Remote ischemic conditioning influences mitochondrial dynamics. Shock. 2016;45(2):192–7. doi: 10.1097/SHK.0000000000000500. [DOI] [PubMed] [Google Scholar]
- 29.Sharp WW, Fang YH, Han M, Zhang HJ, Hong Z, Banathy A, et al. Dynamin-related protein 1 (Drp1)-mediated diastolic dysfunction in myocardial ischemia-reperfusion injury:therapeutic benefits of Drp1 inhibition to reduce mitochondrial fission. FASEB J. 2014;28(1):316–26. doi: 10.1096/fj.12-226225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Veeranki S, Tyagi SC. Mdivi-1 induced acute changes in the angiogenic profile after ischemia-reperfusion injury in female mice. Physiol Rep. 2017;5(11):e13298. doi: 10.14814/phy2.13298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zepeda R, Kuzmicic J, Parra V, Troncoso R, Pennanen C, Riquelme JA, et al. Drp1 loss-of-function reduces cardiomyocyte oxygen dependence protecting the heart from ischemia-reperfusion injury. J Cardiovasc Pharmacol. 2014;63(6):477–87. doi: 10.1097/FJC.0000000000000071. [DOI] [PubMed] [Google Scholar]
- 32.Din S, Mason M, Volkers M, Johnson B, Cottage CT, Wang Z, et al. Pim-1 preserves mitochondrial morphology by inhibiting dynamin-related protein 1 translocation. Proc Natl Acad Sci U S A. 2013;110(15):5969–74. doi: 10.1073/pnas.1213294110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qi D, Young LH. AMPK:energy sensor and survival mechanism in the ischemic heart. Trends Endocrinol Metab. 2015;26(8):422–9. doi: 10.1016/j.tem.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331(6016):456–61. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13(2):132–41. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dyck JR, Lopaschuk GD. AMPK alterations in cardiac physiology and pathology:enemy or ally? J Physiol. 2006;574(Pt 1):95–112. doi: 10.1113/jphysiol.2006.109389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kudo N, Gillespie JG, Kung L, Witters LA, Schulz R, Clanachan AS, et al. Characterization of 5'AMP-activated protein kinase activity in the heart and its role in inhibiting acetyl-CoA carboxylase during reperfusion following ischemia. Biochim Biophys Acta. 1996;1301(1-2):67–75. doi: 10.1016/0005-2760(96)00013-6. [DOI] [PubMed] [Google Scholar]
- 38.Jaswal JS, Gandhi M, Finegan BA, Dyck JR, Clanachan AS. Inhibition of p38 MAPK and AMPK restores adenosine-induced cardioprotection in hearts stressed by antecedent ischemia by altering glucose utilization. Am J Physiol Heart Circ Physiol. 2007;293(2):H1107–14. doi: 10.1152/ajpheart.00455.2007. [DOI] [PubMed] [Google Scholar]
- 39.Capano M, Crompton M. Bax translocates to mitochondria of heart cells during simulated ischaemia:involvement of AMP-activated and p38 mitogen-activated protein kinases. Biochem J. 2006;395(1):57–64. doi: 10.1042/BJ20051654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bairwa SC, Parajuli N, Dyck JR. The role of AMPK in cardiomyocyte health and survival. Biochim Biophys Acta. 2016;1862(12):2199–210. doi: 10.1016/j.bbadis.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 41.Ge W, Guo R, Ren J. AMP-dependent kinase and autophagic flux are involved in aldehyde dehydrogenase-2-induced protection against cardiac toxicity of ethanol. Free Radic Biol Med. 2011;51(9):1736–48. doi: 10.1016/j.freeradbiomed.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li C, Sun W, Gu C, Yang Z, Quan N, Yang J, et al. Targeting ALDH2 for therapeutic interventions in chronic pain-related myocardial ischemic susceptibility. Theranostics. 2018;8(4):1027–41. doi: 10.7150/thno.22414. [DOI] [PMC free article] [PubMed] [Google Scholar]