Abstract
Lithium has been previously demonstrated to alleviate cognitive impairment caused by neurodegenerative diseases and acute brain injuries; however, the specific mechanism remains elusive. In the present study, the C57BL/6 mouse model of spatial cognitive impairment induced by repeated cerebral ischemia-reperfusion was established. Morris water maze test was performed to evaluate the levels of spatial cognitive impairment. Nissl staining was used to observe any morphological alterations, whilst western blotting was performed to measure the expression levels of microtubule-associated protein light chain 3 (LC3) and Beclin1 in addition to mTOR phosphorylation. LiCl was found to significantly improve spatial learning and memory impairments according to data from the Morris water maze test. Nissl staining indicated that LiCl inhibited neuronal damage in the CA1 region of the hippocampus. Additionally, LiCl increased mTOR phosphorylation, reduced beclin1 expression and reduced the LC3 II/I expression ratio. Taken together, these findings suggest that LiCl may alleviate the spatial cognitive impairment induced by repeated cerebral ischemia-reperfusion. This observation may be attributed to the inhibition of excessive autophagy by LiCl through mTOR signaling activation.
Keywords: lithium chloride, spatial cognitive impairment, repeated cerebral ischemia-reperfusion mouse model
Introduction
Cerebral ischemia-reperfusion (IR) injury is a complex pathophysiological process that primarily occurs during transient ischemic attack, cardiac arrest, shock and severe head trauma (1). Due to the high metabolic rates, neurons in the hippocampal CA1 region are particularly vulnerable to the deleterious effects of ischemic insult, resulting in further damage to learning and memory (2). Although the exact mechanism of which remains unclear, a number of pathological processes, including disturbances in energy metabolism and oxidative stress have been reported to be involved in neuronal damage, which are mainly caused by excessive levels of reactive oxygen species (ROS), excitotoxicity, necrotic and apoptotic cell death (3-6).
Autophagy is a process in which dysfunctional organelles and proteins are degraded, in a manner that is dependent on the type of lysosome involved. Under physiological conditions, autophagy operating at basal levels ensures that damaged organelles and abnormally aggregated proteins are removed (7). However, aberrant regulation of autophagy has been previously associated with neurodegenerative disorders, stroke, cancer and heart diseases, where excessive activation of autophagy can trigger cell death (8). Cellular processes in neurons are highly dynamic, such that cell growth, synaptic formation or synaptic plasticity are highly dependent on the adequate regulation of protein synthesis and degradation (9). However, excessive autophagy can trigger extensive degradation of essential proteins and organelles, leading to the collapse of cellular functions. Accumulating evidence have suggested that excessive autophagy induced by cerebral IR is detrimental to neurons that eventually leads to neuronal cell death (10-12). Additionally, as a consequence of prolonged, elevated cell stress, it could be hypothesized that excessive activation of autophagy is persistent. Therefore, prevention of excessive autophagy in cerebral IR-induced hippocampal neuronal damage may alleviate cognitive impairment.
Lithium, a commonly applied therapeutic agent for depression (13), has been demonstrated to exert protective effects on neuronal cells both in vitro and in vivo (14). A previous study has reported that lithium chloride can ameliorate deficits in spatial learning and memory induced by repeated cerebral IR injury by increasing Akt and GSK3β phosphorylation in addition to increasing BDNF expression in hippocampal neurons (15,16). Therefore, the present study was designed to investigate whether LiCl can alleviate cognitive deficits as a result of repeated cerebral IR by targeting the autophagy signaling pathway in mouse models, where the potential mechanism was explored.
Materials and methods
Animals
Male C57BL/6 mice (n=120, weight, 22-26 g; age, 10-12 weeks) were purchased from Beijing Vital River Laboratory Animal Technology Company (license no. SCXK 2013-011). All animal usage, humane endpoints and procedures complied with the laboratory animal management regulations of the Ministry of Science and Technology in China [1988] no. 134 (17-19). Ethics approval was obtained from the Ethics Committee of Hebei General Hospital (approval no. 201909).
Animal health and behavior were monitored twice daily, once in the morning and once in the evening. The animals were kept in the Animal Center of Hebei People's Hospital (Shijiazhuang, China) under 12-h light: Dark cycle at an ambient temperature of 22-25˚C and humidity range of 30-70%. Anesthesia (50 mg/kg pentobarbital sodium, intraperitoneal) was applied prior to model establishment and before the animals were sacrificed for specimen collection.
Repeated cerebral IR model
Repeated cerebral IR was induced according to the protocols described previously with some modifications (15), GCI was achieved by isolation of the common carotid arteries through a ventral midline incision in the neck, followed by bilateral occlusion of the arteries using cotton thread for 5 min. Tension on the cotton thread was removed for 10 min and then initiated again for another 5 min. At the end of the occlusion, the cotton thread was completely removed, the arteries were visually inspected for reflow and the midline incision was sutured. Sham-operated animals underwent a similar procedure, with the exception of arterial occlusion. The mice were anesthetized by an intraperitoneal injection with 50 mg/kg pentobarbital sodium, following which they were subjected to cerebral ischemia for 20 min by bilateral common carotid artery occlusion, with this treatment repeated three more times at 10-min intervals. Sham-operated mice underwent identical surgical procedures but their bilateral common carotids were exposed and not occluded. Body temperature was maintained at 37.0±0.5˚C throughout the operation. All moribund animals were provided with pain relief and none were found dead following surgery.
Experimental design
The present study was divided into two parts.
Time-course expression of mTOR, p-mTOR, microtubule-associated protein light chain 3(LC3) II/I and Beclin1 in the hippocampus following repeated cerebral IR. In total, 72 mice were randomly assigned into six groups after repeated cerebral IR: i) Sham-control; ii) day 1; iii) day 3; iv) day 7; v) day 14; and vi) day 28 (n=8 in each group). The mice were euthanized 1, 3, 7, 14 and 28 days following model establishment, where the number of mice that survived surgery in each group was 12, 11, 12, 10 and 11, respectively. In total, 6 mice were randomly selected from each group for experimentation, the remaining mice were used for subsequent analyses.
LiCl treatment and Morris water maze test. LiCl (Sigma-Aldrich; Merck KGaA) was dissolved in normal saline (NS) and was injected intraperitoneally. A total of 48 mice were divided into 4 groups, with n=12 for each group that underwent the following treatment: i) Sham group, where the mice received an equal volume of NS for 14 days; ii) vehicle group, where the mice received an equal volume of NS for 14 days following repeated cerebral IR; iii) Pre-Li group, where the mice received 84 mg/kg LiCl for 7 days before repeated cerebral IR and then were treated with NS for 14 d after cerebral IR; and iv) Li group, where the mice received 2 mmol/kg LiCl for 14 days after repeated cerebral IR. The optimal concentration of LiCl was determined as previously described (16). The number of mice that survived surgery was 12 in the sham operation group, 11 in the model group, 11 in the Pre-Li group and 10 in the Li group, where intolerance to surgery was the cause of death. Mice in each group were euthanized following the completion of the water maze test. The duration of the entire experiment was 20 days, consisting of 14 days after model establishment followed by 6 days of Morris water maze testing.
Morris water maze test
The Morris water maze test is a widely applied procedure for assessing cognitive impairment associated with the hippocampal region (20). The maze consisted of a large circular pool that was 120 cm in diameter, 60 cm in height and 45 cm in depth. The temperature of the water was maintained at 23±1˚C. Within the maze, a platform was located at the center of the north-east quadrant of the water tank, positioned ~1 cm below the water surface. To start each experiment, the mouse was gently placed in the water at the edge of a randomly selected quadrant but not the north-east quadrant, with its nose pointing toward the wall. The time taken for the mouse to navigate to the platform was automatically recorded using the DigBehv Animal Behavior Video-tracking System (JLBehv-MWMG-1; Shanghai Jiliang Software Technology Co., Ltd.). Each experiment was considered as complete when the mouse mounts the platform or at 60 sec regardless. If the mice failed to reach the platform within 60 sec, they were guided manually to the platform and allowed to stay on it for 30 sec. Its escape latency, the time taken to reach the platform, was accepted as 60 sec. The experiments were conducted six times daily for five consecutive days.
On the sixth day of the test, a probe trial was conducted without the platform. At the start of the experiment, the mice were placed in a quadrant which did not contain the platform. The mice were allowed to swim freely for 60 sec in the pool. The time spent in the target quadrant where the platform had been located was recorded.
Histopathology
Immediately after the behavioral tests, mice were anesthetized with pentobarbital sodium followed by transcardial perfusion with 4% paraformaldehyde. Following perfusion, the brain tissues were immediately removed and immersed in 4% paraformaldehyde at 4˚C, for 48 h before being embedded in paraffin. Coronal brain sections were subsequently cut into 5-µm thick sections and underwent Nissl staining with 1% toluidine blue at 37˚C for 10 min, according to manufacturer's protocols (cat. no. C0117; Beyotime Institute of Biotechnology). Two slides that were selected from the same site of each mouse were observed under light microscopy (Nikon 50i; Nikon).
Western blot analysis
Anesthetized mice (n=6 at each time point in each group) were first sacrificed by cervical dislocation. The hippocampal tissues were then removed, frozen in liquid nitrogen and stored at -80˚C until protein extraction for western blot analysis. Tissues were first homogenized in ice-cold RIPA buffer (Beijing Solarbio Science & Technology Co., Ltd.) and incubated on ice for 30 min, which were then centrifuged at 4˚C at a speed of 12,000 x g for 10 min. The dialyzed supernatants were obtained after centrifugation. Protein concentration was determined using the Bicinchoninic acid method (Pierce; Thermo Fisher Scientific, Inc.). A total of 40 µg of protein were electrophoresed on a 7-12% SDS-polyacrylamide gel at 80 V for 2 h and then transferred 2 h onto PVDF membranes (EMD Millipore). Membranes were blocked with 5% skimmed milk for 2-4 h at room temperature and then incubated overnight with the following primary antibodies at 4˚C: mTOR (1:2,000 dilution; Signalway Antibody LLC; cat. no. 41187), p-mTOR (1:1,000 dilution; Anbo Biotechnology Co., Ltd.; cat. no. ab109268), LC3B (1:200 dilution; Abgent, Inc.; cat. no. AP1806a) and Beclin1 (1:200 dilution; Abgent, Inc.; cat. no. AP52755). The next day, following three rounds of washing with TBS supplemented with 10% Tween-20 (TBST), the membranes were incubated with the anti-rabbit horseradish peroxidase-conjugated secondary antibody (1:10,000 dilution; Proteintech Group Inc., cat. no. HRP-60008) for 2 h at room temperature. Following a further three rounds of washing with TBST and one round of TBS wash, the protein on the membranes were detected using the ECL method (High sensitive ECL luminescence reagent; Sangon Biotech Co., Ltd.; cat. no. C500044), where β-actin (Proteintech Group, Inc.; cat. no. HRP-60008) was served as a loading control. The bands were scanned and analyzed using the ImageJ analysis software (version 1.30v; National Institutes of Health).
Statistical analysis
Data were expressed as the mean ± standard error of the mean and processed using the GraphPad Prism 8.0 (GraphPad Software, Inc.) software. For the Morris water maze test, latencies to find the platform were analyzed using repeated-measures ANOVA followed by Tukey's test for multiple comparisons among different groups. All the other data were analyzed using one-way analysis of variance (ANOVA) followed by Tukey's test for intergroup comparisons. P<0.05 was considered to indicate a statistically significant difference.
Results
LiCl treatment attenuates learning and memory impairment in repeated cerebral ischemia-reperfusion mouse models
Following the final LiCl injection, all mice were trained in the Morris water maze for 5 days consecutively to assess their learning capabilities, followed by memory examination on the final day prior to sacrifice. Mice in the vehicle group required significantly more time to find the platform (P<0.01) compared with those in the sham-operated group. Mice in the Pre-Li and Li groups demonstrated shorter mean latencies compared with those in the vehicle group (P<0.05, Pre-Li vs. Li groups; P<0.01, Pre-Li or Li group vs. the vehicle group) on day 3. Over the next two days, mice in the Pre-Li and Li groups exhibited significantly shorter escape latencies compared with those in the vehicle group (both P<0.01).
During the probe trial without the platform, the vehicle group spent significantly less time in the target quadrant compared with those in the sham-operated group (P<0.01). Compared with the vehicle group, mice in the Pre-Li group spent significantly more time in the target quadrant (P<0.01). Although mice in the Li group also spent more time in the target quadrant compared with mice in vehicle group, no significant difference was observed between the two groups (Fig. 1B).
Figure 1.
Morris water maze experiment data, showing the effect of LiCl treatment on spatial cognitive impairment in mice following repeated cerebral ischemia. (A) Escape latency, showing the time taken for the mice to find the hidden platform. For repeated-measures ANOVA, all mice exhibited progressive declines in the escape latency, where the main factors of day [F(4,160)=72.736; P<0.01] and group [F(3,40)=46.731; P<0.01] were found to be statistically significant. (B) Time spent in the target quadrant in the probe trail. n=12 for sham group, n=11 for vehicle and Pre-Li groups, and n=10 for Li group, F(3,40)=22.636. *P<0.01 vs. Sham and #P<0.01 vs. Vehicle. Li, LiCl.
LiCl treatment reverses morphologic alterations in the repeated cerebral IR mice
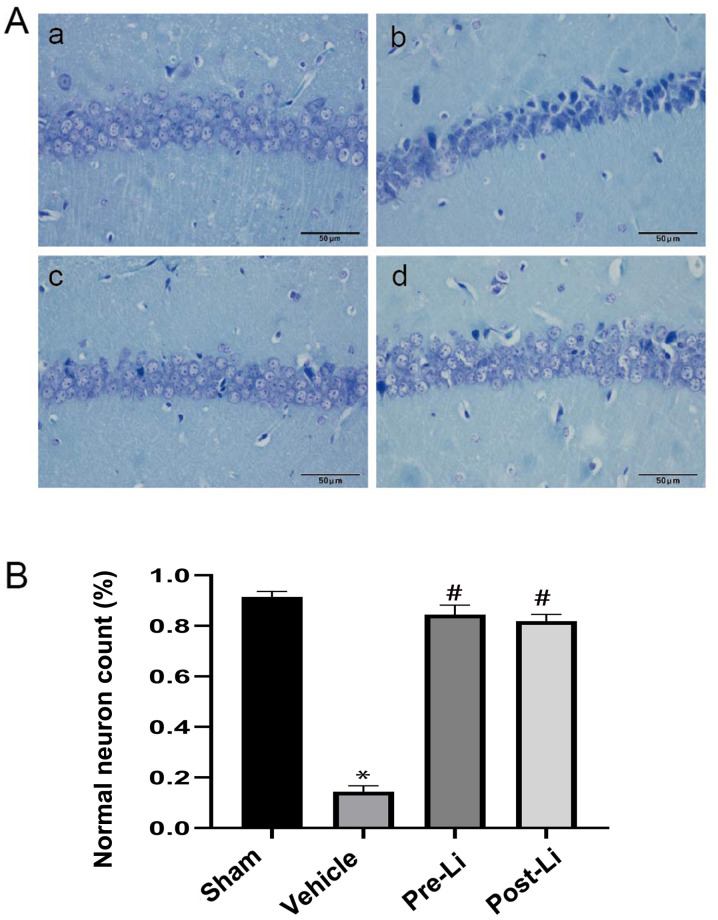
Nissl staining results demonstrated that the pyramidal neurons in the hippocampus CA1 region in the sham group were tightly ranked in order, where the neurons were clear and moderate in size, synonymous with normal microstructure (Fig. 2A-a). Compared with the sham group, hippocampal samples from the vehicle group exhibited fewer pyramidal neurons that are loosely arranged with neuronal shrinkage (Fig. 2A-b). Administration of LiCl reversed the morphologic changes and increased the number of normal neurons (Fig. 2).
Figure 2.
Effects of LiCl on morphological changes induced by repeated cerebral ischemia in the hippocampal CA1 area. (A) Representative images of the morphological changes in the hippocampal CA1 region. (a) Sham group. (b) The vehicle groups. (c) The Pre-Li group and (d) the Li group. Scale bar, 50 µm. (B) Quantification of the number of normal neurons over the total number of cells in each group. Data are presented as the ratio of normal neurons/total cells. *P<0.01 vs. Sham and #P<0.01 vs. vehicle. Li, LiCl.
Expression of LC3II/I, Beclin1 and mTOR phosphorylation after repeated cerebral IR in mice
Western blotting was used to measure the expression levels of LC3II/I and Beclin1 in addition to mTOR phosphorylation after repeated cerebral ischemia. There was no marked difference in total mTOR expression between the six groups (Fig. 3). mTOR phosphorylation was significantly increased in both pre-Li and Li treatment groups compared with that in the sham group (P<0.01). The gradual increase in LC3II/I and Beclin1 expression suggested that autophagy was in progress, which rose to the highest level on day 14 but was reduced on day 28 (Fig. 3). The reductions in LC3II/I and Beclin1 expression 28 days after model establishment compared with those on day 14 suggested the occurrence of hippocampal repair, but the level of autophagy remained higher compared with that in the sham group (Fig. 3).
Figure 3.
Effects of repeated cerebral ischemia on the expression of proteins associated with autophagy in mouse hippocampal tissues. (A) Representative western blotting images and (B) quantitative analysis of LC3II/I ratios, Beclin1 expression and (C) mTOR phosphorylation in hippocampal tissues from mice in the Sham group and 1, 3, 7, 14 and 28 days after repeated ischemia. β-actin was used as internal control. *P<0.01 vs. Sham.
LiCl regulates p-mTOR phosphorylation, Beclin1 expression and the LC3II/I ratio in the mouse hippocampus
The LC3II/I ratio, Beclin1 expression and p-mTOR phosphorylation were examined using western blot analysis 14 days after repeated cerebral IR. p-mTOR phosphorylation was significantly increased in the Pre-Li and Li groups compared with that in the vehicle group (both P<0.01; Fig. 4). The LC3II/I ratio and Beclin1 expression were significantly reduced in the Pre-Li and Li groups compared with that in the vehicle group (both P<0.01; Fig. 4).
Figure 4.
Effects of LiCl on the expression of proteins associated with autophagy in mouse hippocampal tissues following repeated cerebral ischemia. (A) Representative western blot images and (B) quantitative analysis of LC3II/I ratios, Beclin1 expression and (C) mTOR phosphorylation 14 days after repeated cerebral IR in the hippocampus, in the presence or absence of LiCl treatment. β-actin was used as an internal control. *P<0.01 vs. Sham and #P<0.01 vs. vehicle. Li, LiCl.
Discussion
Although a number of studies have previously demonstrated that cerebral IR may induce excessive autophagy in the early stages (21-23), whether mechanisms associated with autophagy serve a persistent role in cognitive impairment as a result of repeated cerebral IR remains unknown. Therefore, in the present study, a repeated cerebral IR mouse model was used to monitor the long-term dynamic changes in the expression of autophagy markers. In a previous study (15), 2 mmol/kg LiCl has been demonstrated to exert protective effects against spatial cognitive impairment in mouse repeated cerebral IR injury models by increasing Akt and GSK3β phosphorylation in the hippocampus. A subsequent study demonstrated that pre- and post-treatment with LiCl can alleviate spatial learning and memory impairment (16). In the present study, pre-administration with LiCl at a dose of 2 mmol/kg significantly protected against impaired learning and memory impairment as indicated by markedly shorter escape latencies and longer time spent in the target quadrant. However, post-treatment with 2 mmol/kg LiCl partially alleviated spatial cognitive impairment with slightly shorter escape latencies, whilst both LiCl pre- and post-treatment effectively increased mTOR phosphorylation and inhibited autophagy.
Although repeated cerebral ischemia attack followed by reperfusion injury does not result in limb movement disorder, damage to the neurons in the hippocampus can occur (24). The hippocampus is known as the predominant location for the regulation of learning and memory, where neuronal damage is closely associated with cognitive impairment (25). The C57Bl/6 mouse model used for the present study are particularly vulnerable to brain ischemia due to poorly-developed posterior communicating arteries (26-28). In previous studies (15,16,29), 20-min cerebral ischemia for three times at 10-min intervals induced neuronal injury in the hippocampus, resulting in impaired learning and memory. For the present study, identical models were established, in which mice in the repeated cerebral IR group exhibited longer escape latencies and spent significantly less time in the target quadrant in the Morris water maze test compared with those in the sham group, suggesting that the successful model can be applied to evaluate the effects of LiCl on spatial cognition following repeated cerebral IR.
Although the mood stabilizing effects of lithium is well documented, a number of studies have suggested that the benefits of lithium can also be extended to increased protection against cognitive impairment induced by acute cerebrovascular disease (30-34). Additional studies have also demonstrated that treatment or pre-treatment with lithium improved impairments in spatial learning and memory as a result of global cerebral IR (35,36). In the present study, pre-Li treatment significantly improved the impaired learning and memory deficits which were consistent with the aforementioned studies, but Li treatment only partially alleviated the cognitive deficits. This difference could be due to the treatment time (16). In a previous study (15), it was demonstrated that repeated cerebral IR mice treated with 2 mmol/kg LiCl for 28 days significantly reduced escape latency and increased time spent in the target quadrant in Morris water maze experiments. Coupling this previous observation with data from the present study, LiCl treatment may be an effective protective agent against cognitive impairment in a time-dependent manner.
In spite of controversies regarding the role of autophagy in neuronal cell death, accumulating evidence have reported that autophagy is activated in focal or global cerebral ischemia or hypoxia-ischemia models in rats and mice (10,37,38). Beclin1 knockdown has been demonstrated to reduce infarct volume, histological injury and neurological deficits induced by focal cerebral ischemia in adult rats (39). These findings support the notion that autophagy serves a detrimental role in acute cerebral ischemia (40). Yang et al (41) found that inhibition of autophagy by 3-methyladenine (3-MA) could markedly reduce infarct size, edema formation and neurological deficits after permanent middle cerebral artery occlusion. However, the extent to which autophagy is involved in repeated cerebral IR-induced cognitive impairment has not been elucidated in those previous studies. In the present study, a time course experiment was performed by repeating cerebral ischemia for 20 min, with 10 min reperfusions three times. The animals were then euthanized as various time points (1, 3, 7, 14 and 28 days) after the treatment and hippocampal tissue samples were collected to measure the expression of proteins associated with autophagy. The expression of Beclin1 and LC3-II in the hippocampus increased significantly from day 1 to day 28 after cerebral IR, with maximal induction observed on day 14. This observation suggest that increased autophagy is linked with neuron damage in the hippocampus and subsequent impairments in spatial cognitive function following cerebral IR. Following LiCl administration, autophagy was reduced to a certain extent along with a reduction in the population of dying neurons; however, it remains unclear whether the reduction of autophagy was the sole cause of this effect.
The mTOR signaling pathway is a central regulator of protein synthesis and is considered to be a key controller of cell growth, cell survival and autophagy (42-44). In mammalian cells, mTOR assembles into two functionally distinct protein complexes-mTORC1 and mTORC2. mTORC1 can serve as a regulator of autophagy and is highly sensitive to rapamycin (45). Although the role of mTOR in ischemic injury is unclear, mTOR has been previous demonstrated to exhibit neuroprotective properties (46,47). In focal cerebral IR models of neonatal rats, 3-MA exerted neuroprotective effects even after 4 h ischemia (48). The present study revealed that mTOR phosphorylation in the hippocampus increased from days 1 to 28 following cerebral IR. Additionally, LiCl treatment significantly increased mTOR phosphorylation whilst reducing the ratio of LC3II/I and Beclin1 expression, suggesting that mTOR may serve as a therapeutic target in cases of repeated cerebral IR injury. However, by contrast, induction of autophagy by upregulating mTOR phosphorylation has been previously reported to promote neuronal survival (49). It could be hypothesized that the discrepancy between these findings may be associated with distinct cell types and models across different studies. In animal experiments, the mice were treated with LiCl prior to the establishment of IR model with the aim of minimizing the off-target effects of model establishment whilst maximizing the potential effects of LiCl (16). However, application of this methodology in clinical practice remains to be explored and requires further validation.
For future studies, supplementing a group of positive controls treated with autophagic inhibitors, to detect the mechanism of autophagy in repeated IR-induced injury, could be used to further assist in elucidating the potential mechanism of lithium chloride on autophagy.
In summary, the present study suggests that excessive autophagy can contribute to neuronal injury in a repeated cerebral IR mouse model. Additionally, LiCl inhibits excessive autophagy by activating mTOR signaling and ameliorating repeated IR-induced the neuronal injury in the hippocampus. LiCl may serve as a promising therapeutic agent for the treatment of cognitive impairment caused by cerebral IR injury.
Acknowledgements
Not applicable.
Funding
This project was supported by the National Natural Science Foundation of China (grant no. 81241037), the Natural Science Foundation of Hebei (grant no. H2013307046) and the Major Medical Scientific Research Subject of Hebei (grant no. zd2013005).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
YX designed the study, performed the experiments mainly including the surgery, behavioral test and the western blot, carried out the statistical analysis, and prepared the manuscript. MF, WJ, WAL and YJ were involved in the experiments such as the surgeries and behavioral tests. YD, XJ, JX and NM performed data collection. PL was responsible for the supervision of the entire project. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Ethics approval was obtained from the Ethics Committee of Hebei General Hospital (Shijiazhuang, China; approval no. 201909).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Chatauret N, Badet L, Barrou B, Hauet T. Ischemia-reperfusion: From cell biology to acute kidney injury. Prog Urol. 2014;24 (Suppl 1):S4–S12. doi: 10.1016/S1166-7087(14)70057-0. [DOI] [PubMed] [Google Scholar]
- 2.Erfani S, Khaksari M, Oryan S, Shamsaei N, Aboutaleb N, Nikbakht F, Jamali-Raeufy N, Gorjipour F. Visfatin reduces hippocampal CA1 cells death and improves learning and memory deficits after transient global ischemia/reperfusion. Neuropeptides. 2015;49:63–68. doi: 10.1016/j.npep.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Huang XP, Tan H, Chen BY, Deng CQ. Combination of total astragalus extract and total Panax notoginseng saponins strengthened the protective effects on brain damage through improving energy metabolism and inhibiting apoptosis after cerebral ischemia-reperfusion in mice. Chin J Integr Med. 2017;23:445–452. doi: 10.1007/s11655-015-1965-0. [DOI] [PubMed] [Google Scholar]
- 4.Guo C, Wang S, Duan J, Jia N, Zhu Y, Ding Y, Guan Y, Wei G, Yin Y, Xi M, Wen A. Protocatechualdehyde protects against cerebral ischemia-reperfusion-induced oxidative injury via protein kinase Cepsilon/Nrf2/HO-1 Pathway. Mol Neurobiol. 2017;54:833–845. doi: 10.1007/s12035-016-9690-z. [DOI] [PubMed] [Google Scholar]
- 5.Zhang S, Zhang Y, Li H, Xu W, Chu K, Chen L, Chen X. Antioxidant and anti-excitotoxicity effect of Gualou Guizhi decoction on cerebral ischemia/reperfusion injury in rats. Exp Ther Med. 2015;9:2121–2126. doi: 10.3892/etm.2015.2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yaidikar L, Thakur S. Punicalagin attenuated cerebral ischemia-reperfusion insult via inhibition of proinflammatory cytokines, up-regulation of Bcl-2, down-regulation of Bax, and caspase-3. Mol Cell Biochem. 2015;402:141–148. doi: 10.1007/s11010-014-2321-y. [DOI] [PubMed] [Google Scholar]
- 7.Shintani T, Klionsky DJ. Autophagy in health and disease: A double-edged sword. Science. 2004;306:990–995. doi: 10.1126/science.1099993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kourtis N, Tavernarakis N. Autophagy and cell death in model organisms. Cell Death Differ. 2009;16:21–30. doi: 10.1038/cdd.2008.120. [DOI] [PubMed] [Google Scholar]
- 9.Lee JA. Neuronal autophagy: A housekeeper or a fighter in neuronal cell survival? Exp Neurobiol Mar. 2012;21:1–8. doi: 10.5607/en.2012.21.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi R, Weng J, Zhao L, Li XM, Gao TM, Kong J. Excessive autophagy contributes to neuron death in cerebral ischemia. CNS Neurosci Ther. 2012;18:250–260. doi: 10.1111/j.1755-5949.2012.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rami A, Langhagen A, Steiger S. Focal cerebral ischemia induces upregulation of Beclin 1 and autophagy-like cell death. Neurobiol Dis. 2008;29:132–141. doi: 10.1016/j.nbd.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Wen YD, Sheng R, Zhang LS, Han R, Zhang X, Zhang XD, Han F, Fukunaga K, Qin ZH. Neuronal injury in rat model of permanent focal cerebral ischemia is associated with activation of autophagic and lysosomal pathways. Autophagy. 2008;4:762–769. doi: 10.4161/auto.6412. [DOI] [PubMed] [Google Scholar]
- 13.Machado-Vieira R, Zanetti MV, DE Sousa RT, Soeiro-DE-Souza MG, Moreno RA, Busatto GF, Gattaz WF. Lithium efficacy in bipolar depression with flexible dosing: A six-week, open-label, proof-of-concept study. Exp Ther Med. 2014;8:1205–1208. doi: 10.3892/etm.2014.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, Yang Q, Bennett C, Harada Y, Stankunas K, et al. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by MAPK and GSK3 to regulate cell growth. Cell. 2006;126:955–968. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 15.Fan M, Song C, Wang T, Li L, Dong Y, Jin W, Lu P. Protective effects of lithium chloride treatment on repeated cerebral ischemia-reperfusion injury in mice. Neurol Sci. 2015;36:315–321. doi: 10.1007/s10072-014-1943-x. [DOI] [PubMed] [Google Scholar]
- 16.Fan M, Jin W, Zhao H, Xiao Y, Jia Y, Yin Y, Jiang X, Xu J, Meng N, Lv P. Lithium chloride administration prevents spatial learning and memory impairment in repeated cerebral ischemia-reperfusion mice by depressing apoptosis and increasing BDNF expression in hippocampus. Behav Brain Res. 2015;291:399–406. doi: 10.1016/j.bbr.2015.05.047. [DOI] [PubMed] [Google Scholar]
- 17.Ogden BE, Pang William W, Agui T, Lee BH. Laboratory animal laws, regulations, guidelines and standards in China mainland, Japan, and Korea. ILAR J. 2016;57:301–311. doi: 10.1093/ilar/ilw018. [DOI] [PubMed] [Google Scholar]
- 18.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. Osteoarthritis Cartilage. 2012;20:256–260. doi: 10.1111/j.1939-165X.2012.00418.x. [DOI] [PubMed] [Google Scholar]
- 19. Science and Technology in China. Nature 162: 17, 1948. [Google Scholar]
- 20.Vorhees CV, Williams MT. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang JY, Xia Q, Chu KT, Pan J, Sun LN, Zeng B, Zhu YJ, Wang Q, Wang K, Luo BY. Severe global cerebral ischemia-induced programmed necrosis of hippocampal CA1 neurons in rat is prevented by 3-methyladenine: A widely used inhibitor of autophagy. J Neuropathol Exp Neurol. 2011;70:314–322. doi: 10.1097/NEN.0b013e31821352bd. [DOI] [PubMed] [Google Scholar]
- 22.Zheng Y, Hou J, Liu J, Yao M, Li L, Zhang B, Zhu H, Wang Z. Inhibition of autophagy contributes to melatonin-mediated neuroprotection against transient focal cerebral ischemia in rats. J Pharmacol Sci. 2014;12:354–364. doi: 10.1254/jphs.13220fp. [DOI] [PubMed] [Google Scholar]
- 23.Li L, Tian J, Long MK, Chen Y, Lu J, Zhou C, Wang T. Protection against experimental stroke by Ganglioside GM1 is associated with the inhibition of autophagy. PLoS One. 2016;11(e0144219) doi: 10.1371/journal.pone.0144219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chuang EH, Iwasaki K, Mishima K, Egashira N, Fujiwara M. Repeated cerebral ischemia induced hippocampal cell death and impairments of spatial cognition in the rat. Life Sci. 2002;72:609–619. doi: 10.1016/s0024-3205(02)02269-5. [DOI] [PubMed] [Google Scholar]
- 25.Voss JL, Bridge DJ, Cohen NJ, Walker JA. A Closer look at the hippocampus and memory. Trends Cogn Sci. 2017;21:577–588. doi: 10.1016/j.tics.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wellons JC III, Sheng H, Laskowita DT, Mackensen GB, Pearlstein RD, Warner DS. A comparison of strain-related susceptibility in two murine recovery models of global cerebral ischemia. Brain Res. 2000;868:14–21. doi: 10.1016/s0006-8993(00)02216-2. [DOI] [PubMed] [Google Scholar]
- 27.Fujii M, Hara H, Meng W, Vonsattel JP, Huang Z, Moskowitz MA. Strain-related differences in susceptibility to transient forebrain ischemia in SV-129 and C57black/6 mice. Stroke. 1997;28:1805–1810. doi: 10.1161/01.str.28.9.1805. [DOI] [PubMed] [Google Scholar]
- 28.Maeda K, Hata R, Hossmann KA. Regional metabolic disturbances and cerebrovascular anatomy after permanent middle cerebral artery occlusion in C57black/6 and SA129 mice. Neurobiol Dis. 1999;6:101–108. doi: 10.1006/nbdi.1998.0235. [DOI] [PubMed] [Google Scholar]
- 29.Wang T, Lv P, Jin W, Zhang H, Lang J, Fan M. Protective effect of donepezil hydrochloride on cerebral ischemia/reperfusion injury in mice. Mol Med Rep. 2014;9:509–514. doi: 10.3892/mmr.2013.1823. [DOI] [PubMed] [Google Scholar]
- 30.Fiorentini A, Rosi MC, Grossi C, Luccarini I, Casamenti F. Lithium improves hippocampal neurogenesis, neuropathology and cognitive functions in APP mutant mice. PLoS One. 2010;5(e14382) doi: 10.1371/journal.pone.0014382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Contestabile A, Greco B, Ghezzi D, Tucci V, Benfenati F, Gasparini L. Lithium rescues synaptic plasticity and memory in Down syndrome mice. J Clin Invest. 2013;123:348–361. doi: 10.1172/JCI64650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li N, Zhang X, Dong H, Zhang S, Sun J, Qian Y. Lithium ameliorates LPS-induced astrocytes activation partly via inhibition of toll-like receptor 4 expression. Cell Physiol Biochem. 2016;38:714–725. doi: 10.1159/000443028. [DOI] [PubMed] [Google Scholar]
- 33.Zhu ZF, Wang QG, Han BJ, William CP. Neuroprotective effect and cognitive outcome of chronic lithium on traumatic brain injury in mice. Brain Res Bull. 2010;83:272–277. doi: 10.1016/j.brainresbull.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 34.Gold AB, Herrmann N, Lanctot KL. Lithium and its neuroprotective and neurotrophic effects: Potential treatment for post-ischemic stroke sequelae. Curr Drug Targets. 2011;12:243–255. doi: 10.2174/138945011794182764. [DOI] [PubMed] [Google Scholar]
- 35.Yan XB, Hou HL, Wu LM, Liu J, Zhou JN. Lithium regulates hippocampal neurogenesis by ERK pathway and facilitates recovery of spatial learning and memory in rats after transient global cerebral ischemia. Neuropharmacology. 2007;53:487–495. doi: 10.1016/j.neuropharm.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 36.Bian Q, Shi T, Chuang DM, Qian Y. Lithium reduces ischemia-induced hippocampal CA1 damage and behavioral deficits in gerbils. Brain Res. 2007;1184:270–276. doi: 10.1016/j.brainres.2007.09.054. [DOI] [PubMed] [Google Scholar]
- 37.Adhami F, Liao G, Morozov YM, Schloemer A, Schmithorst VJ, Lorenz JN, Dunn RS, Vorhees CV, Wills-Karp M, Degen JL, et al. Cerebral ischemia-hypoxia induces intravascular coagulation and autophagy. Am J Pathol. 2006;169:566–583. doi: 10.2353/ajpath.2006.051066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li WL, Yu SP, Chen D, Yu SS, Jiang YJ, Genetta T, Wei L. The regulatory role of NF-κB in autophagy-like cell death after focal cerebral ischemia in mice. Neuroscience. 2013;244:16–30. doi: 10.1016/j.neuroscience.2013.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang Y, Gao K, Hu Z, Li W, Davies H, Ling S, Rudd JA, Fang M. Autophagy upregulation and apoptosis downregulation in DAHP and triptolide treated cerebral ischemia. Mediators Inflamm. 2015;2015(120198) doi: 10.1155/2015/120198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng YQ, Liu JX, Li XZ, Xu L, Xu YG. RNA interference- mediated downregulation of Beclin1 attenuates cerebral ischemic injury in rats. Acta Pharmacol Sin. 2009;30:919–927. doi: 10.1038/aps.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang Z, Zhong L, Zhong S, Xian R, Yuan B. Hypoxia induces microglia autophagy and neural inflammation injury in focal cerebral ischemia model. Exp Mol Pathol. 2015;98:219–224. doi: 10.1016/j.yexmp.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 42.Laplante M, Asbatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou J, Tan SH Nicolas V, Bauvy C, Yang ND, Zhang J, Xue Y, Codogno P, Shen HM. Activation of lysosomal function in the course of autophagy via mTORC1 suppression and autophagosome-lysosome fusion. Cell Res. 2013;23:508–523. doi: 10.1038/cr.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wullschleger S, Loewith R, Hall MN. mTOR signaling in growth control and disease. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 45.Jung CH, Ro SH, Cao J, Otto NM, Kim DH. mTOR regulation of autophagy. FEBS Lett. 2010;584:1287–1295. doi: 10.1016/j.febslet.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chi OZ, Mellender SJ, Barsoum S, Liu X, Damito S, Weiss HR. Effects of rapamycin pretreatment on blood-brain barrier disruption in cerebral ischemia-reperfusion. Neurosci Lett. 2016;620:132–136. doi: 10.1016/j.neulet.2016.03.053. [DOI] [PubMed] [Google Scholar]
- 47.Fu L, Huang L, Cao C, Yin Q, Liu J. Inhibition of AMP-activated protein kinase alleviates focal cerebral ischemia injury in mice: Interference with mTOR and autophagy. Brain Res. 2016;1650:103–111. doi: 10.1016/j.brainres.2016.08.035. [DOI] [PubMed] [Google Scholar]
- 48.Puyal J, Vaslin A, Mottier V, Clarke PG. Postischemic treatment of neonatal cerebral ischemia should target autophagy. Ann Neurol. 2009;66:378–389. doi: 10.1002/ana.21714. [DOI] [PubMed] [Google Scholar]
- 49.Wang PR, Wang JS, Zhang C, Song XF, Tian N, Kong LY. Huang-Lian-Jie-Du-Decotion induced protective autophagy against the injury of cerebral ischemia/reperfusion via MAPK-mTOR signaling pathway. J Ethnopharmacol. 2013;149:270–280. doi: 10.1016/j.jep.2013.06.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.