Abstract
Reduced systolic/diastolic blood pressure (BP) by >40/20 mmHg defines initial orthostatic hypotension (IOH). Rapid resolution of hypotension and lightheadedness follows, but tachycardia may be prolonged. We aimed to examine IOH in controls and patients with postural tachycardia syndrome (POTS) using indices of spontaneous fluctuations of heart rate (HR) and systolic BP as measures of cardiac baroreflex differences. We recruited otherwise healthy IOH patients without POTS (n = 20, 16 ± 3 yr), healthy volunteers (n = 32, 17 ± 3 yr), and POTS patients (n = 39, 17 ± 4 yr). Subjects were instrumented for electrocardiography and beat-to-beat BP. After 10 min supine, subjects stood for 5 min. Following supine recovery, subjects underwent 70° head-up tilt for 10 min to test for POTS. BP, HR, and time, referenced to standing, were measured at events during standing: minimum BP, BP recovery, peak HR, HR minimum, and steady state. Baseline HR and BP were higher in POTS compared with healthy groups. IOH occurred in 13% of controls and 51% of POTS patients. The BP minimum was lower in POTS. Parasympathetic modulation of cardiac baroreflex was decreased in all POTS and control-IOH subjects. Sympathetic indices were increased. Events following BP minimum occurred progressively later in all POTS and control-IOH subjects compared with non-IOH controls. IOH is more frequent in POTS than in controls with a lower minimum BP. POTS has markedly reduced heart rate variability and baroreflex, indicating reduced HR buffering of BP. POTS-IOH and control-IOH subjects had similar peak HR despite decreased minimum BP in POTS. IOH data indicate modest parasympathetic and cardiovagal baroreflex deficits in control-IOH subjects. Parasympathetic deficits are more severe in all POTS patients.
NEW & NOTEWORTHY Significant initial orthostatic hypotension (IOH) occurs in ~50% of postural tachycardia syndrome (POTS) patients and 13% of controls. Heart rate and blood pressure recovery are prolonged in IOH sustaining lightheadedness; IOH is more prevalent and severe in POTS. Altered cerebral blood flow and cardiorespiratory regulation are more prevalent in POTS. Altered heart rate variability and baroreflex gain may cause nearly instantaneous lightheadedness in POTS. IOH alone fails to confer a strong probability of POTS.
Keywords: autonomic nervous system, cardiac baroreflex, initial orthostatic hypotension, postural tachycardia syndrome
INTRODUCTION
Orthostasis (standing upright) shifts an estimated 500–700 mL from central stores to below the diaphragm into the legs and splanchnic vascular beds (36). The initial circulatory response comprises a brief decrease in blood pressure (BP) and reflexive increase in heart rate (HR) such that a BP minimum, or nadir, occurs near 10 s post-stand, and BP is typically restored during the 30 s of standing (50). A normal 10-s lag in sympathetic vasoconstriction accounts for the BP nadir and recovery: the activation of arterial and cardiopulmonary baroreflexes produces afferent signals conducted through the solitary tract, ultimately reaching the rostral ventrolateral medulla, where first preganglionic and then postganglionic sympathetic efferents are stimulated, and that signal transduced to produce arterial vasoconstriction and active splanchnic venoconstriction restoring BP. Parasympathetic withdrawal produces a rapid onset of tachycardia (2, 7). The changes of BP are so sufficiently rapid that they are often missed by sphygmomanometry, requiring use of beat-to-beat blood pressure monitoring such as finger volume photoplethysmography. However, the tachycardia may be more prolonged (15, 16).
The transient fall of BP, if sufficiently large, has been designated initial orthostatic hypotension (IOH) (55). It is transient and distinguished from true sustained orthostatic hypotension (OH), in which BP progressively falls without recovery after standing (14). The diagnosis of IOH is often suggested by a characteristic history of lightheadedness immediately on standing, with rapid recovery and the absence of sustained lightheadedness or orthostatic hypotension (12). Active standing is the diagnostic test of choice by duplicating real-world signs and symptoms. Tilt-table testing usually produces a lesser fall in BP (39) and is not as helpful in diagnosing IOH.
IOH is a transient form of orthostatic intolerance (OI) defined as “difficulty tolerating the upright posture because of symptoms or signs that abate when returned to supine” (44). Signs and symptoms include lightheadedness, impending loss of consciousness, visual difficulties, headache, weakness, hypotension, pallor, and diaphoresis (20). Chronic OI is defined by at least 3 mo of OI. Clinically significant IOH is defined by an exaggerated transient decrease in BP exceeding 40 mmHg systolic or 20 mmHg diastolic BP upon rapid standing from supine or squatting position (55). Reflex tachycardia accompanies hypotension. Patients fulfilling criteria for clinically significant IOH typically complain of brief marked lightheadedness but most often recover spontaneously. A few may experience loss of consciousness after standing, made likely by immediate walking and then stopping abruptly (55). Less severe symptoms often accompany smaller changes in BP = attenuated IOH; these symptoms are common in healthy children taking the form of a short-lived “rush” upon standing, rapidly becoming asymptomatic (3). In our laboratory, using the criteria stated above (7), we estimate that attenuated IOH or significant IOH occur in ~50% of healthy youngsters. Arguably, it is the most common form of OI. This is consistent with a study of healthy Japanese and Swedish schoolchildren that showed an average fall of systolic BP by 22 mmHg within 6–15 s of standing, with BP normalizing within 10–20 s after standing (49). An estimated 20–25% of their children fulfilled significant IOH criteria.
Postural tachycardia syndrome (POTS) is another form of chronic OI with chronic orthostatic symptoms plus persistently excessive upright tachycardia in the absence of sustained hypotension (34, 43). Symptoms occur daily, and the illness is often present for months before the diagnosis is made (22, 33, 42). Excessive upright tachycardia, the hallmark of POTS, is defined in adults by an increase of HR to >120 beats/min or an increase in HR exceeding 30 beats/min during a 10-min tilt or standing test (30). An increase exceeding 40 beats/min during a tilt test is required for the diagnosis in children less than 19 yr old (37). Active standing has not yet been validated as a test for POTS in children, although it is often used in screening younger patients. Validation of active standing in the young has been complicated by incomplete description of the early standing response in POTS, which may differ significantly in extent and time course from healthy controls.
We hypothesize that the time course of HR and BP in IOH depends on parasympathetic deficits that may differ markedly between POTS and control subjects, leading to delays in the immediate response (7) and HR recovery (21) produced by vagal dysregulation.
METHODS
Subjects.
We performed a prospective study over a 2-yr period at the Center for Hypotension, which is an outpatient facility of the Department of Pediatrics at New York Medical College, Valhalla, New York. We recruited 39 consecutive POTS patients (36 female), with a history of OI, tachycardia, and no history of recent transient loss of consciousness, ranging in age from 13– 23 yr [17.3 ± 3.3 (SD)]. We also recruited 32 healthy, nonfainting control subjects (29 females), ranging in age from 14–22 yr [17.6 ± 3.9 (SD)]. To obtain useful statistics and complement the small number of IOH healthy patients that we obtained from our healthy subjects, we added data from 20 healthy POTS-free, IOH patients randomly selected from a prior study (47) (18 females, 16.2 ± 3.2 yr) of healthy volunteers (see section titled Comparing IOH in POTS to IOH in healthy controls for rationale). POTS patients were referred for suspected POTS with OI lasting longer than 6 mo. POTS was confirmed by 70° upright tilt performed during the testing day (see below). POTS subjects were either therapeutically naïve or were weaned off medications that affect the autonomic nervous system for at least 2 wk before being studied.
The medical history of patients with POTS was obtained, and each underwent a physical examination with electrocardiography. Many also had echocardiography or prolonged electrocardiographic monitoring if deemed necessary to exclude cardiac disease. Patients with POTS had daily orthostatic symptoms during prolonged standing. No POTS patient had signs of cardiovascular disease, systemic disease, or transient infection. Patients on long-term bed rest were also excluded.
We also recruited control subjects from age-, body mass index-, and sex-matched volunteers for standing tests in whom subsequent 70° upright tilt for 10 min was normal. Control subjects reported no clinical illness and no orthostatic intolerance and had never fainted. Approximately 50% reported mild dizziness upon rapid standing from the supine position. There were no competitive athletes.
A tentative diagnosis of IOH could be made for many POTS and healthy control subjects based on clinical history; diagnostic features encompassed predisposing situations. For example, prolonged supine rest or standing from a squatting position, followed by rapidly standing upright, produced lightheadedness, reduced BP, and increased HR standing in absence of prodromal signs or symptoms, with rapid recovery of BP and symptoms while remaining standing.
Exclusion criteria for participation included infectious or systemic illness, competitive athletic training, recent long-term bed rest, hypertension, use of nicotine-containing products, or pregnancy within the last year. Prior medication, if any, was discontinued for at least 2 wk before participation in this study. No subjects were taking neurally active or vasoactive drugs at the time of evaluation.
All subjects refrained from caffeine and xanthine-containing products for at least 72 h before testing. All subjects were instructed to fast for at least 3 h before testing. This study was approved by the Institutional Review Board of New York Medical College. All subjects 18 or older signed an informed consent form; those younger than 18 yr old assented to participate, and their parent or legal guardian signed an informed consent form.
Instrumentation.
All subjects were instrumented in a similar fashion by the same operator. During instrumentation, subjects lay supine on an electronic motorized tilt table with a footboard (Colin Medical Instruments Corp., San Antonio, TX). An electrocardiograph measured HR from the beat-to-beat cardiac electrical interval. Beat-to-beat BP was measured using finger arterial photoplethysmography (Finometer; FMS, Amsterdam, The Netherlands) placed on the right middle or index finger and corrected for tilt angle using ModelFlow software (6). Finometer data were calibrated to brachial artery pressure and intermittently checked against oscillometric BP measurements. Signals were acquired at 200 samples/s, multiplexed, and analog-to-digital converted using custom software. These measurements are routine during our tilt-table studies. Data were analyzed off line.
Protocol.
Subjects arrived at our center at 9:00 AM and were prepared for study while supine on the tilt table. After being instrumented, subjects remained awake and supine for at least 30 min to acclimate. Baseline HR and BP data were then collected for 10 min in the supine position to use for heart rate variability (HRV) and blood pressure variability (BPV) calculations using transfer function analysis. After supine data collection, subjects stood up over 5 s and remained standing for 5 min while HR and BP were continuously monitored. Five minutes was chosen because we have not observed vasovagal syncope over this time period, and it gave sufficient standing time to allow for a steady HR steady-state trend to establish. Some patients fulfilled criteria for IOH during our standing procedure with duplication of real-world OI symptoms. After 5 min, subjects returned to supine for 30-min recovery, during which baseline HR and BP were restored. Patients and control subjects were then tilted upright to 70° for 10 min without pharmacologic provocation to determine coexistent POTS. IOH is often poorly reproduced on upright tilt (50, 52, 55). The POTS diagnosis required symptoms of OI during tilt with an increase of HR exceeding 30 beats/min in patients >19 yr old or exceeding 40 beats/min in patients <19 yr old during upright tilt (30, 37). Symptoms and signs abated once patients were placed supine. Only patients with POTS and free of vasovagal syncope or true orthostatic hypotension during tilt testing were retained for this study in the POTS group. Only healthy control subjects without POTS and free of vasovagal syncope or orthostatic hypotension during tilt testing were retained in the control group of this study.
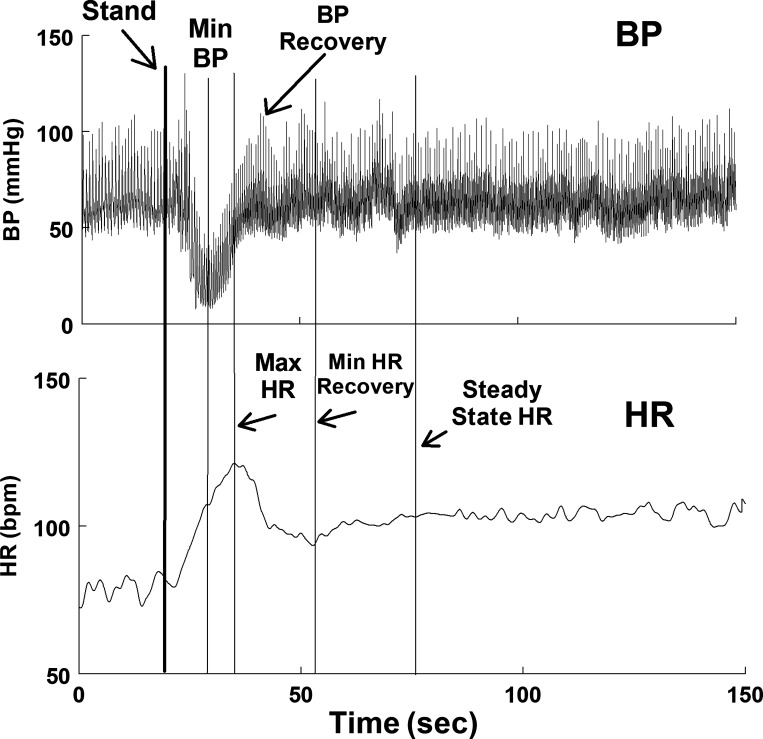
We compared data for control subjects and POTS patients at baseline and at the occurrence of events depicted in Fig. 1, which shows the changes in BP and HR in a representative healthy individual with IOH. Parameters were as follows:
Fig. 1.
Representative phasic blood pressure (BP; top) and time-averaged heart rate (HR; bottom) are shown for a control subject who had initial orthostatic hypotension (IOH). Events are as follows: stand = standing initiate; min BP =the nadir or lowest blood pressure achieved after standing; peak HR = the maximum HR achieved after standing; BP recovery = the first local maximum of BP after standing; min HR (HR recovery) = the lowest HR after standing; steady state HR = HR increases after HR recovery until it achieves a steady slope. bpm, beats/min.
Stand = standing initiated;
Min BP = the nadir or lowest blood pressure achieved after standing;
Peak HR = the maximum HR achieved after standing;
BP recovery = the first local maximum of BP after standing;
Min HR (HR recovery) = the lowest HR after standing;
Steady trend HR = HR increases after HR recovery; at onset steady trend slope turns downward. Steady trend heart rate was computed by the running slope differences/t test technique of Bin et al. (1). Graphs also included a point midway between peak HR and min HR, designated as HR½ min.
Heart rate and blood pressure variability.
Custom software calculated HRV and BPV during the baseline period based on the RR interval and BP signals, respectively. These methods assume stationary, linear, time-independent data that pertain during baseline but not during standing or tilt. Prior to variability calculations, data were resampled at 2 Hz. For power spectra analysis, an autoregressive model based on the methods of Montano et al. (24) and Pagani et al. (28) was used. Each signal was recorded as a time series of discrete point events. An impulse train of corresponding pulses with equal intervals was created from each signal (4). Software completed the autoregression and computed the power spectra (18, 32). The Yule–Walker equation, modified by the Levinson–Durbin algorithm and succeeded by Anderson’s test, allowed the order of the model used to be chosen such that Akaike’s final prediction error was minimized. The resultant interval spectrum was transformed to the spectrum of counts via division by the mean signal sequence (4, 18). The defined frequency band total power (0.01–0.40 Hz) was used to calculate individual spectral powers for the very low frequency (VLF, 0.01–0.05 Hz), low frequency (LF, 0.05–0.15 Hz), and high frequency (HF, 0.15–0.40 Hz) power bands. Only LF and HF were tabulated. We used the ratio of LF RR interval power to LF BP power (the α-index) to describe changes in the cardiovagal baroreflex sensitivity. HF RR interval corresponds to the respiratory sinus arrhythmia and is strictly vagal dependent. LF BP power corresponds to arterial vasomotion and is an index of sympathetic activity (27). LF RR interval power depends on sympathetic-dependent LF BP power transduced by the cardiovagal baroreflex (23). Our laboratory has experience using variability measurements (25, 40, 41, 46).
Data analysis.
Averaged HR, BP, and time from standing ± standard deviation were tabulated for each event using a moving average window with a 3-s aperture. The start of standing was a reference point (the 0 of time). Graphical data showed change in heart rate (ΔHR ± standard error of the mean) using baseline HR as the 0 reference and calculated for each of the defined events and reported in units of beats per minute. Change in systolic BP (ΔBP ± standard error of the mean) used baseline systolic BP as the 0 reference and is reported in units of mmHg.
Statistical methods.
Time of occurrence of events and HR and BP in absolute units at the events are shown in Tables 1–3; ΔHR and ΔBP at the events are shown in Figures 2–4. The data shown in Tables 1–3 were used to determine whether there were differences between POTS and controls at events. Comparison of POTS and controls over the time-ordered events was analyzed as a repeated measures ANOVA, with time-ordered events (i.e., periods) considered as a fixed effect. Three dependent variables were considered: heart rate (HR), systolic blood pressure (BP), and time (in seconds) to achieve each event. Each dependent variable was assessed in a separate model. For these models, we assumed a covariance structure of compound symmetry. To determine whether the pattern of responses over the periods differed between POTS patients, IOH patients, and controls, we evaluated a group-by-period interaction term. P values for the interaction term were adjusted using the Greenhouse–Geisser correction. If the interaction term was significant, post hoc comparisons of the study groups were accomplished using Scheffe’s test to control for multiple comparisons. Significance was established at P < 0.05. To reduce interrater variability, all data were collected and analyzed by the same investigator throughout the entire study. Results were calculated using GraphPad Prism version 4.0.
Table 1.
All POTS and controls
| Event | POTS (n = 39) |
Healthy Controls (n = 32) |
||||||
|---|---|---|---|---|---|---|---|---|
| Time | SBP | DBP | HR | Time | SBP | DBP | HR | |
| Baseline | 121 ± 12* | 56 ± 9 | 69 ± 11 | 111 ± 9 | 54 ± 5 | 73 ± 5 | ||
| Stand | 0 | 121 ± 15 | 55 ± 9 | 84 ± 12 | 0 | 114 ± 12 | 56 ± 8 | 77 ± 11 |
| BP nadir | 14 ± 1 | 79 ± 27* | 34 ± 13 | 111 ± 13 | 11 ± 4 | 86 ± 14 | 42 ± 7 | 110 ± 9 |
| HR peak | 24 ± 17* | 122 ± 31 | 61 ± 20 | 124 ± 13 | 16 ± 5 | 112 ± 21 | 56 ± 13 | 120 ± 12 |
| BP recovery | 28 ± 12 | 150 ± 28* | 75 ± 16* | 103 ± 16* | 23 ± 6 | 137 ± 22 | 63 ± 12 | 90 ± 21 |
| HR recovery | 50 ± 25* | 133 ± 20 | 63 ± 14 | 88 ± 14* | 27 ± 9 | 131 ± 19 | 59 ± 10 | 75 ± 13 |
| Steady trend | 98 ± 40* | 127 ± 16 | 69 ± 10 | 108 ± 15* | 57 ± 28 | 124 ± 12 | 62 ± 9 | 91 ± 17 |
Time of event, blood pressure (BP), and heart rate (HR) data are shown as means ± SD; n = no. of patients. DBP, diastolic blood pressure; POTS, postural tachycardia syndrome; SBP, systolic blood pressure
P < 0.05 compared with controls.
Table 3.
No initial orthostatic hypotension
| Event | Non-IOH POTS (n = 19) |
Non-IOH Controls (n = 28) |
||||||
|---|---|---|---|---|---|---|---|---|
| Time | SBP | DBP | HR | Time | SBP | DBP | HR | |
| Baseline | 124 ± 13* | 58 ± 9 | 76 ± 13 | 112 ± 11 | 57 ± 9 | 73 ± 8 | ||
| Stand | 0 | 124 ± 13 | 57 ± 8 | 91 ± 23* | 0 | 114 ± 11 | 55 ± 10 | 77 ± 10 |
| BP nadir | 14 ± 6 | 104 ± 19* | 45 ± 11 | 110 ± 11 | 12 ± 4 | 86 ± 14 | 42 ± 7 | 109 ± 8 |
| HR peak | 23 ± 6* | 139 ± 26* | 54 ± 21 | 124 ± 17 | 16 ± 5 | 109 ± 21 | 55 ± 12 | 118 ± 11 |
| BP recovery | 26 ± 14 | 155 ± 28* | 79 ± 17* | 107 ± 16* | 22 ± 6 | 135 ± 21 | 63 ± 13 | 89 ± 21 |
| HR recovery | 54 ± 37* | 151 ± 25* | 71 ± 9 | 92 ± 17* | 28 ± 9 | 129 ± 19 | 59 ± 11 | 72 ± 12 |
| Steady trend | 99 ± 64* | 137 ± 18* | 64 ± 10 | 108 ± 20* | 58 ± 29 | 124 ± 13 | 62 ± 10 | 91 ± 18 |
Time of event, blood pressure (BP), and heart rate (HR) data are shown as means ± SD; n = no. of patients. DBP, diastolic blood pressure; IOH, initial orthostatic hypotension; POTS, postural tachycardia syndrome; SBP, systolic blood pressure.
P < 0.05 compared with controls.
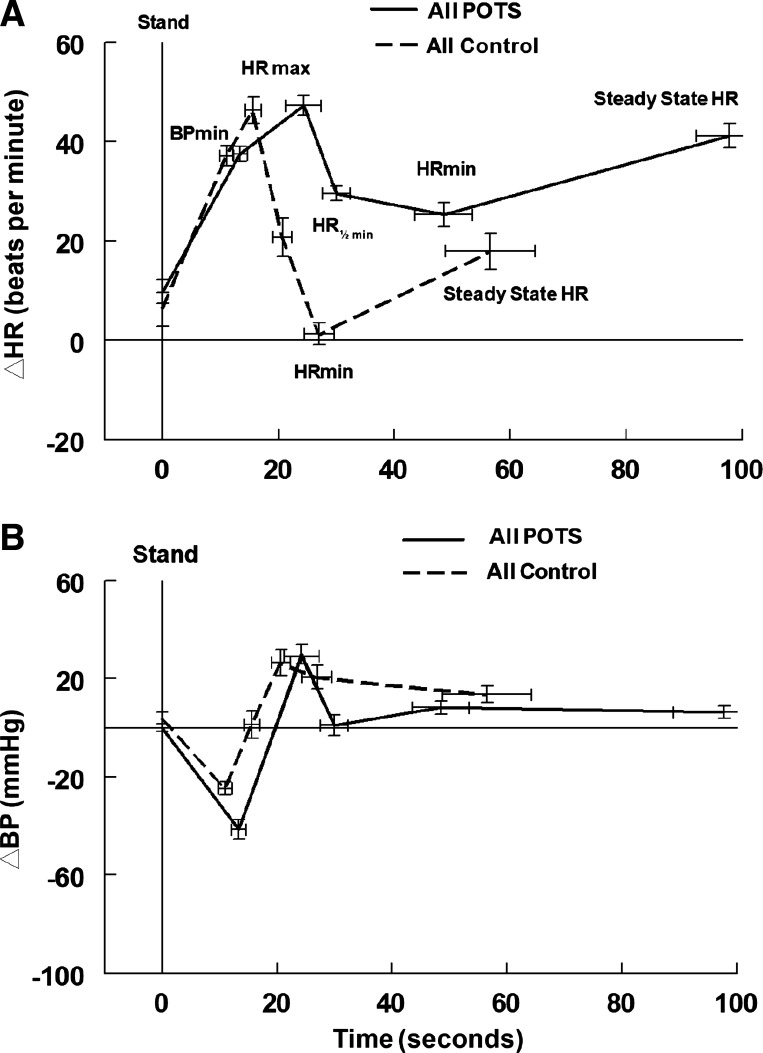
Fig. 2.
Changes in heart rate (HR) and systolic blood pressure (BP) in all postural tachycardia syndrome (POTS) and control subjects. Change in heart rate (ΔHR) with standing (A) and change in systolic BP (ΔBP) (B) in all POTS with or without initial orthostatic hypotension (IOH; all POTS, solid line) and all controls with or without IOH (all control, dashed line). All events after minimum BP (BPmin) occurred later in POTS than in controls. Only the minimum decrease in systolic BP was significantly lower in POTS. HR½ min, midway point between peak HR (HRmax) and minimum HR (HRmin).
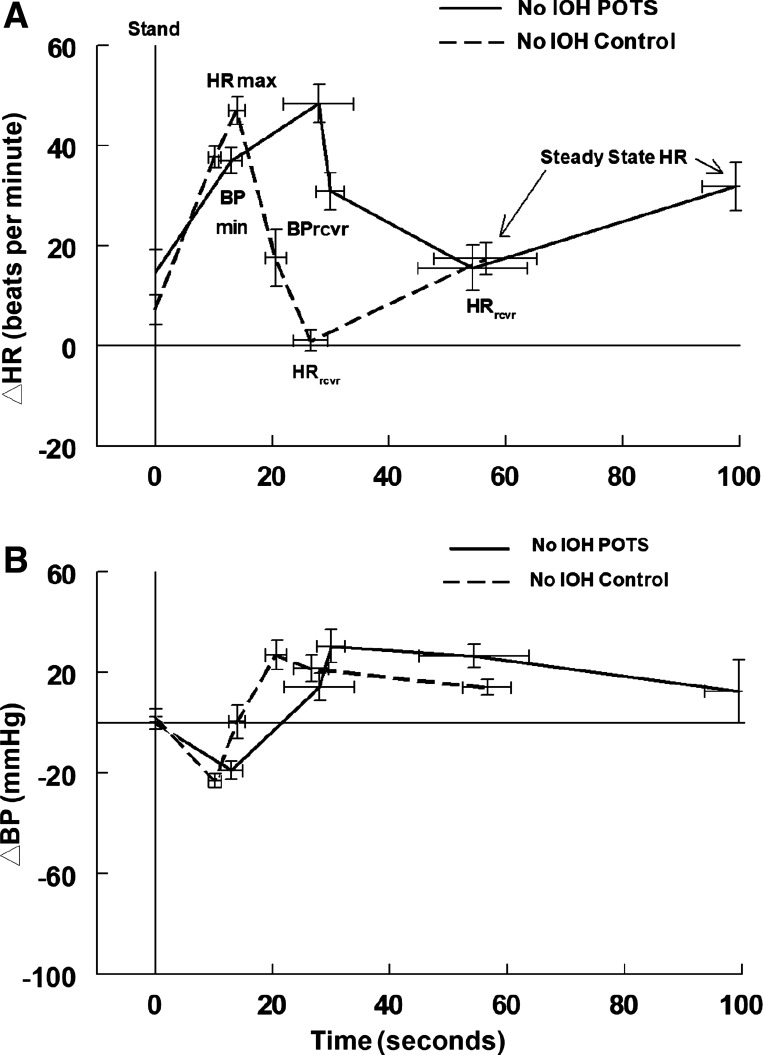
Fig. 4.
Changes in heart rate (HR) and systolic blood pressure (BP) in postural tachycardia syndrome (POTS) and control subjects without initial orthostatic hypotension (No IOH). Change in heart rate (ΔHR) with standing (A) and change in systolic BP (ΔBP) (B) in POTS without IOH (No IOH POTS, solid line) and controls without IOH (No IOH control, dashed line). Minimum BP (BPmin), peak HR (HRmax), recovery BP (BPrcvr), recovery HR (HRrcvr), and time to steady-state HR are prolonged in POTS. HRrcvr and steady-state HR are increased in POTS, but earlier HRs are similar, as are all systolic BPs.
RESULTS
Timing of events is prolonged in POTS compared with controls, and the blood pressure nadir is lower in POTS.
Table 1 shows event times, BP ± SD, and HR ± SD as dependent variables using the ordinal event scale in all consecutive POTS and all consecutive healthy control subjects unselected for IOH. Event timing was significantly prolonged, and HR was increased in POTS after the HR peak. BP at the nadir was decreased in POTS compared with control, whereas the systolic BP at HR peak and BP recovery were increased. Figure 2 depicts these findings using time on the abscissa and depicting ∆HR and ∆BP as functions of time. As can be seen, the time course of ∆HR for POTS and controls is different, with an extended time to HR recovery and steady state in POTS.
Significant IOH is more common in POTS.
The average change in systolic BP from baseline to nadir was 41.4 ± 4.1 mmHg (SE) in unselected POTS patients who had significant IOH compared with 27.4 ± 3.8 mmHg for controls. By count, 20 of 39 (~51%) unselected POTS patients had significant IOH compared with only 4 of 32 (12.5%) controls that had IOH. Thus, approximately half of unselected POTS patients have IOH, whereas 1 of 8 of controls have IOH. We found that significant IOH prolongs HR recovery time most in POTS and recovery to steady state most in healthy IOH patients.
Comparing IOH in POTS to IOH in healthy controls.
Table 2 and Fig. 3 compare IOH in POTS patients with IOH in control subjects. Because the study of consecutive controls yielded 4 patients with IOH, we supplemented those 4 control-IOH from the current sample by adding data obtained from 20 age- and sex-matched IOH-controls subsampled from healthy patients in a prior study using the same methods (47).
Table 2.
Initial orthostatic hypotension
| Event | POTS with IOH (n = 20) |
Controls with IOH (n = 24) |
||||||
|---|---|---|---|---|---|---|---|---|
| Time | SBP | DBP | HR | Time | SBP | DBP | HR | |
| Baseline | 119 ± 13* | 58 ± 9 | 75 ± 13* | 110 ± 17 | 56 ± 9 | 68 ± 11 | ||
| Stand | 0 | 119 ± 15 | 54 ± 9 | 82 ± 12* | 0 | 119 ± 13 | 60 ± 11 | 73 ± 12 |
| BP nadir | 14 ± 7 | 59 ± 14* | 25 ± 10 | 110 ± 11 | 12 ± 4 | 66 ± 15 | 30 ± 10 | 104 ± 14 |
| HR peak | 21 ± 11 | 109 ± 28 | 54 ± 21 | 123 ± 13 | 23 ± 6 | 107 ± 22 | 56 ± 21 | 121 ± 13 |
| BP recovery | 30 ± 14 | 148 ± 23* | 74 ± 14* | 99 ± 14* | 26 ± 7 | 132 ± 19 | 65 ± 13 | 89 ± 17 |
| HR recovery | 43 ± 24 | 136 ± 19 | 66 ± 12 | 85 ± 14* | 43 ± 26 | 126 ± 20 | 63 ± 14 | 76 ± 15 |
| Steady trend | 97 ± 43 | 124 ± 19 | 64 ± 10 | 101 ± 11* | 90 ± 40 | 127 ± 16 | 69 ± 10 | 91 ± 15 |
Time of event, blood pressure (BP), and heart rate (HR) data are shown as means ± SD; n = no. of patients. DBP, diastolic blood pressure; IOH, initial orthostatic hypotension; POTS, postural tachycardia syndrome; SBP, systolic blood pressure.
P < 0.05 compared with controls.
Fig. 3.
Changes in heart rate (HR) and systolic blood pressure (BP) in postural tachycardia syndrome (POTS) and control subjects with initial orthostatic hypotension (IOH). Change in heart rate (ΔHR) with standing (A) and change in systolic BP (ΔBP) (B) in POTS with IOH (IOH POTS, solid line) and controls with IOH (IOH control, dashed line). The timing of all events was similar for POTS and controls. Only the minimum decrease in systolic BP (BPmin) is significantly lower in POTS. HR½ min, midway point between peak HR (HRmax) and minimum HR (HRmin).
In IOH-POTS patients, BP nadir remained lower, and BP recovery was higher at BP recovery. Events occurred at similar times, but HR remained consistently higher in POTS. ∆HR was similar for either group, as shown in Fig. 3.
Does increased percentage of IOH in POTS account for prolonged recovery?
We wondered whether the increased percentage of IOH in POTS accounts for the mean prolongation of recovery events and BP differences in POTS. To address this question, we removed patients with IOH from both control and POTS samples of Table 1 and compared results in these POTS-no IOH versus control-no IOH in Table 3 and Fig. 4. Absolute systolic BP was uniformly higher in POTS-no IOH compared with control-no IOH, and times to events were prolonged in POTS. Timing of events was similar in POTS with or without IOH.
Do measures of parasympathetic and sympathetic measures account for time course and blood pressure changes?
We used measures of HR and BP variability to investigate autonomic and particularly parasympathetic modulation in POTS patients and controls with or without IOH. Table 4 shows measures of total HRV and BPV, the square root of the ratio of HR power to BP power (the α-index of baroreflex gain, with squared coherence >0.5), and HF-HRV (measure of respiratory sinus arrhythmia) reflect parasympathetic activity. Total BPV and LF-BPV have been used as measures of sympathetic activation (27). All vagal indices (HRV, HF-HRV, and α-index) are low for all POTS patients with or without IOH and signify a loss of vagal modulation of HR. Smaller decreases in HRV, HF-HRV, and α-index are observed in IOH-controls. This may help to account for the slowed fall of HR following the HR peak in IOH-controls. BPV and LF-BPV were increased for all IOH, indicating enhanced modulation of sympathetic activity.
Table 4.
Spectral analysis of heart rate and blood pressure variability
| All Controls | All POTS | IOH Controls | IOH POTS | Non-IOH Controls | Non-IOH POTS | |
|---|---|---|---|---|---|---|
| n | 32 | 39 | 24 | 20 | 29 | 19 |
| Total HRV | 3,531 ± 458 | 1,068 ± 148* | 2,387 ± 645* | 1,047 ± 178* | 4,577 ± 652 | 1,091 ± 153* |
| LF HRV | 976 ± 128 | 439 ± 88* | 940 ± 127 | 395 ± 104* | 921 ± 149 | 372 ± 72* |
| HF HRV | 1,729 ± 326 | 401 ± 60* | 1,354 ± 455 | 414 ± 72* | 2,594 ± 390 | 346 ± 70* |
| Total BPV | 9.68 ± 1.17 | 12.15 ± 1.63 | 12.15 ± 1.49* | 13.39 ± 1.49* | 9.11 ± 1.71 | 10.49 ± 1.49 |
| LF BPV | 2.64 ± 0.30 | 3.44 ± 0.20* | 3.66 ± 0.22* | 3.92 ± 0.42* | 2.08 ± 0.25 | 2.98 ± 0.39 |
| HF BPV | 1.39 ± 0.15 | 1.18 ± 0.15 | 1.57 ± 0.12 | 1.25 ± 0.16 | 1.39 ± 0.20 | 1.27 ± 0.20 |
| α-Index | 20.1 ± 1.3 | 11.2 ± 0.7* | 15.4 ± 0.9* | 11.1 ± 1.1* | 20.9 ± 1.6 | 11.1 ± 0.8* |
Data are means ± SE; n = no. of patients. BPV, blood pressure variability; HF, high frequency; HRV, heart rate variability; LF, low frequency; IOH, initial orthostatic hypotension; POTS, postural tachycardia syndrome;
P < 0.05 different from all controls.
DISCUSSION
The probability of IOH in POTS exceeds the probability of IOH in healthy controls.
The central finding of this study is that IOH occurs much more frequently and to a lower systolic BP in POTS patients than in controls. The incidence of significant IOH in POTS patients was >50%, compared with 13% in controls.
This raises the possibility of whether IOH could be a marker for POTS. Bayes theorem (19) contradicts that hypothesis:
Let P(POTS, IOH) be the joint probability of having POTS and IOH. In terms of prior and conditional probabilities, Bayes theorem states:
where
Thus, the odds of having POTS given IOH on a standing test are quite low.
Increased HRV-LF suggests sympathetic activation during IOH-POTS and IOH-controls.
BP nadir and BP recovery occur over a similar time course in POTS and controls with significant IOH, although BP at baseline and recovery were increased in POTS compared with controls. A potential reason for this is enhanced BPV, specifically LF BPV, indicating an increase in the modulation of sympathetic influence of BP (13) for all IOH. IOH is also associated with reduced modulation of cardiac parasympathetic tone in controls, evidenced by a decrease in α-index of cardiovagal baroreflex gain. HRV and HF-HRV are reduced to a far lesser extent for IOH controls compared with non IOH controls. Thus, IOH appears to relate to decreased modulation of parasympathetic tone and increased modulation of sympathetic tone compared with the control state without IOH.
Minimum blood pressure (at the nadir) is lower in POTS-IOH.
The BP and ∆BP at nadir were lower for POTS-IOH compared with control-IOH. This may decrease cerebral blood flow (11). Because data indicate that systemic vascular resistance is enhanced in POTS while upright (13) and central blood volume is reduced compared with controls (48), it is probable that lower BP at nadir results from relative or absolute central hypovolemia in POTS compared with controls, along with lower cardiovagal baroreflex gain and thus lower capability to buffer rapid changes in BP with suitable changes in HR. We hypothesize that reduced BP may produce reduced carotid and cerebral blood flow (26) contributing to reduced blood flow to the carotid body. Potentially, a state of “stagnant ischemia” sensitizes the carotid body chemoreflex producing hyperventilation (5).
Increased heart rate in IOH.
Our findings also show that HR increments during rapid standing in young healthy patients with IOH are excessive (ΔHR > 40 beats/min) and thus fall into the range associated with POTS for a short period of time (47), even though there is no POTS by history or by upright tilt testing. Such control-IOH subjects have a similar increase in HR of shorter duration than POTS patients. However, absolute HR is higher in POTS except for nadir and BP recovery. In our experience, healthy subjects often report dizziness immediately after standing but rarely spontaneously complain of lightheadedness during an interview unless specifically asked. Such youngsters are often not referred for orthostatic intolerance because of paucity of reported symptoms.
Autonomic dysfunction in IOH?
IOH in the young has often been thought to be an expected consequence of the normal physiological changes that occur as an immediate response to rapid shifts of blood by gravity. Indeed, small changes in BP occur in most healthy volunteers upon standing. Symptomatic IOH patients have decreased BP at the nadir and a slightly prolonged BP recovery (26.4 ± 0.9 vs. 21.3 ± 1.5 s), which suggest autonomic deficits of adrenergic vasoconstriction or hypovolemia (53, 54). However, a striking finding is the overall reduced “speed of heart rate recovery in response to orthostatic challenge” considered to be a defect of parasympathetic control (21). Defective parasympathetic control is an independent predictor of mortality in older adults (17, 21). Taken together with changes in heart rate variability and baroreflex gain, we infer that greatly reduced speed of HR recovery observed in these young IOH patients confers a risk of parasympathetic autonomic dysfunction that has not previously been considered (45).
IOH in the young may additionally represent a defect in the cardiovagal baroreflex (29) given that the maximum change in heart rate for IOH patients was not significantly larger than control values (53 ± 3 vs. 46 ± 3 beats/min, P = 0.06), whereas the minimum of systolic BP was larger (45 ± 3 vs. 25 ± 3 mmHg, P < 0.0001). A larger change in systolic BP in IOH does not give rise to a larger change in HR, implying reduced cardiovagal gain.
IOH in older adults can reflect incipient neurogenic orthostatic hypotension or serve as a prelude to autonomic dysfunction (9, 10). The effects of age can be confounded by other autonomic problems or by medications that interfere with adrenergic vasoconstriction, splanchnic venoconstriction, or blood volume (54). These factors are not a consideration in the younger population of IOH patients in the current study.
Comparison of IOH in controls with POTS.
The likelihood of IOH is influenced by the presence of POTS and differs from POTS in at least three ways: 1) tachycardia, while unsustained in control-IOH, is sustained in POTS; 2) hypotension occurs, albeit fleetingly in IOH but not in POTS unless there is IOH; and 3) the clinical history of IOH in controls and POTS without IOH is quite different.
Thus, although IOH and POTS are both causes of chronic orthostatic intolerance, the IOH connotes transient upright hypotension with recovery and unsustained postural tachycardia. The diagnosis of POTS connotes sustained postural tachycardia without hypotension. The current paper implies that the co-occurrence of IOH in POTS is indeed common, but IOH alone fails to confer a strong probability of POTS. The consequences of IOH in POTS or control subjects are beyond the scope of this paper.
Limitations.
Findings are reported for POTS and IOH patients without other coexistent forms of OI and are thus not generalizable to this wider group. Data are for a restricted age group and cannot be generalized to different ages. Small numbers of IOH were found in our unselected control group. However, the incidence of IOH in larger control groups is similar (2, 13, 14).
GRANTS
Funding for this project was provided by grants RO1 HL 112736 and RO1 HL 134674 from the National Heart Lung and Blood Institute (NHLBI).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.M.S. conceived and designed research; J.M.S., A.K., M.B.O.-S., C.T., and M.S.M. performed experiments; J.M.S., A.K., M.B.O.-S., P.V., and M.S.M. analyzed data; J.M.S., M.B.O.-S., P.V., C.T., and M.S.M. interpreted results of experiments; J.M.S. and M.S.M. prepared figures; J.M.S. drafted manuscript; J.M.S. and M.S.M. edited and revised manuscript; J.M.S., A.K., M.B.O.-S., P.V., C.T., and M.S.M. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank the participants for their time and efforts in the completion of this project. We also thank Mr. Tyler Fialkoff and Ms. Brianna Tuma-Marcella for their technical help in performing experiments and data collection.
REFERENCES
- 1.Bin Z, Li J, Sun C, Zhou X. A new statistical method for detecting trend turning. Theor Appl Climatol 138: 201–213, 2019. doi: 10.1007/s00704-019-02817-9. [DOI] [Google Scholar]
- 2.Borst C, Wieling W, van Brederode JF, Hond A, de Rijk LG, Dunning AJ. Mechanisms of initial heart rate response to postural change. Am J Physiol Heart Circ Physiol 243: H676–H681, 1982. doi: 10.1152/ajpheart.1982.243.5.H676. [DOI] [PubMed] [Google Scholar]
- 3.Dambrink JH, Imholz BP, Karemaker JM, Wieling W. Circulatory adaptation to orthostatic stress in healthy 10-14-year-old children investigated in a general practice. Clin Sci (Lond) 81: 51–58, 1991. doi: 10.1042/cs0810051. [DOI] [PubMed] [Google Scholar]
- 4.DeBoer RW, Karemaker JM, Strackee J. Comparing spectra of a series of point events particularly for heart rate variability data. IEEE Trans Biomed Eng 31: 384–387, 1984. doi: 10.1109/TBME.1984.325351. [DOI] [PubMed] [Google Scholar]
- 5.Del Pozzi AT, Schwartz CE, Tewari D, Medow MS, Stewart JM. Reduced cerebral blood flow with orthostasis precedes hypocapnic hyperpnea, sympathetic activation, and postural tachycardia syndrome. Hypertension 63: 1302–1308, 2014. doi: 10.1161/HYPERTENSIONAHA.113.02824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dyson KS, Shoemaker JK, Arbeille P, Hughson RL. Modelflow estimates of cardiac output compared with Doppler ultrasound during acute changes in vascular resistance in women. Exp Physiol 95: 561–568, 2010. doi: 10.1113/expphysiol.2009.050815. [DOI] [PubMed] [Google Scholar]
- 7.Ewing DJ, Hume L, Campbell IW, Murray A, Neilson JM, Clarke BF. Autonomic mechanisms in the initial heart rate response to standing. J Appl Physiol 49: 809–814, 1980. doi: 10.1152/jappl.1980.49.5.809. [DOI] [PubMed] [Google Scholar]
- 9.Finucane C, O’Connell MD, Donoghue O, Richardson K, Savva GM, Kenny RA. Impaired orthostatic blood pressure recovery is associated with unexplained and injurious falls. J Am Geriatr Soc 65: 474–482, 2017. doi: 10.1111/jgs.14563. [DOI] [PubMed] [Google Scholar]
- 10.Finucane C, O’Connell MD, Fan CW, Savva GM, Soraghan CJ, Nolan H, Cronin H, Kenny RA. Age-related normative changes in phasic orthostatic blood pressure in a large population study: findings from The Irish Longitudinal Study on Ageing (TILDA). Circulation 130: 1780–1789, 2014. doi: 10.1161/CIRCULATIONAHA.114.009831. [DOI] [PubMed] [Google Scholar]
- 11.Fitzgibbon-Collins LK, Noguchi M, Heckman GA, Hughson RL, Robertson AD. Acute reduction in cerebral blood velocity on supine-to-stand transition increases postural instability in young adults. Am J Physiol Heart Circ Physiol 317: H1342–H1353, 2019. doi: 10.1152/ajpheart.00360.2019. [DOI] [PubMed] [Google Scholar]
- 12.Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, Cheshire WP, Chelimsky T, Cortelli P, Gibbons CH, Goldstein DS, Hainsworth R, Hilz MJ, Jacob G, Kaufmann H, Jordan J, Lipsitz LA, Levine BD, Low PA, Mathias C, Raj SR, Robertson D, Sandroni P, Schatz IJ, Schondorf R, Stewart JM, van Dijk JG. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Auton Neurosci 161: 46–48, 2011. doi: 10.1016/j.autneu.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Furlan R, Heusser K, Minonzio M, Shiffer D, Cairo B, Tank J, Jordan J, Diedrich A, Gauger P, Zamuner AR, Dipaola F, Porta A, Barbic F. Cardiac and vascular sympathetic baroreflex control during orthostatic pre-syncope. J Clin Med 8: 1434, 2019. doi: 10.3390/jcm8091434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldstein DS, Sharabi Y. Neurogenic orthostatic hypotension: a pathophysiological approach. Circulation 119: 139–146, 2009. doi: 10.1161/CIRCULATIONAHA.108.805887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imholz BP, Settels JJ, van der Meiracker AH, Wesseling KH, Wieling W. Non-invasive continuous finger blood pressure measurement during orthostatic stress compared to intra-arterial pressure. Cardiovasc Res 24: 214–221, 1990. doi: 10.1093/cvr/24.3.214. [DOI] [PubMed] [Google Scholar]
- 16.Imholz BP, Wieling W, van Montfrans GA, Wesseling KH. Fifteen years experience with finger arterial pressure monitoring: assessment of the technology. Cardiovasc Res 38: 605–616, 1998. doi: 10.1016/S0008-6363(98)00067-4. [DOI] [PubMed] [Google Scholar]
- 17.Kannankeril PJ, Le FK, Kadish AH, Goldberger JJ. Parasympathetic effects on heart rate recovery after exercise. J Investig Med 52: 394–401, 2004. doi: 10.1136/jim-52-06-34. [DOI] [PubMed] [Google Scholar]
- 18.Kay S, Marple SL. Spectrum analysis—a modern perspective. Proc IEEE 69: 1380–1419, 1981. doi: 10.1109/PROC.1981.12184. [DOI] [Google Scholar]
- 19.López Puga J, Krzywinski M, Altman N. Points of significance: Bayes’ theorem. Nat Methods 12: 277–278, 2015. doi: 10.1038/nmeth.3335. [DOI] [PubMed] [Google Scholar]
- 20.Low PA, Opfer-Gehrking TL, McPhee BR, Fealey RD, Benarroch EE, Willner CL, Suarez GA, Proper CJ, Felten JA, Huck CA, Corfits JL. Prospective evaluation of clinical characteristics of orthostatic hypotension. Mayo Clin Proc 70: 617–622, 1995. doi: 10.4065/70.7.617. [DOI] [PubMed] [Google Scholar]
- 21.McCrory C, Berkman LF, Nolan H, O’Leary N, Foley M, Kenny RA. Speed of heart rate recovery in response to orthostatic challenge. Circ Res 119: 666–675, 2016. doi: 10.1161/CIRCRESAHA.116.308577. [DOI] [PubMed] [Google Scholar]
- 22.Medow MS, Stewart JM, Sanyal S, Mumtaz A, Sica D, Frishman WH. Pathophysiology, diagnosis, and treatment of orthostatic hypotension and vasovagal syncope. Cardiol Rev 16: 4–20, 2008. doi: 10.1097/CRD.0b013e31815c8032. [DOI] [PubMed] [Google Scholar]
- 23.Moak JP, Goldstein DS, Eldadah BA, Saleem A, Holmes C, Pechnik S, Sharabi Y. Supine low-frequency power of heart rate variability reflects baroreflex function, not cardiac sympathetic innervation. Heart Rhythm 4: 1523–1529, 2007. doi: 10.1016/j.hrthm.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montano N, Ruscone TG, Porta A, Lombardi F, Pagani M, Malliani A. Power spectrum analysis of heart rate variability to assess the changes in sympathovagal balance during graded orthostatic tilt. Circulation 90: 1826–1831, 1994. doi: 10.1161/01.CIR.90.4.1826. [DOI] [PubMed] [Google Scholar]
- 25.Ocon AJ, Medow MS, Taneja I, Clarke D, Stewart JM. Decreased upright cerebral blood flow and cerebral autoregulation in normocapnic postural tachycardia syndrome. Am J Physiol Heart Circ Physiol 297: H664–H673, 2009. doi: 10.1152/ajpheart.00138.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogoh S, Hirasawa A, Raven PB, Rebuffat T, Denise P, Lericollais R, Sugawara J, Normand H. Effect of an acute increase in central blood volume on cerebral hemodynamics. Am J Physiol Regul Integr Comp Physiol 309: R902–R911, 2015. doi: 10.1152/ajpregu.00137.2015. [DOI] [PubMed] [Google Scholar]
- 27.Okamoto LE, Raj SR, Gamboa A, Shibao CA, Arnold AC, Garland EM, Black BK, Farley G, Diedrich A, Biaggioni I. Sympathetic activation is associated with increased IL-6, but not CRP in the absence of obesity: lessons from postural tachycardia syndrome and obesity. Am J Physiol Heart Circ Physiol 309: H2098–H2107, 2015. doi: 10.1152/ajpheart.00409.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pagani M, Lombardi F, Guzzetti S, Rimoldi O, Furlan R, Pizzinelli P, Sandrone G, Malfatto G, Dell’Orto S, Piccaluga E. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ Res 59: 178–193, 1986. doi: 10.1161/01.RES.59.2.178. [DOI] [PubMed] [Google Scholar]
- 29.Parati G, Di Rienzo M, Mancia G. How to measure baroreflex sensitivity: from the cardiovascular laboratory to daily life. J Hypertens 18: 7–19, 2000. doi: 10.1097/00004872-200018010-00003. [DOI] [PubMed] [Google Scholar]
- 30.Plash WB, Diedrich A, Biaggioni I, Garland EM, Paranjape SY, Black BK, Dupont WD, Raj SR. Diagnosing postural tachycardia syndrome: comparison of tilt testing compared with standing haemodynamics. Clin Sci (Lond) 124: 109–114, 2013. doi: 10.1042/CS20120276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roberts R, Mullis C. Spectral estimation. In: Digital Signal Processing. Reading, MA: Addison-Wesley, 1987, p. 483–565. [Google Scholar]
- 33.Schondorf R, Low PA. Idiopathic postural orthostatic tachycardia syndrome: an attenuated form of acute pandysautonomia? Neurology 43: 132–137, 1993. doi: 10.1212/WNL.43.1_Part_1.132. [DOI] [PubMed] [Google Scholar]
- 34.Sheldon RS, Grubb BP II, Olshansky B, Shen WK, Calkins H, Brignole M, Raj SR, Krahn AD, Morillo CA, Stewart JM, Sutton R, Sandroni P, Friday KJ, Hachul DT, Cohen MI, Lau DH, Mayuga KA, Moak JP, Sandhu RK, Kanjwal K. 2015 heart rhythm society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm 12: e41–e63, 2015. doi: 10.1016/j.hrthm.2015.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheriff DD, Nådland IH, Toska K. Role of sympathetic responses on the hemodynamic consequences of rapid changes in posture in humans. J Appl Physiol (1985) 108: 523–532, 2010. doi: 10.1152/japplphysiol.01185.2009. [DOI] [PubMed] [Google Scholar]
- 37.Singer W, Sletten DM, Opfer-Gehrking TL, Brands CK, Fischer PR, Low PA. Postural tachycardia in children and adolescents: what is abnormal? J Pediatr 160: 222–226, 2012. doi: 10.1016/j.jpeds.2011.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sprangers RL, Wesseling KH, Imholz AL, Imholz BP, Wieling W. Initial blood pressure fall on stand up and exercise explained by changes in total peripheral resistance. J Appl Physiol (1985) 70: 523–530, 1991. doi: 10.1152/jappl.1991.70.2.523. [DOI] [PubMed] [Google Scholar]
- 40.Stewart JM. Autonomic nervous system dysfunction in adolescents with postural orthostatic tachycardia syndrome and chronic fatigue syndrome is characterized by attenuated vagal baroreflex and potentiated sympathetic vasomotion. Pediatr Res 48: 218–226, 2000. doi: 10.1203/00006450-200008000-00016. [DOI] [PubMed] [Google Scholar]
- 41.Stewart JM. Intravenous cannulation of adolescents does not affect the modulation of autonomic tone assessed by heart rate and blood pressure variability. Clin Auton Res 10: 7–12, 2000. doi: 10.1007/BF02291383. [DOI] [PubMed] [Google Scholar]
- 42.Stewart JM. Chronic orthostatic intolerance and the postural tachycardia syndrome (POTS). J Pediatr 145: 725–730, 2004. doi: 10.1016/j.jpeds.2004.06.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stewart JM. Common syndromes of orthostatic intolerance. Pediatrics 131: 968–980, 2013. doi: 10.1542/peds.2012-2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stewart JM, Boris JR, Chelimsky G, Fischer PR, Fortunato JE, Grubb BP, Heyer GL, Jarjour IT, Medow MS, Numan MT, Pianosi PT, Singer W, Tarbell S, Chelimsky TC; Pediatric Writing Group of the American Autonomic Society . Pediatric disorders of orthostatic intolerance. Pediatrics 141: e20171673, 2018. doi: 10.1542/peds.2017-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stewart JM, Clarke D. “He’s dizzy when he stands up”: an introduction to initial orthostatic hypotension. J Pediatr 158: 499–504, 2011. doi: 10.1016/j.jpeds.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stewart JM, Erb M, Sorbera C. Heart rate variability and the outcome of head-up tilt in syncopal children. Pediatr Res 40: 702–709, 1996. doi: 10.1203/00006450-199611000-00009. [DOI] [PubMed] [Google Scholar]
- 47.Stewart JM, Javaid S, Fialkoff T, Tuma-Marcella B, Visintainer P, Terilli C, Medow MS. Initial orthostatic hypotension causes (transient) postural tachycardia. J Am Coll Cardiol 74: 1271–1273, 2019. doi: 10.1016/j.jacc.2019.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stewart JM, Pianosi P, Shaban MA, Terilli C, Svistunova M, Visintainer P, Medow MS. Postural hyperventilation as a cause of postural tachycardia syndrome: increased systemic vascular resistance and decreased cardiac output when upright in all postural tachycardia syndrome variants. J Am Heart Assoc 7: e008854. , 2018. doi: 10.1161/JAHA.118.008854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tanaka H, Thulesius O, Borres M, Yamaguchi H, Mino M. Blood pressure responses in Japanese and Swedish children in the supine and standing position. Eur Heart J 15: 1011–1019, 1994. doi: 10.1093/oxfordjournals.eurheartj.a060622. [DOI] [PubMed] [Google Scholar]
- 50.van Wijnen VK, Finucane C, Harms MPM, Nolan H, Freeman RL, Westerhof BE, Kenny RA, Ter Maaten JC, Wieling W. Noninvasive beat-to-beat finger arterial pressure monitoring during orthostasis: a comprehensive review of normal and abnormal responses at different ages. J Intern Med 282: 468–483, 2017. doi: 10.1111/joim.12636. [DOI] [PubMed] [Google Scholar]
- 52.van Wijnen VK, Harms MP, Go-Schön IK, Westerhof BE, Krediet CT, Stewart J, Wieling W. Initial orthostatic hypotension in teenagers and young adults. Clin Auton Res 26: 441–449, 2016. doi: 10.1007/s10286-016-0382-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Wijnen VK, Harms MPM, Wieling W. Orthostatic hypotension in the first minute after standing up: what is the clinical relevance and do symptoms matter? Hypertension 71: 816–818, 2018. doi: 10.1161/HYPERTENSIONAHA.118.10609. [DOI] [PubMed] [Google Scholar]
- 54.van Wijnen VK, Hove DT, Finucane C, Wieling W, van Roon AM, Ter Maaten JC, Harms MPM. Hemodynamic mechanisms underlying initial orthostatic hypotension, delayed recovery and orthostatic hypotension. J Am Med Dir Assoc 19: 786–792, 2018. doi: 10.1016/j.jamda.2018.05.031. [DOI] [PubMed] [Google Scholar]
- 55.Wieling W, Krediet CT, van Dijk N, Linzer M, Tschakovsky ME. Initial orthostatic hypotension: review of a forgotten condition. Clin Sci (Lond) 112: 157–165, 2007. doi: 10.1042/CS20060091. [DOI] [PubMed] [Google Scholar]