Abstract
Based on a prior anesthetized model, we developed an unanesthetized model to evaluate the effects of hypoglossal nerve stimulation (HNS) during sleep. We prepared three rabbits with injections of hyaluronic acid in the base of tongue to produce upper airway obstruction followed by HNS implant. Two rabbits were saline controls, and one, a passive control. Measures were sleep, airflow, effort, oxygen saturation, and heart rate. HNS with electrodes around the right hypoglossal nerve were adjusted to a level without behaviorally disturbing the animal. During HNS stimulation in the tongue-base injected rabbits, obstructive apneas and hypopneas of intermediate (3 to 7 cycles of respiratory effort) or longer (≥8 cycles) duration were largely eliminated while less clinically relevant shorter events (<3) were unaffected, and oxygen saturation was improved. Control animals exhibited no intermediate or long events. In this model HNS can relieve induced sleep apnea, without disturbing the animal: however, despite being non-canine and of substantial size, the model has its challenges.
NEW & NOTEWORTHY This report describes a rabbit model for testing the impact of hypoglossal nerve stimulation (HNS) on obstructive apneas. Obstructive sleep apnea (OSA) is induced by injecting hyaluronic acid (as a filler) into the base of the tongue. HNS reduced the length and rate of obstructions and improved oxygenation during sleep. Our efforts with this model advanced understanding of the complexities of this OSA preclinical model for neurostimulation reversal of sleep-disordered breathing.
Keywords: animal models, nerve stimulation, sleep apnea
INTRODUCTION
Obstructive sleep apnea (OSA) is a disorder characterized by pauses in breathing during sleep. The most common cause is upper airway obstruction, collapse of one or more pharyngeal sites. Recovery requires a transient increase in muscle tone to open the airway and take an unobstructed breath. The consequences are a fragmented sleep pattern which can manifest as daytime sleepiness, headache, decreased ability to concentrate, hypertension, metabolic disorders, cardiovascular morbidity, and psychological changes (24).
Some anatomically based assessments for OSA directly target pharyngeal sites of obstruction, and surgical treatments range in invasiveness, and the ability to correct upper airway obstruction (10, 19). Other treatments that target skeletal structures also span a range of invasiveness with variable efficacy, and include hyoid suspension and maxilla-mandibular advancement (MMA) (12). While MMA is effective for a defined patient cohort it is major surgery that is not suitable for most patients with OSA. One surgical option is a tracheotomy, which bypasses the sites of obstruction, but it is rarely utilized due to changes in lifestyle and body image. Oral devices that advance the mandible or reposition the tongue during sleep can be effective but generally require at least partially intact dentition in all four quadrants (21). Continuous positive-airway pressure (CPAP) restores upper airway patency in patients with severe OSA by application of intraluminal pressure to dilate/stent a collapsible pharyngeal segment (9), but is not uniformly tolerated (22). For those in whom human OSA is associated with obesity, weight loss through diet and exercise or bariatric surgery is an option although patient compliance remains a challenge for most (4).
At the onset of an upper airway (obstructive) apnea there is a reduction in phasic and tonic muscle tone to a level that no longer supports pharyngeal patency (15). A more recent therapeutic approach employs electrical stimulation of cranial nerve XII (CN XII–the hypoglossal nerve which provides motor innervation to the tongue musculature) to counteract a deficit in muscle tone with sleep. Upper airway stimulation by the hypoglossal nerve device (HNS) (Inspire Medical Systems, Golden Valley, MN) is an FDA-approved therapy shown to improve OSA symptoms by advancing the tongue and expanding primarily the retrolingual airway. Through soft tissue connections the retropalatal airway is often expanded as well (16). Thus, HNS improves pharyngeal airway patency during sleep in humans (7). Neurotherapy is most effective in those with particular pharyngeal anatomy, and clinically the Inspire device is dependent on more distal electrode placement on the medial branch of the nerve to effectively target tongue protrusors (20, 23). There is a need for a great deal more research on this therapeutic modality, and hence there is a need for appropriate animal models.
In developing an animal model of OSA, one has to distinguish other efforts toward cause from the perspective of the unique upper airway of humans—anatomy that makes people almost singularly susceptible to upper airway collapse from the consequences. To date most animal models focus on the consequences of apnea (hypoxemia or arousals) without OSA actually being present (6). Mice and rats have central apneas but do not commonly exhibit obstructive apneas (5, 8). Looking for animals with a causal pathway, the rodent, dog, and cat have a pharyngeal airway anatomy which is comparatively rigid with short velum-to-larynx distance, and greater separation of the pharynx from the oral tongue (8). The hyoid bone in these species is firmly attached by cartilage to the styloid process and thyroid cartilage. All of these anatomic features result in a less collapsible airway (19). Although HNS was found to be effective at reversing obstruction in a dog, the way the obstruction was created in the first place was by using creating negative pressure via suction from below (25).
We recently turned to a rabbit model as this species has an upper airway pharyngeal structure, a floating hyoid bone, and a hypoglossal nerve branching pattern similar to that seen in humans (1). Importantly, the rabbit upper airway is shown by others to be susceptible to collapse during sleep following fluid (of varying types) injections to the base of the tongue (3, 27) or muscle paralysis (2, 11).
In a prior study, we assessed the effects of HNS (3) and sciatic nerve stimulation (17) on the upper airway and respiratory dynamics in an anesthetized rabbit model. The animal was instrumented for assessment of upper airway resistance. HNS successfully improved upper airway patency whereas sciatic nerve stimulation did not. For the rabbit model to be relevant to OSA, there is a need for an unanesthetized animal model. Here, we hypothesized that we could produce a chronic unanesthetized OSA model via tongue-base injection, and that HNS would alleviate and/or prevent the induced OSA, thereby improving blood oxygenation, without awakening the rabbit. Thus we demonstrate a method for inducing sleep disordered breathing in the rabbit and then alleviating the condition.
METHODS
Surgical and experimental procedures were approved by the Louis Stokes Cleveland VA Medical Center (LSCVAMC) Institutional Animal Care and Use Committee (IACUC). New Zealand White male rabbits (Charles River, Waltham MA) weighing 2.8–3.2 kg were used in this study. Male rabbits were chosen because they do not have a prominent dewlap outside the anterior pharyngeal wall, thus making them a better model for the human condition. Rabbits were kept in humidity and temperature controlled facility with a 12:12-h light:dark cycle, where they had ad libitum access to food and water. Body temperatures were measured while the animals were in the facility but not during the experimental procedures. Six animals were equally divided between a treatment group and controls. There were three active treatment animals with hyaluronic acid injections in the tongue-base and with HNS devices implanted. For control animals, two had tongue injections of saline without an electrode implant, and one animal had neither injection nor implant.
Base of tongue injections.
In the preliminary studies in the anesthetized rabbit, we found that injection of saline either into the tongue base or into the palate would produce upper airway obstruction relieved by manual hyoid advancement or by hypoglossal muscle stimulation (3). Adding bulk creates a model that is consistent with clinical observations that tongue size (fat more than muscle) is particularly important in the pathogenesis of OSA in people (18). In the present study, the rabbit was briefly anesthetized with propofol intravenously (1.4 mg/kg), and then ~0.5 mL hyaluronic acid (Juvéderm, Madison, NJ) was injected directly into the tongue base. The objective of this injection was to produce partial obstruction during sleep. Two to three injections spaced two to three weeks apart were needed to produce audible upper airway stertor during sleep. Following the final injection, the rabbits recovered for 2 wk and then underwent survival surgery for implantation of the HNS electrode.
Survival surgery and hypoglossal nerve electrode placement.
The night before the surgery, the rabbit was premedicated with cefovecin sodium (Zoetis, Parsippany, NJ) 0.045 mg/lb. Prior to midnight, the animal was provided with food and water ad libitum but was not fed after midnight (NPO). On the morning of surgery, the animal was given buprenorphine 0.02 mg/kg sc 20 min before the start of surgery. During surgical preparation, a venous catheter was placed in the ear. A surgical plane of anesthesia was achieved with propofol 14 mg/kg iv. Just before the initial incision, localized subcutaneous marcaine 4 mg/kg was applied to ensure regional analgesia.
Custom nerve cuff electrodes were developed for the rabbit CNXII (Ardiem Medical, Indiana, PA). The internal cuff diameter was either 0.75 or 1.0 mm. Each cuff contained four platinum iridium (PtIr) cathodes. Cathodes were 0.5 mm × 1.0 mm and positioned ~90° apart around the perimeter of, and near one edge of the cuff. A single, circumferential anode with a length of 1 mm was positioned near the other edge of the cuff. The lead length was 40 cm.
The surgical procedure was performed with binocular magnification. A 3-cm vertical incision was made in the ventral midline. The dissection was directed lateral to the laryngotracheal complex and medial to the vascular sheath. The greater horn of the hyoid was identified and just deep to that structure the main trunk of the hypoglossal nerve could be reliably found. Using fine microdissection technique, the nerve was carefully mobilized distally until it separated into medial (which innervates the genioglossus) and lateral branches. The vasa-nervorum were left intact. The mylohyoid muscle was retracted to allow for distal dissection of the medial branch which was mobilized sufficiently to allow for atraumatic stimulation of the nerve. A 1-mm nerve cuff was placed around the main trunk of the hypoglossal nerve before the nerve arborized. A strain relief loop was created, and the lead was sutured to fascia, adjacent to the hyoid bone. A 0.75-mm electrode was placed around the medial branch just distal to where it takes off as a distinct branch from the main trunk (see Fig. 1). Cuff leads were tunneled subcutaneously to the back of the neck where they emerged into a tether lead. Externalized leads were placed in a steel wire reinforced lined pouch. The leads were tested for continuity at multiple stages (before tunneling, and before and after the tether was in place). Following surgery, the unterminated leads were stripped of insulation using hot tweezers. Each lead was crimped into a gold-plated ITT Cannon pin and pushed into a head stage for easy connection to the stimulator. When not in use, the externalized leads and pouch were secured with an animal harness.
Fig. 1.
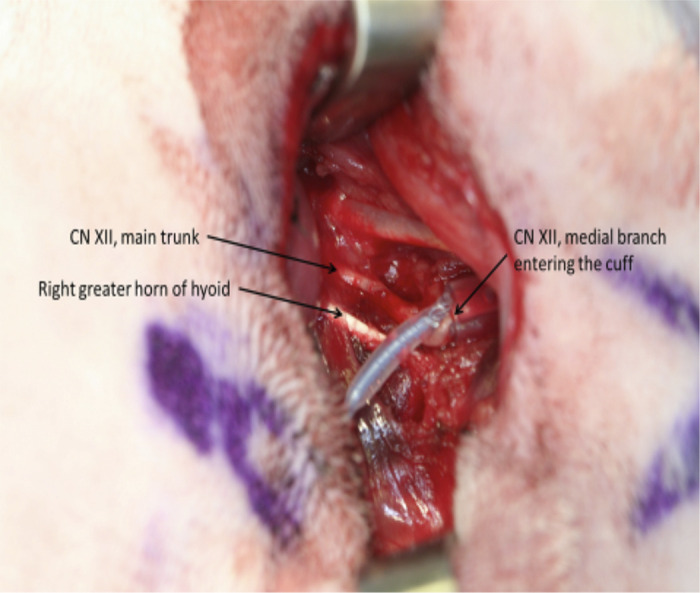
Surgical exposure in the right neck of a rabbit (magnification 3×). The main trunk of the hypoglossal nerve (CN XII) is noted, as is its relationship to the greater horn (cornu) of the hyoid bone. Also shown is a 0.75-mm electrode wrapped around the medial branch of the hypoglossal nerve as it moves anteriorly (rostrally) to innervate the genioglossus muscle. The 1.0-mm cuff has not yet been placed around the main trunk of the nerve.
Following surgery, each rabbit was recovered and provided routine care. Carprofen 4 mg/kg sc (NSAID) was administered for 2–3 days. Fluids were provided subcutaneously at a rate of 60 ml·kg−1·day−1. Following the HNS placement, the rabbits did not lose weight during convalescence from the HNS surgery.
Nerve stimulation titration.
The animals were given 4 wk to heal from the HNS implantation at which time rabbits were intermittently tested for electrode impedance. When impedance had reached an acceptably low level a test of the HNS system was done. On a day before the unanesthetized experiment, the rabbit was anesthetized with propofol 14 mg/kg iv. A programmable stimulator delivering a charge-balanced cathode-first stimulus (standard stimulation paradigm in clinical practice) was attached to the percutaneous leads. The stimulus pulse width was set to 250 μs and the pulse amplitude was increased in 0.1-mA increments starting from 0.1 mA until a response in the tongue was observed. The electrodes to both the main trunk of the hypoglossal nerve as well as the medial branch (innervating the genioglossus—a protrusor) were tested separately. The pulse amplitude that first elicited a response as well as the response elicited were noted. Each of the four cathodes in each cuff was tested independently. The channel that elicited tongue protrusion at the lowest pulse amplitude was determined and used for unanesthetized experiments.
Unanesthetized monitoring.
Prior to the laboratory study, animals were monitored videographically for 3–6 days to determine the optimal time for monitoring for sleep. A trail camera with movement tracker was used to monitor the cage. Over a 24-h time period the movement detector in control animals showed no variation over time, and visual inspection showed inactivity for ~30 min each hour with short bouts of activity being common and associated with environmental noises (air conditioning and ventilation cycles, door opening and closing even down the hall, etc.). In the filler injected animals two periods of prolonged inactivity occurred between 4:00 and 6:00 AM and 11:00 AM to 3:00 PM. As a result, all animals were brought into the recording laboratory for 2 h between 10:30 AM and 2:30 PM.
Respiratory flow at the nose, respiratory effort, electroencepholograms (EEGs), an electrocardiogram (ECG) and heart rate, and blood oxygenation (SpO2) were collected during each experiment, as was a trigger signal from the nerve stimulator. All data were collected and sampled at 2,500 Hz. using the multichannel MP150 data-acquisition system and AcqKnowledge 4.4 software (Biopac Systems, Goleta, CA).
Regions of the rabbit’s head, haunch, and chest were shaved. To collect EEG and heart rate data, gold-plated cup electrodes filled with electrode gel were placed over the C3-C4 regions, bilaterally, and referenced to the A1 skin patch. To collect ECG data, gel electrodes were attached to the rabbit’s haunch, and left and right chest. EEG and ECG electrodes were connected to 3 bioelectric modules. To collect SpO2 data, an infrared sensor was placed on the ear or on the shaved haunch—whichever had the best pulsatile signal—and attached to the Biopac DC amplifier. Respiratory flow was measured via a nasal cannula which was inserted into the rabbit’s nostrils. One end of the cannula was attached to an oxygen supply with a flow regulator, so that if there was sustained hypoxemia (>90% for 3–4 min) during the study oxygen could be delivered by cannula; this was required by the animal safety committee but no animal needed supplemental oxygen during the monitoring protocol. A small heavily perforated (low resistance) muzzle was placed on the rabbit to hold the nasal cannula in place and prevent dislodgement.
The rabbit was placed in 2 × 3 × 2 ft acrylic locking box with a hinged lid (a plethysmography chamber). The box had 2 small holes in it that allowed for rubber tubing to be inserted into the box to gather flow data and deliver oxygen. The flow transducer was attached to a differential amplifier (DA100C). The cannula was attached to a pressure transducer (TSD160A) and differential amplifier (Biopac DC amplifier (TSD160A). A reduction or zero pressure differential from the nasal cannula indicated that the rabbit could be classified as having an apnea (>70% reduction in flow) or hypopnea (>30% reduction). An additional opening in the box allowed wires to be passed into the box from the stimulator to the electrodes and could be opened if required. The stimulator cables were attached to the cuff electrode selected during the nerve stimulation titration and the cuff anode. The hole through which the stimulator cables passed was sealed with a self-sealing plastic film. The programmable stimulator was set to a pulse frequency of 40 Hz, a pulse width of 250 μs, and a pulse amplitude that ranged from 0.7 to 2.4 mA (for either the main CN XII trunk or medial branch) based on the nerve stimulation titration described earlier. Stimulus was controlled via a laptop computer.
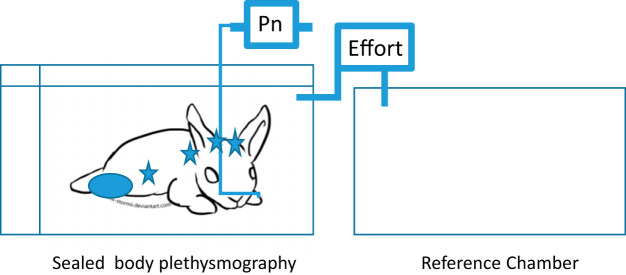
During data collection, the rabbit remained in the acrylic box (Fig. 2). To indicate effort, the air pressure in the sealed chamber was sampled by a differential flow transducer, referenced to a second, sealed, empty, equally sized chamber. Persistence of a pressure differential in the box at the frequency of breathing and an absence of pressure swings (flow) at the nose indicated obstruction. If there were no such nasal fluctuations but and flow differences between the chambers, this would be called a nonobstructive (central) apnea.
Fig. 2.
The experimental setup. The drawing shows a schema of the experimental setup. The drawing illustrates the body box setup with the recording and reference chamber. The stars represent the general electrode placement for EEG, oximeter, and ECG. Pressure at the nares (Pn) by nasal cannula was to monitor flow. Effort was recorded as the pressure of the box is referenced to an empty box of the same size.
At the start of each session, there was a period of up to 10 min during which we allowed the rabbit to acclimate to the box and/or stimulation. Following acclimation, the rabbit underwent consecutive 30-min sessions. The first session recorded for 30 min with the stimulator turned off; the second was with the stimulator turned on. If time permitted (in the animals’ natural tranquil period), the sequence was repeated. In 2 chronic animals who could not sleep for more than 10 min at a time in the mandated 2-h recording window, we premedicated with diazepam 0.5–1 mg/kg to collect data in the experimental setup. The diazepam left the animal in a mildly drowsy state, but arousable with intentional noise.
Data analysis.
Post hoc analysis of the data was performed to quantify sleep and wake. This was determined by analysis of the EEG in combination with notes on the behavioral state of the animal recorded every 5 min. Heart rate was recorded as an average over 5 min and a summary provided for each animal (mean ± SD). Throughout the experiment, the pressure and flow were analyzed to determine respiratory rate in wake or sleep (mean ± SD), and inspected for pauses in breathing. Breathing pauses were categorized as obstructive if flow was absent or partially obstructive if there was a reduction in flow by <30% with persistence of effort. If breathing effort was absent on the plethysmograph, then the pause was defined as nonobstructive. Human sleep apnea is categorized by the lack of flow for at least 2.5 respiratory cycles. We created categories of severity based on pauses using the number of missed respiratory efforts. Fewer than 3 pauses with effort cycles was categorized as short, from 3 to 7 effort cycles as intermediate, and ≥8 efforts as long. Number and severity of apneas were compared from periods without stimulation to periods with stimulation. The SpO2 profile from these periods was also compared. Differences with P < 0.05 were considered significant.
RESULTS
Great care was required to maintain the animal in a calm unagitated state during handling. Even during brief periods of anesthesia a careful balance was struck by matching the depth of anesthesia to the invasiveness of the procedure (whether a tongue base injection or a HNS implantation). Carefully managing recovery to alleviate any discomfort improved and shortened acclimation to the procedures. The volume of tongue base injection was selected based on what the animal could tolerate (0.5 cc hyaluronic acid). Even after implementing the aforementioned factors injection produced a substantial change in behavior in food and water consumption that lasted as long as 3–4 days required subcutaneous fluids and resulted in a 5–10% weight loss. This completely resolved with weight restoration 4–7 days following injection. One animal required 2 injections and 2 animals required 3 injections to create audible obstruction (snoring and stertor) observed during sleep. In addition to hearing audible snoring the results presented below represent data collected during which two other conditions were met. First, an animal had to sleep for at least 30 continuous minutes. Second, for rabbits implanted with the HNS the implantation was successful if impedances were sufficiently low (4.6 ± 0.6 kΩ for the main CNXII trunk and 4.0 ± 0.3 kΩ for the medial branch) and stimulation could be performed without animal discomfort.
The useable data examined and presented here were collected from 3 rabbits who had received two to three injections of the tongue base (having exhibited audible snoring during sleep) as well as HNS devices. Two rabbits that received saline injections without stimulator implantation (active control), and 1 animal in which no injections were performed (passive control) were just monitored during sleep. Figure 3 illustrates pauses according to breathing rate in which there is absence of airflow but persistence of respiratory efforts.
Fig. 3.
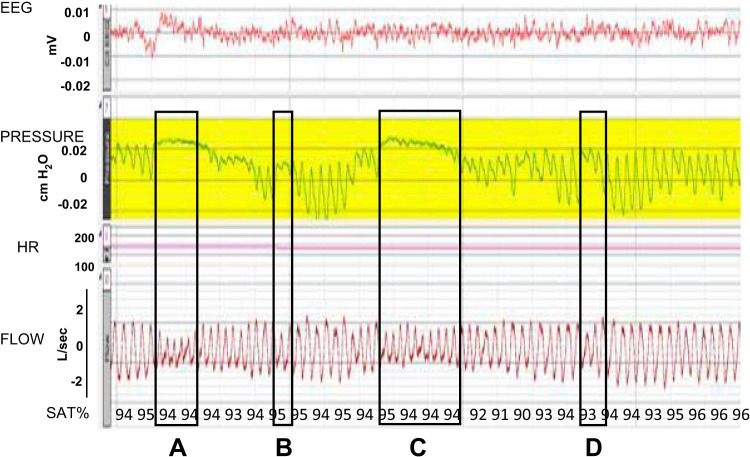
Examples of sleep disordered breathing with definition of pause length. A strip chart recording in seconds illustrating four of the core measures (from the top) of EEG, pressure (nasal airflow), heart rate (HR), box flow (breathing efforts), and oxygen saturation (SAT %). The four boxed areas illustrate pauses of varying lengths as defined by the effort signal. The first (A) is an intermediate, ~7 breaths, pause; the second (B) is a short pause, ~2 cycles; the third (C) is a long pause with ~9 breaths pause; and the last (D) is a short pause (2 effort cycles). Only the rabbits with hyaluronic acid injections exhibited intermediate and long cycle pause.
Based upon visual inspection of the plethysmography output signals no animals exhibited breathing pauses during wakefulness. During sleep, the control animals (passive and active) had only brief pauses, <1 apnea per 30 min. Resting respiratory rate and heart rate during quiet wakefulness and sleep in control animals was 114 breaths/min (range 98–128) when not sniffing and 169 beats/min (range 170–184), respectively, and during sleep 83 breaths/min (range 81–85) and 165 beats/min (range: 160–171), respectively. In the injected rabbits, values for respiratory rate were 124 breaths/min (range 114–130), when not sniffing, and heart rate was 189 beats/min (range 185–196) during wakefulness; corresponding values during quiet sleep were 104 breaths/min (range 50–67) and 178 beats/min (range 162–195).
For rabbits receiving the hyaluronic acid injections, there was variability in the severity of upper airway obstruction during sleep between the three animals. Two of the animals had mostly hypopneas of intermediate length (3–7 effort cycles) at a rate of 8–11 per 30 min of sleep with only occasional hypopneas characterized as long (≥8 effort cycles) at a rate of 1–3 per 30 min of sleep; in both of these animals, these moderate and longer events were no longer prominent in the 3rd and 4th sessions. One of the experimental animals showed a more significant response to the filler injection and HNS treatment. For this animal, Fig. 4 illustrates the effects of stimulation on the pause rate during sleep. During stimulation, the rates for short and moderate length pauses fell, and there were no long pauses at all.
Fig. 4.
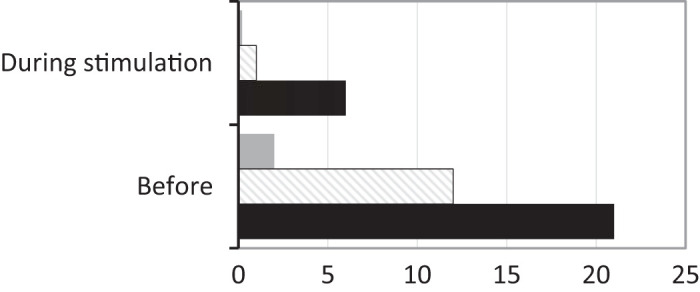
Event rates without and with HNS. This bar graph illustrates in the experimental animal with the sleep disordered breathing the effects during stimulation on the pause rate, in 30 min of sleep in each condition. The bars represent the number of pauses before and during stimulation: short (<3 effort cycles without airflow) indicated with black bars, intermediate (3–7 effort cycles without airflow) with diagonal lines and long (≥8 effort cycles without airflow) by gray bars. Long and intermediate events were largely eliminated.
Oxygen saturation values in general during wakefulness were high and similar among all animals (≥96%): in animals with filler injection, values were consistently 96% while in those not injected saturation levels were 97–99%. During sleep the mean O2 saturation of animals with hyaluronic acid injections averaged 96% (range 95–97%) as compared with 98% (range: 96–99%) for control animals.
Stimulus to the main trunk of the hypoglossal nerve had little discernable affect whereas stimulating the medial branch had notable affects that could be readily observed. During HNS of the medial nerve the median oxygen saturation increased from 95.1% to 95.3% (Fig. 5). When sampling the oxygenation at multiple time points before and during stimulation and averaging the values, the difference was found to be significant (P < 0.001, ANOVA). Further, the percent of observations at 95% oxygen saturation decreased from 35% before stimulation to 28% during stimulation (P < 0.0001, test of two proportions). The degree of oxygen desaturation, even in the most severe of the experimental animals, does not indicate hypoxemia to any significant degree.
Fig. 5.
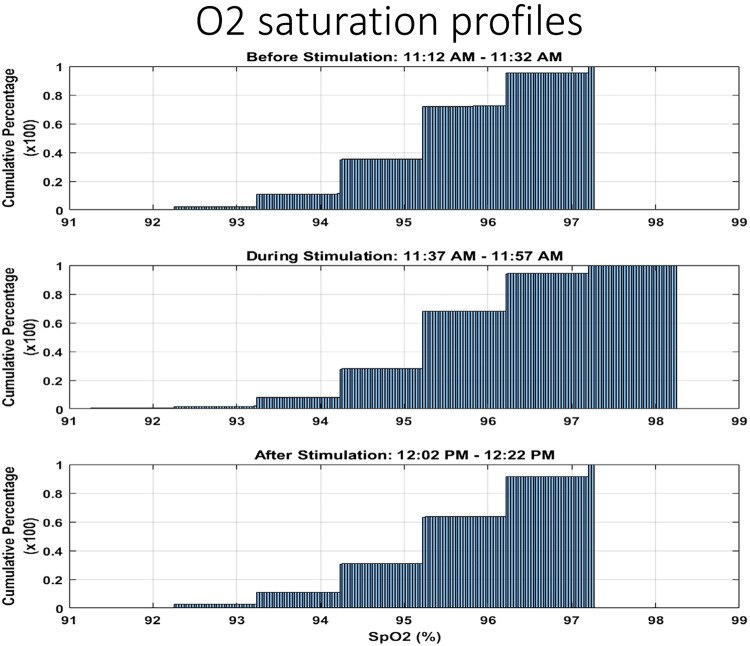
Effects on oxygen saturation of stimulation. The three histograms illustrate oxygen saturation profiles before (top), during (middle), and after (bottom) a stimulation trial. Each illustrates a 20-min period sample during sleep. Sleep efficiency was ~85%. The oxygen saturation profile improved. The steplike appearance is the rounding function of saturation values.
DISCUSSION
This study explores the feasibility of creating a chronic unanesthetized rabbit model of upper airway obstructions during sleep. The sleep apnea is induced by injecting hyaluronic acid into the base of the tongue. We confirm a prior report that uses polyacrylamide gel injections in the soft palate in an attempt to cause upper airway obstruction (13) and another injecting silicone into the tongue base (27). In our study, tongue base injection was used to recreate retrolingual obstruction which was thought to be a more appropriate anatomic model for evaluating HNS efficacy. As previously noted, we have prior successful experience with this model (2). Animals developed apneas and hypopneas of varying lengths related to airway obstruction during sleep that were not present in wakefulness. The approach we report does not produce a predictable number of events, but all those with filler injections experienced sleep pauses of all types. No pauses of >2–3 respiratory cycles were evident during sleep or wakefulness in control animals. Also, we show that it is possible to stimulate the hypoglossal nerve (medial branch) and in doing so, alleviate pathological upper airway obstructions without behaviorally disturbing the animal or its ability to enter or maintain sustained sleep. This HNS improved oxygen saturation values over time. Shorter pauses in breathing, which were not associated with desaturations, were only slightly influenced by HNS. The effects last up to 6 mo.
The use of an animal model like the rabbit has been employed before. The report of producing OSA by paralysis of the genioglossus muscle is not useful for a study of HNS (11). Our model was based upon prior reports of the effect of synthetics injection in anesthetized and unanesthetized animals (13). In the Yu et.al. study (27) using silicone, 1 mo following injection rabbits had a mean of 29 hypopneas/hour and a mean of 10 apneas per hour. Three months following injection the animals had 28 hypopneas per hour and 10 apneas per hour, demonstrating durability. We chose not to use silicone due to a tendency for the material to unpredictably diffuse away from the targeted area over prolonged periods of time. Our choice of hyaluronic acid also produced a durable result 0.5 and 6 mo after injection.
As the rabbit is an obligate nasal breather there was no need for an oral measure of flow. The indication of effort was made using a measure of the differential pressure between the box housing the animal and a reference box. This is not the usual adiabatic method (the seal was not hermetic) but the air seal was sufficient such that the pressure differential that existed allowed for reliable measurements of respiratory-related excursions. In larger animals like the rabbit this is possible, as compared with rodents. Animal metrics for an apnea are not defined in the literature, so we took the approach of defining pause length in terms of breathing frequency (respiratory rate). The human definition is based on an assumption that a cessation of airflow ≥2.5 respiratory cycles (~10 s) is likely to have consequences on sleep and oxygenation (ASSM definitions), and post-sigh apneas are excluded. In this study we report pauses >1 respiratory cycles as clinically relevant events and divided these into short (<3 cycles), intermediate (3–7 cycles), and long (≥8 cycles). The effect of hypoglossal nerve stimulation was to reduce disproportionately the longer pauses in breathing that were associated with respiratory efforts, and less so the short ones which more often occurred after a sigh.
The effects of HNS are highly likely the result of efferent (motor) innervation. Although CNXII has been found to carry some afferent fibers (there are cerebellar nucleo-olivary projections from the sensory component to other cranial nerves) these are not likely to have mediated a reduction in upper airway resistance (14). This is consistent with our work in the anesthetized animal in which sensory innervation via the sciatic nerve did not effect a reduction in upper airway resistance while HNS (medial branch) did lower resistance. Interestingly, sciatic nerve stimulation did produce a change in respiratory drive while HNS stimulation did not (17). Another reason to attribute the reduction in OSA symptoms directly to HNS in this model is the absence of behavioral or macro-EEG evidence for arousal. The increase in overall oxygen saturation that occurred with stimulation was independent of greater wakefulness.
The effect of stimulation was not only to directly increase the nadir and median of oxygen saturation but also the amount of time at higher oxygen saturations. Speculatively, other pathological mechanisms like partial upper airway obstruction during sleep or even chronic upper airway partial obstruction could have produced hypoventilation and an increase in arterial carbon dioxide levels resulting in a decrease in alveolar oxygen levels and hypoxemia. For this matter there was no significant difference in respiratory frequency or oxygen saturation (relative to baseline) in comparison to control animals; however, tidal volume was not quantified so that we could not estimate possible hypoventilation. This result, in our opinion, represents the reverse of both mechanisms for mild hypoxemia—some hypoventilation and obstructive events—before and after HNS periods. The oxygen saturation values in these animals however were generally above a concerning range, as seen in the human condition.
This model and study do have limitations. The most significant is that the rabbit is a prey animal, with an inherently high vigilance level. The environment in the animal research facility turned out to be a critical factor for success—any noise in the room vent, outside in the hall, or in adjacent rooms would wake up or startle a sleeping animal. Compared with a dog, which can be studied more easily in sleep and with stimulation (25, 26), a rabbit was (in our hands) less likely to remain asleep without a very quiet environment or a pharmacological agent. We did not formally explore sedation as a part of this model; however, our experience was that, with a considerable amount of attention to regulating the rabbit’s environment, neither breathing nor oxygen saturation appeared to be significantly affected by animal’s high level of vigilance. It should be noted that values for breathing and heart rates are higher than in the normal uninstrumented and unrestrained rabbit, in controls as well as injected animals. However, in trialing the GABA agonist we found that this did permit the rabbit to achieve sustained sleep for the study period, alleviating some of the difficulty in creating a better “tranquil” environment. Additionally, we believe that the same breed of rabbits obtained from different vendors have varying levels of “vigilance” or acceptance of the laboratory and animal resource environment. Rabbits from one vender were docile and accustomed to human contact while those from another were not.
We offer the following suggestions to others who might want to continue this work. Future experiments should employ implanted wireless telemetry for all signals to obviate the need for wires. Prolonged recordings in the home cage setting could minimize the need for pharmacological aids to encourage sedation and sleep. An experienced staff is needed to interact with each rabbit in a consistent manner, and experiments should be conducted in sound-proof or sound-reduced environment.
Finally, for the model to be more reliable it will need to generate a substantially greater number of events. This will require a considerable amount of time allowing the animal to habituate to its home environment. Remote wireless and continuous monitoring will then likely facilitate capture of natural sleep-wake behavior and examination of off-target effects on blood pressure or cardiovascular outcomes or to replicate the closed-loop feedback control technology used clinically. The Inspire Medical device uses an intermittent stimulation pattern triggered by intrathoracic pressure (20). Other devices in development use different cuff electrode configurations and numbers as well as proprietary stimulation patterns without respiratory feedback to cause a reduction in upper airway resistance. We would suggest that modeling work and study designs for stimulation be tested in the rabbit as a means of better understanding treatment effects. One obstacle however would be downscaling the size of the more complex electrodes to adapt it to the rabbit size.
What we have learned from this and other studies of neurostimulation is that such approaches in the rabbit model are feasible but that each intervention has its distinct impact on respiratory drive and upper airway function. HNS reduces upper airway resistance but does not substantially change drive to the diaphragm of intercostal (chest wall) muscles. Sciatic nerve stimulation increases drive in both upper airway and chest wall muscles, but the effects on upper airway resistance are negligible. In contract, pacing of the phrenic nerve or diaphragm muscle pacing does not change tone in the upper airway muscles, so that activation of the chest wall muscles can produce an apnea. An ideal neurostimulation would both increase drive and decrease upper airway resistance, preferably through one access point.
Conclusions.
This study establishes proof-of-concept in an unanesthetized rabbit model for creating OSA via an injection of hyaluronic acid into the base of tongue and then stimulating the hypoglossal nerve as a means for relieving the chronic upper airway obstruction. It is consistent with our prior studies in anesthetized animals, and with other reports of unanesthetized rabbit models. While it provides one example of a preclinical approach, it is not a high-throughput model and takes dedicated expertise in handling the species as well as the instrumentation.
GRANTS
This work was funded in part by NIH-SPARC Award NIA-STTR R41AG047658 and the VA Rehabilitation Service SPiRE Pilot Award 1I21RX002041.
DISCLOSURES
K. P. Strohl is a site PI for the ADHERE post-FDA approval registry for the Inspire System. No conflicts of interest, financial or otherwise, are declared by the other authors.
AUTHOR CONTRIBUTIONS
M.S., J.P.B., and K.P.S. conceived and designed research; M.S., J.G., J.P.B., and K.P.S. performed experiments; M.S. and K.P.S. analyzed data; M.S. interpreted results of experiments; M.S., J.P.B., and K.P.S. prepared figures; M.S., J.G., and J.P.B. edited and revised manuscript; M.S., J.G., J.P.B., and K.P.S. approved final version of manuscript; K.P.S. drafted manuscript.
REFERENCES
- 1.Ateş S, Karakurum E, Dursun N. Origin, course and distribution of the hypoglossal nerve in the New Zealand rabbit (Oryctolagus cuniculus L). Anat Histol Embryol 40: 360–364, 2011. doi: 10.1111/j.1439-0264.2011.01080.x. [DOI] [PubMed] [Google Scholar]
- 2.Bellemare F, Pecchiari M, Bandini M, Sawan M, D’Angelo E. Reversibility of airflow obstruction by hypoglossus nerve stimulation in anesthetized rabbits. Am J Respir Crit Care Med 172: 606–612, 2005. doi: 10.1164/rccm.200502-190OC. [DOI] [PubMed] [Google Scholar]
- 3.Benderro GF, Gamble J, Schiefer MA, Baskin JZ, Hernandez Y, Strohl KP. Hypoglossal nerve stimulation in a pre-clinical anesthetized rabbit model relevant to OSA. Respir Physiol Neurobiol 250: 31–38, 2018. doi: 10.1016/j.resp.2018.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carneiro-Barrera A, Díaz-Román A, Guillén-Riquelme A, Buela-Casal G. Weight loss and lifestyle interventions for obstructive sleep apnoea in adults: Systematic review and meta-analysis. Obes Rev 20: 750–762, 2019. doi: 10.1111/obr.12824. [DOI] [PubMed] [Google Scholar]
- 5.Chopra S, Polotsky VY, Jun JC. Sleep Apnea Research in Animals. Past, Present, and Future. Am J Respir Cell Mol Biol 54: 299–305, 2016. doi: 10.1165/rcmb.2015-0218TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis EM, O’Donnell CP. Rodent models of sleep apnea. Respir Physiol Neurobiol 188: 355–361, 2013. doi: 10.1016/j.resp.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Decker MJ, Haaga J, Arnold JL, Atzberger D, Strohl KP. Functional electrical stimulation and respiration during sleep. J Appl Physiol (1985) 75: 1053–1061, 1993. doi: 10.1152/jappl.1993.75.3.1053. [DOI] [PubMed] [Google Scholar]
- 8.Drager LF, Polotsky VY, O’Donnell CP, Cravo SL, Lorenzi-Filho G, Machado BH. Translational approaches to understanding metabolic dysfunction and cardiovascular consequences of obstructive sleep apnea. Am J Physiol Heart Circ Physiol 309: H1101–H1111, 2015. doi: 10.1152/ajpheart.00094.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med 188: 996–1004, 2013. doi: 10.1164/rccm.201303-0448OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Epstein LJ, Kristo D, Strollo PJ Jr, Friedman N, Malhotra A, Patil SP, Ramar K, Rogers R, Schwab RJ, Weaver EM, Weinstein MD; Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine . Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med 5: 263–276, 2009. doi: 10.5664/jcsm.27497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee MC, Lee CH, Hong SL, Kim SW, Lee WH, Lim JY, Joe S, Yoon IY, Kim JW. Establishment of a rabbit model of obstructive sleep apnea by paralyzing the genioglossus. JAMA Otolaryngol Head Neck Surg 139: 834–840, 2013. doi: 10.1001/jamaoto.2013.4001. [DOI] [PubMed] [Google Scholar]
- 12.Lin HC, Friedman M, Chang HW, Gurpinar B. The efficacy of multilevel surgery of the upper airway in adults with obstructive sleep apnea/hypopnea syndrome. Laryngoscope 118: 902–908, 2008. doi: 10.1097/MLG.0b013e31816422ea. [DOI] [PubMed] [Google Scholar]
- 13.Lu HY, Dong F, Liu CY, Wang J, Liu Y, Xiao W. An animal model of obstructive sleep apnoea-hypopnea syndrome corrected by mandibular advancement device. Eur J Orthod 37: 284–289, 2015. doi: 10.1093/ejo/cju041. [DOI] [PubMed] [Google Scholar]
- 14.Popratiloff AS, Streppel M, Gruart A, Guntinas-Lichius O, Angelov DN, Stennert E, Delgado-García JM, Neiss WF. Hypoglossal and reticular interneurons involved in oro-facial coordination in the rat. J Comp Neurol 433: 364–379, 2001. doi: 10.1002/cne.1145. [DOI] [PubMed] [Google Scholar]
- 15.Remmers JE, deGroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol 44: 931–938, 1978. doi: 10.1152/jappl.1978.44.6.931. [DOI] [PubMed] [Google Scholar]
- 16.Safiruddin F, Vanderveken OM, de Vries N, Maurer JT, Lee K, Ni Q, Strohl KP. Effect of upper-airway stimulation for obstructive sleep apnoea on airway dimensions. Eur Respir J 45: 129–138, 2015. doi: 10.1183/09031936.00059414. [DOI] [PubMed] [Google Scholar]
- 17.Schiefer M, Gamble J, Strohl KP. Sciatic nerve stimulation and its effects on upper airway resistance in the anesthetized rabbit model relevant to sleep apnea. J Appl Physiol (1985) 125: 763–769, 2018. doi: 10.1152/japplphysiol.00225.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwab RJ, Pasirstein M, Kaplan L, Pierson R, Mackley A, Hachadoorian R, Arens R, Maislin G, Pack AI. Family aggregation of upper airway soft tissue structures in normal subjects and patients with sleep apnea. Am J Respir Crit Care Med 173: 453–463, 2006. doi: 10.1164/rccm.200412-1736OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strohl KP, Butler JP, Malhotra A. Mechanical properties of the upper airway. Compr Physiol 2: 1853–1872, 2012. doi: 10.1002/cphy.c110053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strollo PJ Jr, Soose RJ, Maurer JT, de Vries N, Cornelius J, Froymovich O, Hanson RD, Padhya TA, Steward DL, Gillespie MB, Woodson BT, Van de Heyning PH, Goetting MG, Vanderveken OM, Feldman N, Knaack L, Strohl KP; STAR Trial Group . Upper-airway stimulation for obstructive sleep apnea. N Engl J Med 370: 139–149, 2014. doi: 10.1056/NEJMoa1308659. [DOI] [PubMed] [Google Scholar]
- 21.Sutherland K, Vanderveken OM, Tsuda H, Marklund M, Gagnadoux F, Kushida CA, Cistulli PA. Oral appliance treatment for obstructive sleep apnea: an update. J Clin Sleep Med 10: 215–227, 2014. doi: 10.5664/jcsm.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weaver TE, Sawyer AM. Adherence to continuous positive airway pressure treatment for obstructive sleep apnoea: implications for future interventions. Indian J Med Res 131: 245–258, 2010. [PMC free article] [PubMed] [Google Scholar]
- 23.Woodson BT, Gillespie MB, Soose RJ, Maurer JT, de Vries N, Steward DL, Baskin JZ, Padhya TA, Lin HS, Mickelson S, Badr SM, Strohl KP, Strollo PJ Jr; STAR Trial Investigators . Randomized controlled withdrawal study of upper airway stimulation on OSA: short- and long-term effect. Otolaryngol Head Neck Surg 151: 880–887, 2014. doi: 10.1177/0194599814544445. [DOI] [PubMed] [Google Scholar]
- 24.Yaggi HK, Strohl KP. Adult obstructive sleep apnea/hypopnea syndrome: definitions, risk factors, and pathogenesis. Clin Chest Med 31: 179–186, 2010. doi: 10.1016/j.ccm.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 25.Yoo PB, Durand DM. Effects of selective hypoglossal nerve stimulation on canine upper airway mechanics. J Appl Physiol (1985) 99: 937–943, 2005. doi: 10.1152/japplphysiol.00652.2004. [DOI] [PubMed] [Google Scholar]
- 26.Yoo PB, Sahin M, Durand DM. Selective stimulation of the canine hypoglossal nerve using a multi-contact cuff electrode. Ann Biomed Eng 32: 511–519, 2004. doi: 10.1023/B:ABME.0000019170.74375.fb. [DOI] [PubMed] [Google Scholar]
- 27.Yu MS, Jung NR, Choi KH, Choi K, Lee BJ, Chung YS. An animal model of obstructive sleep apnea in rabbit. Laryngoscope 124: 789–796, 2014. doi: 10.1002/lary.24398. [DOI] [PubMed] [Google Scholar]