Abstract
The skeletal muscle is an integrated multicomponent system with complex dynamics of continuous myoelectrical activation of various muscle types across time scales to facilitate muscle coordination among units and adaptation to physiological states. To understand the multiscale dynamics of neuromuscular activity, we investigated spectral characteristics of different muscle types across time scales and their evolution with physiological states. We hypothesized that each muscle type is characterized by a specific spectral profile, reflecting muscle composition and function, that remains invariant over time scales and is universal across subjects. Furthermore, we hypothesized that the myoelectrical activation and corresponding spectral profile during certain movements exhibit an evolution path in time that is unique for each muscle type and reflects responses in muscle dynamics to exercise, fatigue, and aging. To probe the multiscale mechanism of neuromuscular regulation, we developed a novel protocol of repeated squat exercise segments, each performed until exhaustion, and we analyzed differentiated spectral power responses over a range of frequency bands for leg and back muscle activation in young and old subjects. We found that leg and back muscle activation is characterized by muscle-specific spectral profiles, with differentiated frequency band contribution, and a muscle-specific evolution path in response to fatigue and aging that is universal across subjects in each age group. The uncovered universality among subjects in the spectral profile of each muscle at a given physiological state, as well as the robustness in the evolution of these profiles over a range of time scales and states, reveals a previously unrecognized multiscale mechanism underlying the differentiated response of distinct muscle types to exercise-induced fatigue and aging.
NEW & NOTEWORTHY To understand coordinated function of distinct fibers in a muscle, we investigated spectral dynamics of muscle activation during maximal exercise across a range of frequency bands and time scales of observation. We discovered a spectral profile that is specific for each muscle type, robust at short, intermediate, and large time scales, universal across subjects, and characterized by a muscle-specific evolution path with accumulation of fatigue and aging, indicating a previously unrecognized multiscale mechanism of muscle tone regulation.
Keywords: aging, fatigue, muscle fibers, spectral power, time scales
INTRODUCTION
The skeletal muscle is a complex system composed of multiple muscle fibers that respond individually and differently to a myriad of environmental influences (75). According to their specific myosin heavy chain expression, muscle fiber types range from slow/oxidative to fast/glycolytic (7, 8, 63) and present particular frequency profiles in response to fatigue. The literature on the use of frequency domain parameters assessing skeletal muscle fatigue is extensive. However, there is limited research focusing on the evolution in time of the spectral power profile of frequency bands representing different muscle fibers activation and the specific contribution of different muscle fiber frequency bands in response to exercise-induced fatigue and aging. Thus, the underlying multiscale regulatory mechanism remains not understood.
Mean frequency and median (center) frequency are the traditionally utilized physiological measures to evaluate skeletal muscle fatigue in electromyographical (EMG) signals (12, 67). However, the lack of reproducibility of such frequency domain measures for different muscle groups across subjects and experimental protocols raises questions regarding their clinical utility in assessing skeletal muscle function (10, 58, 80). Moreover, these traditional measures cannot provide complete information on how the spectral profiles of muscle activation are modulated as a consequence of fatigue. For instance, fatigue-related decrease in EMG center frequency could be provoked by an increase in low-frequency power, a decrease in high-frequency power, or a combination of both (3).
An alternative method to assess muscle fatigue is to measure responses in the spectral power of different EMG frequency bands (10, 16, 23, 49, 65, 72, 77). Because muscle fatigue elicits specific changes in the spectral power for different EMG frequencies (15, 73), frequency band analyses enable more detailed characterization of the response of different muscle fibers in a given muscle as well as of different muscle types. Investigating separately the spectral intensity of low- and high-frequency EMG components has helped to determine the different contribution levels of slow- and fast-twitch muscle fibers (31), with recent applications to muscle fatigability (24, 26), diagnosis of patellofemoral pain syndrome (23), changes in voluntary effort (61), and joint positional variability (49). Furthermore, EMG frequency content and related spectral power characteristics have been utilized to study age-associated changes in neuromuscular control and assess sarcopenic muscle function (10).
Previous works in the field have mainly focused on separate “snapshots in time” to quantify spectral power characteristics of frequency bands and did not investigate how spectral profiles of muscle activation in different muscle types evolve in time in response to fatigue and age-related neuromuscular degeneration. However, fatigue- and age-induced physiological adaptations of skeletal muscle and muscle fibers continuously evolve as a result of soft-assembled states dwelling at different time scales and levels of biological system organization (36, 41, 81). Earlier studies have identified the presence of long-range power law correlations in wrist locomotion (37, 40) and gait dynamics (30) and related cardiovascular variables (44, 86) with invariant behavior at different time scales, indicating the presence of multiscale mechanisms underlying neural regulation of locomotion (1, 38). Therefore, because muscle activation is necessary for locomotion, and given that locomotion is characterized by scale-invariant characteristics over a broad range of time scales, we hypothesize that muscle activation will also exhibit scale-invariant profiles. More specifically, we hypothesize that 1) there is a particular evolution process in time that underlies muscle activation and related spectral power characteristics, 2) each muscle type is characterized by a spectral profile hat exhibits a muscle-specific evolution of different frequency bands in response to exercise-induced fatigue, 3) different muscle fibers within a given muscle are associated with specific time evolution paths of their spectral profiles, 4) spectral profiles of muscle activation exhibit similar characteristics across time scales, and 5) whereas the functional form of the spectral profile characterizing muscle activation may be similar in young and old subjects, old subjects exhibit a different evolution path with less pronounced increase of spectral power in response to exercise and fatigue. Establishing consistency in the spectral power profiles of different muscle types and robust evolution paths of these profiles over a range of time scales for all subjects in a given age group would reveal a universal behavior related to a basic mechanism of muscle tone regulation in response to exercise-induced fatigue.
To test our hypotheses, we developed a protocol that allowed us to identify and track simultaneously the evolution of the spectral power profiles of different muscle types and to study the multiscale mechanism underlying the differentiated response of different frequency bands to exercise-induced fatigue in young and old adults. Given that previous research on frequency banding mainly considered muscles in an isolated manner and by means of simple movements over short time segments, and because of the need to establish the relative contribution of trunk muscles together with leg muscles during more complex tasks and how it changes over prolonged periods of extended and repeated exercises (76), we used a protocol that included repeated long-squat exercise segments performed until exhaustion and interspersed by rest segments. The squat test can be considered as an administrable and reliable tool to simultaneously assess the activation of different muscle types and to measure the physical status in both young (5, 53) and old subjects (90). We collected EMG data from two different muscle types: the erector spinae back muscle composed of slow oxidative type I muscle fibers (9) and the vastus lateralis leg muscle composed of higher percentage of fast glycolytic type II muscle fibers (64, 83), both of which showed high myoelectrical activity during squats but with different levels of activation and contribution to the exercise effort (46).
Accordingly, we investigated the leg [vastus lateralis (VL)] and back [erector spinae (ES)] muscle spectral power profiles and their time evolution during three consecutive squat tests performed until exhaustion and four interspersed rest segments in healthy young and old adults. By quantifying the contribution of differentiated frequency bands to the spectral profile of the leg and back muscle, our study focused on the evolution of these profiles in response to accumulated and residual fatigue over long, intermediate, and short time scales, i.e., across consecutive exercise and rest segments, within exercise segments, and for a single squat movement.
METHODS
Participants and Inclusion Criteria
To determine the sample size for this study, a power analysis was conducted using G∗Power 3.1 (22). Previous research assessing fatigue effects on repeated exercise performed until exhaustion (25) has reported large effect sizes. Thus, using an effect size of d = 1.2, α < 0.05, power (1 − β) = 0.80, we estimated a minimum sample size = 20. Accordingly, 14 healthy young adults (6 males and 8 females: age 22.19 ± 13.56 yr, height 174.69 ± 10 cm, and mean body mass 66.81 ± 13.39 kg) and seven healthy old adults (3 males and 4 females: age 56.2 ± 2.95 yr, height 169 ± 11.93 cm, and mean body mass 73.42 ± 11.09 kg) were recruited to participate in the study. With the aim of ensuring a homogenous sample, participants were recruited strictly according to the following inclusion criteria: 1) aged 20–30 yr (healthy young adults group) or 50–60 yr (healthy old adults group), 2) BMI (in kg/m2) >18.5 and <30, 3) normal physical activity >5 and <10 h/wk, but without sport specialization, and 4) blood pressure <140/90 mmHg. Exclusion criteria consisted of 1) intake of prescribed drugs that could affect muscle strength, such as corticosteroids, 2) current or previous injury, either during the previous period before testing or at any other moment, going against the study protocol, and 3) any other condition that may have prevented the performance of an exercise protocol until exhaustion. The experiment was approved by the Clinical Research Ethics Committee of the Sports Administration of Catalonia and carried out according to the Helsinki Declaration. Before taking part in the study, participants read the study description and risks and signed an informed consent form (88).
Study Design and Test Protocol
In our protocol the participants visited the laboratory for two different sessions, separated by a 2-day interval. During the first session (i.e., familiarization), participants practiced the squat test until they were able to execute the movement according to the protocol (see study test protocol below). In the second session, participants performed the study test protocol.
We specifically selected the squat exercise because it demands the coordinated activity between lower back and leg muscles and has been recognized as a functional and safe movement that closely resembles complex everyday tasks (14). Furthermore, the squat is one of the most traditional resistance exercises used to enhance performance in sports and in lower-limb rehabilitation processes, as it develops powerful muscles that are activated during many functional tasks, such as running or jumping (21). Because the focus of this article is to identify spectral profiles of muscle activation that are specific for each muscle type and to track the evolution of this muscle-specific spectral profiles in response to exercise-induced fatigue, we utilized a maximal (i.e., squats performed until exhaustion) instead of a submaximal squat test. The use of a submaximal squat test would not provoke sufficiently high levels of muscle fatigue necessary to investigate changes in the spectral profiles of leg and back muscle activation and how these profiles evolve in the process of exercise from short to large time scales.
The protocol is composed of the following consecutive segments: 1) a 10-min rest period in supine position (rest 1), 2) a squat test performed until exhaustion (exercise 1), 3) a 10-min rest period in supine position (rest 2), 4) a squat test performed until exhaustion (exercise 2), 5) a 10-min rest period in the supine position (rest 3), 6) a squat test performed until exhaustion (exercise 3), and 7) a 10-min rest period in the supine position (rest 4).
Rest segments.
During rests 1, 2, 3, and 4, participants lay down in a supine position on a massage table. With the aim of avoiding joint compression and facilitating relaxation, we placed a pillow under the participants’ knees. Furthermore, we located another pillow under the back to avoid contact between the back electrodes and the table.
Exercise segments.
During exercises 1, 2, and 3, participants performed a squat test until exhaustion. The squat tests are performed according to the following instructions: “Place feet a little wider than shoulder-width apart. Extend the arms out straight. Initiate movement by inhaling and unlocking the hips, slightly bringing them back. Keep sending hips backward as the knees begin to flex. Squat down until touching the rope. Return to standing position. Repeat until exhaustion.” The rope was adjusted to a height where the participants’ thighs were parallel to the ground at the bottom of their squat. Participants were instructed to keep their chests up and weight over the heels and to not allow their knees to fall into a valgus position (5, 53, 78). Given that the back squat is used much more commonly compared with its front squat variation (89), and since the front squat requires higher ankle mobility (loss of ankle dorsiflexion is a common feature in young and old populations; see Ref. 69), the back squat was selected for the current study. The pace of the squat was controlled by means of a metronome (MetroTimer version 3.3.2, ONYX Apps), using a 3:3 tempo (3 s down and 3 s up, so 1 single squat lasts 6 s). The squat test was finished when participants were not able to squat down/up anymore or, alternatively, when they could not maintain the prescribed squat tempo.
The repetition of three consecutive squat tests performed until exhaustion allowed us to identify the effects of acute fatigue on the leg and back spectral power profiles and to track the evolution of the spectral power profiles with gradual accumulation of fatigue within each exercise segment. The short 10-min resting periods in our protocol led to only a partial recovery after a maximal squat test and allowed us to quantify the effects of residual fatigue reflected on spectral power profiles of leg and back muscle activation across consecutive exercise segments. Whereas acute fatigue occurs when the energy consumption exceeds the muscle aerobic capacity and a large fraction of the required energy has to come from anaerobic metabolism (11), residual fatigue is characterized by neuromechanical and biochemical alterations (e.g., decrease in maximal force) provoked by previous exercise (29).
Electromyography Acquisition
Participants were asked to wear appropriate clothing for access to the electromyography (EMG) electrode placement sites. Before the mounting of the EMG electrodes, the participants’ skin was shaved and cleaned using alcohol and left to dry for 60 s to reduce the myoelectrical impedance according to the SENIAM guidelines (33). The following muscles were investigated simultaneously during the whole study test protocol: left (VL-L) and right vastus lateralis (VL-R) and left (ES-L) and right erector spinae (ES-R). The placement of the surface electrodes (Ag/AgCl bipolar surface electrodes; Sorimex, Toruń, Poland) was also carried out according to the recommendations of the SENIAM organization and the Cram Guidelines (13). More specifically, vastus lateralis electrodes were placed at 2/3 on the line from the anterior spina iliaca superior to the lateral side of the patella, and the erector spinae electrodes were located at a two-finger width lateral from the spinous process of vertebra L1. After the electrodes were secured, a quality check was performed to ensure EMG signal validity. The aforementioned muscles were selected since they presented the highest myoelectrical activity during body weight squat (46).
EMG Signal Processing and Data Analysis
We recorded data using Biopac MP36 (Biopac Systems, Inc., Goleta, CA) and processed them by means of Matlab (Mathworks, Natick, MA). Raw data were recorded at a sample frequency of 500 Hz and filtered online using a 5- to 250-Hz band-pass filter. Furthermore, we used a notch filter with a width of 1 Hz at the frequency of 50 Hz (i.e., 49.5–50.5 Hz) to remove line interference.
The procedure we followed to process the data for the current research was composed of three main steps over longer to shorter time scales. Whereas the first step focused on the overall spectral power changes across the whole study test protocol (i.e., larger time scale), the second and third steps aimed at performing a more in-depth analysis on the changes within exercise segments (i.e., shorter time scales). All of the analyses were carried out separately for both young and old groups.
The first step was to study spectral power S(f) distribution profile for both leg and back muscles and its evolution across different rest and exercise segments (i.e., large time scales) (Fig. 1). We extracted spectral power for each muscle (i.e., VL-L, VL-R, ES-L, and ES-R) and segment (i.e., rests 1, 2, 3, and 4 and exercises 1, 2, and 3), considering a 2-s time window with an overlap of 1 s. For each time window, we computed spectral power across all frequencies. Given that no remarkable differences were observed between left and right leg and back in the current study, we show only the results for VL-R and ES-R. Next, to quantify the results observed in the spectral power distribution curves (Figs. 2 and 3), we computed the total spectral power (f) for each muscle and exercise/rest segment (Fig. 4), summing up the power across all frequencies:
where fi are all frequencies considered in our spectral analysis. We obtained a value for the spectral power for each 0.5 Hz in the 5- to 250-Hz range; therefore, N = 500. Furthermore, to elucidate the contribution of different frequencies, we then subdivided the spectrum of frequencies in the following bands: 5–25 Hz, 25–50 Hz, 50–150 Hz, 150–250 Hz; then, we took the average spectral power corresponding to the frequency bins of 0.5 Hz in each frequency band (Fig. 5):
where fi are all of the frequencies in each frequency band binned in bins of 0.5 Hz. Note that because the frequency bands have different width, we used the average spectral power <S(f)> instead of the sum. The aforementioned frequency bands were selected according to the shape of the empirical spectral power distribution observed in Figs. 2 and 3 and relate to earlier studies of different muscle fiber types (31).
Fig. 1.
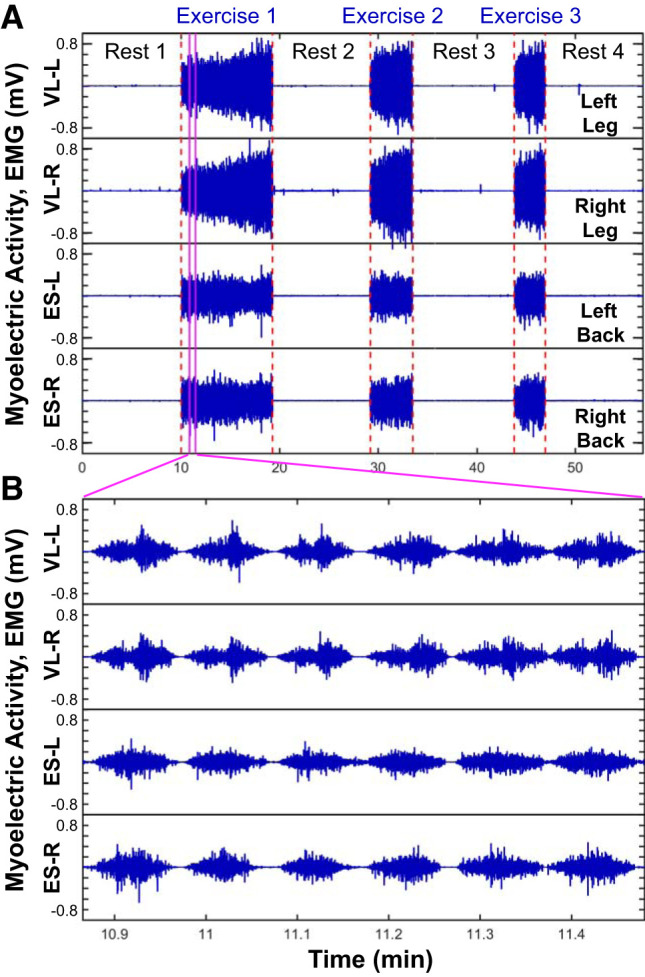
Leg and back muscle myoelectrical activity during exercise at different time scales. Evolution of myoelectrical activity represented by electromyographical (EMG) amplitude profiles at large time scale of consecutive exercise segments (A) and short time scale of individual and consecutive squats (B), comparing the right (VL-R) and left leg vastus lateralis muscle (VL-L) with the right (ES-R) and left back erector spinae (ES-L), for a typical young subject. The exercise protocol is composed by 3 consecutive squat tests, each performed until exhaustion, separated by 10-min rest periods in the supine position; EMG data are recorded with 500-Hz sampling frequency. Leg and back muscles show different EMG amplitude profiles at both large (A) and short (B) time scales. A: within and across exercise segments, the leg EMG amplitude gradually increases, reflecting the effect of fatigue, in contrast to the back muscle, where the EMG amplitude does not change significantly. Note also the higher initial EMG amplitude at the beginning of exercises 2 and 3 compared with exercise 1 for the leg muscle, indicating residual fatigue, an effect that is not present for the back muscle. B: markedly different EMG amplitude profiles are observed for the leg and back muscles at short time scales of a few seconds, associated with individual squats, with bimodal profile for the leg muscle with 2 phases corresponding to the down (smaller amplitudes) and up (larger amplitude) squat movements. The observed differences between the leg and back muscles in the EMG amplitude profiles at both short and large time scales and the profile evolution with fatigue reflect different muscle fiber structure and role to squat movement. These empirical observations motivate our hypothesis that distinct muscles have specific spectral power profiles, with different roles of muscle fibers to the spectral power of high- and low-frequency components, and muscle-specific trajectory for the evolution of spectral profiles across short and long time scales in response to exercise-induced fatigue and aging.
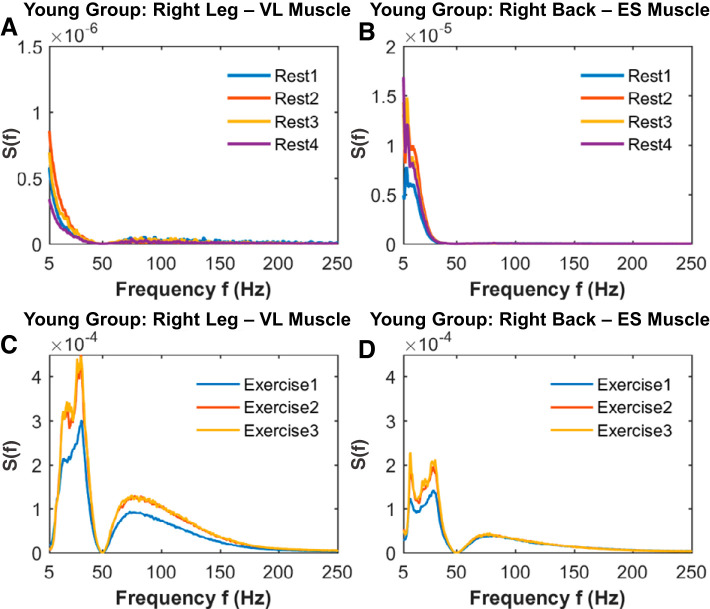
Fig. 2.
Leg and back muscle spectral power density profiles and their evolution across rest and exercise segments in young subjects. Group average spectral power S(f) distribution curves for the right leg vastus lateralis (VL) muscle and right back erector spinae (ES) muscle for consecutive rest (A and B) and exercise (C and D) segments of squat tests (see methods). Whereas for the resting periods leg and back muscle exhibit similar spectral behavior, their electromyographical (EMG) dynamics during exercise are characterized by different S(f) curve profiles, indicating a clear relationship between muscle type and spectral power. This relationship is consistently present for all individual subjects in the group (not shown), demonstrating that each muscle type is characterized by a robust spectral power distribution shape. However, a significant increase in S(f) is observed with accumulation of fatigue from exercise 1 to exercise 2 for both and leg and back muscle, preserving the muscle-specific profile of S(f). Note that the leg muscle response to exercise and fatigue is quantified by spectral power, with a factor of 2 higher compared with the back muscle. Spectral power was obtained after a 50-Hz notch filter was applied to EMG signals to remove power grid line interference and was computed for each muscle type and exercise rest segment in a 2-s moving window with 1-s overlap. Results for the left leg and left back muscle are consistent with the young group averaged results shown for the right leg and right back muscle.
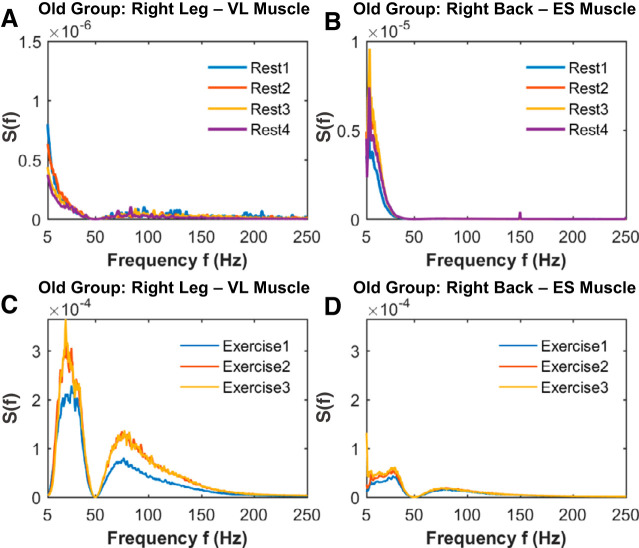
Fig. 3.
Leg and back muscle spectral power density profiles and their evolution across rest and exercise segments in old subjects. Group average spectral power S(f) distribution curves for the right leg vastus lateralis (VL) muscle and right back erector spinae (ES) muscle for consecutive rest (A and B) and exercise (C and D) segments of squat tests (see methods). For the leg muscle, a similar response to rest (B) and exercise (D), with similar spectral power evolution with accumulated fatigue, is observed in the old subjects compared with the young group shown in Fig. 2. In contrast, the back muscle exhibits a more reduced spectral response to rest (B) and exercise (D) and fatigue in old subjects compared with the young group. Electromyographical (EMG) signal preprocessing, spectral power calculation, and group averaging are performed as in Fig. 2 (see methods). Results for the left leg and left back muscle are consistent with the old group averaged results shown for the right leg and right back muscle in Fig. 2.
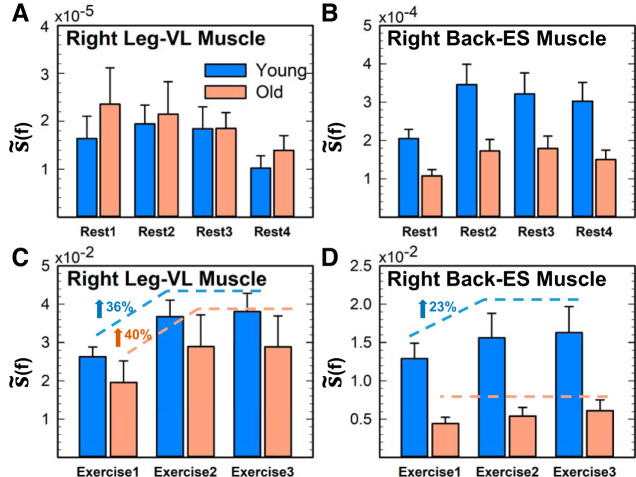
Fig. 4.
Leg and back muscle total spectral power for rest and exercise in young and old subjects. Bar charts representing group averaged total spectral power [i.e., area under the S(f) curves in Figs. 2 and 3] for right leg vastus lateralis (VL) muscle (A and C) and right back erector spinae (ES) muscle (B and D) for consecutive rest and exercise segments. With transition from rest to exercise, both leg and back muscles’ total spectral power increases 10−2 to 10−3 times in young and old subjects. A and C: no significant differences are observed for the total spectral power of the leg muscle activation between young and old subjects for both rest and exercise. B and D: in contrast to the leg muscle, the total power of back muscle is significantly higher in young subjects for both rest and exercise (comparing young vs. old: Student’s t test, P < 0.02 for rest periods and P < 0.01 for exercise periods). C: leg muscle activation in both age groups shows significant effect of accumulated fatigue. with 36% increase in spectral power for young subjects and 40% increase for old subjects from exercise 1 to exercise 2 (ANOVA repeated-measures test; P < 0.01 for both groups). D: in contrast, the back muscle exhibits significant evolution in spectral power across exercise segments, with a 23% increase from exercise 1 to exercise 2 only for the young subjects (ANOVA repeated-measures: P = 0.04 for young, P = 0.07 for old). Error bars indicate SE. Results for the left leg and left back muscles are consistent with the results shown for the right leg and right back muscles shown.
Fig. 5.
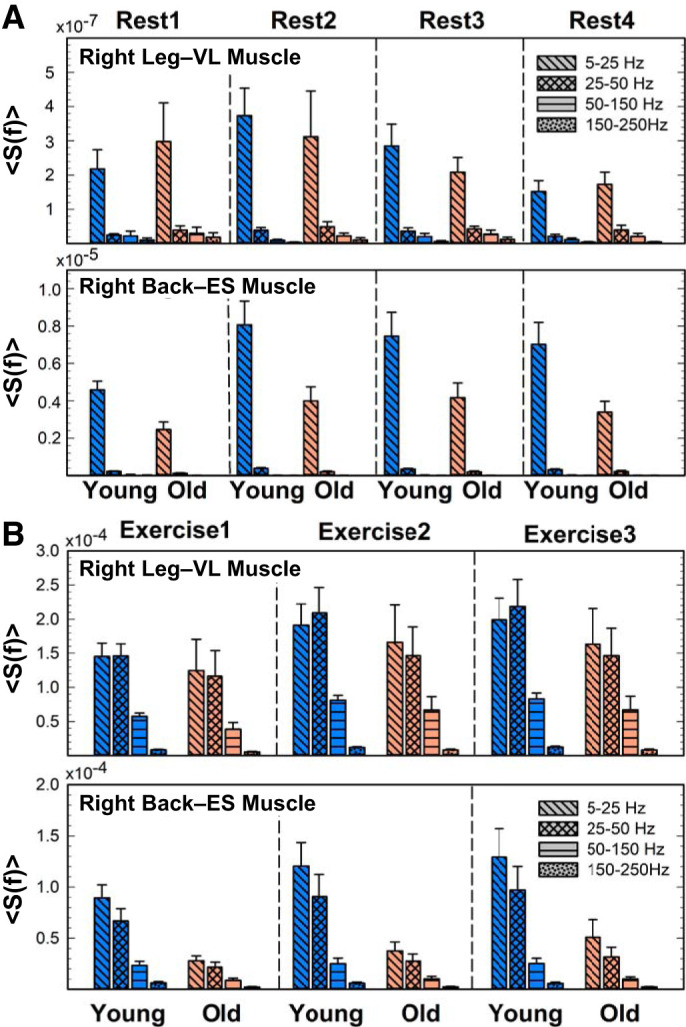
Frequency bands’ contribution to the spectral power profile and their evolution at large time scales of consecutive exercise and rest segments. Spectral profiles show distinct contributions of different frequency bands to the total power of leg [vastus lateralis (VL)] and back [erector spinae (ES)] muscles for rest (A) and exercise (B). Myoelectrical activity exhibits markedly different spectral profiles for rest vs. exercise. A: at rest, the low-frequency (5–25 Hz) band is the only contribution to total spectral power. B: in contrast, for exercise, the low- (5–25 Hz) and intermediate-frequency (25–50 Hz) bands dominate the spectral power, with significant contribution from the high-frequency (50–150 Hz) band. The observed differences between rest and exercise, which are present for both leg and back muscles, indicate that each physiological state is characterized by a specific spectral profile. Significant reduction in spectral contribution from the intermediate-frequency (25–50 Hz) band compared with the low-frequency (5–25 Hz) band in back muscle for consecutive exercise segments (Student’s t test, P < 0.002), which is not observed for the leg muscle (Student’s t test, P > 0.2), demonstrates muscle-differentiated response to exercise and fatigue accumulation. For consecutive exercise segments, spectral power increases (with ∼30%) proportionally for all frequency bands in both young and old groups, reflecting the common response across the entire frequency range to accumulated fatigue. Results for the left leg and left back muscles are consistent with the results shown for the right leg and right back muscles shown. The consistency of spectral profiles among subjects from a given group (young/old) at a given physiological state (rest/exercise) and the robustness of these profiles for repeated rest and exercise segments indicate a universality behavior related to a basic mechanism of muscle tone regulation. Results for the left leg and left back muscle are consistent with the results shown for the right leg and right back muscle shown.
The second step of our analysis is to study the spectral power profile evolution within exercise segments (i.e., intermediate time scales) by means of a spectrogram (Fig. 6). With the aim of further clarifying the contribution of different frequencies, we consider in this case 10 × 10 Hz frequency bands, from 5 to 200 Hz. The 200- to 250-Hz range was removed given the lack of activity observed in the previous figures. As in the previous step, we consider a 2-s time window with 1-s overlap. For each time window and frequency band, we calculated the sum (f) of all the power across all frequencies within that frequency band. Each node in Fig. 6 is assigned a color and represents the power inside the corresponding time window and frequency band. To facilitate visual comparison among exercise segments and between age groups, the same color bar ranges are used in the different subplots. The maximal power in the color bar corresponds to the highest power value obtained during the three exercise segments. To quantify the results shown in Fig. 6, we specifically compared the beginning versus the end of each exercise segment. To this end, we considered a 1-min segment after the first 30 s (i.e., beginning) and a 1-min segment before the very last 12 s (i.e., end) of each exercise segment. The first 30 s (i.e., 5 squats) were not considered since participants needed an average of two or three repetitions to get synchronized with the metronome. The last 12 s (i.e., 2 squats) were also not considered given the high instability that typically characterized the very last squats of the exercise segments due to exhaustion. We computed spectral power using a 2-s time window with an overlap of 1 s for both beginning and end. We considered three of the four original frequency bands (i.e., 5–25 Hz, 25–50 Hz, and 50–150 Hz). The 150- to 250-Hz band was removed from the analysis given its reduced activity in previous steps. We consider the average <S(f)> of the spectral power of all frequencies inside each frequency band (Figs. 7–10, right). To probe detailed characteristics of the spectral power profiles and the specific contribution of different muscle fibers in response to fatigue and aging, we also considered 34 frequency bands each with a width of 4 Hz (from 4 to 44 Hz and from 56 to 152 Hz) for both the beginning and end of each exercise segment. The 44- to 56-Hz range is not included because of the notch filter at 50 Hz, which modifies the EMG signal, altering the spectral power of frequencies ∼50 Hz (i.e., 49.5–50.5 Hz). Because the detailed frequency bands have the same width of 4 Hz, we next calculated the sum of the spectral power (f) for all frequency bins of 0.5 Hz inside each frequency band (Figs. 7–10, left). Note that, given the lack of remarkable differences between exercise 2 and 3, exercise 2 is not shown from Fig. 6 onward.
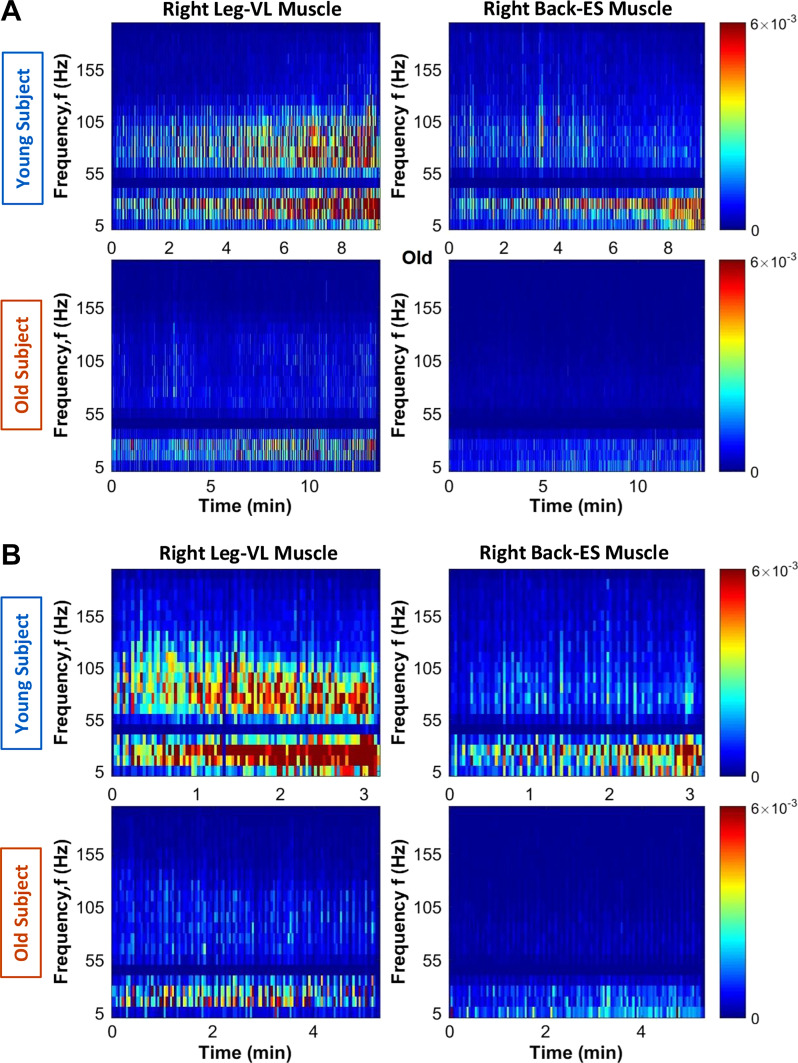
Fig. 6.
Evolution of spectral power at intermediate time scales in the course of exercise. Spectrograms of electromyographical (EMG) signals (500-Hz sampling frequency) obtained from the right leg [vastus lateralis (VL)] and the right back [erector spinae (ES)] muscles of a representative young and an old subject during a single exercise segment (∼10 min of a squat test performed until exhaustion). Spectral power is calculated in moving time windows of 2 s with 1-s overlap over the entire duration of the exercise segment (x-axis) and in frequency bands of 10 Hz (y-axis) over the entire range of physiologically relevant frequencies (5–200 Hz; y-axis). Spectrograms are colored coded with warm colors, indicating periods of high spectral power. With progression of the exercise, there is a pronounced increase both in spectral power level and in the range of active frequency bands in response to effort and accumulation of fatigue, a clear evolution in the spectral profile from fewer active frequency bands with relatively lower spectral power at the beginning of exercise to a broader range of frequency bands activated at higher levels when exhaustion is approached at the end of the exercise. Notably, these fatigue effects are more pronounced for the leg compared with the back muscle (A, top); back muscle spectral power in high-frequency bands (>55 Hz) does not increase in the course of exercise, and response to fatigue with widening range of active frequency bands and increased power is observed only for low and intermediate frequencies. In old subjects, the evolution in spectrogram characteristics with accumulation of fatigue is less pronounced for the leg muscle, (A, bottom) and is not present for the back muscle. The differences between the leg and back muscle spectral profiles and their evolution with fatigue at intermediate time scales during exercise are consistent with the behavior at large time scales, where back muscle total spectral power remains unchanged for repeated exercise segments in Fig. 4. Spectrograms obtained for the left leg and left back muscles (not shown) are consistent with the results for the right leg and right back muscles across all the subjects in the young and old groups.
Fig. 7.
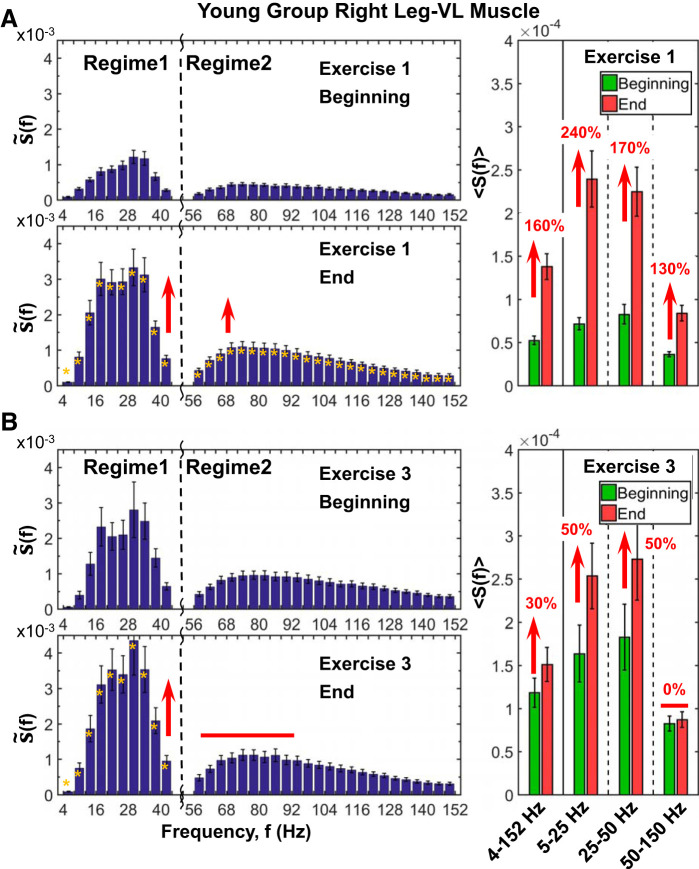
Leg muscle spectral profile, its evolution with accumulated fatigue at intermediate time scales during exercise, and response to residual fatigue at large time scales across consecutive exercise segments. Detailed spectral power distribution profiles of right leg [vastus lateralis (VL)] muscle activation presented in frequency bands of 4 Hz for periods of 1 min (10 squats) at the beginning and end of the 1st (A) and 3rd (B) exercise segments (see protocol in methods). Profiles represent S(f) group average, and error bars show SE for group average power in every 4-Hz bin. Yellow stars at left indicate the frequency bands with statistically significant differences in spectral power between beginning and end. Profiles are characterized by 2 separate regimes with different responses to accumulating fatigue in the course of exercise (A, left): regime 1, corresponding to low- (5–25 Hz) and intermediate-frequency (25–50 Hz) bands with dramatic 200% in spectral power (Wilcoxon matched-pairs test, P < 0.003) at the end of the exercise, and regime 2, including high-frequency bands (50–150 Hz) with less pronounced increase (∼100%, Wilcoxon matched-pairs test P < 0.001) in spectral power (A; right). Similar S(f) profile with 2 frequency regimes exhibiting distinct responses to accumulated fatigue is observed also during exercise 3 (B, left), but with much higher (100%) starting total spectral power compared with the start of exercise 1, reduced response (∼50% increase; Wilcoxon matched-pairs test, P < 0.002) in low (25–50 Hz) and intermediate (25–50 Hz) bands, and no change in the power of high frequencies (50–150 Hz) (B, right), all due to residual fatigue from previous exercise segments. The observed evolution in the S(f) profile from the beginning to the end of an exercise and with consecutive exercise segments reflects a differentiated role of leg muscle fibers with different contributions in response to exercise-induced fatigue. Note that the observed evolution in the spectral profile of the leg muscle at intermediate time scales within an exercise in response to accumulated fatigue (shown here) is consistent with the results shown for the change in spectral power in the leg spectral power at large time scales of consecutive exercise segments (Fig. 5B), where the power of all the frequency bands increases from exercise 1 to exercise 2 but does not change from exercise 2 to exercise 3 due to the effects of residual fatigue.
Fig. 10.
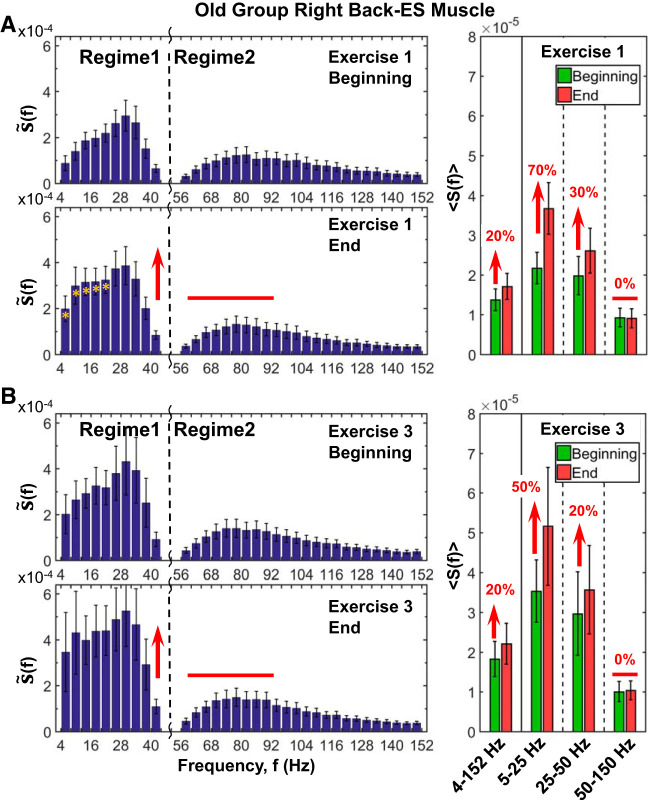
Effects of aging on back muscle spectral power response to accumulated fatigue at intermediate time scales during exercise and to residual fatigue at large time scales across consecutive exercise segments. Group average results for old subjects shown are obtained following the same experimental protocol, data processing, analysis procedure, and statistical tests as shown for the group of young subjects in Fig. 8 (see methods). Error bars indicate SE. Yellow stars at left indicate the frequency bands, with statistically significant differences in spectral power between beginning and end. A: detailed analysis of the contribution of different frequency bands to the right back muscle [erector spinae (ES)] spectral power shows S(f) profile shape and profile evolution during exercise that are consistently similar for individual subjects in both old (A) and young (Fig. 8A) groups. The back muscle spectral power profile exhibits 2 distinct frequency regimes with 1) different starting levels of power at the beginning of exercise, where regime 1 of low (5–25 Hz) and intermediate (25–50 Hz) frequencies has higher power (A, right) compared with regime 2 of high (50–150 Hz) frequencies, and 2) with different patterns of evolution in response to accumulation of fatigue with progression of exercise, where the power in regime 1 significantly increases (Student’s t test P < 0.01), whereas regime 2 remains unchanged (Student’s t test P = 0.7). The spectral power profile of the back muscle and its evolution with fatigue in the old group (A) are consistent with the young subjects in Fig. 8, indicating universality in myoelectrical activation during exercise. Note that the spectral power increase in regime 1 at the end of exercise 1 is less pronounced in old subjects (70% increase for 5–25 Hz and 30% for 25–50 Hz; A, right; Student’s t test, P < 0.01) compared with young subjects (140% increase for 5–25 Hz and 70% for 25–50 Hz; Fig. 8A, right panel; Wilcoxon matched-pairs test P < 0.002), indicating reduced response of back muscle activation in old subjects with accumulation of fatigue at intermediate time scales within an exercise. B: spectral power profile characteristics with 2 distinct frequency regimes and their evolution with fatigue in exercise 3 for the old group. S(f) behavior during exercise 3 is similar to exercise 1. Spectral power in regime 1 at the beginning of exercise 3 is significantly higher compared with exercise 1 (65% increase; Student’s t test, P < 0.04) due to residual fatigue from exercise 1 and exercise 2. Note that the 65% elevation in spectral power of regime 1 due to residual fatigue at the beginning of exercise 3 compared with the beginning of exercise 1 is less pronounced for the old group than for the young subjects (100% increase; Student’s t test, P < 0.04; see Fig. 8B, right), indicating reduced back muscle response to residual fatigue in old subjects. In contrast, the spectral power level in regime 2 at the beginning of exercise 3 does not increase compared with the beginning of exercise 1, indicating differentiated response of frequency regimes to residual fatigue that reflects specific back muscle fibers composition. As in exercise 1, there is a markedly different response of regime 1 and regime 2 to accumulation of fatigue during exercise 3 with significant increase in power of regime 1 from beginning to end of exercise (Student’s t test P < 0.05) and no change in the power of regime 2. However, the increase in regime 1 power during exercise 3 is less pronounced compared with exercise 1 (50% in the 5- to 25-Hz and 20% in the 25- to 50-Hz bands in exercise 3 compared with 70% and 30% correspondingly in exercise 1 for old subjects; A and B, right), indicating reduced back muscle response to accumulation of fatigue during exercise 3 due to the existing residual fatigue from exercises 1 and 2. Same effect on spectral power response in regime 1 (but with a larger amplitude) is also observed for young subjects with 80% (5–25 Hz) and 40% (25–50 Hz) increase in exercise 3 compared with 140% and 70%, respectively, in exercise 1 (Fig. 8 A and B, right). In addition to this muscle type differentiation based on aging effect, the results in A and B show a remarkable dissociation in response to fatigue of muscle fiber within the back muscle, as represented by different frequency bands; whereas there is a clear response to both accumulated and residual fatigue in regime 1, there is no change in frequency regime 2 (a behavior observed for both young and old subjects).
The last step of our analysis was to identify the spectral power profiles during single squat movements (i.e., short time scales) and analyze the evolution of these profiles from the beginning to end of the exercise segments. Thus, we took five squats from the beginning (i.e., squat 6 to 10) and five squats from the end (i.e., the last 5 squats, without considering the very last 2 squats) of exercise 1. We next divided each squat into two parts: down (3 s, lengthening contraction) and up (3 s, shortening contraction). Then, we computed the spectral power for both down and up (beginning and end), considering a 1-s time window with an overlap of 0.5 s. Similarly to the previous step, we considered both the three original frequency bands (5–25 Hz, 25–50 Hz, and 50–150 Hz) and the 34 frequency bands (from 4 to 44 Hz and from 56 to 152 Hz, each 4 Hz). We next computed the sum (f) (34 frequency bands; Figs. 11 and 12) or the average (<S(f)>; 4 original frequency bands; Figs. 13, A and B, and 14, A and B) of the power across all frequencies inside each frequency band. Finally, to study the evolution of lengthening and shortening contractions from beginning to end of exercise, we obtain the ratios <S(f)>end/<S(f)>begin (Figs. 13C and 14C). The ratios are obtained dividing down phase beginning by down phase end and up phase beginning by up phase end values of the averaged spectral power for each frequency band.
Fig. 11.
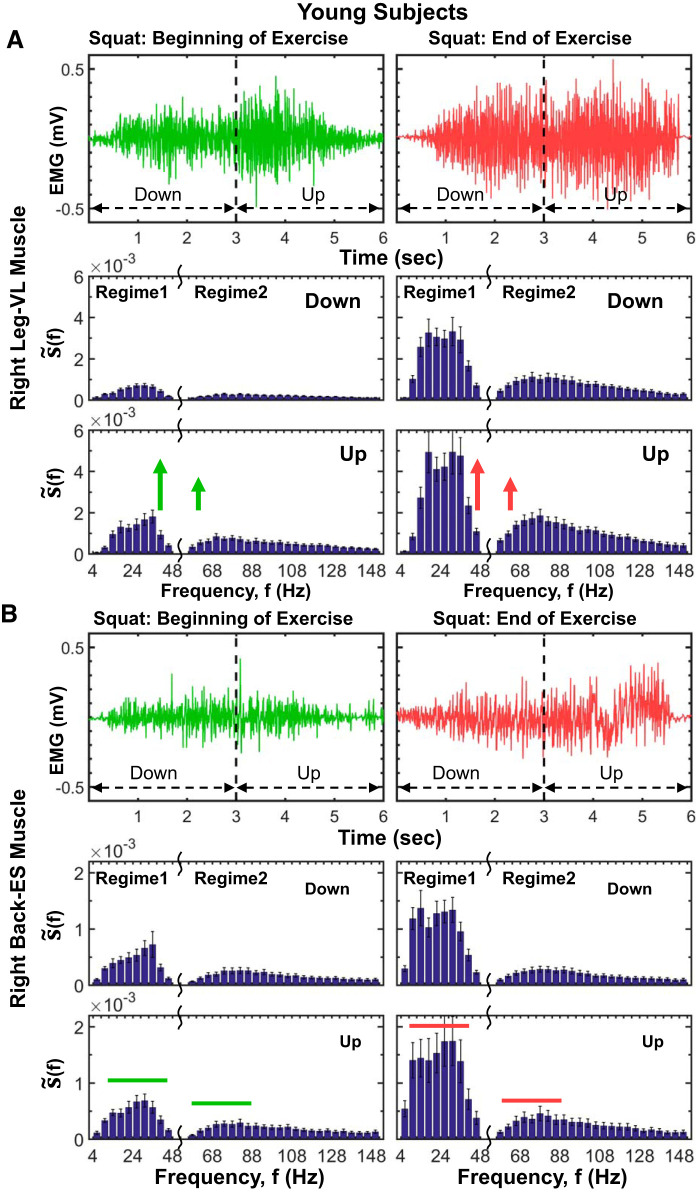
Leg and back muscle profiles in myoelectrical activity and spectral power at short time scales of a single squat movement and response to accumulated fatigue during exercise. A, top: myoelectrical activity profiles of right leg muscle [vastus lateralis (VL)] for the down and up phases of a single squat at the beginning and end of exercise 1 (see methods) for a representative young subject. Whereas at the beginning of exercise the EMG amplitude clearly increases with transition from down to up phase within a squat, leading to a bimodal profile, with progression of exercise the amplitude in both squat phases increases due to fatigue accumulation, and the transition from down to up phase in each squat becomes less pronounced. A, bottom: detailed leg muscle spectral profile (f) (presented in frequency bands of 4 Hz; see methods) and its evolution at short time scales from down to up phase within a squat at the beginning (left) and end (right) of exercise 1. Shown is the group average for all young subjects, where the spectral profile of each subject is derived from 5 separate squat movements at the beginning and end of exercise 1 (methods). Error bars in plots represent the group average SE. As for intermediate and large time scales, the leg muscle spectral profile is characterized by 2 distinct frequency regimes: regime 1 of low- (5–25 Hz) and intermediate-frequency (25–50 Hz) bands and regime 2 of high-frequency (50–150 Hz) band, with higher concentration of spectral power in regime 1 during the down phase and larger increase of spectral power in regime 1 compared with regime 2 in the up phase of the squat (A, bottom left). This evolution in spectral power profile with down/up transition within a single squat is consistently observed across subjects in the young group and shows a differentiated response of distinct leg muscle fibers represented by different frequency bands, where slow muscle fiber (regime 1) generates the dominant contribution to the spectral power increase in the up phase, reflecting specific role of different muscle fibers during lengthening (down phase) and shortening (up phase) muscle contractions. This characteristic spectral profile and its down/up phase transition is robust, as it is present for consecutive squat movements, albeit with increasing level of total spectral power in response to accumulated fatigue at the end of the exercise (A, bottom right). Notably, the leg muscle spectral profile and its evolution observed at short time scales within a single squat movement are also observed at intermediate time scales during separate exercise segments (Figs. 7 and 9) and at large time scales of consecutive exercise segments (Fig. 5B). B, top: myoelectrical activity of right back erector spinae (ES) exhibits an electromyographical (EMG) amplitude profile that is unimodal, with no down/up transition in squats at the beginning and end of exercise, in contrast to the bimodal amplitude profile of the right leg VL muscle (A, top). B, bottom: back muscle spectral power profiles for the down and up phases of squat movements at the beginning of exercise and spectral power evolution in response to fatigue at the end of exercise. Same protocol, data analysis, and group averaging procedures are performed as in A. As the leg muscle in A, the spectral profile of the back muscle is characterized with 2 distinct frequency regimes with a similar effect of fatigue accumulation, represented by an elevated level of total spectral power at the end of exercise. However, in contrast to the leg muscle, the back muscle spectral power does not increase with transition from the down to up phase of the squat. Episodes of increase in the back muscle spectral power with down/up transition within each squat at both the beginning and end of exercise, as well as the smaller rate of increase in total power with accumulation of fatigue at the end of exercise, reflect the secondary role back muscle plays in squat movements compared with leg muscle. Our findings for left leg (VL) and left back (ES) muscles (not shown here) are consistent with the results shown in A and B.
Fig. 12.
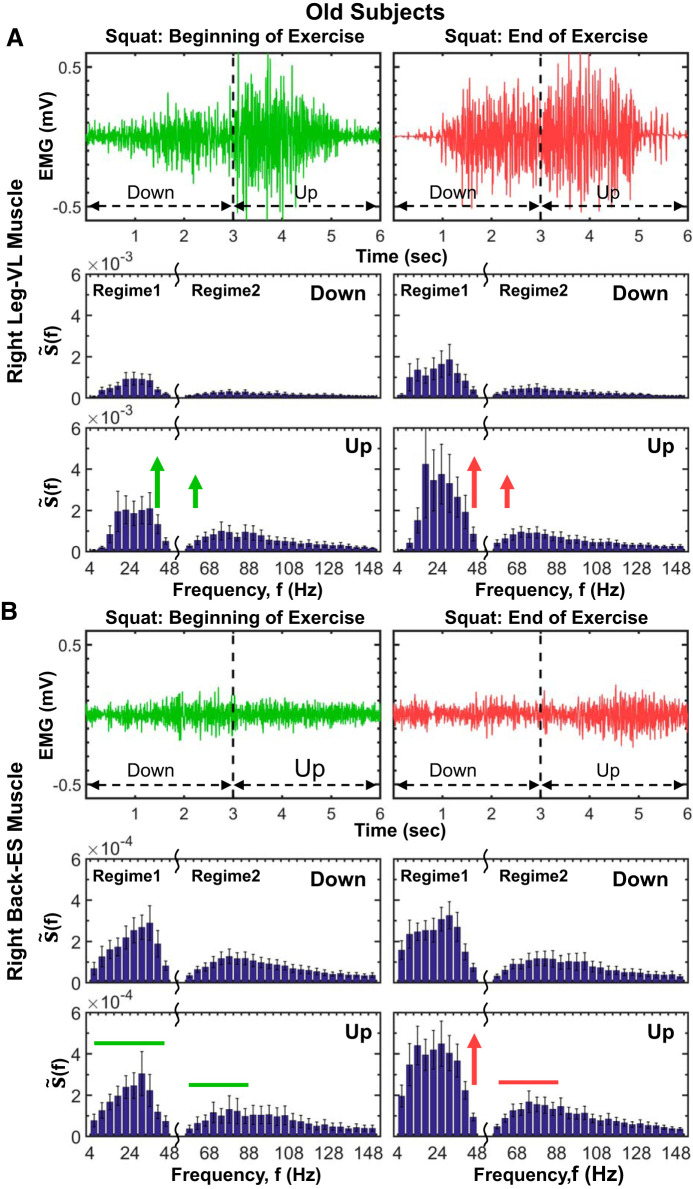
Aging effects on leg and back muscle myoelectrical activity and evolution of spectral power profiles at short time scales of a single squat. Myoelectrical activity profiles of a single squat movement (down and up phase) of a representative old subject for the right leg muscle [vastus lateralis (VL); A, top] and the right back muscle [erector spinae (ES); B, top] at the beginning and end of an exercise segment (see methods). The leg muscle myoelectrical activity during a single squat exhibits a bimodal profile, with lower EMG amplitude during the down squat phase, transition to a higher amplitude during the up phase, and significant increase in the electromyographical (EMG) amplitude of the entire squat movement with progression of exercise due to accumulated fatigue, the same general behavior as observed for the young group in Fig. 11. In contrast to the leg, the back muscle EMG squat profile is unimodal, with no difference in the EMG amplitude between the down and up squat phase, and less pronounced increase in the EMG amplitude with accumulation of fatigue at the end of exercise. Note that these characteristics of the leg and back muscle EMG amplitude profiles during squat movements (i.e., bimodal vs. unimodal EMG profiles) and how the EMG profiles change with progression of exercise are consistently observed for both old (A, top, and B, top) as well as for young subjects (Fig. 11), indicating strong association between the EMG amplitude profile and muscle type. The spectral power profile of the leg muscle (VL) for old subjects is characterized by 2 frequency regimes during both down and up squat phases, with a transition to higher spectral power during the up phase (A, bottom), consistent with the young group (Fig. 11). With progression of exercise, total power increases during both down and up phases, preserving the general shape of the spectral profile (A, bottom), an effect of accumulation of fatigue that is less pronounced for the down phase in old subjects (∼80% increase in down phase power from beginning to end of exercise; Wilcoxon matched-pairs test, P < 0.05) compared with young subjects (∼300% increase in down phase power from beginning to end of exercise; Wilcoxon matched-pairs test P < 0.01; see Fig. 11A), that can be related to an increase in connective tissue in the muscle due to aging (see Leg and Back Muscle Myoelectrical Activity and Spectral Power Profiles at Short Time Scales of a Single Squat Movement for physiological interpretation). Similarly to the right leg VL muscle, the spectral profile of the right back ES muscle exhibits 2 frequency regimes with higher concentrations of power in regime 1, but without an increase in spectral power with transition from down to up squat phase (B, bottom), a behavior consistently observed also in young subjects for the back muscle (Fig. 11B). In contrast to the young group (Fig. 11B, bottom), the down phase of the back muscle spectral power in old subjects does not change from beginning to end of exercise, indicating an aging effect of reduced back muscle activation in response to fatigue that can be attributed to increased connective tissue in the muscle (same aging effect, but less pronounced, is present for the leg muscle; see A, bottom, and Leg and Back Muscle Myoelectrical Activity and Spectral Power Profiles at Short Time Scales of a Single Squat Movement). Note the dramatic decline (∼1 decade) in the back muscle total spectral power of a single squat in old subjects compared with the young, indicating a reduction in back muscle mass as a result of sarcopenia. Same protocol, data analysis, and group averaging procedures are performed for the old subjects in A and B as for the young group in Fig. 11. Results for the left leg and left back muscle (not shown) are consistent with the results for the right leg and right back muscle shown.
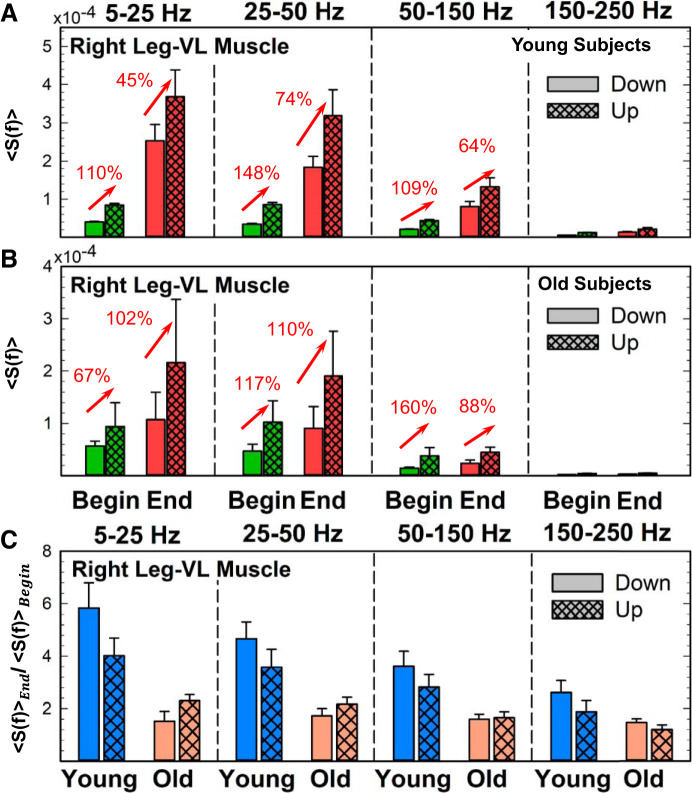
Fig. 13.
Leg muscle spectral power transition from the down to up phase of a single squat movement and response to accumulated fatigue during exercise. Right leg muscle [vastus lateralis (VL)] spectral power transition from the down to up phase during a squat at the beginning and end of exercise 1 in young (A) and old subjects (B). A: spectral power significantly increases from the down to up phase at the beginning of exercise in young subjects for low (5–25 Hz), intermediate (25–50 Hz), and high (50–150 Hz) bands (Wilcoxon matched-pairs test P < 0.05), reflecting the presence of the stretch/shorten cycle, the increase in muscle length (down phase), followed immediately by a shortening of the muscle (up phase). The characteristic down/up transition in the leg muscle spectral power is also present for consecutive squat movements; however, the down/up spectral power increase is less pronounced at the end compared with the beginning of exercise (red arrows in A) due to the bigger increase in the down phase spectral power at the end of exercise compared with the spectral power increase in the up phase (see Fig. 11A, bottom), indicating a high level of accumulated fatigue. Accordingly, young subjects exhibit a significantly higher ratio of the down phase spectral power at the end vs. beginning of exercise compared with the same ratio for the up phase (Wilcoxon matched-pairs test comparing the end-begin for the down squat vs. the up squat phase gives P < 0.02; C). As for the young group (A), the down/up spectral power transition is also present for old subjects (Wilcoxon matched-pairs test gives P < 0.05 for all the frequency bands; B), but with a reduced change in the down phase spectral power from beginning to end of exercise compared with young subjects (see Fig. 12A, bottom), reflecting reduced leg muscle response during lengthening contractions with aging. As a result, the down phase ratio in the old group is close to 1 for all the frequency bands (C). Error bars in plots represent the group average SE. Red arrows indicate statistically significant changes in spectral power from down to up squat phase. Results for the left leg (not shown) are consistent with the results for the right leg muscle shown.
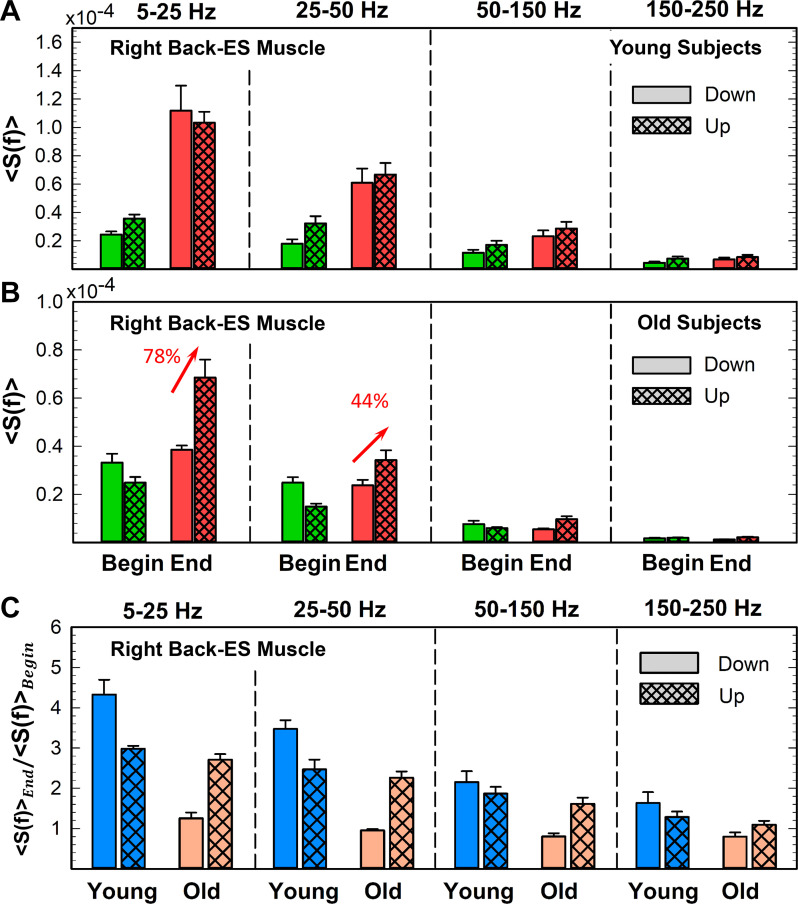
Fig. 14.
Back muscle spectral power transition from the down to up phase of a single squat movement and response to accumulated fatigue during exercise. Right back muscle [erector spinae (ES)] spectral power transition from the down and up phase during a squat at the beginning and end of exercise 1 in young (A) and old subjects (B). In contrast to the leg muscle (see Fig. 13), the back muscle spectral power for young subjects (A) does not increase with transition from the down to up phase for any frequency band either at the beginning or end of exercise, reflecting the different role back muscle plays during the squat compared with the leg muscle; whereas force generation is the primary function of the leg muscle, the main role of the back muscle is trunk stabilization. The back muscle spectral power profile and its lack of down/up transition is also observed with accumulation of fatigue at the end of exercise, but with an increased total power that is more pronounced in the down than in the up phase (see Fig. 11B, bottom). Accordingly, young subjects exhibit a higher ratio of the down/phase spectral power at the end vs. beginning of exercise compared with the same ratio for the up phase (Wilcoxon matched-pairs test comparing end/beginning ratio for the down squat phase vs. the same ratio for the up squat phase gives P < 0.01; C). As for the young group (A), back muscle spectral power for old subjects (B) does not increase with transition from the down to up squat phase at the beginning of exercise (Wilcoxon matched-pairs test comparing down vs. up phase at the beginning of exercise gives P > 0.3 for all frequency bands). However, with progression of exercise, there is a significant increase in total spectral power only for the up phase (Wilcoxon matched-pairs test comparing the up phase at the beginning vs. end of exercise gives P < 0.02 for the low- and intermediate-frequency bands; also see Fig. 12B, bottom). Similarly to the leg muscle in old subjects (Fig. 13B), the down phase spectral power for the back muscle (B) does not increase from beginning to end of exercise, indicating that reduced back muscle response to accumulated fatigue during the lengthening contractions of the squat movement and the down phase ratio or the old group (C) is close to 1 for all frequency bands. Error bars in plots represent the group average SE. Red arrows indicate statistically significant changes in spectral power from down to up squat phase. Results for the left back (not shown) are consistent with the results for the right back muscle shown.
The selection of a short 1-min time period (Figs. 7–10; intermediate time scales) or five squats (Figs. 11–14; short time scales) at the beginning and end of each exercise segment allows for more accurate quantification of the spectral profiles of the leg and back muscle activation for different physiological states within exercise segments: absence of fatigue at the beginning of exercise 1, residual fatigue at the beginning of exercises 2 and 3, and maximal level of fatigue accumulation at the end of each exercise segment. Selection of longer time periods within exercise segments would lead to reduced accuracy in the analysis to quantify association of distinct physiological states with the spectral power profiles of muscle activation for different muscle types and age groups; absence of fatigue can be accurately tested only at the beginning of exercise 1, residual fatigue only at the beginning of exercise 2 and 3, and maximum level of accumulation of fatigue only at the end of exercise 3. Alternatively, a choice of shorter time periods to quantify these physiological states would not provide sufficient data to perform reliable analyses.
Statistical Tests
Statistical analyses are performed using SPSS (version 23; SPSS, Inc.). All data were tested for normality by using a Shapiro-Wilk test. To analyze the spectral power frequency bands evolution across exercises 1, 2, and 3 (Figs. 4 and 5), we performed a repeated-measures ANOVA with Bonferroni post hoc correction separately for each muscle. To assess the changes within exercise segments (i.e., beginning versus end; Figs. 7–10) and during a single squat movement (down versus up squat phase; Figs. 11–14), we used Student’s t test. The between-group comparison (young versus old) was computed by means of an independent Student’s t-test. Alternatively, in the case of non-Gaussian distribution, we used a Friedman ANOVA (across exercise segments), a Wilcoxon matched-pairs test (within exercise segments and during a single squat movement), or a Mann-Whitney U matched-pair test (between age groups). We use an α-level of 0.05 for all statistical tests.
RESULTS
Performance
The number of squats significantly decreased across the three exercise segments in both the young (123.50 ± 41.73, 53.64 ± 19.95, and 47.54 ± 23.39 in exercises 1, 2, and 3, respectively; Friedman ANOVA test; χ2 = 22.29; df = 2; P < 0.001) and the old groups (65.23 ± 36.82, 39.51 ± 24.02, and 32.00 ± 20.85, in exercises 1, 2, and 3, respectively; Friedman ANOVA test; χ2 = 14.00; df = 2; P = 0.001). Specifically, the number of squats was significantly reduced from exercise 1 to exercise 2 (Wilcoxon matched-pairs test; Z = 3.29, P = 0.01 for the young, and Z = 2.36, P = 0.01 for the old group). In the between-group comparison, the young subjects performed significantly more squats than the old group only in exercise 1 (Mann-Whitney U test; U = 13.00, P = 0.006).
Spectral Power Distribution of Leg and Back muscle Activation for Consecutive Rest and Exercise Segments
The leg and back muscles showed different EMG amplitude profiles at both large and intermediate and short time scales (Fig. 1). Within and across exercise segments, there was a progressive increment in the EMG amplitude of leg, reflecting the effect of fatigue, in contrast to the back muscle, where the EMG amplitude did not remarkably change. Note also the higher initial EMG amplitude at the beginning of exercises 2 and 3 compared with exercise 1 for the leg muscle, indicating residual fatigue, an effect that was not present for the back muscle (Fig. 1A). The leg and back muscles also showed markedly distinct EMG amplitude profiles at short time scales of a few seconds associated with individual squats. Whereas the leg muscle presented a bimodal profile with two phases corresponding to the down (smaller amplitudes) and up (larger amplitude) squat movements, the back muscle showed a unimodal EMG profile (Fig. 1B). The observed differences at both short and large time scales between the leg and back muscles in the EMG amplitude profiles and its evolution with fatigue indicated different muscle fiber structure and role during the squat. These empirical observations motivated our hypothesis that distinct muscles have specific spectral power profiles, with different contribution of muscle fibers to the spectral power of high- and low-frequency components, and muscle-specific spectral power evolution profiles across short and large time scales in response to exercise-induced fatigue and aging.
To test our hypothesis, we first obtained the spectral power distribution for the leg and back muscles during rest and exercise and for both young and old subjects. Our analysis shows that the leg and back muscles have different spectral power profiles, according to their specific histochemical properties and distinct role during the squat movement (Fig. 2). Specifically, whereas both leg and back spectral profiles exhibit a major contribution of low frequencies, the leg spectral profile is also characterized by a more remarkable contribution of higher frequencies compared with the back muscle. These muscle spectral profiles are also different during rest and exercise, indicating specific muscle fiber contribution during different physiological states (rest versus exercise). Note also that both leg and back spectral profiles are preserved in the old group, but with a reduced total power with age, reflecting the typical decline in muscle mass (sarcopenia) and strength in old adults (91).
Leg and Back Muscle Spectral Power Profiles and Their Evolution at Large Time Scales Across Consecutive Rest and Exercise Segments
We next asked the question whether the spectral power profiles of the leg and back muscles evolve and change at large time scales across consecutive rest and exercise segments. We remarkably found that the shape of the spectral profiles does not change across rest (Fig. 2, a and B) and exercise segments (Fig. 2, C and D); however, there is a marked vertical shift to higher spectral power across exercise and rest segments. According to the performance results (see methods), this S(f) vertical shift present in both leg and back muscle is more pronounced between exercise 1 and 2 than from exercise 2 to 3 and reflects the response to residual fatigue. Moreover, we investigated whether the observed spectral profiles in the leg and back muscle and their evolution with aging are different (Fig. 3). We observed that young and old subjects showed similar evolution in the leg and back muscle spectral profiles. These observations indicated that the spectral power profiles for both leg and back muscle are robust, as they are consistently reproduced at a given physiological state (rest/exercise) and muscle (leg/back) and are preserved across rest and exercise segments.
Rest segments.
To quantify the results observed in Figs. 1 and 2, we compute the total spectral power for each muscle during the four rest segments (Fig. 4, A and B). A significant effect of accumulated fatigue is only observed on the right back muscle total power in both young (ANOVA repeated measures; F = 3.98, df = 3; P = 0.02; exercise 1 vs. exercise 2, P = 0.02) and old (F = 5.92; df = 3; P = 0.005; exercise 1 vs. exercise 2, P = 0.03) groups. Furthermore, to elucidate the contribution of low- and high-frequency components to the total spectral power, we quantify the power in the following frequency bands 5–25 Hz, 25–50 Hz, 50–150 Hz, and 150–250 Hz and their evolution across rest segments (Fig. 5A). A significant effect of accumulated fatigue is mainly shown in the low-frequency 5–25 Hz band (F = 3.47, df = 3; P < 0.02), indicating a clear dominance of the low frequencies during the four rest periods. Note that the other frequency bands were mostly absent in both the right leg [vastus lateralis (VL-R)] and right back [erector spinae (ES-R)] muscles. According to the size principle of motor recruitment (32, 56), type I muscle fibers are recruited at rest and during light exercise, provoking a clear dominance of low frequencies during rest segments. As shown in Fig. 5A, the 5- to 25-Hz band is ∼102 times higher in the ES-R compared with the VL-R. A feasible explanation could be related to the different fiber composition in VL and ES muscles; whereas ES is a postural muscle mainly composed of type I fibers (9), VL has a higher percentage of type II fibers (64, 83). This means that the ES might present higher muscle tone at rest, provoking an increased spectral power, specifically at low frequencies. When comparing between age groups, the 5- to 25-Hz band is significantly higher in the young group during the four rest segments, specifically in the ES-R (t = 1.72, P = 0.04; Fig. 5a). No significant differences are shown between age groups regarding the VL-R.
Exercise segments.
With the aim of quantifying the leg and back spectral power evolution across exercise segments observed in Fig. 2, we next computed the total spectral power for each muscle during the three consecutive exercise segments (Fig. 4, C and D). With transition from rest to exercise, both the right leg (vastus lateralis; VL-R) and the right back (erector spinae; ES-R) total spectral power increase 102 to 103 times in young and old subjects. The VL-R activation in both age groups shows significant effects of accumulated fatigue, with 36% increase in spectral power for young (ANOVA repeated measures: F = 9.78, df = 2, P = 0.001; exercise 1 vs. exercise 2, P = 0.01) and 40% increase for old subjects from exercise 1 to exercise 2 (F = 9.06, df = 2, P = 0.004; exercise 1 vs. exercise 2, P = 0.04). In contrast, the ES-R exhibits significant evolution in spectral power across exercise segments, with a 23% increase from exercise 1 to exercise 2 only for the young subjects (ANOVA repeated measures: F = 4.72, df = 2, P = 0.03; exercise 1 vs. exercise 2, P = 0.04). No significant differences are observed for the total spectral power of the VL-R activation between young and old subjects during exercise. However, the ES-R total power is significantly higher in young than in old subjects (Student’s t test P < 0.01). The reduced ES-R power evolution in the old group might be explained by the typical decline in lumbar extensors strength, which has been observed from the 3rd to 6th decade of life (20, 77). Given the usual higher myoelectrical activity (i.e., higher contribution) of VL compared with ES muscle during squats (46), total power is 100% higher in the VL-R compared with the ES-R in both young and old groups.
As for the previous subsection (rest segments), we also identify the specific contribution of different frequency bands to the VL-R and ES-R total power (shown in Fig. 4, C and D) for consecutive exercise segments (Fig. 5B). Spectral power increases (∼30%) proportionally for all frequency bands in both young and old groups, reflecting a common response across the entire frequency range. Specifically, a significant effect of accumulated fatigue on VL-R is observed in all frequency bands in the young group (ANOVA repeated measures: df = 2, P < 0.03) and only in the 5- to 25-Hz and 25- to 50-Hz bands in the old group (ANOVA repeated measures: F = 7.11, df = 2, P = 0.09; and F = 5.35, df = 2, P = 0.02, respectively). In contrast to the VL-R, a significant effect of accumulated fatigue on the ES-R is observed in 5- to 25-Hz and 25- to 50-Hz bands (ANOVA repeated measures: F = 4.57, df = 2, P = 0.02; and F = 4.52, df = 2, P = 0.02, respectively) for the young subjects and only in the 5- to 25-Hz band for the old subjects (ANOVA repeated measures: F = 4.72, df = 2, P = 0.04). According to the size principle of motor recruitment (32, 56), during exercise segments both the 5- to 25-Hz and 25- to 50-Hz bands dominate and the 50- to 150-Hz band is remarkably present, indicating the recruitment of faster motor neurons with higher excitation thresholds (84), compared with the rest segments. Furthermore, given the distinct muscle fiber composition of VL and ES muscles, a significant reduction in spectral contribution in the intermediate-frequency band (25–50 Hz) compared with the low-frequency band (5–25 Hz) is observed in the ES-R for the thee exercise segments (Student’s t test: t = 5.04, P < 0.001; t = 3.95, P = 0.002; and t = 4.02, P = 001), an effect that is not observed for the VL-R. As shown in previous studies, the high- and low-frequency contents within the EMG seem to be associated with the recruitment of fast and slow motor units, respectively (4, 34, 68, 85). More specifically, the shape and conduction velocity of the motor unit action potentials are determined by the intrinsic attributes of muscles fibers (6, 84) that make up each motor unit, forming the basis for the spectral properties in the EMG. Thus, because ES is mainly composed of type I fibers (9), which seem to be recruited below 40–50 Hz (17, 87), there is a less pronounced response in the 25- to 50-Hz band, and no evolution is observed in the 50- to 150-Hz and 150- to 250-Hz bands.
The consistency of the spectral power profiles among subjects from a given group (young/old) and at a given physiological state (rest/exercise) and muscle (leg/back) and the robustness of these profiles at large time scales for repeated rest and exercise segments indicate a universal behavior related to a basic mechanism of muscle tone regulation.
Leg and Back Muscle Spectral Profiles and Their Evolution at Intermediate Time Scales Within a Single Exercise Segment
Furthermore, we ask the question of whether the robustness of the leg and back spectral profiles observed at large time scales in both young and old groups (Figs. 4 and 5), is preserved at intermediate time scales during exercise 1 with accumulation of fatigue (Figs. 7A, 8A, 9A, and 10A). Moreover, we test how residual fatigue from exercise 1 and exercise 2 affects the shape and evolution of such spectral profiles during exercise 3 (Fig. 7B, 8B, 9B, and 10B).
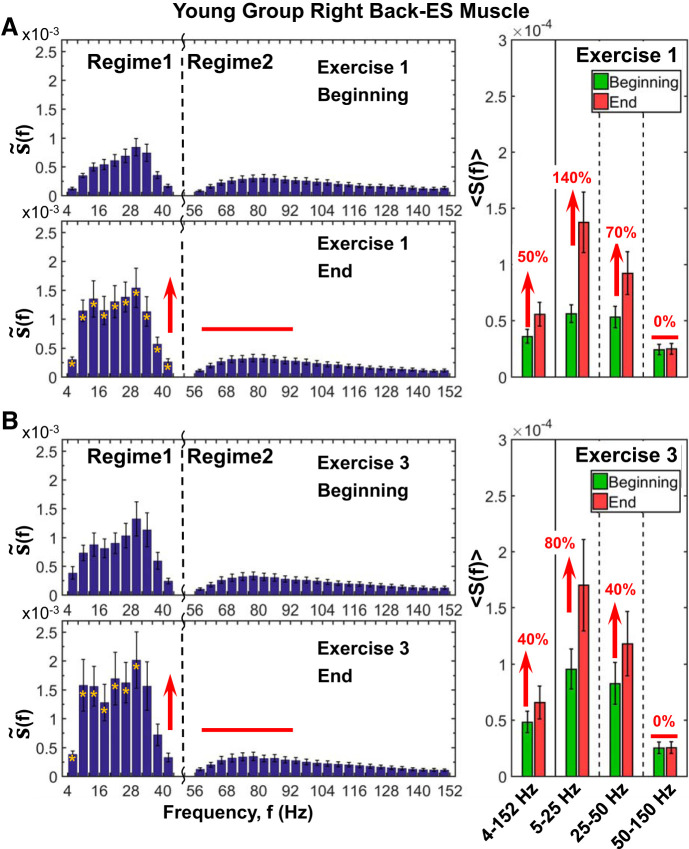
Fig. 8.
Back muscle spectral profile, its evolution with accumulated fatigue at intermediate time scales during exercise, and response to residual fatigue at large time scales across consecutive exercise segments. Detailed spectral power distribution profiles of right back [erector spinae (ES)] muscle activation presented in frequency bands of 4 Hz for periods of 1 min (10 squats) at the beginning and end of exercise 1 (A) and exercise 3 (B) segments (see protocol in methods). Profiles are obtained following the same procedure as in Fig. 7. Similarly to leg muscle activation, the back spectral power also increases with fatigue accumulation during exercise (A, left), an effect that is less pronounced compared with the leg muscle and concentrated only in regime 1: 140% increase in S(f) in the low (5–25 Hz) band and 70% in the intermediate (25–50 Hz) band (Wilcoxon matched-pairs test, P > 0.02) (A, right). This evolution in S(f) profile in the course of exercise is consistently observed also for exercise 3 (B, left), albeit with higher starting spectral power at the beginning of exercise compared with exercise 1, with less pronounced effect in regime 1 (B, right), due to residual fatigue from previous exercise segments. Episodes of response to fatigue in the high frequencies (50–150 Hz) of regime 2 of the back muscle spectral profile reflect different muscle fiber composition and their different contributions to squat movement compared with the leg muscle. Notably, the evolution of the back muscle spectral profile with accumulated fatigue at intermediate time scales within an exercise (shown here) is remarkably consistent with observed spectral power evolution at large time scales of consecutive exercise segments (Fig. 5B) where the power of low and intermediate frequencies significantly increases, whereas high-frequency (>50 Hz) contribution to spectral power remains unchanged. Yellow stars at left indicate the frequency bands with statistically significant differences in spectral power between beginning and end.
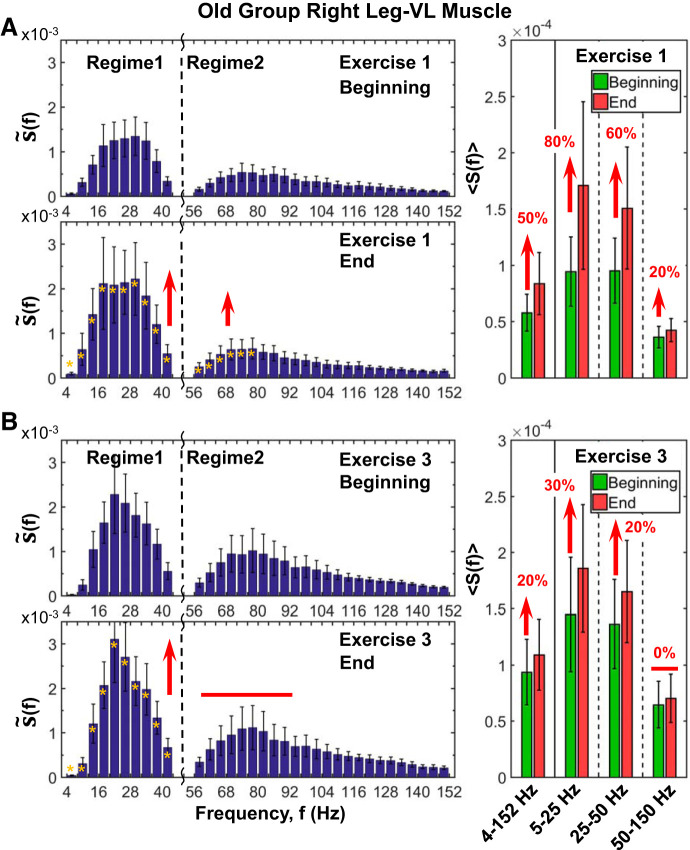
Fig. 9.
Effects of aging on leg muscle spectral power response to accumulated fatigue at intermediate time scales during exercise and to residual fatigue at large time scales across consecutive exercise segments. The results are obtained following the same experimental protocol, data processing, and analysis procedure and statistical tests as shown for the group of young subjects in Fig. 7 (see methods). Error bars indicate SE. Yellow stars at left indicate the frequency bands, with statistically significant differences in spectral power between beginning and end. A: detailed analysis of the right leg muscle [vastus lateralis (VL)] spectral power contribution of different frequency bands in old subjects shows a very similar shape of the S(f) profile as well as a similar profile evolution from beginning to end of the exercise for individual subjects and their group average in both young (Fig. 7A) and old groups. The spectral power profile S(f) exhibits 2 distinct regimes of frequency bands with 1) different starting levels of power at the beginning of exercise, with higher power for regime 1 of low (5–25 Hz) and intermediate (25–50 Hz) frequencies and lower power for regime 2 of high (50–150 Hz) frequencies), and 2) distinct evolution of frequency regimes in response to accumulation of fatigue with progression of exercise, with more pronounced increase in power for regime 1 compared with regime 2. The observed leg muscle spectral power profile and its evolution with fatigue in the old group is consistent with the leg muscle spectral response of young subjects in Fig. 7, indicating universality in myoelectrical activation during exercise. However, in contrast to the young group, the spectral power in regime 1 of low- and intermediate-frequency bands in old subjects starts at significantly higher levels at the beginning of exercise 1 (with ∼30%, Mann-Whitney U test P < 0.05), indicating significantly elevated initial muscle tone after rest 1 as an effect of aging. Note also that the spectral power increase in regime 1 at the end of exercise 1 is less pronounced (80% for 5–25 Hz and 60% for 25–50 Hz in old vs. 240% and 170%, respectively, in young subjects), indicating a reduced response of the leg muscle activation in old subjects with accumulation of fatigue at intermediate time scales within an exercise. B: similar S(f) profile characteristics and evolution to exercise 1 are also observed for exercise 3. Significantly elevated spectral power in regimes 1 and 2 at the beginning of exercise 3 compared with exercise 1 (Wilcoxon matched-pairs test P < 0.04), with the same level of elevation for the young (Fig. 7b, right) and the old group (right), indicates an effect of residual fatigue from exercises 1 and 2 that is the same for both groups. This residual fatigue effect leads to a reduced response to accumulated fatigue during exercise 3 compared with exercise 1: less pronounced increase of 30% in the 5- to 25-Hz and 20% in the 25- to 50-Hz band in exercise 3 compared with 80% and 60% correspondingly in exercise 1 for old subjects (A and B, right) and ∼50% increase for the 5- to 25-Hz and 25- to 50-Hz bands for exercise 3 compared with 240% and 170%, respectively, for exercise 1 in young subjects (Fig. 7, A and B, right). Note also a similar level of power response in the regime 1 frequency bands at the end of exercise 3 and exercise 1 corresponding to the maximum leg muscle capacity (all exercise segments are performed until full exhaustion; see methods), which is significantly lower in old (A and B, right) compared with young subjects (Fig. 7, A and B, right) (Mann-Whitney U test, P < 0.04). In contrast to regime 1, there is no change in the spectral power of regime 2 of high (50–150 Hz) frequencies. Such episodes of increased evolution in regime 2 during exercise 3 are in contrast to regime 1, where there is a pronounced evolution resulting from residual fatigue at the beginning of exercise 3. This effect is not seen for exercise 1, where there is no effect of residual fatigue and where regime 2 spectral power increases with accumulation of fatigue during the exercise. This behavior of regime 2 in response to accumulated fatigue during exercise and in response to residual fatigue (exercise 3) is consistently observed in both the young (Fig. 7B) and old groups.
Leg muscle spectral power evolution with accumulation of fatigue.
With progression of exercise in the young group, there is a clear evolution in the leg muscle (vastus lateralis; VL-R) spectral profile from fewer active frequency bands (mainly low frequencies, <50 Hz) with relatively lower spectral power at the beginning of exercise 1 to a broader range of frequency bands (both low and high frequencies, >50 Hz) at higher levels when approaching exhaustion at the end of the exercise (Fig. 6A). As stated by previous authors (56), motor units are recruited from smallest to largest. This means that performing submaximal squat contractions results mainly in the recruitment of lower threshold motor units that innervate type I fibers, but increasing fatigue leads to the recruitment of higher threshold motor units that innervate type II muscle fibers. Accordingly, VL-R spectral profiles in the young group are characterized by two separate regimes with different responses to accumulating fatigue in the course of exercise (Fig. 7A, left). Regime 1, containing low (5–25 Hz) and intermediate (25–50 Hz) frequency bands, increases dramatically, ≤200%, from the beginning to the end of exercise (Wilcoxon matched-pairs test: Z = 3.30, P = 0.001; and Z = 2.93, P = 0.003). Regime 2, which includes high-frequency bands (50–150 Hz), shows a less pronounced increase in spectral power at the end of exercise (∼100%; Wilcoxon matched-pairs test: Z = 3.29, P = 0.001). Thus, a clear dominance of lower frequencies as the muscle becomes fatigued can be observed. According to previous research, the main factor leading to the low-frequency dominance profile is the changes in the shapes of the motor unit action potentials, which are caused primarily by a reduction in the conduction velocities of the active fibers (3, 62). In turn, the conduction velocities are largely influenced by intracellular pH (60) and acidosis developed during high-intensity exercise. The maximal squat test is a highly demanding exercise in which elevated levels of acidosis are accumulated when exhaustion is approached (42).
Regarding the VL-R spectral power evolution within exercise 1 in the old group (Fig. 9A), a very similar S(f) profile and evolution from the beginning to the end of the exercise (in both individual subject and group average) can be observed compared with the young group (Fig. 7A). The spectral power profile also exhibits two distinct regimes of frequency bands (Fig. 9A, left), with 1) different starting levels of power at the beginning of exercise and 2) a distinct response to accumulation of fatigue with progression of exercise. Concretely, there is a more pronounced increase in the low (5–25 Hz) and intermediate (25–50 Hz) bands in regime 1 (Wilcoxon matched-pairs test: Z = 2.02, P = 0.04 and Z = 2.12, P = 0.03) compared with high frequencies (50–150 Hz) in regime 2 (Z = 1.63, P = 0.04). The observed leg muscle spectral power profile and its evolution with fatigue in the old group (Fig. 9A) is consistent with the leg muscle spectral response of young subjects in Fig. 7A, indicating universality in myoelectrical activation during exercise. However, in contrast to the young group, the spectral power of low- and intermediate-frequency bands (regime 1) in old subjects starts at significantly higher levels at the beginning of exercise 1 (with ∼30%; Mann-Whitney U test: U = 51.00, P = 0.04), indicating a reduced frequency range response during exercise. Furthermore, whereas the increase in regime 1 at the end of exercise is less pronounced (50% for 5–25 Hz and 50% for 25–50 Hz in old vs. 220% and 110%, respectively, in young subjects), the increase in high frequencies (50–150 Hz) in regime 2 is completely absent in the old group. The overall reduced spectral power and the lack of activity at higher frequencies in the old compared with the young group might be explained by the reduction in the discharge rate of motor neurons in old adults (48) and by sarcopenia, the normal decline of skeletal muscle and strength during aging (2, 91). Sarcopenia involves primarily type II muscle fibers, which motor units seem to fire above 50 Hz and up to 100–140 Hz (28, 87).
Leg muscle spectral power response to residual fatigue.
A very similar spectral profile is observed for the leg muscle (vastus lateralis; VL-R) in the young group within exercise 3 (Fig. 7B) compared with exercise 1 (Fig. 7A). However, the existent significantly elevated spectral power in regimes 1 and 2 at the beginning of exercise 3 compared with exercise 1 (Wilcoxon matched-pairs test: Z = 2.90, P = 0.04) indicates the presence of residual fatigue from exercise 1 and 2. This residual fatigue effect leads to a reduced response to accumulated fatigue during exercise 3 compared with exercise 1 in young subjects: ∼50% increase for the 5- to 25-Hz and 25- to 50-Hz bands for exercise 3 (Wilcoxon matched-pairs test: Z = 3.15, P = 0.002; and Z = 3.17, P = 0.001) compared with 240% and 170%, respectively, for exercise 1 (Fig. 7). Note also that the level of power response in the regime 1 frequency bands at the end of exercise 3, corresponding to the maximum leg muscle capacity, is similar to that of exercise 1. In contrast to regime 1, no significant increments from beginning to end during exercise 3 are detected in the high-frequency (50–150 Hz) band in regime 2 in young subjects. The lack of increment at high frequencies in exercise 3 might be related to the greater fatigability characterizing type II fibers (11, 63).
The aforementioned behavior in regime 1 and regime 2 for young subjects, in response to accumulated fatigue during exercise 3 and in response to residual fatigue, is consistently observed in the old group (Fig. 9B). As in the young group, the power at the beginning of exercise 3 (Fig. 9B) is increased ∼50% compared with exercise 1 (Fig. 9A). However, the increase from the beginning to the end of exercise 3 is less pronounced in the old group (30% increase for the 5- to 25-Hz and 20% for the 25- to 40-Hz bands, Wilcoxon matched pairs test: Z = 2.37, P = 0.02; and Z = 2.21, P = 0.03) compared with young subjects, indicating a reduced response of VL-R activation during exercise 3.
Note that the observed evolution in the VL-R spectral profile at intermediate time scales during exercise (Figs. 6, 7, and 9) in response to accumulated fatigue is consistent with the results shown for the change in the VL-R spectral power at large time scales across consecutive exercise segments (Figs. 4, C and D, and 5B), where the power increases from exercise 1 to exercise 2 but does not change from exercise 2 to exercise 3 due to the effects of residual fatigue.
Back muscle spectral power evolution with accumulation of fatigue.
As for the leg muscle (VL-R), the back muscle (erector spinae; ES-R) spectral profile is also characterized by two distinct frequency regimes with different responses to accumulating fatigue in the course of exercise (Fig. 8A). However, in contrast to the leg muscle, ES-R spectral power in high-frequency bands (>55 Hz) does not increase during exercise, and response to fatigue with widening range of active frequency bands and increased power is observed only for low and intermediate frequencies (Fig. 6A). Specifically, a significant effect of accumulated fatigue from beginning to end in the young group is only observed in regime 1: 140% increase in S(f) in the low (5–25 Hz) and 70% in the intermediate (25–50 Hz) band (Wilcoxon matched-pairs test: Z = 3.05, P = 0.02; and Z = 2.42, P = 0.01) (Fig. 8A). The lack of change in the high frequencies (50–150 Hz) in regime 2 for the back spectral profile shows a remarkable dissociation in response to fatigue of back muscle fibers represented by different frequency bands, given the specific back muscle fiber composition (9).
The spectral power profile of the back muscle and its evolution with fatigue in the old group (Fig. 10A) are consistent with the young subjects in Fig. 8A, indicating universality in myoelectrical activation during exercise. However, there is an overall remarkable reduction (10 times lower) compared with the young group, and the S(f) evolution in spectrogram characteristics with accumulation of fatigue is mostly absent. As shown in Fig. 10A, S(f) increases from beginning to end are only observed in the 5- to 25-Hz band (70% increase; Student’s t test: t = 3.51, P = 0.01), reflecting reduced response of back muscle activation in old subjects with accumulation of fatigue at intermediate time scales. These results are in agreement with previous research (49), where an inability to activate tibialis anterior from 30 to 60 Hz was found in old compared with young adults. As the erector spinae, tibialis anterior is mainly composed by type I fibers (43). As explained in previous sections, lumbar muscle degeneration and the reduced force levels in the elderly (54) might be responsible for these results.
Back muscle spectral power response to residual fatigue.
A very similar spectral profile for the back muscle [erector spinae (ES)] is observed in exercise 3 (Fig. 8B) compared with exercise 1 (Fig. 8A). However, starting spectral power at the beginning of exercise 3 is higher compared with exercise 1 for low frequencies (5–25 Hz; 100% increase) and intermediate frequencies (25–50 Hz; 60% increase) in regime 1 due to residual fatigue from previous exercise segments. Accordingly, ES-R spectral power increase from the beginning to the end of exercise 3 is less pronounced than in exercise 1 [80% increase in the low (5–25 Hz) and 40% in the intermediate (25–50 Hz) bands in regime 1; Wilcoxon matched-pairs test: Z = 2.73, P = 0.006; and Z = 2.01, P = 0.004) (Fig. 8B]. No changes are observed in the high (50–150 Hz) band in regime 2.
Regarding the old group (Fig. 10B), the 65% elevation in spectral power of regime 1 due to residual fatigue at the beginning of exercise 3, compared with the beginning of exercise 1, is less pronounced than for the young subjects (100% increase; Fig. 8B, right). Furthermore, similarly to exercise 1, there is a markedly different response of regime 1 and regime 2 to the accumulation of fatigue during exercise 3 in the old group. However, the increase in regime 1 power during exercise 3 is less pronounced compared with exercise 1 [50% in the 5- to 25-Hz (P = 0.04) and 20% in the 25- to 50-Hz bands; Fig. 10, A, right, and B, right], indicating reduced back muscle response to accumulation of fatigue during exercise 3 due to the existing residual fatigue from exercises 1 and 2.
Notably, the evolution of the ES-R spectral profile with accumulated fatigue at intermediate time scales within an exercise (Figs. 6, 8, and 10) is remarkably consistent with the observed spectral power evolution at large time scales of consecutive exercise segments (Fig. 5B). Specifically, the changes on the ES-R power are mainly concentrated in the low (5–25 Hz) and intermediate (25–50 Hz) bands in region 1, whereas the evolution of the high-frequency (50–150 Hz) band in regime 2 is completely absent.
Leg and Back Muscle Myoelectrical Activity and Spectral Power Profiles at Short Time Scales of a Single Squat Movement
In previous sections, we demonstrate that the leg and back spectral profiles in both young and old groups are robust, as they are consistently reproduced at large (Figs. 4 and 5) and intermediate time scales (Figs. 6–10). Finally, we tested the robustness of such spectral profiles at short time scales of a single squat movement and their response to accumulated fatigue during exercise 1 (Figs. 11–14). Specifically, we identified the leg and back spectral profiles for both down and up squat phases characterized by different types of muscle contractions (lengthening and shortening contractions, respectively).
Leg muscle activation and spectral power evolution during down and up squat phases.
Whereas at the beginning of exercise 1 the EMG amplitude of the leg muscle (vastus lateralis; VL-R) clearly increases with transition from down to up phase within a squat, leading to a bimodal profile, with progression of exercise the amplitude in both squat phases increases due to fatigue accumulation, and the transition from down to up phase in each squat becomes less pronounced (Fig. 11A, top). As at intermediate and large time scales, the VL-R spectral profile in young subjects is characterized by two distinct frequency regimes, regime 1 of low- (5–25 Hz) and intermediate-frequency (25–50 Hz) bands and regime 2 of the high-frequency (50–150 Hz) band, with a higher concentration of spectral power in regime 1 during the down phase and a larger increase of spectral power in regime 1 compared with regime 2 in the up phase of the squat (Fig. 11A, bottom). Specifically, spectral power significantly increases from down to up at the beginning of exercise for both low and intermediate frequencies in regime 1 (Wilcoxon matched-pairs test: Z = 3.18, P = 0.001), and high frequencies in regime 2 (Z = 3.18, Z = 0.001) (Fig. 13A). A feasible interpretation for the overall higher spectral power in the up compared with down phase may be related to two different aspects. First, as described in a previously detailed review (18), EMG amplitude during lengthening contractions is lower than during shortening contractions. This reduction is due to a greater force capacity of muscle during lengthening contractions, which in turn leads to a diminished motor unit recruitment and discharge rate. Second, the higher spectral power observed in up could also be influenced by the existing stretch/shorten cycle in the transition from down to up, that is, the increase in muscle length followed immediately by a shortening of the muscle (18, 66).
The characteristic VL-R spectral profile and its down/up phase transition observed in the young group is robust, as it is also present for consecutive squat movements, albeit with increased total power in response to accumulated fatigue at the End of Exercise 1 (Fig. 11A, bottom). Note that this down/up increment in spectral power is always higher at the beginning compared with the end of exercise (Fig. 13A, red arrows). The reduced down phase spectral power due to the typical reduction in neuromuscular activity associated with lengthening contractions (18) may be responsible for the higher increment in the spectral power from the down to up phase at the beginning (i.e., no fatigue condition) compared with the end of exercise. The down/up spectral power increment at the end of exercise (Fig. 13A, red arrows) is lower given the high level of accumulated fatigue, which provokes elevated myoelectrical activity not only in the up but also in the down squat phase. Accordingly, young subjects exhibit a significantly higher ratio of the down phase spectral power at the end versus beginning of exercise [<S(f)>end/S(f)>begin] compared with the same ratio for the up phase (Wilcoxon matched-pairs test comparing end versus beginning of exercise for the down squat phase versus the up squat phase gives Z = 2.20, P < 0.02; Fig. 13C).
As for the old group (Fig. 11A, bottom), similar spectral profiles with transition from the down/up phase for a single squat and similar response to accumulated fatigue are observed for the VL-R compared with young subjects (Fig. 12A, bottom), albeit with reduced change in the down phase spectral power from beginning to end (300% and 80% increase in down phase power from beginning to end of exercise in young and old subjects, respectively). As a result, the down phase ratio [<S(f)>end/S(f)>begin] in the old group is close to 1 for all the frequency bands (Fig. 13C), and a feasible explanation for the reduced down phase spectral power evolution during exercise may be related to the preservation of eccentric muscle strength in older adults due to the noncontractile and structural properties intrinsic to the muscle. More specifically, the accumulation of connective tissue within the muscles with age increases passive stiffness, which might offer a mechanical advantage during lengthening contractions (55, 71). In turn, such an aging effect could have also affected VL-R activation in the down squat phase, leading to decreased down spectral power evolution during exercise.
Back muscle activation and spectral power evolution during down and up squat phases.
In contrast to the bimodal EMG amplitude profile of the leg (VL-R) muscle, myoelectrical activity of the right back erector spinae (ES-R) muscle exhibits a unimodal EMG amplitude profile, with no down/up transition in squats (Fig. 11B, top). The spectral profile of the ES-R is characterized by two distinct frequency regimes with a similar effect of fatigue accumulation, represented by an elevated total power at the end of exercise for both the down and up phases (Fig. 11B, bottom). However, in contrast to the leg muscle, the back muscle spectral power does not increase with transition from the down to the up phase of the squat for any frequency band, either at the beginning or the end of exercise (Fig. 14A). The lack of down/up transition in the back spectral power, as well as the smaller rate of increase in the total power with accumulation of fatigue at the end of exercise, reflects the different role ES plays during the squat compared with VL. Whereas force generation is the primary function of the VL, the main role of the back muscle is trunk stabilization (83).
Regarding the old group (Fig. 12B), similar spectral profiles with two frequency regimes and no transition from down/up phase within a single squat are observed as for the young subjects. However, note the dramatic decline (almost 1 decade) of a single squat total spectral power of the ES-R in old subjects compared with the young. Similarly to the VL-R down phase for the old group (Fig. 13B), the ES-R down spectral power does not increase from the beginning to the end of exercise with accumulation of fatigue for any frequency band (Fig. 14B). Accordingly, the down phase ratio [i.e., S(f)end/S(f)begin] is ∼1 for all the frequency bands in old subjects (Fig. 14C), indicating reduced back muscle response during lengthening contractions.
Notably, the leg and back muscle spectral profiles and their evolution observed at short time scales during a single squat movement are also observed at intermediate time scales during separate exercise segments (Figs. 7–10) and at large time scales of consecutive exercise segments (Figs. 4, C and D, and 5B).
DISCUSSION
The present study investigates the leg and back muscle spectral power profiles and their evolution during three consecutive extended squat tests performed until exhaustion and four interspersed rest periods over a range of large, intermediate, and short time scales across repeated exercise and rest segments, within exercise segments, and during a single squat movement. In summary, 1) we identify the spectral profiles for both leg and back muscle activation with their muscle-specific evolution in response to fatigue according to the distinct histochemical properties and role of each muscle during the squat movement, 2) both low- and high-frequency regimes within the spectral profiles of leg and back muscle exhibit a muscle-specific evolution path, 3) leg and back muscle spectral power profiles reveal similar characteristics over a range of time scales, and 4) young and old subjects exhibit similar form of the spectral profiles for leg and back muscle activation. However, old subjects are characterized by different evolution path with less pronounced increase of spectral power in response to exercise and fatigue. The observed spectral power profiles for the leg and back muscle show universality, as they are consistently reproduced for subjects in each age group (young or old) for each muscle type (leg or back) at a given physiological state (rest or exercise) and are robust since the general form of the profiles is preserved across exercise segments. Our approach offers new insights into a previously unrecognized mechanism of multiscale organization and integration of different frequency bands that represents coordinated activity among distinct types of muscle fibers in generating global muscle tone and its modulation in response to exercise-induced fatigue and aging.
We uncover a particular spectral profile for both leg VL muscle and back ES muscle and how it changes in response to accumulated fatigue during exercise segments and to residual fatigue for consecutive exercise segments. Specifically, we show the following differences between muscles: 1) with increasing fatigue, the VL spectral power increases for both low and intermediate frequencies in regime 1 (<50 Hz) and for high frequencies in regime 2 (>50 Hz), whereas in contrast the ES spectral power mainly increases for low and intermediate frequencies in regime 1 (<50 Hz); 2) an overall reduced spectral response to fatigue is observed for the ES compared with the VL muscle; and 3) higher spectral power in the up phase of each squat compared with the down squat phase is observed for the VL, whereas in contrast no differences are observed between the up and down squat phases for the ES muscle. The reported differences in the spectral profile of the leg and back muscle activation and the differentiated response of different frequency bands to exercise and fatigue of these two muscle types may result from muscle-specific histochemical properties (muscle fiber content) and the distinct role during the squat movement of the VL and ES muscles. According to their fatigue and contractile characteristics, motor units are classified into three major types, slow fatigue‐resistant, fast fatigue‐resistant, and fast fatigable motor units, which typically consist of type I, type IIA, and type IIX muscle fibers (7, 8, 50, 63, 87). Whereas the back ES muscle is mainly composed of type I slow/oxidative fibers (9, 79), the leg VL muscle contains a higher percentage of type II fast/glycolytic fibers (64, 83). Furthermore, although both leg and back muscles show high myoelectrical activity during squats, their role during the movement is different; whereas force generation is the primary function of VL, the main role of the ES muscle is trunk stabilization (83).
Our findings demonstrate that the different muscle fibers composing a certain muscle show a universal spectral profile and evolution in response to exercise-induced fatigue, as it can be robustly observed over a range from large time scales (i.e., within and across exercise segments; minutes to hours) to short time scales (during a single squat movement; seconds) in both young and old subjects. Human physiology presents a remarkable amount of distinct rhythms at all organismic levels (61) that are coupled and coordinated with each other over several magnitudes of time scales (30, 37, 40, 41, 44) to generate integrated physiological functions associated with different systems (1, 39, 51). Exercise-induced physiological adaptations evolve as a consequence of soft-assembled states dwelling on different time scales and levels of biological systems organization (36). For instance, whereas at a kinematic level exercise-related adaptations dwell at short time scales of seconds to minutes (35), at a performance level the changes occur at scales of days, weeks, and months. Thus, further research is warranted to investigate the changes in muscle spectral profiles at much larger time scales (weeks or months) as a consequence of a training intervention.
Our approach reveals a universal spectral power behavior across subjects and age groups in response exercise-induced fatigue. However, the following differences between age groups should be highlighted: 1) overall reduced spectral power evolution in the high-frequency regime 2 (>50 Hz) for old subjects and 2) less pronounced spectral power evolution for the back ES muscle in old subjects across and within exercise segments compared with the young group. A feasible explanation for these divergences may stem from the reduction in the discharge rate of motor neurons in old adults (48) and from sarcopenia-related normal decline in skeletal muscle and strength during aging (57, 91). Sarcopenia involves primarily type II muscle fibers, which fire above 50 Hz (28, 87). The reduced back ES muscle spectral power evolution in the old group might be related to that reported here absence of ES spectral power changes during the down squat phase (i.e., lengthening contractions) with the course of exercise. Such an effect could be explained by the accumulation of intramuscular connective tissue with aging, which significantly increases passive stiffness (71). The lack of spectral power evolution of the back ES muscle during the down squat phase with accumulation of fatigue is a physiologically relevant finding, since lengthening contractions appear to be of key importance for the absorption of kinetic forces during the descent phase of a fall impact in old individuals, which could decrease the risk of hip fracture (74).
Given that sarcopenia mainly affects type II muscle fibers, and since the leg VL contains higher proportion of type II fibers than the back ES muscle, one would expect a larger effect of aging for the leg VL muscle. However, our results show the opposite, namely, a more pronounced aging effect for the ES muscle. Our findings demonstrate that the mechanisms underlying muscular changes during aging are specific to individual muscle types, as also indicated in earlier works on human limb and trunk muscles (19, 59). Furthermore, the reduced ES spectral power could have been provoked by other reasons aside from sarcopenia in type II muscle fibers. We note that the cause of decreased back muscle function with aging can also be attributed to the reduction in quality and quantity of muscle excitability and contractility (52, 77, 82), which are in turn related to changes in the volume of intramuscular adipose tissues, histological composition, and motor neuron signaling with aging (27). Accordingly, a pronounced decline in back muscle strength is typically observed after the 6th decade of life (20).
The experimental and analytic framework presented here with focus on quantifying the spectral power profile of different muscle types and its evolution during exercise to investigate the multiscale mechanism underlying the differentiated response of distinct frequency bands to fatigue accumulation provides physiologically relevant information and new insights on how different muscle fibers composing the skeletal muscle respond to exercise-induced fatigue and aging. From a practical point of view, our approach can be utilized to assess and quantify the efficiency of training programs. Because spectral power profiles of muscle activation and their response to exercise-induced fatigue are specific for each muscle type, tracking changes in the spectral profiles alongside other physiological markers could help quantify more precisely physiological adaptations after exercise and training interventions and may assist coaches with selection of the most appropriate training programs. For instance, the lack of changes in a given frequency band for a certain muscle after a training period could indicate that the previous intervention was not effective to provoke adaptations in the corresponding muscle fibers. This might be particularly relevant for exercise and training programs targeting specific muscle fibers in elderly subjects (47), cancer patients (31), or patients with neurodegenerative disorders (45), where the activation and training of type II muscle fibers is very important in preventing muscle atrophy and strength degradation. Further research is needed to 1) confirm the universality of results over larger cohorts of subjects, 2) investigate muscle spectral profiles under different clinical conditions (e.g., acute and chronic muscle injuries, neuromuscular disorders, etc.), and 3) identify muscle spectral power responses to distinct types of training programs (e.g., resistance, endurance).
The findings reported here should be considered in the context of certain limitations, including the limited number of subjects in both the young and old group. Nevertheless, since participants performed three consecutive squat tests, each with a duration of 6–12 min, the utilized protocol provides a sufficient number of squat movements necessary to perform reliable analyses of the spectral power profiles and to assess effects of accumulated and residual fatigue (see Performance). Because the limited number subjects in each age group, we were unable to study the effects of sex difference. Furthermore, we note that the magnitude of EMG signals may be influenced by variations in skin thickness and subcutaneous tissues among subjects. Given that elderly subjects present higher body fat mass compared with young adults (70), the observed differences across age groups might be partially influenced by different body fat levels in the two groups. However, as described in earlier studies (77), this artifact has been shown to mainly affect the EMG signal amplitude and has relatively little effect on the functional form of the signal spectral power distribution in the frequency domain. Finally, the present study is not supported by kinematic data, and ankle, knee, and hip angles are not examined throughout the down and up squat phases.
Conclusions
In summary, we investigated leg and back muscle activation during repeated maximal exercise. We found that leg and back muscle dynamics were characterized by muscle-specific spectral power profiles, where different frequency bands exhibited a differentiated response to accumulation of fatigue within exercise segments and to residual fatigue across repeated exercise segments. The established universality in general spectral power profile characteristics among subjects in both young and old groups indicated the presence of a previously unrecognized multiscale mechanism underlying the distinct response of different muscle types to exercise-induced fatigue and effects of aging. The consistency of the spectral power profiles among subjects (young or old group) at a given physiological state (rest or exercise) and muscle type (leg or back), as well as the robustness in the evolution of these profiles at large time scales for consecutive exercise segments (hours), intermediate time scales within exercise segments (minutes), and at short time scales during a single squat movement (seconds), reveals a universal behavior related to a basic multiscale mechanism of muscle tone regulation that integrates the activation of different muscle fibers types across frequency bands. To our knowledge, this is the first research uncovering differentiated response and evolution across time scales of the leg and back spectral power profiles in healthy young and old adults with accumulation of fatigue during exercise.
GRANTS
This work was supported by research grants to PChI from the following agencies: the W. M. Keck Foundation (http://www.wmkeck.org), National Institutes of Health (NIH Grant 1R01-HL098437, https://www.nih.gov/), US-Israel Binational Science Foundation (BSF Grant 2012219, http://www.bsf.org.il/BSFPublic/), and Office of Naval Research (ONR Grant 000141010078, https://www.onr.navy.mil/). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.G.-R. and P.C.I. conceived and designed research; S.G.-R. and C.S. performed experiments; S.G.-R., R.R., J.W.J.L.W., C.S., and P.C.I. analyzed data; S.G.-R., R.R., J.W.J.L.W., C.S., and P.C.I. interpreted results of experiments; R.R., J.W.J.L.W., and P.C.I. prepared figures; S.G.-R. and P.C.I. drafted manuscript; S.G.-R., R.R., J.W.J.L.W., C.S., and P.C.I. edited and revised manuscript; P.C.I. approved final version of manuscript.
REFERENCES
- 1.Ashkenazy Y, Hausdorff JM, Ivanov PC, Stanley HE. A stochastic model of human gait dynamics. Physica A 316: 662–670, 2002. doi: 10.1016/S0378-4371(02)01453-X. [DOI] [Google Scholar]
- 2.Barreiro E, Salazar-Degracia A, Sancho-Muñoz A, Gea J. Endoplasmic reticulum stress and unfolded protein response profile in quadriceps of sarcopenic patients with respiratory diseases. J Cell Physiol 234: 11315–11329, 2019. doi: 10.1002/jcp.27789. [DOI] [PubMed] [Google Scholar]
- 3.Beck TW, Stock MS, Defreitas JM. Shifts in EMG spectral power during fatiguing dynamic contractions. Muscle Nerve 50: 95–102, 2014. doi: 10.1002/mus.24098. [DOI] [PubMed] [Google Scholar]
- 4.Beck TW, von Tscharner V, Housh TL, Cramer JT, Weir JP, Malek MH, Mielke M. Time/frequency events of surface mechanomyographic signals resolved by nonlinear scaled wavelets. Biomed Signal Process Control 3: 255–266, 2008. doi: 10.1016/j.bspc.2008.01.005. [DOI] [Google Scholar]
- 5.Bianco A, Lupo C, Alesi M, Spina S, Raccuglia M, Thomas E, Paoli A, Palma A. The sit up test to exhaustion as a test for muscular endurance evaluation. Springerplus 4: 309, 2015. doi: 10.1186/s40064-015-1023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buchthal F, Dahl K, Rosenfalck P. Rise time of the spike potential in fast and slowly contracting muscle of man. Acta Physiol Scand 87: 261–269, 1973. doi: 10.1111/j.1748-1716.1973.tb05389.x. [DOI] [PubMed] [Google Scholar]
- 7.Burke RE. The structure and function of motor units. In: Disorders of Voluntary Muscle (7th ed.), edited by Karpati G, Hilton D, and Griggs RC). Cambridge, UK: Cambridge University Press, 2001, p. 3–21. [Google Scholar]
- 8.Burke RE, Levine DN, Tsairis P, Zajac FE III. Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol 234: 723–748, 1973. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cagnie B, Dhooge F, Schumacher C, De Meulemeester K, Petrovic M, van Oosterwijck J, Danneels L. Fiber typing of the erector spinae and multifidus muscles in healthy controls and back pain patients: a systematic literature review. J Manipulative Physiol Ther 38: 653–663, 2015. doi: 10.1016/j.jmpt.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Cardozo AC, Gonçalves M, Dolan P. Back extensor muscle fatigue at submaximal workloads assessed using frequency banding of the electromyographic signal. Clin Biomech (Bristol, Avon) 26: 971–976, 2011. doi: 10.1016/j.clinbiomech.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Cheng AJ, Place N, Westerblad H. Molecular basis for exercise-induced fatigue: the importance of strictly controlled cellular Ca2+ handling. Cold Spring Harb Perspect Med 8: a029710, 2018. doi: 10.1101/cshperspect.a029710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cifrek M, Medved V, Tonković S, Ostojić S. Surface EMG based muscle fatigue evaluation in biomechanics. Clin Biomech (Bristol, Avon) 24: 327–340, 2009. doi: 10.1016/j.clinbiomech.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Criswell E. Cram’s Introduction to Surface Electromyography. Sudbury, MA: Jones and Barltett Publishers, 2011. [Google Scholar]
- 14.Contreras B, Vigotsky AD, Schoenfeld BJ, Beardsley C, Cronin J. A comparison of gluteus maximus, biceps femoris, and vastus lateralis electromyography amplitude in the parallel, full, and front squat variations in resistance-trained females. J Appl Biomech 32: 16–22, 2016. doi: 10.1123/jab.2015-0113. [DOI] [PubMed] [Google Scholar]
- 15.De Luca CJ. Myoelectrical manifestations of localized muscular fatigue in humans. Crit Rev Biomed Eng 11: 251–279, 1984. [PubMed] [Google Scholar]
- 16.Dolan P, Mannion AF, Adams MA. Fatigue of the erector spinae muscles. A quantitative assessment using “frequency banding” of the surface electromyography signal. Spine 20: 149–159, 1995. doi: 10.1097/00007632-199501150-00005. [DOI] [PubMed] [Google Scholar]
- 17.Dreibati B, Lavet C, Pinti A, Poumarat G. Influence of electrical stimulation frequency on skeletal muscle force and fatigue. Ann Phys Rehabil Med 53: 266–277, 2010. doi: 10.1016/j.rehab.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Duchateau J, Enoka RM. Neural control of shortening and lengthening contractions: influence of task constraints. J Physiol 586: 5853–5864, 2008. doi: 10.1113/jphysiol.2008.160747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dutta C, Hadley EC. The significance of sarcopenia in old age. J Gerontol A Biol Sci Med Sci 50A, Special: 1–4, 1995. doi: 10.1093/gerona/50A.Special_Issue.1. [DOI] [PubMed] [Google Scholar]
- 20.Eagan MS, Sedlock DA. Kyphosis in active and sedentary postmenopausal women. Med Sci Sports Exerc 33: 688–695, 2001. doi: 10.1097/00005768-200105000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Escamilla RF. Knee biomechanics of the dynamic squat exercise. Med Sci Sports Exerc 33: 127–141, 2001. doi: 10.1097/00005768-200101000-00020. [DOI] [PubMed] [Google Scholar]
- 22.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39: 175–191, 2007. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- 23.Ferrari D, Kuriki HU, Silva CR, Alves N, Mícolis de Azevedo F. Diagnostic accuracy of the electromyography parameters associated with anterior knee pain in the diagnosis of patellofemoral pain syndrome. Arch Phys Med Rehabil 95: 1521–1526, 2014. doi: 10.1016/j.apmr.2014.03.028. [DOI] [PubMed] [Google Scholar]
- 24.Fidalgo-Herrera A, Miangolarra-Page J, Carratalá-Tejada M. Traces of muscular fatigue in the rectus femoris identified with surface electromyography and wavelets on normal gait. Physiother Theory Pract: 1–15, 2020. doi: 10.1080/09593985.2020.1725945. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Retortillo S, Javierre C, Hristovski R, Ventura JL, Balagué N. Cardiorespiratory coordination in repeated maximal exercise. Front Physiol 8: 387, 2017. doi: 10.3389/fphys.2017.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.González-Izal M, Rodríguez-Carreño I, Malanda A, Mallor-Giménez F, Navarro-Amézqueta I, Gorostiaga EM, Izquierdo M. sEMG wavelet-based indices predicts muscle power loss during dynamic contractions. J Electromyogr Kinesiol 20: 1097–1106, 2010. doi: 10.1016/j.jelekin.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 27.Goubert D, Oosterwijck JV, Meeus M, Danneels L. Structural changes of lumbar muscles in non-specific low back pain: a systematic review. Pain Physician 19: E985–E1000, 2016. [PubMed] [Google Scholar]
- 28.Grimby L, Hannerz J, Hedman B. Contraction time and voluntary discharge properties of individual short toe extensor motor units in man. J Physiol 289: 191–201, 1979. doi: 10.1113/jphysiol.1979.sp012732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hader K, Rumpf MC, Hertzog M, Kilduff LP, Girard O, Silva JR. Monitoring the athlete match response: can external load variables predict post-match acute and residual fatigue in soccer? A systematic review with meta-analysis. Sports Med Open 5: 48, 2019. doi: 10.1186/s40798-019-0219-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hausdorff JM, Ashkenazy Y, Peng CK, Ivanov PC, Stanley HE, Goldberger AL. When human walking becomes random walking: fractal analysis and modeling of gait rhythm fluctuations. Physica A 302: 138–147, 2001. doi: 10.1016/S0378-4371(01)00460-5. [DOI] [PubMed] [Google Scholar]
- 31.Hegedus A, Trzaskoma L, Soldos P, Tuza K, Katona P, Greger Z, Zsarnoczky-Dulhazi F, Kopper B. Adaptation of fatigue affected changes in muscle EMG frequency characteristics for the determination of training load in physical therapy for cancer patients. Pathol Oncol Res 26: 1129–1135, 2020. doi: 10.1007/s12253-019-00668-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henneman E, Somjen G, Carpenter DO. Excitability and inhibitability of motoneurons of different sizes. J Neurophysiol 28: 599–620, 1965. doi: 10.1152/jn.1965.28.3.599. [DOI] [PubMed] [Google Scholar]
- 33.Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol 10: 361–374, 2000. doi: 10.1016/S1050-6411(00)00027-4. [DOI] [PubMed] [Google Scholar]
- 34.Hodson-Tole EF, Wakeling JM. Variations in motor unit recruitment patterns occur within and between muscles in the running rat (Rattus norvegicus). J Exp Biol 210: 2333–2345, 2007. doi: 10.1242/jeb.004457. [DOI] [PubMed] [Google Scholar]
- 35.Hristovski R, Balagué N. Fatigue-induced spontaneous termination point--nonequilibrium phase transitions and critical behavior in quasi-isometric exertion. Hum Mov Sci 29: 483–493, 2010. doi: 10.1016/j.humov.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 36.Hristovski R, Venskaitytė E, Vainoras A, Balagué N, Vazquez P. Constraints-controlled metastable dynamics of exercise-induced psychobiological adaptation. Medicina (Kaunas) 46: 447–453, 2010. doi: 10.3390/medicina46070064. [DOI] [PubMed] [Google Scholar]
- 37.Hu K, Ivanov PC, Chen Z, Hilton MF, Stanley HE, Shea SA. Non-random fluctuations and multi-scale dynamics regulation of human activity. Physica A 337: 307–318, 2004. doi: 10.1016/j.physa.2004.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu K, Scheer FA, Ivanov PC, Buijs RM, Shea SA. The suprachiasmatic nucleus functions beyond circadian rhythm generation. Neuroscience 149: 508–517, 2007. doi: 10.1016/j.neuroscience.2007.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ivanov PC, Nunes Amaral LA, Goldberger AL, Stanley HE. Stochastic feedback and the regulation of biological rhythms. Europhys Lett 43: 363–368, 1998. doi: 10.1209/epl/i1998-00366-3. [DOI] [PubMed] [Google Scholar]
- 40.Ivanov PC, Hu K, Hilton MF, Shea SA, Stanley HE. Endogenous circadian rhythm in human motor activity uncoupled from circadian influences on cardiac dynamics. Proc Natl Acad Sci USA 104: 20702–20707, 2007. doi: 10.1073/pnas.0709957104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ivanov PC, Ma QD, Bartsch RP, Hausdorff JM, Nunes Amaral LA, Schulte-Frohlinde V, Stanley HE, Yoneyama M. Levels of complexity in scale-invariant neural signals. Phys Rev E Stat Nonlin Soft Matter Phys 79: 041920, 2009. doi: 10.1103/PhysRevE.79.041920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Izquierdo M, González-Izal M, Navarro-Amezqueta I, Calbet JAL, Ibañez J, Malanda A, Mallor F, Häkkinen K, Kraemer WJ, Gorostiaga EM. Effects of strength training on muscle fatigue mapping from surface EMG and blood metabolites. Med Sci Sports Exerc 43: 303–311, 2011. doi: 10.1249/MSS.0b013e3181edfa96. [DOI] [PubMed] [Google Scholar]
- 43.Jaworowski A, Porter MM, Holmbäck AM, Downham D, Lexell J. Enzyme activities in the tibialis anterior muscle of young moderately active men and women: relationship with body composition, muscle cross-sectional area and fibre type composition. Acta Physiol Scand 176: 215–225, 2002. doi: 10.1046/j.1365-201X.2002.t01-2-01004.x. [DOI] [PubMed] [Google Scholar]
- 44.Karasik R, Sapir N, Ashkenazy Y, Ivanov PC, Dvir I, Lavie P, Havlin S. Correlation differences in heartbeat fluctuations during rest and exercise. Phys Rev E Stat Nonlin Soft Matter Phys 66: 062902, 2002. doi: 10.1103/PhysRevE.66.062902. [DOI] [PubMed] [Google Scholar]
- 45.Kelly NA, Ford MP, Standaert DG, Watts RL, Bickel CS, Moellering DR, Tuggle SC, Williams JY, Lieb L, Windham ST, Bamman MM. Novel, high-intensity exercise prescription improves muscle mass, mitochondrial function, and physical capacity in individuals with Parkinson’s disease. J Appl Physiol (1985) 116: 582–592, 2014. doi: 10.1152/japplphysiol.01277.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khaiyat OA, Norris J. Electromyographic activity of selected trunk, core, and thigh muscles in commonly used exercises for ACL rehabilitation. J Phys Ther Sci 30: 642–648, 2018. doi: 10.1589/jpts.30.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kienbacher T, Habenicht R, Starek C, Mair P, Wolf M, Paul B, Riegler S, Kollmitzer J, Ebenbichler G. The potential use of spectral electromyographic fatigue as a screening and outcome monitoring tool of sarcopenic back muscle alterations. J Neuroeng Rehabil 11: 106, 2014. doi: 10.1186/1743-0003-11-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klass M, Baudry S, Duchateau J. Age-related decline in rate of torque development is accompanied by lower maximal motor unit discharge frequency during fast contractions. J Appl Physiol (1985) 104: 739–746, 2008. doi: 10.1152/japplphysiol.00550.2007. [DOI] [PubMed] [Google Scholar]
- 49.Kwon M, Baweja HS, Christou EA. Ankle variability is amplified in older adults due to lower EMG power from 30-60 Hz. Hum Mov Sci 31: 1366–1378, 2012. doi: 10.1016/j.humov.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 50.Lieber RL, Fridén J. Selective damage of fast glycolytic muscle fibres with eccentric contraction of the rabbit tibialis anterior. Acta Physiol Scand 133: 587–588, 1988. doi: 10.1111/j.1748-1716.1988.tb08446.x. [DOI] [PubMed] [Google Scholar]
- 51.Lo CC, Amaral LN, Havlin S, Ivanov PC, Penzel T, Peter JH, Stanley HE. Dynamics of sleep-wake transitions during sleep. Europhys Lett 57: 625–631, 2002. doi: 10.1209/epl/i2002-00508-7. [DOI] [Google Scholar]
- 52.Macaluso A, De Vito G. Muscle strength, power and adaptations to resistance training in older people. Eur J Appl Physiol 91: 450–472, 2004. doi: 10.1007/s00421-003-0991-3. [DOI] [PubMed] [Google Scholar]
- 53.McAllister M, Costigan P. Evaluating movement performance: What you see isn’t necessarily what you get. Hum Mov Sci 64: 67–74, 2019. doi: 10.1016/j.humov.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 54.McKay MJ, Baldwin JN, Ferreira P, Simic M, Vanicek N, Burns J; 1000 Norms Project Consortium . Normative reference values for strength and flexibility of 1,000 children and adults. Neurology 88: 36–43, 2017. doi: 10.1212/WNL.0000000000003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Melo RC, Takahashi AC, Quitério RJ, Salvini TF, Catai AM. Eccentric torque-producing capacity is influenced by muscle length in older healthy adults. J Strength Cond Res 30: 259–266, 2016. doi: 10.1519/JSC.0000000000001047. [DOI] [PubMed] [Google Scholar]
- 56.Mendell LM. The size principle: a rule describing the recruitment of motoneurons. J Neurophysiol 93: 3024–3026, 2005. doi: 10.1152/classicessays.00025.2005. [DOI] [PubMed] [Google Scholar]
- 57.Mitchell WK, Williams J, Atherton P, Larvin M, Lund J, Narici M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front Physiol 3: 260, 2012. doi: 10.3389/fphys.2012.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moffroid MT, Haugh LD, Haig AJ, Henry SM, Pope MH. Endurance training of trunk extensor muscles. Phys Ther 73: 3–10, 1993. doi: 10.1093/ptj/73.1.3. [DOI] [PubMed] [Google Scholar]
- 59.Monemi M, Eriksson PO, Eriksson A, Thornell LE. Adverse changes in fibre type composition of the human masseter versus biceps brachii muscle during aging. J Neurol Sci 154: 35–48, 1998. doi: 10.1016/S0022-510X(97)00208-6. [DOI] [PubMed] [Google Scholar]
- 60.Mortimer JT, Magnusson R, Petersén I. Conduction velocity in ischemic muscle: effect on EMG frequency spectrum. Am J Physiol 219: 1324–1329, 1970. doi: 10.1152/ajplegacy.1970.219.5.1324. [DOI] [PubMed] [Google Scholar]
- 61.Moser M, Penter R, Fruehwirth M, Kenner T. Why life oscillates–biological rhythms and health. Conf Proc IEEE Eng Med Biol Soc 2006: 424–428, 2006. doi: 10.1109/IEMBS.2006.259562. [DOI] [PubMed] [Google Scholar]
- 62.Muanjai P, Mickevicius M, Sniečkus A, Sipavičienė S, Satkunskiene D, Kamandulis S, Jones DA. Low frequency fatigue and changes in muscle fascicle length following eccentric exercise of the knee extensors. Exp Physiol 105: 502–510, 2020. doi: 10.1113/EP088237. [DOI] [PubMed] [Google Scholar]
- 63.Murgia M, Toniolo L, Nagaraj N, Ciciliot S, Vindigni V, Schiaffino S, Reggiani C, Mann M. Single muscle fiber proteomics reveals fiber-type-specific features of human muscle aging. Cell Reports 19: 2396–2409, 2017. doi: 10.1016/j.celrep.2017.05.054. [DOI] [PubMed] [Google Scholar]
- 64.Nederveen JP, Ibrahim G, Fortino SA, Snijders T, Kumbhare D, Parise G. Variability in skeletal muscle fibre characteristics during repeated muscle biopsy sampling in human vastus lateralis. Appl Physiol Nutr Metab 45: 368–375, 2020. doi: 10.1139/apnm-2019-0263. [DOI] [PubMed] [Google Scholar]
- 65.Neto OP, Baweja HS, Christou EA. Increased voluntary drive is associated with changes in common oscillations from 13 to 60 Hz of interference but not rectified electromyography. Muscle Nerve 42: 348–354, 2010. doi: 10.1002/mus.21687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nicol C, Avela J, Komi PV. The stretch-shortening cycle : a model to study naturally occurring neuromuscular fatigue. Sports Med 36: 977–999, 2006. doi: 10.2165/00007256-200636110-00004. [DOI] [PubMed] [Google Scholar]
- 67.Phinyomark A, Thongpanja S, Hu H, Phukpattaranont P, Limsakul C. The usefulness of mean and median frequencies in electromyography analysis. In: Computational Intelligence in Electromyography Analysis: A Perspective on Current Applications and Future Challenges, edited by Naik G. Rijeka, Croatia: InTech, 2012, p. 195–220. [Google Scholar]
- 68.Qi L, Wakeling JM, Ferguson-Pell M. Spectral properties of electromyographic and mechanomyographic signals during dynamic concentric and eccentric contractions of the human biceps brachii muscle. J Electromyogr Kinesiol 21: 1056–1063, 2011. doi: 10.1016/j.jelekin.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 69.Rabin A, Kozol Z. Utility of the overhead squat and forward arm squat in screening for limited ankle dorsiflexion. J Strength Cond Res 31: 1251–1258, 2017. doi: 10.1519/JSC.0000000000001580. [DOI] [PubMed] [Google Scholar]
- 70.Reinders I, Visser M, Schaap L. Body weight and body composition in old age and their relationship with frailty. Curr Opin Clin Nutr Metab Care 20: 11–15, 2017. doi: 10.1097/MCO.0000000000000332. [DOI] [PubMed] [Google Scholar]
- 71.Roig M, Macintyre DL, Eng JJ, Narici MV, Maganaris CN, Reid WD. Preservation of eccentric strength in older adults: Evidence, mechanisms and implications for training and rehabilitation. Exp Gerontol 45: 400–409, 2010. doi: 10.1016/j.exger.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Roman-Liu D, Konarska M. Characteristics of power spectrum density function of EMG during muscle contraction below 30%MVC. J Electromyogr Kinesiol 19: 864–874, 2009. doi: 10.1016/j.jelekin.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 73.Roy SH, De Luca CJ, Casavant DA. Lumbar muscle fatigue and chronic lower back pain. Spine 14: 992–1001, 1989. doi: 10.1097/00007632-198909000-00014. [DOI] [PubMed] [Google Scholar]
- 74.Sandler R, Robinovitch S. An analysis of the effect of lower extremity strength on impact severity during a backward fall. J Biomech Eng 123: 590–598, 2001. doi: 10.1115/1.1408940. [DOI] [PubMed] [Google Scholar]
- 75.Schwartz LM. Skeletal muscles do not undergo apoptosis during either atrophy or programmed cell death-revisiting the myonuclear domain hypothesis. Front Physiol 9: 1887, 2019. doi: 10.3389/fphys.2018.01887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Siddiqi A, Arjunan SP, Kumar DK. Age-associated changes in the spectral and statistical parameters of surface electromyogram of tibialis anterior. BioMed Res Int 2016: 1–6, 2016. doi: 10.1155/2016/7159701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ajit Singh DK, Bailey M, Lee R. Strength and fatigue of lumbar extensor muscles in older adults. Muscle Nerve 44: 74–79, 2011. doi: 10.1002/mus.21998. [DOI] [PubMed] [Google Scholar]
- 78.Swinton PA, Lloyd R, Keogh JW, Agouris I, Stewart AD. A biomechanical comparison of the traditional squat, powerlifting squat, and box squat. J Strength Cond Res 26: 1805–1816, 2012. doi: 10.1519/JSC.0b013e3182577067. [DOI] [PubMed] [Google Scholar]
- 79.Tsuboi H, Nishimura Y, Sakata T, Ohko H, Tanina H, Kouda K, Nakamura T, Umezu Y, Tajima F. Age-related sex differences in erector spinae muscle endurance using surface electromyographic power spectral analysis in healthy humans. Spine J 13: 1928–1933, 2013. doi: 10.1016/j.spinee.2013.06.060. [DOI] [PubMed] [Google Scholar]
- 80.van Dieën JH, Heijblom P. Reproducibility of isometric trunk extension torque, trunk extensor endurance, and related electromyographic parameters in the context of their clinical applicability. J Orthop Res 14: 139–143, 1996. doi: 10.1002/jor.1100140122. [DOI] [PubMed] [Google Scholar]
- 81.Vázquez P, Hristovski R, Balagué N. The path to exhaustion: time-variability properties of coordinative variables during continuous exercise. Front Physiol 7: 37, 2016. doi: 10.3389/fphys.2016.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, Simonsick EM, Harris TB. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci 60: 324–333, 2005. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 83.Vromans M, Faghri PD. Functional electrical stimulation-induced muscular fatigue: Effect of fiber composition and stimulation frequency on rate of fatigue development. J Electromyogr Kinesiol 38: 67–72, 2018. doi: 10.1016/j.jelekin.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 84.Wakeling JM. Patterns of motor recruitment can be determined using surface EMG. J Electromyogr Kinesiol 19: 199–207, 2009. doi: 10.1016/j.jelekin.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 85.Wakeling JM, Rozitis AI. Spectral properties of myoelectric signals from different motor units in the leg extensor muscles. J Exp Biol 207: 2519–2528, 2004. doi: 10.1242/jeb.01042. [DOI] [PubMed] [Google Scholar]
- 86.Wang J, Ning X, Ma Q, Bian C, Xu Y, Chen Y. Multiscale multifractality analysis of a 12-lead electrocardiogram. Phys Rev E Stat Nonlin Soft Matter Phys 71: 062902, 2005. doi: 10.1103/PhysRevE.71.062902. [DOI] [PubMed] [Google Scholar]
- 87.Wernbom M, Aagaard P. Muscle fibre activation and fatigue with low-load blood flow restricted resistance exercise-An integrative physiology review. Acta Physiol (Oxf) 228: e13302, 2020. doi: 10.1111/apha.13302. [DOI] [PubMed] [Google Scholar]
- 88.World Medical Association WMA Declaration of Taipei on Ethical Considerations Regarding Health Databases and Biobanks. https://www.wma.net/policies-post/wma-declaration-of-taipei-on-ethical-considerations-regarding-health-databases-and-biobanks/ [8 Apr 2020]. [DOI] [PubMed]
- 89.Yavuz HU, Erdağ D, Amca AM, Aritan S. Kinematic and EMG activities during front and back squat variations in maximum loads. J Sports Sci 33: 1058–1066, 2015. doi: 10.1080/02640414.2014.984240. [DOI] [PubMed] [Google Scholar]
- 90.Yeh CP, Huang HC, Chang Y, Chen MD, Hsu M. The reliability and validity of a modified squat test to predict cardiopulmonary fitness in healthy older men. BioMed Res Int 2018: 1–7, 2018. doi: 10.1155/2018/4863454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zampieri S, Mosole S, Löfler S, Fruhmann H, Burggraf S, Cvečka J, Hamar D, Sedliak M, Tirptakova V, Šarabon N, Mayr W, Kern H. Physical exercise in aging: nine weeks of leg press or electrical stimulation training in 70 years old sedentary elderly people. Eur J Transl Myol 25: 237–242, 2015. doi: 10.4081/ejtm.2015.5374. [DOI] [PMC free article] [PubMed] [Google Scholar]