Abstract
Near-infrared spectroscopy (NIRS) provides a simple and reliable measure of skeletal muscle oxidative capacity; however, its relationship to aerobic fitness and sex are unclear. We hypothesized that NIRS-derived oxidative capacity in the vastus lateralis (VL) and medial gastrocnemius (MG) would be correlated with indices of aerobic fitness and independent of sex. Twenty-six participants (13 males, 13 females) performed ramp- and step-incremental tests to volitional exhaustion on separate days to establish maximal oxygen uptake (V̇o2max), peak power output (PPO), lactate threshold (LT), gas exchange threshold (GET), respiratory compensation point (RCP), and maximal fat oxidation (MFO). Data were normalized to lean body mass to account for sex-based differences in body composition. Exercise tests were preceded by duplicate measurements of NIRS-derived oxidative capacity on the VL and MG muscles (i.e., repeated arterial occlusions following a brief set of muscle contractions). Skeletal muscle oxidative capacity for the VL (means ± SD: 21.9 ± 4.6 s) and MG (22.5 ± 6.1 s) were similar but unrelated (r2 = 0.03, P = 0.39). Skeletal muscle oxidative capacity for the VL, but not the MG (P > 0.05 for all variables), was significantly correlated with V̇o2max (r2 = 0.24; P = 0.01), PPO (r2 = 0.23; P = 0.01), LT (r2 = 0.23; P = 0.01), GET (r2 = 0.23; P = 0.01), and RCP (r2 = 0.27; P = 0.006). MFO was not correlated with VL or MG skeletal muscle oxidative capacity (P > 0.05). Females (54.9 ± 4.5 mL·kg LBM−1·min−1) and males (56.0 ± 6.2 mL·kg LBM−1·min−1), matched for V̇o2max (P = 0.62), had similar NIRS-derived oxidative capacities for VL (20.7 ± 4.4 vs. 23.2 ± 4.6 s; P = 0.18) and MG (24.4 ± 6.8 vs. 20.5 ± 4.8 s; P = 0.10). Overall, NIRS-derived skeletal muscle oxidative capacity in VL is indicative of aerobic fitness and independent of sex in humans.
NEW & NOTEWORTHY Near-infrared spectroscopy (NIRS) can be used to measure skeletal muscle oxidative capacity. Here, we demonstrated that NIRS-derived skeletal muscle oxidative capacity of the vastus lateralis was independent of sex, reliable across and within days, and correlated with maximal and submaximal indices of aerobic fitness, including maximal oxygen uptake, lactate threshold, and respiratory compensation point. These findings highlight the utility of NIRS for investigating skeletal muscle oxidative capacity in females and males.
Keywords: fat oxidation, lactate threshold, maximal oxygen uptake, reliability, vastus lateralis
INTRODUCTION
Oxidative capacity, the maximal rate at which skeletal muscle utilizes oxygen to meet energetic demands, is primarily assessed using in vitro techniques such as high-resolution respirometry and mitochondrial enzyme content/activity (30) or in vivo techniques such as 31P magnetic resonance spectroscopy (31P-MRS) to quantify phosphocreatine (PCr) recovery kinetics postexercise (29). These techniques, although widely used and highly insightful, are relatively expensive to perform, dependent on highly trained personnel and equipment, invasive (biopsies), and restrictive (31P-MRS), which limit their utility. Recently, a simpler, noninvasive, in vivo measure of skeletal muscle oxidative capacity using near-infrared spectroscopy (NIRS) has emerged (2, 36, 58). This technique involves monitoring the oxygenation status of the muscle with NIRS during transient, repeated arterial occlusions that follow a brief exercise stimulus (2, 55, 58). With blood flow impeded via arterial occlusion, changes in deoxygenated hemoglobin and myoglobin (HHb), oxygenated hemoglobin and myoglobin (O2Hb), or tissue saturation index (TSI) reflect muscle oxygen consumption (mV̇o2). The kinetics of mV̇o2 recovery postexercise, which follow an exponential time course, are indicative of muscle oxidative capacity: faster mV̇o2 recovery indicates greater oxidative capacity and vice versa (36). This technique is reliable (45, 49) and has been validated through comparisons with standard in vitro (44) and in vivo (47) measures of skeletal muscle oxidative capacity. Yet, because it is a relatively new technique, the utility of NIRS-derived muscle oxidative capacity to exercise physiology research is unclear.
While sex influences skeletal muscle metabolism (52), data regarding its influence on skeletal muscle oxidative capacity, as measured by transmission electron microscopy, PCr recovery kinetics, and mitochondrial respiration, enzyme activity, and protein content, are equivocal (8, 9, 25, 34, 35, 42, 53, 54). One explanation for discrepancies across studies is the influence of body composition on aerobic fitness: as females tend to have higher proportions of body fat compared with males with the same body mass index (BMI) (20), leading to lower relative maximal oxygen uptake (V̇o2max) values (mL·kg−1·min−1) in females than males who could otherwise be considered similarly fit (52). To account for this difference in body composition, V̇o2max is typically normalized to lean body mass (LBM) to isolate the influence of sex while one studies metabolism (12, 52). Given that V̇o2max is related to standard measures of skeletal muscle oxidative capacity (15, 19, 21), appropriately matching males and females for aerobic fitness is essential for examining sex-based differences in this trait. Yet, some studies reporting indices of skeletal muscle oxidative capacity in females and males have not ensured similar V̇o2max across sexes (34, 42, 54), whereas others reported similar V̇o2max across sexes without accounting for differences in body composition (8, 25, 35). Potentially due to differences in V̇o2max (mL·kg LBM−1·min−1), some studies indicate that indices of skeletal muscle oxidative capacity are similar between females and males (25, 42, 54), whereas others indicate they are different (8, 34, 35). In at least two studies that normalized V̇o2max to LBM, indices of skeletal muscle oxidative capacity were similar in females and males (9, 53). Using NIRS to compare muscle oxidative capacity between females and males, matched for aerobic fitness, will help resolve whether there are sex-based differences in skeletal muscle oxidative capacity. Furthermore, determining whether NIRS-derived skeletal muscle oxidative capacity is influenced by sex will also expand the potential utility of the method.
While exercise training increases muscle oxidative capacity and maximal and submaximal indices of aerobic fitness (7, 40), the extent to which NIRS-derived oxidative capacity is associated with whole-body measures of aerobic fitness has received little attention. Strong, positive associations exist between parameters of whole-body aerobic fitness [e.g., V̇o2max, lactate threshold (LT), and fat oxidation] and in vitro (19, 21, 39) and in vivo (31, 32) measures of muscle oxidative capacity. Similarly, two studies demonstrated that the mean NIRS-derived muscle oxidative capacities of the vastus lateralis (VL) (6) and gastrocnemius (28) muscles were higher in trained compared with untrained individuals; however, we are unaware of any study that has examined correlations between NIRS-derived muscle oxidative capacity and maximal and submaximal measures of aerobic fitness in individuals with a broad, continuous range of fitness levels (i.e., without excluding participants with intermediate fitness).
Thus the primary purposes of the current study were to determine 1) whether there are sex-based differences in NIRS-derived muscle oxidative capacity and 2) the relationship between NIRS-derived muscle oxidative capacity and whole-body measures of aerobic fitness. A secondary purpose was to determine the reliability of our specific protocol. We hypothesized that NIRS-derived skeletal muscle oxidative capacity in the VL and medial gastrocnemius (MG) would be 1) similar between males and females matched for aerobic fitness, 2) associated with maximal and submaximal measures of aerobic fitness, and 3) reliable within and across days.
METHODS
Ethical Approval
Experimental protocols were approved by the Conjoint Health Research Ethics Board of the University of Calgary (18-1511). Before testing, participants were informed of the study’s purpose, procedures, and potential risks and benefits, and provided written consent.
Participants
We sought to recruit young (18–40 yr) males and females of varying aerobic fitness. A total of 29 participants (13 males, 16 females) completed this study; however, only data from 26 participants (13 males, 13 females) were included in the final analysis because oxidative capacity values were uninterpretable for either the VL (n = 2) or MG (n = 1). To be included, participants were required to pass a physical activity readiness screen (CSEP Get Active Questionnaire), be non-obese (BMI ≤30 kg/m2), not pregnant and eumenorrheic (females), nonsmoking, and healthy (i.e., free of medical conditions and not taking medications likely to affect study results). Female participants completed testing during the follicular phase (days 5–9) of the menstrual cycle.
Experimental Design
On three separate occasions, participants arrived at the laboratory in the morning following an overnight fast and having avoided vigorous exercise, caffeine, and alcohol for at least 24, 12, and 12 h, respectively. During the first visit, participants underwent body composition testing [LBM and adipose tissue thickness (ATT)], isokinetic maximal voluntary contraction (MVC) determination, and familiarization with the NIRS-derived muscle oxidative capacity protocol. For the subsequent two experimental visits, NIRS-derived muscle oxidative capacity tests were performed in duplicate on both the VL and MG muscles, with the order of assessment randomized. During the first visit, muscle oxidative capacity measures were followed by a step-incremental exercise test to exhaustion on an electromagnetically braked cycle ergometer to determine maximal fat oxidation (MFO), FATmax, and LT. During the second visit, all NIRS-derived muscle oxidative capacity tests were repeated, to assess reliability across days, and followed by a ramp incremental test to determine gas exchange threshold (GET) and respiratory compensation point (RCP). As both tests were maximal, V̇o2max was determined as the greatest 30-s rolling average of V̇o2 for either exercise test.
Measures
Body composition.
Participants underwent a whole-body dual-energy X-ray absorptiometry (DXA) scan (Horizon DXA system, Hologic, Marlborough, MA) to determine LBM. A B-mode ultrasound (EnVisor Ultrasound system, Philips, Amsterdam, The Netherlands) was used to accurately position the NIRS probe over each muscle belly, determine local ATT, and verify that the cuff pressure occluded blood flow. To compare males and females, all measures of whole-body fitness [i.e., V̇o2max, peak power output (PPO), LT, GET, RCP, MFO, and FATmax] were normalized to LBM (12, 52).
Isokinetic maximal voluntary contractions.
Resistance for the NIRS-derived oxidative capacity protocol was based on isokinetic MVCs of each muscle of interest, using an isokinetic dynamometer (Cybex 6000, CSMI, Stoughton, MA). The chair and dynamometer were positioned to align the relevant joint with the dynamometer axis of rotation. MVC determination protocols were identical for each muscle, with the exception of the speeds of contraction (90°/s for knee extension and 60°/s for plantar flexion), which were selected to match the frequency of contractions performed during the muscle oxidative capacity protocol (i.e., 0.5 Hz). MVC trials were repeated until a plateau in peak torque was reached (3–5 trials). The highest torque value for each muscle was recorded as the MVC.
NIRS-derived oxidative capacity.
NIRS was combined with a brief exercise stimulus and repeated, transient arterial occlusions to measure oxidative capacity of the VL and MG muscles. The present protocol was adapted from previous studies (28, 44, 45, 47, 49). The NIRS probe (Portamon, Artinis Medical Systems, Elst, The Netherlands) emitted light at 760- and 850-nm wavelengths through three channels penetrating the muscle tissue at depths of 15, 17.5, and 20 mm. Values of HHb and O2Hb were sampled at a continuous rate of 10 Hz. For both muscles, the NIRS probe was taped within muscle boundaries established through ultrasound assessment and covered to block external light. Probe locations over both muscles were outlined with an indelible marker to ensure reliable probe placement across visits. A blood pressure cuff attached to a rapid cuff inflator (Rapid Cuff Inflation System, D. E. Hokanson, Inc., Bellevue WA) was placed proximal to the NIRS probe. Each testing session was preceded by a 5-min occlusion (i.e., physiological calibration) to identify the maximal and minimal HHb and O2Hb values. After allowing the NIRS signal to stabilize (2–3 min), participants performed a set of 20 isotonic knee extension (15% MVC) or plantar flexion (10% MVC) repetitions at 0.5 Hz, using the same dynamometer setup as during MVC determination. Immediately following exercise, a series of 17 5-s arterial occlusions (250 mmHg), interspersed by 10 s of recovery, was delivered over 4 min. Two muscle oxidative capacity trials were performed consecutively for each muscle on each experimental visit, with the second test beginning once HHb and O2Hb stabilized (~5 min). A total of four muscle oxidative capacity measures were performed per muscle.
NIRS-derived oxidative capacity data analysis.
Raw NIRS data were collected with Oxysoft software (Artinis Medical Systems) and exported onto a custom Microsoft Excel template for organization and initial analysis. Similar to Ryan et al. (45), data were corrected for changes in blood volume; however, a modified correction was applied to the O2Hb and HHb data using the following equations:
where t = time (s); βt = the % O2 saturation at time t (i.e., βt = |O2Hbt| / (|O2Hbt| + |HHbt|); tHb = total hemoglobin and myoglobin. Compared with the correction formulas from Ryan et al., which sum the O2Hb and HHb signals, the present equations adjust the instantaneous changes in O2Hb and HHb signals using the instantaneous change in oxygenated and deoxygenated tHb, respectively. The changes in O2Hb and HHb are then added to the previously corrected O2Hb or HHb value. Differences between these two approaches are small unless the change in tHb is large or βt deviates considerably from 50% (Supplemental Fig. S1; all Supplemental Material is available at https://doi.org/10.6084/m9.figshare.12568340). All O2Hb and HHb data hereafter correspond to the corrected values. Although TSI data have also been used to assess mV̇o2 recovery kinetics (1), we measured mV̇o2 recovery kinetics using the HHb signal, as this method is independent of exercise intensity (43) and has been validated against PCr recovery kinetics (47) and mitochondrial respiration (44).
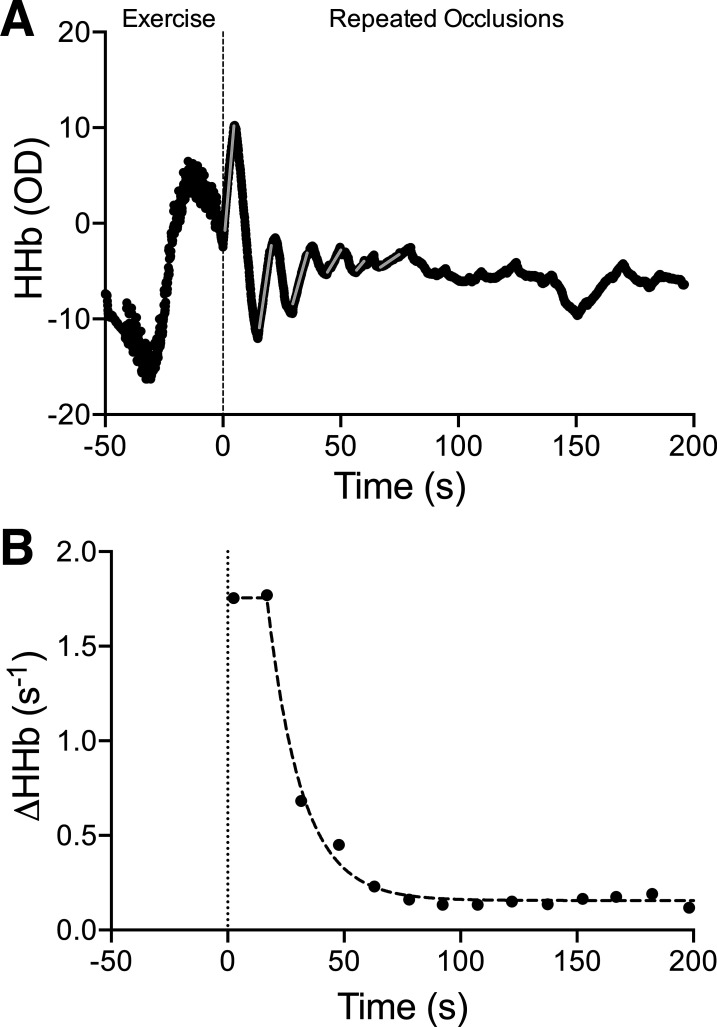
For each occlusion, the slope of the HHb data (i.e., ΔHHb; see Fig. 1) was calculated using a linear regression over a 3-s span of data, which was manually selected to maximize slope fit and avoid erroneous data during the occlusion (i.e., before full cuff inflation or movement artifacts). Only slopes with a good fit (r2 ≥0.75) were used to fit the monoexponential decay equation. In the event that values from one of the three channels were of low quality (i.e., slope values did not resemble a monoexponential curve), that channel was excluded from analysis. The ΔHHb values, averaged across all included channels, were then plotted against time and fit with a monoexponential decay equation:
where y(t) = relative mV̇o2 during arterial occlusions (i.e., ΔHHb) at time t; t = time; A1 = the difference in mV̇o2 between end of exercise (e.g., y-intercept) and the plateau; Plateau = the mV̇o2 at which the curve stabilized; and τ = the time constant, taken as the measure of oxidative capacity. Data were cleaned before curve fitting to remove invalid values or outliers (i.e., low initial ΔHHb (11), or incomplete occlusions). Specifically, the initiation of the monoexponential decay curve was identified, and data points preceding this starting point were removed. Most often, this rule led to removing one or more of the initial points up to the highest value present from the test. Occasionally, the highest point was removed when the distance between it and the next point was much less than the distance between the next two points. Points within the plateau were only removed if they dissociated from the curve or plateau enough to suggest that the point was invalid. Raters were blinded to participant fitness while analyzing curves, and all tests were independently analyzed by two raters. When a discrepancy between raters was present, the test was reevaluated by both researchers together to reach a consensus. Tests were averaged within each day and across the 2 days to give an overall oxidative capacity value for each participant for all analyses other than reliability.
Fig. 1.
Representative data from a single near-infrared spectroscopy (NIRS)-derived muscle oxidative capacity test. A: deoxygenated hemoglobin signal (HHb) during exercise and the repeated occlusion protocols, with occlusion slopes indicated by grey lines. B: change in HHb from each occlusion in A is plotted against time and fit to a monoexponential decay equation, ignoring low initial values. The time constant, τ, is taken as the skeletal muscle oxidative capacity. Note that the first data point was removed, meaning 16 slopes were used for curve fitting (see methods).
Incremental Test Data Collection
Ventilatory and gas exchange variables were sampled breath-by-breath by a metabolic cart (Quark CPET, Cosmed, Rome, Italy). Gas analyzers were calibrated using a precision-analyzed gas mixture of known concentration (15.90% O2, 4.95% CO2, and balanced N2). A turbine flowmeter was calibrated using a 3-L syringe, as per manufacturer recommendations. Heart rate (HR) was monitored and recorded continuously using a chest strap HR monitor (Garmin, HRM-Dual, Schaffhausen, Switzerland).
Step-Incremental Test Protocol
A step-incremental test was performed to exhaustion to determine V̇o2max, FATmax, MFO, and LT. Participants sat on the cycle ergometer for ~5 min before starting baseline measurements. The baseline ventilatory, gas exchange, and blood lactate concentration ([La−]b) measurements were taken during a 3-min resting period (i.e., no cycling, 0 W) using a portable blood lactate analyzer (Lactate Plus, Nova Biomedical, Cheshire, UK). Following baseline measures, cycling power output (PO) increased by 25 W every 3 min until participants reached volitional failure or could no longer maintain a cadence above 60 rpm despite strong verbal encouragement. Participants were blinded to cycling PO and test duration and were instructed to cycle at a consistent cadence between 75 and 90 rpm throughout the test. [La−]b samples were collected during the last minute of each 3-min stage. Once [La−]b exceeded baseline values (rise >0.5 mmol/L), subsequent samples were collected in duplicate [intraclass correlation (ICC) = 0.96], and values were averaged for each stage. Upon termination of the test, participants recovered for 10 min, before completing a verification bout that involved cycling until exhaustion at 25 W above the final stage reached during the step-incremental test (33). On average, these tests lasted 29.7 ± 7.8 min (range: 18.2–45.7 min), and the validation stage lasted 137 ± 30 s (range: 79–212 s).
Ramp-Incremental Test Protocol
A ramp-incremental test was performed to exhaustion to determine V̇o2max, PPO, GET, and RCP. After a 4-min warm-up at 50 W, the PO was gradually increased by 30 W/min (i.e., 1 W every 2 s) until participants reached volitional failure or could no longer maintain a cadence above 60 rpm despite strong verbal encouragement. Participants were blinded to cycling PO and test duration and instructed to cycle at a consistent cadence between 75 and 90 rpm throughout the entire test. A validation test was not performed for the ramp incremental tests because previous studies demonstrated no benefit of the additional test (37). On average, these tests lasted 12.7 ± 2.3 min (range: 9.0–17.7 min).
Gas Exchange Data Analysis
The V̇o2 mean response time was determined from the ramp-incremental exercise test. Briefly, V̇o2 was plotted against time and then aligned so that time “zero” corresponded to the onset of the ramp portion of the test. A linear fit of both baseline and ramp V̇o2 data were used to identify and remove data ±3 SD from the local mean. V̇o2 data were then linearly interpolated to 1-s intervals, and the best fit of a double-linear function was applied from the last 2 min of the baseline to the GET using nonlinear least squares regression (Origin, Origin Laboratory, Northampton, MA). The slope of the first segment of the double-linear was fixed at “0,” and the breakpoint reflected the mean response time (24). The V̇o2 data were left-shifted by the mean response time to identify the POs aligned with GET and RCP (23).
To determine the GET and RCP, gas exchange, and ventilatory measures were plotted against V̇o2 for each ramp-incremental test. The GET was determined as the breakpoint in breath-by-breath V̇co2 vs. V̇o2 (i.e., the point at which V̇co2 began to increase out of proportion to V̇o2). This breakpoint coincided with a systematic rise in V̇E to V̇o2 and end-tidal PO2, whereas V̇E/V̇co2 and end-tidal Pco2 were stable (4, 18). The RCP was determined as the point at which end-tidal Pco2 began to fall after an isocapnic buffering phase, observed by examining the V̇E/V̇co2 and V̇E/V̇o2 relationships, as well as the second breakpoint in the V̇E-to-V̇o2 relationship (18, 57). V̇o2max was determined as the highest V̇o2 from a 30-s rolling average during either exercise test. While V̇o2max could theoretically be underestimated in relatively short or long tests, this is unlikely (17). Also note that the ICC for the V̇o2max/LBM values from the two tests was 0.91, indicating similar values across tests. The highest PO recorded at the end of the ramp-incremental test was deemed the PPO, as this value always exceeded the highest PO of the stage test.
Substrate oxidation.
Fat oxidation rates were calculated over the final 2 min of each stage of the step-incremental test using a stoichiometric equation (22):
MFO was identified as the highest calculated rate of fat oxidation, and the corresponding exercise intensity was recorded as FATmax.
Lactate threshold.
LT was determined as the breakpoint in [La-]b vs. PO, representing a departure of [La−]b from a linear baseline, using an R script (Lactate-R), as previously described (38). While other [La−]b-derived measures were available, only LT is reported because the various measures were highly correlated with each other (data not reported).
Diet Standardization
Based on individual preferences and calculations of basal metabolic rate (16) (activity factor of 1.49), participants were provided with individualized meals (Heart to Home Meals, Brampton ON) and snacks, representing one-third of estimated daily energy expenditure (kcal distribution: 59 ± 2% carbohydrate, 27 ± 2% fat, and 15 ± 1% protein), to consume the evening before the assessment of MFO to reduce the effects of diet on substrate utilization.
Statistical Analysis
Statistical analysis was performed in SPSS (Version 25, IBM, Armonk, NY). Differences in age, physical characteristics, measures of whole-body aerobic fitness (V̇o2max, PPO, LT, GET, RCP, MFO, and FATmax), and oxidative capacity between males and females were assessed using independent t tests. Relationships between measures of whole-body aerobic fitness and NIRS-derived oxidative capacity were assessed using linear regression. Day-to-day and test-retest reliability (within each day) were assessed for each muscle. Bland–Altman plots were constructed by plotting the mean of the two compared values against the difference (e.g., test 1 – test 2), and 95% limits of agreement were calculated (1.96 * SD of the difference). Bias was assessed by a one-sample t test between the mean difference and zero. Furthermore, two-way, random intraclass correlation coefficients for absolute agreement and the average coefficients of variation (CV) and typical errors (TE) were calculated. CVs were also calculated for each sex. Results were considered statistically significant at P < 0.05, and data are presented as means ± SD.
RESULTS
Participant Characteristics
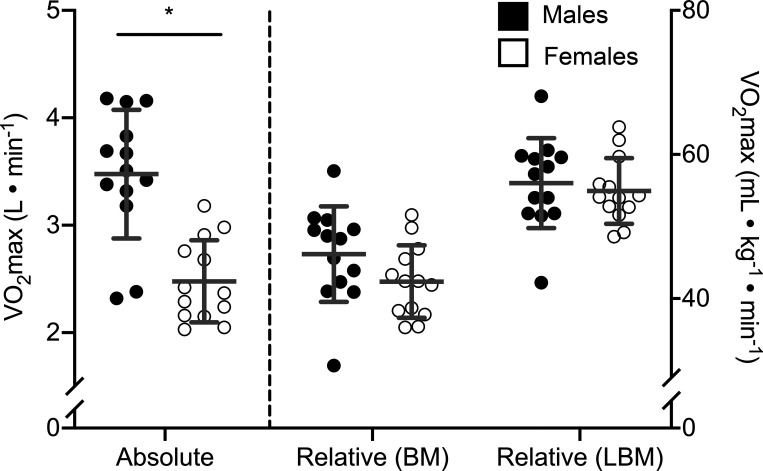
Twenty-six participants (13 females, 13 males) were included in data analysis (Table 1). Compared with females, males had a significantly greater height, body mass, LBM, and BMI and lower body fat percentage, whereas ATT for the VL and MG were not different between sexes (Table 1). While males had a greater absolute V̇o2max than females, differences between sexes were not significant when V̇o2max was normalized to total body weight and were further reduced when V̇o2max was normalized to LBM (Table 1; Fig. 2). When normalized to LBM, the LT (M: 3.2 ± 1.0 W/kg LBM; F: 2.9 ± 0.8 W/kg LBM; P = 0.46), GET (M: 30.2 ± 4.3 mL·kg LBM−1·min−1; F: 33.6 ± 6.1 mL·kg LBM−1·min−1; P = 0.12), RCP (M: 41.6 ± 5.2 mL·kg LBM−1·min−1; F: 43.8 ± 6.8 mL·kg LBM−1·min−1; P = 0.35), MFO (M: 8.0 ± 1.6 mg·kg LBM−1·min−1; F: 9.2 ± 1.8 mg·kg LBM−1·min−1; P = 0.08), FATmax (M: 1.28 ± 1.02 W/kg LBM; F: 1.36 ± 0.75 W/kg LBM; P = 0.81), and PPO (M: 5.8 ± 0.8 W/kg LBM; F: 6.0 ± 0.6 W/kg LBM; P = 0.41) were similar between males and females.
Table 1.
Participant characteristics subdivided by sex
| Females (n = 13) |
Males (n = 13) |
Comparison (P value) |
|
|---|---|---|---|
| Age, yr | 24.7 ± 3.6 | 25.3 ± 4.9 | 0.72 |
| Height, cm | 165.2 ± 6.1 | 178.5 ± 6.8 | <0.001 |
| Body mass, kg | 58.7 ± 7.2 | 75.3 ± 7.8 | <0.001 |
| Lean body mass, kg | 45.1 ± 5.2 | 61.9 ± 7.2 | <0.001 |
| Body mass index, kg/m2 | 21.5 ± 1.9 | 23.6 ± 2.2 | 0.013 |
| Body fat, % | 19.3 ± 4.0 | 14.2 ± 3.7 | 0.003 |
| VL adipose tissue thickness, cm | 0.73 ± 0.22* | 0.60 ± 0.16 | 0.10 |
| MG adipose tissue thickness, cm | 0.48 ± 0.12 | 0.41 ± 0.09 | 0.12 |
| V̇o2max, mL·kg LBM−1·min−1 | 54.9 ± 4.5 | 56.0 ± 6.2 | 0.62 |
Data are presented as means ± SD. VL, vastus lateralis; MG, medial gastrocnemius; V̇o2max, maximal oxygen uptake.
For this measure, n = 12 females.
Fig. 2.
Maximal oxygen uptake (V̇o2max) of males and females in absolute units and normalized to body mass (BM) and lean body mass (LBM). Each circle represents 1 individual, the central horizontal line is the mean, and each error bar represents 1 SD. *Statistically significant difference between groups (P < 0.05) based on an independent t test; n = 26 (13 females, 13 males) for each comparison.
NIRS-Derived Oxidative Capacity and Sex
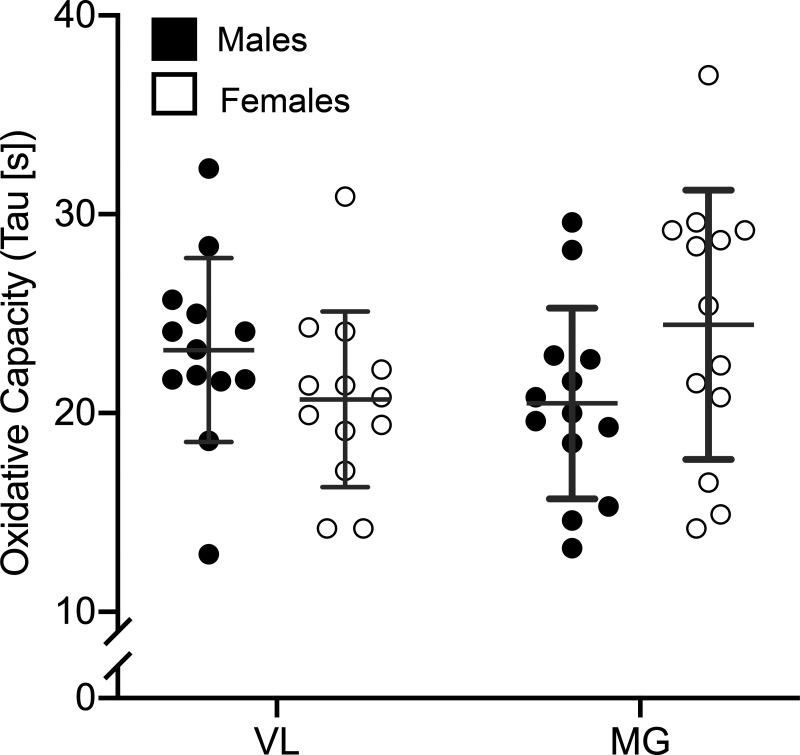
A representative trace of the HHb signal during the oxidative capacity protocol, and a plot of the resulting slopes of each occlusion vs. time, are shown in Fig. 1. The NIRS-derived oxidative capacity was similar in males and females for the VL (M: 23.2 ± 4.6 s; F: 20.7 ± 4.4 s; P = 0.18) and MG (M: 20.5 ± 4.8 s; F: 24.4 ± 6.8 s; P = 0.10) (Fig. 3).
Fig. 3.
Near-infrared spectroscopy (NIRS)-derived skeletal muscle oxidative capacity of the vastus lateralis (VL) and medial gastrocnemius (MG) from males and females. Each circle represents 1 individual, the central horizontal line is the mean, and each error bar represents 1 SD. There were no statistically significant differences between sexes, as determined by independent t tests; n = 26 (13 females, 13 males) for each comparison.
Physiological Correlates of NIRS-Derived Oxidative Capacity
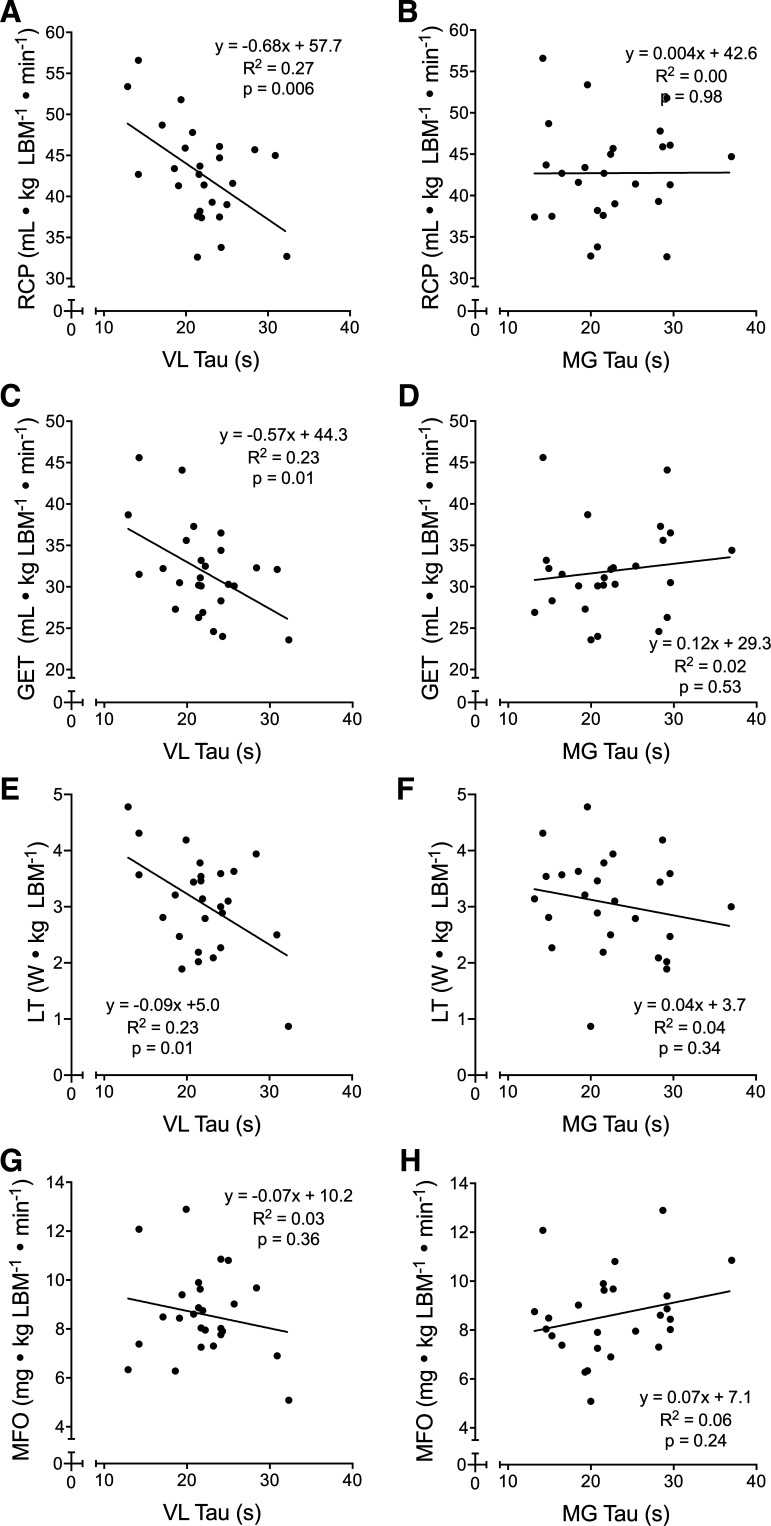
Oxidative capacity values for the VL and MG were 21.9 ± 4.6 and 22.5 ± 6.1 s, respectively, with no difference (P = 0.70) or association (r2 = 0.03, P = 0.39) between muscles. Correlations between maximal and submaximal physiological variables and NIRS-derived oxidative capacity from each muscle are shown in Figs. 4 and 5, respectively. There was no significant relationship between muscle oxidative capacity and FATmax in either muscle (VL: R2 = 0.01, P = 0.76; MG: R2 = 0.03, P = 0.58).
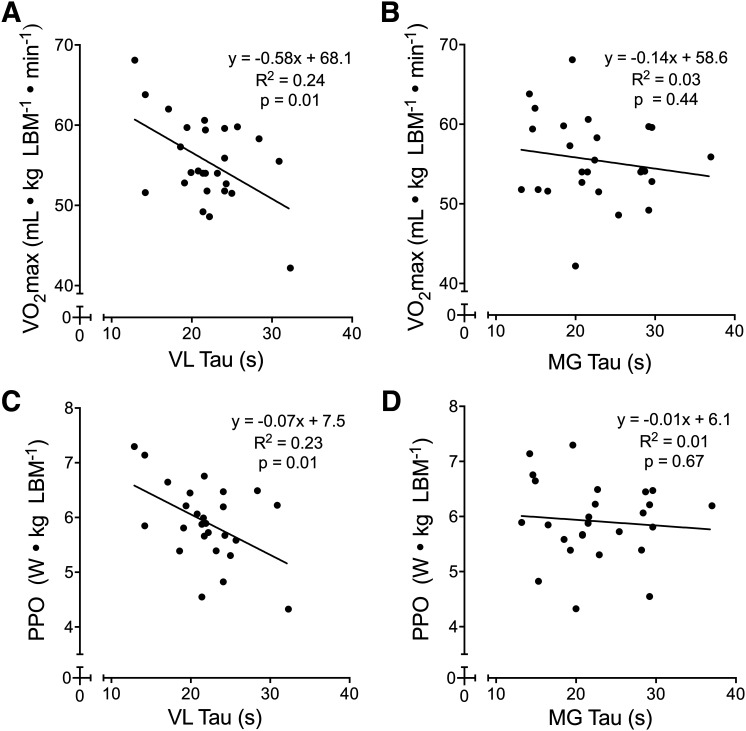
Fig. 4.
Scatterplots showing the relationships between maximal aerobic fitness variables and near-infrared spectroscopy (NIRS)-derived skeletal muscle oxidative capacity in the vastus lateralis (VL) and medial gastrocnemius (MG). The VL oxidative capacity was significantly correlated with maximal oxygen uptake (V̇o2max) and peak power output (PPO; A and C), whereas the MG oxidative capacity was not correlated with either variable (B and D). For all plots, n = 26 (13 females, 13 males). Linear regression equations, coefficients of determination (R2), and P values are displayed for each plot.
Fig. 5.
Scatterplots of various indices of submaximal aerobic fitness and near-infrared spectroscopy (NIRS)-derived skeletal muscle oxidative capacity in the vastus lateralis (VL) and medial gastrocnemius (MG). The VL oxidative capacity was significantly correlated with the respiratory compensation point (RCP), gas exchange threshold (GET), and lactate threshold (LT) (A, C, and E) but not maximal rates of fat oxidation (MFO; G). The MG oxidative capacity was not correlated with any of these variables (B, D, F, and H). For all plots, n = 26 (13 females, 13 males). Linear regression equations, coefficients of determination (R2), and P values are displayed for each plot.
Reliability of NIRS-Derived Oxidative Capacity
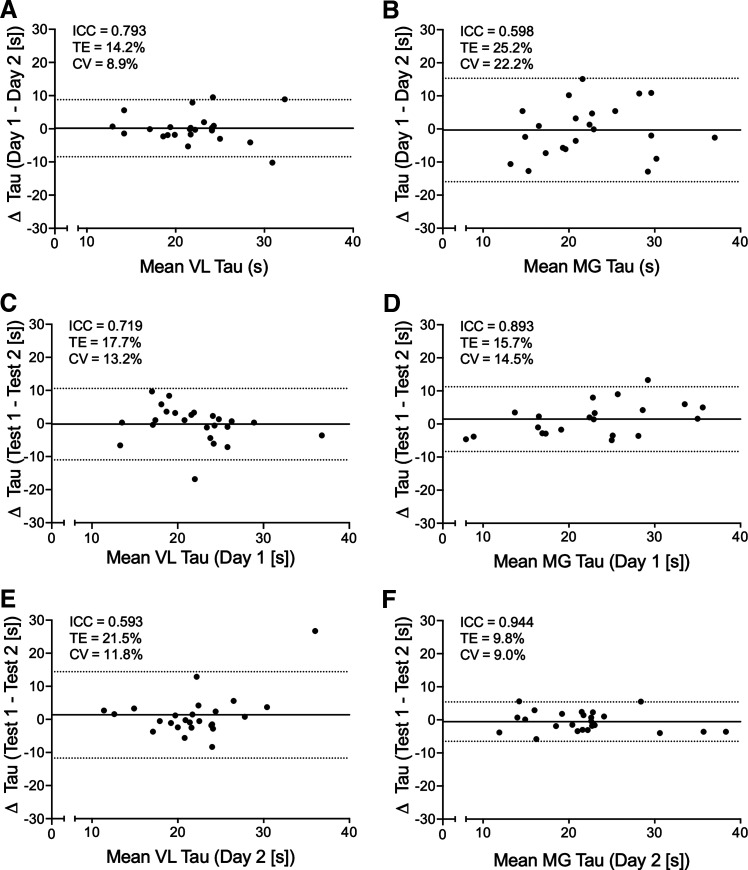
From the 26 participants included in the analysis, a total of 104 NIRS-derived oxidative capacity tests were performed for each muscle. Oxidative capacity values were derived from 95.2% (99/104) of VL tests and 88.5% (92/104) of MG tests. Bland–Altman plots showing day-to-day and test-retest reliability are displayed in Fig. 6, including ICCs, average CVs, and TE. No bias was present within or across days (P > 0.05). Sex-specific CV were comparable for VL [day 1–day 2 (M: 9.1%; F: 8.7%), day 1 test-retest (M: 10.2%; F: 16.8%), and day 2 test-retest (M: 9.3%; F: 14.1%)] and MG [day 1–day 2 (M: 27.6%; F: 14.3%), day 1 test-retest (M: 15.6%; F: 13.2%), and day 2 test-retest (M: 7.2%; F: 11.0%)].
Fig. 6.
Bland–Altman plots showing day-to-day (A and B) and test-retest reliability (C–F) for near-infrared spectroscopy (NIRS)-derived skeletal muscle oxidative capacity (tau) of the vastus lateralis (VL) and medial gastrocnemius (MG). The mean difference is shown with the solid line, and 95% limits of agreement with the dotted lines. Intraclass correlation coefficients (ICC), typical error (TE), and the mean coefficient of variation (CV) are also reported in each panel. Sample size varied from 21 to 26 depending on the number of test pairs available.
DISCUSSION
Consistent with our hypotheses, NIRS-derived skeletal muscle oxidative capacity in the VL was similar between males and females (matched for V̇o2max/LBM) and correlated well with V̇o2max, PPO, LT, GET, and RCP. There was moderate-to-good day-to-day and moderate-to-excellent test-retest reliability (27) for the protocol used in this study, with no indication of bias for VL and MG oxidative capacities. In contrast to our hypotheses, NIRS-derived skeletal muscle oxidative capacity in the VL was not associated with FATmax or MFO, and none of the maximal or submaximal parameters of whole-body aerobic fitness correlated with MG oxidative capacity. Thus the major novel finding from this experiment is that NIRS-derived oxidative capacity of the VL is a valid and reliable indicator of aerobic fitness in humans that is independent of sex.
Sex-Based Differences
Existing literature on sex-based differences in skeletal muscle oxidative capacity is limited and conflicting, and no study, to our knowledge, has used NIRS to compare skeletal muscle oxidative capacity between females and males. In the present study, VL and MG muscle oxidative capacities were not different between groups of females and males matched for aerobic fitness. Although we reported a significant difference in the mean absolute V̇o2max between males and females, the difference was not significant when V̇o2max was expressed relative to total body mass or LBM, which is the standard for comparing metabolic outcomes between female and males (52).
Although in conflict with reported sex-based differences in certain measures of intrinsic mitochondrial function (8, 34), the findings of the present study support the lack of a sex effect when one examines typical measures of oxidative capacity (9), including measures against which NIRS-derived oxidative capacity has been validated (44, 47). Our findings are consistent with comparisons of 31P-MRS-derived PCr recovery constants between males and females following maximal isometric dorsiflexor (42) and constant work rate knee extensor exercise (3). Two other studies examining the gastrocnemius muscle found no sex-based differences in mitochondrial content, respiration, or oxidative enzyme activity, determined through skeletal muscle biopsy analysis (10, 54). Males and females compared in these studies were matched based on physical activity (54) or current athletic participation (10). Although it is possible there was similar aerobic fitness across sexes, the results are difficult to interpret without matching for physiological outcomes. Alternatively, studies examining mitochondrial content in the VL have produced conflicting results. For example, one study reported greater mitochondrial volume density in females than males but did not account for LBM when matched for fitness (35). It is possible that females in that study had a higher V̇o2max/LBM than males, which complicates interpretations. In disagreement with that study, and in accordance with the present findings, Tarnopolsky and colleagues (53) reported similar mitochondrial volume density and mitochondrial enzyme activity (9) in females and males matched for V̇o2max/LBM. Intrinsic mitochondrial respiration in isolated mitochondria was higher in females compared with males, when matched for V̇o2max relative to total body mass, with females having similar intrinsic mitochondrial respiration as trained males with a greater V̇o2max (+32%). The authors noted that an equally large, and unlikely, difference in body fat percentage would be needed to dismiss the similarity as a sign of greater mitochondrial function in females. Nevertheless, as NIRS-derived oxidative capacity measures the muscle in vivo, we would not necessarily expect sex differences in intrinsic mitochondrial properties to be detectable in the present study. Interestingly, mass-specific mitochondrial respiration (O2 flux relative to muscle sample weight) is consistently similar between females and males across several studies (8, 34, 35).
Taken together with the results of other studies, and with similar day-to-day and test-retest reliability across sexes, the possibility of sex-based differences in NIRS-derived skeletal muscle oxidative capacity should not be used as a reason to exclude females from future experiments.
Oxidative Capacity and Maximal/Submaximal Fitness
In the VL, but not the MG, the recovery of muscle oxygen consumption postexercise was quicker in individuals with higher aerobic fitness, assessed as V̇o2max and PPO (normalized to LBM), indicating a positive relationship between whole-body aerobic capacity and skeletal muscle oxidative capacity. Several other studies using NIRS to measure skeletal muscle oxidative capacity have reported greater oxidative capacity in endurance-trained individuals compared with untrained participants in the VL (6) and MG (28), and improved oxidative capacity in wrist flexor muscles following 4 wk of progressive endurance training (46). Although Lagerwaard et al. (28) observed a correlation between V̇o2peak and the NIRS-derived oxidative capacity in the lateral gastrocnemius muscle, they excluded participants with moderate fitness levels (i.e., V̇o2max = 47–57 mL·kg−1·min−1), obscuring the overall relationship between the two variables. In contrast, the present study recruited subjects across a continuous range of fitness levels. NIRS-derived skeletal muscle oxidative capacity itself has been associated with standard in vitro (44) and in vivo (47) measures of skeletal muscle oxidative capacity. Similarly, the relationships between NIRS-based oxidative capacity and V̇o2max and PPO are consistent with previous studies that demonstrated relationships between V̇o2max (15, 19, 21) and LT (19) with mitochondrial respiration in VL muscle fibers and between V̇o2max and quadriceps PCr resynthesis postexercise, determined through 31P-MRS (51). While we did not identify a significant relationship between MG oxidative capacity and V̇o2max or PPO, a significant relationship has previously been observed between whole-body V̇o2max and PCr recovery kinetics in the gastrocnemius muscle following 90 s of isometric plantarflexion exercise (45, 70, and 100% MVC) (31). As the 31P-MRS technique has shown good agreement with NIRS-derived oxidative capacity (47), we cannot explain this discrepancy.
NIRS-derived skeletal muscle oxidative capacity, again in the VL but not the MG, was correlated with LT, GET, and RCP, submaximal indices of aerobic fitness. These relationships are likely explained by the relationships between skeletal muscle mitochondria content and submaximal aerobic fitness (21). Given that the GET and LT are indicative of the same threshold (4), our findings for GET and LT agree with a previously established relationship between mitochondria respiratory capacity (VL) and LT determined from a step-incremental cycling test (19). This is reasonable, as NIRS-derived oxidative capacity has previously been shown to be associated with in vitro measures of skeletal muscle mitochondrial respiratory capacity (44). Similarly, the V̇o2 associated with RCP is equal to the V̇o2 achieved at the maximal lactate steady state (MLSS), the highest exercise intensity for which blood lactate concentration is stable (23). Although we are unaware of any studies explicitly linking mitochondrial content with MLSS, the relationship between NIRS-derived oxidative capacity and RCP suggests that the oxidative capacity of skeletal muscle is likely a determinant of MLSS. In contrast with our findings, we expected stronger relationships between oxidative capacity and submaximal measures of aerobic fitness compared with maximal.
Neither VL nor MG oxidative capacities were associated with MFO, which is typically higher in aerobically trained vs. untrained individuals (26, 56). Although against our hypothesis and a previous study (13), our results align with the results of Nordby et al. (39), which demonstrated that in vitro measures of skeletal muscle oxidative enzyme activity and whole-body peak fat oxidation, determined from a graded-exercise test similar to our protocol, were not correlated. Interestingly, those authors reported a correlation between muscle oxidative capacity and whole-body fat oxidation measured during a prolonged submaximal exercise test, which they attributed to a difference in hormonally mediated substrate delivery between continuous and graded-exercise protocols. Thus future studies should examine whether the NIRS-derived measure of muscle oxidative capacity also correlates with fat oxidation during prolonged exercise.
Despite having similar mean values, no relationship was found between the oxidative capacities of the VL and MG in our sample of 26 adults, suggesting that the VL and MG oxidative capacities are not interchangeable. While the MG (or lateral gastrocnemius) is commonly assessed in studies utilizing NIRS-derived muscle oxidative capacity (28, 43, 45, 47, 49), the VL is commonly assessed in studies using muscle biopsy-derived measures of oxidative capacity (8, 19, 21, 39, 44). Given that the quadriceps muscle group contributes significantly more to cycling PO than the gastrocnemius (5, 14, 41), the lack of a relationship between the oxidative capacities of the two muscle groups in the present study leads us to propose that future studies related to cycling assess the oxidative capacity of the VL instead of, or in addition to, the gastrocnemius.
Reliability
The protocol used to measure muscle oxidative capacity in the present study was based largely on previous studies from McCully and colleagues (28, 44, 45, 47, 49); however, our exercise stimulus and occlusion protocols were slightly different, as we attempted to normalize exercise intensity across individuals. Therefore, the methods used here required an assessment of reliability. Both VL and MG protocols demonstrated moderate to good day-to-day and moderate-to-excellent test-retest reliability (27), with no indication of bias. Notably, the sex-specific CV values reflected the CV values from the full data set. Similarly, previous protocols using NIRS in conjunction with short-duration plantarflexion exercise have found comparable test-retest repeatability (mean CV = 8.1% for channel 1 and 6.9% for channel 2) in measures of MG oxidative capacity (47). The results of the present study are in close agreement with Ryan et al. (45), who found similar day-to-day reproducibility of measures of VL oxidative capacity (mean CV = 10%) following short-duration electrical stimulation. It has been shown that measures of oxidative capacity, using NIRS, are independent of the frequency, intensity, and type of contraction (43) and seem to be independent of the duration and number of cuff occlusions performed (43, 47, 50). Therefore, the slight differences in protocols between the present and past studies should have minimal effects on measurement validity. Furthermore, the overall reliability of the present VL and MG NIRS measurements reinforces the practicality of the exercise and occlusion protocols conducted in this study for assessing skeletal muscle oxidative capacity. To put the present data in perspective, the test-retest CVs for oxidative capacity measured with 31P-MRS and citrate synthase activity were 3.4 (29) and 15.3% (48), respectively.
Limitations
While the NIRS-derived method of assessing skeletal muscle oxidative capacity is promising, it has some important limitations that warrant comment. First, for tau values to reflect skeletal muscle oxidative capacity, the exercise stimulus must maximize mitochondrial enzyme activity and the oxygen supply cannot be limiting (2). Here, we chose an exercise stimulus that was highly similar to previous studies (43, 46, 47); however, because the procedure is noninvasive, rates of oxidative phosphorylation and microvascular partial pressures of oxygen cannot be ascertained and are therefore not available for reassurance. Second, the most appropriate analysis method is presently unclear, as previous studies have used different approaches and have not published the full details of their analysis procedures. Like previous studies, we selected 3-s slopes for each occlusion (43), with the aim of optimizing slope fit; however, we also omitted outliers (i.e., points that did not reflect monoexponential decay) from individual mV̇o2 recovery curves in a blinded manner to improve the overall fit of these curves. Often, it was the slope from the first occlusion that had to be removed because it was equal to or lower than the second slope. This finding could be indicative of an oxygen limitation (1), but this limitation is unlikely given the relatively low-intensity rhythmic contractions that were performed. Alternatively, it may also indicate a contribution of glycolysis to PCr recovery during the first few seconds of recovery, which has been reported for higher intensity contractions (11). As slopes were removed in a blinded manner, this strategy should not have impacted the overall results. We are unaware of any previous studies that omitted individual slopes; however, in our hands, curves were rarely perfect (e.g., r2 = >0.95), even after removal of erroneous slopes. Future studies should provide full details of their analysis strategy to permit the formation of guidelines and also improve reproducibility across research groups.
Conclusions
Overall, NIRS-derived skeletal muscle oxidative capacity in the VL was correlated with V̇o2max, PPO, LT, GET, and RCP, but not MFO or FATmax, whereas NIRS-derived MG oxidative capacity was not associated with any whole-body measures of aerobic fitness. For both VL and MG, skeletal muscle oxidative capacity was independent of sex and reliable within and across days. Based on the present data, in conjunction with the available literature, we suggest that the NIRS-derived measure of oxidative capacity is a valid and reliable technique to investigate questions related to skeletal muscle physiology in humans.
GRANTS
This research was funded by internal funding from the University of Calgary and a Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant to M. J. MacInnis (RGPIN-2018-06424). T. R. Tripp was funded by an NSERC CGS-M scholarship.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.T.B., T.R.T., J.Z., and M.J.M. conceived and designed research; A.T.B., T.R.T., J.Z., and M.J.M. performed experiments; A.T.B., T.R.T., J.Z., and M.J.M. analyzed data; A.T.B., T.R.T., J.Z., and M.J.M. interpreted results of experiments; A.T.B., T.R.T., J.Z., and M.J.M. prepared figures; A.T.B., T.R.T., J.Z., and M.J.M. drafted manuscript; A.T.B., T.R.T., J.Z., and M.J.M. edited and revised manuscript; A.T.B., T.R.T., J.Z., and M.J.M. approved final version of manuscript.
REFERENCES
- 1.Adami A, Cao R, Porszasz J, Casaburi R, Rossiter HB. Reproducibility of NIRS assessment of muscle oxidative capacity in smokers with and without COPD. Respir Physiol Neurobiol 235: 18–26, 2017. doi: 10.1016/j.resp.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adami A, Rossiter HB. Principles, insights, and potential pitfalls of the noninvasive determination of muscle oxidative capacity by near-infrared spectroscopy. J Appl Physiol (1985) 124: 245–248, 2018. doi: 10.1152/japplphysiol.00445.2017. [DOI] [PubMed] [Google Scholar]
- 3.Barker AR, Welsman JR, Fulford J, Welford D, Armstrong N. Muscle phosphocreatine kinetics in children and adults at the onset and offset of moderate-intensity exercise. J Appl Physiol (1985) 105: 446–456, 2008. doi: 10.1152/japplphysiol.00819.2007. [DOI] [PubMed] [Google Scholar]
- 4.Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol (1985) 60: 2020–2027, 1986. doi: 10.1152/jappl.1986.60.6.2020. [DOI] [PubMed] [Google Scholar]
- 5.Brennan SF, Cresswell AG, Farris DJ, Lichtwark GA. The effect of cadence on the mechanics and energetics of constant power cycling. Med Sci Sports Exerc 51: 941–950, 2019. doi: 10.1249/MSS.0000000000001863. [DOI] [PubMed] [Google Scholar]
- 6.Brizendine JT, Ryan TE, Larson RD, McCully KK. Skeletal muscle metabolism in endurance athletes with near-infrared spectroscopy. Med Sci Sports Exerc 45: 869–875, 2013. doi: 10.1249/MSS.0b013e31827e0eb6. [DOI] [PubMed] [Google Scholar]
- 7.Burgomaster KA, Howarth KR, Phillips SM, Rakobowchuk M, Macdonald MJ, McGee SL, Gibala MJ. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J Physiol 586: 151–160, 2008. doi: 10.1113/jphysiol.2007.142109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardinale DA, Larsen FJ, Schiffer TA, Morales-Alamo D, Ekblom B, Calbet JAL, Holmberg HC, Boushel R. Superior intrinsic mitochondrial respiration in women than in men. Front Physiol 9: 1133, 2018. doi: 10.3389/fphys.2018.01133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carter SL, Rennie CD, Hamilton SJ, Tarnopolsky MA. Changes in skeletal muscle in males and females following endurance training. Can J Physiol Pharmacol 79: 386–392, 2001. doi: 10.1139/y01-008. [DOI] [PubMed] [Google Scholar]
- 10.Costill DL, Daniels J, Evans W, Fink W, Krahenbuhl G, Saltin B. Skeletal muscle enzymes and fiber composition in male and female track athletes. J Appl Physiol 40: 149–154, 1976. doi: 10.1152/jappl.1976.40.2.149. [DOI] [PubMed] [Google Scholar]
- 11.Crowther GJ, Kemper WF, Carey MF, Conley KE. Control of glycolysis in contracting skeletal muscle. II. Turning it off. Am J Physiol Endocrinol Metab 282: E74–E79, 2002. doi: 10.1152/ajpendo.2002.282.1.E74. [DOI] [PubMed] [Google Scholar]
- 12.Cureton KJ. Matching of male and female subjects using VO2 max. Res Q Exerc Sport 52: 264–268, 1981. doi: 10.1080/02701367.1981.10607865. [DOI] [PubMed] [Google Scholar]
- 13.Dandanell S, Meinild-Lundby AK, Andersen AB, Lang PF, Oberholzer L, Keiser S, Robach P, Larsen S, Rønnestad BR, Lundby C. Determinants of maximal whole-body fat oxidation in elite cross-country skiers: role of skeletal muscle mitochondria. Scand J Med Sci Sports 28: 2494–2504, 2018. doi: 10.1111/sms.13298. [DOI] [PubMed] [Google Scholar]
- 14.Ericson MO. Mechanical muscular power output and work during ergometer cycling at different work loads and speeds. Eur J Appl Physiol Occup Physiol 57: 382–387, 1988. doi: 10.1007/BF00417980. [DOI] [PubMed] [Google Scholar]
- 15.Gifford JR, Garten RS, Nelson AD, Trinity JD, Layec G, Witman MA, Weavil JC, Mangum T, Hart C, Etheredge C, Jessop J, Bledsoe A, Morgan DE, Wray DW, Rossman MJ, Richardson RS. Symmorphosis and skeletal muscle V̇O2 max : in vivo and in vitro measures reveal differing constraints in the exercise-trained and untrained human. J Physiol 594: 1741–1751, 2016. doi: 10.1113/JP271229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henry CJ. Basal metabolic rate studies in humans: measurement and development of new equations. Public Health Nutr 8, 7A: 1133–1152, 2005. doi: 10.1079/PHN2005801. [DOI] [PubMed] [Google Scholar]
- 17.Iannetta D, de Almeida Azevedo R, Keir DA, Murias JM. Establishing the V̇o2 versus constant-work-rate relationship from ramp-incremental exercise: simple strategies for an unsolved problem. J Appl Physiol (1985) 127: 1519–1527, 2019. doi: 10.1152/japplphysiol.00508.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iannetta D, Inglis EC, Fullerton C, Passfield L, Murias JM. Metabolic and performance-related consequences of exercising at and slightly above MLSS. Scand J Med Sci Sports 28: 2481–2493, 2018. doi: 10.1111/sms.13280. [DOI] [PubMed] [Google Scholar]
- 19.Ivy JL, Withers RT, Van Handel PJ, Elger DH, Costill DL. Muscle respiratory capacity and fiber type as determinants of the lactate threshold. J Appl Physiol 48: 523–527, 1980. doi: 10.1152/jappl.1980.48.3.523. [DOI] [PubMed] [Google Scholar]
- 20.Jackson AS, Stanforth PR, Gagnon J, Rankinen T, Leon AS, Rao DC, Skinner JS, Bouchard C, Wilmore JH. The effect of sex, age and race on estimating percentage body fat from body mass index: The Heritage Family Study. Int J Obes Relat Metab Disord 26: 789–796, 2002. doi: 10.1038/sj.ijo.0802006. [DOI] [PubMed] [Google Scholar]
- 21.Jacobs RA, Lundby C. Mitochondria express enhanced quality as well as quantity in association with aerobic fitness across recreationally active individuals up to elite athletes. J Appl Physiol (1985) 114: 344–350, 2013. doi: 10.1152/japplphysiol.01081.2012. [DOI] [PubMed] [Google Scholar]
- 22.Jeukendrup AE, Wallis GA. Measurement of substrate oxidation during exercise by means of gas exchange measurements. Int J Sports Med 26, Suppl 1: S28–S37, 2005. doi: 10.1055/s-2004-830512. [DOI] [PubMed] [Google Scholar]
- 23.Keir DA, Fontana FY, Robertson TC, Murias JM, Paterson DH, Kowalchuk JM, Pogliaghi S. Exercise intensity thresholds: identifying the boundaries of sustainable performance. Med Sci Sports Exerc 47: 1932–1940, 2015. doi: 10.1249/MSS.0000000000000613. [DOI] [PubMed] [Google Scholar]
- 24.Keir DA, Paterson DH, Kowalchuk JM, Murias JM. Using ramp-incremental V̇O2 responses for constant-intensity exercise selection. Appl Physiol Nutr Metab 43: 882–892, 2018. doi: 10.1139/apnm-2017-0826. [DOI] [PubMed] [Google Scholar]
- 25.Kent-Braun JA, Ng AV. Skeletal muscle oxidative capacity in young and older women and men. J Appl Physiol (1985) 89: 1072–1078, 2000. doi: 10.1152/jappl.2000.89.3.1072. [DOI] [PubMed] [Google Scholar]
- 26.Kiens B. Effect of endurance training on fatty acid metabolism: local adaptations. Med Sci Sports Exerc 29: 640–645, 1997. doi: 10.1097/00005768-199705000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 15: 155–163, 2016. [Erratum in J Chiropr Med 16: 346, 2017.] doi: 10.1016/j.jcm.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lagerwaard B, Keijer J, McCully KK, de Boer VCJ, Nieuwenhuizen AG. In vivo assessment of muscle mitochondrial function in healthy, young males in relation to parameters of aerobic fitness. Eur J Appl Physiol 119: 1799–1808, 2019. doi: 10.1007/s00421-019-04169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lanza IR, Bhagra S, Nair KS, Port JD. Measurement of human skeletal muscle oxidative capacity by 31P-MR spectroscopy: a cross-validation with in vitro measurements. J Magn Reson Imaging 34: 1143–1150, 2011. doi: 10.1002/jmri.22733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larsen S, Nielsen J, Hansen CN, Nielsen LB, Wibrand F, Stride N, Schroder HD, Boushel R, Helge JW, Dela F, Hey-Mogensen M. Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J Physiol 590: 3349–3360, 2012. doi: 10.1113/jphysiol.2012.230185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larson-Meyer DE, Newcomer BR, Hunter GR, Hetherington HP, Weinsier RL. 31P MRS measurement of mitochondrial function in skeletal muscle: reliability, force-level sensitivity and relation to whole body maximal oxygen uptake. NMR Biomed 13: 14–27, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 32.McCully KK, Fielding RA, Evans WJ, Leigh JS Jr, Posner JD. Relationships between in vivo and in vitro measurements of metabolism in young and old human calf muscles. J Appl Physiol (1985) 75: 813–819, 1993. doi: 10.1152/jappl.1993.75.2.813. [DOI] [PubMed] [Google Scholar]
- 33.Midgley AW, Carroll S. Emergence of the verification phase procedure for confirming ‘true’ VO(2max). Scand J Med Sci Sports 19: 313–322, 2009. doi: 10.1111/j.1600-0838.2009.00898.x. [DOI] [PubMed] [Google Scholar]
- 34.Miotto PM, McGlory C, Holloway TM, Phillips SM, Holloway GP. Sex differences in mitochondrial respiratory function in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 314: R909–R915, 2018. doi: 10.1152/ajpregu.00025.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montero D, Madsen K, Meinild-Lundby AK, Edin F, Lundby C. Sexual dimorphism of substrate utilization: differences in skeletal muscle mitochondrial volume density and function. Exp Physiol 103: 851–859, 2018. doi: 10.1113/EP087007. [DOI] [PubMed] [Google Scholar]
- 36.Motobe M, Murase N, Osada T, Homma T, Ueda C, Nagasawa T, Kitahara A, Ichimura S, Kurosawa Y, Katsumura T, Hoshika A, Hamaoka T. Noninvasive monitoring of deterioration in skeletal muscle function with forearm cast immobilization and the prevention of deterioration. Dyn Med 3: 2, 2004. doi: 10.1186/1476-5918-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murias JM, Pogliaghi S, Paterson DH. Measurement of a true V̇O2max during a ramp incremental test is not confirmed by a verification phase. Front Physiol 9: 143, 2018. doi: 10.3389/fphys.2018.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Newell J, Higgins D, Madden N, Cruickshank J, Einbeck J, McMillan K, McDonald R. Software for calculating blood lactate endurance markers. J Sports Sci 25: 1403–1409, 2007. doi: 10.1080/02640410601128922. [DOI] [PubMed] [Google Scholar]
- 39.Nordby P, Saltin B, Helge JW. Whole-body fat oxidation determined by graded exercise and indirect calorimetry: a role for muscle oxidative capacity? Scand J Med Sci Sports 16: 209–214, 2006. doi: 10.1111/j.1600-0838.2005.00480.x. [DOI] [PubMed] [Google Scholar]
- 40.Perry CG, Heigenhauser GJ, Bonen A, Spriet LL. High-intensity aerobic interval training increases fat and carbohydrate metabolic capacities in human skeletal muscle. Appl Physiol Nutr Metab 33: 1112–1123, 2008. doi: 10.1139/H08-097. [DOI] [PubMed] [Google Scholar]
- 41.Raasch CC, Zajac FE, Ma B, Levine WS. Muscle coordination of maximum-speed pedaling. J Biomech 30: 595–602, 1997. doi: 10.1016/S0021-9290(96)00188-1. [DOI] [PubMed] [Google Scholar]
- 42.Russ DW, Lanza IR, Rothman D, Kent-Braun JA. Sex differences in glycolysis during brief, intense isometric contractions. Muscle Nerve 32: 647–655, 2005. doi: 10.1002/mus.20396. [DOI] [PubMed] [Google Scholar]
- 43.Ryan TE, Brizendine JT, McCully KK. A comparison of exercise type and intensity on the noninvasive assessment of skeletal muscle mitochondrial function using near-infrared spectroscopy. J Appl Physiol (1985) 114: 230–237, 2013. doi: 10.1152/japplphysiol.01043.2012. [DOI] [PubMed] [Google Scholar]
- 44.Ryan TE, Brophy P, Lin CT, Hickner RC, Neufer PD. Assessment of in vivo skeletal muscle mitochondrial respiratory capacity in humans by near-infrared spectroscopy: a comparison with in situ measurements. J Physiol 592: 3231–3241, 2014. doi: 10.1113/jphysiol.2014.274456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ryan TE, Erickson ML, Brizendine JT, Young HJ, McCully KK. Noninvasive evaluation of skeletal muscle mitochondrial capacity with near-infrared spectroscopy: correcting for blood volume changes. J Appl Physiol (1985) 113: 175–183, 2012. doi: 10.1152/japplphysiol.00319.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ryan TE, Southern WM, Brizendine JT, McCully KK. Activity-induced changes in skeletal muscle metabolism measured with optical spectroscopy. Med Sci Sports Exerc 45: 2346–2352, 2013. doi: 10.1249/MSS.0b013e31829a726a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ryan TE, Southern WM, Reynolds MA, McCully KK. A cross-validation of near-infrared spectroscopy measurements of skeletal muscle oxidative capacity with phosphorus magnetic resonance spectroscopy. J Appl Physiol (1985) 115: 1757–1766, 2013. doi: 10.1152/japplphysiol.00835.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sahl RE, Morville T, Kraunsøe R, Dela F, Helge JW, Larsen S. Variation in mitochondrial respiratory capacity and myosin heavy chain composition in repeated muscle biopsies. Anal Biochem 556: 119–124, 2018. doi: 10.1016/j.ab.2018.06.029. [DOI] [PubMed] [Google Scholar]
- 49.Southern WM, Ryan TE, Reynolds MA, McCully K. Reproducibility of near-infrared spectroscopy measurements of oxidative function and postexercise recovery kinetics in the medial gastrocnemius muscle. Appl Physiol Nutr Metab 39: 521–529, 2014. doi: 10.1139/apnm-2013-0347. [DOI] [PubMed] [Google Scholar]
- 50.Sumner MD, Beard S, Pryor EK, Das I, McCully KK. Near infrared spectroscopy measurements of mitochondrial capacity using partial recovery curves. Front Physiol 11: 111, 2020. doi: 10.3389/fphys.2020.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takahashi H, Inaki M, Fujimoto K, Katsuta S, Anno I, Niitsu M, Itai Y. Control of the rate of phosphocreatine resynthesis after exercise in trained and untrained human quadriceps muscles. Eur J Appl Physiol Occup Physiol 71: 396–404, 1995. doi: 10.1007/BF00635872. [DOI] [PubMed] [Google Scholar]
- 52.Tarnopolsky MA. Sex differences in exercise metabolism and the role of 17-beta estradiol. Med Sci Sports Exerc 40: 648–654, 2008. doi: 10.1249/MSS.0b013e31816212ff. [DOI] [PubMed] [Google Scholar]
- 53.Tarnopolsky MA, Rennie CD, Robertshaw HA, Fedak-Tarnopolsky SN, Devries MC, Hamadeh MJ. Influence of endurance exercise training and sex on intramyocellular lipid and mitochondrial ultrastructure, substrate use, and mitochondrial enzyme activity. Am J Physiol Regul Integr Comp Physiol 292: R1271–R1278, 2007. doi: 10.1152/ajpregu.00472.2006. [DOI] [PubMed] [Google Scholar]
- 54.Thompson JR, Swanson SA, Casale GP, Johanning JM, Papoutsi E, Koutakis P, Miserlis D, Zhu Z, Pipinos II. Gastrocnemius mitochondrial respiration: are there any differences between men and women? J Surg Res 185: 206–211, 2013. doi: 10.1016/j.jss.2013.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van Beekvelt MC, Colier WN, Wevers RA, Van Engelen BG. Performance of near-infrared spectroscopy in measuring local O2 consumption and blood flow in skeletal muscle. J Appl Physiol (1985) 90: 511–519, 2001. doi: 10.1152/jappl.2001.90.2.511. [DOI] [PubMed] [Google Scholar]
- 56.Venables MC, Achten J, Jeukendrup AE. Determinants of fat oxidation during exercise in healthy men and women: a cross-sectional study. J Appl Physiol (1985) 98: 160–167, 2005. doi: 10.1152/japplphysiol.00662.2003. [DOI] [PubMed] [Google Scholar]
- 57.Whipp BJ, Davis JA, Wasserman K. Ventilatory control of the ‘isocapnic buffering’ region in rapidly-incremental exercise. Respir Physiol 76: 357–367, 1989. doi: 10.1016/0034-5687(89)90076-5. [DOI] [PubMed] [Google Scholar]
- 58.Willingham TB, McCully KK. In vivo assessment of mitochondrial dysfunction in clinical populations using near-infrared spectroscopy. Front Physiol 8: 689, 2017. doi: 10.3389/fphys.2017.00689. [DOI] [PMC free article] [PubMed] [Google Scholar]