Abstract
High-grade soft-tissue sarcoma (STS) is a highly malignant neoplasm with a poor overall prognosis. Numerous prognostic factors determine tumor progression and patient outcomes. Various immune-associated cells identified in the tumor microenvironment have important roles in various tumor types. The present study was performed to evaluate the expression of immune-associated genes and to elucidate the association between these genes and the prognosis in high-grade STS. A total of 12 formalin-fixed, paraffin-embedded tissue samples of high-grade STS were subjected to gene expression analysis using the NanoString nCounter® System and another 35 samples were used for immunohistochemistry. For comparative analysis, the patients were divided into two groups according to overall survival (OS). The expression levels of 770 genes were first analyzed using the nCounter® PanCancer Immune Profiling Panel. Immunohistochemistry was then performed for the most significantly altered genes. Subsequently, the association between gene expression and prognosis of high-grade STS was evaluated. Of the 770 immune-associated genes analyzed, several genes were identified as being differentially expressed between the two groups. Based on gene expression levels and fold change, 13 representative genes were identified; 7 of the 13 candidate genes (C3, CD36, DOCK9, FCER2, FOS, HLA-DRB4 and NCAM1) were significantly overexpressed in the poor prognosis group, while the other 6 immune-associated genes (BIRC5, DUSP4, FOXP3, HLA-DQA1, HLA-DQB1 and LAG3) were increased in the good prognosis group. By immunohistochemistry, the expression of the 13 immune-associated genes was confirmed to be significantly different between the two groups. Expression of HLA-DQA1, HLA-DQB1 and HLA-DRB4 was observed in 74.3, 34.3 and 48.6% of tumors, respectively. HLA-DQA1 and HLA-DQB1 were significantly decreased, whereas HLA-DRB4 was significantly increased in the poor prognosis group. Of note, expression of HLA-DQA1 was associated with a significantly longer OS (P=0.028). In conclusion, HLA-DQA1 expression was significantly associated with long-term survival and may therefore be an immune biomarker for good prognosis in high-grade STS.
Keywords: soft-tissue sarcoma, immune, biomarker, prognosis, human leukocyte antigen
Introduction
Soft tissue sarcomas (STS) are a rare type of malignant tumor that account for 1% of all cancers in adults. They are a heterogeneous group, comprising >50 different types (1,2). The diagnosis of STS is based on the morphologic pattern and immunohistochemistry (IHC), in addition to traditional H&E staining. An increasing number of specific genetic aberrations, including chromosomal translocations, gene amplification and mutations, have been identified in various types of sarcoma. These results led to a recommendation to perform molecular tests, including reverse transcription PCR, fluorescence in situ hybridization and next-generation sequencing (3,4). Histologic diagnosis is important in predicting the outcome of STS. Sarcoma grading is also widely used as a predictor of outcome in the majority of cases of STS and several different grading systems have been developed (2,5,6). Irrespective of the system used, high-grade STS is associated with a higher rate of metastasis and a lower survival rate, and identification of the grade may influence clinical decisions made during primary tumor treatment (7). The 5-year survival rate (5YSR) of STS is ~65%, but the 5YSR of high-grade, advanced-stage STS is only 10-20% (1). Although surgical resection is the primary treatment method, conventional chemotherapy is generally used in patients with high-grade and/or advanced-stage STS. In high-risk patients, the tumor is frequently not completely resected and the prognosis is poor, despite additional treatment. Patients also frequently suffer from treatment-associated toxicity (8). These data emphasize the urgent requirement for novel therapies (9).
Numerous types of immune-associated cell detected in the tumor microenvironment, as well as tumor cells themselves, exhibit genetic mutations that are essential for the prognostication or treatment of the different subtypes of STS (10). Studies have indicated that immunotherapy is effective for the treatment of solid tumors. Immune checkpoint inhibitors targeting the programmed cell death-1 (PD-1)/PD-1 ligand 1 (PD-L1) signaling axis are being explored as a novel treatment modality in STS, and evaluation of PD1- and PD-L1-positivity in STS may provide the criteria necessary for determining the suitability of patients for PD-1-based immunotherapy (11,12).
Analysis of immune-associated genes in the tumor microenvironment in STS may thus be helpful in identifying markers for the prognostication and management of STS. In the present study, the expression levels of immune-associated genes in high-grade STS were evaluated using the NanoString nCounter® PanCancer Immune Profiling Panel, which is a novel multiplex gene expression panel designed to quantitate 770 genes from different immune cell types and populations covering both adaptive and innate immune responses, common checkpoint inhibitors, tumor-specific antigens and housekeeping genes (13), and tumor-specific immune targets were identified. The selected genes were subsequently confirmed/further evaluated through additional immunohistochemistry using high-grade STS samples embedded in paraffin blocks and their clinicopathological significance was assessed.
Materials and methods
Patients and tissue samples
High-grade STS tissue samples were obtained by surgical resection between January 1998 and December 2013 at Pusan National University Hospital (Busan, Korea). The included cases were of high-grade STS, comprising pleomorphic tumor cells and advanced tumor stages. Diagnoses were confirmed by pathological analysis using the diagnostic criteria defined by the World Health Organization. Each case was evaluated according to the French Federation of Cancer Centers (FNCLCC) sarcoma group grading system and the staging system of the American Joint Committee on Cancer (2). A total of 35 formalin-fixed, paraffin-embedded blocks were selected for immunohistochemistry and 12 were subjected to gene expression analysis using the NanoString nCounter® System (Nanostring Technologies, Inc.). All cases were FNCLCC grade 2 or 3 high-grade STS; the subtypes were as follows: Undifferentiated pleomorphic sarcoma (n=27), high-grade myxofibrosarcoma (n=5), leiomyosarcoma (n=2) and rhabdomyosarcoma (n=1). Clinical information was obtained from the patients' medical records. Chemotherapy was given following surgical resection, consisting of mesna, doxorubicin and ifosfamide (mesna 1.2 g/m2/day, doxorubicin 25 mg/m2/day and intravenous ifosfamide 2.0 g/m2/day on days 1-3; repeated every 3 weeks). Overall survival (OS) was calculated from the date of surgery to the date of death or the last follow-up visit. For the comparative analysis, patients were divided into two categories, a good prognosis group and a poor prognosis group, depending on survival of the follow-up period.
Written informed consent for the use of the resected tissues and clinical data was obtained from the patients and approval was obtained from the Institutional Ethics Board of Pusan National University Hospital (Busan, Korea), prior to use of any materials. The biospecimens and data used for this study were provided by the Biobank of Pusan National University Hospital, a member of the Korea Biobank Network.
NanoString nCounter® PanCancer Immune Profiling Panel for gene expression analysis. Formalin-fixed paraffin blocks were selected from 12 high-grade STS cases for gene expression analysis using the nCounter® PanCancer Immune Profiling Panel (Nanostring Technologies, Inc.), a unique 770-multiplex gene expression panel that determines the human immune response in all cancer types. The 12 high-grade STS cases included 7 cases belonging to the good prognosis group and 5 cases in which death occurred during the follow-up period. Total RNA was extracted using the RNeasy mini prep kit (Qiagen, Inc.). RNA yield and purity were assessed using a DS 11 Spectrophotometer (Denovix Inc.) and an RNA quality check was performed using Fragment Analyzer™ (Advanced Analytical Technologies). Total RNA (100 ng) was assayed using the nCounter Digital Analyzer (Nanostring Technologies, Inc.) according to the manufacturer's protocol. Total RNA was processed using the digital multiplexed nCounter® human mRNA expression assay and the Human Pancancer Immune Profiling Panel Kit (Nanostring Technologies, Inc.). Hybridizations were performed by combining 5 µl of each RNA sample with 8 µl of nCounter® Reporter probes in the hybridization buffer and 2 µl of nCounter® Capture probes (for a total reaction volume of 15 µl) overnight at 65˚C for 18 h. Excess probes were removed by two-step magnetic bead-based purification with the nCounter® Prep Station. Specific target molecules were then quantified on the nCounter® Digital Analyzer by counting the individual fluorescent barcodes and assessing the target molecules. For each assay, a high-density scan encompassing 280 fields of view was performed. The data were collected using the nCounter® Digital Analyzer after images of the immobilized fluorescent reporters were acquired in the sample cartridge with a charge-coupled device camera.
mRNA data anaylsis was performed using the freely available nSolver™ analysis software (version 4.0.70; NanoString Technologies, Inc.) with the mRNA profiling data normalized to the expression of housekeeping genes. R software was used for the analysis. Changes of >2-fold (upregulation or downregulation) were considered significant. Genes that exhibited a fold change of >2 and P<0.05 between the two groups were selected. A heat map of gene expression for genes that were differentially expressed between the good and poor prognostic groups was then plotted.
Immunohistochemical staining and analysis
Sections were transferred to poly-L-lysine-coated glass slides, dewaxed in xylene and rehydrated in ethanol. Staining was performed using BondMax autostainer and reagents (Vision Biosystems). Deparaffinization was performed automatically in the autostainer with BondWash solution at 72˚C for 30 min. Slides were then sequentially incubated with Epitope Retrieval Solution 1 (Leica Microsystems) for 20 min at 100˚C, peroxide-blocked for 5 min and incubated with primary antibody for 15 min, post-primary reagent for 8 min and polymer for 8 min. Antibodies to human leukocyte antigen (HLA)-DQA1 (rabbit monoclonal; ab128959; Abcam), HLA-DQB1 (rabbit polyclonal; ab224600; Abcam) and HLA-DRB4 (rabbit monoclonal; ab140612; Abcam) were used as primary antibodies. Human tonsil tissue was used as a positive control, as it is known to express these markers.
For all primary antibodies, the samples were considered positive if cells exhibited cytoplasmic and/or membranous staining. As the immunohistochemical scoring method, the combined positive score (CPS) was determined. The CPS was estimated by adding up the number of positively stained cells (tumor cells, lymphocytes, macrophages), dividing the result by the total number of viable tumor cells and multiplying by 100. Immunohistochemical results were considered positive for expression of HLA-DQA1, HLA-DQB1 and HLA-DRB4 if the CPS was ≥1 and considered negative when the CPS was <1(14).
Statistical analysis
Statistical analysis was performed using SPSS 22.0 software (IBM, Corp.). The associations between OS and the expression of HLA-DQA1, HLA-DQB1 and HLA-DRB4 were assessed using Pearson's χ2 test, as well as Kaplan-Meier curves with the log-rank test. P<0.05 was considered to indicate a statistically significant difference.
Results
NanoString nCounter® PanCancer Immune Profiling
The 12 patients selected for gene expression analysis ranged in age from 50 to 86 years (mean, 56 years). The male:female ratio was 7:5. Patient follow-up was conducted from the date of surgery until final follow-up date identified in medical records; the date of death or the last visit to the hospital. The follow-up period ranged from 4 to 122 months (mean, 68.3 months). In total, 7 patients survived during the follow-up period and were classified as the good prognosis group, while 5 died and were classified as the poor prognosis group (Table I).
Table I.
Twelve cases applied to NanoString nCounter® PanCancer Immune Profiling.
| Case no. | Prognosis group | Histological diagnosis | Site | AJCC stage | FU data |
|---|---|---|---|---|---|
| 1 | Good | UPS | Upper extremity | II | Survival |
| 2 | Good | UPS | Lower extremity | II | Survival |
| 3 | Good | UPS | Lower extremity | III | Survival |
| 4 | Good | UPS | Lower extremity | II | Survival |
| 5 | Good | UPS | Lower extremity | III | Survival |
| 6 | Good | UPS | Lower extremity | II | Survival |
| 7 | Good | UPS | Lower extremity | III | Survival |
| 8 | Poor | HG myxofibrosarcoma | Upper extremity | II | Death |
| 9 | Poor | UPS | Lower extremity | III | Death |
| 10 | Poor | UPS | Lower extremity | III | Death |
| 11 | Poor | HG myxofibrosarcoma | Lower extremity | II | Death |
| 12 | Poor | UPS | Upper extremity | III | Death |
UPS, undifferentiated pleomorphic sarcoma; HG, high grade; AJCC, American Joint Committee on Cancer; FU, follow up.
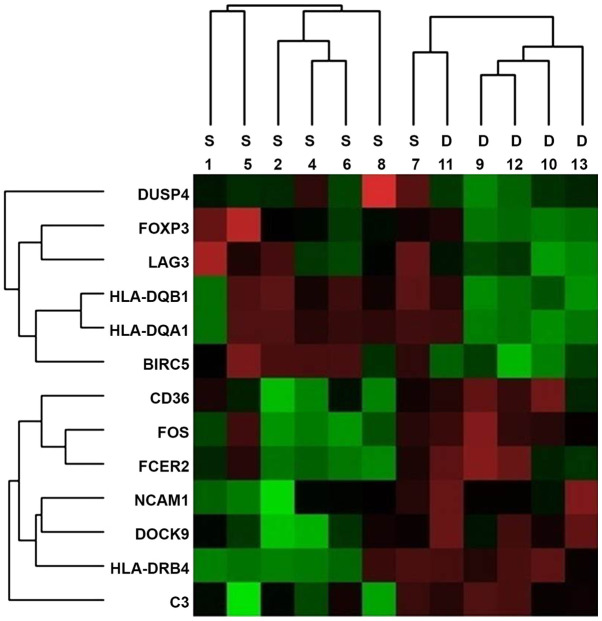
Nanostring nCounter® Analysis was performed on all samples and gene expression levels were compared between the two groups. In the panel of 770 immune-associated genes in the nCounter® Analysis, a number of genes was determined to be differentially expressed between the two groups and 13 representative genes were selected based on gene expression levels and fold changes (Fig. 1). Of the 13 candidate genes, 7 [HLA-DRB4, neural cell adhesion molecule (NCAM)1, CD36, CD3, FOS, Fc fragment of IgE receptor II (FCER2) and dedicator of cytokines (DOCK)9] exhibited a significant increase in the poor prognosis group (Table II). By contrast, the expression of 6 immune-associated genes [HLA-DQA1, HLA-DQB1, lymphocyte activation gene 3 (LAG3), forkhead box P3 (FOXP3), baculoviral IAP repeat containing 5 (BIRC5) and dual specificity phosphatase (DUSP)4] was increased in the good prognosis group (Table III). The associated molecules are classified according to the primary function of each gene: Antigen processing and presentation (HLA-DRB4, HLA-DQA1 and HLA-DQB1), adhesion molecule (NCAM1), transporter function (CD36), regulation of immune response (C3, FCER2 and LAG3), cytokine (FOXP3), cell cycle (BIRC5) and others (FOS, DOCK9 and DUSP4).
Figure 1.
Heat map generated from mRNA expression data reflecting 13 representative genes that were differentially expressed between the S and D prognostic groups. Red color indicates highly expressed genes. Groups: S, good prognosis; D, poor prognosis. HLA-DRB4, human leukocyte antigen; NCAM1, neural cell adhesion molecule; CD36; CD3, FOS, FCER2, Fc fragment of IgE receptor II; DOCK9, dedicator of cytokines 9; HLA-DQA1, HLA-DQB1, LAG3, lymphocyte antivation gene 3; FOXP3, forkhead box P3; BIRC5, baculoviral IAP repeat containing 5; DUSP4, dual specificity phosphatase 4.
Table II.
Selected genes with increased expression in the poor prognostic group according to the NanoString nCounter® analysis.
| Gene name | mRNA/probe ID | Fold change | Gene annotation | P-value |
|---|---|---|---|---|
| HLA-DRB4 | NM_021983.4:194 | 56.93 | Antigen processing and presentation | 0.011 |
| NCAM1 | NM_000615.5:1620 | 6.12 | Adhesion molecules | 0.046 |
| CD36 | NM_001001548.2:705 | 5.24 | Transporter function | 0.016 |
| C3 | NM_000064.2:4396 | 3.61 | Immune response regulation | 0.046 |
| FOS | NM_005252.2:1475 | 3.51 | Transcription factor | 0.010 |
| FCER2 | NM_002002.4:420 | 3.06 | Immune response regulation | 0.043 |
| DOCK9 | NM_001130048.1:1020 | 2.17 | Immune cell function | 0.016 |
HLA, human leukocyte antigen; NCAM1, neural cell adhesion molecule 1; FCER2, Fc fragment of IgE receptor II; DOCK9, dedicator of cytokines 9.
Table III.
Six genes showing significant expression in the good prognostic group by the NanoString nCounter® analysis.
| Gene name | mRNA/probe ID | Fold change | Gene annotation | P-value |
|---|---|---|---|---|
| HLA-DQA1 | NM_002122.3:261 | 77.78 | Antigen processing and presentation | 0.026 |
| HLA-DQB1 | NM_002123.3:384 | 28.94 | Antigen processing and presentation | 0.022 |
| LAG3 | NM_002286.5:1735 | 2.91 | Immune response regulation T-cell function | 0.015 |
| FOXP3 | NM_014009.3:1230 | 2.41 | Transcriptional regulation T-cell function | 0.020 |
| BIRC5 | NM_001168.2:1215 | 2.38 | G2 phase and G2/M transition | 0.001 |
| DUSP4 | NM_057158.2:3115 | 2.18 | Innate immune response | 0.043 |
HLA, human leukocyte antigen; LAG3, lymphocyte activation gene 3; FOXP3, forkhead box P3; BIRC5, baculoviral IAP repeat containing 5; DUSP4, dual specificity phosphatase 4.
Immunohistochemical analysis
The subjects for immunohistochemical confirmation included 35 patients with high-grade STS, including 19 males and 16 females with an average age of 66.2 years (range, 39-86 years). The tumors were located in a lower limb in 25 patients, an upper limb in 8 patients and the trunk in 2 patients. A total of 26 patients had tumor sizes of ≤10 cm, while the other patients had tumor sizes of >10 cm. The average follow-up period was 69.1 months (range, 4-150 months). As the clinical information was obtained from the patients' medical records, the OS was calculated from the date of surgery to the date of mortality or last follow-up visit. In total, 15 patients survived the follow-up period and were classified as the good prognosis group, while 20 patients who died of the disease were classified as the poor prognosis group. The clinicopathological data of this cohort are summarized in Table IV.
Table IV.
Clinicopathological features of 35 patients whose high-grade STS tumors were subjected to immunohistochemical analysis.
| Item | Total (n=35) | Good prognosis group (n=15) | Poor prognosis group (n=20) | P-value |
|---|---|---|---|---|
| Age (years, mean ± SD) | 66.2±12.5 | 62.3±9.7 | 67.9±13.9 | 0.275 |
| Sex | 0.182 | |||
| Male | 16 | 6 | 13 | |
| Female | 19 | 9 | 7 | |
| Tumor location | 0.296 | |||
| Upper extremity | 8 | 0 | 8 | |
| Lower extremity | 25 | 14 | 11 | |
| Trunk | 2 | 1 | 1 | |
| Tumor size (cm) | 0.244 | |||
| ≤10 | 26 | 13 | 13 | |
| >10 | 9 | 2 | 7 | |
| AJCC stage | 0.489 | |||
| Ⅱ | 23 | 11 | 12 | |
| Ⅲ | 11 | 4 | 7 | |
| IV | 1 | 0 | 1 | |
| Histological diagnosis | 0.696 | |||
| UPS | 27 | 11 | 16 | |
| High-grade myxofibrosarcoma | 5 | 3 | 2 | |
| Leiomyosarcoma | 2 | 1 | 1 | |
| Rhabdomyosarcoma | 1 | 0 | 1 |
Groups: Good prognosis, alive at last follow-up visit; poor prognosis, died of disease during the follow-up period (mean, 69.1 months; range, 4-150 months). SD, standard deviation; UPS, undifferentiated pleomorphic sarcoma; AJCC, American Joint Committee on Cancer.
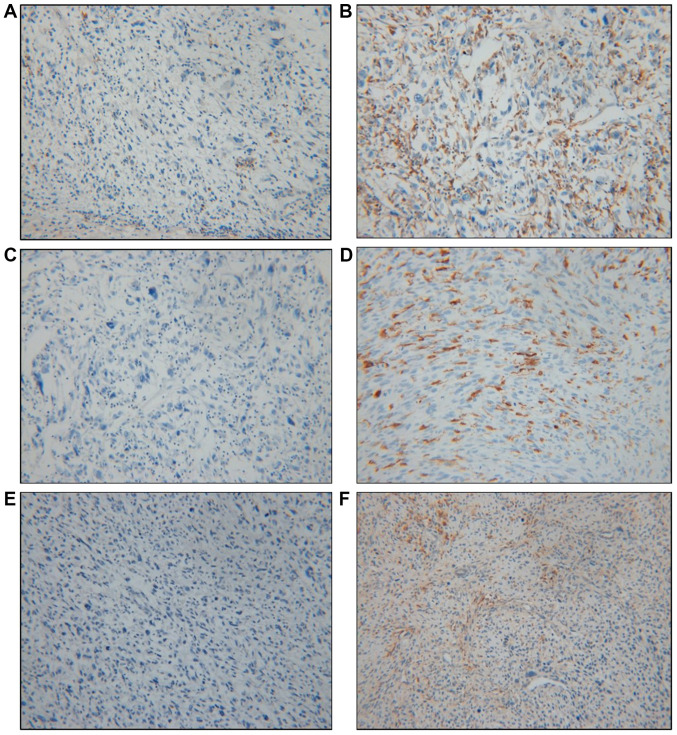
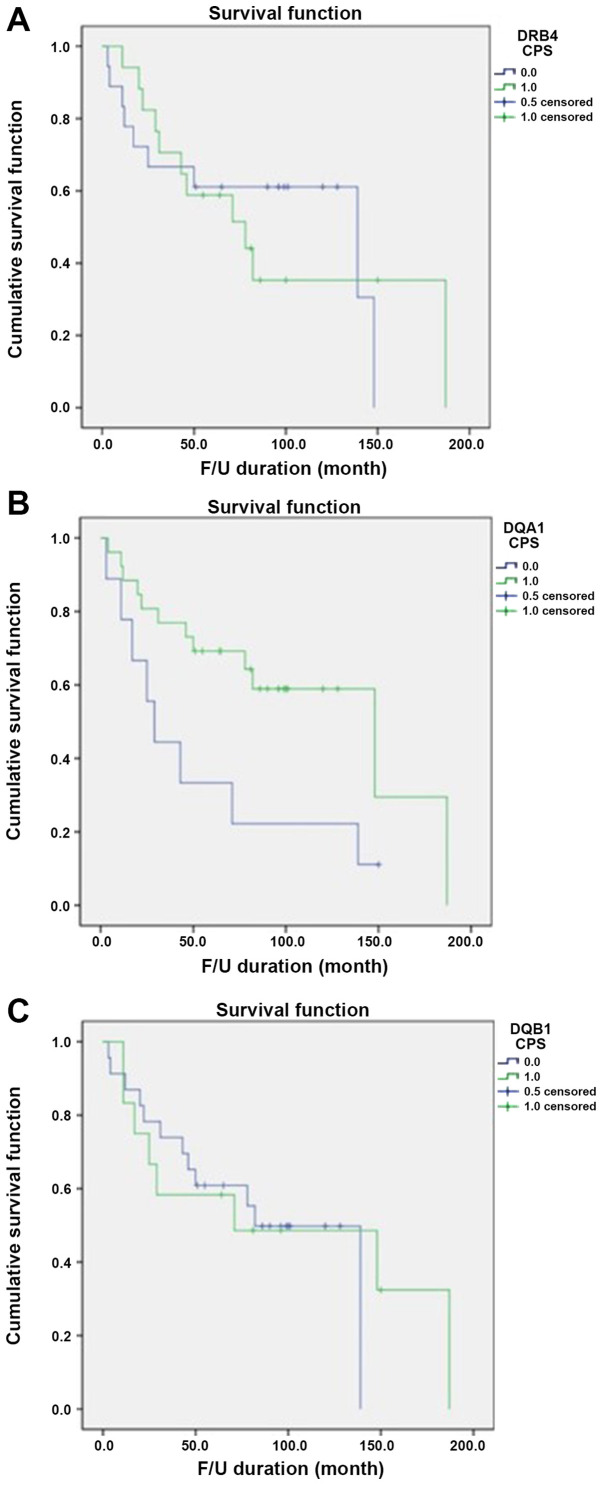
Based on data obtained from the Nanostring nCounter® analysis, HLA-DQA1, HLA-DQB1 and HLA-DRB4 exhibited the greatest differences in fold change and were selected for further immunohistochemical study using paraffin-embedded blocks of samples from the 35 patients. The immunohistochemical data were considered positive if the cells were either cytoplasmic or membranous stained; the results were analyzed by determining the CPS (12) (Fig. 2). Positive expression of HLA-DQA1, HLA-DQB1 and HLA-DRB4 was observed in 74.3% (26/35), 34.3% (12/35) and 48.6% (17/35) of tumors, respectively (Table V). The immunohistochemical results of the 12 cases subjected to the Nanostring nCounter® Analysis were consistent with the results of the gene expression analysis. For HLA-DQA1, 26 (74.3%) patients exhibited positive expression in their tumor tissues, comprising 14 cases (14/26, 53.9%) in the good prognosis group and 12 cases (12/26, 46.1%) in the poor prognosis group. In the group with negativity for HLA-DQA1, only 1 (1/9, 11.1%) patient survived, while 8 (8/9, 88.9%) died of their disease. There was a statistically significant difference between the groups stratified by HLA-DQA1 expression and prognosis, with an increased survival rate in the positive expression group (P=0.028). HLA-DQB1 exhibited positive expression in 12 (34.3%) patients, 4 (33.3%) in the good prognosis group and 8 (66.7%) in the poor prognosis group, but there was no significant difference between the groups stratified by HLA-DQB1 expression and prognosis (P=0.489). HLA-DRB4 positivity was observed in 17 (48.6%) patients, with no statistically significant difference between the 6 (35.3%) in the good prognosis group and 11 (64.7%) in the poor prognosis group (P=0.500). Only positive expression of HLA-DQA1 was significantly associated with favorable OS (Fig. 3).
Figure 2.
Representative immunohistochemical staining images for high-grade soft-tissue sarcoma. Brown-stained cells were considered to have positive staining; they were mostly immune cells, including lymphocytes and macrophages. To analyze the results, the CPS was determined. (A) Immunostaining for HLA-DRB4 (CPS<1) was negative in a sample from the good prognosis group. (B) Immunostaining for HLA-DRB4 (CPS≥1) revealed positive staining in a sample from the poor prognosis group. (C) Immunostaining for HLA-DQA1 (CPS<1) was negative in a sample from the poor prognosis group. (D) Immunostaining for HLA-DQA1 (CPS≥1) was positive in a sample from the good prognosis group. (E) Immunostaining for HLA-DQB1 (CPS<1) was negative in a sample from the poor prognosis group. (F) Immunostaining for HLA-DQB1 (CPS≥1) was positive in a sample from the good prognosis group. Magnification, x200. CPS, combined positive score; HLA, human leukocyte antigen.
Table V.
Survival analysis of immunohistochemistry results for HLA-DQA1, HLA-DQB1 and HLA-DRB4.
| Gene/expression status | Good prognosis group (n=15) | Poor prognosis group (n=20) | Total (n=35) | P-value |
|---|---|---|---|---|
| HLA-DQA1 | 0.028 | |||
| CPS (-) | 1 (11.1) | 8 (88.9) | 9 | |
| CPS (+) | 14 (53.9) | 12 (46.1) | 26 | |
| HLA-DQB1 | 0.489 | |||
| CPS (-) | 11 (47.8) | 12 (52.2) | 23 | |
| CPS (+) | 4 (33.3) | 8 (66.7) | 12 | |
| HLA-DRB4 | 0.500 | |||
| CPS (-) | 9(50) | 9(50) | 18 | |
| CPS (+) | 6 (35.3) | 11 (64.7) | 17 |
Values are expressed as n (%). Groups: Good prognosis, alive at last follow-up visit; poor prognosis, died of disease during the follow-up period (mean, 69.1 months; range, 4-150 months). CPS, combined positive score; HLA, human leukocyte antigen.
Figure 3.
Kaplan-Meier survival analysis for expression of HLA-DRB4, HLA-DQA1 and HLA-DQB1 detected via immunohistochemistry. (A) Survival curves by HLA-DRB4 expression indicated no significant association with prognosis (P=0.489). (B) HLA-DQA1 expression was significantly associated with an increase in the survival rate (P=0.028). (C) Survival curves by HLA-DRB1 expression indicated no significant association with prognosis (P=0.500). Blue line, CPS-negative expression group; green line, CPS-positive expression group. CPS, combined positive score; HLA, human leukocyte antigen. Patient follow-up was conducted from the date of surgery until final follow up date identified in medical records; the date of death or the last visit to the hospital. The follow-up period ranged from 4 to 122 months (mean, 68.3 months). CPS, combined positive score; HLA, human leukocyte antigen.
Discussion
High-grade STS has a mortality rate ranging from 40 to 60%. The predictors of survival time in patients with high-grade STS include tumor size, histology, grading, margin status at resection and the presence of pre-surgical metastasis (5). It has been previously indicated that old age affects the prognosis of STS (15), but in the cohort of the present study, with an average age of 66.2 years (range, 39-86 years), the age difference between the two study groups was not statistically significant. The standard treatment of high-grade STS includes surgical resection, with radiation therapy as a supplementary treatment and chemotherapy added if required. Based on the genetic mutations detected in each case, targeted therapy may also be performed (16,17). A previous study reported that co-administration of the monoclonal antibody Olaratumab, which acts against platelet-derived growth factor receptor α, and the traditional chemotherapy agent doxorubicin, increase the overall long-term survival rate (18). A better understanding of prognostic factors is required to determine the prognosis and select the most appropriate treatment regimen for each patient.
A number of studies have reported changes in the immune response and the activity of immune checkpoint inhibitors in STS (9-12). Recent years have seen the emergence of PD-1 and PD-L1 as novel targets in cancer immunotherapy. PD-1 and PD-L1 expression has been detected in tumor tissue and the microenvironment in certain types of STS. The association between their expression levels and clinical outcomes demonstrates the importance of immune checkpoint inhibitors in patients with high-grade STS (11).
In the present study, the expression of diverse immune-associated genes in high-grade STS according to prognosis was investigated and the association between immune-associated genes and survival was further examined using 35 samples of formalin-fixed, paraffin-embedded tissue. The small number of samples may be a limitation of the present study. However, it was attempted to obtain high-grade STS samples with pleomorphic tumor cells and advanced stages, as previous studies using STS samples comprised numerous different histological types of tumor and it affected their results (5,6). This was achieved on the fully automated and highly precise Nanostring nCounter® system designed for gene expression analysis, which accurately detects and quantifies 770 transcripts from 24 different types of immune cell. The system measures transcripts related to the adaptive and innate immune response, common checkpoint inhibitors, tumor-specific antigens and housekeeping genes; it does so using a small amount of mRNA and is based on digital color-coded barcode technology (13). The Nanostring nCounter® Sarcoma Fusion CodeSet was recently introduced in the field of STS research to assess fusion transcripts in formalin-fixed, paraffin-embedded material (19). The custom-designed NanoString nCounter®-based sarcoma assay is highly sensitive and specific in a clinical setting for molecular diagnosis of sarcoma.
The present results revealed significant genetic variations between the survival group (good prognosis group) and the poor prognosis group. The criteria applied to identify significantly differentially expressed genes were P<0.05 and a fold change of >2 between the two groups. A total of 13 genes were selected. Of these, 7 immune-associated genes (C3, CD36, DOCK9, FCER2, FOS, HLA-DRB4 and NCAM1) were significantly increased in the poor prognosis group, while the expression of 6 immune-associated genes (BIRC5, DUSP4, FOXP3, HLA-DQA1, HLA-DQB1 and LAG3) was increased in the good prognosis group.
NCAM1 encodes a cell adhesion protein that is a member of the immunoglobulin superfamily. The encoded protein was reported to be involved in the expansion of T cells and dendritic cells, which have an important role in immune surveillance. Certain studies have indicated that NCAM1 is associated with therapeutic resistance in cancer (20,21). CD36 has a role in immune signaling and is also a scavenger receptor for fatty acid uptake that modulates cell-to-extracellular matrix attachment and TGF-β activation. CD36 has increasingly emerged as a prognostic marker associated with the metastatic process (22). Complement C3 is useful in the development of novel strategies to improve the effectiveness of cancer immunotherapy (23). FCER2 is a B-cell specific antigen with essential roles in B-cell growth and differentiation, as well as the regulation of IgE production, although it remains unknown how this gene affects the development or treatment of cancer (24). DOCK is a family of guanine-nucleotide exchange factors for Rho GTPases and FOS is a subunit of the transcription factor activator protein-1. Their functions in the immune system and cancer remain to be fully elucidated.
BIRC5, also called survivin, is a well-known cancer therapeutic target. BIRC5 has multiple mechanisms of action in immune responses, as well as utilities in molecular cancer diagnostics and therapeutics (25). Survivin peptide immunogen-reactive antibodies exert an additional advantage for survivin immunotherapy. Survivin-specific T-cell reactivity strongly correlates with tumor response and patient survival (26). The present study revealed reduced levels of survivin in the poor prognosis group; hence, the possibility of survivin as a therapeutic target should be considered in cases of high-grade STS. FOXP3 is a member of the forkhead/winged-helix family of transcriptional regulators and is associated with T-cell function. Regulatory T cells expressing the transcription factor FOXP3 have a critical role in the maintenance of immune homeostasis and prevention of autoimmunity and cancer pathogenesis (27). Due to their ability to suppress self-antigen responses, regulatory T cells with FOXP3 may have anti-tumor immune function, whereas increased FOXP3 expression in tumor tissue was reported to be associated with favorable prognosis in certain cancer types. This discrepancy was identified particularly in colorectal cancer (28,29). In the present study, FOXP3 expression was increased in the good prognosis group and decreased in the poor prognosis group of high-grade STS cases. These results emphasize the requirement for accurate assessment of FOXP3 expression in tumor immunity. DUSP4 negatively regulates members of the MAPK superfamily, which are associated with cellular proliferation and differentiation. DUSP4 promotes doxorubicin resistance in gastric cancer through its effects on the epithelial-mesenchymal transition (30).
The proteins encoded by HLA-DRB4, HLA-DQA1 and HLA-DQB1 are associated with antigen processing and presentation. HLA, the human leukocyte antigen, is a group of proteins that are encoded by the major histocompatibility complex (MHC) in humans and is a cell membrane glycoprotein that is expressed on the surface of human nucleated cells. It induces an adaptive immune response against invading antigens by presenting an antigen to T lymphocytes and protects normal cells from the apoptotic function of natural killer cells. The HLA class II gene locus includes 9 types of HLA (HLA-DRA, -DRB1, -DRB3, -DRB4, -DRB5, -DQA1, -DQB1, -DPA1 and -DPB1), and HLA class II DR, DQ and DP molecules are expressed by antigen-presenting cells. HLA genes have been investigated over a period of several decades, and accordingly, relatively much is known about them. HLA-DQA1, HLA-DQB1 and DRB4 are associated with numerous inflammatory and autoimmune diseases, such as celiac disease, Addison disease, idiopathic inflammatory myopathy and Hashimoto's thyroiditis. Previous studies on the associations between HLA class II molecules and human cancers have indicated diverse results. Mahmoodi et al (31) reported that the HLA-DQA1*0301 allele is mainly associated with an increased risk of breast cancer development, whereas HLA-DQB1*0602 appears to protect against early-onset breast cancer. Shen et al (32) recently demonstrated that HLA-DQA1 has an important role in esophageal squamous cell carcinoma (ESCC) progression and may be a biomarker for ESCC diagnosis and prognosis, as well as a potential target for treatment of patients with ESCC.
In the present study, to validate the association between alterations in immune-related genes and patient prognosis, cases of high-grade STS were selected and immunohistochemical analysis was performed for HLA-DQA1, HLA-DQB1 and HLA-DRB4, which indicated the maximum fold-change differences between the two groups. The Nanostring nCounter® system analysis revealed that the expression of HLA-DRB4 was markedly increased in the poor prognosis group, whereas the expression of HLA-DQA1 and HLA-DQB1 was increased in the good prognosis group. Immunohistochemistry further demonstrated positive expression of HLA-DQA1, HLA-DQB1 and HLA-DRB4 in 74.3% (26/35), 34.3% (12/35) and 48.6% (17/35) of cases, respectively. However, only the expression of HLA-DQA1 was significantly associated with survival (P=0.028), suggesting the possibility of its application as a good prognostic factor for high-grade STS. This may be due to increased HLA-DQA1 expression being able to induce a good immune response in tumors or increase the sensitivity to immune checkpoint blockade. The mechanism remains elusive and requires further research.
The results provided by the present study may be meaningful in the research area of immune-biomarker expression in high-grade STS. Although formalin-fixed, paraffin-embedded materials were used, good results were obtained from the Nanostring nCounter® system. Certain immune-related genes were identified and immunohistochemistry was performed to validate the prognostic significance of HLA-DQA1, HLA-DQB1 and HLA-DRB4. The present results indicated that the expression of HLA-DQA1 was significantly associated with long-term survival, suggesting its potential as an immune biomarker of good prognosis in high-grade STS.
Acknowledgements
Not applicable.
Funding
This study was supported by a Biomedical Research Institute Grant, Pusan National University Hospital (grant no. 2017-31).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
JYB and KUC contributed to the conception and design of the study. AK and SJL collected the patients' clinical data. KK, JYK and ISL contributed significantly to the analysis and interpretation of data. SHC helped perform the analysis and was involved in constructive discussions. JIK supervised the study and wrote the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Approval for this study was obtained from the Institutional Ethics of Pusan National University Hospital (Busan, Korea). Informed consent was obtained from all the patients whose tissues were used in this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1. Weiss SW, Goldblum JR and Folpe AL: Enzinger and Weiss's Soft Tissue Tumors. 6th edition. Elsevier, Philadelphia, pp1176, 2014. [Google Scholar]
- 2. WHO classification of tumors: Soft Tissue and Bone Tumors. 5th edition. IARC, 2020. [Google Scholar]
- 3.Meis-Kindblom JM, Bjerkehage B, Böhling T, Domanski H, Halvorsen TB, Larsson O, Lilleng P, Myhre-Jensen O, Stenwig E, Virolainen M, et al. Morphologic review of 1000 soft tissue sarcomas from the Scandinavian Sarcoma Group (SSG) Register: The peer-review committee experience. Acta Orthop Scand Suppl. 1999;285:18–26. doi: 10.1080/17453674.1999.11744818. [DOI] [PubMed] [Google Scholar]
- 4.Italiano A, Di Mauro I, Rapp J, Pierron G, Auger N, Alberti L, Chibon F, Escande F, Voegeli AC, Ghnassia JP, et al. Clinical effect of molecular methods in sarcoma diagnosis (GENSARC): A prospective, multicentre, observational study. Lancet Oncol. 2016;17:532–538. doi: 10.1016/S1470-2045(15)00583-5. [DOI] [PubMed] [Google Scholar]
- 5.Ottaiano A, De Chiara A, Fazioli F, Talamanca AA, Mori S, Botti G, Milano A, Apice G. Biological prognostic factors in adult soft tissue sarcoma. Anticancer Res. 2005;25:4519–4526. [PubMed] [Google Scholar]
- 6.Italiano A, Le Cesne A, Mendiboure J, Blay JY, Piperno-Neumann S, Chevreau C, Delcambre C, Penel N, Terrier P, Ranchere-Vince D, et al. Prognostic factors and impact of adjuvant treatments on local and metastatic relapse of soft-tissue sarcoma patients in the competing risks setting. Cancer. 2014;120:3361–3369. doi: 10.1002/cncr.28885. [DOI] [PubMed] [Google Scholar]
- 7.Coindre JM. Grading of soft tissue sarcomas: Review and update. Arch Pathol Lab Med. 2006;130:1448–1453. doi: 10.1043/1543-2165(2006)130[1448:GOSTSR]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 8.Gronchi A, Frustaci S, Mercuri M, Martin J, Lopez-Pousa A, Verderio P, Mariani L, Valagussa P, Miceli R, Stacchiotti S, et al. Short, full-dose adjuvant chemotherapy in high-risk adult soft tissue sarcomas: A randomized clinical trial from the Italian Sarcoma Group and the Spanish Sarcoma Group. J Clin Oncol. 2012;30:850–856. doi: 10.1200/JCO.2011.37.7218. [DOI] [PubMed] [Google Scholar]
- 9.van der Graaf WT, Blay JY, Chawla SP, Kim DW, Bui-Nguyen B, Casali PG, Schöffski P, Aglietta M, Staddon AP, Beppu Y, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012;379:1879–1886. doi: 10.1016/S0140-6736(12)60651-5. [DOI] [PubMed] [Google Scholar]
- 10.Raj S, Miller LD, Triozzi PL. Addressing the adult soft tissue sarcoma microenvironment with intratumoral immunotherapy. Sarcoma. 2018;12(9305294) doi: 10.1155/2018/9305294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Budczies J, Mechtersheimer G, Denkert C, Klauschen F, Mughal SS, Chudasama P, Bockmayr M, Johrens K, Endris V, Lier A, et al. PD-L1 (CD274) copy number gain, expression, and immune cell infiltration as candidate predictors for response to immune checkpoint inhibitors in soft-tissue sarcoma. Oncoimmunology. 2017;6(e1279777) doi: 10.1080/2162402X.2017.1279777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sorbye SW, Kilvaer T, Valkov A, Donnem T, Smeland E, AlShibli K, Bremnes RM, Busund LT. Prognostic impact of lymphocytes in soft tissue sarcomas. PLoS One. 2011;6(e14611) doi: 10.1371/journal.pone.0014611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cesano A. nCounter(®) pancancer immune profiling panel (Nanostring technologies, Inc., Seattle WA) J Immunother Cancer. 2015;3(42) doi: 10.1186/s40425-015-0088-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kulangara K, Zhang N, Corigliano E, Guerrero L, Waldroup S, Jaiswal D, Ms MJ, Shah S, Hanks D, Wang J, et al. Clinical utility of the combined positive score for programmed death ligand-1 expression and the approval of Pembrolizumab for treatment of gastric cancer. Arch Pathol Lab Med. 2019;143:330–337. doi: 10.5858/arpa.2018-0043-OA. [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto K, Nishimura S, Hara Y, Oka N, Tanaka H, Iemura S, Akagi M. Clinical outcomes of patients with primary malignant bone and soft tissue tumor aged 65 years or older. Exp Ther Med. 2019;17:888–894. doi: 10.3892/etm.2018.7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wardelmann E, Schildhaus HU, Merkelbach-Bruse S, Hartmann W, Reichardt P, Hohenberger P, Büttner R. Soft tissue sarcoma: From molecular diagnosis to selection of treatment. Pathological diagnosis of soft tissue sarcoma amid molecular biology and targeted therapies. Ann Oncol. 2010;21 (Suppl 7):vii265–vii269. doi: 10.1093/annonc/mdq381. [DOI] [PubMed] [Google Scholar]
- 17.Reichardt P. Soft tissue sarcomas, a look into the future: Different treatments for different subtypes. Future Oncol. 2014;10 (Suppl 8):S19–S27. doi: 10.2217/fon.14.116. [DOI] [PubMed] [Google Scholar]
- 18.Tap WD, Jones RL, Van Tine BA, Chmielowski B, Elias AD, Adkins D, Agulnik M, Cooney MM, Livingston MB, Pennock G, et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: An open-label phase 1b and randomised phase 2 trial. Lancet. 2016;388:488–497. doi: 10.1016/S0140-6736(16)30587-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang KTE, Goytain A, Tucker T, Karsan A, Lee CH, Nielsen TO, Ng TL. Development and evaluation of a pan-sarcoma fusion gene detection assay using the NanoString nCounter platform. J Mol Diagn. 2018;20:63–77. doi: 10.1016/j.jmoldx.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Sykes SM. NCAM1 supports therapy resistance and LSC function in AML. Blood. 2019;133:2247–2248. doi: 10.1182/blood-2019-03-901017. [DOI] [PubMed] [Google Scholar]
- 21.Jiang C, Zhao W, Qin M, Jin M, Chang L, Ma X. CD56-chimeric antigen receptor T-cell therapy for refractory/recurrent rhabdomyosarcoma: A 3.5-year follow-up case report. Medicine (Baltimore) 2019;98(e17572) doi: 10.1097/MD.0000000000017572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Enciu AM, Radu E, Popescu ID, Hinescu ME, Ceafalan LC. Targeting CD36 as biomarker for metastasis prognostic: How far from translation into clinical practice? Biomed Res Int. 2018;4(7801202) doi: 10.1155/2018/7801202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng W, McKenzie JA, Hwu P. Complementing T-cell function: An inhibitory role of the complement system in T-cell-mediated antitumor immunity. Cancer Discov. 2016;6:953–955. doi: 10.1158/2159-8290.CD-16-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laitinen T, Ollikainen V, Lázaro C, Kauppi P, de Cid R, Antó JM, Estivill X, Lokki H, Mannila H, Laitinen LA, Kere J. Association study of the chromosomal region containing the FCER2 gene suggests it has a regulatory role in atopic disorders. Am J Respir Crit Care Med. 2000;161:700–706. doi: 10.1164/ajrccm.161.3.9810056. [DOI] [PubMed] [Google Scholar]
- 25.Li F, Aljahdali I, Ling X. Cancer therapeutics using survivin BIRC5 as a target: What can we do after over two decades of study? J Exp Clin Cancer Res. 2019;38(368) doi: 10.1186/s13046-019-1362-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Becker JC, Andersen MH, Hofmeister-Müller V, Wobser M, Frey L, Sandig C, Walter S, Singh-Jasuja H, Kämpgen E, Opitz A, et al. Survivin-specific T-cell reactivity correlates with tumor response and patient survival: A phase-II peptide vaccination trial in metastatic melanoma. Cancer Immunol Immunother. 2012;61:2091–2103. doi: 10.1007/s00262-012-1266-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wing JB, Tanaka A, Sakaguchi S. Human FOXP3+ regulatory T cell heterogeneity and function in autoimmunity and cancer. Immunity. 2019;50:302–316. doi: 10.1016/j.immuni.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 28.deLeeuw RJ, Kost SE, Kakal JA, Nelson BH. The prognostic value of FoxP3+ tumor-infiltrating lymphocytes in cancer: A critical review of the literature. Clin Cancer Res. 2012;18:3022–3029. doi: 10.1158/1078-0432.CCR-11-3216. [DOI] [PubMed] [Google Scholar]
- 29.Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, Platell C, Iacopetta B. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27:186–192. doi: 10.1200/JCO.2008.18.7229. [DOI] [PubMed] [Google Scholar]
- 30.Kang X, Li M, Zhu H, Lu X, Miao J, Du S, Xia X, Guan W. DUSP4 promotes doxorubicin resistance in gastric cancer through epithelial-mesenchymal trantision. Oncotarget. 2017;8:94028–94039. doi: 10.18632/oncotarget.21522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahmoodi M, Nahvi H, Mahmoudi M, Kasaian A, Mohagheghi MA, Divsalar K, Nahavandian B, Jafari A, Ansarpour B, Moradi B, et al. HLA-DRB1,-DQA1 and -DQB1 allele and haplotype frequencies in female patients with early onset breast cancer. Pathol Oncol Res. 2012;18:49–55. doi: 10.1007/s12253-011-9415-6. [DOI] [PubMed] [Google Scholar]
- 32.Shen FF, Pan Y, Li JZ, Zhao F, Yang HJ, Li JK, Gao ZW, Su JF, Duan LJ, Lun SM, et al. High expression of HLA-DQA1 predicts poor outcome in patients with esophageal squamous cell carcinoma in Northern China. Medicine (Baltimore) 2019;98(e14454) doi: 10.1097/MD.0000000000014454. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.