Abstract
Background
Ambulatory wireless video electroencephalography (AEEG) is the method of choice to discriminate epileptic seizures from other nonepileptic episodes. However, the influence of prior general anesthesia (GA), sedation, or antiseizure drug (ASD) on the diagnostic ability of AEEG is unknown.
Hypothesis/Objectives
The use of sedation/GA or ASD treatment before AEEG recording may affect the diagnostic ability of AEEG and the time to first abnormality on AEEG.
Animals
A total of 108 client‐owned dogs undergoing ambulatory AEEG for paroxysmal episodes.
Methods
Retrospective cohort study. Proportions of diagnostic AEEG and time to first abnormality were compared between dogs that received sedation/GA or neither for instrumentation as well as dogs receiving at least 1 ASD and untreated dogs.
Results
Ambulatory EEG was diagnostic in 60.2% of all dogs including 49% of the sedation/GA dogs and 68% of dogs that received neither (odds ratio [OR], 2.25; 95% confidence interval [CI], 1.02‐5.00; P = .05). The AEEG was diagnostic in 51% of dogs receiving at least 1 ASD and 66% of untreated dogs (OR, 1.95; 95% CI, 0.9‐4.3; P = .11). No difference was found in time to first abnormality between sedation/GA or neither or ASD‐treated or untreated dogs (P = .1 and P = .3 respectively). Ninety‐five percent of dogs had at least 1 abnormality within 277 minutes.
Conclusion and Clinical Importance
Sedation/GA and concurrent ASD administration were not identified as confounding factors for decreasing AEEG diagnostic capability nor did they delay the time to first abnormality. A 4‐hour minimal recording period is recommended.
Keywords: EEG, epilepsy, movement disorder, paroxysmal episode, veterinary neurology
Abbreviations
- AEEG
ambulatory wireless video electroencephalography
- ASD
antiseizure drug
- CI
confidence interval
- GA
general anesthesia
1. INTRODUCTION
Differentiating epileptic seizures from other paroxysmal episodes is important in clinical practice to ensure that appropriate treatment is started. Currently, the distinction mainly relies on clinical history and videos of the episode, which have shown low interobserver agreement on the presence or absence of an epileptic seizure and the nature of the seizure. 1 Recording the electrical activity of the brain by electroencephalography (EEG) represents the most objective way of differentiating an epileptic seizure from another paroxysmal episode. 2 , 3 , 4 Although not all seizures present with abnormal electrical discharges on EEG and some electrical discharges are not associated with epilepsy, this test is the standard of care in human medicine for investigation of paroxysmal disorders. 4 , 5 Electroencephalography is not commonly performed in veterinary medicine for several technical reasons such as difficulty to place and maintain several functional electrodes as well as the need for special training to interpret it with ease. This impractical clinical application of EEG in veterinary medicine partly may explain the lack of protocol standardization, which makes it difficult to compare findings between different recordings. 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 Nonetheless, EEG has proven useful in veterinary medicine to detect epileptic activity that was not clinically observable. 17 , 18 In recent years, the use of ambulatory video EEG (AEEG) has been reported in dogs. 10 This procedure enables recording a patient's brain electrical activity while allowing video capture of the paroxysmal episode. It therefore potentially can increase the diagnostic yield of AEEG because it does not rely solely on detection of interictal discharges but also allows diagnosis of nonepileptic disorders if the AEEG shows no abnormal discharges while the patient experiences an episode. 10 This technique however does not improve the practicality of electrode placement and thus general anesthesia (GA) or sedation commonly are used to place the electrodes before recording. However, GA frequently is used to treat refractory status epilepticus and therefore may have a suppressive effect on epileptic discharges. 19 Some drugs used for sedation also may have some anti‐ or proepileptic effects that could affect the diagnostic capability of AEEG. 20 , 21 , 22 , 23 Similarly, the fact that dogs often are started on an antiseizure drug (ASD) before AEEG is performed may change the probability of recording epileptic discharges on AEEG. 24 , 25
Our objective was to evaluate the use of sedation/GA or ASD treatment for electrode placement before recording as confounding factors for not achieving a diagnosis on AEEG in dogs presenting for paroxysmal episodes. We hypothesized that sedation/GA for electrode placement or use of an ASD may change the probability of establishing a diagnosis on AEEG and may delay the time taken to observe at least 1 abnormality or episode during the recording.
2. MATERIALS AND METHODS
Medical records of dogs that underwent AEEG examination were reviewed from 5 academic and private veterinary referral hospitals. All AEEG were performed with owner consent during investigation of paroxysmal episodes. Dogs were included if they had a history of paroxysmal episodes (suspected epileptic seizures or suspected nonepileptiform episode, referred to as “episode of interest”) and had an AEEG performed. Dogs for which the AEEG was uninterpretable because of major artifacts were excluded from the study. Dogs that underwent AEEG for status epilepticus monitoring or that were obtunded or comatose because of a severe intracranial pathology were excluded from this study because they would not have been able to behave freely and show paroxysmal episodes. Dogs also were excluded if there was no mention about having received sedation, GA, or ASD in their record. For dogs that underwent multiple AEEG, only the first recording for initial investigation of paroxysmal episodes was analyzed. Breed, sex, age, abnormal episode frequency, and ASD treatment at the time of recording were retrieved from the medical records. A subset of these dogs was described in a previous study evaluating the diagnostic capability of wireless AEEG but not evaluating the effect of sedation/GA and ASD on the diagnostic yield of AEEG. 10
Wireless AEEG with synchronized video was recorded using a Trackit MK3 AEEG/Polygraphy recorder (Lifelines Neurodiagnostic Systems, Troy, Illinois) according to a previously published protocol. 10 Number of electrodes varied according to the size of the patient's head with small patients having fewer electrodes placed. 10 Because it is an ambulatory system, dogs were awake and behaving as they would normally during AEEG recording.
The AEEG recording duration was not standardized, and AEEG recordings were ended when the patient removed the electrodes, episodes of interest were observed and allowed a diagnosis or when the patient was discharged from the hospital. The AEEG was digitally saved and reviewed retrospectively by 2 board‐certified veterinary neurologists who were not blinded to the nature of the episodes. A subset of AEEG was reviewed by a pediatric neurophysiologist if a conclusion on the diagnosis was not reached by the veterinary neurologists.
The AEEG was classified as diagnostic either if it confirmed epileptic seizures by showing ictal or interictal epileptiform discharges (epileptic seizure) as defined previously, 26 or if it recorded the episode of interest on the synchronized video and showed no abnormal discharge on AEEG during the episode (nonepileptiform paroxysmal episode). Ictal or interictal epileptiform discharges included single or trains of focal or generalized spikes, sharp waves, spike waves, polyspikes, and polyspike waves. 26 The AEEG was classified as nondiagnostic if no episode and no abnormal ictal or interictal discharges were recorded. Hence, a patient suspected to have experienced epileptic seizures based on history and clinical assessment but that did not show any episode or ictal or interictal epileptiform discharge during AEEG was classified as having a nondiagnostic AEEG. Moreover, the time to first abnormality was recorded as the duration of time elapsed between the beginning of recording and the first epileptiform discharge noted on AEEG or the first paroxysmal episode observed on video.
To compare the effect of sedation and GA on diagnostic capability of AEEG, patients were divided into 2 groups depending whether or not they had received any drugs for sedation/GA for electrode placement before AEEG recording. Because many sedative drugs were used before GA, dogs that received sedation and dogs that received GA were grouped together. For dogs in the sedation/GA group, AEEG was recorded long enough to allow recovery (return to ambulation) from sedation/GA to ensure that part of the recordings was performed on awake ambulatory dogs. To compare the effect of prior ASD treatment on diagnostic capability, patients were allocated into 2 groups depending on whether or not they were receiving any ASD at the time of AEEG recording.
Simple descriptive statistics were performed on the study population signalment, proportion of epileptic patients, time to first abnormality, and duration of AEEG in each group. Continuous variables were evaluated for normality using the Shapiro‐Wilk test and examination of residuals. For normally distributed variables, Student's t tests or analysis of variance (ANOVA) was used. For variables that were not normally distributed, a log‐transformation was applied before using a Student's t test or ANOVA. If the log‐transformed variables were not normally distributed, a nonparametric Mann‐Whitney U test was used. Proportions were compared using Fisher's exact tests. Episode frequency was described as minutely, hourly, daily, weekly, monthly, or annually and compared between groups using a Cochran‐Armitage test for trends. The proportion of diagnostic AEEG in each group (sedation/GA or not and ASD or not) was compared using exact conditional logistic regression and calculation of an odds ratio. Synergy between administration of sedation/GA and ASD was evaluated by conducting a multivariate analysis that included both presence/absence of sedation/GA and presence/absence of ASD in the statistical model. Tukey's post hoc correction for multiple comparisons was used. The correlation between episode frequency and time to first abnormality was assessed using a Spearman correlation. Statistical significance was defined as P < .05. Statistical tests were performed using SAS 9.3 (SAS Institute Inc, Cary, North Carolina).
3. RESULTS
One hundred and eight dogs underwent AEEG for investigation of paroxysmal episodes and were included in the study. Fifty‐four different breeds were represented. Females accounted for 45% of the population. Age ranged from 3 months to 15 years (average, 5.7 years; median, 5 years). Comparison of signalment between groups is provided in Tables 1 and 2.
TABLE 1.
Comparison of clinical characteristics between dogs that received sedation/general anesthesia (GA) for electrode placement and dogs that received neither
| Variable | Sedation/GA | No sedation/GA | P value |
|---|---|---|---|
| Age (average, years) | 6 | 5.3 | .57 |
| Sex | 48% (22/45) female | 43% (27/63) female | .24 |
| % of patient diagnosed with epileptic seizures | 59% (13/22) | 51% (22/43) | .61 |
| Duration of electroencephalography (mean, hours) | 9.3 | 2.3 | 4.28 × 10−8 |
TABLE 2.
Comparison of clinical characteristics between dogs that received at least 1 concurrent antiseizure drug (ASD) and dogs that received none
| Variable | Antiseizure drug | No ASD | P value |
|---|---|---|---|
| Age (average, years) | 5.7 | 5.8 | .93 |
| Sex | 39% (17/44) female | 48% (30/62) female | .43 |
| % of patient diagnosed with epileptic seizures | 87% (20/23) | 34% (14/41) | .0001 |
| Duration of electroencephalography (mean, hours) | 5.9 | 4.7 | .06 |
Sixty‐three dogs did not receive sedation or GA whereas 45 dogs received sedation or GA or both for electrode placement. Information about ASDs was available for 107 dogs of which 62 were not receiving any ASDs at the time of AEEG recording and 45 were receiving at least 1 ASD (Figure 1). The ASDs included phenobarbital, potassium bromide, zonisamide, levetiracetam, imepitoin, gabapentin, diazepam, and clorazepate used alone or in various combinations. Paroxysmal episodes investigated were: suspected epileptic seizures, idiopathic head tremors (head bobbing), compulsive tail chasing, myoclonus, episodic stiffness, compulsive tongue licking, fly biting, collapse episodes, trance‐like episodes, jaw chattering episodes, episodic drooling, episodic aggression, and possible rapid eye movement (REM) sleep disorder.
FIGURE 1.
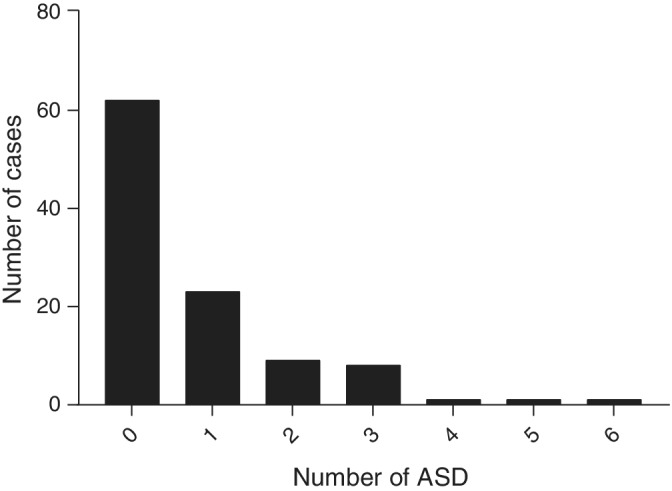
Distribution of antiseizure drug (ASD) treatment in dogs evaluated for paroxysmal episodes with electroencephalography (EEG)
Episode frequency ranged from at least 1 episode every minute (minutely) to 1 episode per year on average (yearly). Most dogs in both the sedation/GA and no sedation/GA groups had episodes that occurred daily (Figure 2). No statistically significant difference in episode frequency distribution was identified between the 2 groups (P = .08, Cochran‐Armitage test). Similarly, most dogs experienced daily episodes regardless of whether or not they were receiving ASD and no significant difference in the episode frequency distribution was found between the 2 groups (P = .4, Cochran‐Armitage test, Figure 3).
FIGURE 2.
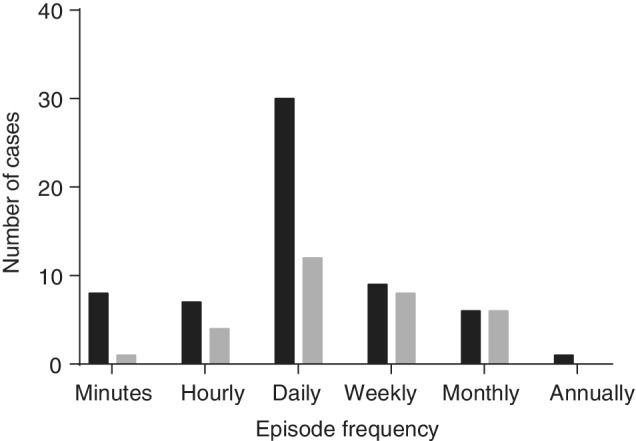
Distribution of paroxysmal episode frequency between dogs that received sedation/general anesthesia (GA) for electrode placement (gray) and dogs that received neither (black). Paroxysmal episode frequency was estimated from the medical records for the event of interest
FIGURE 3.
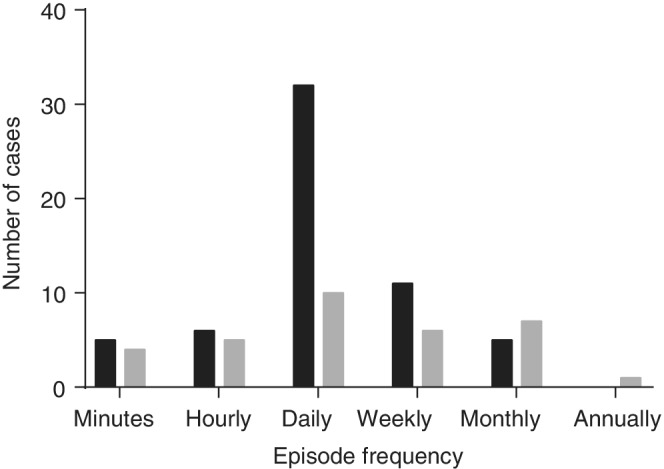
Distribution of paroxysmal episode frequency between dogs that received at least one concurrent antiseizure drug (ASD; gray) and dogs that received none (black). Paroxysmal episode frequency was estimated from the medical records for the event of interest
The AEEG recording was longer for dogs that received sedation/GA compared to dogs that received neither (mean, 9.3 hours and 2.3 hours, respectively, P = 4 × 10−8, Mann‐Whitney U test).
Overall, diagnostic AEEG was obtained in 65/108 dogs (60.2%) with 35 dogs confirmed to be experiencing epileptic seizures (53.8%) and 30 experiencing other paroxysmal episodes (46.2%). For patients confirmed to experience epileptic seizures based on the presence of interictal or ictal epileptiform discharges on AEEG, diagnoses included genetic epilepsy, suspected genetic epilepsy, epilepsy of unknown cause, and structural epilepsy (meningoencephalitis of unknown etiology, distemper encephalitis, neoplasia). For patients experiencing nonepileptiform paroxysmal episodes, diagnoses included: compulsive behaviors, Border Collie collapse, peripheral myoclonus, trance‐like syndrome of Bull Terriers, and REM sleep disorder. More dogs diagnosed with epileptic seizures on AEEG were concurrently receiving an ASD compared to dogs diagnosed with non‐epileptiform paroxysmal discharges (58.82% versus 10%, respectively, P = 6 × 10 −5, Fisher's exact test).
To evaluate the effect of sedation and GA on the diagnostic capability of AEEG, we compared the proportion of diagnostic AEEG as well as the time to reach a diagnosis (first abnormality or episode seen on AEEG) between dogs that received sedation or GA and dogs that received neither for electrode placement. A diagnosis was reached in 22/45 dogs that received sedation/GA (49%; 95% confidence interval [CI], 34%‐64%) and 43/63 dogs that received neither (68%; 95% CI, 55%‐79%) representing an odds ratio (OR) of 2.25 (95% CI, 1.02‐5.00; P = .05).
Median time to first abnormality on AEEG was 28 minutes in our sample and ranged from 1 to 1013 minutes. The overall proportion of diagnostic AEEG over time is represented in Figure 4. For dogs that received sedation/GA, the median was 39.5 minutes (95% CI, 2‐249; mean, 97 minutes; range: 1‐1013 minutes) whereas it was 13 minutes (95% CI, 1‐277; mean, 47 minutes; range: 1‐458 minutes) for dogs that received neither, resulting in a difference of 26.5 minutes (P = .1).
FIGURE 4.
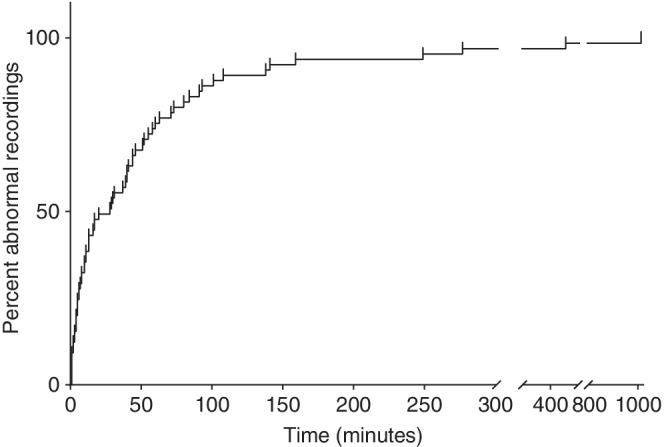
Proportion of ambulatory wireless video electroencephalography (AEEG) that have shown at least one electrical abnormality or recorded one paroxysmal episode on video over time
We then evaluated the effect of concurrent ASD administration of the diagnostic capability of AEEG in a similar fashion. A diagnosis was reached in 23/45 dogs receiving at least 1 ASD (51%; 95% CI, 36%‐66%) and in 41/62 dogs not receiving any ASD (66%; 95% CI, 53%‐78%) resulting in an OR of 1.95 (95% CI, 0.89‐4.3; P = .1).
Median time to first abnormality was 10 minutes for dogs receiving an ASD (95% CI, 1‐159 minutes; mean, 51 minutes; range: 1‐277 minutes) and 31 minutes for dogs not receiving any (95% CI, 2‐458 minutes; mean, 73 minutes; range: 1‐1013 minutes) resulting in a difference of 21 minutes (P = .3).
To evaluate a potential synergistic effect of sedation/GA and concurrent ASD administration on proportion of diagnostic AEEG, we performed a multivariate analysis by including both factors in our statistical model. No statistical difference was found between groups for both proportion of diagnostic AEEG (P = .1) and time to first abnormality (P = .8).
Finally, we evaluated the effect of episode frequency on the time to first abnormality on AEEG. When grouping all of the dogs together, a slight correlation was observed, with an increase in time to first abnormality as the episode frequency decreased (r = 0.32; P = .01, Spearman correlation). Subgroup analysis determined that this correlation was stronger in the sedation/GA group (r = 0.70; P = .003, Spearman Correlation) whereas no correlation was observed for dogs that received neither (r = 0.19; P = .2, Spearman correlation).
4. DISCUSSION
Electroencephalography is rarely used clinically to differentiate epileptic seizures from nonepileptic paroxysmal episodes in dogs, mostly because of technical difficulties and lack of a standardized protocol. 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 More specifically, the confounding factors that influence the diagnostic capability of electroencephalography are not known. Our study evaluated the effect of sedation/GA for electrode placement and concurrent ASD treatment on the diagnostic capability of AEEG and did not detect a strong association between the use of sedation/GA or concurrent use of ASD and the diagnostic capability of AEEG. Sixty‐eight percent of dogs that did not receive sedation/GA had a diagnostic AEEG compared to 49% of dogs that had received sedation/GA for instrumentation (P = .05). To understand this result in a clinical context, we calculated an absolute risk increase of 19% (95% CI, 0.78%‐38%). Therefore, the number of patients needed to harm is 5.16 (95% CI, 2.63‐128.33) which means that, on average, sedation/GA used for electrode placement may prevent a diagnostic AEEG in 1 patient for every 5 patients that receive sedation/GA. Although guidelines on what is acceptable on that topic are not available, it could be acceptable to use sedation/GA for electrode placement, especially if instrumentation for AEEG recording is challenging because of an uncooperative patient. Moreover, the differences in time to first abnormality observed were relatively small compared to the total length of AEEG recording and may not be clinically relevant. Again, no specific guidelines exist on recommended AEEG duration for veterinary patients. However, in our study, median AEEG recording time for dogs that received sedation/GA and did not receive a diagnosis (ie, where the diagnosis potentially was missed because of sedation/GA) was 4.75 hours with a mean of 9.01 hours (range, 0.83‐32.5 hours). Because the difference in time to first abnormality observed between the 2 groups was only 26.5 minutes (P = .1), which is 10 times shorter than the median recording time, this finding suggests that the difference may not be clinically relevant. These data suggest that GA or sedation may be used with more confidence to facilitate AEEG electrode placement provided that the AEEG recording extends beyond recovery from sedation/GA. Similarly, starting an ASD before performing an AEEG may not substantially impair our ability to differentiate between epileptic seizures or other paroxysmal episodes. Here, the absolute risk increase is 15.02% (95% CI, 3.75%‐33.78%) which gives a number of patients needed to harm of 6.66. Therefore, on average, starting an ASD before AEEG recording may prevent obtaining a diagnostic AEEG in 1 of every 7 patients. This estimate is consistent with previous research findings because ASD do not completely suppress interictal epileptiform discharges in patients that still experience seizures and are not likey to suppress paroxysmal episodes that are not seizures, allowing the clinician to reach a diagnosis with AEEG. 27 , 28 , 29
Three dogs in our population received 4 to 6 ASD (1 dog received 4, 1 dog received 5, and 1 dog received 6) and therefore probably represent refractory epileptic patients because they continued to have weekly episodes. The dog that received 6 ASD had interictal epileptiform discharges on AEEG, which allowed a diagnosis. The other 2 dogs did not have any epileptiform discharge nor did they experience seizures during the recording and therefore were classified as having a nondiagnostic AEEG.
These data support further questions about the potential use of AEEG as a monitoring tool for epileptic patients. However, levetiracetam previously has been shown to decrease ictal epileptiform discharges in a Rhodesian ridgebacks with juvenile myoclonic epilepsy and absence seizures. 30 Our study was not designed to evaluate the role of AEEG as a monitoring tool for epileptic patients because dogs that were diagnosed with nonepileptic paroxysmal episodes using AEEG had been pre‐emptively receiving ASD treatment before AEEG recording. We also did not compare the decrease in ictal or interictal epileptiform discharges in dogs by comparing paired AEEG before and after an ASD because our goal was to evaluate the effect of ASD on the overall diagnostic capability of AEEG. To answer this specific question, a prospective study comparing the frequency of ictal or interictal epileptiform discharges on AEEG before and after starting an ASD in epileptic dogs would be warranted.
An interesting finding of our study was the time to first AEEG abnormality observed, where this abnormality was either an episode of interest on video or an epileptiform pattern on AEEG. Overall, 95% of AEEG showed at least 1 abnormality within 277 minutes whereas a 30‐minute recording showed at least 1 abnormality in only 35/65 dogs (53.4%). Other studies that have performed short‐term electroencephalography (mean duration <45 minutes) while under sedation/GA in dogs with suspected epileptic seizures have reported diagnostic rates of 20 to 86%, 8 , 11 , 15 , 31 , 32 which is consistent with our findings. Although a previous study did not find any association between AEEG diagnosis and duration of recording, 10 this result may have been because some recordings were continued even if a diagnosis was reached, thereby decreasing the association between AEEG diagnosis and duration of AEEG recording. In our study, a plateau was reached between 2 and 3 hours of recording, and 95% of dogs showed at least 1 abnormality within 277 minutes of recording, which argues for a minimum recording time of >4 hours (Figure 4). Moreover, this time to first abnormality is related to the episode frequency, with a strong correlation for dogs receiving sedation/GA and an increase in time to first abnormality as the episode frequency decreases.
Limitations of our study are mainly a consequence of its retrospective nature. The AEEG recordings were not standardized across dogs, which may have influenced capability to reach a diagnosis. The number of electrodes, for example, varied among dogs and throughout the recording because some were lost with time. It is unknown however if the number of electrodes influences AEEG diagnostic capability. Also, the time since the last episode was not taken into account because it was not available in the majority of dogs and this factor could influence the probability of witnessing an event during AEEG recording. Other factors that may have influenced AEEG recording and that were not investigated are skull shape and masticatory muscle mass, which may decrease detection of the electrical signal originating from the cortex. Several sedative drugs and GA protocols were used, and all dogs receiving either a sedative drug or GA were placed in the sedation/GA group. Although different drugs may have different pro‐ or antiepileptic properties, this grouping allowed sufficient power for the study. Although the duration of recording was not associated with the probability of obtaining a diagnostic EEG in another study, it could have been the case in our study. 10 However, the recordings were substantially longer in the sedation/GA group whereas the proportion of diagnostic AEEG was lower (49% versus 68%) albeit with a P‐value of .05. Moreover, our study did not include brain disorders that result in persistent behavioral modifications such as certain metabolic encephalopathies because we selected only patients with intermittent paroxysmal episodes.
An inherent limitation to the study of epilepsy using AEEG is that this test is neither sensitive nor specific for the diagnosis of paroxysmal disorders. Particularly for our study, false positives could have influenced our results if more false positives occurred in 1 of our groups. Although unknown in dogs, normal children can have waves that resemble epileptiform discharges and therefore result in false positives for a diagnosis of epilepsy. 33 Moreover, the sensitivity and specificity of AEEG has been evaluated at 63 and 95%, respectively, in humans presented after 1 suspected seizure. 34 It therefore is possible that some patients in our study showed AEEG patterns mistaken for interictal epileptiform discharges and were misclassified as having a diagnostic AEEG. Given the high specificity of AEEG in humans, the probability of a false‐positive AEEG likely is low in our study. The specificity of AEEG has not been evaluated in dogs yet because no normal dogs have been evaluated using awake AEEG. However, electroencephalography recordings have been performed in normal dogs under sedation/GA. One study reported that at least 1 epileptiform discharge could be recorded during electroencephalography in 9/19 (47.37%) healthy beagle dogs undergoing sedation with medetomidine. 32 However, no epileptiform discharges were recorded in 45 healthy dogs under GA or in 10 healthy beagles undergoing electroencephalography under GA and activation procedures. 11 , 35 Another study recorded some epileptiform discharges in 1/6 clinically healthy Finnish Spitz under medetomidine sedation. 31 The variability in the rate of false positives observed among these different studies may be associated with differences in the protocols for electroencephalography recording or in the population of dogs studied in 1 study 32 because it is higher than the false positive rate reported in humans. 34
Although we studied AEEG recordings from 108 dogs, our study may lack power to statistically differentiate between the small differences in the proportions of diagnostic AEEG recordings found in our study. A post hoc analysis indicated we could detect a proportion of 39.2% diagnostic AEEG in the sedation/GA group with a power of 80% and a risk of type I error (α) of .05, which is lower than the proportion detected in our study. However, because the differences found probably are not clinically relevant, statistically confirming smaller differences by increasing sample size may not provide more practically useful information.
The dogs were not randomized between groups which may have introduced bias in our study. To evaluate for such bias, a prospective study, ideally with AEEG recording before and after GA, should be performed. Such a paired, prospective study also would allow quantification of any decrease in diagnostic AEEG associated with sedation/GA or ASD treatment because our study only investigated their role as potential risk factors for not obtaining a diagnostic AEEG, which does not seem to be the case in our study population.
No blinding of treatment group was performed while reviewing the AEEG. Because the AEEG were reviewed with video synchronization which was used for diagnosis, it would have been difficult to blind the AEEG evaluators to treatment groups. The findings used to classify an AEEG as diagnostic or not were objective (ie, presence or absence of epileptiform discharges or paroxysmal episode of interest, time to first abnormality seen), but lack of blinding may have introduced some bias.
Finally, we used the presence of interictal epileptiform discharges as well as the recording of an episode of interest to classify an AEEG as diagnostic. In theory, only recording an episode of interest can differentiate between an epileptic seizure or a nonepileptic paroxysmal event. In human medicine, interictal epileptiform discharges are very specific for epileptic seizures. 5 These findings have not yet been evaluated in dogs.
In conclusion, we did not identify a clinically relevant decrease in the diagnostic capability of AEEG with the use of sedation/GA or concurrent ASD nor was sedation/GA or concurrent ASD associated with a clinically relevant increase in time to the first observed abnormality. The findings suggest that using these protocols may be acceptable for electrode placement before AEEG recording, provided that AEEG is performed while dogs are awake and able to display the episode of interest. The duration of recording probably should be >4 hours to increase the chance of recording an episode of interest or epileptiform discharges on the AEEG, but the required time is dependent on the frequency of the episode of interest, especially if sedation or GA is used.
Parmentier T, Monteith G, Cortez MA, et al. Effect of prior general anesthesia or sedation and antiseizure drugs on the diagnostic utility of wireless video electroencephalography in dogs. J Vet Intern Med. 2020;34:1967–1974. 10.1111/jvim.15856
REFERENCES
- 1. Packer R, Berendt M, Bhatti S, et al. Inter‐observer agreement of canine and feline paroxysmal event semiology and classification by veterinary neurology specialists and non‐specialists. BMC Vet Res. 2015;11(1):39 10.1186/s12917-015-0356-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kumar‐Pelayo M, Oller‐Cramsie M, Mihu N, Harden C. Utility of video‐EEG monitoring in a tertiary care epilepsy center. Epilepsy Behav. 2013;28(3):501‐503. 10.1016/j.yebeh.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 3. Watemberg N, Tziperman B, Dabby R, Hasan M, Zehavi L, Lerman‐Sagie T. Adding video recording increases the diagnostic yield of routine electroencephalograms in children with frequent paroxysmal events. Epilepsia. 2005;46(5):716‐719. 10.1111/j.1528-1167.2005.50004.x. [DOI] [PubMed] [Google Scholar]
- 4. Nordli DR. Usefulness of video‐EEG monitoring. Epilepsia. 2006;47(s1):26‐30. 10.1111/j.1528-1167.2006.00656.x. [DOI] [PubMed] [Google Scholar]
- 5. Goodin Douglas S, Aminoff Michael J. Does the interictal EEG have a role in the diagnosis of epilepsy? Lancet. 1984;323(8381):837‐839. 10.1016/S0140-6736(84)92281-5. [DOI] [PubMed] [Google Scholar]
- 6. Pellegrino F. Canine electroencephalographic recording technique: findings in normal and epileptic dogs. Clin Neurophysiol. 2004;115(2):477‐487. 10.1016/S1388-2457(03)00347-X. [DOI] [PubMed] [Google Scholar]
- 7. Hasegawa D. Diagnostic techniques to detect the epileptogenic zone: pathophysiological and presurgical analysis of epilepsy in dogs and cats. Vet J. 2016;215:64‐75. 10.1016/j.tvjl.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 8. Berendt M, Høgenhaven H, Flagstad A, Dam M. Electroencephalography in dogs with epilepsy: similarities between human and canine findings. Acta Neurol Scand. 1999;99(5):276‐283. 10.1111/j.1600-0404.1999.tb00676.x. [DOI] [PubMed] [Google Scholar]
- 9. Cauduro A, Dondi M, Favole P, Opreni M, Simonetto LA, Lorenzo V. Artifacts during short‐term Interictal electroencephalographic recording in dogs. J Am Anim Hosp Assoc. 2017;53(2):80‐89. 10.5326/JAAHA-MS-6486. [DOI] [PubMed] [Google Scholar]
- 10. James FMK, Cortez MA, Monteith G, et al. Diagnostic utility of wireless video‐electroencephalography in unsedated dogs. J Vet Intern Med. 2017;31(5):1469‐1476. 10.1111/jvim.14789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jaggy A, Bernardini M. Idiopathic epilepsy in 125 dogs: a long‐term study. Clinical and electroencephalographic findings. J Small Anim Pract. 1998;39(1):23‐29. 10.1111/j.1748-5827.1998.tb03665.x. [DOI] [PubMed] [Google Scholar]
- 12. Steiss JE. A survey of current techniques in veterinary electrodiagnostics: EEG, spinal evoked and brainstem auditory evoked potential recording. Vet Res Commun. 1988;12(4–5):281‐288. 10.1007/BF00343246. [DOI] [PubMed] [Google Scholar]
- 13. Tepper LC, Shores A. Electroencephalographic recordings in the canine: effects of low dose medetomidine or dexmedetomidine followed by atipamezole. Open J Vet Med. 2014;04(02):7‐13. 10.4236/ojvm.2014.42002. [DOI] [Google Scholar]
- 14. Holliday TA, Williams DC. Interictal paroxysmal discharges in the electroencephalograms of epileptic dogs. Clin Tech Small Anim Pract. 1998;13(3):132‐143. 10.1016/S1096-2867(98)80034-0. [DOI] [PubMed] [Google Scholar]
- 15. Brauer C, Kästner SBR, Rohn K, Schenk HC, Tünsmeyer J, Tipold A. Electroencephalographic recordings in dogs suffering from idiopathic and symptomatic epilepsy: diagnostic value of interictal short time EEG protocols supplemented by two activation techniques. Vet J. 2012;193(1):185‐192. 10.1016/j.tvjl.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 16. Ákos P, Thalhammer J, Leschnik M, Halász P. Electroencephalographic examination of epileptic dogs under propofol restraint. Acta Vet Hung. 2012;60(3):309‐324. 10.1556/AVet.2012.026. [DOI] [PubMed] [Google Scholar]
- 17. Cuff DE, Bush WW, Stecker MM, Williams DC. Use of continuous electroencephalography for diagnosis and monitoring of treatment of nonconvulsive status epilepticus in a cat. J Am Vet Med Assoc. 2014;244(6):708‐714. 10.2460/javma.244.6.708. [DOI] [PubMed] [Google Scholar]
- 18. Granum LK, Bush WW, Williams DC, Stecker MM, Weaver CE, Werre SR. Prevalence of electrographic seizure in dogs and cats undergoing electroencephalography and clinical characteristics and outcome for dogs and cats with and without electrographic seizure: 104 cases (2009–2015). J Am Vet Med Assoc. 2019;254(8):967‐973. 10.2460/javma.254.8.967. [DOI] [PubMed] [Google Scholar]
- 19. Blades Golubovic S, Rossmeisl JH. Status epilepticus in dogs and cats, part 1: etiopathogenesis, epidemiology, and diagnosis: status epilepticus: etiopathogenesis and diagnosis. J Vet Emerg Crit Care. 2017;27(3):278‐287. 10.1111/vec.12605. [DOI] [PubMed] [Google Scholar]
- 20. Chaitanya G, Arivazhagan A, Sinha S, et al. Dexmedetomidine anesthesia enhances spike generation during intra‐operative electrocorticography: a promising adjunct for epilepsy surgery. Epilepsy Res. 2015;109:65‐71. 10.1016/j.eplepsyres.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 21. Lee W‐L, Hablitz JJ. Effect of APV and ketamine on epileptiform activity in the CA1 and CA3 regions of the hippocampus. Epilepsy Res. 1990;6(2):87‐94. [DOI] [PubMed] [Google Scholar]
- 22. Meyer S, Shamdeen MG, Kegel B, et al. Effect of propofol on seizure‐like phenomena and electroencephalographic activity in children with epilepsy vs children with learning difficulties. Anaesthesia. 2006;61(11):1040‐1047. 10.1111/j.1365-2044.2006.04782.x. [DOI] [PubMed] [Google Scholar]
- 23. Zijlmans M, Huiskamp GM, Cremer OL, Ferrier CH, van Huffelen AC, Leijten FSS. Epileptic high‐frequency oscillations in intraoperative electrocorticography: the effect of propofol. Epilepsia. 2012;53(10):1799‐1809. 10.1111/j.1528-1167.2012.03650.x. [DOI] [PubMed] [Google Scholar]
- 24. Fredsø N, Sabers A, Toft N, Møller A, Berendt M. A single‐blinded phenobarbital‐controlled trial of levetiracetam as mono‐therapy in dogs with newly diagnosed epilepsy. Vet J. 2016;208:44‐49. 10.1016/j.tvjl.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 25. Stabile F, van Dijk J, Barnett CR, De Risio L. Epileptic seizure frequency and semiology in dogs with idiopathic epilepsy after initiation of imepitoin or phenobarbital monotherapy. Vet J. 2019;249:53‐57. 10.1016/j.tvjl.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 26. St. Louis EK, Frey LC, Britton JW, et al. Electroencephalography (EEG): An Introductory Text and Atlas of Normal and Abnormal Findings in Adults, Children, and Infants. Chicago, IL: American Epilepsy Society; 2016. http://www.ncbi.nlm.nih.gov/books/NBK390354/. Accessed September 27, 2019. [PubMed] [Google Scholar]
- 27. Libenson MH, Caravale B. Do antiepileptic drugs differ in suppressing interictal epileptiform activity in children? Pediatr Neurol. 2001;24(3):214‐218. 10.1016/S0887-8994(00)00271-X. [DOI] [PubMed] [Google Scholar]
- 28. Guida M, Iudice A, Bonanni E, Giorgi FS. Effects of antiepileptic drugs on interictal epileptiform discharges in focal epilepsies: an update on current evidence. Expert Rev Neurother. 2015;15(8):947‐959. 10.1586/14737175.2015.1065180. [DOI] [PubMed] [Google Scholar]
- 29. Selvitelli MF, Walker LM, Schomer DL, Chang BS. The relationship of interictal epileptiform discharges to clinical epilepsy severity: a study of routine electroencephalograms and review of the literature. J Clin Neurophysiol. 2010;27(2):87‐92. 10.1097/WNP.0b013e3181d64b1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wielaender F, James FMK, Cortez MA, et al. Absence seizures as a feature of juvenile myoclonic epilepsy in Rhodesian ridgeback dogs. J Vet Intern Med. 2018;32(1):428‐432. 10.1111/jvim.14892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jeserevics J, Viitmaa R, Cizinauskas S, et al. Electroencephalography findings in healthy and Finnish Spitz dogs with epilepsy: visual and background quantitative analysis. J Vet Intern Med. 2007;21:1299‐1306. [DOI] [PubMed] [Google Scholar]
- 32. Wrzosek M, Ives JR, Karczewski M, Dziadkowiak E, Gruszka E. The relationship between epileptiform discharges and background activity in the visual analysis of electroencephalographic examinations in dogs with seizures of different etiologies. Vet J. 2017;222:41‐51. 10.1016/j.tvjl.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 33. Mizrahi EM. Avoiding the pitfalls of EEG interpretation in childhood epilepsy. Epilepsia. 1996;37(s1):S41‐S51. 10.1111/j.1528-1157.1996.tb06021.x. [DOI] [PubMed] [Google Scholar]
- 34. Geut I, Weenink S, Knottnerus ILH, van Putten MJAM. Detecting interictal discharges in first seizure patients: ambulatory EEG or EEG after sleep deprivation? Seizure. 2017;51:52‐54. 10.1016/j.seizure.2017.07.019. [DOI] [PubMed] [Google Scholar]
- 35. Brauer C, Kästner SBR, Schenk HC, Tünsmeyer J, Tipold A. Electroencephalographic recordings in dogs: prevention of muscle artifacts and evaluation of two activation techniques in healthy individuals. Res Vet Sci. 2011;90(2):306‐311. 10.1016/j.rvsc.2010.06.004. [DOI] [PubMed] [Google Scholar]


