Abstract
Background
Neutrophil extracellular traps (NETs), webs of DNA and citrullinated histones extruded from activated neutrophils cause transfusion‐related acute lung injury. Supernatants of stored red blood cell (RBC) units might promote NETosis in neutrophils from the units or from transfusion recipients.
Hypotheses
(1) NETs form during storage of canine RBC, (2) leukoreduction (LR) before storage of RBC reduces NETosis, and (3) supernatant from stored, nonleukoreduced (NLR) RBC units induces NETosis in healthy canine neutrophils modeling transfusion recipients.
Animals
Six healthy purpose‐bred research dogs were utilized for blood donation.
Methods
Prospective controlled study. RBC units were collected from each dog, aseptically divided into 2 equal subunits, 1 of which was leukoreduced, and stored for 42 days. Stored units were sampled biweekly for quantification of NET markers citrullinated histone H3 (Western blot) and cell‐free DNA (cfDNA) (DNA dye binding). Unit supernatants were applied ex vivo to canine neutrophils and extracellular DNA release representing NETosis was assessed.
Results
Markers of NETs increased during RBC storage (cfDNA P < .0001 and citrullinated H3 P = .0002) and were higher in NLR than LR units (day 42 LR cfDNA 0.34 ± 0.82 ng/mL vs day 42 NLR 1361.07 ± 741.00 ng/mL, P < .0001; day 42 LR citrullinated H3 0.19 ± 0.13 AU vs NLR 0.57 ± 0.34 AU, P = .007). Isolated neutrophils did not form NETs when exposed to stored canine RBC supernatant.
Conclusions and Clinical Importance
NETosis occurs in stored canine NLR RBC units, and is attenuated by LR before storage. NETs might be mediators of transfusion reactions.
Keywords: cfDNA, histone, leukoreduction, transfusion reaction
Abbreviations
- cfDNA
cell‐free DNA
- IMHA
immune‐mediated hemolytic anemia
- LR
leukoreduced
- NETs
neutrophil extracellular traps
- NLR
nonleukoreduced
- RBCs
red blood cells
- TRALI
transfusion‐related acute lung injury
1. INTRODUCTION
Transfusion of blood products can be a life‐saving treatment in many companion animal diseases. However, life‐threatening transfusion reactions can occur. Incidence rates of overt transfusion reactions in dogs are up to 28%, and this is likely an underestimation. 1 , 2 Among the most common adverse events that occur with compatible blood transfusions are febrile nonhemolytic reactions and, at least in people, transfusion‐related acute lung injury (TRALI). 3 Transfusion of stored blood can induce inflammation, which is undesirable in dogs that are already in a proinflammatory state as a result of their underlying disease processes. Prestorage leukoreduction (LR) helps eliminate post‐transfusion inflammation in dogs. 4 Indeed, LR of RBC units is standard of care in human medicine and is mandated in much of Europe as numerous studies have documented that the risk of acute transfusion reaction is reduced by prestorage LR of blood units. 5 , 6 , 7 LR is not standardly employed in veterinary blood banks.
Neutrophil extracellular traps (NETs) might be key players in mediating nonleukoreduced (NLR) blood induced transfusion reactions. NETs are webs of DNA, nucleosomes, histones, and granular proteins extruded from activated neutrophils for antimicrobial purposes, but they can also be proinflammatory and prothrombotic. 8 , 9 , 10 , 11 If NETs were transfused into dogs, they could cause thrombosis and inflammation in the recipient. NETs have been implicated in the pathogenesis of TRALI in murine models, NET biomarkers are elevated in human patients with TRALI, and inhibiting NETosis results in lung protection in experimental TRALI. 12 , 13 NETs can form in stored human RBC units and LR of units before storage attenuates their formation. 14 NETs can form in stored blood via several mechanisms including RBC release of neutrophil‐activating proinflammatory lipid mediators and heme, a NET inducer. 15 , 16 Given the postulated role of NETs in the pathogenesis of TRALI and their potential to cause other damage in transfusion recipients, documenting the generation of NETs in stored canine RBC and NETosis attenuation by LR would support the need for LR to become standard in veterinary medicine.
The first objective of the present study was to determine if NETs form during the 42 day storage period of canine RBC units and if LR prevents this process. We analyzed stored RBC units for the presence of cell‐free DNA (cfDNA), a nonspecific NET marker, and citrullinated histones, a specific NET marker as NETosis requires histone citrullination. 17 Secondly, we aimed to investigate if application of supernatant from stored canine RBC could induce NETosis in isolated healthy dog neutrophils, modeling recipient neutrophils. We hypothesized that stored canine RBC units would develop NETs as well as the ability to induce NETosis over 42 day storage conditions and that these processes would be attenuated by prestorage LR.
2. MATERIALS AND METHODS
2.1. Animals
Six purpose bred research dogs were included if they met the criteria of weighing 17 kg or greater and had no underlying disease as determined by a complete physical exam and screening bloodwork (complete blood count and serum chemistry panel). The study population consisted of 4 intact male dogs and 2 intact female dogs with body weights ranging from 17 to 25.6 kg. This study was approved by the local Institutional Animal Care and Use Committee and dogs were cared for as outlined in the NIH Guide for the Care and Use of Laboratory Animals.
2.2. Blood collection and leukoreduction
Research dogs were sedated with dexmedetomidine (2‐10 μg/kg) (Dexdomitor, Zoetis, Parsippany, New Jersey) and butorphanol (0.05‐0.2 mg/kg) (Torbugesic, Zoetis, Parsippany, New Jersey) IM. Four hundred and fifty milliliters of whole blood were collected via sterile atraumatic jugular venipuncture from all 6 dogs into standard triple collection systems containing citrate‐phosphate‐dextrose‐adenine for anticoagulation (Teruflex Blood Bag System, Terumo Corporation, Tokyo, Japan). The sedation was reversed with 0.02 to 0.1 mg/kg atipamazole (Anti‐sedan, Zoetis, Parsippany, New Jersey) IM. Whole blood units were centrifuged at 2493.1 g at 4°C for 32 minutes. Plasma was extracted. Optisol preservative was added to the RBCs and mixed. Using the Optisol bag and the RBC bag and while maintaining a closed system, the RBCs were then separated into 2 equal half‐units for storage and allowed to cool for 4 hours at 4°C. One of these half‐units was randomly selected to undergo LR using a commercial LR filter (BPF Leukocyte Reduction Filtration System, Haemonetics Manufacturing, Inc., Covina, California). This was defined as day 0. An aliquot (4 mL) was obtained pre and post filtration for evaluation of cell counts. LR was confirmed by leukocyte count using 2 methods: complete blood counts using an automated hematology analyzer (ADVIA 2120i Hematology System, Siemens Medical Solutions USA Inc, Malvern, Pennsylvania) and use of a commercial propidium iodide‐based leukocyte labeling kit (BD Leucocount, BD Biosciences, San Jose, California), according to the manufacturer's instructions, followed by flow cytometric analysis (BD FACSCanto flow cytometer, BD Biosciences, San Jose, California). This assay has been previously validated in canine RBC units. 18 Leukocyte counts from NLR units were reported from the automated hematology analyzer whereas leukocyte counts from the LR units were reported from the flow cytometric method, as these counts were below the limit of detection of the automated hematology analyzer. 19 Successful LR was defined per human blood banking standards as less than 5 × 106 white cells per unit. 20
As previously described, EDTA blood was collected at a later time point from each research dog for neutrophil isolation in order to test whether RBC unit supernatant induces NETosis in isolated canine neutrophils. 21 , 22
2.3. Sample preparation
LR and NLR RBC units were stored at 4°C for 42 days and sampled (15 mL) on days 0, 14, 28 and 42. Samples were collected using sterile sampling ports (HEMO‐TAP Blood Bag Spike, Utah Medical Products, Inc, Midvale, Utah) under a sterile laminar flow hood and centrifuged at 2500g for 20 minutes at 4°C without deceleration to generate cell‐free supernatants; the supernatants were removed and these were then centrifuged a second time at 10000g for 5 minutes at room temperature with deceleration to rid samples of any cellular debris. Supernatants were stored at −80°C for a maximum of 175 days before batched analysis of NET markers and ability of unit supernatants to induce NETosis in isolated canine neutrophils. On day 42, the final day of sampling, 1 mL of blood from each unit was cultured aerobically and anaerobically to ensure lack of bacterial contamination of units during their sampling and storage.
2.4. Sample analysis for free hemoglobin
Hemolysis can be generated during filtration of red cells and could be a confounding factor in our analysis. Therefore, hemolysis of the units at each time point was quantified by means of a Drabkin's assay according to the manufacturer's instructions. Briefly, samples were incubated with Drabkin's reagent (Ricca Chemical Company, Arlington, Texas) for 30 minutes in the dark to convert hemoglobin to cyanmethemoglobin. Absorbance at 540 nm was measured on a Versamax microplate reader (Molecular Devices, Sunnyvale, California). A540 was converted to concentration using a standard curve of bovine hemoglobin (Sigma Aldrich, St. Louis, Missouri).
2.5. Sample analysis for NET markers
Two assays were performed on sample supernatants from each LR and NLR unit at each time point to assess for evidence of NET formation during RBC storage, quantification of cell‐free DNA and Western blot analysis for citrullinated histone H3.
2.5.1. Quantification of cell‐free DNA (cfDNA)
Using previously described methods for sensitive detection of DNA, DNA from supernatants was first purified using a commercial DNA purification kit (QIAamp DNA Mini and Blood Mini kit, Qiagen, Valencia, California). 23 Purified DNA was then diluted, mixed with PicoGreen dye (Quant‐iT PicoGreen dye, Molecular Probes, Inc., Eugene, Oregon) to label DNA according to manufacturer's instructions, and fluorescence was detected with a microplate spectrofluorometer (Synergy HT, BioTek Instruments, Winooski, Vermont). DNA concentrations in samples were calculated based on a standard curve generated by fluorometric analysis of solutions containing known DNA concentrations (Quant‐iT PicoGreen kit, Molecular Probes, Inc., Eugene, Oregon).
2.5.2. Western blot analysis for citrullinated histone H3
Citrullinated histones in sample supernatants were measured by Western blot as a specific indicator of NETosis as previously described. 24 Briefly, sample supernatants were mixed with Laemmli buffer with DTT and then subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis. Immunoblotting was performed with an antihistone H3, citrulline R2 + R8 + R17 (Abcam, Cambridge, Massachusetts) followed by a horseradish peroxidase conjugated secondary antibody (Pierce, Rockford, Illinois). Chemiluminescence detection was performed by using Pierce ECL‐2 substrate (Thermo Fisher, Waltham, Massachusetts). Films were developed using a standard photographic procedure and quantitative analysis of detected bands was carried out by densitometer scanning using GelDoc XR+ and ImageLab software (BioRad Laboratories, Hercules, California). Citrullinated histone H3 purified from septic canine abdominal fluid was used as a standard for densitometry. Septic abdominal fluid was selected for this purpose since NETosis and circulating citrullinated H3 have been well‐documented in both naturally occurring sepsis and animal models of sepsis, and NETs and citrullinated H3 have been previously documented in canine septic abdominal fluid. 25 , 26 Specificity of the commercial anticitrullinated histone H3 antibody was confirmed via nano liquid chromatography with tandem mass spectrometry as described in Supporting file 1.
2.6. Assessment of RBC supernatant ability to induce NETosis in recipient neutrophils
As a model of ability of blood product to induce NETosis in recipient neutrophils, sample supernatants from day 0 and day 42 were applied ex vivo to isolated canine neutrophils. Neutrophils were isolated from EDTA blood of 6 healthy research dogs as previously described. 21 , 22 Briefly, erythrocytes were removed by dextran sedimentation before separation of granulocytes by a Histopaque 1077 gradient (Sigma Aldrich, St. Louis, Missouri). Remaining erythrocytes were removed by brief osmotic lysis before neutrophils were washed in PBS, counted and resuspended in PBS after filtration through a 70 μm sterile filter. For a final concentration of 250 000 cells/mL per well, 5 × 104 cells/well, counted using a hemocytometer, were added to a sterile, tissue culture treated 96 well plate (Microplate, Corning, Tewksbury, Massachusetts) containing Roswell Park Memorial Institute medium (HyClone, Logan, Utah) with 0.5% fetal calf serum (Gibco, Gaithersburg, Maryland) to a final well volume of 200 μL.
For 6 dogs, 20 μL of supernatant collected from LR and NLR blood at day 0 and day 42 were added to the test wells containing isolated neutrophils described above (10% dilution), with each condition set up in triplicate. For 1 dog, 5% and 25% dilutions were also prepared. As a positive control, cells from each dog were also stimulated with the known NET agonist, phorbol 12‐myristate 13‐acetate (PMA; final concentration 7 μM) (Sigma Aldrich, St. Louis, Missouri) and as a negative control, nonstimulated wells in which stimulants were replaced by an equal volume of PBS were also set‐up. To determine if citrate from the anticoagulant in blood units could be reducing cell viability, supernatants collected from a blood bag filled with PBS were also added to neutrophils (10% dilution). To control for DNA or interfering substances in supernatants, blank wells were prepared by adding supernatants to acellular wells.
Cells were incubated for 3 hours at 39°C before addition of the nucleic acid dye, Sytox Green (Molecular Probes, Inc., Eugene, Oregon) to a final concentration of 6 μM. After 15 minutes incubation at 39°C, fluorescence (ex 504 nm/em 523 nm) was measured using a microplate reader (Synergy HT, BioTek Instruments, Winooski, Vermont). Viable cells are impermeable to Sytox green, so fluorescence reflects extracellular DNA and dead or dying cells. Values were blanked by subtracting the fluorescence of acellular wells containing the same stimulant, and a fold change in Sytox green fluorescence calculated by dividing the average fluorescence of test wells by the average fluorescence of nonstimulated neutrophils from the same dog.
2.7. Data analysis and sample size calculation
A sample size calculation was performed using data from the literature comparing nucleosome, a NET marker, formation in human LR and NLR RBC units; this showed that in order to obtain an 80% power with a type I error rate of 5%, 6 LR and 6 NLR units were needed. 14 Response variables were analyzed using repeated measures analysis of variance. Treatment, time and their interaction were fixed effects, whereas animal was the subject of repeated measures. DNA data were log transformed before analyses. When significance was discovered, individual comparisons between time points were made by Tukey's t‐test with adjustments for multiple comparisons [Statistical Analysis System (SAS) 9.3, SAS Institute, Inc, Cary, North Carolina]. A P‐value of <.05 was considered significant. The ability of unit supernatants from all time points to induce NETosis in healthy dog neutrophils was similarly analyzed.
Correlations between test parameters (hemoglobin, cfDNA and citrullinated histones) were assessed by using the Spearman correlation.
Cell counts of RBC units pre and post filtration failed D'Agostino & Pearson omnibus normality testing; cell counts pre and post filtration were thus compared with Mann‐Whitney U test and are reported as median (range) (Prism 6.0, GraphPad Software, Inc, La Jolla, California).
3. RESULTS
3.1. Efficacy of LR filter
Leukoreduction was greater than 99% effective at removing leukocytes and platelets in every case. In NLR blood units, the median leukocyte count was 12.23 × 103/μL (range: 9.62‐17.24 × 103/μL) whereas for LR blood units the median residual leukocyte count was 0.09 cells/μL (range: 0.0‐0.23 cells/μL) (P = .002). In NLR blood units, the median platelet count was 273 × 103/μL (range: 185‐338 × 103/μL) whereas for LR blood units the median residual platelet count was 2.5 × 103/μL (range: 1.0‐4.0 × 103/μL) (P = .002). The median volume of blood that was lost by the process of filtration was 33.5 mL (range 30‐35 mL), an average volume loss of 20.3% among the LR units (data not shown). Although hemolysis occurred in stored units over time (hemoglobin increased over time in both groups, P < .0001), there was no difference in hemoglobin present in supernatants in LR compared to NLR units (P = .20) (Figure 1). Blood units all cultured negative for bacterial contamination at the conclusion of the study.
FIGURE 1.
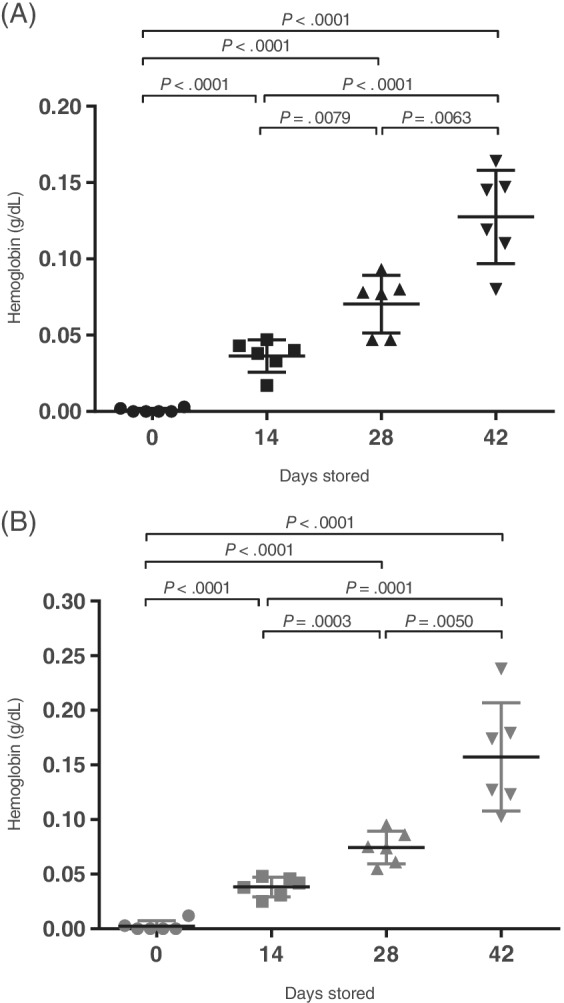
Packed red cell unit supernatant hemoglobin levels. Hemoglobin in supernatants collected throughout the 42 day storage period of nonleukoreduced (NLR, A) and leukoreduced (LR, B) canine packed red blood cell units (n = 6). For pairwise comparisons between timepoints within a given unit type that achieved significance, P values are reported with associated lines indicating which time points were compared. If no line and value are reported, the comparison did not achieve significance. *P values are for comparisons between NLR and LR groups at each time point. Lines represent mean values with error bars representing SD
3.2. Release of NET markers with storage
We evaluated whether neutrophils release extracellular DNA during accepted RBC storage times of 42 days. There was significantly more cfDNA (Figure 2) present in the supernatants of NLR samples at days 14 (P < .0001), 28 (P = .0014), and 42 (P < .0001) compared to day 0 with approximately 1000‐fold more cfDNA in the day 42 NLR units than in the baseline samples. There was no significant increase in cfDNA over time in the LR group. LR units contained only 0.34 ng/mL (± 0.82 ng/mL) DNA after 42 days compared to 1361.07 ± 741.00 ng/mL (mean ± SD) in the NLR units at 42 days (P < .0001). Similarly, there was significantly more citrullinated histone H3 (Figure 3) measured in supernatants of NLR samples at days 28 (P = .002) and 42 (P = .003) compared to day 0. Although Histone H3 increased in the LR group from day 0 to day 28 (P = .002), from day 14 to 28 (P = .01), and from day 0 to day 42 (P = .02), citrullinated histone H3 was approximately 3‐fold higher in NLR compared to LR samples on days 28 (P = .006) and 42 (P = .007). The results of a representative Western blot for citrullinated histone H3 are depicted in Figure 4.
FIGURE 2.
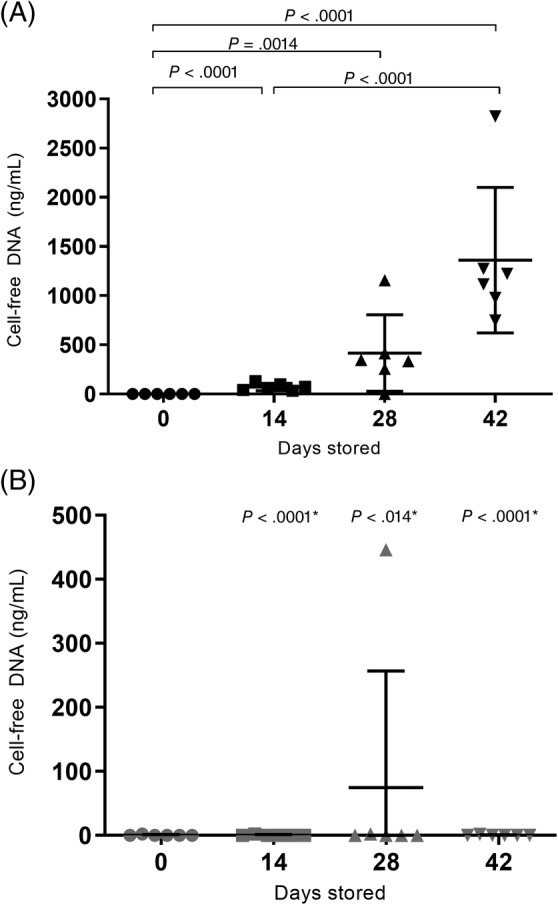
Packed red cell unit supernatant cell‐free DNA. Cell‐free DNA (cfDNA) in supernatants collected throughout the 42 day storage period of nonleukoreduced (NLR, A) and leukoreduced (LR, B) canine packed red blood cell units (n = 6). For pairwise comparisons between timepoints within a given unit type that achieved significance, p values are reported with associated lines indicating which time points were compared. If no line and value are reported, the comparison did not achieve significance. *P values are for comparisons between NLR and LR groups at each time point. Lines represent mean values with error bars representing SD
FIGURE 3.
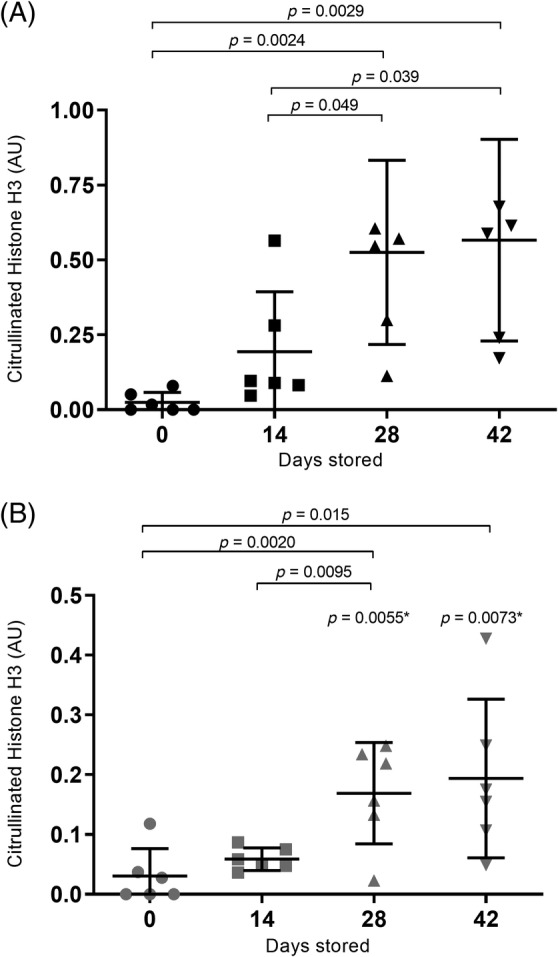
Packed red cell unit supernatant citrullinated histone H3. Citrullinated histone H3 measured in supernatants collected throughout the 42 day storage period of nonleukoreduced (NLR, A) and leukoreduced (LR, B) canine packed red blood cell units (n = 6). For pairwise comparisons between timepoints within a given unit type that achieved significance, P values are reported with associated lines indicating which time points were compared. If no line and value are reported, the comparison did not achieve significance. *P values are for comparisons between NLR and LR groups at each time point. Units are arbitrary absorption units. Lines represent mean values with error bars representing SD. A given dog's LR and NLR unit samples from all timepoints were analyzed on 1 gel/western blot. All gels/blots were performed in parallel and the same positive control was run on each gel against which all other samples were normalized
FIGURE 4.
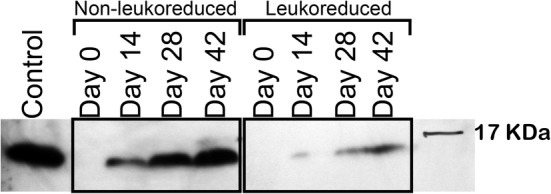
Citrullinated H3 in supernatants of stored canine packed red blood cell units. Representative Western blot analysis of citrullinated histone H3 in 1 pair of canine nonleukoreduced (NLR) and leukoreduced (LR) packed red blood cell units from the same dog over the 42 day storage period. The 17.4 kDa protein ladder band is shown. The labeled positive control is citrullinated histone H3 purified from canine septic abdominal fluid. The entire width of the blot is included, but the area of the gel above the 21 kDa molecular marker has been cropped out of the image for clarity
Using the Spearman correlation, cfDNA and citrullinated histone H3 results were significantly correlated (r = 0.58; P < .0001), suggesting that both assays measure NETs. There was a weak but significant correlation between hemoglobin and cfDNA (r = 0.32; P = .02) and strong correlation between hemoglobin and citrullinated histone H3 (r = 0.73; P < .0001).
3.3. Ability of unit supernatants to induce NETosis
Capacity of RBC unit supernatant to induce NETosis was assessed by applying supernatant to isolated canine neutrophils and quantifying DNA release via Sytox Green fluorescence. One dog was excluded from analysis because of interference from the unit supernatant resulting in Sytox Green fluorescence in the acellular controls. There was an effect of time on supernatant induced DNA release (P = .01), but this effect was weak [1.9‐fold increase, which is within assay variation (data not shown)] and was not prevented by LR (P = .39) (Figure 5). Confirming that neutrophils performed as expected in these experiments, those stimulated with PMA resulted in a median fold increase compared to unstimulated neutrophils of 21.9 (14.4‐39.1).
FIGURE 5.
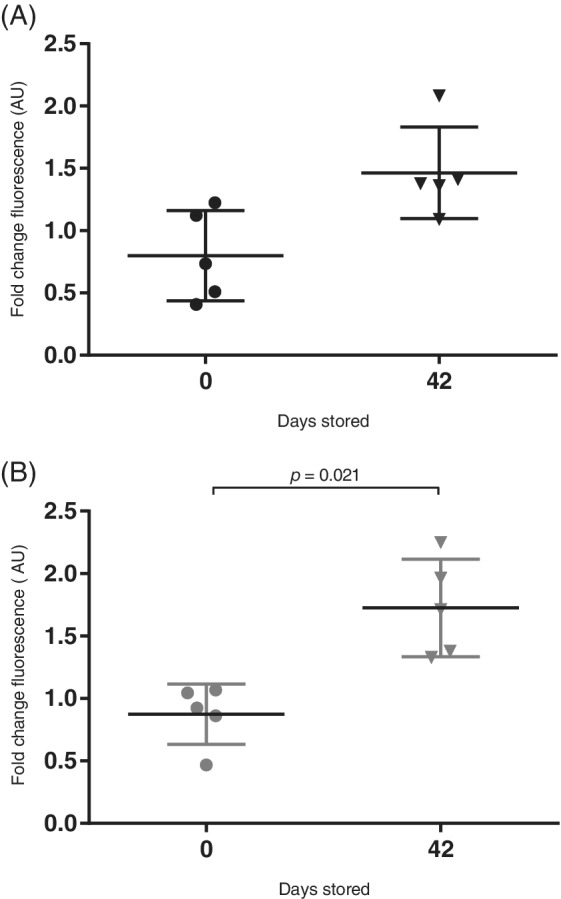
DNA release from healthy canine neutrophils evaluated by fluorescence. Neutrophils isolated from healthy dog blood were incubated with and without supernatants from nonleukoreduced (NLR, A) and leukoreduced (LR, B) stored packed red blood cell units and Sytox green added to detect extracellular DNA. Data is presented as fold change in fluorescence compared to unstimulated neutrophils (mean ± SD). Supernatants from 5 NLR and LR units were assessed for ability to induce DNA release from isolated canine neutrophils. Assays were performed in triplicate
4. DISCUSSION
The results of this study provide evidence that markers of NETs, both cfDNA and citrullinated histones, increase over time in stored NLR canine RBC units. Combined, these data suggest that NETosis occurs in stored canine RBC units, and that this process is attenuated by LR before storage. We also found that stored RBC supernatant does not induce NETosis in isolated neutrophils suggesting that stored blood does not induce NETosis in the recipient's own neutrophils. Since NETs can be prothrombotic and injurious to tissue, our documentation of NET formation in stored blood units and the ability to abrogate the process with LR provides support for advising leukoreduction in veterinary blood banking.
NETosis is a process by which activated neutrophils liberate DNA decorated with granular proteins in order to trap bacteria. However, exposed DNA and granular proteins can be damaging to tissues and can be prothrombotic. 9 , 10 , 11 , 12 , 27 NETs are also important in the pathogenesis of several inflammatory diseases. 12 , 27 Transfusion of NET material is unlikely to be beneficial to hospitalized dogs, especially given that dogs requiring transfusions are likely to have systemic inflammation because of their underlying disease. 4
NETosis requires neutrophil stimulation. Several mediators of NETosis accumulate in stored RBC that could induce NETosis. We documented that storage of both LR and NLR units resulted in significant accumulation of free hemoglobin over time and heme‐related molecules are known to be strong inducers of NETs. 16 Indeed, in this study hemoglobin and cfDNA and hemoglobin and citrullinated histone H3 were correlated. Though hemoglobin increased in LR units as well as NLR units, LR would not have had sufficient remaining neutrophils to allow for significant NETosis. NET induction by interleukin‐8 (IL‐8) is another plausible mechanism of NET generation in stored NLR blood. IL‐8 increases in NLR canine pRBC units during a 35 day storage period, but does not increase in LR units. 28 , 29 IL‐8 is a well‐established NETosis inducer. 8 Additionally, activated platelets can induce NETosis 30 and NETs can activate platelets, 27 thus the effect of LR we observed could be attributed to both the filter's platelet and leukocyte reducing effects.
TRALI is a severe form of acute respiratory distress syndrome that is the leading cause of transfusion‐related deaths in people according to the Food and Drug Administration's surveillance data. 31 Increases in circulating NET biomarkers in TRALI human patients' blood and presence of NETs in the alveoli of mice and people with TRALI suggest there could be a key role for NETs in TRALI pathogenesis. 12 , 13 Although the incidence of TRALI in veterinary medicine is likely much lower than it is in human medicine 32 or might not occur at all, 1 the proinflammatory nature of NETs makes them likely players in other transfusion reactions as well. 9 , 10 , 11 Transfusion of NLR RBC to healthy dogs induces significant inflammation which is eliminated by LR of the RBC units before storage. 4 In human blood banking, it is well established that LR reduces the incidence of both transfusion reactions, especially febrile nonhemolytic reactions, and inflammatory responses 33 , 34 , 35 , 36 and this has led LR to become standard practice in much of Europe and Canada. 37 Based on our study results, reduction of NETosis in stored blood units could contribute to the beneficial effects of LR. This warrants further study of NET markers in dogs before and after transfusion with LR and NLR blood.
Our results are especially intriguing in light of recent transfusion and NETosis data in canine immune‐mediated hemolytic anemia (IMHA). Markers of NETs are increased in dogs with IMHA and cfDNA might predict outcome in dogs with IMHA. 24 , 38 Given that NETs are prothrombotic, 39 , 40 and that thrombosis is the leading cause of morbidity and mortality in IMHA, 41 , 42 NETosis could contribute to the development of IMHA thrombosis. Transfusion of aged pRBCs increased death risk in dogs with hemolysis, most of which were dogs with IMHA. 43 In light of our current study's documentation that NET material accumulates in aged NLR blood, we can speculate that NETosis might have occurred in the aged stored units, leading to infusion of NET material into recipients, contributing to their prothrombotic state and increased mortality.
We were unable to clearly document that stored blood supernatants can induce NETosis in canine neutrophils. Although we identified a statistically significant increase in the fold change of Sytox Green fluorescence after neutrophil incubation with day 42 supernatants (suggesting DNA release), the effect was weak and, in our experience, within the variation of this assay. Thus while NETosis appears to occur in NLR stored blood units, supernatants of stored blood, at least in our ex vivo system, did not definitively induce NETosis in freshly isolated neutrophils but might have with longer incubation time.
Though not a study objective, we learned during the study that the LR filters employed (BPF Leukocyte Reduction Filtration System, Haemonetics Manufacturing, Inc., Covina, CA), though efficacious in removing white blood cells from canine packed RBC units, caused marked hemolysis if RBCs were not allowed to cool before filtration. While the optimal postcollection cool time was not investigated here, we found 4 hours at 4°C to be an adequate time to reduce hemolysis in our supernatant samples. This observation is interesting because the manufacturer of the LR filter states that human blood can be filtered without hemolysis immediately after donation. We speculate that cooling increased blood viscosity, slowing filtration and decreasing shear force on the canine RBCs. 44 There might be fundamental differences in the blood cells of canines that make them more susceptible to lysis after blood donation and immediate leukoreduction. When using a different LR filter with canine blood, cooling the blood for several hours before leukoreduction improved filtration efficacy. 45 However, most studies utilizing newer leukoreduction filters do not allow the blood to cool before filtration. Whether or not prefiltration cooling enhances leukoreduction efficacy or reduces prestorage hemolysis of red cells was not investigated in this study but further investigation is warranted to ensure the best adaptation possible of these human products to canine blood.
We recognize that our study has limitations. The cfDNA assay is quantitative and demonstrated both significant accumulation of cfDNA with RBC storage and attenuation of this process by LR. However, cfDNA can accumulate with any form of cell death including necrosis and apoptosis, 46 , 47 and is not specific for NETosis. We employed the citrullinated histone H3 assay to provide mechanistic information regarding the source of the cfDNA. The citrullinated H3 western blots were limited in that we did not have a gel loading control since we could not be certain another protein like albumin or immunoglobulin would be stable in the pRBC units over the 42 day 4°C storage time. For this reason, although citrullinated histone H3 is specific for NETosis, the data obtained from this assay is only semiquantitative. However, the correlation between our cfDNA levels and our citrullinated histone H3 data suggests that the cfDNA source is NETs. Furthermore, cfDNA, regardless of its origin, has inflammatory and prothrombotic potential and thus transfusing blood containing cfDNA from any source could be detrimental. 48 Nevertheless, a specific and quantitative NET assay such as one that detects granular protein‐DNA complexes would be desirable. Though such an assay exists in human medicine, no such assay exists for dogs and in our experience the human assays do not work with canine samples (data not shown). 13
A second study limitation was a possible sample labeling error between 2 samples. We suspect, based on individual dog data, that 1 dog's day 28 LR sample was mislabeled as another dog's day 28 NLR sample. The tube labeled LR showed an unexpected increase in cfDNA on day 28 (446.1 ng/mL) that was not seen in the day 14 (0 ng/mL) or the day 42 (0 ng/mL) time point from this dog. This likely increased the variance in our data. Despite this suspected error, there were still significantly more cfDNA and citrullinated histone H3 in NLR compared to LR units at that time point and across all time points.
In our assessment of the ability of RBC unit supernatants to induce NETosis in healthy canine neutrophils, we did not assess neutrophil viability before incubation with supernatant. However, our method of isolating neutrophils consistently yields 96% to 98% viable cells. Although not assessing isolated neutrophil viability is a shortcoming of this study that we acknowledge, given that our positive (PMA stimulated) and negative (unstimulated) cells performed as expected, we do not believe any neutrophil viability issues impacted results.
In summary, we demonstrated that cfDNA and citrullinated histone H3, markers of NETs, significantly increase over the standard storage life of NLR packed red blood cell units; leukoreduction significantly reduced this effect. This provides further evidence of the inflammatory and prothrombotic potential of stored red cells and the potential beneficial effects of leukoreduction. To our knowledge, no studies investigating the benefit of leukoreduced blood products to clinical veterinary patients have been published. Nevertheless, we believe that this work suggests that LR of blood products has potential to reduce transfusion‐induced inflammation, particularly in products stored 14 days or longer and when transfusing dogs that already have a proinflammatory and prothrombotic disease like IMHA. If LR can reduce infusion of NETs into recipients and thereby reduce recipient inflammation and thrombotic risk, it could improve overall transfusion safety in veterinary medicine.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
This study was performed with permission from the Iowa State University IACUC (protocol 8‐15‐8079‐K).
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Supplementary File 1 Supporting Information
ACKNOWLEDGMENTS
The work was supported by an Iowa State University Veterinary Clinical Sciences Research Incentive Grant. The work was presented at the 2017 ACVIM Forum, National Harbor, MD, in Abstract form. We acknowledge Haemonetics for donation of the leukoreduction filters used in this study, the ISU Office of Biotechnology Flow Cytometry Facility for their assistance in quantifying white blood cells, Dr Matthew Ellinwood and Ms Jackie Jens for providing the dogs used in this study, and Dr Unity Jeffery for her scientific guidance. We also thank Dr Anne Hale for her guidance and expertise in blood banking. BD Leucount Kits were generously provided at a discount in order to perform research validating their use in dogs.
McQuinn ER, Smith SA, Viall AK, Wang C, LeVine DN. Neutrophil extracellular traps in stored canine red blood cell units. J Vet Intern Med. 2020;34:1894–1902. 10.1111/jvim.15876
Funding information Iowa State University Veterinary Clinical Sciences Research Incentive Grant
REFERENCES
- 1. Holowaychuk MK, Leader JL, Monteith G. Risk factors for transfusion‐associated complications and nonsurvival in dogs receiving packed red blood cell transfusions: 211 cases (2008‐2011). J Am Vet Med Assoc. 2014;244:431‐437. [DOI] [PubMed] [Google Scholar]
- 2. Kerl ME, Hohenhaus AE. Packed red blood cell transfusions in dogs: 131 cases (1989). J Am Vet Med Assoc. 1993;202:1495‐1499. [PubMed] [Google Scholar]
- 3. Eder AF, Chambers LA. Noninfectious complications of blood transfusion. Arch Pathol Lab Med. 2007;131:708‐718. [DOI] [PubMed] [Google Scholar]
- 4. McMichael MA, Smith SA, Galligan A, et al. Effect of leukoreduction on transfusion‐induced inflammation in dogs. J Vet Intern Med. 2010;24:1131‐1137. [DOI] [PubMed] [Google Scholar]
- 5. van der Meer PF, Gratama JW, van Delden CJ, et al. Comparison of five platforms for enumeration of residual leucocytes in leucoreduced blood components. Br J Haematol. 2001;115:953‐962. [DOI] [PubMed] [Google Scholar]
- 6. Dzik WH, Anderson JK, O'Neill EM, et al. A prospective, randomized clinical trial of universal WBC reduction. Transfusion. 2002;42:1114‐1122. [DOI] [PubMed] [Google Scholar]
- 7. Rajesh K, Harsh S, Amarjit K. Effects of prestorage leukoreduction on the rate of febrile nonhemolytic transfusion reactions to red blood cells in a tertiary care hospital. Ann Med Health Sci Res. 2015;5:185‐188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532‐1535. [DOI] [PubMed] [Google Scholar]
- 9. Martinod K, Demers M, Fuchs TA, et al. Neutrophil histone modification by peptidylarginine deiminase 4 is critical for deep vein thrombosis in mice. Proc Natl Acad Sci U S A. 2013;110:8674‐8679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Geiger S, Holdenrieder S, Stieber P, et al. Nucleosomes as a new prognostic marker in early cerebral stroke. J Neurol. 2007;254:617‐623. [DOI] [PubMed] [Google Scholar]
- 11. Kessenbrock K, Krumbholz M, Schonermarck U, et al. Netting neutrophils in autoimmune small‐vessel vasculitis. Nat Med. 2009;15:623‐625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thomas GM, Carbo C, Curtis BR, et al. Extracellular DNA traps are associated with the pathogenesis of TRALI in humans and mice. Blood. 2012;119:6335‐6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Caudrillier A, Kessenbrock K, Gilliss BM, et al. Platelets induce neutrophil extracellular traps in transfusion‐related acute lung injury. J Clin Invest. 2012;122:2661‐2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fuchs TA, Alvarez JJ, Martinod K, Bhandari AA, Kaufman RM, Wagner DD. Neutrophils release extracellular DNA traps during storage of red blood cell units. Transfusion. 2013;53:3210‐3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Silliman CC, Moore EE, Kelher MR, Khan SY, Gellar L, Elzi DJ. Identification of lipids that accumulate during the routine storage of prestorage leukoreduced red blood cells and cause acute lung injury. Transfusion. 2011;51:2549‐2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kono M, Saigo K, Takagi Y, et al. Heme‐related molecules induce rapid production of neutrophil extracellular traps. Transfusion. 2014;54:2811‐2819. [DOI] [PubMed] [Google Scholar]
- 17. Leshner M, Wang S, Lewis C, et al. PAD4 mediated histone hypercitrullination induces heterochromatin decondensation and chromatin unfolding to form neutrophil extracellular trap‐like structures. Front Immunol. 2012;3:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Viall AK, LeVine DN. Performance of a Nageotte hemocytometer method and a flow cytometric assay for residual leukocyte quantification in leukoreduced canine packed red blood cells. J Vet Emerg Crit Care. 2020;30:272‐278. [DOI] [PubMed] [Google Scholar]
- 19. Strobel J, Antos U, Zimmermann R, Eckstein R, Zingsem J. Comparison of a new microscopic system for the measurement of residual leucocytes in apheresis platelets with flow cytometry and manual counting. Vox Sang. 2014;107:233‐238. [DOI] [PubMed] [Google Scholar]
- 20. United States Department of Health and Human Services. Guidance for industry . Pre‐Storage Leukocyte Reduction of Whole Blood and Blood Components Intended for Transfusion. Rockville, MD: Food and Drug Administration:2012. [Google Scholar]
- 21. Jeffery U, Kimura K, Gray R, Lueth P, Bellaire B, LeVine D. Dogs cast NETs too: canine neutrophil extracellular traps in health and immune‐mediated hemolytic anemia. Vet Immunol Immunopathol. 2015;168:262‐268. [DOI] [PubMed] [Google Scholar]
- 22. Jeffery U, Gray RD, LeVine DN. A simple fluorescence assay for quantification of canine neutrophil extracellular trap release. J Vis Exp. 2016;54726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen JA, Meister S, Urbonaviciute V, et al. Sensitive detection of plasma/serum DNA in patients with systemic lupus erythematosus. Autoimmunity. 2007;40:307‐310. [DOI] [PubMed] [Google Scholar]
- 24. Lawson C, Smith SA, O'Brien M, et al. Neutrophil extracellular traps in plasma from dogs with immune‐mediated hemolytic anemia. J Vet Intern Med. 2018;32:128‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Camicia G, Pozner R, de Larranaga G. Neutrophil extracellular traps in sepsis. Shock. 2014;42:286‐294. [DOI] [PubMed] [Google Scholar]
- 26. Li RHL, Johnson LR, Kohen C, Tablin F. A novel approach to identifying and quantifying neutrophil extracellular trap formation in septic dogs using immunofluorescence microscopy. BMC Vet Res. 2018;14:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fuchs TA, Brill A, Duerschmied D, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A. 2010;107:15880‐15885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Corsi R, McMichael MA, Smith SA, et al. Cytokine concentration in stored canine erythrocyte concentrates. J Vet Emerg Crit Care (San Antonio). 2014;24:259‐263. [DOI] [PubMed] [Google Scholar]
- 29. Purcell SL, Claus M, Hosgood G, Smart L. Effect of leukoreduction on concentrations of interleukin‐8, interleukin‐1beta, and tumor necrosis factor‐alpha in canine packed red blood cells during storage. Am J Vet Res. 2015;76:969‐974. [DOI] [PubMed] [Google Scholar]
- 30. Clark SR, Ma AC, Tavener SA, et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463‐469. [DOI] [PubMed] [Google Scholar]
- 31. Center for Biologics Evaluation and Research (CBER), Food and Drug Administration (FDA) . Fatalities reported to FDA following blood collection and transfusion. Annual Summary for Fiscal Year 2016; 2016.
- 32. Thomovsky EJ, Bach J. Incidence of acute lung injury in dogs receiving transfusions. J Am Vet Med Assoc. 2014;244:170‐174. [DOI] [PubMed] [Google Scholar]
- 33. Paglino JC, Pomper GJ, Fisch GS, Champion MH, Snyder EL. Reduction of febrile but not allergic reactions to RBCs and platelets after conversion to universal prestorage leukoreduction. Transfusion. 2004;44:16‐24. [DOI] [PubMed] [Google Scholar]
- 34. King KE, Shirey RS, Thoman SK, Bensen‐Kennedy D, Tanz WS, Ness PM. Universal leukoreduction decreases the incidence of febrile nonhemolytic transfusion reactions to RBCs. Transfusion. 2004;44:25‐29. [DOI] [PubMed] [Google Scholar]
- 35. Yazer MH, Podlosky L, Clarke G, Nahirniak SM. The effect of prestorage WBC reduction on the rates of febrile nonhemolytic transfusion reactions to platelet concentrates and RBC. Transfusion. 2004;44:10‐15. [DOI] [PubMed] [Google Scholar]
- 36. Izbicki G, Rudensky B, Na'amad M, Hershko C, Huerta M, Hersch M. Transfusion‐related leukocytosis in critically ill patients. Crit Care Med. 2004;32:439‐442. [DOI] [PubMed] [Google Scholar]
- 37. Bianchi M, Vaglio S, Pupella S, et al. Leucoreduction of blood components: an effective way to increase blood safety? Blood Transfus. 2016;14:214‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jeffery U, Ruterbories L, Hanel R, LeVine DN. Cell‐free DNA and DNase activity in dogs with immune‐mediated hemolytic anemia. J Vet Intern Med. 2017;31:1441‐1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jeffery U, LeVine DN. Canine neutrophil extracellular traps enhance clot formation and delay lysis. Vet Pathol. 2018;55:116‐123. [DOI] [PubMed] [Google Scholar]
- 40. Martinod K, Wagner DD. Thrombosis: tangled up in NETs. Blood. 2014;123:2768‐2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Carr AP, Panciera DL, Kidd L. Prognostic factors for mortality and thromboembolism in canine immune‐mediated hemolytic anemia: a retrospective study of 72 dogs. J Vet Intern Med. 2002;16:504‐509. [DOI] [PubMed] [Google Scholar]
- 42. Klein MK, Dow SW, Rosychuk RA. Pulmonary thromboembolism associated with immune‐mediated hemolytic anemia in dogs: ten cases (1982‐1987). J Am Vet Med Assoc. 1989;195:246‐250. [PubMed] [Google Scholar]
- 43. Hann L, Brown DC, King LG, Callan MB. Effect of duration of packed red blood cell storage on morbidity and mortality in dogs after transfusion: 3,095 cases (2001‐2010). J Vet Intern Med. 2014;28:1830‐1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dzik S. Leukodepletion blood filters: filter design and mechanisms of leukocyte removal. Transfus Med Rev. 1993;7:65‐77. [DOI] [PubMed] [Google Scholar]
- 45. Brownlee L, Wardrop KJ, Sellon RK, Meyers KM. Use of a prestorage leukoreduction filter effectively removes leukocytes from canine whole blood while preserving red blood cell viability. J Vet Intern Med. 2000;14:412‐417. [DOI] [PubMed] [Google Scholar]
- 46. Lichtenstein AV, Melkonyan HS, Tomei LD, Umansky SR. Circulating nucleic acids and apoptosis. Ann N Y Acad Sci. 2001;945:239‐249. [DOI] [PubMed] [Google Scholar]
- 47. Hamaguchi S, Akeda Y, Yamamoto N, et al. Origin of circulating free DNA in sepsis: analysis of the CLP mouse model. Mediators Inflamm. 2015;2015:614518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Borissoff JI, Joosen IA, Versteylen MO, et al. Elevated levels of circulating DNA and chromatin are independently associated with severe coronary atherosclerosis and a prothrombotic state. Arterioscler Thromb Vasc Biol. 2013;33:2032‐2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary File 1 Supporting Information


