Abstract
Background
Cardiac sarcoidosis (CS) is increasingly being recognized in the last two decades. The diagnosis of CS depends on clustering of multiple symptoms, investigations and demonstration of a non-caseating granuloma on histopathology. Serum Angiotensin Converting Enzyme (SACE) level, one of the serological markers, is often elevated in systemic sarcoidosis. However, the yield of SACE level among patients with isolated or predominant CS is unclear. We conducted a retrospective study to assess the prevalence of elevated SACE level among patients with proven CS.
Materials and methods
From our Granulomatous myocarditis (GM) registry, 45 biopsy proven CS patients were enrolled. Inclusion criteria: Clinical diagnosis of CS [HRS definition + Lymph Node biopsy/Endomyocardial biopsy (non-caseating granuloma)]. Exclusion criteria - Other causes of GM like cardiac tuberculosis (TB culture/AFB smear -positive) and patients taking medications affecting SACE level.
Results
Among 143 GM cases, 45 CS were analyzed. Mean age:42 ± 11 years (Range 22–63 years, 19 females). With our laboratory reference of SACE (Normal range: 20–70 U/L), 3 out of 45 (6.7%) patients of CS had elevated SACE. In a comparative analysis we found, Erythrocyte Sedimentation Rate (ESR) and High sensitive-C Reactive Protein (Hs-CRP) are much more sensitive, although not specific for CS. Patients with pulmonary involvement more often had elevated SACE level.
Conclusion
Serum ACE is elevated only in approximately 6.7% of patients with biopsy proven CS. Hence, it is insensitive serological tool for diagnosis of CS even in the active phase of the disease. In contrast, ESR and Hs-CRP emerges to be more sensitive markers of active CS.
Keywords: Serum angiotensin converting enzyme, ACE, SACE, Cardiac sarcoidosis, Acute phase reactants
1. Introduction
Sarcoidosis is a systemic granulomatous disease of unknown etiology with a heterogeneous presentation, clinical course and outcome. Majority of patients have lung and intra-thoracic lymph node involvement. In most cases, sarcoidosis is subacute and self-limiting; however, significant morbidity and mortality may result from pulmonary fibrosis or cardiac or neurological involvement [1,2]. Clinically evident involvement of heart has been noted in at least 2–7% of patients with sarcoidosis but occult involvement is much higher (>20%) [[3], [4], [5]]. The incidence of cardiac sarcoidosis is much higher in Japanese patients (50–78% in necropsy studies) [[6], [7], [8]]. In Japan, cardiac involvement is the leading cause of death due to sarcoidosis, accounting for 77–85% of mortality [9,10].
Although there are no globally accepted guideline/criteria for cardiac sarcoidosis, the recent HRS consensus statement [11] on CS is useful for making a clinical diagnosis of CS. There is a constant search for a serological marker for diagnosis of CS [12]. Use of Serum Angiotensin Converting Enzyme (SACE) as a marker of active sarcoidosis was proposed by Liberman et al. [13]. Subsequent reports advocated it as useful index of disease activity and treatment response [14,15]. Presently, SACE levels are regularly used as one of the diagnostic modalities for extracardiac sarcoidosis. Serum levels of Angiotensin Converting Enzyme (ACE) were found to be increased in approximately 60% of all sarcoid patients [1]. But there is paucity of data regarding its utility in sarcoidosis presenting only with cardiac symptoms like ventricular tachycardia (VT), ventricular dysfunction or conduction system disease. The literature also provides varied results regarding the yields of elevated SCAE in CS. Thus, we conducted a retrospective study to assess the sensitivity and usefulness of this serological marker for making a diagnosis of CS.
2. Materials and methods
This is a retrospective study was conducted in the setting of a tertiary care institute. Data was retrieved from the Granulomatous Myocarditis Registry maintained at our institute with the approval of the Institutional Ethics Committee. We enrolled patients with clinical cardiac sarcoidosis. We looked at the sensitivity of elevated SACE level in cardiac sarcoidosis.
2.1. Inclusion criteria
-
➢
Clinical diagnosis of CS + biopsy showing non-caseating granuloma [Lymph node (LN) biopsy/endomyocardial (EM) biopsy] [according to HRS consensus statement [11]] and having SACE level measurement at diagnosis.
2.2. Exclusion criteria
-
➢
Other causes of granulomatous myocarditis like TB (TB culture positive or AFB smear positive).
-
➢
Patients who already had received prednisolone/steroids (that reduces SACE otherwise)
-
➢
Patients on Angiotensin Converting Enzyme (ACE) inhibitors (which can falsely give lower SACE results)
-
➢
Having other confounding disease which can falsely alter SACE levels:
a. Diseases which increase SACE level- Cirrhosis, Hyperthyroidism.
b. Diseases which decrease SACE level- Hypothyroid, Cystic fibrosis, Emphysema.
Diagnosis of CS was made according to HRS consensus criteria [11]. All had cardiac 18FDG PET-CT and/or cardiac MRI, as a screening for cardiac involvement due sarcoidosis. Subsequently, all of them who had either LN biopsy or EM biopsy suggestive of CS were included in the study. Serum ACE was done in all. Many of them had measured ESR and Hs-CRP levels at the initial presentation and follow up.
2.3. Serum ACE
ACE is a glycoprotein enzyme normally secreted by monocytes, macrophages, and pulmonary endothelial cells into the bloodstream, and is responsible for the physiological conversion of angiotensin I to angiotensin II, which is very important for normal blood pressure control. ACE is also ectopically produced by epithelioid and giant cells in sarcoid granulomas [16,17]. Besides serum, ACE activity has also been assessed in bronchoalveolar lavage (BAL) fluid, cerebrospinal fluid (CSF) and urine of patients with sarcoidosis, with elevation described in the former two biological fluids [[18], [19], [20]]. Insertion/deletion (I/D) polymorphisms of the ACE gene and angiotensin II receptor 1 (AT2R1) gene are confounding factors that can affect SACE activity [[21], [22], [23]]. Therefore, evaluation of SACE activity can be improved by genotyping patients for ACE I/D polymorphisms. However, it is not always practically feasible to perform genotyping of ACE gene. Due to the likelihood of variability it is important to have proper cutoff range as well.
2.4. Methodology of standardization
In our lab the SACE level was measured with reagent and standard FAPGG {N-[3 (-2 Furyl) acrylol]-L-phenylalanyl-glycyl-glycine} method (Company: BULHAMANN) on Beckman DXC 800 instrument. The linearity of this is up to 100 U/L and the coefficient of variation is 7.8%. The Internal quality control is done by kit provided controls (Low & High). In our lab, the globally accepted cutoff of international standard is followed. Initially few samples were collected from the proven sarcoid cases and controls to validate the normal biological range in our population. It was found to be matching with the international standard (coefficient of variation). The Biological Reference Range is 20–70 U/L. The SACE reagent on the machine is regularly calibrated on monthly basis to avoid technical/lab errors. Calibration is done by kit provided ‘calibrator’ and is validated with peer group by running ‘External Quality assurance programme’ (Biorad Monthly Immunoassay Eqas).
2.5. Statistical analysis
Continuous variables are represented as mean and standard deviation where data follows normal distribution, otherwise as median with range. Categorical variables are represented as frequencies and percentages. The statistical significance in the difference in the outcome variables between the groups and was assessed by chi square test and Fisher exact test. Data was analyzed using R studio.
3. Results
We analyzed 143 cases of clinical CS from our granulomatous myocarditis (GM) registry who presented between Feb 2008 to Dec 2017. Among only the biopsy proven CS cases, 45 patients had measured SACE level at presentation who met the inclusion criteria (Flow chart – Fig. 1).
Fig. 1.
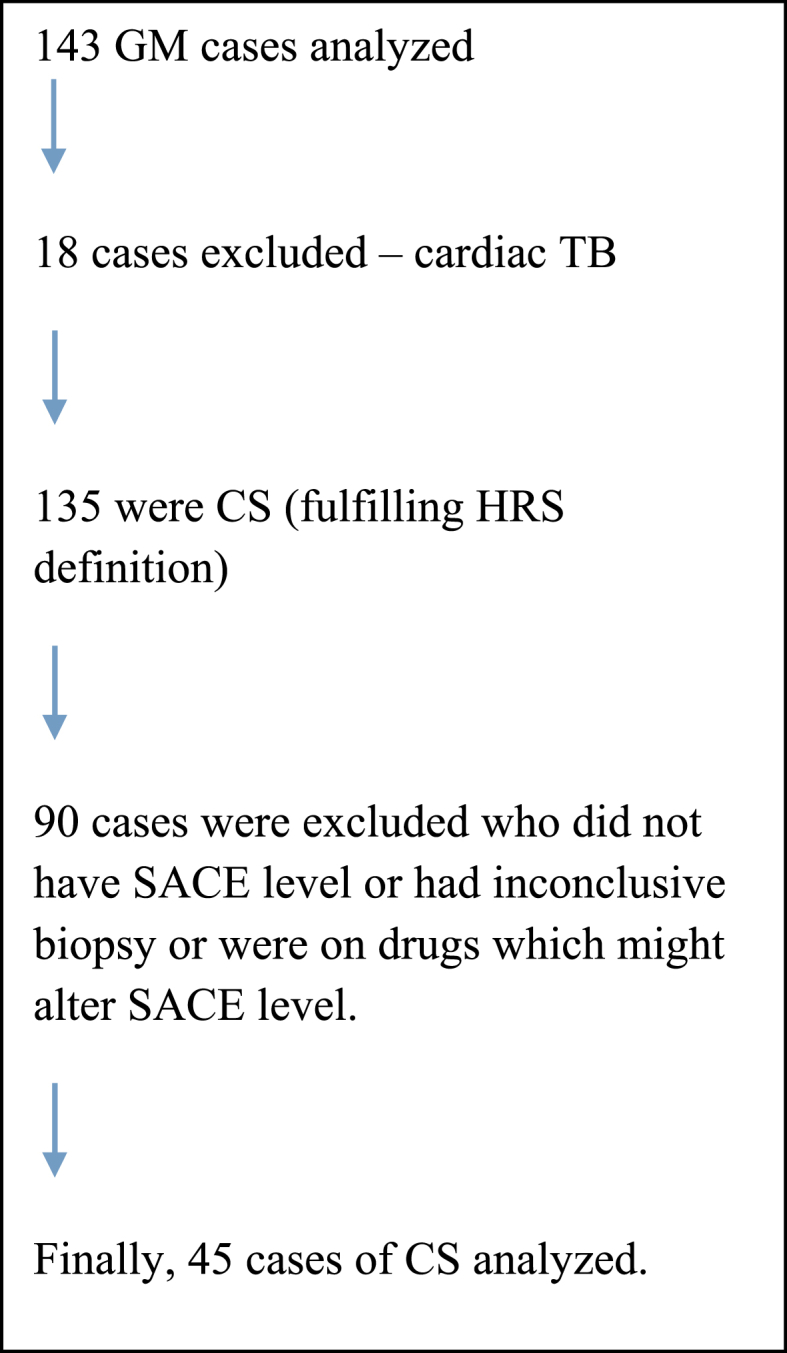
Inclusion and analysis of CS cases.
Baseline characteristics are describing in Table 1.
Table 1.
Baseline characteristics.
| Parameter | Value |
|---|---|
| Mean age at presentation (years) | 42 ± 11 yrs (range:22–63 years) |
| Sex (M/F) | 26/19 |
| LV ejection fraction (mean ± SD) | 38.3 ± 12% |
| PET/MRI/Both | 30/4/11 |
| Extra-cardiac involvement | 6/45 |
3.1. Presentation
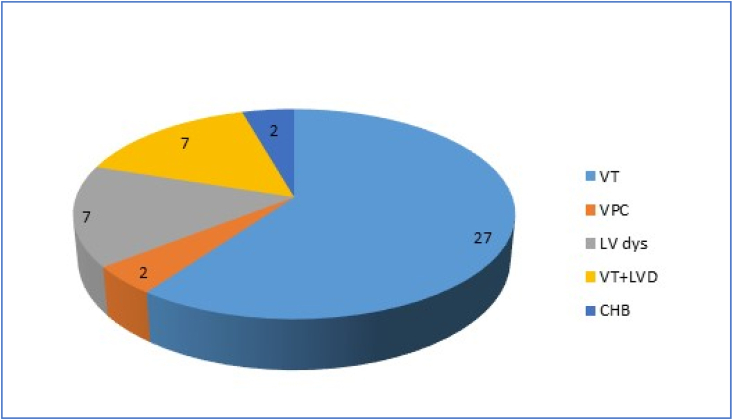
Among these 45 patients, predominant presentation was ventricular arrhythmia (VA) and heart failure [Left ventricular dysfunction (LVD) or Right ventricular (RV) dysfunction (RVD)]. Among the 45 cases sustained ventricular tachycardia (VT) was present in 27; VPC (ventricular premature complexes) in 2; LVD (±RVD) in 7; and VT + LVD in another 7. High grade electrical conduction defect was seen in 2 out of 45 [both had complete heart block (CHB)] [ Fig. 2].
Fig. 2.
Clinical presentation in proportion.
3.2. SACE level
All of these 45 patients had measured SACE level at presentation. With the standard reference range used by our lab for SACE of 20–70 U/L, only 3 patients (6.7%) had elevated SACE level and would have qualified for having CS, if this was made essential criteria for diagnosis. Hence, it shows that the prevalence of elevated SACE is certainly very low in our cohort. The sensitivity of test was only 6.7%, keeping tissue diagnosis as gold standard. Among the 45 patients, 7 cases had more than one value of SACE measurement during their clinical deterioration/non-improvement. Interestingly, there was no evidence of increasing trend in SACE level despite clinical worsening.
Among the three patients having elevated SACE in our cohort, 2 had significant pulmonary involvement. The other patient had severe LV as well as RV systolic involvement. Among the cohort of 45 cases, 4 patients had pulmonary involvement. The yield of elevated SACE among that cohort is higher [50%, 2 of 4 of cases with pulmonary involvement].
3.3. ESR and HsCRP
For a comparative analysis, we also measured ESR and Hs-CRP level at presentation among many of these 45 patients. ESR and Hs CRP level were available at initial diagnosis in 25 and 27 patients, respectively.
According to our lab reference (with a cut off of 10 mm/h), the sensitivity of elevated ESR was 84% (21/25). The mean ESR was 22 mm/h. Hs-CRP (normal range = 0-5mcg/ml) was elevated in 81% (22/27) patients of proven CS. The mean HsCRP was 6.4 mcg/ml. In the same cohort it was also found that after disease specific treatment there was a downward trend of these serological markers, which could be used for monitoring of treatment response.
4. Discussion
ACE is produced by epithelioid cells that are derived from recently-activated macrophages in granuloma. It was found that SACE is an appropriate representative of whole-body granuloma [24]. Relationship between disease chronicity and high-resolution CT scan patterns [25] has also been reported. Silverstein et al. [26] reported that there was an inverse relationship between disease duration and ACE level. In this study, however, we did not look into the chronicity of the disease. Some investigators had shown that there is no correlation between ACE level and sarcoidosis prognosis [27] and this marker merely demonstrates an overall granuloma burden as a whole, rather than isolated lung involvement [28,29]. Based on these findings we hypothesized that low prevalence of elevated SACE in our cohort might be due to localized granuloma (in myocardium ± LN) in this subset of patients.
In an older series by Studdy et al., the positive and negative predictive values of SACE were found to be 84% and 74%, respectively [30]. In our series, it was far lower. We believe, in past the diagnosis of more disseminated sarcoidosis was predominantly made and there was significant under-diagnosis of single organ-limited disease (like cardiac limited sarcoid). This would be one of the causes of higher yield of elevated SACE in those earlier series. In our cohort, only 6 patients have extracardiac manifestations and 33% (2/6) of them had increased SACE level, much higher than the overall cohort (6.7%). Among the cases with concomitant pulmonary involvement the prevalence of elevated SACE was 50% (2 out of 4). The other patent having abnormal SACE, had severe LV and RV involvement with abundant PET uptake (SUV max = 9.8). This further supports the hypothesis of proportional elevation of SACE in cases with high granuloma burden.
In contrast to earlier papers [31,32], our cohort interestingly had lesser proportion of patients with AV block, which could be multifactorial. The geographic distribution might have played a role with genotypic variation in this south-east Asian territory leading to different phenotypic expression. Moreover, many of the relatively young AV block cases in India are not investigated thoroughly and are simply treated with permanent pacemaker implantation, hence, not reaching us. In contrast, being a tertiary care referral arrhythmia center, it is possible that the VT patients were preferentially referred to us.
SACE levels can be also influenced by ACE gene polymorphisms and genotype corrected reference values might improve interpretation [21,22], which is not always feasible. Although we did not perform genetic polymorphism testing for ACE, we postulate the genetic difference in Indian population might also contribute to very low yield of elevated SACE in our patients. The rarity of lung involvement also probably has contributed to the low yield. We did not find it suitable for treatment response either. As compared to SACE, blood ESR and Hs-CRP were far more sensitive markers for the diagnosis of CS. Like several other studies [12] this study supported that the acute phase reactants are useful for monitoring response to immunosuppressant therapy.
5. Conclusion
This study finds, although SACE levels are elevated in a sizable proportion of systemic sarcoidosis, the prevalence among cardiac sarcoidosis is very low. The yield of elevated SACE is also significantly lower than older studies. ESR and hs-CRP are much more sensitive markers for diagnosis at initial presentation, although certainly not specific. However, they are more useful in monitoring treatment response.
From this retrospective study, we conclude that SACE is a very insensitive tool for diagnosing CS and defies the usual belief of using SACE level as a screening tool for clinching the diagnosis or ruling out CS. This is not useful for diagnosis even in the active phase of the disease.
6. Limitations
-
1
This is a retrospective study. The inherent limitations of retrospective studies would have been present. We have included only biopsy proven cases to minimize confounding bias of presumptive CS cases.
-
2
The sample size is moderate, however, in regards to isolated/predominant cardiac sarcoidosis with tissue diagnosis, this is one of the largest cohorts to the best of our knowledge.
-
3
As our center is actively involved in managing complex arrhythmia and heart failure, there might be a referral bias for cases with predominant cardiac involvement. Hence, the proportion of patients with extra-cardiac involvement might have been less in our cohort.
-
4
Among the 143 cases screened, only 45 cases could be included who had all clinical data including ACE level report, hence were not consecutive. This might have resulted in an unintentional selection bias.
Disclosures
None.
Declaration of competing interest
None.
Acknowledgements
We are grateful to Mr. Sudhir Kumar for his inputs in statistical calculation. We also convey our regards to Mrs. Swapna Nalla and Ms. Kalpana for helping us in data collection. We thank Mrs. Venkatlaxmi Lavishetty in helping us to develop the manuscript.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
References
- 1.Iannuzzi M.C., Rybicki B.A., Teirstein A.S. Sarcoidosis. N Engl J Med. 2007;357(21):2153–2165. doi: 10.1056/NEJMra071714. [DOI] [PubMed] [Google Scholar]
- 2.Fleming H.A. Sarcoid heart disease. Br Heart J. 1974;36(1):54–68. doi: 10.1136/hrt.36.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kinney E.L., Jackson G.L., Reeves W.C., Zelis R. Thallium-scan myocardial defects and echocardiographic abnormalities in patients with sarcoidosis without clinical cardiac dysfunction. An analysis of 44 patients. Am J Med. 1980;68(4):497–503. doi: 10.1016/0002-9343(80)90292-2. [DOI] [PubMed] [Google Scholar]
- 4.Tellier P., Paycha F., Antony I. Reversibility by dipyridamole of thallium-201 myocardial scan defects in patients with sarcoidosis. Am J Med. 1988;85(2):189–193. doi: 10.1016/s0002-9343(88)80340-1. [DOI] [PubMed] [Google Scholar]
- 5.Fahy G.J., Marwick T., McCreery C.J., Quigley P.J., Maurer B.J. Doppler echocardiographic detection of left ventricular diastolic dysfunction in patients with pulmonary sarcoidosis. Chest. 1996;109(1):62–66. doi: 10.1378/chest.109.1.62. [DOI] [PubMed] [Google Scholar]
- 6.Yazaki Y., Isobe M., Hiramitsu S. Comparison of clinical features and prognosis of cardiac sarcoidosis and idiopathic dilated cardiomyopathy. Am J Cardiol. 1998;82(4):537–540. doi: 10.1016/s0002-9149(98)00377-4. [DOI] [PubMed] [Google Scholar]
- 7.Yazaki Y., Isobe M., Hiroe M. Central Japan Heart Study Group Prognostic determinants of long-term survival in Japanese patients with cardiac sarcoidosis treated with prednisone. Am J Cardiol. 2001;88(9):1006–1010. doi: 10.1016/s0002-9149(01)01978-6. [DOI] [PubMed] [Google Scholar]
- 8.Iwai K., Sekiguti M., Hosoda Y. Racial difference in cardiac sarcoidosis incidence observed at autopsy. Sarcoidosis. 1994;11(1):26–31. [PubMed] [Google Scholar]
- 9.Sekiguchi M., Numao Y., Imai M., Furuie T., Mikami R. Clinical and histopathological profile of sarcoidosis of the heart and acute idiopathic myocarditis. Concepts through a study employing endomyocardial biopsy. I. Sarcoidosis. Jpn Circ J. 1980;44(4):249–263. doi: 10.1253/jcj.44.249. [DOI] [PubMed] [Google Scholar]
- 10.Tachibana T., Ohmori F., Ueda E. Clinical study on cardiac sarcoidosis. Ann N Y Acad Sci. 1986;465:530–542. doi: 10.1111/j.1749-6632.1986.tb18530.x. [DOI] [PubMed] [Google Scholar]
- 11.Birnie D.H., Sauer W.H., Bogun F. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014;11:1305–1323. doi: 10.1016/j.hrthm.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 12.Ahmadzai H., Wakefield D., Thomas P.S. The potential of the immunological markers of sarcoidosis in exhaled breath and peripheral blood as future diagnostic and monitoring techniques. Inflammopharmacology. 2011;19(2):55–68. doi: 10.1007/s10787-011-0079-3. [DOI] [PubMed] [Google Scholar]
- 13.Lieberman J. Elevation of serum angiotensin-converting enzyme (ACE) in sarcoidosis. Am J Med. 1975;59:365–372. doi: 10.1016/0002-9343(75)90395-2. (Erratum) [DOI] [PubMed] [Google Scholar]
- 14.Sequeira Winston, Stinar Donald. Serum angiotensin-converting enzyme levels in sarcoid arthritis. Arch Intern Med. 1986;146(1):125–127. [PubMed] [Google Scholar]
- 15.Jean Weaver L., Solliday Norman H., Celic Lillian, Cugell David. Serial observations of angiotensin-converting enzyme and pulmonary function in sarcoidosis. Arch Intern Med. 1981;141(7):931–934. [PubMed] [Google Scholar]
- 16.Kasahara Y., Ashihara Y. Colorimetry of angiotensin-I converting enzyme activity in serum. Clin Chem. 1981;27:1922–1925. [PubMed] [Google Scholar]
- 17.Yasar Z., Ozgul M.A., Cetinkaya E., Kargi A., Gul S., Talay F. Angiotensin-converting enzyme as a predictor of extrathoracic involvement of sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2016 Jan 18;32(4):318–324. [PubMed] [Google Scholar]
- 18.Allen R.K., Pierce R.J., Barter C.E. Angiotensin-converting enzyme in bronchoalveolar lavage fluid in sarcoidosis. Sarcoidosis. 1992;9(1):54–59. [PubMed] [Google Scholar]
- 19.Bridel C., Courvoisier D.S., Vuilleumier N., Lalive P.H. Cerebrospinal fluid angiotensin-converting enzyme for diagnosis of neurosarcoidosis. J Neuroimmunol. 2015 Aug 15;285:1–3. doi: 10.1016/j.jneuroim.2015.05.020. [DOI] [PubMed] [Google Scholar]
- 20.Tahmoush A.J., Amir M.S., Connor W.W. CSF-ACE activity in probable CNS neurosarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2002;19(3):191–197. [PubMed] [Google Scholar]
- 21.Biller H., Zissel G., Ruprecht B. Genotype-corrected reference values for serum angiotensin-converting enzyme. Eur Respire J. 2006;28:1085–1090. doi: 10.1183/09031936.00050106. [DOI] [PubMed] [Google Scholar]
- 22.Biller H., Ruprecht B., Gaede K.I., Müller-Quernheim J., Zissel G. Gene polymorphisms of ACE and the angiotensin receptor AT2R1 influence serum ACE levels in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2009;26(2):139–146. [PubMed] [Google Scholar]
- 23.Lopez-Sublet M., Caratti di Lanzacco L., Danser A.H.J., Lambert M., Elourimi G., Persu A. Focus on increased serum angiotensin-converting enzyme level: from granulomatous diseases to genetic mutations. Clin Biochem. 2018 Sep;59:1–8. doi: 10.1016/j.clinbiochem.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 24.Selroos Biochemical markers in sarcoidosis. Crit Rev Clin Lab Sci. 1986;24(3):185–216. doi: 10.3109/10408368609110273. [DOI] [PubMed] [Google Scholar]
- 25.Kahkouee S., Samadi K., Alai A., Abedini A., Rezaiian L. Serum ACE level in sarcoidosis patients with typical and atypical HRCT manifestation. Pol J Radiol. 2016;81:458–461. doi: 10.12659/PJR.897708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silverstein E., Friedland J., Kitt M., Lyons H.A. Increased serum angiotensin converting enzyme activity in sarcoidosis. Isr J Med Sci. 1977 Oct;13(10):995–1000. [PubMed] [Google Scholar]
- 27.Ziegenhagen M.W., Benner U.K., Zissel G. Sarcoidosis: TNF-alpha release from alveolar macrophages and serum level of sIL-2R are prognostic markers. Am J Respir Crit Care Med. 1997;156(5):1586–1592. doi: 10.1164/ajrccm.156.5.97-02050. [DOI] [PubMed] [Google Scholar]
- 28.Westall G.P., Stirling R.G., Cullinan P. fourth ed. BC Decker; Hamilton, London, England: 2003. Interstitial lung disease; pp. 332–386. [Google Scholar]
- 29.Klech H., Kohn H., Kummer F. Sensitivity and specificity of 67-gallium scintigraphy: serum ACE levels, chest roentgenography and blood lymphocyte subpopulations. Chest. 1982;82:732–738. doi: 10.1378/chest.82.6.732. [DOI] [PubMed] [Google Scholar]
- 30.Studdy P.R., Bird R. Serum angiotensin converting enzyme in sarcoidosis--its value in present clinical practice. Ann Clin Biochem. 1989 Jan;26(Pt 1):13–18. doi: 10.1177/000456328902600102. [DOI] [PubMed] [Google Scholar]
- 31.Kandolin R., Lehtonen J., Airaksinen J. Cardiac sarcoidosis: epidemiology, characteristics, and outcome over 25 years in a nationwide study. Circulation. 2015;131(7):624–632. doi: 10.1161/CIRCULATIONAHA.114.011522. [DOI] [PubMed] [Google Scholar]
- 32.Chapelon-Abric C., de Zuttere D., Duhaut P. Cardiac sarcoidosis: a retrospective study of 41 cases. Medicine (Baltim) 2004;83(6):315–334. doi: 10.1097/01.md.0000145367.17934.75. [DOI] [PubMed] [Google Scholar]