Abstract
Aims
The latest evidence in the incidence of central retinal artery occlusion (CRAO) is needed to support the development of novel treatments as orphan drugs. However, up-to-date information on the incidence of CRAO in the ageing or aged population is limited. We aimed to investigate the nationwide epidemiological and clinical characteristics of CRAO in Japan, using nationwide health insurance claims data.
Methods
We analysed a total of 16 069 762 claims data in the sampling dataset of the National Database of Health Insurance Claims and Specific Health Checkups of Japan (NDB), which is the nationwide health insurance claims database of 127 million whole Japanese individuals. CRAO was identified using the International Classification of Diseases 10th edition diagnostic code H34.1. The crude incidence rates and age-standardised incidence rates of CRAO, according to the standard age-structure population of the WHO, were calculated.
Results
The crude incidence rate of CRAO in Japan was 5.84 (95% CI, 5.71 to 5.97) per 100 000 person-years. With respect to the sex-related incidence, the rate was higher 1.40 times in men than in women (6.85 (95% CI, 6.65 to 7.06) vs 4.88 (95% CI, 4.71 to 5.05), p<0.001). The age-standardised incidence rate was 2.53 (95% CI, 2.29 to 2.76) per 100 000 person-years.
Conclusions
The crude incidence rate of CRAO was higher in Japan than in other countries, as reported previously, reflecting the Japanese population structure as a super-aged society. These findings can be helpful for the development of appropriate healthcare policies to address the increasing incidence of CRAO with the ageing population.
Keywords: ophthalmology, vetreoretinal, epidemiology, public health
Strengths and limitations of this study.
The current study is the first nationwide, population-based epidemiological study of central retinal artery occlusion (CRAO) in an ageing or aged society.
The age-standardised incidence rates of CRAO, according to the standard age-structure population of the WHO, were calculated to compare with previous reports.
While the current study has the advantage owing to the exclusiveness of the dataset in the insured healthcare service of whole Japanese population with 127 million individuals, it has limitations in estimation of the accurate incidence of CRAO: potential underestimation for a small portion of cases excluded from the National Database due to Japanese health insurance system and potential overestimation in double counting of cases between outpatient and inpatient data.
Introduction
Central retinal artery occlusion (CRAO) is a retinal vascular occlusive disease that results in ophthalmic emergencies likely to cause sudden-onset vision loss.1 Despite its significant potential health impact, there are no established treatments to improve visual acuity for CRAO,2–6 other than a few promising early treatment options.7 8 Accordingly, novel treatment modalities are needed; in this context, up-to-date data on the incidence of CRAO are needed to provide a theoretical basis for the development of such modalities.9 However, there are only few studies on CRAO and these are limited to before 2011 and reported low incidence rates.3 10 11 The most recent and largest study reported data from before 2011 and covered less than 50 million people, who were not included in the ageing society.11
The National Database of Health Insurance Claims and Specific Health Checkups of Japan (NDB) developed by the Japanese Ministry of Health, Labor and Welfare (MHLW) contains entire healthcare insurance claims data of almost all (≥95%) medical treatments in Japan.12 After 2011, more than 1.7 billion records from 127 million individuals are registered annually into the NDB.13 14 MHLW started providing access to this data after a full anonymisation process for research purposes and decision making on health policies.13 14 Given the universal coverage health insurance system of Japan,15 the NDB represents a powerful tool to explore nationwide trends regarding CRAO.
The current study aimed to investigate the latest nationwide incidence of CRAO in Japan, a super-aged society, using the NDB sampling dataset during 2011 and 2015.
Methods
Study design
The need for informed consent was waived owing to the use of legally anonymised data. This nationwide survey obtained permission from the MHLW to use the health insurance claims data in the NDB.
This study investigated the incidence of CRAO according to the Strengthening the Reporting of Observational Studies in Epidemiology16 and the REporting of studies Conducted using Observational Routinely collected health Data statement guidelines.17
NDB sampling dataset
The National Database (NDB) contains detailed information of almost all medical claims data in Japan, including the diagnosis and medical treatments such as prescription, injection, examination and surgery for both outpatients and inpatients. As a sampling dataset, randomly selected claims data covering 1% of all outpatients and 10% of all inpatients in January, April, July and October from 2011 to 2015 were obtained from the MHLW for research purposes (available at https://www.mhlw.go.jp/file/06-Seisakujouhou-12400000-Hokenkyoku/0000164248.pdf, in Japanese, accessed 28 February 2020).18 19 In the current study, a total of 16 069 762 claims data as an NDB sampling dataset from the entire NDB dataset were analysed. The details of the NDB sampling dataset, including the data handling, is described in the online supplemental methods.
bmjopen-2020-041104supp001.pdf (168KB, pdf)
Definition of primary inpatient and secondary inpatient
A substantial percentage of patients with CRAO were reported to have other major active diseases, including acute myocardial infarction, symptomatic carotid stenosis and giant cell arteritis, and to develop CRAO even during hospitalisation.20 21 In the following incidence count process for CRAO, since it is necessary to separately calculate inpatient data of those who were hospitalised primarily for CRAO purposes and of those who developed CRAO while hospitalised for other diseases, we defined them as primary inpatient and secondary inpatient, respectively, as detailed in the online supplemental methods.
Incidence of CRAO in the NDB sampling dataset
CRAO was determined according to the International Classification of Diseases, 10th edition diagnostic code of H34.1. The onset was determined according to the month when the claim data were filed, and the month of CRAO onset was accordingly considered as the index month. Because almost all patients in the Japanese medical system visit outpatient departments before hospital admission, outpatient data alone should be enough to identify the incidence of CRAO. However, some of the inpatient data must be added in rare exceptional cases detailed in the online supplemental methods: 20% addition for emergently admitted inpatients on the day of first consultation without referral (primary inpatients) and 100% addition for patients hospitalised for other diseases at the onset of CRAO (secondary inpatients). The estimated total CRAO patients is calculated by multiplying the number of outpatients by 300 plus 20% of the primary inpatients and 100% of the secondary inpatients by 30, because the NDB sampling dataset covers 1/300 of all outpatient claims data and 1/30 of all inpatient claims data.
Population at risk
The Current Population Estimates as of 1 October 2013 by the Japanese Ministry of Internal Affairs and Communications were used to define the entire population and each sub-group population as the population at risk (available at https://www.stat.go.jp/english/data/jinsui/2.html, accessed 28 February 2020).
Incidence rate of CRAO
The incidence rates for age stratified by 5-year categories and sex during the study period were determined by dividing the number of people who developed CRAO with the total population at risk within each group. The age-standardised incidence rate of CRAO was calculated according to the standard age-structure world population of the WHO for 2000–2025.22 The incidence rates of CRAO in age subgroups, that is, ≥50, 60 and 70 years were also calculated.
Statistical analyses
All statistical analyses were performed using R V.3.4.1 (R Foundation for Statistical Computing, Vienna, Austria). All values are presented with 95% CIs based on the Poisson distribution.
Patient and public involvement
Patients were not involved in the design, analyses and interpretation of this study.
Results
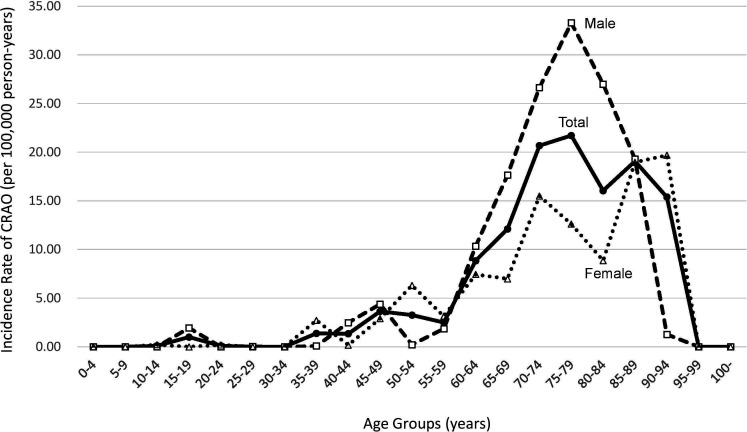
A total of 119 outpatients, 132 primary inpatients and 23 secondary inpatients with CRAO were identified throughout the 5-year study period. Table 1 shows the number of CRAO cases identified in the current dataset and the estimated incidence of CRAO stratified by age. The incidence of CRAO was higher 1.40 times in men than in women (6.85 (95% CI, 6.65 to 7.06) vs 4.88 (95% CI, 4.71 to 5.05) per 100 000 person-years, p<0.001). The incidence of CRAO was also higher in men than in women in most age groups (figure 1). Among the patients with clinically diagnosed CRAO, 13% (estimated inpatients: 4650=(132+23)×30/estimated total:37 182) were hospitalised and supposedly treated or examined intensively.
Table 1.
Frequencies, estimated numbers and incidence rates of clinically diagnosed CRAO in Japan from 2011 to 2015, as determined from the National Database of the Health Insurance Claims and Specific Health Checkups of Japan
| Age group (years) | Japanese population* | Total | Male | Female | Male:female ratio |
|||||||||||||||||
| Total | Male | Female | Out patient |
Primary inpatients† | Secondary inpatients‡ | Estimated total§ | Incidence rate |
95% CI | Out patient |
Primary inpatients† | Secondary inpatients‡ | Estimated total§ |
Incidence rate |
95% CI | Out patient |
Primary inpatients† | Secondary inpatients‡ | Estimated total§ |
Incidence Rate |
95% CI | ||
| 0–4 | 5238 | 2684 | 2554 | 0 | 0 | 0 | 0 | 0.00 | 0.00 | 0 | 0 | 0 | 0 | 0.00 | 0.00 | 0 | 0 | 0 | 0 | 0.00 | 0.00 | NA |
| 5–9 | 5361 | 2743 | 2618 | 0 | 0 | 0 | 0 | 0.00 | 0.00 | 0 | 0 | 0 | 0 | 0.00 | 0.00 | 0 | 0 | 0 | 0 | 0.00 | 0.00 | NA |
| 10–14 | 5790 | 2967 | 2823 | 0 | 0 | 1 | 30 | 0.10 | 0.02 to 0.19 | 0 | 0 | 0 | 0 | 0.00 | 0.00 | 0 | 0 | 1 | 30 | 0.21 | 0.04 to 0.38 | 0 |
| 15–19 | 6047 | 3098 | 2949 | 1 | 0 | 0 | 300 | 0.99 | 0.74 to 1.24 | 1 | 0 | 0 | 300 | 1.94 | 1.45 to 2.43 | 0 | 0 | 0 | 0 | 0.00 | 0.00 | NA |
| 20–24 | 6204 | 3181 | 3023 | 0 | 0 | 1 | 30 | 0.10 | 0.02 to 0.17 | 0 | 0 | 0 | 0 | 0.00 | 0.00 | 0 | 0 | 1 | 30 | 0.20 | 0.04 to 0.36 | 0 |
| 25–29 | 6871 | 3506 | 3365 | 0 | 1 | 0 | 6 | 0.02 | −0.01 to 0.05 | 0 | 1 | 0 | 6 | 0.03 | −0.03 to 0.10 | 0 | 0 | 0 | 0 | 0.00 | 0.00 | NA |
| 30–34 | 7624 | 3867 | 3757 | 0 | 0 | 0 | 0 | 0.00 | 0.00 | 0 | 0 | 0 | 0 | 0.00 | 0.00 | 0 | 0 | 0 | 0 | 0.00 | 0.00 | NA |
| 35–39 | 9059 | 4592 | 4467 | 2 | 4 | 0 | 624 | 1.38 | 1.14 to 1.62 | 0 | 3 | 0 | 18 | 0.08 | 0.00 to 0.16 | 2 | 1 | 0 | 606 | 2.71 | 2.23 to 3.20 | 0.03 |
| 40–44 | 9667 | 4888 | 4779 | 2 | 3 | 1 | 648 | 1.34 | 1.11 to 1.57 | 2 | 1 | 0 | 606 | 2.48 | 2.04 to 2.92 | 0 | 2 | 1 | 42 | 0.18 | 0.06 to 0.29 | 14.11 |
| 45–49 | 8405 | 4228 | 4177 | 5 | 5 | 0 | 1530 | 3.64 | 3.23 to 4.05 | 3 | 4 | 0 | 924 | 4.37 | 3.74 to 5.00 | 2 | 1 | 0 | 606 | 2.90 | 2.39 to 3.42 | 1.51 |
| 50–54 | 7733 | 3870 | 3863 | 4 | 5 | 1 | 1260 | 3.26 | 2.86 to 3.66 | 0 | 2 | 1 | 42 | 0.22 | 0.07 to 0.36 | 4 | 3 | 0 | 1218 | 6.31 | 5.51 to 7.10 | 0.03 |
| 55–59 | 7732 | 3840 | 3892 | 3 | 6 | 1 | 966 | 2.50 | 2.15 to 2.85 | 1 | 4 | 1 | 354 | 1.84 | 1.41 to 2.27 | 2 | 2 | 0 | 612 | 3.14 | 2.59 to 3.70 | 0.59 |
| 60–64 | 9665 | 4740 | 4925 | 14 | 8 | 1 | 4278 | 8.85 | 8.26 to 9.45 | 8 | 3 | 1 | 2448 | 10.33 | 9.41 to 11.24 | 6 | 5 | 0 | 1830 | 7.43 | 6.67 to 8.19 | 1.39 |
| 65–69 | 8700 | 4183 | 4517 | 17 | 18 | 2 | 5268 | 112.11 | 11.38 to 12.84 | 12 | 15 | 0 | 3690 | 17.64 | 16.37 to 18.92 | 5 | 3 | 2 | 1578 | 6.99 | 6.22 to 7.76 | 2.53 |
| 70–74 | 7597 | 3537 | 4060 | 25 | 29 | 6 | 7854 | 20.68 | 19.65 to 21.70 | 15 | 20 | 3 | 4710 | 26.63 | 24.93 to 28.33 | 10 | 9 | 3 | 3144 | 15.49 | 14.28 to 16.70 | 1.72 |
| 75–79 | 6301 | 2772 | 3529 | 22 | 20 | 4 | 6840 | 21.71 | 20.56to 22.86 | 15 | 14 | 1 | 4614 | 33.29 | 31.14 to 35.44 | 7 | 6 | 3 | 2226 | 12.62 | 11.44 to 13.79 | 2.64 |
| 80–84 | 4763 | 1889 | 2874 | 12 | 17 | 4 | 3822 | 16.05 | 14.91 to 17.19 | 8 | 10 | 3 | 2550 | 27.00 | 24.66 to 29.34 | 4 | 7 | 1 | 1272 | 8.85 | 7.76 to 9.94 | 3.05 |
| 85–89 | 2925 | 970 | 1955 | 9 | 10 | 1 | 2790 | 19.08 | 17.49 to 20.66 | 3 | 6 | 0 | 936 | 19.30 | 16.53 to 22.06 | 6 | 4 | 1 | 1854 | 18.97 | 17.04 to 20.90 | 1.02 |
| 90–94 | 1216 | 283 | 933 | 3 | 6 | 0 | 936 | 15.39 | 13.19 to 17.60 | 0 | 3 | 0 | 18 | 1.27 | −0.04 to 2.59 | 3 | 3 | 0 | 918 | 19.68 | 16.83 to 22.52 | 0.06 |
| 95–99 | 350 | 61 | 289 | 0 | 0 | 0 | 0 | 0.00 | 0.00 | 0 | 0 | 0 | 0 | 0.00 | 0.00 | 0 | 0 | 0 | 0 | 0.00 | 0.00 | NA |
| >100 | 55 | 7 | 48 | 0 | 0 | 0 | 0 | 0.00 | 0.00 | 0 | 0 | 0 | 0 | 0.00 | 0.00 | 0 | 0 | 0 | 0 | 0.00 | 0.00 | NA |
| Total | 127 303 | 61 906 | 65 397 | 119 | 132 | 23 | 37 182 | 5.84 | 5.71 to 5.97 | 68 | 86 | 10 | 21 216 | 6.85 | 6.65 to 7.06 | 51 | 46 | 13 | 15 966 | 4.788 | 4.71 to 5.05 | 1.40 |
*Japanese population data (as of 1 October 2013) were based on the Japanese Ministry of Internal Affairs and Communications (unit: 1000 people).
†Primary inpatients represent those admitted primarily for CRAO.
‡Secondary inpatients represent those who developed CRAO while hospitalised for other diseases The incidence of CRAO in outpatients, primary inpatients, secondary inpatients or estimated total are each the sum of 20 months (4 months/year×5 years).
§The estimated total CRAO patients is calculated by multiplying the number of outpatients by 300 plus 20% of the primary inpatients and 100% of secondary inpatients by 30, because the NDB sampling dataset covers 1/300 of all outpatient claims data and 1/30 of all inpatient claims data. The outpatients later becoming inpatients account for 20% of primary inpatients data. The incidence rate is reported per 100 000 person-years.
CRAO, central retinal artery occlusion; NA, not applicable.
Figure 1.
Incidence rate of central retinal artery occlusion per 100 000 person-years (2011–2015) by age group.
Table 2 shows the incidence rates of CRAO per 100 000 person-years. The crude incidence of CRAO in Japan was 5.84 (95% CI, 5.71 to 5.97) for the overall population, 6.85 (95% CI, 6.65 to 7.06) for men and 4.88 (95% CI, 4.71 to 5.05) for women. The age-standardised incidence rate was 2.53 (95% CI, 2.29 to 2.76). Meanwhile, the incidence rates were 11.93, 15.29 and 19.17 in those aged ≥50, 60 and 70 years, respectively. The male-to-female ratios in those aged ≥50 years, ≥60 years and ≥70 years were 1.56, 1.85 and 1.96, respectively. The incidence rate of CRAO increased as the age increased.
Table 2.
Incidence rates of CRAO per 100 000 person-years in the current and previous studies
| Overall | Male | Female | ||||
| Incidence rate | 95% CI | Incidence rate | 95% CI | Incidence rate | 95% CI | |
| Crude | ||||||
| Japan (current study) | ||||||
| Overall | 5.84 | 5.71 to 5.97 | 6.85 | 6.65 to 7.06 | 4.88 | 4.71 to 5.05 |
| Aged ≥50 years | 11.93 | 11.64 to 12.21 | 14.81 | 14.34 to 15.27 | 9.49 | 9.14 to 9.83 |
| Aged ≥60 years | 15.29 | 14.92 to 15.67 | 20.57 | 19.91 to 21.22 | 11.09 | 10.66 to 11.52 |
| Aged ≥70 years | 19.17 | 18.61 to 19.73 | 26.95 | 25.91 to 28.00 | 13.76 | 13.13 to 14.38 |
| Olmsted County in the USA* | 1.33 | 0.60 to 1.71 | ||||
| South Korea* | 1.80 | 1.74 to 1.86 | 2.15 | 2.05 to 2.24 | 1.47 | 1.39 to 1.54 |
| Croatia* | 0.7 | 0.2 to 1.7 (range) |
||||
| Age-standardised | ||||||
| Japan (current study) | 2.53 | 2.29 to 2.76 | ||||
| Olmsted County in USA* | 1.87 | 1.31 to 2.43 | 2.78 | 1.69 to 3.86 | 1.15 | 0.60 to 1.71 |
| South Korea* | 2.06 | |||||
| South Korea in 2020 (projected)* |
2.37 | |||||
| South Korea in 2030 (projected)* |
3.63 | |||||
Discussion
Japan, which has a population of over 120 million, is a super-aged society by the WHO definition, with people aged ≥65 years accounting for over 21% of the total population. Accordingly, healthcare policies on ageing-related diseases are important. However, recent data on the incidence of CRAO, which can be helpful in developing health-related policies, are lacking in Japan. To our best knowledge, the current study is the first nationwide, population-based epidemiological study of CRAO in an ageing or aged society. We found that the crude incidence rates and age-standardised incidence rates of CRAO were 5.84 and 2.53 per 100 000 person-years, respectively. These results provide recent instrumental evidence on the incidence of clinically diagnosed CRAO in Japan and can thus be used as theoretical basis for developing appropriate treatment modalities.
The crude incidence rate of CRAO was 5.84 (95% CI, 5.71 to 5.97) per 100 000 person-years, which was relatively higher than rates in previous reports.3 10 11 The differences may be due to the chronological differences of the research periods. However, compared with the age-standardised incidence rates of the WHO (2000–2025) standard population, the current study found a 2.53 (95% CI, 2.29 to 2.76) incidence rate. The age-standardised incidence rate is comparable to that of South Korea (2.06) and Olmsted County (1.87)3 11 and to projections in the Korean population in 2020 (2.37) and 2030 (3.63) per 100 000 person-years.11 We also found that the incidence rate of CRAO increased with increased age, consistent with previous studies.3 11 The incidence rate of CRAO also increased in those aged 50, 60 and ≥70 years. Collectively, our results and those of previous studies indicate that CRAO is more common in the elderly. Further, the high crude incidence of CRAO in the Japanese, who are rapidly ageing, suggests that the incidence of CRAO will increase in all ageing countries.
In the total estimated CRAO incidence calculation, 20% of primary inpatient CRAO patients were added to outpatient CRAO incidence, referring to the results of the Patient’s Behavior Survey conducted by MHLW as described in the online supplemental methods. Because the 20% figure, however, was not sufficiently validated, we also calculated 0% and 100% primary inpatient CRAO addition to the outpatient CRAO as the possible range. The crude incidence rate of CRAO from 5.72 to 6.34, while the age-adjusted incidence rate of CRAO ranged from 2.47 to 2.74. These possible ranges were mostly within the 95% CI of the most likelihood model with a 20% figure. Indeed the 20% figure was obtained from a survey for all diseases, not for a CRAO-specific survey. If more specific and accurate reports are available in the future, the incidence rate of CRAO will need to be recalculated.
In the current study, only 13% of the patients with clinically diagnosed CRAO were hospitalised and supposedly treated or examined intensively. Also in the past report, only 18% of retina specialists pursue a hospital-based evaluation, defined as hospital admission or emergency room evaluation, while 75% of neurologists did.23 American Heart Association and the American Stroke Association define retinal artery occlusion as an ischaemic stroke and amaurosis fugax as a retinal transient ischaemic attack, and recommend imaging and evaluating all these patient for treatable conditions such as carotid stenosis and atrial fibrillation in the guidelines.24 25 Despite the recommendations in the guidelines, ophthalmologists in both USA and Japan were not fully aware of the need for systemic examination after CRAO.
While the current study has the advantage owing to the exclusiveness of the dataset in the insured healthcare service of a country, some limitations must be acknowledged when interpreting the findings. First, the accuracy of the CRAO diagnosis based on the ICD-10 codes was not sufficient, which is inherent to studies using claims data. Ideally, we would have validated the CRAO diagnosis by the medical records and then performed the research with high diagnostic accuracy. Second, a small portion of cases, under 5% of all insured healthcare services of Japan, are not included in the NDB database,12 which may lead to an underestimation of the actual incidence of CRAO. Third, because a hashed personal identification number was provided only in the outpatient data, we could not exclude any potential preexisting cases of CRAO nor eliminate the overlap between outpatient and inpatient data, although we considered it in the calculation of incidence (online supplemental methods). This might lead to overestimation of the incidence of CRAO. Finally, characteristics of the NDB sampling dataset, including full anonymisation and strict control over access, prevented more detailed discussions. The available data were limited to 2011–2015, which was a barrier to comparison with the previous reports. In addition to more accurate estimation of CRAO incidence, risk factor evaluation for CRAO or accuracy assessments in CRAO diagnosis by stratified specialty could be performed beyond the achievement of consolidated personal identification number and relaxation of access restrictions.
In conclusion, the current study provides the nationwide incidence rate of CRAO in Japan, a super-aged society, based on 5-year population-based NDB sampling dataset. The crude incidence of CRAO in Japan was 5.84 (95% CI, 5.71 to 5.97) per 100 000 person-years and was higher than in other countries and areas as reported previously, reflecting the Japanese population structure as a super-aged society. These findings can be helpful for the development of appropriate healthcare policies to address the increasing incidence of CRAO with the ageing population.
Supplementary Material
Footnotes
AK and HT contributed equally.
Contributors: HOI had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. AK, HT and HOI conceptualised and designed the data. HOI involved in acquisition of data. AK, HT, HOI, MM, SH and AT involved in analysis, or interpretation of data. AK and HT involved in drafting of the manuscript. HOI, MM, SH and AT involved in critical revision of the manuscript for important intellectual content. HOI and AT involved in administrative, technical or material support. AT involved in supervision.
Funding: This study was supported in part by the Translational Research Network Programme and Rare/Intractable Disease Project of the Japan Agency for Medical Research and Development (AMED) (grant number: 18lm0203012h0002 to HOI).
Disclaimer: The funding organisation had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
Competing interests: HOI reports receiving grants from the Japan Agency for Medical Research and Development during the conduct of the study.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication: Not required.
Ethics approval: This study was approved by the Institutional Review Board and by the Ethics Committee of Kyoto University Hospital and Kyoto University Graduate School of Medicine (No. R2405). All investigations were conducted according to the tenets of the Declaration of Helsinki and its later amendments.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data may be obtained from a third party and are not publicly available. After the authorised research period, the raw data of the NDB sampling data must be returned to the MHLW. Thus, researchers have access only to protocols and programme code but no longer to the raw data. Thus the data sharing would be limited to the programme code. Those who want to access raw data needs to apply to MHWL.
References
- 1.Hayreh SS, Zimmerman MB. Central retinal artery occlusion: visual outcome. Am J Ophthalmol 2005;140:376.e1–376.e. 10.1016/j.ajo.2005.03.038 [DOI] [PubMed] [Google Scholar]
- 2.Ahn SJ, Kim JM, Hong J-H, et al. Efficacy and safety of intra-arterial thrombolysis in central retinal artery occlusion. Invest Ophthalmol Vis Sci 2013;54:7746–55. 10.1167/iovs.13-12952 [DOI] [PubMed] [Google Scholar]
- 3.Leavitt JA, Larson TA, Hodge DO, et al. The incidence of central retinal artery occlusion in Olmsted County, Minnesota. Am J Ophthalmol 2011;152:820–3. 10.1016/j.ajo.2011.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Menzel-Severing J, Siekmann U, Weinberger A, et al. Early hyperbaric oxygen treatment for nonarteritic central retinal artery obstruction. Am J Ophthalmol 2012;153:454–9. 10.1016/j.ajo.2011.08.009 [DOI] [PubMed] [Google Scholar]
- 5.Rumelt S, Dorenboim Y, Rehany U. Aggressive systematic treatment for central retinal artery occlusion. Am J Ophthalmol 1999;128:733–8. 10.1016/S0002-9394(99)00359-1 [DOI] [PubMed] [Google Scholar]
- 6.Schumacher M, Schmidt D, Jurklies B, et al. Central retinal artery occlusion: local intra-arterial fibrinolysis versus conservative treatment, a multicenter randomized trial. Ophthalmology 2010;117:1367–75. 10.1016/j.ophtha.2010.03.061 [DOI] [PubMed] [Google Scholar]
- 7.Mac Grory B, Lavin P, Kirshner H, et al. Thrombolytic therapy for acute central retinal artery occlusion. Stroke 2020;51:687–95. 10.1161/STROKEAHA.119.027478 [DOI] [PubMed] [Google Scholar]
- 8.Ikeda HO, Muraoka Y, Hata M, et al. Safety and effectiveness of a novel neuroprotectant, KUS121, in patients with non-arteritic central retinal artery occlusion: an open-label, non-randomized, first-in-humans, phase 1/2 trial. PLoS One 2020;15:e0229068. 10.1371/journal.pone.0229068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murakami M, Narukawa M. Matched analysis on orphan drug designations and approvals: cross regional analysis in the United States, the European Union, and Japan. Drug Discov Today 2016;21:544–9. 10.1016/j.drudis.2016.02.016 [DOI] [PubMed] [Google Scholar]
- 10.Ivanisević M, Karelović D. The incidence of central retinal artery occlusion in the district of split, Croatia. Ophthalmologica 2001;215:245–6. 10.1159/000050868 [DOI] [PubMed] [Google Scholar]
- 11.Park SJ, Choi N-K, Seo KH, et al. Nationwide incidence of clinically diagnosed central retinal artery occlusion in Korea, 2008 to 2011. Ophthalmology 2014;121:1933–8. 10.1016/j.ophtha.2014.04.029 [DOI] [PubMed] [Google Scholar]
- 12.Sugihara T, Yasunaga H, Matsui H, et al. Regional clinical practice variation in urology: usage example of the open data of the National database of health insurance claims and specific health checkups of Japan. Int J Urol 2019;26:303–5. 10.1111/iju.13840 [DOI] [PubMed] [Google Scholar]
- 13.Kato G. History of the secondary use of national database of health insurance claims and specific health checkups of Japan (NDB). Trans Jap Soc Med Biol Eng 2017;55:143–50. [Google Scholar]
- 14.Matsuda S, Fujimori K. The claim database in Japan. Asian Pac J Dis Manag 2014;6:55–9. 10.7223/apjdm.6.55 [DOI] [Google Scholar]
- 15.Ikegami N, Yoo B-K, Hashimoto H, et al. Japanese universal health coverage: evolution, achievements, and challenges. Lancet 2011;378:1106–15. 10.1016/S0140-6736(11)60828-3 [DOI] [PubMed] [Google Scholar]
- 16.Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Epidemiology 2007;18:805–35. 10.1097/EDE.0b013e3181577511 [DOI] [PubMed] [Google Scholar]
- 17.Benchimol EI, Smeeth L, Guttmann A, et al. The reporting of studies conducted using observational Routinely-collected health data (record) statement. PLoS Med 2015;12:e1001885. 10.1371/journal.pmed.1001885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hiragi S, Sato N, Uchino E, et al. Association between the size of healthcare facilities and the intensity of hypertension therapy: a cross-sectional comparison of prescription data from insurance claims data. Hypertens Res 2020. 10.1038/s41440-020-00549-2. [Epub ahead of print: 15 Sep 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sato M, Kondoh E, Iwao T, et al. Nationwide survey of severe postpartum hemorrhage in Japan: an exploratory study using the National database of health insurance claims. J Matern Fetal Neonatal Med 2019;32:3537–42. [DOI] [PubMed] [Google Scholar]
- 20.Lavin P, Patrylo M, Hollar M, et al. Stroke risk and risk factors in patients with central retinal artery occlusion. Am J Ophthalmol 2018;196:96–100. 10.1016/j.ajo.2018.08.027 [DOI] [PubMed] [Google Scholar]
- 21.Park SJ, Choi N-K, Yang BR, et al. Risk and risk periods for stroke and acutemyocardial Infarction in patients withcentral retinal artery Occlusion. Ophthalmology 2015;122:2336–43. 10.1016/j.ophtha.2015.07.018 [DOI] [PubMed] [Google Scholar]
- 22.Ahmad OB, Boschi Pinto C, Lopez AD. Age standardization of rates: a new who standard, 2001: 10–12. [Google Scholar]
- 23.Abel AS, Suresh S, Hussein HM, et al. Practice patterns after acute embolic retinal artery occlusion. Asia Pac J Ophthalmol 2017;6:37–9. 10.22608/APO.201690 [DOI] [PubMed] [Google Scholar]
- 24.Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American heart Association/American stroke association. Stroke 2013;44:2064–89. 10.1161/STR.0b013e318296aeca [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack. Stroke 2014;45:2160–236. 10.1161/STR.0000000000000024 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-041104supp001.pdf (168KB, pdf)