Abstract
Arsenic trioxide has shown a strong anti-tumor effect with little toxicity when used in the treatment of acute promyelocytic leukemia (APL). An effect on glioma has also been shown. Its mechanisms include regulation of apoptosis and autophagy; promotion of the intracellular production of reactive oxygen species, causing oxidative damage; and inhibition of tumor stem cells. However, glioma cells and tissues from other sources show different responses to arsenic trioxide. Researchers are working to enhance its efficacy in anti-glioma treatments and reducing any adverse reactions. Here, we review recent research on the efficacy and mechanisms of action of arsenic trioxide in the treatment of gliomas to provide guidance for future studies.
Keywords: Arsenic trioxide, Glioma, Anti-cancer mechanism
Introduction
Glioma is the most common primary malignant tumor of the central nervous system, accounting for about 50–60% of intracranial tumors. Its invasion and recurrence rates are significantly higher than those of other intracranial tumors [1]. Due to its invasive growth, it generally cannot be completely surgically removed [2]. Despite advances in clinical treatment methods, the prognosis for patients remains poor [3]. New therapeutic strategies, including novel drugs, are needed to effectively inhibit the proliferation, invasion and metastasis of glioma cells and treat this malignant cancer type.
Elemental arsenic is insoluble in water and acid. It has almost no toxicity, but when exposed to air, it readily oxidizes to highly toxic arsenic trioxide. Traditional Chinese medicine has applied arsenic and arsenic-containing medicines to treat a variety of intractable diseases since ancient times [4], but the toxicity has limited its medical applications. Recent studies have shown that arsenic trioxide has an inhibitory effect on acute promyelocytic leukemia (APL), a hematological tumor, and on solid tumors including liver [5], lung [6] and breast cancer [7].
In glioma, arsenic trioxide exerts its anti-cancer effects via regulation of apoptosis and autophagy; its impact on the cell cycle; promotion of the production of intracellular reactive oxygen species (ROS), which results in oxidative damage; inhibition of tumor stem cells; and enhancement of the effects of radiotherapy and chemotherapy. However, glioma cells and tissues from various sources have different responses to arsenic trioxide, and it has some limitations and side effects in tumor therapy [8–10]. Research in this area is increasingly focused on combinations of arsenic trioxide with other drugs or methods to enhance its anti-glioma efficacy and reduce adverse reactions.
Arsenic trioxide induces programmed cell death
There are two forms of programmed cell death, apoptosis and autophagy [11, 12]. Apoptosis is governed by two common pathways: the extrinsic path way initiated by the binding of death ligands to death receptors on the plasma membrane; and the intrinsic pathway, where cellular oxidative stress leads to a loss of mitochondrial integrity and their destruction [13]. Both pathways activate caspase-3.
Autophagy is characterized by the formation of acidic vesicular organelles in the cytoplasm [14]. This causes the destruction of the nucleus and the collapse of cytoplasmic or ganelles. Autophagy can prevent toxic accumulation of cell waste, protect organelles and maintain cell survival. It has also been shown to promote the development of tumor cells and has been implicated in chemotherapy resistance, although it can also inhibit tumor growth in some cases [15].
Similar stimuli can induce apoptosis, autophagy or both, possibly because apoptosis and autophagy have common effector proteins (including Bcl-2, Bcl-xl, Mcl-1, ATG5 and p53) and upstream pathways (including PI3K/Akt/mTOR, NF-κB and MAPK) [16]. Therefore, apoptosis and autophagy may be related or even regulated by the same trigger in the anti-glioma mechanism of arsenic trioxide [17, 18].
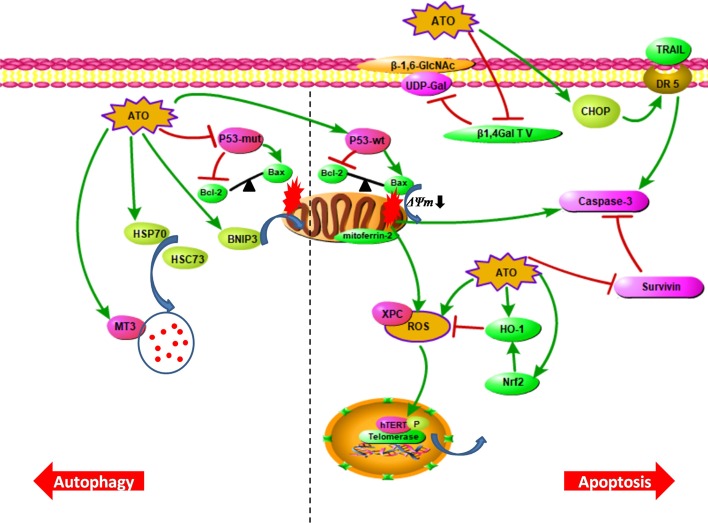
In experiments with six malignant glioma cell lines (U373, U87MG, U251, GB1, A-172 and T98G), Kanzaw et al. showed that treatment with a low concentration of arsenic trioxide (2 μM) caused glioma cell death but could not induce apoptosis. U373 cells showed the loss of microvilli and the presence of a large number of autophagic vacuoles. The same researchers studied the occurrence of autophagy and found that arsenic trioxide upregulated mitochondrial cell death protein BNIP3, thereby opening mitochondrial permeability transition pores and destroying the integrity of the mitochondrial membrane [19].An inhibitor of caspase-3 could not prevent cell death induced by arsenic trioxide, but apoptosis occurred when the autophagy inhibitor bafilomycin A1 was used in combination with arsenic trioxide [20]. Therefore, at low concentrations of arsenic trioxide, if autophagy is inhibited, apoptosis occurs as an alternate pathway.
Similar studies have also shown that inhibiting cathepsin L (CatL) activity partially reduces lysosomal activity, thus affecting autophagy. This can also cause the transition from autophagy to apoptosis in U87MG cells that were treated with a low concentration of arsenic trioxide (2 μM).The drug effect is more obvious in multicellular spheroids than in cell monolayers [21].
The effects of arsenic trioxide on apoptosis and autophagy in glioma may be affected by the drug concentration and the p53 gene type. As arsenic trioxide-induced apoptosis in glioma requires complete p53 protein function, autophagy may become the dominant form of programmed death when apoptosis is inhibited [22]. In a wild-type p53 U87MG cell line, arsenic trioxide at concentrations higher than 4 μM was shown to induce apoptosis [23, 24]. It could also significantly induce the autophagic death of p53-mutant U118 cells. However, even at a high concentration (50 μM), it could not induce apoptosis or cause the activation of aspases-3, -8 and -9. Blocking the apoptosis pathway mediated by p53 may thus be one of the mechanisms by which autophagy is induced by arsenic trioxide [25, 26].
Further study showed that the induction of autophagy in glioma cells may also be related to the heat shock response. After treatment of U118 with arsenic trioxide, the expression of the heat shock protein HSP70 increased. In addition, the HSP70-family protein HSC73 bound to soluble proteins and initiated their transport to lysosomes or lysosomal compartments, resulting in complete protein degradation [27].
However,other reports have been inconsistent with the above-mentioned results (Table 1). For example, Chiu et al. found that arsenic trioxide had both apoptotic and autophagic effects on U118 cells, related to the inhibition of survivin [28]. Zhao et al. studied the expression profiles of apoptosis- and cell cycle-related genes in two glioma cell lines (U87MG and T98G) of different p53 types treated with arsenic trioxide (2 μM). The results suggested that arsenic trioxide inhibited the growth of U87MG cells expressing wild-type p53 mainly by affecting the expression of genes involved in cell cycle arrest, stress and toxicity. Furthermore, it inhibited the growth of p53-mutant T98G cells mainly by upregulating the apoptosis pathway mediated by Bcl-2 and tumor necrosis factor. They also found that arsenic trioxide was more likely to cause apoptosis of p53-mutant cells [29]. A Separate study have shown that 2 μM arsenic trioxide can induce apoptosis in U87MG, U251, SHG44 and C6 glioma cells in a dose-dependent manner [30].
Table 1.
Studies on arsenic trioxide-induced glioma cell death
| First author | Cell line | Does (μM) | Time | Findings | Refs |
|---|---|---|---|---|---|
| Kanzawa | U373, U87MG, U251, GB1, A-172, T98G | 1,2,4 | 72 h | Autophagy | [19, 20] |
| Eun Hee Kim | U373MG, U87MG, U251MG, U343, U251N, T98G | 4,5,6 | 24 h | No apoptosis | [30] |
| Ye Cheng | U87MG, U251, SHG44, C6 | 2,4,8 | 48 h | Apoptosis | [17] |
| Naomi Haga | A172, T98G | 1,10,50 | 48 h | A172: apoptosis; T98G: no apoptosis | [40] |
| Yuanyuan Sun | C6, 9L | 0.5–8 | 24,48,72 h | Apoptosis | [41] |
| Primon, M | U87MG | 2,5,10,20 | 72 h | 2 μM:autophagy; More than 10 μM: apoptosis | [21] |
| Karsy, M | U87MG | 0.5,1,2,4,16 | 24 h | More than 4 μM: apoptosis | [23] |
| Majid Zaki Dizaji | U87MG | 1,2 | 48 h | Apoptosis | [63] |
| G.-B. WANG | U87MG | 4,6,8 | 72 h | Apoptosis | [24] |
| Shiguang Zhao | U87MG,T98G | 1,2,4,8 | 12,24,48 h | Apoptosis, the effect of T98 is greater than U87 | [28] |
| Hui-Wen Chiu | U118MG | 2,4,6,8 | 12,18,24,36 h | Autophagy and apoptosis | [25] |
| Tain-Junn Cheng | U118 | 1,5,10,25,50 | 24 h | Autophagy | [24] |
| Hui-Wen Chiu | U118-MG | 2 | 24 h | Autophagy | [27] |
| Yuanyan Wei | SHG44 | 2,4,6 | 24 h | Apoptosis | [36] |
In conclusion, further study is needed to determine whether apoptosis or autophagy of glioma cells is induced by arsenic trioxide. However, regardless of the p53 type of gliomas, low-dose arsenic trioxide (1–2 μM) can induce programmed cell death in vitro, and it is effective as a single or combined therapy for glioma [23].
There are other mechanisms by which arsenic trioxide regulates programmed cell death in glioma cells. Some studies have found that 5 μM arsenic trioxide can upregulate the expression of the death receptor DR5 and facilitate its binding to tumor necrosis factor-related apoptotic ligand (TRAIL). This leads to both the cleavage of pro-caspase-3 (the precursor of caspase-3) and apoptosis via extrinsic pathways. The upregulation of DR5 induced by arsenic trioxide may be mediated by CCAAT/enhancer-binding protein homologous protein (CHOP) [31]. CHOP is stimulated by DNA damage and is one of the most strongly induced genes during endoplasmic reticulum stress. However, no DNA damage was detected in the cited study, possibly due to the low dose of arsenic trioxide, which is known to have no cytotoxic effect on human normal glial cells. This could be attributed to the low expression of DR4 and DR5 in normal glial cells, which would make them insensitive to apoptosis induced by TRAIL [32].
Apoptosis is also thought to be related to glycosylation. The sugar chain structure of cell surface proteins has an important role in regulating cell survival [33–35]. Arsenic trioxide can affect apoptosis by reducing the expression of a member of the β1,4-galactosyltransferase family: β1,4 GalT V, which galactosylates the β1,6-GlcNAc branch of N-glycans [36, 37].
In cells treated with arsenic trioxide (6 μM) for 48 h, about 40% of the water-soluble arsenic present was found to exist in the metallothionein (MT) protein component. MTs can bind to metal elements, thereby protecting cells from arsenic toxicity. Further studies of the interactions between arsenic trioxide and MT subtypes, with different doses or durations of treatment, indicated that the expression of the MT gene in each subtype increased or decreased to a varying degree. The mRNA expression levels of MT1X, MT1F and MT2A increased the most (up to 13 times), whereas those of MT1A, MT1E and MT3 decreased or remained unchanged. The increase in expression of MT1X, MT1F and MT2A may be one of the mechanisms by which glioma cells resist the toxicity caused by arsenic trioxide therapy. On the other hand, MT3 has low metal inducibility and is capable of rapid binding and release of metals. Its isoform is mainly expressed in the brain and it is necessary for the normal lysosomal function of astrocytes. In astrocytes with MT3 deletion, the activities of several lysosomal enzymes decreased significantly, and the death of glioma cells induced by oxidative stress was substantially weakened [38]. After treatment with arsenic trioxide (7 μM) for 24 h, MT3 expression increased. Therefore, the involvement of MT3 in autophagic cell death induced by arsenic trioxide cannot be ruled out (Fig. 1) [39].
Fig. 1.
The mechanisms of glioma cell death induced by arsenic trioxide. Apoptosis can be induced via external pathways and through internal pathways in glioma cell lines containing wild-type p53. Arsenic trioxide can also cause cell death in glioma through autophagy
Arsenic trioxide acts through oxidative damage
Compared with other organs, the brain seems to be particularly sensitive to ROS stress. Although it accounts for only 2% of bodyweight, it consumes up to 20% of the body’s oxygen supply. Large amounts of ROS are produced in the brain tissue during oxidative phosphorylation [40]. Since tumor cells are very sensitive to the stress response to ROS, treatments that affect ROS levels may also affect tumors.
The mechanisms by which arsenic trioxide induces apoptosis include stimulate on of ROS production, mitochondrial aggregation, Bax oligomerization, dissipation of membrane potential, and mitochondrial membrane collapse, followed by the release of apoptotic factors, the caspase cascade, and, finally, cell death. Increased ROS levels were detected in both A172 and T98G cell lines treated with 50 μM arsenic trioxide. However, there was no mitochondrial aggregation, membrane potential dissipation or Bax oligomerization in these T98G cells. The mitochondria of T98G cells may be less sensitive and responsive to oxidative stress, and thus resistant to arsenic trioxide–induced mitochondrially mediated apoptosis [41].
A study using rat C6 and 9L cell lines showed that 5 μM arsenic trioxide could strongly inhibit cell viability and induce apoptosis by downregulating the expression of Bcl-2 and upregulating the expression of Bax. This change is related to the mitochondrial damage caused by the production of intracellular ROS. However, the inhibition rate for normal rat glial cells was less than 10% of that for rat glioma cells [42].
While arsenic trioxide increased the production of ROS in glioma cells, it also increased the expression of heme oxygenase-1 (HO-1) and its upstream effector Nrf2. HO-1 can protect against oxidative stress and antagonize the effects of ROS. Therefore, inhibiting the expression of the gene encoding HO-1 or Nrf2 can enhance the oxidative damage caused by arsenic trioxide [43].
Iron plays an important part in the production of ROS by mitochondria [44]. Mitoferrin-2 is a mitochondrial protein that mediates ferrous transport through the mitochondrial inner membrane. The production of excess mitochondrial ROS requires more iron to pass through the mitochondrial membrane. In U87MG and T98G cells treated with arsenic trioxide, expression levels of mitoferrin-2 increased four- to fivefold, while ROS production and apoptosis decreased in the cells with low expression of mitoferrin-2. Silencing mitoferrin-2 led to a decrease in arsenic trioxide cytotoxicity. Therefore, the mitoferrin-2 transporter could participate in arsenic trioxide-induced cytotoxicity by promoting the production of ROS [45].
Other studies have shown that arsenic trioxide could induce the expression of XPC (xeroderma pigmentosum, group C) in U87 cells. XPC may be involved in the regulation of the intracellular redox dynamic balance. When it was silenced, U87 cells were more sensitive to arsenic trioxide [46].
Telomeres are a special DNA structure located at the ends of chromosomes, which are gradually shortened with each cell division. It enables cells to avoid proliferative disorder by stabilizing the telomeres, especially through overexpression of its catalytic subunit hTERT, which is found in 85% of cancers, including glioblastoma [47]. Arsenic trioxide treatment causes DNA double-strand breaks due to the related increase in intracellular ROS production. This induces phosphorylation of hTERT and its subsequent translocation from the nucleus to the cytoplasm, and dysfunction of telomerase function, resulting in apoptosis, cell cycle arrest and other cellular changes (Fig. 1) [30].
Arsenic trioxide causes G1 or G2/M phase arrest
In most solid tumor cells, arsenic trioxide induces G1 or G2/M phase arrest of the cell cycle [48]. Cell cycle arrest before mitosis occurs in response to DNA damage, enabling cells to repair damage and preventing genetic errors from being transmitted to daughter cells [49]. It may be the main response to such damage, occurring before the decision to repair or die. In glioma cells treated with a low concentration of arsenic trioxide (2 μM), the cell cycle was blocked in G2/M phase [20], and the levels of cyclinB1, aurora kinase A, and phosphorylated aurora kinase A decreased in a dose-dependent manner. There was no significant change in the levels of cyclinD1, indicating no G1/S phase block [30]. This may be because arsenic trioxide increases the expression of wild-type p53, which can inhibit the cdc25C promoter, which in turn inhibits the activation of cyclinB1, resulting in G2/M phase arrest [50].
The influence of arsenic trioxide on microRNAs (miRNAs)
The anti-cancer effects of arsenic trioxide may be related at least in part to epigenetic regulation of miRNAs. It may alter the expression levels of a large number of miRNAs with important roles in cancer–related signaling pathways by affecting DNA demethylation [51]. Many of these miRNAs are considered to be tumor suppressor genes or oncogenes.
Shidfar et al. treated U87 cells with 4 μM arsenic trioxide for 48 h and applied gene chip technology to analyze the changes inexpression of 88 cancer-related miRNAs. They found that 60 miRNAs showed increased expression and 28 showed decreased expression [52]. Nine miRNAs were significantly upregulated (i.e., a greater than four-fold increase in expression). The most strongly upregulated miRNAs were miR-215 and miR-96, with differential expression rates of about 13- and ninefold, respectively. In addition, a fourfold decrease in expression levels was detected for three miRNAs. Overall, the upregulation of miRNA was more significant, and more were upregulated than downregulated. A large proportion of the significantly upregulated miRNAs (including miR–215, -96, -126, -149, -193a-5p and -183) had inhibitory effects on tumors and metastasis. However, of the significantly downregulated miRNAs, only two (miR-27b and miR-100) have reported carcinogenic effects.
In a separate study, Wang et al. treated U251 cells with arsenic trioxide and showed that it directly inhibited potassium hERG channels at a post-transcriptional level by upregulating miR-133b, thereby causing cell apoptosis [53].
Inhibitory effects of arsenic trioxide on glioma stem cells
To some extent, the recurrence of glioblastoma is mediated by glioblastoma stem cells (GSCs). Arsenic trioxide can reduce the expression of stem cell markers (SOX2, CD133) of GSCs and inhibit the Hedgehog and Notch pathways, thus reducing their sphere forming ability, inhibiting their DNA repair ability, and reducing drug resistance [54]. A combination with (–)-gossypol can enhance this inhibitory effect [55].
Bureta et al. compared the effects of temozolomide combined either with arsenic trioxide or with the Hedgehog pathway inhibitor vismodegib. Both combinations increased the anti-glioma effect of temozolomide, with similar effects, and inhibited the Hedgehog pathway in glioblastoma [56].
When U87MG, U251MG and U373MG GSC cells were treated with arsenic trioxide, the protein levels of Notch1 and Hes1 decreased significantly, indicating that it inhibited the survival and proliferation of tumor stem cells by blocking the activity of the Notch signaling pathway [57]. A further study targeting the Notch pathway showed that treatment with 2 μM arsenic trioxide initially hindered the proliferation of neurospheres, after which the cells could recover. However, treatment with 4 μM arsenic trioxide reduced the recovery of neurosphere formation ability and significantly reduced the formation of tumors. In addition, when the remaining neurospheres were isolated and replicated, it was found that the cells treated with 4 μM arsenic trioxide no longer had the ability to renew themselves because they could not form secondary neurospheres. This phenomenon was related to the inhibition of phosphorylation and activation of AKT and STAT3 via the blocking of Notch signaling [58].
Currently, promyelocytic leukemia (PML) protein is the main target in the treatment of APL, which is the most common use of arsenic trioxide [59, 60]. In vivo and in vitro experiments showed that arsenic trioxide can reduce the expression of PML in GSCs, leading to the ubiquity in degradation of c-Myc and thus inhibiting GSCs [61].
GBM can be divided into four molecular subtypes: proneuronal, neural, classical and mesenchymal. GSCs are key promoters of the occurrence and evolution of GBM and lead to drug resistance and recurrence of tumors. The mesenchymal subtype is more invasive and has a poorer prognosis than the proneuronal subtype [62]. Bell et al. proposed that the different subtypes of GSC have different responses to arsenic trioxide, with a better therapeutic effect in proneuronal than in mesenchymal. This may be related to the overexpression of many oncogenes caused by the activation of MNK1-eIF4E signaling in the mesenchymal subtype. When arsenic trioxide and a signaling pathway inhibitor were used together, the drug resistance of mesenchymal glioblastoma could be reversed [63].
Enhanced anti-glioma effect of arsenic trioxide combined with other drugs or methods
Combined treatment strategies have become the most common approach to overcoming malignant tumor resistance to anti-cancer drugs. Arsenic trioxide has potential in combined strategies but its toxicity and side effects are an issue.
A combination of arsenic trioxide and silibinin was shown to synergistically inhibit U87 metabolic activity, cell proliferation, and gelatinase A and B activities; increase cell apoptosis; decrease the mRNA levels of cathepsin B, urokinase type plasminogen activator, matrix metallopeptidase 2 (MMP-2), MMP-9, survivin, Bcl2 and CA9; and upregulate caspase-3 mRNA expression [64]. The mechanism of the sensitization of cells to arsenic trioxide by chrysin and silybin was studied using the A172 cell line. The results showed that chrysin enhanced the cytotoxicity of arsenic trioxide by inducing glutathione (GSH) depletion and promoting arsenic accumulation.
GSH is the most important intracellular antioxidant. Its depletion can increase the toxicity of arsenic in various cell types via mechanisms including increased oxidative stress and free active arsenic concentration, loss of arsenic-bound mercaptan, and reduction in the detoxification by GSH–dependent methylation and the binding of arsenic to GSH. Silibinin sensitization did not affect GSH levels but doubled the accumulation of arsenic. Accumulation of arsenic may be related to the inhibition of the ATP-binding cassette transporter and multidrug resistance-associated protein, which would slow down the excretion of arsenic and increase its accumulation [65]. It was also found that butylthionine sulfoxide, an inhibitor of GSH biosynthesis, could deplete intracellular GSH and had a synergistic effect with arsenic trioxide in the C6 cell line [66].
Lin et al. found that berberine could enhance the arsenic trioxide-mediated inhibition of the migration and invasiveness of glioma by reducing the activation of protein kinase C signaling and MMP-2 in the extracellular matrix [67].
Mesbahi et al. found that the most common genetic change in malignant glioma was the amplification of epidermal growth factor receptors (EGFR, ERRB1 or HER1). Arsenic trioxide treatment alone could induce the activation of the EGFR signaling pathway in U87MG and A172 cells. Over activation of this pathway may be directly related to resistance to arsenic trioxide as a drug. When erlotinib, an inhibitor of EGFR, was used in combination with arsenic trioxide, ROS levels increased and there were significant synergistic effects on survival, proliferation and migration as well as on the cell cycle [68]. The activation of downstream products of EGFR is related to the regulation of c-Myc. A combination of arsenic trioxide and c-Myc inhibitors can also inhibit glioma [69].
Many researchers have also studied the combined effects of arsenic trioxide and radiotherapy. Ning et al. treated U87MG and SNB75 cells with a combination of radiotherapy and arsenic trioxide in vivo and in vitro. The results showed that arsenic trioxide sensitized glioma to radiation, with the greatest effect when it was administered 4 h after radiotherapy. However, the effects of arsenic trioxide alone or before radiotherapy were limited and this form of radio sensitization was time and sequence dependent [70, 71]. This may be because pre-administration leads to vascular closure, which lasts long enough for the tumor cells to produce a compensatory antioxidant response to both arsenic trioxide and the ROS produced by radiotherapy. By contrast, post-radiation administration of arsenic trioxide as a maintenance therapy may induce further damage through ROS and potentially reduce the ability of the tumor cells to repair the radiation-induced damage [72, 73].
A study of the influence of 2 μM arsenic trioxide and 4 Gy irradiation on U118-MG cells found that the proportion of mitotic cells increased significantly. Arsenic trioxide may inhibit the activation of G2-phase DNA damage checkpoints, thereby allowing cells with DNA damage to enter mitosis from the G2 phase. Entering mitosis in the presence of DNA damage leads to centro some inactivation and obstruction of chromosome separation, delaying the mitotic process and inducing mitotic abnormalities. This could lead to mitotic arrest and subsequent autophagy in malignant glioma cells. This combination could also induce autophagy of U118–MG cells by inhibiting PI3K/Akt and activating ERK1/2 signaling pathways [26].
A clinical trial of arsenic trioxide in glioma treatment
Before 2000, clinical trials of arsenic trioxide focused on the treatment of APL. After treatment with a dose of 0.15 mg/kg/day for 12 to 39 days, most patients with APL achieved complete remission [74]. After its approval as a chemotherapeutic drug for APL by the U.S. Food and Drug Administration in 2000, trials were carried out to determine the clinical effects of arsenic trioxide on various solid tumors, including liver cancer [75], renal cell carcinoma [76], germ cell tumors [77], urothelial tumors [78], pancreatic cancer [79], breast cancer [80], head and neck cancers [81], and colon cancer [82, 83]. Based on the results, arsenic trioxide alone was not recommended as a chemotherapeutic drug for solid tumors.
Phase I and II clinical trials of glioma therapies were conducted at Northwestern University in Chicago to select the best combination dosage of arsenic trioxide, temozolomide and radiotherapy. When arsenic trioxide (0.2 mg/kg) was added to the standard glioma treatment regimen of temozolomide combined with chemotherapy, the survival rate of patients with anaplastic astrocytoma increased, but there was no significant improvement in patients with malignant glioblastoma [84, 85]. This may have been related to the presence of the blood–tumor barrier and the blood–brain barrier (BBB). Although there is evidence that gliomas involve partial BBB rupture or that a small amount of arsenic trioxide can pass through the BBB, this has not been shown to be sufficient for a curative effect. The low permeability of the BBB to drugs, including arsenic trioxide greatly limits the treatment of glioma. Increasing the ability of arsenic trioxide to pass through the BBB is key to improving clinical treatment.
Transmittance of arsenic trioxide to the BBB
In the in vitro BBB model constructed by Tao et al., the transport ratio of arsenic trioxide was 2.21 ± 0.19%. In vivo, the elimination half-life of arsenic trioxide at the end of treatment was 4.41 ± 0.87 h, and the area under the drug-time curve was 0.18 ± 0.03 μg h/ml. After 24 h of intravenous injection, the arsenic trioxide uptake rate in glioma was 0.8 ± 0.3% ID/g, and the mean residence time was 6.17 ± 1.29 h. In this study, polyacrylic acid (PAA) was grafted onto mesoporous silica nanoparticle (MSN) cores to form MSN lipid capsule nanoparticles modified by self-assembled angiopep-2 (amino acid residues TFFYGGSRGKRNNFKTEEY). These could be loaded with arsenic trioxide (ANG-LP-PAA-MSN@As2O3).Owing to the specific recognition and binding of the BBB by angiopep-2, and the high expression of low-density lipoprotein related receptor 1 in glioma cells, the ability of arsenic trioxide to cross the BBB and target glioma increased, and pH-sensitive release [86]. The same research group used polyamidoamine (PAMAM) as a nano-carrier, which was connected to arginine glycine aspartic acid (RGD) for tumor targeting and loaded with arsenic trioxide to form RGDyC mPEG PAMAM/arsenic trioxide. Therapeutic drug delivery could also be achieved by combining arsenic trioxide with integrin αvβ3 [87].
Conclusions
Although arsenic trioxide exerts anti-glioma effects through a variety of mechanisms, multidrug resistance and the existence of the BBB render glioma insensitive to it. In addition, serious adverse reactions to high dose arsenic trioxide limit its clinical applications. Therefore, it is necessary to further study the efficacy on glioma of a combination of drugs with arsenic trioxide as well as the efficacy of drugs encapsulated with arsenic trioxide carriers.
In addition, prior to preclinical trials and clinical application, precision medicine principles should be used to guide experimental research. It is essential that we search for tissue markers of glioma that are sensitive to arsenic trioxide. Finally, most studies to date have been conducted in vitro. Therefore, more preclinical and clinical studies should be conducted to elucidate the anti-cancer effects of arsenic trioxide in the treatment of glioma.
Acknowledgements
Not applicable.
Abbreviations
- APL
Acute promyelocytic leukemia
- BBB
Bood–brain barrier
- CHOP
CCAAT/enhancer binding protein homologous protein
- EGFR
Epidermal growth factor receptor
- GSH
Glutathione
- GSCs
Glioblastoma stem cells
- HO-1
Heme oxygenase-1
- MMP
Matrix metallopeptidase
- MSN
Mesoporous silica nanoparticles
- MT
Metallothionein
- PAA
Polyacrylic acid
- PAMAM
Polyamidoamine
- RGD
Arginine–glycine–aspartic acid
- ROS
Reactive oxygen species
- TRAIL
Tumor necrosis factor-related apoptotic ligand
- XPC
Xeroderma pigmentosum, group C
Authors’ contributions
YF was a major contributor to writing the manuscript. ZZ read and approved the final manuscript. Both authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 81971639).
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Price RL, Chiocca EA. Evolution of malignant glioma treatment: from chemotherapy to vaccines to viruses. Neurosurgery. 2014;61(Suppl 1):74–83. doi: 10.1227/NEU.0000000000000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Becker KP, Yu J. Status quo––standard–of–care medical and radiation therapy for glioblastoma. Cancer J. 2012;18(1):12–19. doi: 10.1097/PPO.0b013e318244d7eb. [DOI] [PubMed] [Google Scholar]
- 3.Milano MT, Johnson MD, Sul J, Mohile NA, Korones DN, Okunieff P, et al. Primary spinal cord glioma: a Surveillance, Epidemiology, and End Results database study. J Neurooncol. 2010;98(1):83–92. doi: 10.1007/s11060-009-0054-7. [DOI] [PubMed] [Google Scholar]
- 4.Liu J, Lu Y, Wu Q, Goyer RA, Waalkes MP. Mineral arsenicals in traditional medicines: orpiment, realgar, and arsenolite. J Pharmacol Exp Ther. 2008;326(2):363–368. doi: 10.1124/jpet.108.139543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang X, Jia S, Yang S, Yang Y, Yang T, Yang Y. Arsenic trioxide induces G2/M arrest in hepatocellular carcinoma cells by increasing the tumor suppressor PTEN expression. J Cell Biochem. 2012;113(11):3528–3535. doi: 10.1002/jcb.24230. [DOI] [PubMed] [Google Scholar]
- 6.Walker AM, Stevens JJ, Ndebele K, Tchounwou PB. Evaluation of arsenic trioxide potential for lung cancer treatment: assessment of apoptotic mechanisms and oxidative damage. J Cancer Sci Ther. 2016;8(1):1–9. doi: 10.4172/1948-5956.1000379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chow SK, Chan JY, Fung KP. Inhibition of cell proliferation and the action mechanisms of arsenic trioxide (As2O3) on human breast cancer cells. J Cell Biochem. 2004;93(1):173–187. doi: 10.1002/jcb.20102. [DOI] [PubMed] [Google Scholar]
- 8.Hai JJ, Gill H, Tse HF, Kumana CR, Kwong YL, Siu CW. Torsade de Pointes during oral arsenic trioxide therapy for acute promyelocytic leukemia in a patient with heart failure. Ann Hematol. 2015;94(3):501–503. doi: 10.1007/s00277-014-2174-1. [DOI] [PubMed] [Google Scholar]
- 9.Alamolhodaei NS, Shirani K, Karimi G. Arsenic cardiotoxicity: an overview. Environ Toxicol Pharmacol. 2015;40(3):1005–1014. doi: 10.1016/j.etap.2015.08.030. [DOI] [PubMed] [Google Scholar]
- 10.Jiao YH, Zhang Q, Pan LL, Chen XY, Lei KL, Zhao J, et al. Rat liver mitochondrial dysfunction induced by an organic arsenical compound 4–(2–nitrobenzaliminyl) phenyl arsenoxide. J Membr Biol. 2015;248(6):1071–1078. doi: 10.1007/s00232-015-9818-5. [DOI] [PubMed] [Google Scholar]
- 11.Bursch W, Hochegger K, Torok L, Marian B, Ellinger A, Hermann RS. Autophagic and apoptotic types of programmed cell death exhibit different fates of cytoskeletal filaments. J Cell Sci. 2000;113(Pt 7):1189–1198. doi: 10.1242/jcs.113.7.1189. [DOI] [PubMed] [Google Scholar]
- 12.Bursch W, Ellinger A, Gerner C, Frohwein U, Schulte-Hermann R. Programmed cell death (PCD)* Apoptosis, autophagic PCD, or others? Ann N Y Acad Sci. 2000;926:1–12. [DOI] [PubMed]
- 13.Beurel E, Jope RS. The paradoxical pro– and anti–apoptotic actions of GSK3 in the intrinsic and extrinsic apoptosis signaling pathways. Prog Neurobiol. 2006;79(4):173–189. doi: 10.1016/j.pneurobio.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schweichel JU, Merker HJ. The morphology of various types of cell death in prenatal tissues. Teratology. 1973;7(3):253–266. doi: 10.1002/tera.1420070306. [DOI] [PubMed] [Google Scholar]
- 15.White E. The role for autophagy in cancer. J Clin Invest. 2015;125(1):42–46. doi: 10.1172/JCI73941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dalby KN, Tekedereli I, Lopez-Berestein G, Ozpolat B. Targeting the prodeath and prosurvival functions of autophagy as novel therapeutic strategies in cancer. Autophagy. 2010;6(3):322–329. doi: 10.4161/auto.6.3.11625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng Y, Qiu F, Ikejima T. Molecular mechanisms of oridonin–induced apoptosis and autophagy in murine fibrosarcoma L929 cells. Autophagy. 2009;5(3):430–431. doi: 10.4161/auto.5.3.7896. [DOI] [PubMed] [Google Scholar]
- 18.Liu B, Cheng Y, Zhang B, Bian HJ, Bao JK. Polygonatum cyrtonema lectin induces apoptosis and autophagy in human melanoma A375 cells through a mitochondria-mediated ROS-p38-p53 pathway. Cancer Lett. 2009;275(1):54–60. doi: 10.1016/j.canlet.2008.09.042. [DOI] [PubMed] [Google Scholar]
- 19.Kanzawa T, Zhang L, Xiao L, Germano IM, Kondo Y, Kondo S. Arsenic trioxide induces autophagic cell death in malignant glioma cells by upregulation of mitochondrial cell death protein BNIP3. Oncogene. 2005;24(6):980–991. doi: 10.1038/sj.onc.1208095. [DOI] [PubMed] [Google Scholar]
- 20.Kanzawa T, Kondo Y, Ito H, Kondo S, Germano I. Induction of autophagic cell death in malignant glioma cells by arsenic trioxide. Cancer Res. 2003;63(9):2103–2108. [PubMed] [Google Scholar]
- 21.Primon M, Huszthy PC, Motaln H, Talasila KM, Torkar A, Bjerkvig R, et al. Cathepsin L silencing enhances arsenic trioxide mediated in vitro cytotoxicity and apoptosis in glioblastoma U87MG spheroids. Exp Cell Res. 2013;319(17):2637–2648. doi: 10.1016/j.yexcr.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 22.Hoyer-Hansen M, Jaattela M. Autophagy: an emerging target for cancer therapy. Autophagy. 2008;4(5):574–580. doi: 10.4161/auto.5921. [DOI] [PubMed] [Google Scholar]
- 23.Karsy M, Albert L, Murali R, Jhanwar-Uniyal M. The impact of arsenic trioxide and all-trans retinoic acid on p53 R273H–codon mutant glioblastoma. Tumour Biol. 2014;35(5):4567–4580. doi: 10.1007/s13277-013-1601-6. [DOI] [PubMed] [Google Scholar]
- 24.Wang GB, Liu JH, Hu J, Xue K. Mechanism of As2O3 induces apoptosis of glioma U87 cells. Eur Rev Med Pharmacol Sci. 2017;21(21):4875–4881. [PubMed] [Google Scholar]
- 25.Cheng TJ, Wang YJ, Kao WW, Chen RJ, Ho YS. Protection against arsenic trioxide–induced autophagic cell death in U118 human glioma cells by use of lipoic acid. Food Chem Toxicol. 2007;45(6):1027–1038. doi: 10.1016/j.fct.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 26.Chiu HW, Ho SY, Guo HR, Wang YJ. Combination treatment with arsenic trioxide and irradiation enhances autophagic effects in U118-MG cells through increased mitotic arrest and regulation of PI3K/Akt and ERK1/2 signaling pathways. Autophagy. 2009;5(4):472–483. doi: 10.4161/auto.5.4.7759. [DOI] [PubMed] [Google Scholar]
- 27.Ng G, Huang J. The significance of autophagy in cancer. Mol Carcinog. 2005;43(4):183–187. doi: 10.1002/mc.20097. [DOI] [PubMed] [Google Scholar]
- 28.Chiu HW, Ho YS, Wang YJ. Arsenic trioxide induces autophagy and apoptosis in human glioma cells in vitro and in vivo through downregulation of survivin. J Mol Med (Berl) 2011;89(9):927–941. doi: 10.1007/s00109-011-0763-1. [DOI] [PubMed] [Google Scholar]
- 29.Zhao S, Zhang J, Zhang X, Dong X, Sun X. Arsenic trioxide induces different gene expression profiles of genes related to growth and apoptosis in glioma cells dependent on the p53 status. Mol Biol Rep. 2008;35(3):421–429. doi: 10.1007/s11033-007-9102-6. [DOI] [PubMed] [Google Scholar]
- 30.Cheng Y, Li Y, Ma C, Song Y, Xu H, Yu H, et al. Arsenic trioxide inhibits glioma cell growth through induction of telomerase displacement and telomere dysfunction. Oncotarget. 2016;7(11):12682–12692. doi: 10.18632/oncotarget.7259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim EH, Yoon MJ, Kim SU, Kwon TK, Sohn S, Choi KS. Arsenic trioxide sensitizes human glioma cells, but not normal astrocytes, to TRAIL-induced apoptosis via CCAAT/enhancer-binding protein homologous protein–dependent DR5 up-regulation. Cancer Res. 2008;68(1):266–275. doi: 10.1158/0008-5472.CAN-07-2444. [DOI] [PubMed] [Google Scholar]
- 32.Song JH, Bellail A, Tse MC, Yong VW, Hao C. Human astrocytes are resistant to Fas ligand and tumor necrosis factor–related apoptosis-inducing ligand-induced apoptosis. J Neurosci. 2006;26(12):3299–3308. doi: 10.1523/JNEUROSCI.5572-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perillo NL, Pace KE, Seilhamer JJ, Baum LG. Apoptosis of T cells mediated by galectin-1. Nature. 1995;378(6558):736–739. doi: 10.1038/378736a0. [DOI] [PubMed] [Google Scholar]
- 34.Jiang CC, Chen LH, Gillespie S, Kiejda KA, Mhaidat N, Wang YF, et al. Tunicamycin sensitizes human melanoma cells to tumor necrosis factor–related apoptosis-inducing ligand-induced apoptosis by up-regulation of TRAIL-R2 via the unfolded protein response. Cancer Res. 2007;67(12):5880–5888. doi: 10.1158/0008-5472.CAN-07-0213. [DOI] [PubMed] [Google Scholar]
- 35.Xu YY, Lu Y, Fan KY, Shen ZH. Apoptosis induced by all-trans retinoic acid in N-acetylglucosaminyltransferase V repressed human hepatocarcinoma cells is mediated through endoplasmic reticulum stress. J Cell Biochem. 2007;100(3):773–782. doi: 10.1002/jcb.21088. [DOI] [PubMed] [Google Scholar]
- 36.Jiang J, Shen J, Wu T, Wei Y, Chen X, Zong H, et al. Down-regulation of beta1,4-galactosyltransferase V is a critical part of etoposide-induced apoptotic process and could be mediated by decreasing Sp1 levels in human glioma cells. Glycobiology. 2006;16(11):1045–1051. doi: 10.1093/glycob/cwl027. [DOI] [PubMed] [Google Scholar]
- 37.Wei Y, Liu D, Ge Y, Zhou F, Xu J, Chen H, et al. Down-regulation of beta1,4GalT V at protein level contributes to arsenic trioxide-induced glioma cell apoptosis. Cancer Lett. 2008;267(1):96–105. doi: 10.1016/j.canlet.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 38.Lee SJ, Koh JY. Roles of zinc and metallothionein-3 in oxidative stress-induced lysosomal dysfunction, cell death, and autophagy in neurons and astrocytes. Mol Brain. 2010;3(1):30. doi: 10.1186/1756-6606-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Falnoga I, Zelenik Pevec A, Slejkovec Z, Znidaric MT, Zajc I, Mlakar SJ, et al. Arsenic trioxide (ATO) influences the gene expression of metallothioneins in human glioblastoma cells. Biol Trace Elem Res. 2012;149(3):331–339. doi: 10.1007/s12011-012-9431-8. [DOI] [PubMed] [Google Scholar]
- 40.Gerlach M, Ben-Shachar D, Riederer P, Youdim MB. Altered brain metabolism of iron as a cause of neurodegenerative diseases? J Neurochem. 1994;63(3):793–807. doi: 10.1046/j.1471-4159.1994.63030793.x. [DOI] [PubMed] [Google Scholar]
- 41.Haga N, Fujita N, Tsuruo T. Involvement of mitochondrial aggregation in arsenic trioxide (As2O3)-induced apoptosis in human glioblastoma cells. Cancer Sci. 2005;96(11):825–833. doi: 10.1111/j.1349-7006.2005.00114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun Y, Wang C, Wang L, Dai Z, Yang K. Arsenic trioxide induces apoptosis and the formation of reactive oxygen species in rat glioma cells. Cell Mol Biol Lett. 2018;23:13. doi: 10.1186/s11658-018-0074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu Y, Liang Y, Zheng T, Yang G, Zhang X, Sun Z, et al. Inhibition of heme oxygenase-1 enhances anti-cancer effects of arsenic trioxide on glioma cells. J Neurooncol. 2011;104(2):449–458. doi: 10.1007/s11060-010-0513-1. [DOI] [PubMed] [Google Scholar]
- 44.Imeryuz N, Tahan V, Sonsuz A, Eren F, Uraz S, Yuksel M, et al. Iron preloading aggravates nutritional steatohepatitis in rats by increasing apoptotic cell death. J Hepatol. 2007;47(6):851–859. doi: 10.1016/j.jhep.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 45.Wang C, Chen X, Zou H, Chen X, Liu Y, Zhao S. The roles of mitoferrin-2 in the process of arsenic trioxide-induced cell damage in human gliomas. Eur J Med Res. 2014;19:49. doi: 10.1186/s40001-014-0049-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu SY, Wen CY, Lee YJ, Lee TC. XPC silencing sensitizes glioma cells to arsenic trioxide via increased oxidative damage. Toxicol Sci. 2010;116(1):183–193. doi: 10.1093/toxsci/kfq113. [DOI] [PubMed] [Google Scholar]
- 47.Shay JW, Keith WN. Targeting telomerase for cancer therapeutics. Br J Cancer. 2008;98(4):677–683. doi: 10.1038/sj.bjc.6604209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Y, Qu X, Qu J, Zhang Y, Liu J, Teng Y, et al. Arsenic trioxide induces apoptosis and G2/M phase arrest by inducing Cbl to inhibit PI3K/Akt signaling and thereby regulate p53 activation. Cancer Lett. 2009;284(2):208–215. doi: 10.1016/j.canlet.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 49.Wahl GM, Carr AM. The evolution of diverse biological responses to DNA damage: insights from yeast and p53. Nat Cell Biol. 2001;3(12):E277–E286. doi: 10.1038/ncb1201-e277. [DOI] [PubMed] [Google Scholar]
- 50.Chen J. The Cell-Cycle Arrest and Apoptotic Functions of p53 in Tumor Initiation and Progression. Cold Spring Harb Perspect Med. 2016;6(3):a026104. doi: 10.1101/cshperspect.a026104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou X, Sun H, Ellen TP, Chen H, Costa M. Arsenite alters global histone H3 methylation. Carcinogenesis. 2008;29(9):1831–1836. doi: 10.1093/carcin/bgn063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shidfar F, Ghaffari SH, Tavoosidana G, Hosseini E, Alimoghaddam K, Ghavamzadeh A. Arsenic trioxide Alters the MicroRNA Expression Profile of U87 glioblastoma. Anticancer Agents Med Chem. 2015;16(2):247–258. doi: 10.2174/1871520615666150629100752. [DOI] [PubMed] [Google Scholar]
- 53.Wang J, Li Y, Jiang C. MiR–133b contributes to arsenic–induced apoptosis in U251 glioma cells by targeting the hERG channel. J Mol Neurosci. 2015;55(4):985–994. doi: 10.1007/s12031-014-0455-8. [DOI] [PubMed] [Google Scholar]
- 54.Ding D, Lim KS, Eberhart CG. Arsenic trioxide inhibits Hedgehog, Notch and stem cell properties in glioblastoma neurospheres. Acta Neuropathol Commun. 2014;2:31. doi: 10.1186/2051-5960-2-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Linder B, Wehle A, Hehlgans S, Bonn F, Dikic I, Rodel F, et al. Arsenic trioxide and (–)–gossypol synergistically target glioma stem-like cells via inhibition of hedgehog and notch signaling. Cancers (Basel). 2019;11(3). [DOI] [PMC free article] [PubMed]
- 56.Bureta C, Saitoh Y, Tokumoto H, Sasaki H, Maeda S, Nagano S, et al. Synergistic effect of arsenic trioxide, vismodegib and temozolomide on glioblastoma. Oncol Rep. 2019;41(6):3404–3412. doi: 10.3892/or.2019.7100. [DOI] [PubMed] [Google Scholar]
- 57.Zhen Y, Zhao S, Li Q, Li Y, Kawamoto K. Arsenic trioxide-mediated Notch pathway inhibition depletes the cancer stem-like cell population in gliomas. Cancer Lett. 2010;292(1):64–72. doi: 10.1016/j.canlet.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 58.Wu J, Ji Z, Liu H, Liu Y, Han D, Shi C, et al. Arsenic trioxide depletes cancer stem-like cells and inhibits repopulation of neurosphere derived from glioblastoma by downregulation of Notch pathway. Toxicol Lett. 2013;220(1):61–69. doi: 10.1016/j.toxlet.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 59.Zhang XW, Yan XJ, Zhou ZR, Yang FF, Wu ZY, Sun HB, et al. Arsenic trioxide controls the fate of the PML–RARalpha oncoprotein by directly binding PML. Science. 2010;328(5975):240–243. doi: 10.1126/science.1183424. [DOI] [PubMed] [Google Scholar]
- 60.Kaiming C, Sheng Y, Zheng S, Yuan S, Huang G, Liu Y. Arsenic trioxide preferentially binds to the ring finger protein PML: understanding target selection of the drug. Metallomics. 2018;10(11):1564–1569. doi: 10.1039/c8mt00202a. [DOI] [PubMed] [Google Scholar]
- 61.Zhou W, Cheng L, Shi Y, Ke SQ, Huang Z, Fang X, et al. Arsenic trioxide disrupts glioma stem cells via promoting PML degradation to inhibit tumor growth. Oncotarget. 2015;6(35):37300–37315. doi: 10.18632/oncotarget.5836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Segerman A, Niklasson M, Haglund C, Bergstrom T, Jarvius M, Xie Y, et al. Clonal variation in drug and radiation response among glioma-initiating cells is linked to proneural-mesenchymal transition. Cell Rep. 2016;17(11):2994–3009. doi: 10.1016/j.celrep.2016.11.056. [DOI] [PubMed] [Google Scholar]
- 63.Bell JB, Eckerdt F, Dhruv HD, Finlay D, Peng S, Kim S, et al. Differential response of glioma stem cells to arsenic trioxide therapy is regulated by MNK1 and mRNA translation. Mol Cancer Res. 2018;16(1):32–46. doi: 10.1158/1541-7786.MCR-17-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dizaji MZ, Malehmir M, Ghavamzadeh A, Alimoghaddam K, Ghaffari SH. Synergistic effects of arsenic trioxide and silibinin on apoptosis and invasion in human glioblastoma U87MG cell line. Neurochem Res. 2012;37(2):370–380. doi: 10.1007/s11064-011-0620-1. [DOI] [PubMed] [Google Scholar]
- 65.Gulden M, Appel D, Syska M, Uecker S, Wages F, Seibert H. Chrysin and silibinin sensitize human glioblastoma cells for arsenic trioxide. Food Chem Toxicol. 2017;105:486–497. doi: 10.1016/j.fct.2017.04.035. [DOI] [PubMed] [Google Scholar]
- 66.Klauser E, Gulden M, Maser E, Seibert S, Seibert H. Additivity, antagonism, and synergy in arsenic trioxide–induced growth inhibition of C6 glioma cells: effects of genistein, quercetin and buthionine–sulfoximine. Food Chem Toxicol. 2014;67:212–221. doi: 10.1016/j.fct.2014.02.039. [DOI] [PubMed] [Google Scholar]
- 67.Lin TH, Kuo HC, Chou FP, Lu FJ. Berberine enhances inhibition of glioma tumor cell migration and invasiveness mediated by arsenic trioxide. BMC Cancer. 2008;8:58. doi: 10.1186/1471-2407-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mesbahi Y, Zekri A, Ahmadian S, Alimoghaddam K, Ghavamzadeh A, Ghaffari SH. Targeting of EGFR increase anti–cancer effects of arsenic trioxide: promising treatment for glioblastoma multiform. Eur J Pharmacol. 2018;820:274–285. doi: 10.1016/j.ejphar.2017.12.041. [DOI] [PubMed] [Google Scholar]
- 69.Yoshimura Y, Shiino A, Muraki K, Fukami T, Yamada S, Satow T, et al. Arsenic trioxide sensitizes glioblastoma to a myc inhibitor. PLoS ONE. 2015;10(6):e0128288. doi: 10.1371/journal.pone.0128288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ning S, Knox SJ. Optimization of combination therapy of arsenic trioxide and fractionated radiotherapy for malignant glioma. Int J Radiat Oncol Biol Phys. 2006;65(2):493–498. doi: 10.1016/j.ijrobp.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 71.Ning S, Knox SJ. Increased cure rate of glioblastoma using concurrent therapy with radiotherapy and arsenic trioxide. Int J Radiat Oncol Biol Phys. 2004;60(1):197–203. doi: 10.1016/j.ijrobp.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 72.Lew YS, Kolozsvary A, Brown SL, Kim JH. Synergistic interaction with arsenic trioxide and fractionated radiation in locally advanced murine tumor. Cancer Res. 2002;62(15):4202–4205. [PubMed] [Google Scholar]
- 73.Lew YS, Brown SL, Griffin RJ, Song CW, Kim JH. Arsenic trioxide causes selective necrosis in solid murine tumors by vascular shutdown. Cancer Res. 1999;59(24):6033–6037. [PubMed] [Google Scholar]
- 74.Soignet SL, Maslak P, Wang ZG, Jhanwar S, Calleja E, Dardashti LJ, et al. Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N Engl J Med. 1998;339(19):1341–1348. doi: 10.1056/NEJM199811053391901. [DOI] [PubMed] [Google Scholar]
- 75.Lin CC, Hsu C, Hsu CH, Hsu WL, Cheng AL, Yang CH. Arsenic trioxide in patients with hepatocellular carcinoma: a phase II trial. Invest New Drugs. 2007;25(1):77–84. doi: 10.1007/s10637-006-9004-9. [DOI] [PubMed] [Google Scholar]
- 76.Vuky J, Yu R, Schwartz L, Motzer RJ. Phase II trial of arsenic trioxide in patients with metastatic renal cell carcinoma. Invest New Drugs. 2002;20(3):327–330. doi: 10.1023/a:1016270206374. [DOI] [PubMed] [Google Scholar]
- 77.Beer TM, Tangen CM, Nichols CR, Margolin KA, Dreicer R, Stephenson WT, et al. Southwest Oncology Group phase II study of arsenic trioxide in patients with refractory germ cell malignancies. Cancer. 2006;106(12):2624–2629. doi: 10.1002/cncr.21925. [DOI] [PubMed] [Google Scholar]
- 78.Lin CC, Pu YS, Hsu CH, Keng HY, Cheng AL, Yang CH. Acute encephalopathy following arsenic trioxide for metastatic urothelial carcinoma. Urol Oncol. 2008;26(6):659–661. doi: 10.1016/j.urolonc.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 79.Kindler HL, Aklilu M, Nattam S, Vokes EE. Arsenic trioxide in patients with adenocarcinoma of the pancreas refractory to gemcitabine: a phase II trial of the University of Chicago Phase II Consortium. Am J Clin Oncol. 2008;31(6):553–556. doi: 10.1097/COC.0b013e318178e4cd. [DOI] [PubMed] [Google Scholar]
- 80.Lai YL, Chang HH, Huang MJ, Chang KH, Su WH, Chen HW, et al. Combined effect of topical arsenic trioxide and radiation therapy on skin-infiltrating lesions of breast cancer-a pilot study. Anticancer Drugs. 2003;14(10):825–828. doi: 10.1097/00001813-200311000-00008. [DOI] [PubMed] [Google Scholar]
- 81.Huilgol NG. A phase I study to study arsenic trioxide with radiation and hyperthermia in advanced head and neck cancer. Int J Hyperthermia. 2006;22(5):391–397. doi: 10.1080/02656730600722685. [DOI] [PubMed] [Google Scholar]
- 82.Ardalan B, Subbarayan PR, Ramos Y, Gonzalez M, Fernandez A, Mezentsev D, et al. A phase I study of 5-fluorouracil/leucovorin and arsenic trioxide for patients with refractory/relapsed colorectal carcinoma. Clin Cancer Res. 2010;16(11):3019–3027. doi: 10.1158/1078-0432.CCR-09-2590. [DOI] [PubMed] [Google Scholar]
- 83.Podolsky L, Oh M, Subbarayan PR, Francheschi D, Livingstone A, Ardalan B. 5–Fluorouracil/Leucovorin and arsenic trioxide for patients with refractory/relapsed colorectal carcinoma: a clinical experience. Acta Oncol. 2011;50(4):602–605. doi: 10.3109/0284186X.2010.524934. [DOI] [PubMed] [Google Scholar]
- 84.Kumthekar P, Grimm S, Chandler J, Mehta M, Marymont M, Levy R, et al. A phase II trial of arsenic trioxide and temozolomide in combination with radiation therapy for patients with malignant gliomas. J Neurooncol. 2017;133(3):589–594. doi: 10.1007/s11060-017-2469-x. [DOI] [PubMed] [Google Scholar]
- 85.Grimm SA, Marymont M, Chandler JP, Muro K, Newman SB, Levy RM, et al. Phase I study of arsenic trioxide and temozolomide in combination with radiation therapy in patients with malignant gliomas. J Neurooncol. 2012;110(2):237–243. doi: 10.1007/s11060-012-0957-6. [DOI] [PubMed] [Google Scholar]
- 86.Tao J, Fei W, Tang H, Li C, Mu C, Zheng H, et al. Angiopep-2-conjugated "Core–Shell" hybrid nanovehicles for targeted and pH-triggered delivery of arsenic trioxide into glioma. Mol Pharm. 2019;16(2):786–797. doi: 10.1021/acs.molpharmaceut.8b01056. [DOI] [PubMed] [Google Scholar]
- 87.Lu Y, Han S, Zheng H, Ma R, Ping Y, Zou J, et al. A novel RGDyC/PEG co-modified PAMAM dendrimer-loaded arsenic trioxide of glioma targeting delivery system. Int J Nanomed. 2018;13:5937–5952. doi: 10.2147/IJN.S175418. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.