Abstract
Agaricus blazei Murill (AbM) is a mushroom belonging to the Basidiomycetes family, which is believed to have antitumor and antioxidative activities. Proteoglycans and ergosterol are considered the key compounds of AbM for antitumor properties and so are used in complementary and alternative medicine as an anticancer drug. AbM is used to avoid serious side effects that would inevitably affect patients. Currently, the efficacy of AbM against chronic myeloid leukemia (CML) has not been established. The present study aimed to investigate the antitumor activities of the acidic RNA protein complex, FA-2-b-β, extracted from wild edible AbM. The CML K562 cells or primary CML bone marrow (BM) cells were treated with FA-2-b-β at different concentrations and time points. CML cell line proliferation and apoptosis were determined using the CCK-8 assay or Annexin V/propidium iodide (PI) labeling, RT-qPCR and western blotting was performed to determine the involvement of the Wnt/β-catenin-associated apoptotic pathway. The results of the present study demonstrated that FA-2-b-β has a high anti-proliferative potency and strong pro-apoptotic effects. Thus, daily intake of mushrooms containing FA-2-b-β may be an adequate source as an alternative medicine in the management of CML, and may provide useful information for the development of a novel therapeutic target in this area.
Keywords: CML, AbM, Wnt/β-catenin signaling pathway, apoptosis cycle
Introduction
Chronic myeloid leukemia (CML) is a myeloproliferative disorder that stems from myeloid CD34+/CD38–/CD44+ progenitors in the bone marrow (BM) (1). The incomplete differentiation of hematopoietic stem cells in CML results in immature leucocytes in both the BM and peripheral blood (PB) (2). Translocation of the abelson (ABL1) gene at chromosome 9 produces the so called ‘Philadelphia chromosome,’ which is considered the main cause of CML (3). This mutation activates the BCR-ABL1 fusion oncoprotein, which modulates a number of signaling pathways and enables stem cells to avoid cell death. Furthermore, this potent tyrosine kinase (TK) activates different signaling pathways, including the Wnt/β-catenin, Bcl-2 and JAK/STAT MAPK/JNK signaling pathways, that are responsible for the pathogenesis of CML (3–5). Thus, BCR-ABL1 is considered a therapeutic chemotherapy target for CML, using the imatinib class of drugs. However, the main drawback of therapeutic TK inhibitors is the severe side effects exhibited in patients with CML (6), including cardiovascular, pulmonary, gastrointestinal and endocrine toxicities (7), as well as development of resistance (8). Thus, it is essential to investigate alternative agents to minimize the side effects.
Complementary and alternative medicine, extensively used for the treatment of disease (9), may be an alternative approach in decreasing the side effects of patients with cancer undergoing chemotherapy, which compromises patients quality of life (10). Natural compounds are well recognized for the management of several diseases, including CML (11). Mushroom-derived compounds have the ability to act as anti-leukemia, antiviral, antioxidant and anti-inflammatory agents, and exert tumor apoptosis-inducing properties (12). Additionally, it has been reported that chemical substances derived from microorganisms, plants and animals possess anticancer potential (13). Thus, it is of great interest to investigate whether there is anti-CML potential associated with different mushrooms and determine the underlying molecular mechanism of action.
Agaricus blazei Murill (AbM) has been extensively applied in alternative medicine in Brazil since 1960 (11). It has been reported that the putative active functions of AbM lie in relieving atopic dermatitis and decreasing glycosuria (9). Although several polysaccharides contain AbM, including dextran, mannan and heteropolysaccharides (14,15), the molecular mechanism underlying such agents in anticancer activity is yet to be investigated. The most useful compound derived from AbM for anticancer activity is a proteoglucan, acid RNA protein complex, FA-2-b-β, which functions in regulating natural killer cell and macrophage activity (16). In addition, AbM-derived linoleic acid is a bactericidal agent that regulates host immunity, enhancing helper T cell expansion and natural killer cell activation, and helping to decrease the percentage of body fat and visceral fat, as well as blood cholesterol and glucose levels in human volunteers of clinical research (14,17). This evidence strongly supports the use of AbM as a health-promoting supplement for immunomodulation (14,17). Steroids have also been identified as an effective component in AbM against acute monocytic leukemia (18).
The Wnt/β-catenin signaling pathway is involved in embryogenesis, cell development, proliferation, differentiation and self-renewal, and homeostasis of multiple organ systems (19). Thus, dysregulation of the Wnt/β-catenin signaling pathway is closely associated with tumorigenesis (20). For example, upregulation of β-catenin is observed in CML, with the expansion of the BCR/ABL gene (21). Conversely, inhibition of β-catenin expression decreases drug resistance in CML, with delayed entry into the blast phase (22).
However, the ability of AbM to inhibit human leukemia in vitro remains unknown. Thus, the present study aimed to investigate the effect of FA-2-b-β, derived from AbM, in the induction of apoptosis in human leukemia cells in vitro, and considered whether such a potential effect targeted the Wnt/β-catenin signaling pathway. The results of the present study provide insight on naturally-derived compounds in the development of a novel therapeutic target in the management of CML.
Materials and methods
Cell culture and reagents
The CML K562 cells and primary CML BM cells from four female patients and one male patient with CML (mean age, 48 years; age range, 40–56 years) were collected. CML K562 cells were purchased from Lanzhou University Medical School (Lanzhou, China), and primary CML BM cells were purchased from Gansu Provincial People's Hospital (Lanzhou, China). The BM samples from patients with chronic leukemia were extracted and isolated by density gradient centrifugation at 1,000 × g (1,000 r/min) at 4°C for 3 h, using a sterile operating table, after obtaining written informed consent from the patients. The cell density was adjusted to 5×106/ml. Either the K562 or the primary CML BM cells were maintained in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum (FBS; Thermo Fisher Scientific, Inc), at 37°C in an atmosphere with 5% CO2. Sub-culturing of the cells was performed when 80% confluence was reached.
Details of the primary antibodies are as follows: Anti-c-myc antibody (1:1,000; cat. no. 5605; Cell Signaling Technology, Inc.), anti-cyclin D1 antibody (1:1,000; cat. no. 2978; Cell Signaling Technology, Inc.), anti-CD44 antibody (1:1,000; cat. no. 3570; Cell Signaling Technology, Inc.), anti-c-Jun antibody (1:1,000; cat. no. 9165; Cell Signaling Technology, Inc.), anti-MMP antibody (1:1,000; cat. no. 3801; Cell Signaling Technology, Inc.), anti-Lef/Tcf antibody (1:1,000; cat. no. 2203; Cell Signaling Technology, Inc.), anti-Bcl-2 antibody (1:1,000; cat. no. 12789-1-AP; ProteinTech Group, Inc.), anti-Bax antibody (1:1,000; cat. no. ab32503; Abcam), anti-β-actin antibody (1:1,000; cat. no. GB-12001; Beijing Biosynthesis Biotechnology Co., Ltd.), anti-β-catenin antibody (1:1,000; cat. no. ab22656; Abcam). The HRP-linked goat anti-rabbit IgG (1:4,000; cat. no. 7074) or goat anti-mouse IgG (1:1,000; cat. no. 7076) were purchased from Cell Signaling Technology, Inc.
AbM was supplied by the Edible Mushroom Center of Shanxi Agricultural University (Shanxi, China) (23). The RNA-protein complex, FA-2-b-β, was extracted in the School of Chemistry and Chemical Engineering, Lanzhou University (Lanzhou, China), as previously described (24). The purified compounds were obtained from Lanzhou University using ethanol precipitation, gel filtration, DEAE-cellulose and Sephadex G-200 column chromatography (15). The fraction FA-2-b-β was dissolved in distilled water for subsequent experimentation.
Cell proliferation assay
K562 cells or primary CML BM cells (10,000) were seeded into 96-well plates in a total of 100 µl and cultured with FA-2-b-β. FA-2-b-β at the concentrations of 1.2, 1.5, 1.8, 2.1 and 2.4 mg/ml or vehicle only (RPMI-1640) were cultured at 37°C for 24, 48 and 72 h. To prevent medium evaporation, 100 μl PBS was added into each well. Cell proliferation was assessed via the Cell Counting Kit-8 (CCK-8) assay (Dojindo Molecular Technologies, Inc.) according to the manufacturers protocol (25). The solution (10 µl/ml) was added and incubated for 1 h at 37°C. The solution in the wells was aspirated gently following centrifugation at 500 × g for 5 min at 4°C. Following mixing for 10 min, the absorbance was measured at 450 nm using an ELX800 absorbance microplate reader (BioTek Instruments, Inc.). Each assay was repeated five times.
Apoptosis and cell cycle assay
For the apoptosis assay, K562 cells or primary CML BM cells (100,000) were seeded into 6-well plates with different concentrations of FA-2-b-β, at a dose range of 0, 1.2, 1.5, 1.8 and 2.4 mg/ml and time periods of 24 and 48 h. These cells were harvested after 24 or 48 h of treatment and permeabilized using Triton-X, washed and resuspended in binding buffer (50 mM HEPES, 700 mM NaCl, 12.5 mM CaCl2 pH 7.4 in 100 µl) with the addition of 2.5 µl Annexin V (cat. no. R8021) and 5 µl PI (cat. no. EZ2811E231) from an Annexin V-FITC Apoptosis Detection kit (BD Biosciences). Briefly, 100,000 cells were washed with ice-cold PBS, resuspended in 195 µl PBS and stained for 15 min at room temperature with 5 µl FITC conjugated anti-Annexin V antibody. Unbound Annexin V antibody was removed by washing with binding buffer. The percentage of apoptotic K562 cells and primary CML BM cells (Annexin V-positive) was determined by flow cytometry analysis. Apoptotic cells were pre-treated at 37°C in the presence or absence of 3 mmol/l N-acetylcysteine (NAC; BD Biosciences) for cell cycle analysis for 2 h, followed by treatment with different concentrations of FA-2-b-β for 24 or 48 h at 37°C. Subsequently, the supernatant was removed, and the treated cells were washed three times with PBS and fixed using 70% ethanol overnight at 4°C. The cells were washed with PBS buffer, incubated with 5 µl RNase (0.25 mg/ml; BD Biosciences) for 30 min at 37°C. The pelleted cells were resuspended in PBS-diluted (1:100) Annexin V-FITC Apoptosis reagent (50 µg/ml) and incubated for 15 min at room temperature in the dark for flow cytometry (BD FACSuite v1.0.538.41; BD Biosciences).
Reverse transcription-quantitative (RT-q) PCR
Total RNA from K562 and primary CML BM cells was extracted using TRIzol® reagent (Takara Bio, Inc). RNase-free DNase I (Promega Corporation) was used to remove the genomic DNA. RT was performed at 70°C for 5 min using the PrimeScript RT MasterMix Reverse transcriptase cDNA synthesis kit (cat. no. SD2193; Takara Bio, Inc.). The Real Time PCR reaction was performed with SYBRGreen (cat. no. SD3034; Takara Bio, Inc.) using the qPCR Applied Biosystems 7500 Real-time System StepOne PLUS with the following thermocycling conditions: Initial denaturation at 95°C for 4 min, then 40 cycles at 95°C for 15 sec and 60°C for 45 sec. β-actin was used as the internal control for AbM inhibition of apoptosis determining Bcl-2, Bax and β-catenin expression. Primers were designed by Takara Bio, Inc, and are presented in Table I. The expression levels of the aforementioned genes were determined using the 2−ΔΔCq method (26).
Table I.
PCR primer sequences.
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| Actin | TGGCACAAAGCACAATGAA | CTAAGTCATAGTCCGCCTAGAAGC |
| β-catenin | GTGCATCTACACCGACAACTCC | GTTCCACTTGAGCTTGTTCACC |
| Bcl-2 | GGATTGTGGCCTTCTTTGAG | TACCCAGCCTCCGTTATCCT |
| Bax | CCGATTCATCTACCCTGCTG | TGAGCCAATTCCAGAGGCAGT |
Western blot analysis
K562 and primary CML BM cells were seeded into a 6-well plate and incubated with FA-2-b-β for 24/48 h at 37°C. Cells were lysed with SDS lysis buffer (cat. no. P1200; Beijing Solarbio Science & Technology Co., Ltd.) and total protein was obtained. Protein concentration was measured using a bicinchoninic acid protein assay. After protein concentration was measured, equal amounts of protein (10 µg/lane) were separated via 8–12% SDS-PAGE and transferred onto polyvinylidene fluoride membranes (EMD Millipore). Membranes were blocked with 5% skimmed dried milk for 1 h at room temperature, and then incubated with the appropriate primary antibodies overnight at 4°C. After primary antibody incubation, the membranes were washed three times with TBS-Tween (0.05% Tween-20; Boster Biological Technology), and then the corresponding HRP-linked secondary antibody was added for 2 h at room temperature. Apoptosis protein bands were detected using enhanced chemiluminescence (ECL kit; cat. no. P10010; Molecular Biotech Co., Ltd.) according to the manufacturers protocol and analyzed using Image Lab™ software v6.0 (Bio-Rad Laboratories, Inc.).
Statistical analysis
Statistical analysis was performed using SPSS software (version 18; SPSS, Inc.). All quantitative data were analyzed using one-way ANOVA, followed by Dunnett's post hoc multiple comparison test to compare all columns with the control column. Data shown are representative of three independent experiments with cells. Data are presented as the mean ± SD. P<0.05 and P<0.001 were considered to indicate statistically significant differences.
Results
FA-2-b-β inhibits CML cell proliferation
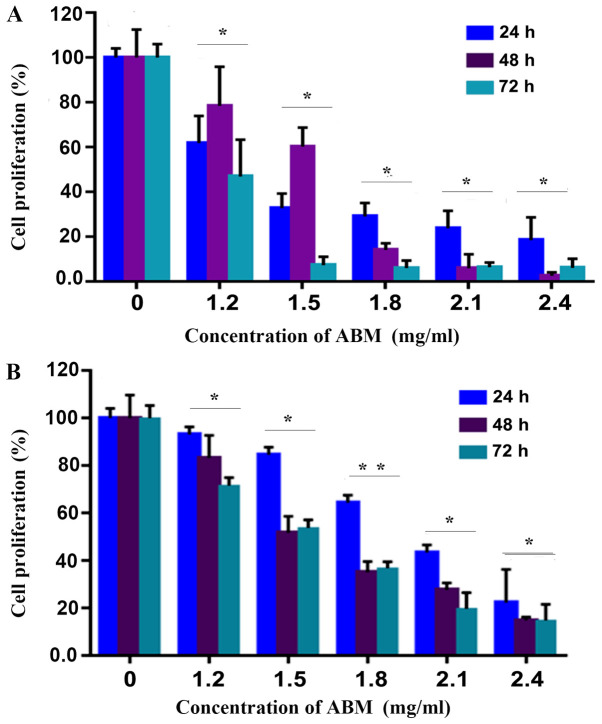
The CCK-8 assay was employed in order to determine if FA-2-b-β was able to inhibit the cell proliferation of CML. FA-2-b-β significantly inhibited proliferation of K562 cells or primary CML BM cells (P<0.05), in a concentration dependent manner (Fig. 1A and B). This effect was enhanced in 1.2 mg/ml of FA-2-b-β over the entire culture period.
Figure 1.
AbM inhibits the cell proliferation of K562 or primary CML BM cells. Cell proliferation was inhibited in (A) K562 or (B) primary CML BM cells at the indicated time points according to the CCK-8 assay. The plots represent the mean ± SD of five replicates (after 24, 48 and 72 h).
FA-2-b-β induces CML apoptosis
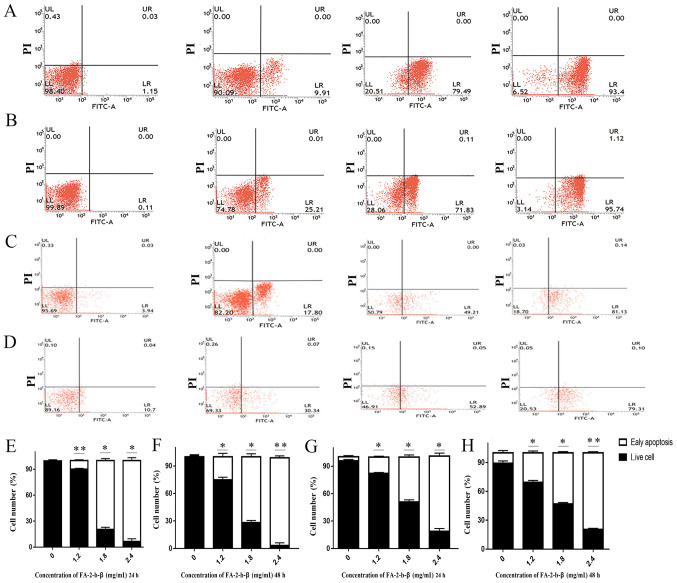
Some of the K562 or primary CML BM cells were completely damaged or inhibited by FA-2-b-β at the dosage used (Fig. 1A and B), but it was unclear if other residual cells were still viable. The apoptosis of the FA-2-b-β treated K562 cells was further determined using flow cytometry. Annexin V-FITC/PI double staining assay was used in order to determine the percentage of cells at different survival status in the entire culture (Fig. 2A-H). The proportion of apoptotic cells increased following treatment with FA-2-b-β. The total apoptotic K562 cells percentages of the control group were 1.15 and 0.11% at 24 and 48 h, respectively. This apoptotic rate was increased to 9.91, 79.49 and 93.40% (P<0.05; Fig. 2E), and 25.21, 71.83 and 95.74% (Fig. 2B) following treatment with FA-2-b-β (at 1.2, 1.8 and 2.4 mg/ml, respectively) for 24 and 48 h, respectively (Fig. 2E-F). The total apoptotic primary CML BM cell percentages of the control group were 3.94 and 10.70% at 24 and 48 h mock treatment, respectively; whereas apoptosis in the primary cells increased to 17.80 and 30.34%, 49.21 and 52.89%, and 81.13 and 79.31% following treatment with FA-2-b-β (at 1.2, 1.8 and 2.4 mg/ml, respectively) for 24 and 48 h, respectively (P<0.05; Fig. 2G and H).
Figure 2.
Flow cytometry analysis reveals AbM increased the apoptotic K562 cells and primary CML BM cells percentages in a concentration-dependent manner. K562 cells and primary CML BM cells were treated with different concentrations of AbM (0, 1.2, 1,8 and 2.4 mg/ml). K562 cells treated with different concentrations of AbM for (A) 24 and (B) 48 h. Primary CML BM cells treated with different concentrations of AbM for (C) 24 and (D) 48 h. Quantification of K562 cells treated with different concentrations of AbM for (E) 24 and (F) 48 h. Quantification of primary CML BM cells treated with different concentrations of AbM for (G) 24 and (H) 48 h. Data shown are representative of three independent experiments with K562 cells and primary CML BM cells. Data are presented as the mean ± SD. *P<0.05; **P<0.01 vs. 0 mg/ml.
FA-2-b-β induced CML cells apoptosis through inhibited G1 phase
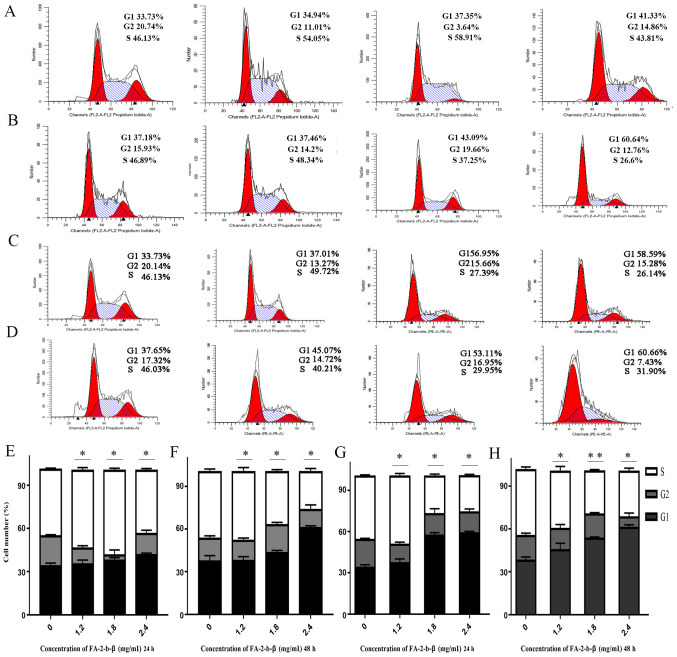
Cell cycle distribution of the K562 cells or primary CML BM cells was evaluated using flow cytometry, in order to determine the mechanisms underlying the cytotoxic activity of FA-2-b-β on the aforementioned cell types. The association between the DNA content and the intensity of the PI was calculated following treatment with FA-2-b-β at 0, 1.2, 1.8 and 2.4 mg/ml, for 24 and 48 h (Fig. 3A-H). There was an increase in the G1 phase, but a partial decrease in the S phase in a dose-dependent manner. The proportion of K562 cells at G1 or S phases was 33.73 and 33.73%, and 46.13 and 46.13%, respectively, with mock treatment for 24 and 48 h, respectively. The proportion of cells in G1 phase increased to 34.94, 37.35 and 41.33% using 1.2, 1.8 and 2.4 mg/ml of FA-2-b-β for 24 h (Fig. 3A), and to 37.01, 56.95 and 58.59% using 1.2, 1.8 and 2.4 mg/ml of FA-2-b-β for 48 h (Fig. 3C); whereas the proportion of cells in S phase partially decreased to 54.05, 58.91 and 43.81% at 24 h (Fig. 3A), and 49.72, 27.39 and 26.14% at 48 h (Fig. 3C). In the primary CML BM cells, the proportion of cells in G1 and S phases were 37.18 and 37.65%, and 46.89 and 46.03%, respectively, with mock treatment for 24 and 48 h, respectively. The proportion of cells in G1 phase increased to 37.46, 43.09 and 60.64% using 1.2, 1.8 and 2.4 mg/ml of FA-2-b-β for 24 h (Fig. 3B), and to 45.07, 53.11 and 60.66% using 1.2, 1.8 and 2.4 mg/ml of FA-2-b-β for 48 h (Fig. 3D). The proportion of cells in S phase decreased to 48.34, 37.25 and 26.6% at 24 h (Fig. 3B), whereas the proportion of cells in S phase partially decreased to 40.21, 29.95 and 31.90% at 48 h (Fig. 3D) in the presence of 1.2, 1.8 and 2.4 mg/ml FA-2-b-β, respectively (Fig. 3A-H).
Figure 3.
AbM promotes K562 cells and primary CML BM cells cycle. AbM induced cell cycle G1 phase arrest. K562 cells and primary CML BM cells were treated with different concentrations of AbM (0, 1.2, 1,8 and 2.4 mg/ml). Representative flow-cytograms are shown. Bar graphs indicate the mean percentages. (A) K562 cells and (B) primary CML BM cells treated with different concentrations of AbM for 24 h. (C) K562 cells and (D) primary CML BM cells treated with different concentrations of AbM for 48 h. Quantification of K562 cells treated with different concentrations of AbM for (E) 24 and (F) 48 h. Quantification of primary CML BM cells treated with different concentrations of AbM for (G) 24 and (H) 48 h. Data shown are representative of three independent experiments. K562 and primary CML BM cells data analysis revealed the proportion of cells in each phase of the cell cycle. Data are presented as the mean ± SD. *P<0.05, **P<0.01 vs. 0 mg/ml.
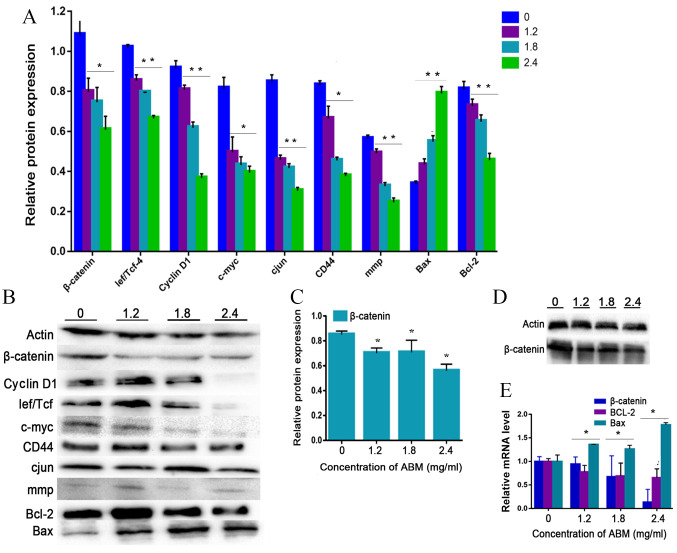
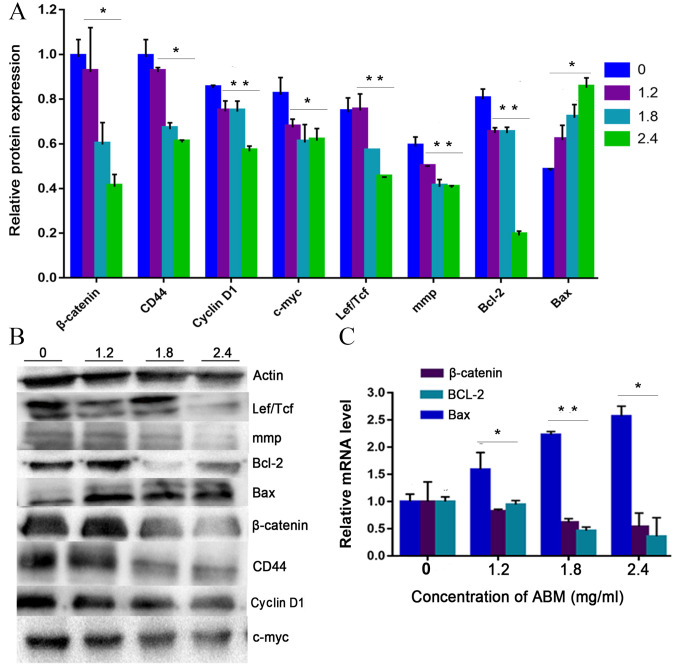
Cyclin D1 is a key cell cycle regulator that is essential for the G1 phase, whereby its expression is closely associated with the development and prognosis of numerous types of cancer. Following FA-2-b-β treatment, the expression of cyclin D1 decreased in a concentration-dependent manner, confirming that G1 phase arrest occurred after FA-2-b-β treatment in K562 cells or primary CML BM cells (Fig. 4A and B or Fig. 5A and B). Thus, FA-2-b-β induced both cell cycle arrest and apoptosis in K562 cells and primary CML BM cells, demonstrating similar findings to those that occur in the patients undergoing chemotherapy (27).
Figure 4.
AbM induces apoptosis of K562 cells. K562 cells were treated with different concentrations of AbM (0, 1.2, 1.8 and 2.4 mg/ml) for 48 h and the expression of apoptosis-associated genes and proteins was determined using western blot analysis for (A and B) 24 h (*P<0.05 and **P<0.001) or (C and D) 48 h and (E) reverse transcription-quantitative PCR. The relative expression levels of β catenin, Bax and Bcl-2 genes in K562 cells were quantified according to the gene expression levels of actin. The relative expression levels of cyclin D1, Lef/Tcf, c-myc, β catenin, CD44, c-Jun, mmp, Bax and Bcl-2 proteins in K562 cells were quantified according to the protein expression levels of actin. Data are representative of three independent experiments and are expressed as the mean ± SD. *P<0.05 vs. 0 mg/ml.
Figure 5.
AbM induces apoptosis of CML cells. Primary CML BM cells were treated with different concentrations of AbM (0, 1.2, 1.8 and 2.4 mg/ml) for 24 h and the expression of apoptosis-related genes and proteins was determined using (A and B) western-blot analysis (*P<0.05 and **P<0.001) and (C) reverse transcription-quantitative PCR. The relative expression levels of β catenin, Bax and Bcl-2 genes in primary CML BM cells were quantified according to the expression levels of actin. The relative expression levels of cyclin D1, Lef/Tcf, c-myc, β catenin, CD44, c-Jun, mmp, Bax and Bcl-2 proteins in primary CML BM cells were quantified according to the expression levels of Actin. Data are representative of three independent experiments and are expressed as the mean ± SD. *P<0.05, **P<0.001.
The present study further tested the hypothesis that FA-2-b-β may exert its effect on CML cells through negative modulation of Wnt/β-catenin signaling. The K562 cells or primary CML BM cells were dose dependently incubated with FA-2-b-β, following which RT-qPCR and western blot analysis were performed to assess the level of Wnt/β-catenin-related apoptosis gene and protein expression at 24 h. The data are presented as the mean of three independent experiments (P<0.05). β-catenin-associated apoptosis protein and gene expression in K562 cells or primary CML BM cells, exposed to FA-2-b-β at 1.2, 1.8 and 2.4 mg/ml for 24 h (Fig. 4A, B and E, and Fig. 5) or 48 h (Fig. 4C and D), was determined. β-catenin expression decreased at 24/48 h in a dose-dependent manner. Comparison was performed in the protein extracts between the drug-treated cells and controls in order to determine if there was an influence of spontaneous fluctuations in the associated apoptosis genes and proteins at each concentration. Actin was also evaluated to allow normalized protein and gene loadings. Bcl-2 and Bax protein and gene expression has been compared with the control cells. Bcl-2 expression decreased whilst Bax expression increased, in a dose-dependent manner. Based on our current study, it was observed that FA-2-b-β decreases cyclin D1 protein expression for binding to the promoter region and decreases β-catenin protein and gene expression for nuclear localization. The present study revealed ~50 or 25% of downregulated β-catenin mRNA in CML cells treated with FA-2-b-β 1.8 or 2.4 mg/ml for 24 h (Figs. 4E and 5C). Thus, FA-2-b-β may stimulate β-catenin via Lef/Tcf trans-inhibition activity, subsequently downregulating cyclin D1 in K562 cells as well as in the primary cells (P<0.05; Figs. 4A and 5A).
Discussion
Chemo-resistance remains a major challenge for clinicians in cancer therapy. Anti-cancer therapy is usually utilized to induce apoptosis or repair defects in cancer cells. Chemo-resistance is partially due to cancer-induced mutations in those genes responsible for apoptotic pathways (28). Thus, inhibition of cell proliferation and/or promotion of cell death are strategies in the management of cancer (29). It is necessary to develop novel therapeutic strategies for the treatment of CML. A significant advantage of drug repositioning over the conventional drug discovery and development process is the rapid implementation of anti-leukemia activity. As natural compounds derived from plants are easily accessible, such materials are routinely used for anti-cancer treatment in China, Japan, Brazil and the United States (30).
AbM has been used for the treatment of numerous types of diseases, including; Diabetes, atherosclerosis, hepatitis, hypercholesterolemia and heart disease (31). Immune-enhancing effects have been demonstrated to occur with AbM, including anti-oxidant, anti-mutagenic and anti-tumor effects (32). The positive mechanism of action of various medicinal plants in acute myeloid leukemia occurs via polysaccharides, which is primarily associated with; inhibition of cell proliferation, invasion and further induction of cancer cell apoptosis (33). Apoptosis is a form of programmed cell death without the accompanied inflammation, a phenomenon that has been a popular topic in anti-cancer research (34). The development of disrupted apoptotic pathways has been demonstrated to be associated with chemo-resistance of leukemia (35). This is consistent with the results of the present study which demonstrated that decreased viabilities of K562 cells or primary CML BM cells occurs in response to treatment with FA-2-b-β. Additionally, the present study observed that FA-2-b-β-treated K562 cells or primary CML BM cells presented morphological characteristics of apoptosis that are associated with downregulation of cyclin Dl and CD44. Cyclin Dl is a target of the Wnt/β-catenin pathway, which is associated with a number of steps of CML development (36). Conversely, cyclin Dl and CD44 are believed to be key genes of the Wnt/β-catenin pathway (36,37). Thus, the results of the present study suggest that FA-2-b-β-induced apoptosis in CML cells utilizes the Wnt/β-catenin pathway. β-catenin is another key mediator in cancer progression, which is involved in the regulation of cell cycle transition and cell apoptosis (38). This is consistent with the results of a previous study which demonstrated that AbM extracts induce apoptotic effects on leukemia cells (39).
c-Jun, c-myc, cyclin D1, CD44 and mmp all contribute to cancer cell proliferation and invasion (40). c-Jun activates β-catenin/Tcf transcription activity via the Wnt/β-catenin pathway, which is reported to be induced in CML (41). Furthermore, β-catenin interacts directly with c-Jun to activate the cyclin D1 and c-myc genes. Currently, a number of β-catenin signaling pathway inhibitors are under investigation, with the aim to disrupt β-catenin activity and its interaction with the transcription factors (42). The present study observed that the effect of FA-2-b-β for anti-cancer activity is via targeting c-myc (Wnt genes). FA-2-b-β decreased β-catenin and c-Jun production, particularly at the G1-S transition which resulted in cell cycle arrest and cell apoptosis at the G0/G1 phase. The results of the present study are supported by a previous study (43), which demonstrated that accumulated β-catenin activates the Wnt signaling pathway. Furthermore, knockout of Wnt signaling decreases β-catenin via the degradation of cytoplasmic β-catenin (44).
Kim et al (45), demonstrated that inhibition of β-catenin reverses the transformed properties in cancer, suggesting that β-catenin plays a key role in oncogenesis. These results may shed light on the development of pharmacological therapeutic targets against tumors. The results of the present study are consistent with others in demonstrating that β-catenin is important in the pathogenesis and progression of CML (46). β-catenin knockout decreases the risk of development of CML (47). Conversely, enhanced Wnt/β-catenin activity is associated with poor clinical outcomes with chemotherapy during ‘blast crisis’ in CML (48), and a meta-analysis revealed that high-dose imatinib achieved a major molecular response at 12 months of therapy (49–51). The present study demonstrated that FA-2-b-β inhibited the proliferation of K562 cells or primary CML BM cells by inducing apoptosis and G1 arrest. Furthermore, the present study revealed that FA-2-b-β suppressed the expression of β-catenin as well as the protein products of Wnt target genes; c-myc, c-Jun and cyclin D1 in the G1 transition. Subsequently, FA-2-b-β treatment contributed to cell cycle arrest during the G1 phase and this observation is supported by a previous study (52). In the absence of Wnt signaling, β-catenin remains low through the degradation of cytoplasmic β-catenin, which prevents induction of the transcription of a number of proliferation-associated genes, including; cyclin D1, c-myc and fibronectin. A decrease in transcription, which would normally cause malignant transformation (53), was detected following downregulation of the protein, β-catenin after treatment with FA-2-b-β. This ultimately inhibits the Wnt signaling pathway, accompanied by decreased cyclin D1, c-myc and CD44 in K562 cells or primary CML BM cells, in a dose-dependent manner. On the other hand, β-catenin is also capable of decreasing cell apoptosis via Bcl-2 or Bax targeting (36). Thus, the results of the present study suggest that the anti-tumor activity of AbM may be due to decreased cellular proliferation, but also the induction of cell apoptosis (54). This is supported by other studies that demonstrate that anti-cancer agents regulate Bcl-2 family members (including Bax or Bcl-2) (55–57). This evidence strongly supports the results of the present study, which demonstrated that FA-2-b-β induced leukemia cell apoptosis in vitro, accompanied by elevated Bax expression and decreased level of Bcl-2. These results suggest that cyclin D1, c-myc, c-Jun and CD44 can drive proliferation in CML as a consequence of β-catenin activity. These data further confirm that the role of β-catenin is indispensable for self-renewal of CML line cells.
The present study presents a number of limitations. First, it would provide more insight to perform the experiment evaluating TK inhibitor-resistant cells and normal BM cells. Secondly, future studies should include the experiment in vivo in order to determine the direct effect of AbM in CML.
FA-2-b-β exhibits anti-cancer potential through modulating different nodes of Wnt/β-catenin signaling pathways, which subsequently affects apoptosis, as well as cell cycling. Therefore, FA-2-b-β has potential for future treatment approaches for CML. The mushroom extract has minimal toxicity in animal models (58) and, to the best of our knowledge is considered safe for human consumption. As Agaricus blazei extract contains natural compounds derived from plants, which can be obtained at relatively low costs for the patients, it is routinely used in a number of clinics throughout China as an anti-cancer therapy.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- AbM
Agaricus blazei Murill
- CML
chronic myeloid leukemia
- BM
bone marrow
- PB
peripheral blood
- ABL1
abelson
- BCR
breakpoint cluster region
- TK
tyrosine kinase
Funding
The present study was funded by Gansu Provincial Department of Science and Technology (grant no. GZK-2013-8) and the Natural Science Foundation of China (grant no. 8156140621).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors contributions
MC and YS managed the design and coordination of the present study. YS provided professional writing. YS, QZ and KY carried out acid RNA protein complex (FA-2-b-β) extraction from Agaricus blazei Murill. LD, ZM, SY and KB performed the in vitro experiments. PY and XZ analyzed the results. All authors read and approved the final published version of this article.
Ethics approval and consent to participate
The present study was approved by the Human Ethics Committee of Gansu Provincial Hospital (approval no. 2019-157). All patients provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bamodu OA, Kuo KT, Yuanc LP, et al. HDAC inhibitor suppresses proliferation and tumorigenicity of drug-resistant chronic myeloid leukemia stem cells through regulation of hsa-miR-196a targeting BCR/ABL1. Exp Cell Res. 2018;370:519–530. doi: 10.1016/j.yexcr.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 2.Zhou H, Mak PY, Mu H, Mak DH, Zeng Z, Cortes J, Liu Q, Andreeff M, Carter BZ. Combined inhibition of β-catenin and Bcr-Abl synergistically targets tyrosine kinase inhibitor-resistant blast crisis chronic myeloid leukemia blasts and progenitors in vitro and in vivo. Leukemia. 2017;31:2065–2074. doi: 10.1038/leu.2017.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiao X, Liu P, Li D, Xia Z, Wang P, Zhang X, Liu M, Liao L, Jiao B, Ren R. Combination therapy of BCR-ABL-positive B cell acute lymphoblastic leukemia by tyrosine kinase inhibitor dasatinib and c-JUN N-terminal kinase inhibition. J Hematol Oncol. 2020;13:80. doi: 10.1186/s13045-020-00912-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deshpande A, Buske C. Knocking the Wnt out of the sails of leukemia stem cell development. Cell Stem Cell. 2007;1:597–598. doi: 10.1016/j.stem.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Zovko A, Yektaei-Karin E, Daniel S, Nilsson A, Wallvik J, Stenke L. Montelukast, a cysteinyl leukotriene receptor antagonist, inhibits the growth of chronic myeloid leukemia cells through apoptosis. Oncol Rep. 2018;40:902–908. doi: 10.3892/or.2018.6465. [DOI] [PubMed] [Google Scholar]
- 6.Braun TP, Eide CA, Druker BJ. Response and resistance to BCR-ABL1-targeted therapies. Cancer Cell. 2020;37:530–542. doi: 10.1016/j.ccell.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vetrie D, Helgason GV, Copland M. The leukaemia stem cell: Similarities, differences and clinical prospects in CML and AML. Nat Rev Cancer. 2020;20:158–173. doi: 10.1038/s41568-019-0230-9. [DOI] [PubMed] [Google Scholar]
- 8.Kumar R, Pereira RS, Zanetti C, et al. Specific, targetable interactions with the microenvironment influence imatinib-resistant chronic myeloid leukemia. Leukemia. 2020;34:2087–2101. doi: 10.1038/s41375-020-0866-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cronan TA, Kaplan RM, Posner L, Blumberg E, Kozin F. Prevalence of the use of unconventional remedies for arthritis in a metropolitan community. Arthritis Rheum. 1989;32:1604–1607. doi: 10.1002/anr.1780321217. [DOI] [PubMed] [Google Scholar]
- 10.AlGhamdi KM, Khurrum H, Al-Natour SH, et al. Use of complementary and alternative medicine among dermatology outpatients: Results from a national survey. J Cutan Med Surg. 2015;19:570–579. doi: 10.1177/1203475415584867. [DOI] [PubMed] [Google Scholar]
- 11.Wu L, Yu J, Chen R, Liu Y, Lou L, Wu Y, Huang L, Fan Y, Gao P, Huang M, et al. Dual inhibition of Bcr-Abl and Hsp90 by C086 potently inhibits the proliferation of imatinib-resistant CML cells. Clin Cancer Res. 2015;21:833–843. doi: 10.1158/1078-0432.CCR-13-3317. [DOI] [PubMed] [Google Scholar]
- 12.Ryan NM, Vertigan AE, Birring SS. An update and systematic review on drug therapies for the treatment of refractory chronic cough. Expert Opin Pharmacother. 2018;19:687–711. doi: 10.1080/14656566.2018.1462795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsai WJ, Yang SC, Huang YL, Chen CC, Chuang KA, Kuo YC. 4-Hydroxy-17-methylincisterol from Agaricus blazei decreased cytokine production and cell proliferation in human peripheral blood mononuclear cells via inhibition of NF-AT and NF-κB activation. Evid Based Complement Alternat Med. 2013;2013:435916. doi: 10.1155/2013/435916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hetland G, Tangen JM, Mahmood F, et al. Antitumor, anti-inflammatory and antiallergic effects of Agaricus blazei mushroom Extract and the related medicinal Basidiomycetes mushrooms, Hericium erinaceus and Grifola frondosa: A review of preclinical and clinical studies. Nutrients. 2020;12:1339. doi: 10.3390/nu12051339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y, Sheng Y, Lu X, Guo X, Xu G, Han X, An L, Du P. Isolation and purification of acidic polysaccharides from Agaricus blazei Murill and evaluation of their lipid-lowering mechanism. Int J Biol Macromol. 2020;157:276–287. doi: 10.1016/j.ijbiomac.2020.04.190. [DOI] [PubMed] [Google Scholar]
- 16.Sun YQ, Guo TK, Xi YM, Chen C, Wang J, Wang ZR. Effects of AZT and RNA-protein complex (FA-2-b-β) extracted from Liang Jin mushroom on apoptosis of gastric cancer cells. World J Gastroenterol. 2007;13:4185–4191. doi: 10.3748/wjg.v13.i31.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin MH, Lee KM, Hsu CY, et al. Immunopathological effects of Agaricus blazei Murill polysaccharides against Schistosoma mansoni infection by Th1 and NK1 cells differentiation. Int Immunopharmacol. 2019;73:502–514. doi: 10.1016/j.intimp.2019.05.045. [DOI] [PubMed] [Google Scholar]
- 18.Ziliotto L, Pinheiro F, Barbisan LF, Rodrigues MAM. Screening for in vitro and in vivo antitumor activities of the mushroom Agaricus Blazei. Nutr Cancer. 2009;61:245–250. doi: 10.1080/01635580802395717. [DOI] [PubMed] [Google Scholar]
- 19.Pak S, Park S, Kim Y, et al. Correction to: The small molecule WNT/β-catenin inhibitor CWP232291 blocks the growth of castration-resistant prostate cancer by activating the endoplasmic reticulum stress pathway. J Exp Clin Cancer Res. 2019;38:440. doi: 10.1186/s13046-019-1451-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin HJ, Huang Y, Huang WL, Feng XL, Peng Y, Zhang XJ, Jin Z, Fan XM. Regulatory mechanism of microRNA and Wnt/β-catenin signaling pathway in tumorigenesis and metastasis. Sci Sin. 2018;45:5–14. [Google Scholar]
- 21.Li ZJ, Qiu LG, Li X, et al. Expression of β-catenin gene in CML and its relationship with bcr/abl. J Exp Hematol. 2007;15:931–935. (In Chinese) [PubMed] [Google Scholar]
- 22.Hu Y, Yu K, Wang G, Zhang D, Shi C, Ding Y, Hong D, Zhang D, He H, Sun L, et al. Lanatoside C inhibits cell proliferation and induces apoptosis through attenuating Wnt/β-catenin/c-Myc signaling pathway in human gastric cancer cell. Biochem Pharmacol. 2018;150:280–292. doi: 10.1016/j.bcp.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 23.Mizuno T, Inagaki R, Kanao T, Hagiwara T, Nakamura T, Ito H, Shimura K, Sumiya T, Asakura A. Antitumor activity and some properties of water-insoluble hetero-glycans from ‘Himematsutake’, the fruiting body of Agaricus blazei Murill. Agr Biol Chem. 1990;54:2897–2905. doi: 10.1080/00021369.1990.10870407. [DOI] [Google Scholar]
- 24.Dong S, Furutani Y, Suto Y, Furutani M, Zhu Y, Yoneyama M, Kato T, Itabe H, Nishikawa T, Tomimatsu H, et al. Estrogen-like activity and dual roles in cell signaling of an Agaricus blazei Murrill mycelia-dikaryon extract. Microbiol Res. 2012;167:231–237. doi: 10.1016/j.micres.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 25.He B, Wang Q, Liu X, et al. A novel HDAC inhibitor chidamide combined with imatinib synergistically targets tyrosine kinase inhibitor resistant chronic myeloid leukemia cells. Biomed Pharmacother. 2020;129:110390. doi: 10.1016/j.biopha.2020.110390. [DOI] [PubMed] [Google Scholar]
- 26.Tang K, Lightner DV. Detection and quantification of infectious hypodermal and hematopoietic necrosis virus in penaeid shrimp by real-time PCR. Dis Aquat Organ. 2001;44:79–85. doi: 10.3354/dao044079. [DOI] [PubMed] [Google Scholar]
- 27.Chen SH, Hsieh YY, Tzeng HE, et al. ABL genomic editing sufficiently abolishes oncogenesis of human chronic myeloid leukemia cells in vitro and in vivo. Cancers (Basel) 2020;12:1399. doi: 10.3390/cancers12061399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryu H, Nam KY, Kim JS, Hwang SG, Song JY, Ahn J. The small molecule AU14022 promotes colorectal cancer cell death via p53-mediated G2/M-phase arrest and mitochondria-mediated apoptosis. J Cell Physiol. 2018;233:4666–4676. doi: 10.1002/jcp.26234. [DOI] [PubMed] [Google Scholar]
- 29.Sun S, Zhang C, Gao J, Qin Q, Zhang Y, Zhu H, Yang X, Yang D, Yan HT. Benzoquinone induces ROS-dependent mitochondria-mediated apoptosis in HL-60 cells. Toxicol Ind Health. 2018;34:270–281. doi: 10.1177/0748233717750983. [DOI] [PubMed] [Google Scholar]
- 30.Taofiq O, Rodrigues F, Barros L, et al. Agaricus blazei Murrill from Brazil: an ingredient for nutraceutical and cosmeceutical applications. Food Funct. 2019;10:565–572. doi: 10.1039/C8FO02461H. [DOI] [PubMed] [Google Scholar]
- 31.Wang H, Fu Z, Han C. The medicinal values of culinary-medicinal royal sun mushroom (Agaricus blazei Murrill) Evid Based Complement Alternat Med. 2013;2013:842619. doi: 10.1155/2013/842619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu S, Li F, Jia S, et al. Drying effects on the antioxidant properties of polysaccharides obtained from Agaricus blazei Murrill. Carbohydr Polym. 2014;103:414–417. doi: 10.1016/j.carbpol.2013.11.075. [DOI] [PubMed] [Google Scholar]
- 33.Zhang F, Song X, Li L, Wang J, Lin L, Li C, Li H, Lv Y, Jin Y, Liu Y, et al. Polygala tenuifolia polysaccharide PTP induced apoptosis in ovarian cancer cells via a mitochondrial pathway. Tumor Biol. 2015;36:2913–2919. doi: 10.1007/s13277-014-2921-x. [DOI] [PubMed] [Google Scholar]
- 34.Schultz DR, Harringto WJ. Apoptosis: Programmed cell death at a molecular level. Semin Arthritis Rheum. 2003;32:345–369. doi: 10.1053/sarh.2003.50005. [DOI] [PubMed] [Google Scholar]
- 35.Mondal A, Banerjee D, Majumder R, Maity TK, Khowala S. Evaluation of in vitro antioxidant, anticancer and in vivo antitumour activity of Termitomyces clypeatus MTCC 5091. Pharm Biol. 2016;54:2536–2546. doi: 10.3109/13880209.2016.1168854. [DOI] [PubMed] [Google Scholar]
- 36.Hou YC, Chao YJ, Tung HL, Wang HC, Shan YS. Coexpression of CD44-positive/CD133-positive cancer stem cells and CD204-positive tumor-associated macrophages is a predictor of survival in pancreatic ductal adenocarcinoma. Cancer. 2014;120:2766–2777. doi: 10.1002/cncr.28774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu Q, Yang Y, Wu S, et al. Evaluation of the correlation of KAI1/CD82, CD44, MMP7 and β-catenin in the prediction of prognosis and metastasis in colorectal carcinoma. Diagn Pathol. 2015;10:176. doi: 10.1186/s13000-015-0411-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yao H, Ashihara E, Maekawa T. Targeting the Wnt/β-catenin signaling pathway in human cancers. Expert Opin Ther Targets. 2011;15:873–887. doi: 10.1517/14728222.2011.577418. [DOI] [PubMed] [Google Scholar]
- 39.Collin-Hansen C, Pedersen SA, Andersen RA, Steinnes E. First report of phytochelatins in a mushroom: induction of phytochelatins by metal exposure in Boletus edulis. Mycologia. 2007;99:161–174. doi: 10.1080/15572536.2007.11832576. [DOI] [PubMed] [Google Scholar]
- 40.Saadeddin A, Babaei-Jadidi R, Spencer-Dene B, Nateri A. The links between Transcription, -catenin/JNK signaling, and carcinogenesis. Mol Cancer Res. 2009;7:1189–1196. doi: 10.1158/1541-7786.MCR-09-0027. [DOI] [PubMed] [Google Scholar]
- 41.Gan X, Wang J, Xi Y, Wu Z, Li Y, Li L. Nuclear Dvl, c-Jun, beta-catenin, and TCF form a complex leading to stabilization of beta-catenin-TCF interaction. J Cell Biol. 2008;180:1087–1100. doi: 10.1083/jcb.200710050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lepourcelet M, Chen YNP, France DS, et al. Small-molecule antagonists of the oncogenic Tcf/beta-catenin protein complex. Cancer Cell. 2004;5:91–102. doi: 10.1016/S1535-6108(03)00334-9. [DOI] [PubMed] [Google Scholar]
- 43.Silverberg E, Lubera J. Cancer statistics, 1987. CA Cancer J Clin. 1987;37:2–19. doi: 10.3322/canjclin.37.1.2. [DOI] [PubMed] [Google Scholar]
- 44.Chairoungdua A, Smith DL, Pochard P, Hull M, Caplan MJ. Exosome release of β-catenin: A novel mechanism that antagonizes Wnt signaling. J Cell Biol. 2010;190:1079–1091. doi: 10.1083/jcb.201002049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim JS, Crooks H, Foxworth A, Waldman T. Proof-of-principle: Oncogenic β-catenin is a valid molecular target for the development of pharmacological inhibitors 1. Mol Cancer Ther. 2002;1:1355–1359. [PubMed] [Google Scholar]
- 46.Liu N, Zang S, Liu Y, et al. FZD7 regulates BMSCs-mediated protection of CML cells. Oncotarget. 2015;7:6175–6187. doi: 10.18632/oncotarget.6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iwasaki H, Akashi K. Springer; Japan: 2016. Identification and biology of CML stem cells. doi:10.1007/978-4-431-55714-2_1. [DOI] [Google Scholar]
- 48.Sun J, Li B, Jia Z, Zhang A, Wang G, Chen Z, Shang Z, Zhang C, Jian C, Yang W. RUNX3 inhibits glioma survival and invasion via suppression of the β-catenin/TCF-4 signaling pathway. J Neurooncol. 2018;140:15–26. doi: 10.1007/s11060-018-2927-0. [DOI] [PubMed] [Google Scholar]
- 49.Hoffmann VS, Hasford J, Deininger M, Cortes J, Baccarani M, Hehlmann R. Systematic review and meta-analysis of standard-dose imatinib vs. high-dose imatinib and second generation tyrosine kinase inhibitors for chronic myeloid leukemia. J Cancer Res Clin Oncol. 2017;143:1311–1318. doi: 10.1007/s00432-017-2385-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ge L, Tian JH, Li YN, Pan JX, Li G, Wei D, Xing X, Pan B, Chen YL, Song FJ, et al. Association between prospective registration and overall reporting and methodological quality of systematic reviews: A meta-epidemiological study. J Clin Epidemiol. 2018;93:45–55. doi: 10.1016/j.jclinepi.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 51.Tian J, Zhang J, Ge L, Yang K, Song F. The methodological and reporting quality of systematic reviews from China and the USA are similar. J Clin Epidemiol. 2017;85:50–58. doi: 10.1016/j.jclinepi.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 52.Sun J, Li B, Jia Z, Zhang A, Wang G, Chen Z, Shang Z, Zhang C, Cui J, Yang W. RUNX3 inhibits glioma survival and invasion via suppression of the β-catenin/TCF-4 signaling pathway. J Neurooncol. 2018;140:15–26. doi: 10.1007/s11060-018-2927-0. [DOI] [PubMed] [Google Scholar]
- 53.Liu ZL, Hu J, Huang ZL, Li H, Liu Y, Feng WL. Influence of AKT inhibitor on Wnt/β-catenin pathway in chronic myeloid leukemia K562 cells. Medical Journal of Chinese People's Liberation Army. 2015;40:710–715. [Google Scholar]
- 54.Zhang L, Xu J, Zhang X, Zhang Y, Wang L, Huang X, Xu Z. The role of tumoral FOXP3 on cell proliferation, migration, and invasion in gastric cancer. Cell Physiol Biochem. 2017;42:1739–1754. doi: 10.1159/000479442. [DOI] [PubMed] [Google Scholar]
- 55.Srivastava R, Cao Z, Nedeva C, et al. BCL-2 family protein BOK is a positive regulator of uridine metabolism in mammals. Proc Natl Acad Sci USA. 2019;116:15469–15474. doi: 10.1073/pnas.1904523116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tajadura V, Hansen MH, Smith J, et al. β-catenin promotes endothelial survival by regulating eNOS activity and flow-dependent anti-apoptotic gene expression. Cell Death Dis. 2020;11:493. doi: 10.1038/s41419-020-2687-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liang J, Wang W, Wei L, Gao S, Wang Y. Oridonin inhibits growth and induces apoptosis of human neurocytoma cells via the Wnt/β-catenin pathway. Oncol Lett. 2018;16:3333–3340. doi: 10.3892/ol.2018.8977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sumiya T, Ikeda Y, Broadmeadow A, May K, Pritchard L, Horne C, Burlinson B. Himematsutake (Iwade Strain 101) extract (ABM-FD): Genetic toxicology and a 3-month dietary toxicity study in rats. Food Chem Toxicol. 2008;46:1949–1959. doi: 10.1016/j.fct.2008.01.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.