Abstract
Diabetes mellitus (DM) is a common disease, but its effect on the prognosis of patients with intrahepatic cholangiocarcinoma (ICC) has not been reported. The aim of the present study was to explore the prognostic significance of diabetes in patients with ICC treated with hepatectomy and to clarify the role of pyruvate kinase M2 (PKM2). A consecutive retrospective cohort of 110 patients with ICC (28 with DM and 82 without DM) who underwent therapeutic hepatectomy was evaluated between January 2006 and January 2011. The clinicopathological characteristics of the two groups and the differences between overall survival (OS) and recurrence-free survival (RFS) were analyzed. The Cox proportional hazards model was further used to identify independent prognostic predictors. PKM2 expression was measured using immunohistochemical staining in tissues collected, after obtaining informed consent. Patients with ICC with DM exhibited significantly lower OS and RFS rates at 1, 3 and 5 years compared with patients with ICC without DM. Cox multivariate analysis revealed that DM was an independent predictor of poor OS and RFS. Additionally, high PKM2 expression was significantly higher in patients with ICC with DM compared with that in patients without DM. Overall, DM was associated with significantly lower OS and RFS rates in patients with ICC. The underlying biological rationale may be attributed to the higher PKM2 expression rate.
Keywords: diabetes mellitus, cholangiocarcinoma, pyruvate kinase M2, Warburg effect, glycolysis
Introduction
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary liver cancer, and its incidence is on the increase. It is a painful disease that poses a great burden to patients (1). Since ICC is resistant to radiotherapy and chemotherapy, surgical resection is considered to be the main treatment (2,3). However, the prognosis in patients with ICC is poor, with an estimated median survival time of 18–39 months and a median 5-year survival rate of 25–40% (2,3).
Over the past 20 years, the global burden of diabetes mellitus (DM) has risen sharply and is expected to affect >500 million adults by 2030, most of whom have type 2 diabetes (4). Previous studies have demonstrated that DM can significantly increase the risk of hepatocellular carcinoma (HCC) and ICC (5,6). Some retrospective studies and meta-analyses identified DM as an independent predictor of poor prognosis in patients with HCC after hepatectomy (7–10). ICC and HCC originate from different cell types; however, obesity, diabetes, hepatitis B and C, drinking and cirrhosis seem to be the main risk factors for both ICC and HCC, suggesting that ICC and HCC share a common pathogenesis mechanism (11). In addition, whether DM is also a prognostic factor of ICC after hepatectomy remains unclear.
Regardless of whether the supply of oxygen is sufficient, rapidly dividing cells convert glucose into lactate to produce ATP, which is known as aerobic glycolysis or the Warburg effect (12,13). The Warburg effect is a widely observed feature of human cancer; in numerous types of tumor, including HCC and ICC, the presence of the Warburg effect is often associated with tumor invasiveness and a poor prognosis (14–16). The key rate limiting enzyme of the Warburg effect is pyruvate kinase M2 (PKM2), a tumor-specific subtype of pyruvate kinase, which catalyzes the synthesis of pyruvate and ATP with phosphoenolpyruvate and ADP as substrates (17,18). Compensatory hyperinsulinemia is an important feature of type 2 DM and is one of the upstream regulators of PKM2 expression (19,20). The effect of DM on PKM2 expression in patients with ICC remains unknown.
The present study performed a retrospective analysis of patients with ICC who underwent hepatectomy at the Second Affiliated Hospital of Chongqing Medical University (Chongqing, China). The aims were to determine whether DM affects the prognosis in patients with ICC after hepatectomy and to provide a scientific basis for further exploration of the specific molecular mechanism.
Materials and methods
Patients and follow-up
A total of 157 patients who underwent hepatectomy for ICC were eligible for inclusion in the present study at the Second Affiliated Hospital of Chongqing Medical University between January 2006 and January 2011. ICC was confirmed by histopathology and the anatomic location of the tumor was determined by review of histopathology, radiology and operation notes. After review, 47 of the 157 patients with ICC were excluded (15 lacked integrated clinical data, 13 lacked prospectively collected follow-up data and 19 had insufficient tissue for investigation). The remaining 110 patients (mean age, 53 years; age range, 28–79 years; 62 males and 48 females; 28 with type 2 DM and 82 without DM) were enrolled in the study. Cancerous and paracancerous tissues were obtained for both the DM and non-DM groups. Paracancerous tissue was defined as normal liver tissue >5 cm away from the tumor.
DM was diagnosed as a fasting plasma glucose level of >7.0 mmol/l (126 mg/dl), or a plasma glucose level of >11.1 mmol/l (200 mg/dl) at 2 h in a 75-g oral glucose tolerance test, or typical DM symptoms (polydipsia, polyuria, overeating, emaciation, fatigue or obesity) together with a casual plasma glucose level of >11.1 mmol/l (200 mg/dl; the normal fasting plasma glucose level is 3.9–6.1 mmol/l) (21).
All the patients were followed up until death or the end of the study in December 2016, with a median follow-up time of 55 months. After hepatectomy, all the patients were followed up every 3 months for 1 year and every 6 months thereafter. Liver function (based on the levels of alanine aminotransferase, aspartate aminotransferase, total bilirubin and albumin), prothrombin time (PT), abdominal ultrasound, chest film and enhanced CT or MRI were collected to monitor the patients. Diagnosis of tumor recurrence (intrahepatic and extrahepatic recurrence) was based on typical imaging findings. Tumor tissues were collected immediately upon resection from patients with ICC, and were subsequently fixed and paraffin-embedded, as described below, for immunohistochemistry.
The present retrospective study was conducted in accordance with the Declaration of Helsinki 2013 edition and national and international guidelines, and was approved by the Ethical Review Committee of the Second Affiliated Hospital of Chongqing Medical University. Written informed consent was provided by all patients.
Immunohistochemical staining
The ICC tissues were fixed in 4% paraformaldehyde at room temperature for 24 h and embedded in paraffin. Immunohistochemical staining of paraffin sections was performed using a two-step protocol. Briefly, the ICC sections (3-µm-thick) were deparaffinized in xylene I for 15 min and xylene II for 15 min at 37°C, and rehydrated in a graded ethanol series (100, 95, 80 and 75% ethanol for 5 min each). Subsequently, antigen retrieval was performed in 10 mmol/l sodium citrate solution (pH 6.0) at 100°C for 15 min, and the samples were cooled for 30 min at room temperature. Endogenous peroxidase activity was inhibited using 3% hydrogen peroxide for 30 min at 37°C, and 5% goat serum (Origene Technologies, Inc.) was used to block non-specific binding for 15 min at 37°C, followed by incubation with a primary rabbit monoclonal anti-PKM2 antibody (1:1,000; cat. no. ab137852; Abcam) at 4°C overnight. Subsequently, the sections were incubated with a secondary anti-rabbit biotin-labelled IgG antibody (1:100; cat. no. SAP-9100; OriGene Technologies, Inc.) at 37°C for 30 min. After washing with PBS, the visualization signal was detected using 3,3′-diaminobenzidine (Boster Biological Technology) and counterstaining was performed using hematoxylin at room temperature for 5 sec. To evaluate PKM2 expression, the slides were assessed independently by two experienced pathologists with minimal interobserver variability. The slides were assessed using an orthotopic light microscope (magnification, ×100; Zeiss AG).
Scoring systems for immunohistochemical staining
A semi-quantitative assessment method score was used. Scoring parameters included the staining intensity (range, 0–3; 0, negative; 1, weak; 2, moderate; and 3, strong) and the percentage of positive cells (range, 1–4; 0, negative or ≤5%; 1, 6–25%; 2, 26–50%; 3, 51–75%; and 4, 76–100%). The staining intensity was based on the color of the positive markers: Light yellow indicated weak staining, brown-yellow indicated moderate staining and dark brown indicated strong staining. The percentage of positive cells and the intensity scores were added to determine the final staining scores. A total score <4 was defined as low PKM2 expression, while a score ≥4 was defined as high PKM2 expression.
Statistical analysis
Statistical analyses were performed using SPSS 22.0 (IBM Corp.). The primary endpoint of the present study was overall survival (OS) after hepatectomy. OS was recorded as the time from the disease diagnosis to death due to any cause. Tumor recurrence-free survival (RFS) was recorded as the time from tumor resection to tumor recurrence. The significance of intergroup differences in continuous data was assessed using an unpaired Student's t-test to analyze the difference of PKM2 expression between ICC patients with DM and without DM, while the significance of differences in categorical data was assessed using the χ2 test or Fisher's exact test (two-tailed). Survival analysis was performed using the Kaplan-Meier method and the log-rank test. Univariate and multivariate analyses were performed using the Cox proportional hazards model to identify independent prognostic factors (multivariate analysis was based on significant results from the univariate analysis). The TNM staging system (22) was used to determine the tumor stage in the analysis of the clinicopathological features and prognosis of patients. Categorical variables are expressed as frequencies (%). The results of the survival analysis are described as hazard ratios (HRs) and 95% CIs. P<0.05 (two-sided) was considered to indicate a statistically significant difference.
Results
Clinicopathological features of patients with and without DM
Between January 2006 and January 2011, 110 patients (28 with type 2 DM and 82 without DM) were included in the present study. The baseline characteristics were similar between the two groups, except that patients in the DM group had a higher frequency of vascular invasion (46.4 vs. 23.2%; P=0.019). There were no significant differences in sex, age, TNM stage, tumor diameter, R0 resection, differentiation degree, lymph node metastasis, intrahepatic metastasis, multiplicity, total bilirubin, alanine aminotransferase, aspartate aminotransferase, albumin and PT (Table I).
Table I.
Statistical differences of clinicopathological characteristics between patients with DM (n=28) and without DM (n=82).
| Variables | DM, n (%) | Non-DM, n (%) | P-value |
|---|---|---|---|
| Sex (male) | 13 (46.4) | 49 (59.8) | 0.220 |
| Age (≥45 years) | 14 (50.0) | 49 (59.8) | 0.368 |
| TNM stage (I–II) | 5 (17.9) | 21 (25.6) | 0.404 |
| Tumor diameter (>5 cm) | 19 (67.9) | 51 (62.2) | 0.591 |
| R0 | 17 (60.7) | 47 (57.3) | 0.753 |
| Differentiation | 0.263 | ||
| Low | 1 (3.6) | 12 (14.6) | |
| Moderate | 23 (82.1) | 62 (75.6) | |
| High | 4 (14.3) | 8 (9.8) | |
| Lymph node metastasis | 10 (35.7) | 25 (30.5) | 0.608 |
| Intrahepatic metastasis | 8 (28.6) | 15 (18.3) | 0.248 |
| Multiplicity | 14 (50.0) | 30 (36.6) | 0.211 |
| Vascular invasion | 13 (46.4) | 19 (23.2) | 0.019a |
| Alanine aminotransferase (>100 IU/l) | 2 (7.1) | 15 (18.3) | 0.229 |
| Aspartate aminotransferase (>100 IU/l) | 5 (17.9) | 18 (22.0) | 0.646 |
| Total bilirubin (>34 µmol/l) | 4 (14.3) | 20 (24.4) | 0.264 |
| Albumin (>35 g/dl) | 23 (82.1) | 72 (87.8) | 0.451 |
| Prothrombin time (>14 sec) | 2 (7.1) | 3 (3.7) | 0.600 |
P<0.05. DM, diabetes mellitus; IU, international unit.
Association of DM and non-DM with OS after hepatectomy in patients with ICC
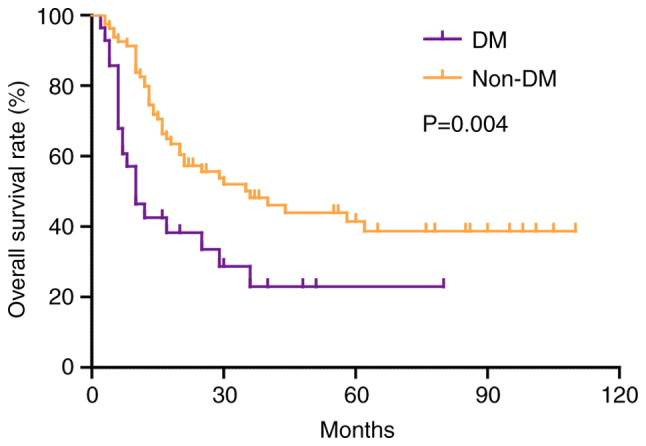
At the end of the follow-up period, 61 (55.5%) patients had died. The median follow-up time was 55 months for all patients. The OS rate of patients with DM was significantly worse than that of patients without DM (P=0.004; Fig. 1). The 1-, 3- and 5-year OS rates for patients with DM were 42.6, 23.0 and 23.0%, which were lower than 83.8, 48.2 and 41.5% in patients without DM, and the median survival time in patients with and without diabetes was 10 and 36 months, respectively (Fig. 1). As shown in Table II, univariate analysis revealed that advanced TNM stage (HR, 0.474; 95% CI, 0.245–0.918; P=0.027), >5 cm tumor diameter (HR, 0.460; 95% CI, 0.260–0.814; P=0.008), R1 resection (HR, 0.515; 95% CI, 0.310–0.857; P=0.011), lymph node metastasis (HR, 2.865; 95% CI, 1.658–4.952; P<0.001), intrahepatic metastasis (HR, 9.266; 95% CI, 4.863–17.657; P<0.001), multiplicity (HR, 6.125; 95% CI, 3.482–10.774; P<0.001), vascular invasion (HR, 5.875; 95% CI, 3.252–10.614; P<0.001), high PKM2 expression (HR, 1.984; 95% CI, 1.177–3.344; P=0.010) and DM (HR, 2.152; 95% CI, 1.255–3.691; P=0.005) were adverse prognostic factors that affected OS in patients with ICC. In addition, multivariate analysis identified the following factors as independent predictors for poor OS: DM (HR, 1.989; 95% CI, 1.084–3.65; P=0.026), high PKM2 expression (HR, 1.364; 95% CI, 1.048–2.948; P=0.007), intrahepatic metastasis (HR, 2.826; 95% CI, 1.288–6.021; P=0.010), multiplicity (HR, 4.004; 95% CI, 1.923–8.336; P<0.001) and vascular invasion (HR, 3.187; 95% CI, 1.516–6.701; P=0.002).
Figure 1.
Kaplan-Meier survival curve analysis of overall survival rate in patients with intrahepatic cholangiocarcinoma according to DM. DM, diabetes mellitus.
Table II.
Univariate and multivariate analysis of risk factors for overall survival.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Sex (male/female) | 1.026 | 0.617–1.706 | 0.922 | |||
| Age (<45/≥45 years) | 0.850 | 0.506–1.428 | 0.540 | |||
| TNM stage (I–II/III–IV) | 0.474 | 0.245–0.918 | 0.027a | 1.003 | 0.462–2.176 | 0.995 |
| Tumor diameter (≤5/>5 cm) | 0.460 | 0.260–0.814 | 0.008b | 0.728 | 0.348–1.527 | 0.402 |
| R0 (R0/R1) | 0.515 | 0.310–0.857 | 0.011a | 0.739 | 0.403–1.354 | 0.327 |
| Differentiation (low) | 1.000 | |||||
| Differentiation (moderate) | 2.193 | 0.873–5.510 | 0.095 | |||
| Differentiation (high) | 1.349 | 0.411–4.423 | 0.622 | |||
| Lymph node metastasis (positive/negative) | 2.865 | 1.658–4.952 | <0.001c | 0.919 | 0.472–1.788 | 0.803 |
| Intrahepatic metastasis (positive/negative) | 9.266 | 4.863–17.657 | <0.001c | 2.826 | 1.288–6.201 | 0.010a |
| Multiplicity (positive/negative) | 6.125 | 3.482–10.774 | <0.001c | 4.004 | 1.923–8.336 | <0.001c |
| Vascular invasion (positive/negative) | 5.875 | 3.252–10.614 | <0.001c | 3.187 | 1.516–6.701 | 0.002b |
| Pyruvate kinase M2 expression (high/low) | 1.984 | 1.177–3.344 | 0.010a | 1.364 | 1.048–2.948 | 0.007b |
| Diabetes mellitus (yes/no) | 2.152 | 1.255–3.691 | 0.005b | 1.989 | 1.084–3.650 | 0.026a |
| Total bilirubin (>34/≤34 µmol/l) | 0.780 | 0.414–1.470 | 0.442 | |||
| Alanine aminotransferase (>100/≤100 IU/l) | 0.940 | 0.477–1.855 | 0.859 | |||
| Prothrombin time (>14/≤14 sec) | 1.533 | 0.556–4.232 | 0.409 | |||
| Albumin (>35/≤35 g/l) | 0.672 | 0.340–1.326 | 0.252 | |||
| Aspartate aminotransferase (>100/≤100 IU/l) | 1.310 | 0.731–2.348 | 0.365 | |||
P<0.05
P<0.01
P<0.001. HR, hazard ratio; IU, international unit.
Association of DM and non-DM with RFS after hepatectomy in patients with ICC
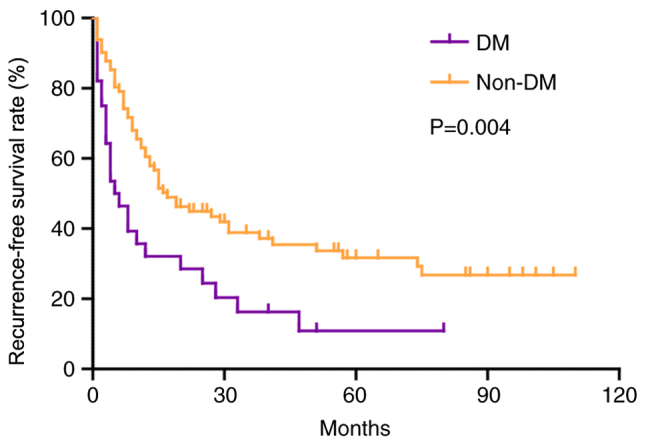
During follow-up, 78 (70.9%) patients experienced tumor recurrence. The RFS rate in patients with DM was significantly worse than that in patients without DM (P=0.004; Fig. 2). The 1-, 3- and 5-year RFS rates for patients with DM were 32.1, 16.3 and 10.9%, which were lower than 60.5, 38.8 and 31.7% in patients without DM, and the median RFS time in patients with and without diabetes was 5 and 17 months, respectively (Fig. 2). As shown in Table III, univariate analysis revealed that lymph node metastasis (HR, 2.664; 95% CI, 1.638–4.331; P<0.001), intrahepatic metastasis (HR, 5.640; 95% CI, 3.143–10.123; P<0.001), multiplicity (HR, 4.427; 95% CI, 2.718–7.212; P<0.001), vascular invasion (HR, 5.100; 95% CI, 3.017–8.619; P<0.001), high PKM2 expression (HR, 2.048; 95% CI, 1.289–3.254; P=0.002) and DM (HR, 1.985; 95% CI, 1.222–3.225; P=0.006) were adverse prognostic factors that affected RFS in patients with ICC. In addition, multivariate analysis identified the following factors as independent predictors for poor OS: DM (HR, 1.784; 95% CI, 1.042–3.053; P=0.035), high PKM2 expression (HR, 1.567; 95% CI, 1.057–3.012; P=0.006), multiplicity (HR, 2.898; 95% CI, 1.564–5.369; P=0.001) and vascular invasion (HR, 2.655; 95% CI, 1.340–5.259; P=0.005).
Figure 2.
Kaplan-Meier survival curve analysis of recurrence-free survival rate in patients with intrahepatic cholangiocarcinoma according to DM. DM, diabetes mellitus.
Table III.
Univariate and multivariate analysis of risk factors for recurrence-free survival.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Sex (male/female) | 0.922 | 0.591–1.440 | 0.722 | |||
| Age (<45/≥45 years) | 1.058 | 0.675–1.660 | 0.805 | |||
| TNM stage (I–II/III–IV) | 0.644 | 0.378–1.099 | 0.107 | |||
| Tumor diameter (≤5/>5 cm) | 0.699 | 0.438–1.118 | 0.135 | |||
| R0 (R0/R1) | 0.602 | 0.382–0.948 | 0.028 | |||
| Differentiation (low) | 1.000 | |||||
| Differentiation (moderate) | 2.052 | 0.938–4.489 | 0.072 | |||
| Differentiation (high) | 1.355 | 0.491–3.742 | 0.557 | |||
| Lymph node metastasis (positive/negative) | 2.664 | 1.638–4.331 | <0.001c | 1.065 | 0.582–1.948 | 0.839 |
| Intrahepatic metastasis (positive/negative) | 5.640 | 3.143–10.123 | <0.001c | 1.517 | 0.722–3.187 | 0.271 |
| Multiplicity (positive/negative) | 4.427 | 2.718–7.212 | <0.001c | 2.898 | 1.564–5.369 | 0.001b |
| Vascular invasion (positive/negative) | 5.100 | 3.017–8.619 | <0.001c | 2.655 | 1.340–5.259 | 0.005b |
| Pyruvate kinase M2 expression (high/low) | 2.048 | 1.289–3.254 | 0.002b | 1.567 | 1.057–3.012 | 0.006b |
| Diabetes mellitus (yes/no) | 1.985 | 1.222–3.225 | 0.006b | 1.784 | 1.042–3.053 | 0.035a |
| Total bilirubin (>34/≤34 µmol/l) | 0.750 | 0.426–1.321 | 0.319 | |||
| Alanine aminotransferase (>100/≤100 IU/l) | 0.857 | 0.463–1.586 | 0.623 | |||
| Prothrombin time (>14/≤14 sec) | 1.134 | 0.414–3.104 | 0.807 | |||
| Albumin (>35/≤35 g/l) | 0.627 | 0.345–1.139 | 0.126 | |||
| Aspartate aminotransferase (>100/≤100 IU/l) | 1.198 | 0.706–2.032 | 0.503 | |||
P<0.05
P<0.01
P<0.001. HR, hazard ratio; IU, international unit.
Association of PKM2 expression in patients with ICC with and without DM
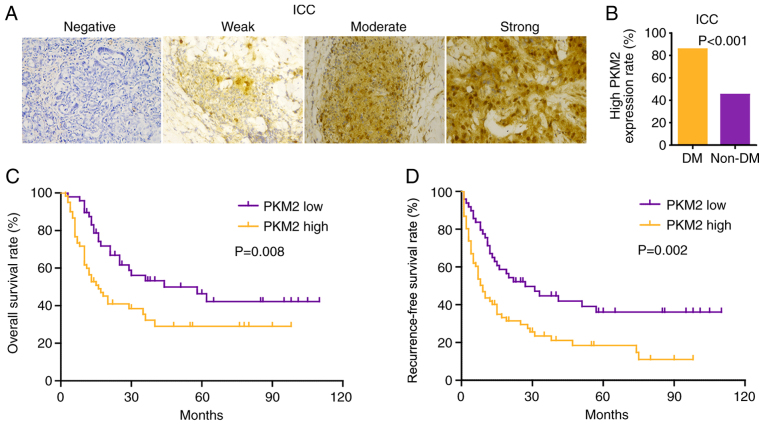
PKM2 expression was mainly concentrated in the cytoplasm and nucleus. Representative images of immunohistochemical staining are shown in Fig. 3A. High PKM2 expression was observed in 61 (55.5%) patients with ICC; among these patients, 85.7% patients had DM, while 45.1% patients did not have DM (Fig. 3B). Similarly to HCC (23), high PKM2 expression was associated with poor OS and RFS (Fig. 3C and D). Notably, the combination of low PKM2 expression and no DM had a favorable prognostic value, while patients with high PKM2 expression and DM had the shortest OS ad RFS time (Fig. 4A and B).
Figure 3.
High PKM2 expression is associated with a poor prognosis in patients with ICC. (A) Immunohistochemical staining of PKM2 expression in ICC tissues. (B) Proportion of high PKM2 expression in patients with ICC with and without DM. Kaplan-Meier survival curve analysis of (C) overall survival rate and (D) recurrence-free survival rate in patients with ICC according to PKM2 expression. Magnification, ×100. DM, diabetes mellitus; ICC, intrahepatic cholangiocarcinoma; PKM2, pyruvate kinase M2.
Figure 4.
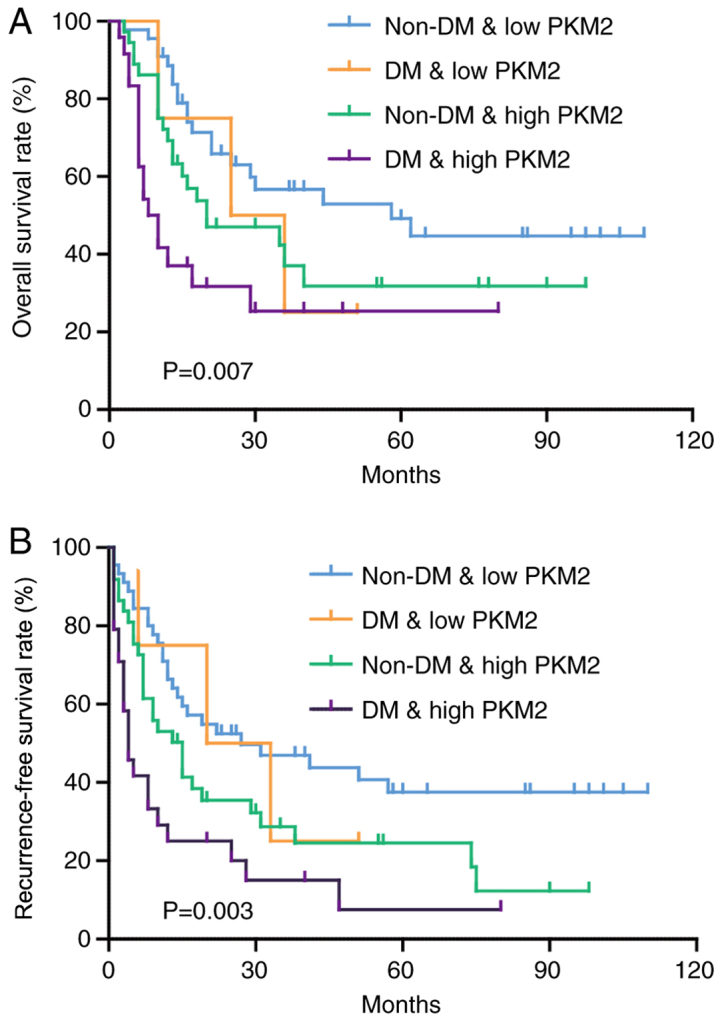
Patients with high PKM2 expression and DM have the shortest survival. (A) Kaplan-Meier survival curve analysis of overall survival rate (%) in patients with ICC according to DM and PKM2 expression. (B) Kaplan-Meier survival curve analysis of Recurrence-free survival rate (%) in patients with ICC according to DM and PKM2 expression. DM, diabetes mellitus; ICC, intrahepatic cholangiocarcinoma; PKM2, pyruvate kinase M2.
Discussion
In the present retrospective study, it was revealed that DM was an independent prognostic factor for survival that significantly affected the OS and RFS rates of patients with ICC. The impact of DM was independent of patient demographics. In addition, patients with DM had a higher PKM2 expression rate than patients without DM, but the mechanism by which DM may regulate PKM2 expression remains to be uncovered. DM has been identified as an independent risk factor for ICC in a number of countries, and routine measurements for γ-glutamyl transferase and/or CA19-9 have been recommended during follow-up for DM to detect ICC at an early stage and expect a good OS (24); however, the effect of DM on the outcome of ICC has been rarely reported (24–27). A retrospective study by Endo et al (28) revealed that DM was a prognostic factor for patients undergoing surgery for ICC; however, only 6 (6/81) patients with ICC had DM, affecting the reliability of the results. In the present cohort, 28 (25.2%) patients had DM, which is similar to a previously reported prevalence (4.9–33.1%) in patients with ICC (29,30). The current multivariate analysis revealed that DM was an independent risk factor for OS and RFS, suggesting that a more rigorous follow-up strategy should be adopted for patients with DM and suitable anti-diabetes treatments may be efficacious in patients with ICC complicated with DM.
PKM2 is widely expressed in cancer and can promote cancer cell proliferation through multiple biological mechanisms (31). In breast cancer, PKM2-Y105D phosphomimetic mutant increases MCF-10a cell colony formation and CD44+/CD24− cancer stem cell population by increasing YY1-associated protein 1 (YAP) nuclear localization (32). ErbB2 is a strong inducer of PKM2-Y105D phosphorylation, which promotes the nuclear localization of YAP and increases the number of tumor stem cells (32). PKM2 binds directly to histone H3 and phosphorylates histone H3 at threonine 11 when EGFR is activated; this phosphorylation is required for the separation of histone deacetylase 3 from cyclin D1 and Myc promoter regions, and subsequent acetylation of histone H3 at lysine 9 (33). PKM2-dependent histone H3 modification serves an important role in EGF-induced cyclin D1 and c-Myc expression, glioma cell proliferation, cell cycle progression and brain tumorigenesis (33). Further analysis of the mechanism of PKM2 revealed that mitomycin 2 (MFN2), a key regulator of mitochondrial fusion, interacted with PKM2, promoted mitochondrial fusion and production of phosphorus oxide, and suppressed glycolysis (34). Additionally, mTOR increases the interaction between PKM2 and MFN2 through phosphorylation of MFN2, and it regulates the effects of PKM2 and MFN2 on glycolysis, mitochondrial fusion and oxidative phosphorylation in hepatocellular carcinoma and lung cancer cells (34). Therefore, the mTOR-MFN2-PKM2 signal axis combines glycolysis with oxygen and phosphorus to regulate the growth of hepatocellular carcinoma and lung cancer cells (34). Insulin can increase PKM2 expression in vitro, but this has not been verified in the clinic (19,20). To the best of our knowledge, the present data indicated for the first time in clinic samples that PKM2 expression was higher in patients with DM than in those without, consistent with previous in vitro results (23). Although the accurate underlying mechanism remains unknown, the current results may partially explain why patients with DM have a poorer survival outcome than patients without DM.
The present study presents some limitations. First, DM treatment can significantly affect long-term survival in patients with HCC (35). Therefore, the same phenomenon may be observed in patients with ICC. However, the DM treatment strategy in the present study was unknown. Second, all patients in the current cohort underwent surgical resection, but these patients represented only a small proportion of all patients with ICC (3). Whether DM also indicates a poor prognosis in patients without surgery remains to be explored.
In summary, the present data revealed that DM was associated with a significantly lower OS rate in patients with ICC. A potential cause may be associated with the abnormal glucose metabolism mediated by PKM2, which should be further investigated.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science Foundation of China (grant no. 81802778).
Availability of data and materials
All data generated and/or analyzed during this study are included in this published article.
Authors' contributions
KF, XY and HW wrote the manuscript. XY and HW were involved in the statistical analysis. XY, HW and JG were involved in clinical data collection. KF, XY, JG and XL were involved in the study design, acquisition of data, financial support and proofreading of the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All experiments were approved by the Ethics Committee of the Second Affiliated Hospital of Chongqing Medical University. Written informed consent was provided by all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Shaib YH, Davila JA, McGlynn K, El-Serag HB. Rising incidence of intrahepatic cholangiocarcinoma in the United States: A true increase? J Hepatol. 2004;40:472–477. doi: 10.1016/j.jhep.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 2.Spolverato G, Kim Y, Alexandrescu S, Popescu I, Marques HP, Aldrighetti L, Clark Gamblin T, Miura J, Maithel SK, Squires MH, et al. Is hepatic resection for large or multifocal intrahepatic cholangiocarcinoma justified? Results from a multi-institutional collaboration. Ann Surg Oncol. 2015;22:2218–2225. doi: 10.1245/s10434-014-4223-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raoof M, Dumitra S, Ituarte PHG, Melstrom L, Warner SG, Fong Y, Singh G. Development and validation of a prognostic score for intrahepatic cholangiocarcinoma. JAMA Surg. 2017;152:e170117. doi: 10.1001/jamasurg.2017.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 5.Yang WS, Shu XO, Gao J, Li HL, Cai H, Yang G, Ji BT, Rothman N, Gao YT, Zheng W, Xiang YB. Prospective evaluation of type 2 diabetes mellitus on the risk of primary liver cancer in Chinese men and women. Ann Oncol. 2013;24:1679–1685. doi: 10.1093/annonc/mdt017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaiteerakij R, Yang JD, Harmsen WS, Slettedahl SW, Mettler TA, Fredericksen ZS, Kim WR, Gores GJ, Roberts RO, Olson JE, et al. Risk factors for intrahepatic cholangiocarcinoma: Association between metformin use and reduced cancer risk. Hepatology. 2013;57:648–655. doi: 10.1002/hep.26092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Komura T, Mizukoshi E, Kita Y, Sakurai M, Takata Y, Arai K, Yamashita T, Ohta T, Shimizu K, Nakamoto Y, et al. Impact of diabetes on recurrence of hepatocellular carcinoma after surgical treatment in patients with viral hepatitis. Am J Gastroenterol. 2007;102:1939–1946. doi: 10.1111/j.1572-0241.2007.01354.x. [DOI] [PubMed] [Google Scholar]
- 8.Ting CT, Chen RC, Chen CC, Liu MH, Chu D, Kuo NW. Diabetes worsens the surgical outcomes in cirrhotic patients with hepatocellular carcinoma. Tohoku J Exp Med. 2012;227:73–81. doi: 10.1620/tjem.227.73. [DOI] [PubMed] [Google Scholar]
- 9.Wang YG, Wang P, Wang B, Fu ZJ, Zhao WJ, Yan SL. Diabetes mellitus and poorer prognosis in hepatocellular carcinoma: A systematic review and meta-analysis. PLoS One. 2014;9:e95485. doi: 10.1371/journal.pone.0095485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang YY, Huang S, Zhong JH, Ke Y, Guo Z, Liu JQ, Ma L, Li H, Ou BN, Li LQ. Impact of diabetes mellitus on the prognosis of patients with hepatocellular carcinoma after curative hepatectomy. PLoS One. 2014;9:e113858. doi: 10.1371/journal.pone.0113858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palmer WC, Patel T. Are common factors involved in the pathogenesis of primary liver cancers? A meta-analysis of risk factors for intrahepatic cholangiocarcinoma. J Hepatol. 2012;57:69–76. doi: 10.1016/j.jhep.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the warburg effect: The metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 14.Yu G, Yu W, Jin G, Xu D, Chen Y, Xia T, Yu A, Fang W, Zhang X, Li Z, Xie K. PKM2 regulates neural invasion of and predicts poor prognosis for human hilar cholangiocarcinoma. Mol Cancer. 2015;14:193. doi: 10.1186/s12943-015-0462-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beyoglu D, Imbeaud S, Maurhofer O, Bioulac-Sage P, Zucman-Rossi J, Dufour JF, Idle JR. Tissue metabolomics of hepatocellular carcinoma: Tumor energy metabolism and the role of transcriptomic classification. Hepatology. 2013;58:229–238. doi: 10.1002/hep.26350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beyoglu D, Idle JR. The metabolomic window into hepatobiliary disease. J Hepatol. 2013;59:842–858. doi: 10.1016/j.jhep.2013.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Israelsen WJ, Dayton TL, Davidson SM, Fiske BP, Hosios AM, Bellinger G, Li J, Yu Y, Sasaki M, Horner JW, et al. PKM2 isoform-specific deletion reveals a differential requirement for pyruvate kinase in tumor cells. Cell. 2013;155:397–409. doi: 10.1016/j.cell.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cortes-Cros M, Hemmerlin C, Ferretti S, Zhang J, Gounarides JS, Yin H, Muller A, Haberkorn A, Chene P, Sellers WR, Hofmann F. M2 isoform of pyruvate kinase is dispensable for tumor maintenance and growth. Proc Natl Acad Sci USA. 2013;110:489–494. doi: 10.1073/pnas.1212780110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Q, Liu X, Yin Y, Zheng JT, Jiang CF, Wang J, Shen H, Li CY, Wang M, Liu LZ, Jiang BH. Insulin regulates glucose consumption and lactate production through reactive oxygen species and pyruvate kinase M2. Oxid Med Cell Longev. 2014;2014:504953. doi: 10.1155/2014/504953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li W, Wang J, Chen QD, Qian X, Li Q, Yin Y, Shi ZM, Wang L, Lin J, Liu LZ, Jiang BH. Insulin promotes glucose consumption via regulation of miR-99a/mTOR/PKM2 pathway. PLoS One. 2013;8:e64924. doi: 10.1371/journal.pone.0064924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Diabetes Association. Standards of medical care in diabetes-2013. Diabetes Care 36 Suppl. 2013;1(Suppl 1):S11–S66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. 7th. Springer; New York, NY: 2010. AJCC Cancer Staging Manual. [Google Scholar]
- 23.Liu Y, Wu H, Mei Y, Ding X, Yang X, Li C, Deng M, Gong J. Clinicopathological and prognostic significance of PKM2 protein expression in cirrhotic hepatocellular carcinoma and non-cirrhotic hepatocellular carcinoma. Sci Rep. 2017;7:15294. doi: 10.1038/s41598-017-14813-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishioka T, Kubo S, Tanaka S, Wakasa K, Takemura S, Kinoshita M, Hamano G, Kuwae Y, Shibata T, Suehiro S. Outcomes of hepatic resection in intrahepatic cholangiocarcinoma patients with diabetes, hypertension, and dyslipidemia: Significance of routine follow-up. Liver Cancer. 2016;5:107–120. doi: 10.1159/000367752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee BS, Park EC, Park SW, Nam CM, Roh J. Hepatitis B virus infection, diabetes mellitus, and their synergism for cholangiocarcinoma development: A case-control study in Korea. World J Gastroenterol. 2015;21:502–510. doi: 10.3748/wjg.v21.i2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu Q, He XD, Yu L, Liu W, Tao LY. The metabolic syndrome and risk factors for biliary tract cancer: A case-control study in China. Asian Pac J Cancer Prev. 2012;13:1963–1969. doi: 10.7314/APJCP.2012.13.5.1963. [DOI] [PubMed] [Google Scholar]
- 27.Huang YJ, Wu AT, Chiou HY, Chuang MT, Meng TC, Chien LN, Yen Y. Interactive role of diabetes mellitus and female sex in the risk of cholangiocarcinoma: A population-based nested case-control study. Oncotarget. 2017;8:6642–6651. doi: 10.18632/oncotarget.14254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Endo I, Gonen M, Yopp AC, Dalal KM, Zhou Q, Klimstra D, D'Angelica M, DeMatteo RP, Fong Y, Schwartz L, et al. Intrahepatic cholangiocarcinoma: Rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008;248:84–96. doi: 10.1097/SLA.0b013e318176c4d3. [DOI] [PubMed] [Google Scholar]
- 29.Jing W, Jin G, Zhou X, Zhou Y, Zhang Y, Shao C, Liu R, Hu X. Diabetes mellitus and increased risk of cholangiocarcinoma: A meta-analysis. Eur J Cancer Prev. 2012;21:24–31. doi: 10.1097/CEJ.0b013e3283481d89. [DOI] [PubMed] [Google Scholar]
- 30.Welzel TM, Graubard BI, El-Serag HB, Shaib YH, Hsing AW, Davila JA, McGlynn KA. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: A population-based case-control study. Clin Gastroenterol Hepatol. 2007;5:1221–1228. doi: 10.1016/j.cgh.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dayton TL, Jacks T, Vander Heiden MG. PKM2, cancer metabolism, and the road ahead. EMBO Rep. 2016;17:1721–1730. doi: 10.15252/embr.201643300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou Z, Li M, Zhang L, Zhao H, Şahin Ö, Chen J, Zhao JJ, Songyang Z, Yu D. Oncogenic kinase-induced PKM2 tyrosine 105 phosphorylation converts nononcogenic PKM2 to a tumor promoter and induces cancer stem-like cells. Cancer Res. 2018;78:2248–2261. doi: 10.1158/0008-5472.CAN-17-2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang W, Xia Y, Hawke D, Li X, Liang J, Xing D, Aldape K, Hunter T, Alfred Yung WK, Lu Z. PKM2 phosphorylates histone H3 and promotes gene transcription and tumorigenesis. Cell. 2012;150:685–696. doi: 10.1016/j.cell.2012.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li T, Han J, Jia L, Hu X, Chen L, Wang Y. PKM2 Coordinates glycolysis with mitochondrial fusion and oxidative phosphorylation. Protein Cell. 2019;10:583–594. doi: 10.1007/s13238-019-0618-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Casadei Gardini A, Faloppi L, De Matteis S, Foschi FG, Silvestris N, Tovoli F, Palmieri V, Marisi G, Brunetti O, Vespasiani-Gentilucci U, et al. Metformin and insulin impact on clinical outcome in patients with advanced hepatocellular carcinoma receiving sorafenib: Validation study and biological rationale. Eur J Cancer. 2017;86:106–114. doi: 10.1016/j.ejca.2017.09.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated and/or analyzed during this study are included in this published article.