Introduction:
Primary mediastinal (thymic) large B-cell lymphoma is a subtype of diffuse large B-cell lymphoma with distinct clinical, molecular and genetic features, many of which overlap with Hodgkin’s lymphoma. Increasingly, initial therapy for these patients has employed dose-dense chemotherapy with or without radiation with excellent results. In patients with relapsed and primary refractory disease, outcomes of second-line therapy followed by consolidation with high-dose therapy and autologous stem cell transplantation remains largely undefined. We reviewed the outcomes of sixty transplant-eligible patients with relapsed or refractory primary mediastinal (thymic) large B-cell lymphoma enrolled on sequential protocols with uniform second-line therapy with intent to consolidate with autologous stem cell transplant. The estimated 3-year overall and event-free survivals for all patients are 61% and 57% respectively, and 68% and 65% respectively for patients proceeding to stem cell transplant. Multivariable analysis of risk factors prior to transplant revealed that an incomplete response to initial therapy, advanced Ann Arbor stage at disease progression, and failure to achieve a partial remission or better to second-line therapy to be independently associated with inferior event-free and overall survival. A risk score based on these variables was able to identify patients who are unlikely to respond to conventional second-line strategies. These results suggest that salvage chemoradiotherapy with intent of subsequent high-dose therapy and autologous stem cell transplant is successful in the majority of patients with relapsed and refractory primary mediastinal (thymic) large B-cell lymphoma. Alternative strategies are warranted for a significant subset of patients with high-risk disease who are unlikely to be cured with this strategy.
Introduction:
Primary mediastinal (thymic) large B-cell lymphoma (PMBL) is a subtype of diffuse large B-cell lymphoma (DLBCL) with distinct clinical, molecular and genetic features. First identified as a unique entity in 1980 [1, 2], our understanding of PMBL continues to be defined as new biologic insights are applied to the clinical treatment paradigm. PMBL accounts for 6–13% of all DLBCL diagnoses and is recognized in both the Revised European-American Classification of Lymphoid Neoplasms (REAL) and World Health Organization (WHO) classifications as a distinct subtype of DLBCL [3, 4]. Manifesting with a clinical presentation similar to that of Hodgkin lymphoma (HL) [5–7], it is now demonstrated that this similarity extends to the molecular level based on gene expression profiling revealing PMBL is more similar to HL than to DLBCL subtypes [8].
At Memorial Sloan Kettering Cancer Center (MSKCC), initial therapy for PMBL patients treated on protocol has employed dose-dense chemotherapy with or without radiation (RT) with excellent results [6]. In patients with relapsed and primary refractory disease, outcomes of patients treated with second-line therapy (SLT) followed by consolidation with high-dose therapy and autologous stem cell transplant (HDT/ASCT) remains largely undefined. Available data suggests a clinical propensity for extranodal disease progression at the time of relapse or primary refractory disease and published outcomes of patients treated with SLT and HDT/ASCT are variable [9–11]. We report MSKCC’s experience with relapsed and refractory PMBL patients who were subsequently treated with second-line therapy (SLT) with intent to consolidate with HDT/ASCT. We assessed outcomes for these patients, including event-free (EFS) and overall survival (OS), and evaluated the effect of key clinicopathologic characteristics on outcomes.
Materials/Methods:
Patient Population:
Sixty transplant-eligible patients from 1989 to 2014 with PMBL who experienced disease progression following front-line anthracycline-based chemotherapy were identified. PMBL was defined based on previously reported clinical criteria [12]: 1) a diagnosis of diffuse mixed cell, diffuse large cell or immunoblastic lymphoma by the haematopathology staff in the Department of Pathology at MSKCC; 2) a B cell phenotype as determined by CD20 positivity and negative staining for T cell markers; and 3) a mediastinal mass of at least 5 cm with or without contiguous extranodal or supraclavicular involvement with no concomitant extra-mediastinal mass greater in size than the primary mediastinal lesion. Biopsy confirmation of relapsed/refractory disease was required, per institution practice. Transplant eligibility was determined by the treating oncologist. Approval for this retrospective review was obtained from the Institutional Review and Privacy Board at MSKCC.
Eligibility for salvage therapy
All patients were staged according to standard Ann Arbor criteria. All patients had adequate cardiac function (left ventricular ejection fraction > 50%) and renal function (serum creatinine ≤ 1.5 mg/dL or creatinine clearance ≥ 60 mL/min or higher) to undergo platinum-based chemotherapy.
Salvage chemotherapy and response criteria
ICE chemotherapy was administered as previously described, either with or without rituximab depending on the era of treatment.[13, 14] Re-staging PET-CT or CT of the chest, abdomen, and pelvis was performed within four weeks of completion of SLT. Response criteria were per the International Harmonization Project (IHP) when pre- and post- SLT PET were available, otherwise by the sum of the product of the diameters (SPD) on CT scan as per the International Working Group [15, 16] and were compared to the reference imaging study performed following completion of first-line therapy.
High-dose therapy and autologous stem cell transplantation
Patients deemed to have chemosensitive disease by their treating oncologist were eligible for HDT/ASCT. All patients undergoing HDT/ASCT were required to have adequate pulmonary function (diffusion capacity greater than 50% of predicted) and liver function (serum bilirubin level less than 2 mg/dL). The choice of the conditioning regimen depended on the patient’s age, the extent of prior therapy, and the clinical trials active at the time of transplantation. Pre-transplant radiotherapy (RT) was administered at the discretion of the primary physician and was delivered to the involved and at-risk regions. Delivered radiation doses ranged from 25.2 to 45Gy.
Statistics:
The endpoints of interest, EFS and OS were assessed from the first day of SLT in the intention-to-treat cohort and day of transplant for the transplanted subset analysis. EFS was defined from the starting point until treatment failure, progression of disease, death from all causes, toxicity from SLT or HDT/ASCT requiring treatment discontinuation, or secondary malignancy, whichever came first, and patients who did not experience any of these events were censored at the date of last follow-up. OS was defined from the starting point until death, and patients who did not die during the study time were censored at the date of last follow-up. Both endpoints were analyzed using the Kaplan-Meier method and Cox proportional hazards models [17].
Factors known during or following SLT that were selected as possible prognostic indicators of PFS or OS included: choice of initial treatment, primary treatment response (relapsed disease vs. primary refractory disease, defined as failure to achieve a CR to front-line therapy), rituximab with initial therapy, radiation treatment with initial therapy, age (younger than 60 vs. 60 or older), Ann Arbor stage (I/II vs. III/IV), disease bulk (less than 10 cm vs. 10 cm or more in longest axis), LDH (normal vs. elevated), KPS (less than 80 vs. 80 or greater), B symptoms, extranodal involvement, second-line IPI score (low or low-intermediate vs. high-intermediate or high), second line age-adjusted IPI (low or low-intermediate vs. high-intermediate/high [18], choice of SLT, rituximab with SLT, radiation treatment following SLT, and chemosensitivity to SLT (defined as CR or PR vs. SD or POD).
Univariable Cox regression was conducted for OS in both ITT and transplanted cohorts using factors known by the starting point of OS. Factors with a univariate p value <0.05 were considered for a multivariable Cox regression model. Clinical significance and ease of practical use were considered when choosing which of the statistically significant factors to include in the final model. Since all prognostic factors were categorical, patients were grouped based on cross-classification of the factors in the final model and the OS were compared among the groups of patients.
All statistical tests were two-sided, and 5% was set as the level of significance. Statistical analyses were performed using R 3.2.0 (R Development Core Team.
Results:
Patient Characteristics:
Pre-SLT treatment characteristics of the sixty patients are shown in Tables 1 and 2. Median age was 35 (range 19–68); 3 patients (5%) were older than 60. 18% of patients received chemotherapy and radiation in the first-line setting whereas 84% received chemotherapy alone. With respect to initial treatment response, 42% of patients had relapsed disease whereas 58% had primary refractory disease. 60% of patients had stage I or II disease at time of progression while 40% had stage III or IV disease. 30% of patients had extranodal involvement. 50% of patients had bulky disease. The median time from initial diagnosis to date of relapsed or refractory disease diagnosis was 6.9 months for all patients (range 0.1 – 3.1 years).
Table 1:
Patient Characteristics before salvage chemotherapy
| All patients | Transplanted | |
|---|---|---|
| Total patients | 60 | 51 |
| Age | ||
| • Median (range) | 35 (19–68) | 35 (19–61) |
| • >60 | 3 (5%) | 1 (2%) |
| Initial Treatment | ||
| • ABVD | 4 (7%) | 4 (8%) |
| • CHOP | 10 (17%) | 6 (12%) |
| • NHL-15 | 20 (33%) | 17 (33%) |
| • R-CHOP | 14 (23%) | 12 (24%) |
| • R-CHOP/ICE | 6 (10%) | 6 (12%) |
| • R-EPOCH | 4 (7%) | 4 (8%) |
| Radiation therapy with primary treatment | ||
| • Yes | 11 (18%) | 8 (16%) |
| • No | 49 (82%) | 43 (84%) |
| Initial Response | ||
| • Relapsed | 25 (42%) | 23 (45%) |
| • Refractory | 35 (58%) | 28 (55%) |
| Disease Bulk at progression | ||
| • Yes | 30 (50%) | 24 (47%) |
| • No | 30 (50%) | 27 (53%) |
| B symptoms at progression | ||
| • Yes | 16 (27%) | 15 (29%) |
| • No | 44 (73%) | 36 (71%) |
| AA stage at progression | ||
| • I-II | 36 (60%) | 31 (61%) |
| • III-IV | 24 (40%) | 20 (39%) |
| LDH at progression | ||
| • Within normal limits | 27 (45%) | 22 (43%) |
| • Elevated | 33 (55%) | 29 (57%) |
| KPS at relapse | ||
| • 80+ | 49 (82%) | 42 (82%) |
| • <80 | 11 (18%) | 9 (18%) |
| Second-line IPI | ||
| • Low | 35 (58%) | 30 (59%) |
| • Low-intermediate | 11 (18%) | 10 (20%) |
| • High-intermediate | 7 (12%) | 7 (14%) |
| • High | 7 (12%) | 4 (8%) |
| Second-line aaIPI | ||
| • Low | 20 (33%) | 16 (31%) |
| • Low-intermediate | 18 (30%) | 16 (31%) |
| • High-intermediate | 16 (27%) | 15 (29%) |
| • High | 6 (10%) | 4 (8%) |
R: rituximab. CHOP: cyclophosphamide, hydroxydaunomycin (doxorubicin), Oncovin (vincristine), prednisone. EPOCH: etoposide, prednisone, Oncovin, cyclophosphamide, hydroxydaunomycin. ICE: ifosfamide, carboplatin, etoposide. ABVD: Adriamycin (doxorubicin), bleomycin, vinblastine, dexamethasone. NHL-15: doxorubicin, vincristine, cyclophosphamide.
Table 2:
Salvage chemotherapy characteristics and response
| All patients (N=60) | Relapsed (N=25) | Refractory (N=35) | |
|---|---|---|---|
| Salvage Therapy | |||
| • R-ICE | 29 (48%) | 9 (36%) | 20 (37%) |
| • ICE | 16 (27%) | 8 (32%) | 8 (23%) |
| • O-DHAP | 5 (8%) | 2 (8%) | 3 (9%) |
| • ICEMAN | 3 (5%) | 3 (12%) | 0 (0%) |
| • Other | 7 (12%) | 3 (12%) | 4 (11%) |
| Response to therapy** | |||
| • CR | 24 (40%) | 14 (56%) | 10 (29%) |
| • PR | 15 (25%) | 6 (24%) | 9 (26%) |
| • SD | 7 (12%) | 1 (4%) | 6 (17%) |
| • POD | 14 (23%) | 4 (16%) | 10 (29%) |
R-ICE: rituximab, ifosfamide, carboplatin, etoposide. O-DHAP: ofatumumab, dexamethasone, high-dose Ara-C (cytarabine), cisplatin. ICEMAN: ifosfamide, carboplatin, etoposide, methotrexate, cytarabine. CR: complete remission. PR: partial remission. SD: stable disease. POD: progression of disease.
Second-line chemotherapy:
Second-line chemotherapy consisted of ICE (33%) and R-ICE (48%) in the majority of cases. The overall response rate (ORR) to SLT was 65%, with 40% achieving a complete response and 25% achieving a partial response. Patients with relapsed disease had a higher overall response rate (80% vs. 54%, p = 0.02) and complete response rate (56 vs 29%, p = 0.02) as compared to patients with primary refractory disease. Patients with early stage disease at time of disease progression had a higher complete response rate (53% vs 21%, p = 0.01) and trend towards higher overall response rate (72% vs 54%, p = 0.07) as compared with patients with advanced stage disease at relapse.
Radiation therapy:
RT was delivered as a component of salvage therapy in all but 6 patients, who had received prior RT as part of initial combined-modality therapy (Table 4). A single patient received both RT in the upfront setting to the mediastinum and left supraclavicular fossa as well as in the salvage setting to the lumbosacral spine as the RT fields did not overlap. Among patients who were treated at MSKCC and had detailed RT fields available for review (n=51), median RT dose delivered was 30Gy (range: 12Gy – 45Gy). The most common RT regimen (n=24) was 30Gy in 20 twice-daily fractions immediately preceding ASCT. Seven patients received ≥39.6Gy (range: 39.6Gy – 45Gy) to the mediastinum. Ten patients received 12 – 15Gy of total body irradiation as a component of their RT plan and a single patient received 12Gy of TBI alone.
Table 4:
Salvage Radiation Therapy Characteristics for Patients with Complete Radiotherapy Records (n=52)
| Median dose | 30 Gy |
| • Range | 12–45 Gy |
| Fractionation | |
| • Daily | 22 (42%) |
| • BID | 30 (58%) |
| RT Fields | |
| • TBI alone | 3 (6%) |
| • TBI component | 10 (19%) |
| • Mantle fields | 2 (4%) |
| • Mediastinum +/− neck | 45 (87%) |
| • Non-mediastinum | 2 (4%) |
HDT and ASCT
Fifty-one (85%) patients proceeded to consolidative autologous stem cell transplant. Of the patients undergoing ASCT, thirty-eight (75%) had chemosensitive disease with 45% achieving a CR and 29% achieving a partial response (PR). Six patients with stable disease and seven patients with progression of disease proceeded to HDT/ASCT. Only two of these thirteen patients remain alive at last follow-up. Both achieved a CR to involved field radiotherapy following SLT failure and proceeded to ASCT thereafter. Only one patient achieving a chemosensitive remission did not proceed to ASCT due to strong patient preference. This patient was treated with consolidative radiotherapy and remained progression-free at last interim follow-up.
The characteristics and conditioning regimens for patients proceeding to HDT/ASCT are listed in Table 3. The majority (92%) of patients received one of five conditioning regimens: BEAM (carmustine, etoposide, cytarabine, melphalan) (57%), IE (ifosfamide, etoposide) (10%), cyclophosphamide and etoposide (8%), CBV (cyclophosphamide, carmustine, etoposide) (7%), or melphalan (5%). RT was administered to 46 (90%) of 51 transplanted patients; of the 5 patients who did not receive pre-SCT RT, all had received radiation with initial therapy and could not receive additional radiation to the mediastinum due to normal tissue tolerances. Three deaths (5.8%) occurred within 100 days of stem cell reinfusion; one due to neutropenic sepsis and multi-organ failure and two due to widespread progression of disease. Notably, no patients in this cohort developed pneumonitis, either following radiotherapy or following HDT/ASCT.
Table 3:
Characteristics and conditioning regimens for patients who underwent HDT/ASCT
| No. patients | |
|---|---|
| Total patients | 51 |
| Radiotherapy prior to conditioning | 46 (90%) |
| Conditioning regimen | |
| • BEAM/BEAC | 29 (57%) |
| • IE + TBI | 6 (12%) |
| • Cyclophosphamide + etoposide | 2 (4%) |
| • Cyclophosphamide + etoposide + TBI | 3 (6%) |
| • CBV | 4 (8%) |
| • Melphalan | 3 (6%) |
| • Other | 4 (8%) |
HDT/ASCT: high dose therapy/autologous stem cell transplant. CR: complete remission, PR: partial remission, SD: stable disease. BEAM: BCNU (carmustine), etoposide, ara-C (cytarabine), melphalan. BEAC: BCNU (carmustine), etoposide, ara-C (cytarabine), cyclophosphamide
IE: ifosfamide, etoposide. CBV: cyclophosphamide, BCNU (carmustine), VP-16 (etoposide).
Nine patients completing second-line chemotherapy did not proceed to HDT/ASCT. One patient with early-stage disease who had not been radiated with upfront therapy received definitive radiotherapy and has remained progression free. A second patient has received a checkpoint inhibitor and remains alive after eighteen months of follow-up. The remaining seven patients have died with a median overall survival of less than one year from initial relapse.
Overall and Progression-free Survival
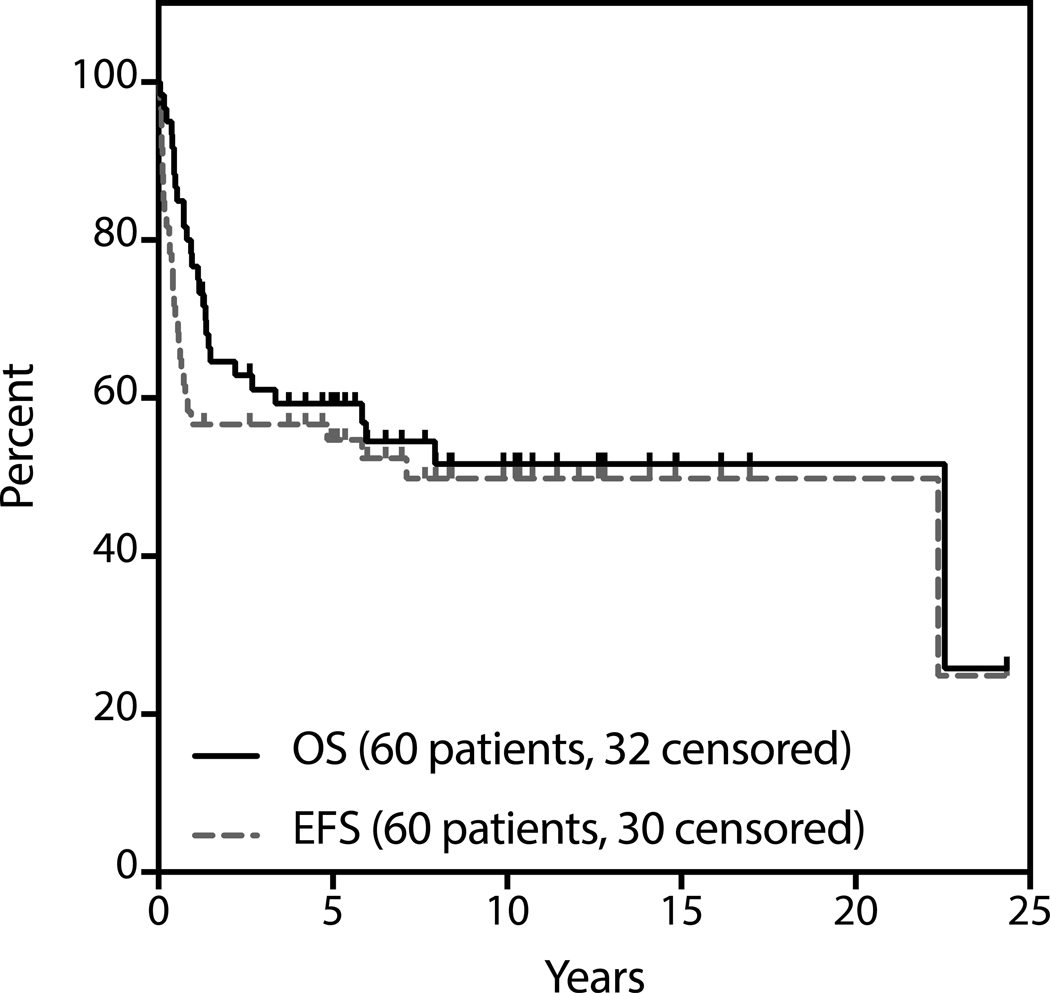
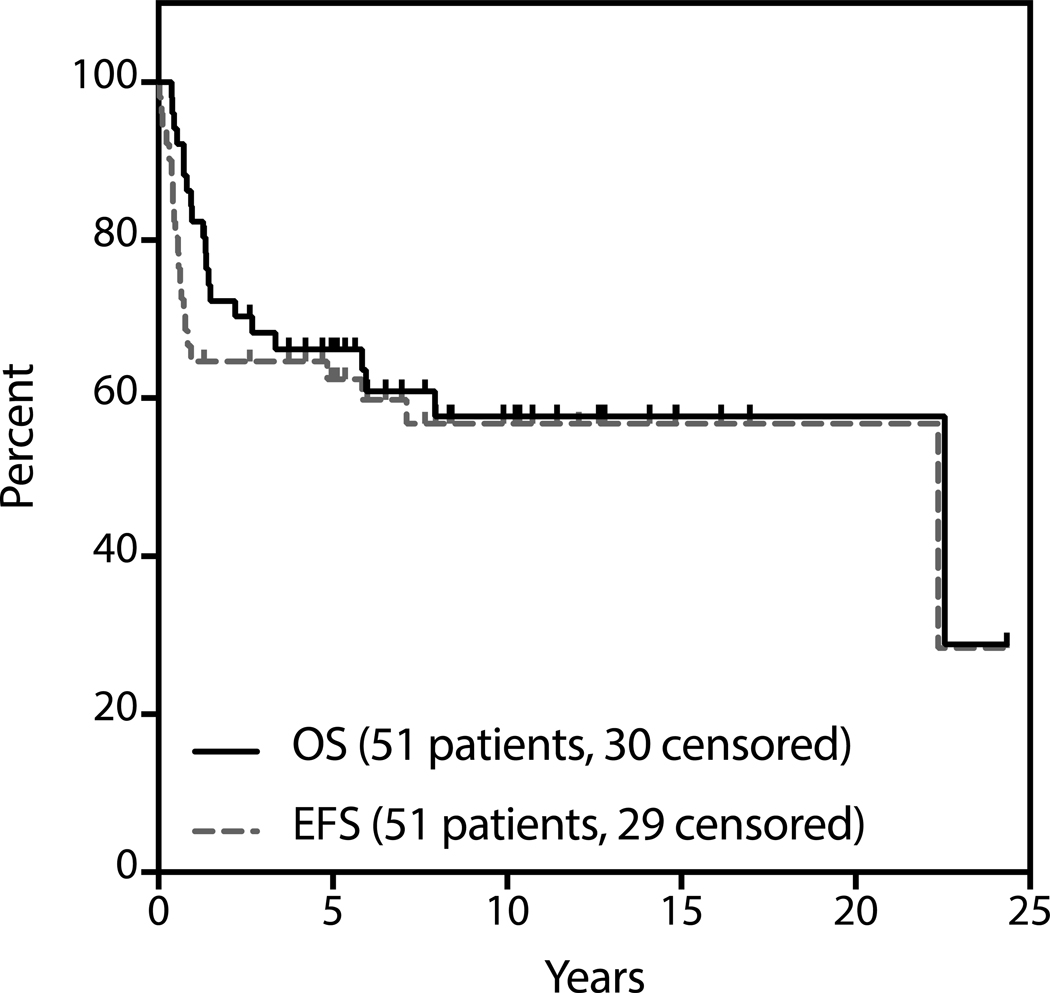
Median follow-up for survivors was 4.8 years (range 0.05 to 24.3). The estimated 3-year OS and EFS are 61% and 57% respectively (Figure 1). With respect to patients proceeding to ASCT, median follow-up for survivors from the date of SCT was 5.6 years (range 0.38 to 24.3 years). The estimated 3-year OS and PFS of patients proceeding to transplant are 65% and 60% respectively (Figure 2). Two of nine patients who did not undergo consolidative transplant after first ST are alive and only one remains event-free at the time of last follow-up.
Figure 1:
Survival analysis by intention-to-treat (N=60).
Figure 2.
Survival analysis of patients who underwent transplantation (N=51).
Prognostic Factors:
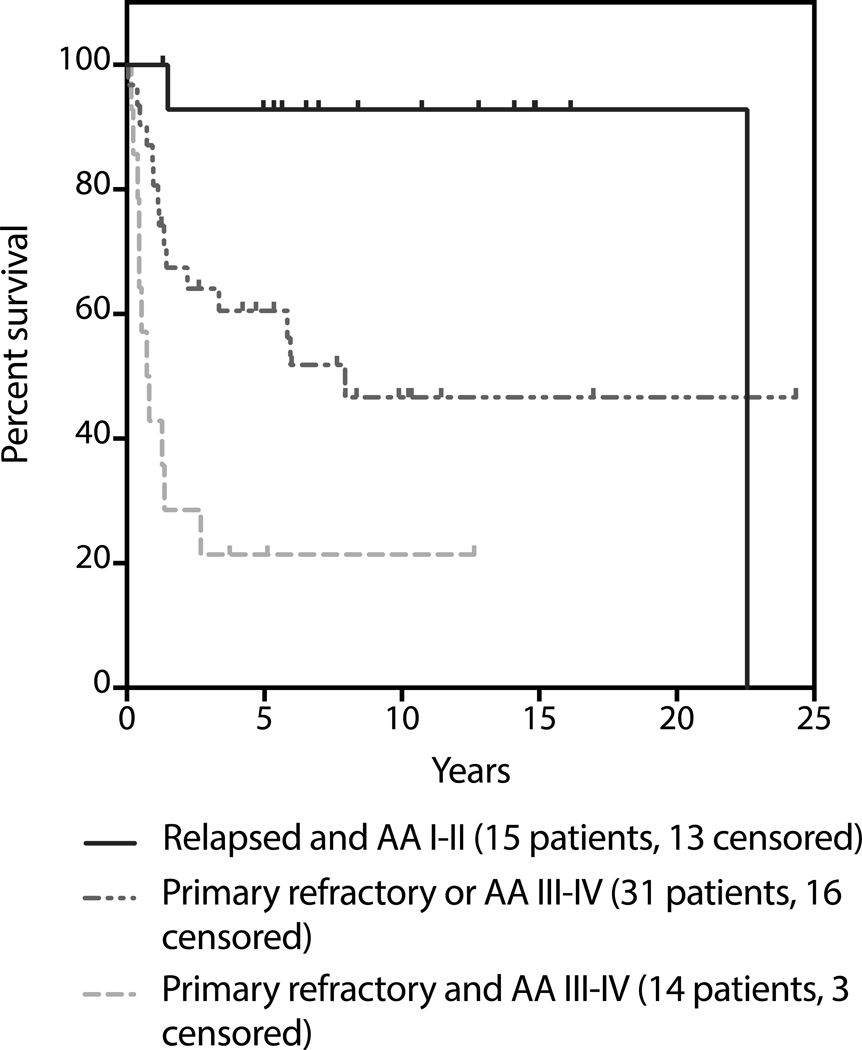
Within both the ITT and transplanted cohorts, univariable and multivariate analysis revealed primary refractory disease and advanced stage disease as pre-SLT risk factors that were independently associated with inferior OS; patients with either or both of these risk factors had a hazard ratio of 2.68 and 6.86 for death, respectively, as compared with patients with neither of these risk factors (Figure 3 and Table 5). Lack of chemosensitive response was also associated with inferior OS in both the ITT and transplanted cohorts; within the transplanted cohort chemorefractory disease carried a hazard ratio of 9.4 for death as compared to chemosensitive disease; only 15% of these patients were still alive at 3 years.
Figure 3: Survival as determined by Ann Arbor stage at relapse and response to first-line therapy.
Figure is truncated at 5 years.
Table 5:
Pre-treatment risk factors associated with OS in multivariate analysis (N=60)
| Number of PFS risk factors | 3y OS | HR for progression (95% CI) |
|---|---|---|
| 0 (relapsed, AA I-II, N=25) | 79% | |
| 1 (primary refractory or AA* III-IV, N=26) | 58% | 2.68 (1.04, 6.91) |
| 2 (primary refractory and AA III-IV, N=9) | 22% | 6.86 (2.27, 20.8) |
AA = Ann Arbor stage
Discussion:
Primary mediastinal (thymic) large B-cell lymphoma is a unique disease entity with mounting evidence for an excellent outcome when upfront dose intensive regimens are employed. Treatment paradigms have generally incorporated a combined modality approach [19–22], although more recently the use of rituximab and omission of radiotherapy have been explored [23, 24].
At the time of disease progression, the standard of care remains second-line therapy followed by HDT/ASCT to maximize potential curability based on the Parma study. However, there is relatively minimal published data regarding this strategy for PMBL; some reports suggest long-term PFS of less than 20% in patients who fail front-line therapy [9] while others have suggested higher cure rates, particularly in those with chemosensitive disease to SLT [28].
We now report our single-institution series of patients with relapsed and refractory PMBL treated with combined-modality SLT and intent to consolidate with HDT/ASCR. Our data demonstrate that this strategy can be effective in the relapsed/refractory PMBL population. Despite being a high risk cohort (58% with primary refractory disease and 40% with stage III or IV disease), SLT was effective in the majority of patients, with an ORR of 65% and CR rate of 40%, and 85% of patients ultimately proceeded to SCT. Ultimately, 57% of all patients and 60% of transplanted patients achieved long-term cures with this strategy. Despite extending over a twenty-five year period, we observed no significant differences in outcomes of our patient cohort over time.
The outcomes seen in this report compare favorably to existing reports of relapsed PMBL demonstrating a long term EFS of less than 50% [9,10]. This is likely due to the fact that in our institution the overwhelming majority of patients were treated with a chemoradiotherapy regimen that included aggressive second-line multiagent chemotherapy. As a result the majority of patients were able to proceed to HDT/ASCR. Notably, the EFS of transplanted patients was similar to existing reports showing an EFS of approximately 60% [29,30].
A number of prognostic variables are informative about this cohort of patients. First, the inclusion of rituximab, either with first or second-line therapy, did not significantly impact outcome; conversely, lack of RT following SLT was associated with inferior event-free survival (p = 0.035), suggesting that RT, administered prior to HDT/ASCT, is an important component of the second-line treatment strategy, although this is somewhat confounded by patient selection. The potential importance of RT in the second-line setting may be amplified in the setting of modern, radiation-sparing upfront treatment strategies, although this remains to be demonstrated.
The single greatest predictor of both EFS and OS in our cohort was chemosensitivity to second-line therapy. Patients achieving less than a PR to SLT had uniformly poor outcomes, even if proceeding to HDT/ASCT; the only chemorefractory patients achieving long-term remissions after failure of SLT achieved a subsequent CR to IFRT prior to HDT/ASCR. This suggests that strategies aimed at improving outcomes in this population should be directed towards improving response to SLT. Furthermore, patients achieving less than a PR to SLT should not proceed to HDT/ASCR, and should likely not receive further chemotherapy; we would recommend either investigational agents (such as PD-1 inhibitors) or definitive radiotherapy in this setting.
Finally, patients with primary refractory and advanced stage disease had significantly inferior outcomes as compared to patients with early stage and relapsed disease. In particular, the outcome of patients with primary refractory advanced stage disease was particularly poor. This was likely due to a significantly lower response rate, suggesting that investigational studies aimed at improving the salvage rate may be particularly appropriate for this population. With this report, we establish a benchmark for second-line therapy in PMBL and identify areas for future development of novel clinical strategies.
Highlights.
Outcomes of relapsed primary mediastinal large B-cell lymphoma are uncharacterized
We reviewed patients treated with second-line treatment and intent to transplant
Our data suggests that this strategy is curative in the majority of patients
A risk score identified patients less likely to be cured with this strategy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Levitt LJ, et al. , Primary non-Hodgkin’s lymphoma of the mediastinum. Cancer, 1982. 50(11): p. 2486–92. [DOI] [PubMed] [Google Scholar]
- 2.Lichtenstein AK, et al. , Primary mediastinal lymphoma in adults. Am J Med, 1980. 68(4): p. 509–14. [DOI] [PubMed] [Google Scholar]
- 3.Harris NL, et al. , World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting-Airlie House, Virginia, November 1997. J Clin Oncol, 1999. 17(12): p. 3835–49. [DOI] [PubMed] [Google Scholar]
- 4.Jaffe ES, et al. , Pathology and genetics of tumors of hematopoietic amd lymphoid tissues World Health Organization classification of tumors, ed. P K and Vol SL vol 3 2001, Lyon: IARC Press. [Google Scholar]
- 5.Abou-Elella AA, et al. , Primary mediastinal large B-cell lymphoma: a clinicopathologic study of 43 patients from the Nebraska Lymphoma Study Group. J Clin Oncol, 1999. 17(3): p. 784–90. [DOI] [PubMed] [Google Scholar]
- 6.Hamlin PA, et al. , Primary mediastinal large B-cell lymphoma: optimal therapy and prognostic factor analysis in 141 consecutive patients treated at Memorial Sloan Kettering from 1980 to 1999. Br J Haematol, 2005. 130(5): p. 691–9. [DOI] [PubMed] [Google Scholar]
- 7.Kirn D, et al. , Large-cell and immunoblastic lymphoma of the mediastinum: prognostic features and treatment outcome in 57 patients. J Clin Oncol, 1993. 11(7): p. 1336–43. [DOI] [PubMed] [Google Scholar]
- 8.Alizadeh AA, et al. , Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature, 2000. 403(6769): p. 503–11. [DOI] [PubMed] [Google Scholar]
- 9.Kuruvilla J, et al. , Salvage chemotherapy and autologous stem cell transplantation are inferior for relapsed or refractory primary mediastinal large B-cell lymphoma compared with diffuse large B-cell lymphoma. Leuk Lymphoma, 2008. 49(7): p. 1329–36. [DOI] [PubMed] [Google Scholar]
- 10.Popat U, et al. , High-dose chemotherapy for relapsed and refractory diffuse large B-cell lymphoma: mediastinal localization predicts for a favorable outcome. J Clin Oncol, 1998. 16(1): p. 63–9. [DOI] [PubMed] [Google Scholar]
- 11.Sehn LH, et al. , Primary diffuse large B-cell lymphoma of the mediastinum: outcome following high-dose chemotherapy and autologous hematopoietic cell transplantation. Blood, 1998. 91(2): p. 717–23. [PubMed] [Google Scholar]
- 12.Cazals-Hatem D, et al. , Primary mediastinal large B-cell lymphoma. A clinicopathologic study of 141 cases compared with 916 nonmediastinal large B-cell lymphomas, a GELA (“Groupe d’Etude des Lymphomes de l’Adulte”) study. American Journal of Surgical Pathology, 1996. 20(7): p. 877–88. [DOI] [PubMed] [Google Scholar]
- 13.Kewalramani T, et al. , Rituximab and ICE as second-line therapy before autologous stem cell transplantation for relapsed or primary refractory diffuse large B-cell lymphoma. Blood, 2004. 103(10): p. 3684–8. [DOI] [PubMed] [Google Scholar]
- 14.Moskowitz CH, et al. , Ifosfamide, carboplatin, and etoposide: a highly effective cytoreduction and peripheral-blood progenitor-cell mobilization regimen for transplant-eligible patients with non-Hodgkin’s lymphoma. J Clin Oncol, 1999. 17(12): p. 3776–85. [DOI] [PubMed] [Google Scholar]
- 15.Cheson BD, et al. , Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol, 1999. 17(4): p. 1244. [DOI] [PubMed] [Google Scholar]
- 16.Cheson BD, et al. , Revised response criteria for malignant lymphoma. J Clin Oncol, 2007. 25(5): p. 579–86. [DOI] [PubMed] [Google Scholar]
- 17.Cox DR, Regression Models and Life-Tables. Journal of the Royal Statistical Society Series B-Statistical Methodology, 1972. 34(2): p. 187-+. [Google Scholar]
- 18.Lerner RE, et al. , The International Prognostic Index assessed at relapse predicts outcomes of autologous transplantation for diffuse large-cell non-Hodgkin’s lymphoma in second complete or partial remission. Biol Blood Marrow Transplant, 2007. 13(4): p. 486–92. [DOI] [PubMed] [Google Scholar]
- 19.Binkley MS, et al. , A single-institution retrospective analysis of outcomes for stage I-II primary mediastinal large B-cell lymphoma treated with immunochemotherapy with or without radiotherapy. Leuk Lymphoma, 2016. 57(3): p. 6048. [DOI] [PubMed] [Google Scholar]
- 20.Martelli M, et al. , [18F]fluorodeoxyglucose positron emission tomography predicts survival after chemoimmunotherapy for primary mediastinal large B-cell lymphoma: results of the International Extranodal Lymphoma Study Group IELSG-26 Study. J Clin Oncol, 2014. 32(17): p. 1769–75. [DOI] [PubMed] [Google Scholar]
- 21.Rieger M, et al. , Primary mediastinal B-cell lymphoma treated with CHOP-like chemotherapy with or without rituximab: results of the Mabthera International Trial Group study. Ann Oncol, 2011. 22(3): p. 664–70. [DOI] [PubMed] [Google Scholar]
- 22.Soumerai JD, et al. , Treatment of primary mediastinal B-cell lymphoma with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone is associated with a high rate of primary refractory disease. Leuk Lymphoma, 2014. 55(3): p. 538–43. [DOI] [PubMed] [Google Scholar]
- 23.Dunleavy K, et al. , Dose-adjusted EPOCH-rituximab therapy in primary mediastinal B-cell lymphoma. N Engl J Med, 2013. 368(15): p. 1408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moskowitz C, et al. , Sequential Dose-Dense RCHOP Followed by ICE Consolidation (MSKCC protocol 01–142) without Radiotherapy for Patients with Primary Mediastinal Large B Cell Lymphoma. Blood, 2010. 116(21): p. 187–188. [Google Scholar]
- 25.Todeschini G, et al. , Primary mediastinal large B-cell lymphoma (PMLBCL): long-term results from a retrospective multicentre Italian experience in 138 patients treated with CHOP or MACOP-B/VACOP-B. Br J Cancer, 2004. 90(2): p. 372–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zinzani PL, et al. , Induction chemotherapy strategies for primary mediastinal large B-cell lymphoma with sclerosis: a retrospective multinational study on 426 previously untreated patients. Haematologica, 2002. 87(12): p. 1258–64. [PubMed] [Google Scholar]
- 27.Adams MJ, et al. , Cardiovascular status in long-term survivors of Hodgkin’s disease treated with chest radiotherapy. J Clin Oncol, 2004. 22(15): p. 3139–48. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez J, et al. , Primary mediastinal large cell lymphoma (PMBL): frontline treatment with autologous stem cell transplantation (ASCT). The GEL-TAMO experience. Hematol Oncol, 2008. 26(3): p. 171–8. [DOI] [PubMed] [Google Scholar]
- 29.Aoki T, et al. , High-dose chemotherapy followed by autologous stem cell transplantation for relapsed/refractory primary mediastinal large B-cell lymphoma. Blood Cancer J, 2015. 5: p. e372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Avivi I, et al. , Autologous stem cell transplantation for primary mediastinal B-cell lymphoma: long-term outcome and role of post-transplant radiotherapy. A report of the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant, 2018. [DOI] [PubMed] [Google Scholar]