Abstract
Objective:
The aim of this study was to characterize continuous renal replacement therapy (CRRT) utilization on extracorporeal membrane oxygenation (ECMO) and to determine the association of both fluid overload (FO) at CRRT initiation and fluid removal during CRRT with mortality in a large multicenter cohort.
Methods:
Retrospective chart review of all children < 18 years of age concurrently treated with ECMO and CRRT from January 1, 2007 to December 31, 2011 at 6 tertiary care children’s hospital. Children treated with hemodialysis or peritoneal dialysis were excluded from the FO analysis.
Measurements and Main Results:
A total of 756 of the 1009 children supported with ECMO during the study period had complete FO data. Of these, 357 (47.2%) received either CRRT or were treated with an in-line filter and thus entered into the final analysis. Survival to ECMO decannulation was 66.4% and survival to hospital discharge was 44.3%. CRRT initiation occurred at median of 1 day (IQR 0, 2) after ECMO initiation. Median FO at CRRT initiation was 20.1% (IQR 5, 40) and was significantly lower in ECMO survivors vs. non-survivors (15.3% vs. 30.5% p= 0.005) and in hospital survivors vs. non-survivors (13.5% vs. 25.9%, p= 0.004). Median FO at CRRT discontinuation was significantly lower in ECMO survivors (23% vs. 37.6% p= 0.002) and hospital survivors vs. non-survivors (22.6% vs. 36.1%, p=0.002). In ECMO survivors, after adjusting for pH at CRRT initiation, non-renal complications, ECMO mode, support type, center, patient age and AKI, FO at CRRT initiation (p = 0.01), and FO at CRRT discontinuation (p = 0.0002) were independently associated with duration of ECMO. In a similar multivariable analysis, FO at CRRT initiation (adjusted adds ratio [aOR] 1.09, 95% CI 1.00–1.18, p=0.045) and at CRRT discontinuation (aOR 1.11, 95% CI 1.03–1.19, p=0.01) were independently associated with hospital mortality.
Conclusions:
In a multicenter pediatric ECMO cohort, this study demonstrates that severe FO was very common at CRRT initiation. We found an independent association between the degree of FO at CRRT initiation with adverse outcomes including mortality and increased duration of ECMO support. The results suggest intervening prior to the development of significant FO may be a clinical therapeutic target and warrants further evaluation.
Keywords: Fluid overload, Continuous Renal Replacement Therapy, ECMO, Extracorporeal Membrane Oxygenation
Introduction
Extracorporeal membrane oxygenation (ECMO) provides essential gas exchange and hemodynamic support for pediatric patients with severe reversible cardiac and respiratory failure. For patients on ECMO, acute kidney injury (AKI) and fluid overload (FO) occur frequently and are associated with increased mortality [1–10]. As such, continuous renal replacement therapy (CRRT) has become an important tool in managing severe AKI in patients undergoing ECMO [2, 11–13].
The Kidney Interventions During Membrane Oxygenation (KIDMO) study group is an international multidisciplinary group of six participating centers that has been established to study AKI, FO, and CRRT in children on ECMO. The KIDMO study group performed a 5-year retrospective study of all children treated with ECMO at each of the 6-member institutions. The initial publications from this cohort clearly showed that AKI and FO both occur commonly in children on ECMO and are associated with adverse outcomes [9, 10]. To date there have been two small single center studies that have evaluated the impact of FO and fluid removal on outcomes in CRRT on pediatric ECMO [14, 15]. These studies showed that the degree of FO at CRRT initiation and degree of fluid accumulation during ECMO, predicted outcomes for children on ECMO. In order to continue to improve outcomes in this population it is critical to better understand the impact of FO in pediatric patients treated with CRRT and ECMO in a large multicenter cohort.
Here, we present a planned secondary analysis from the 5-year KIDMO retrospective cohort of all children requiring ECMO who received CRRT at the 6 participating centers over a 5-year period. The aims of this study are to 1) evaluate the association of degree of FO at CRRT initiation and discontinuation with outcomes and 2) examine the association of the change in FO while on CRRT with outcomes. We hypothesized that the degree of FO at CRRT initiation and at CRRT discontinuation as well as the change in FO while on CRRT would be independently associated with the outcomes of hospital mortality, ECMO mortality, and duration of ECMO.
Methods:
Study Population:
This study is a retrospective observational cohort study of pediatric patients on ECMO at 6 centers from January 1, 2007 to December 31, 2011. All patients < 18 years of age at the time of ECMO initiation were included. Patients for whom data from the retrospective chart review could not be matched with data from the Extracorporeal Life Support Organization (ELSO) international registry, those with inadequate fluid balance data, and those with multiple ECMO runs were excluded. A detailed description of the inclusion/exclusion criteria and study population are outlined in Supplemental Figure 1. Additionally, children treated exclusively with hemodialysis or peritoneal dialysis or for whom renal support therapy was initiated after 21 ECMO study days were not included in the final FO analysis. Investigational Review Board or Research Ethics Board approval for data collection and study, including a waiver of informed consent, was obtained and maintained at each individual participating center.
Data Collection:
Data sources for this study included the ELSO Registry (ELSO, Ann Arbor Michigan) and the retrospective evaluation of individual paper or electronic medical records. Data from the ELSO registry were merged with the retrospective database, utilizing a 4-point matching scheme (ELSO ID, date of birth, date of ECMO initiation, and date of ECMO discontinuation) as previously reported [9]. Data collected from the ELSO registry included: demographic/baseline characteristics, pre-ECMO variables, and ECMO variables including indication, support mode, duration of ECMO, and number of non-renal complications [9]. Medical records were reviewed for AKI and FO-specific variables including all serum creatinine (SCr) values, CRRT variables, detailed FO data before, during, and at ECMO discontinuation (Table 1 and Supplemental Table 1).
Table 1:
Baseline patient characteristics overall and by hospital mortality
| Variable | Overall | Hospital Mortality | p-value | |
|---|---|---|---|---|
| (N = 357) | Yes (n=199) | No (n=158) | ||
| Age (days) | 23 (4 – 317) | 12 (5 – 158) | 96 (2 – 914) | 0.003 |
| Neonates | 187 (52.4) | 122 (61.3) | 65 (41.1) | 0.0002 |
| Hours on ECMO | 165 (96 – 300) | 179 (91 – 330) | 159.5 (96 – 253) | 0.10 |
| Day of ICU of CRRT Initiation | 3 (1 – 9) | 5 (1 – 11) | 2 (1 – 5) | <0.0001 |
| Day of ECMO of CRRT Initiation | 1 (0 – 2) | 1 (0 – 2) | 1 (0 – 2) | 0.08 |
| CRRT Initiation same day as ECMO * | 140 (39.2%) | 73 (36.7%) | 67 (42.4%) | |
| ECMO Mode | ||||
| VA ECMO | 265 (74.2) | 175 (87.9) | 90 (57) | <0.0001 |
| VV ECMO | 92 (25.8) | 24 (12.1) | 68 (43) | |
| OI Median (IQR), (n=235) | 44.9 (27.8 – 65.3) | 47.1 (28.4 – 67.8) | 43 (27.6 – 61.1) | 0.32 |
| pH Median (IQR), (n=340) | 7.19 (7.06 – 7.30) | 7.20 (7.08 – 7.30) | 7.18 (7.05 – 7.30) | 0.97 |
| Pre ECLS Inotropes | 315 (88.2) | 181 (91) | 134 (84.8) | 0.07 |
| Non-Renal Patient Complications | 347 (97.2) | 196 (98.5) | 151 (95.6) | 0.11 |
| AKI | 256 (71.7) | 154 (77.4) | 102 (64.6) | 0.01 |
| Severe AKI (Stage 2 and 3) | 183 (51.3) | 112 (56.3) | 71 (44.9) | 0.03 |
| Primary ECMO Indication | ||||
| Pulmonary | 174 (48.7) | 74 (37.2) | 100 (63.3) | <0.0001 |
| Cardiac | 110 (30.8) | 79 (39.7) | 31 (19.6) | |
| ECPR | 73 (20.5) | 46 (23.1) | 27 (17.1) | |
| Mechanism of CRRT | ||||
| CRRT with Device | 81 (22.7) | 34 (17.1) | 47 (29.8) | 0.005 |
| In Line filter | 276 (77.3) | 165 (82.9) | 111 (70.2) | |
| Center | ||||
| A | 57 (16) | 34 (17.1) | 23 (14.6) | 0.23 |
| B | 16 (4.5) | 12 (6) | 4 (2.5) | |
| C | 20 (5.6) | 13 (6.5) | 7 (4.4) | |
| D | 135 (37.9) | 74 (37.2) | 61 (38.6) | |
| E | 118 (33.1) | 58 (29.2) | 60 (38) | |
| F | 11 (3.1) | 8 (4) | 3 (2) | |
CRRT initiated the same calendar day as ECMO
Abbreviations: ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; CRRT, continuous renal replacement therapy; VA, veno-arterial; VV, veno-venous; OI, oxygenation index; ECLS, extracorporeal cardiac life support; ECPR, extracorporeal cardiopulmonary resuscitation; PD, peritoneal dialysis
Continuous variables presented as median and interquartile range (IQR) and differences between groups examined using Wilcoxon Rank Sum test. Categorical variables presented as counts and percentages, and differences between groups examined using Chi-square/Fisher’s test
Acute kidney injury was defined using the SCr criteria of the Kidney Disease Improving Global Outcome (KDIGO) AKI definition with two modifications. Urine output criteria were not utilized in this study because hourly urine output was not consistently available. The criteria evaluating glomerular filtration rate were not utilized as a large proportion of the study cohort included neonates. Baseline SCr was defined as the lowest SCr in the 3 months prior to ECMO cannulation. In children < 1 month old, the neonatal version of the KDIGO AKI definition was applied. As previously described, two independent investigators defined baseline SCr in all neonates, followed by adjudication by a third investigator to resolve differences [9]. Severe AKI was defined by KDIGO stage 2 or 3. Acute kidney injury and severe AKI were defined as occurring at any time during ECMO. Early CRRT initiation was defined as that which occurred on ECMO day 0 or Day 1. The indication for CRRT were extracted from medical record notes and included FO, FO prevention, AKI, electrolyte abnormality, toxin removal, or other.
Fluid Overload (primary exposure)
Daily cumulative percent FO was determined using daily intake and output for the 28 days prior to ECMO cannulation and the first 21 days following cannulation [16]. Cumulative percent FO was calculated according to previously published definition:
Fluid overload was calculated at multiple time-points (CRRT initiation, CRRT discontinuation). We calculated the change in FO between CRRT initiation and discontinuation as follows:
Change in FO on CRRT = FO% at CRRT Discontinuation - FO % at CRRT Initiation
Outcome:
The primary outcome of this study was hospital mortality. ECMO mortality and duration of ECMO served as secondary outcomes.
Statistical Methods:
Data was summarized as median and interquartile range for continuous variables and group differences were examined using Wilcoxon Rank Sum test. Categorical variables were presented as counts and percentages, and group differences were examined using Chi-Square or Fisher’s Exact test. Fluid overload at CRRT initiation and discontinuation was evaluated as 10% change and distribution of categories of FO at CRRT initiation in relation to ECMO and in-hospital mortality was presented. Association of FO with ECMO morality and in-hospital mortality was examined by developing logistic regression models with and without adjustment for covariates (age, ECMO mode, support type, patient complications, pH and center), and estimates of association were reported as odds ratios (unadjusted [OR] and adjusted [aOR]) and 95%CI. Similarly, unadjusted and adjusted linear regression models were used to examine relationship between 10% absolute increase in FO and ECMO duration. ECMO duration was log transformed because of positive skewness. Results were reported after back transforming the log estimates for ECMO duration in hours, representing relative change in ECMO duration for every 10% increase in FO. Interactions between FO and AKI were also assessed for examining association between FO and outcomes in the total CRRT cohort and in subset of those who survived ECMO. SAS version 9.4 (SAS Institute Inc. Cary, NC) was used and all analyses were conducted as two-sided test with p ≤ 0.05 as statistically significant.
Results:
Patient Characteristics:
A total of 1,009 patients underwent ECMO during the study period. The FO data was available for 756 patients. Of these, the study cohort included 357 (47.2%) patients who received renal support therapy; of those, 276 (77.3%) received CRRT with an inline filter, while 81 (22.7%) received concurrent CRRT with a device. Two hundred and sixty-five (74.2%) patients received veno-arterial ECMO and 92 (25.8%) patients received veno-venous ECMO. There were 187 neonates (52.4%). Median patient age at ECMO initiation was 23 (4, 317) days. Baseline characteristics of the study population are summarized in Table 1 and Supplemental Table 1.
The indications for ECMO in the study cohort were pulmonary (n = 174, 48.7%), extracorporeal cardiopulmonary resuscitation (ECPR) (n = 73, 20.5%), and cardiac (n = 110, 30.8%). The primary indications for the initiation of CRRT in this specific cohort were as follows: 1) FO or FO prevention (n = 302, 84.6%), 2) AKI (n = 40, 11.2%), 3) electrolyte abnormalities (n = 4, 1.1%), 4) toxin removal (n = 4, 1.1%), and 5) other (n = 7, 2%).
Acute kidney injury, as defined by SCr based criteria alone, occurred during the ECMO course in 71.7% (n = 256) of the population. This included 20.4% (n=73) with Stage 1, 24.4% (n = 87) with Stage 2, and 26.9% (n = 96) with Stage 3. A total of 101 patients did not meet the SCr based criteria for AKI. Severe AKI occurred in 51.3% (n = 183) of the population.
Distribution of Fluid Overload Severity during Continuous Renal Replacement Therapy:
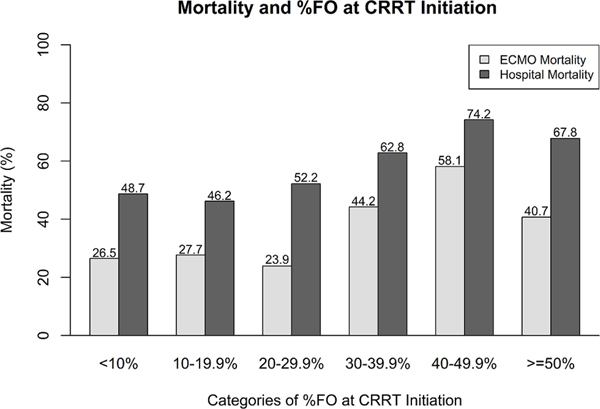
The characteristics of FO in relation to ECMO mortality and hospital mortality is summarized in Table 2. The proportion of patients who died increased with greater fluid overload. A summary of the timing of CRRT initiation in relationship to FO and AKI are included in table 3a and 3b. CRRT was initiated a median of 1 (IQR 0, 2) day after ECMO initiation, with 140 (39.2%) starting CRRT on the same day as ECMO initiation. Early CRRT initiation, defined as that which occurred on day 0 or 1 after ECMO initiation occurred in 67.2% (n=240) of patients.
Table 2:
Fluid Overload Severity Distribution and Association with Mortality
| % FO at CRRT initiation | N | ECMO Mortality | Hospital Mortality | ||
|---|---|---|---|---|---|
| Yes | No | Yes | No | ||
| <10% | 113 | 30 (26.5) | 83 (73.5) | 55 (48.7) | 58 (51.3) |
| 10–19.9% | 65 | 18 (27.7) | 47 (72.3) | 30 (46.2) | 35 (53.8) |
| 20–29.9% | 46 | 11 (23.9) | 35 (76.1) | 24 (52.2) | 22 (47.8) |
| 30–39.9%% | 43 | 19 (44.2) | 24 (55.8) | 27 (62.8) | 16 (37.2) |
| 40–49.9% | 31 | 18 (58.1) | 13 (41.9) | 23 (74.2) | 8 (25.8) |
| > =50% | 59 | 24 (40.7) | 35 (59.3) | 40 (67.8) | 19 (32.2) |
Presented as Count (%)
Table 3a:
Description of Timing of CRRT initiation, Fluid Overload, and Acute Kidney Injury
| ECMO day of CRRT Initiation | %FO at ECMO initiation | % Peak FO on ECMO | AKI | Severe AKI | |||
|---|---|---|---|---|---|---|---|
| Yes (N=256) | No (N=101) | Yes (N=183) | No (N=174) | ||||
| n (%) | Median (IQR) | Median (IQR) | n (%) | n (%) | n (%) | n (%) | |
| 0 (same day) | 140 (39.2) | 11.1 (0, 29.4) | 28.6 (12.6, 50.3) | 101 (39.5) | 39 (38.6) | 75 (41) | 65 (37.4) |
| 1 | 100 (28) | 9.1 (0, 21.7) | 30.6 (17.7, 50) | 72 (28.1) | 28 (27.7) | 49 (26.8) | 51 (29.3) |
| 2 | 47 (13.2) | 14.5 (4.4, 21.9) | 43.1 (25.5, 65) | 34 (13.3) | 13 (12.9) | 26 (14.2) | 21 (12.1) |
| 3 | 20 (5.6) | 10.3 (2.1, 17.5) | 51.5 (29, 67.1) | 14 (5.5) | 6 (5.9) | 12 (6.6) | 8 (4.6) |
| 4 | 12 (3.4) | 13.8 (5.5, 38.6) | 52.8 (23.7, 96.1) | 8 (3.1) | 4 (4) | 7 (3.8) | 5 (2.9) |
| 5 | 6 (1.7) | 14.3 (5.4, 15.4) | 54.4 (44.9, 84.3) | 3 (1.2) | 3 (3) | 3 (1.6) | 3 (1.7) |
| 6 | 9 (2.5) | 2.1 (0, 7.7) | 82.2 (35.3, 128.2) | 6 (2.3) | 3 (3) | 3 (1.6) | 6 (3.5) |
| >=7 | 23 (6.4) | 8 (2.6, 26.6) | 65.8 (43.3, 93.6) | 18 (7) | 5 (5) | 8 (4.4) | 15 (8.6) |
Table 3b:
The Timing of CRRT initiation and Fluid Overload characteristics
| ECMO day of CRRT Initiation | %FO CRRT initiation | %FO CRRT discontinuation |
|---|---|---|
| Median (IQR) | Median (IQR) | |
| 0 (same day) | 11.1 (0, 29.4) | 22.6 (6.7, 44.8) |
| 1 | 18.1 (6, 32.3) | 23.6 (8.4, 40.1) |
| 2 | 35.9 (17.8, 45.1) | 36.4 (8.8, 52.4) |
| 3 | 30.2 (20, 52.5) | 40.1 (11.4, 51.6) |
| 4 | 42.9 (12.7, 71.2) | 35.7 (10.1, 79.5) |
| 5 | 40.9 (28.3, 53.6) | 62.2 (43.9, 84.3) |
| 6 | 42.1 (15.6, 82.2) | 67.6 (12.9, 133.5) |
| >=7 | 51.9 (29.3, 68.5) | 52.5 (37.6, 74.7) |
The median FO at CRRT initiation was 20.1% (IQR 5, 40) and 27.2% (IQR 10, 50.1) at CRRT discontinuation. Median change in FO on CRRT was positive 5.7% (IQR −2.1, 18.7). A negative change in fluid balance on CRRT occurred in 31.8% (n = 99) of patients (Table 4). One hundred and thirteen patients (31.6%) had <10% FO at CRRT initiation, 179 (50.1%) patients had FO ≥ 20% and 133 (37.3%) patients had FO ≥ 30% at CRRT initiation (Table 2). A description of the degree of FO at CRRT initiation, timing of CRRT initiation, and CRRT mechanism (machine vs. in-line filter) by institution is described in Supplemental Table 2 and 3. As expected, the participating institutions differed significantly in all variables including FO at CRRT initiation, FO at CRRT discontinuation, day of ECMO of CRRT initiation, and mechanism of CRRT.
Table 4:
Fluid overload on CRRT among ECMO patients overall and by survival status
| Variable | Overall | ECMO Mortality | p | ||||
|---|---|---|---|---|---|---|---|
| N | n | Yes | n | No | |||
| Fluid Overload at CRRT Initiation (%) | 357 | 20.1 (5, 40) | 120 | 30.5 (9.4, 45.2) | 237 | 15.3 (4, 35.5) | 0.005 |
| Fluid Overload at CRRT Discontinuation/ Death (%) | 312 | 27.2 (10, 50.1) | 107 | 37.6 (13.8, 61.4) | 205 | 23 (8.4, 44.4) | 0.002 |
| Change in Fluid Overload on CRRT (%) | 311 | 5.7 (−2.1, 18.7) | 106 | 8.1 (−0.6, 22.5) | 205 | 5.4 (−3.1, 15.9) | 0.07 |
| Negative Chante in Fluid Overload on CRRT (n (%)) | 311 | 99 (31.8) | 106 | 28 (28.3) | 205 | 71 (71.7) | 0.14 |
| Variable | Overall | Hospital Mortality | p | ||||
| N | n | Yes | n | No | |||
| Fluid Overload at CRRT Initiation (%) | 357 | 201. (5, 40) | 199 | 25.9 (5.9, 44.1) | 158 | 13.5 (4.2, 31) | 0.004 |
| Fluid Overload at CRRT Discontinuation/ Death (%) | 312 | 27.2 (10, 50.1) | 172 | 36.1 (11.2, 61.1) | 140 | 22.6 (7.8, 40.3) | 0.002 |
| Change in Fluid Overload on ECMO (%) | 311 | 5.7 (−2.1, 18.7) | 171 | 7.3 (−1, 20.9) | 140 | 5.2 (−3.2, 15.4) | 0.06 |
| Negative Chante in Fluid Overload on CRRT (n (%)) | 311 | 99 (31.8) | 171 | 47 (47.5) | 140 | 52 (52.1) | 0.07 |
All continuous values are median and interquartile range. p for group differences (y/n) examined using Wilcoxon Rank Sum test. Categorical variables presented as counts and percentages, and differences between groups examined using Chi-square/Fisher’s test.
The outcomes and FO characteristics in the “Early Initiation” are presented in Supplemental Table 3. Briefly those that initiated CRRT early had lower FO at CRRT initiation, lower FO at CRRT discontinuation, greater change in FO on CRRT, and were more likely to have a negative fluid balance on CRRT.
Association of Fluid Overload and Mortality
Survival to ECMO decannulation and hospital discharge was 66.4% (n= 237) and 44.3% (n=158), respectively. Patient characteristics associated with survival are presented for hospital mortality (Table 1) and ECMO mortality (Supplemental Table 1). The univariable associations of different measures of FO at CRRT initiation, CRRT discontinuation, and change in FO on CRRT with mortality are shown in Table 4. The degree of FO at CRRT initiation and discontinuation was associated with both ECMO mortality and hospital mortality (Table 4). Figure 1 shows that in general there was a graded increase in both ECMO and hospital mortality with increasing FO at CRRT initiation (by 10% intervals). There was a sharp increase in mortality at 30% FO at CRRT initiation where the mortality on ECMO appeared to increase. After 10% FO at CRRT initiation, there was a stepwise increased association with hospital mortality. The median change in FO from CRRT initiation to discontinuation was not associated with increased ECMO mortality (5.4% vs. 8.1%, p = 0.07) or hospital mortality (5.2% vs. 7.3% p =0.06). In an unadjusted analysis examining negative change in FO on CRRT (yes/no), only 28.3% (n=28/99) died on ECMO in comparison to 71.7% (n=71/99) surviving to ECMO decannulation (p = 0.14). Similarly, only 47.5% (n=47/99) died prior to hospital discharge in comparison to 52.1% (n=52/99) who survived (p = 0.07) (Table 4). Early CRRT initiation was not associated with ECMO mortality (p = 0.18) or hospital mortality (p = 0.05) (Supplemental Table 3).
Figure 1:
Association of increasing fluid overload at CRRT initiation and mortality.
The univariable logistic regression analysis also showed that each 10% rise in FO at CRRT initiation was associated with increased ECMO mortality (OR 1.07, 95% CI 1.00, 1.14, p = 0.04) and increased hospital mortality (OR 1.09, 95% CI 1.02, 1.17, p = 0.01). The degree of FO at CRRT discontinuation was also associated with increased ECMO mortality (OR 1.08, 95% CI 1.02, 1.15, p = 0.01) and hospital mortality (OR 1.08, 95% CI 1.02, 1.15, p =0.01). The change in FO during CRRT, however, was not associated with ECMO mortality (OR 1.09, 95% CI 0.98, 1.21, p =0.11) or hospital mortality (OR 1.06, 95% CI 0.96, 1.17, p = 0.25). Further, a trend was observed between negative change in FO at CRRT (yes/no) and lower odds for ECMO mortality (OR 0.65, 95% CI 0.39, 1.10, p=0.11) and hospital mortality (OR 0.64. 95% CI 0.39, 1.04, p=0.07).
Multivariable analyses controlling for pH at ECMO initiation, non-renal complications, ECMO mode, support type, center, patient age, and AKI are shown in Table 5. This analysis showed that each 10% increase in FO at CRRT initiation, there was a 9% increase in hospital mortality (OR 1.09, 95% CI 1.00 – 1.18, p = 0.05), but not ECMO mortality (OR 1.05, 95% CI 0.97 – 1.13, p = 0.24). Each 10% increase in the degree of FO at CRRT discontinuation was associated with a 7% increase in ECMO mortality (OR 1.07, 95% CI 1.00 – 1.15, p = 0.04) and an 11% increase in hospital mortality (OR 1.11 CI 1.03 – 1.19, p = 0.01), independent of other factors. Change in FO during CRRT was associated with hospital mortality (OR 1.14, 95% CI 1.01 −1.29, p = 0.04). In a similar model negative change in FO on CRRT (yes/no) was associated with a 53% reduction in ECMO mortality (OR 0.47, 95% CI 0.26 – 0.88, p = 0.02)) and a 63% reduction in hospital mortality (OR 0.37, 95% CI 0.21 – 0.68, p = 0.001.) Multivariable models including severe AKI in lieu of AKI showed similar results. A sensitivity analysis evaluating the impact of an interaction between FO and AKI did not show a significant interaction for ECMO mortality or hospital mortality, so this model was not reported.
Table 5:
Multivariable analysis of association between fluid overload at CRRT initiation, CRRT discontinuation, Change in FO on CRRT, and Negative change in FO on CRRT with ECMO and hospital mortality
| Hospital Mortality | ECMO Mortality | |
|---|---|---|
| OR (95% CI) | OR (95% CI) | |
| FO at CRRT Initiation* | 1.09 (1.00 – 1.18), p=0.05# | 1.05 (0.97 – 1.13), p=0.24 |
| Pediatric ** | 0.47 (0.28 – 0.78) ## | 1.10 (0.65 −1.87) |
| ECMO Mode VV *** | 0.26 (0.13 – 0.52) ## | 0.30 (0.13 – 0.68) ## |
| Support Type: Cardiac **** | 1.53 (0.79 – 2.95) | 1.24 (0.64 – 2.39) |
| Support Type: ECPR **** | 1.26 (0.62 – 2.58) | 1.98 (0.97 – 4.04) |
| Non- Renal Patient Complications | 1.44 (0.32 – 6.60) | 3.59 (0.39 – 32.84) |
| pH at initiation | 1.17 (0.31 – 4.45) | 0.24 (0.06 – 0.96) |
| AKI | 1.51 (0.85 – 2.69) | 0.87 (0.48 – 1.59) |
| Hospital Mortality | ECMO Mortality | |
| OR (95% CI) | OR (95% CI) | |
| FO at CRRT Discontinuation* | 1.11 (1.03 – 1.19), p=0.01# | 1.07 (1.00 – 1.15), p=0.04# |
| Pediatric ** | 0.49 (0.28 – 0.85) # | 1.27 (0.71 – 2.28) |
| ECMO Mode VV *** | 0.22 (0.10 – 0.49) ## | 0.28 (0.11 – 0.71) # |
| Support Type: Cardiac **** | 1.82 (0.89 – 3.73) | 1.18 (0.57 – 2.43) |
| Support Type: ECPR **** | 1.41 (0.65 – 3.06) | 1.93 (0.89 – 4.21) |
| Non- Renal Patient Complications | 1.22 (0.27 – 5.59) | 3.24 (0.35 – 30.40) |
| pH at initiation | 0.49 (0.11 – 2.23) | 0.13 (0.03 – 0.62) # |
| AKI | 1.24 (0.66 – 2.32) | 0.82 (0.43 – 1.58) |
| Hospital Mortality | ECMO Mortality | |
| OR (95% CI) | OR (95% CI) | |
| Change in FO on CRRT* | 1.14 (1.01 – 1.29), p=0.04# | 1.12 (0.99 – 1.27), p=0.06 |
| Pediatric ** | 0.44 (0.25 – 0.77) ## | 1.19 (0.66 – 2.14) |
| ECMO Mode VV *** | 0.22 (0.10 – 0.49) ## | 0.28 (0.11 – 0.71) # |
| Support Type: Cardiac **** | 1.70 (0.83 – 3.47) | 1.10 (0.53 – 2.27) |
| Support Type: ECPR **** | 1.24 (0.58 – 2.66) | 1.76 (0.81 – 3.81) |
| Non- Renal Patient Complications | 1.17 (0.26 – 5.19) | 2.96 (0.32 – 27.63) |
| pH at initiation | 0.42 (0.09 – 1.90) | 0.11 (0.02 – 0.53) # |
| AKI | 1.33 (0.71 – 2.49) | 0.89 (0.46 – 1.73) |
| Negative change in Fluid Overload om CRRT | ||
| OR (95% CI) | OR (95% CI) | |
| Negative change in FO on CRRT | 0.37 (0.21 – 0.68), p=0.001** | 0.47 (0.26 – 0.88), p=0.02* |
| Pediatric ** | 0.42 (0.24 – 0.75) ** | 1.17 (0.65 – 2.11) |
| ECMO Mode VV *** | 0.21 (0.09 – 0.47) ** | 0.27 (0.11 – 0.68) * |
| Support Type: Cardiac **** | 1.73 (0.84 – 3.56) | 1.10 (0.53 – 2.27) |
| Support Type: ECPR **** | 1.16 (0.54 – 2.49) | 1.67 (0.77 – 3.62) |
| Non- Renal Patient Complications | 1.23 (0.28 – 5.43) | 3.08 (0.33 – 28.89) |
| pH at initiation | 0.37 (0.08 – 1.71) | 0.10 (0.02 – 0.50) ** |
| AKI | 1.44 (0.76 – 2.71) | 0.94 (0.48 – 1.82) |
OR; odds ratio, all models adjusted for center in addition
1unit increase represents a 10% increase in FO
Reference neonates,
Reference VA ECMO,
Reference Support type: Pulmonary
p≤0.05;
p≤0.005
An evaluation of the 79 excluded patients after ELSO matching showed that those that were excluded had significantly higher ECMO mortality (49.4% vs 33.6%, p=0.01) and similar in hospital mortality (67.1% vs. 55.7%, p=0.06).
Association Between Fluid Overload, Renal Support Therapy, and ECMO Duration
The median duration of ECMO was 165 hours (IQR 96, 300 hours) in ECMO survivors. In order to evaluate the impact of FO and CRRT on duration of ECMO, only ECMO survivors were included in the analysis (N=237). In bivariate analysis, the degree of FO at CRRT initiation in those who survived through ECMO decannulation was associated with a 6% relative increase in ECMO duration (1.06, 95% CI 1.03 – 1.09, p = <0.0001). Additionally, the degree of FO at CRRT discontinuation was also associated with a 6% relative increase in ECMO duration in this population (1.06, 95% CI 1.03 – 1.08, p = <0.0001). In multivariable analysis (Table 6), both FO at CRRT initiation and at discontinuation were associated with longer ECMO duration (hours), independent of other factors. Specifically, each 10% rise in FO at CRRT initiation was associated with a 3% relative increase in the number of hours on ECMO (p = 0.01); each 10% rise in FO at CRRT discontinuation was associated with a 4% relative increase in the number of hours on ECMO (p = 0.0002) (Table 6). In a sensitivity analysis the significance of the findings of the multivariable analysis did not change in a separate analysis that included non-survivors.
Table 6:
Linear Regression Models Predicting Duration of ECMO in ECMO survivors (N=237)
| Fluid Overload at CRRT Initiation | |||
|---|---|---|---|
| Variable | Relative Change | 95% CI | p-value |
| Fluid Overload at CRRT Initiation * | 1.033 | (1.007 – 1.060) | 0.01 |
| Pediatric** | 1.062 | (0.895 – 1.259) | 0.49 |
| ECMO Mode VV *** | 0.965 | (0.773 – 1.205) | 0.76 |
| Support type: Cardiac**** | 0.629 | (0.501 – 0.789) | <0.0001 |
| Support type: ECPR**** | 0.597 | (0.461 – 0.773) | 0.0001 |
| Non-Renal Patient Complications | 1.957 | (1.282 – 2.987) | 0.002 |
| pH at initiation | 1.280 | (0.817 – 2.004) | 0.28 |
| AKI | 1.304 | (1.079 – 1.576) | 0.01 |
| Fluid Overload at CRRT Discontinuation | |||
| Variable | Relative Change | 95% CI | p-value |
| Fluid Overload on CRRT Discontinuation * | 1.041 | (1.020 – 1.063) | 0.0002 |
| Pediatric** | 1.070 | (0.897 – 1.277) | 0.45 |
| ECMO Mode VV *** | 0.905 | (0.714 – 1.146) | 0.40 |
| Support type: Cardiac**** | 0.693 | (0.546 – 0.897) | 0.003 |
| Support type: ECPR**** | 0.632 | (0.486 – 0.823) | 0.001 |
| Non-Renal Patient Complications | 1.947 | (1.303 – 2.909) | 0.001 |
| pH at initiation | 0.940 | (0.592 – 1.492) | 0.79 |
| AKI | 1.157 | (0.955 – 1.401) | 0.13 |
| Change in Fluid Overload on CRRT | |||
| Variable | Relative Change | 95% CI | p-value |
| Change in Fluid Overload on CRRT (%) * | 1.036 | (0.998 – 1.075) | 0.06 |
| Pediatric** | 1.053 | (0.877 – 1.264) | 0.58 |
| ECMO Mode VV *** | 0.914 | (0.717 – 1.166) | 0.47 |
| Support type: Cardiac**** | 0.678 | (0.530 – 0.866) | 0.002 |
| Support type: ECPR**** | 0.607 | (0.463 – 0.795) | 0.0004 |
| Non-Renal Patient Complications | 2.015 | (1.333 – 3.045) | 0.001 |
| pH at initiation | 0.969 | (0.601 – 1.562) | 0.90 |
| AKI | 1.160 | (0.953 – 1.413) | 0.14 |
| Negative change in Fluid Overload om CRRT | |||
| Variable | Relative Change | 95% CI | p-value |
| Negative change in Fluid Overload (%) on CRRT (Y/N) * | 0.882 | (0.739 – 1.053) | 0.16 |
| Pediatric** | 1.065 | (0.887 – 1.279) | 0.50 |
| ECMO Mode VV *** | 0.917 | (0.719 – 1.171) | 0.49 |
| Support type: Cardiac**** | 0.672 | (0.525 – 0.86) | 0.002 |
| Support type: ECPR**** | 0.598 | (0.456 – 0.785) | 0.0002 |
| Patient Complications | 2.044 | (1.35 – 3.096) | 0.001 |
| pH at initiation | 0.984 | (0.61 – 1.589) | 0.95 |
| AKI | 1.174 | (0.964 – 1.43) | 0.11 |
All models adjusted for center in addition;
Examines per 10% rise in FO
Reference neonates,
Reference VA ECMO,
Reference Support type: Pulmonary
In an evaluation of the interaction between FO and AKI, AKI was not found to be an effect modifier when examining association between FO at CRRT initiation or FO at CRRT discontinuation with ECMO duration in ECMO survivors, However, in total cohort including both survivors and non-survivors, AKI had an influence in modifying the effect of FO at CRRT initiation and FO at CRRT discontinuation on ECMO duration (Supplemental Table 4.) Nevertheless, in those who did not have AKI, FO at CRRT initiation was associated with 7% relative increase in ECMO duration. Similarly, FO at CRRT discontinuation resulted in 7% increase in ECMO duration if AKI was absent. No interaction between AKI and change in FO or negative change in FO was found.
Discussion:
This is the first multicenter evaluation studying the association between the degree of FO, timing of CRRT initiation, and outcome in pediatric ECMO patients. Our study shows that severe FO occurs commonly in children supported by ECMO prior to CRRT initiation and that worse FO at CRRT initiation and at discontinuation is associated with increased mortality and duration of ECMO. In addition, we present information investigating the kinetics of fluid removal after CRRT initiation and the impact on mortality.
The association between FO at CRRT initiation and subsequent mortality is observed in a variety of critical care populations, particularly at levels >10–20% [14, 16–25]. Our current study shows that a cohort of children on ECMO CRRT is initiated at a median FO of nearly 20%, and the degree of FO at CRRT initiation and discontinuation predicted ECMO duration and mortality, adding to the previous work in this field [14, 16–20]. We also show that while children are already 20% FO at CRRT initiation, over a third of patients are above 30% FO. While this amount of excessive fluid distribution is likely multifactorial, including resuscitative fluids required prior to and during cannulation as well as redistribution in the setting of an inflammatory response, the level of FO at CRRT initiation is concerning. A novel finding in our study is that the timing and degree of FO at CRRT initiation differed significantly by institution. In fact, 3 of the centers had a median FO of > 30% at CRRT initiation (Supplemental Table 3). Not surprisingly these 3 institutions were relatively delayed in initiating CRRT. Our data from the current study, coupled with the previous publication describing the epidemiology of FO in children on ECMO, demonstrate that fluid management strategies and, by extension the timing of CRRT initiation, represent a critical opportunity to standardize and improve care for children on ECMO [10]. Furthermore, given the fact that CRRT was initiated relatively early during ECMO course in our study, the clinical issues to address and opportunities for intervention extend beyond the timing of (i.e., when to initiate) CRRT. A critical component of future management guidelines on CRRT during ECMO will need to include guidance on fluid removal strategies (e.g., how aggressively to remove fluid; when to start more aggressive fluid removal; goal-directed fluid removal goals), which will require research to elucidate and evaluate impact on patient outcomes. A multicenter trial evaluating the timing of CRRT initiation, fluid management and the development of protocols for fluid removal is a critical area of need in this population.
The importance of FO has been recognized by the Extracorporeal Life Support Organization (ELSO) and their recommendations include the following: “The goal of fluid management is to return the extracellular volume to normal (dry weight) and maintain it there.” Despite this recommendation, and the recognition of over three decades of data demonstrating the detrimental effects of FO in patients of all ages on ECMO, there remains no consensus or best practices for fluid management on ECMO [1, 2, 5, 9, 26]. The utilization of renal support therapy is one option to manage volume on ECMO, as the addition of in-line CRRT to the ECMO circuit is associated with reduced time on ECMO and mechanical ventilation [27]. In a survey of ELSO centers, the KIDMO study group reported that FO and FO prevention accounted for 59% of the indications for the initiation of CRRT on ECMO [13]. The current study supports these findings as we show that FO or its prevention accounted for 85% of the indications for CRRT initiation.
Despite the primary indication for the initiation of CRRT being to manage FO in 85% of patients, the change in fluid balance between CRRT initiation and discontinuation in our cohort was positive on average. We showed that the patients in the current study had an overall rise in the median degree of FO from 20% FO at CRRT initiation to 30% FO at discontinuation. In multivariable analysis, this change in fluid balance was associated with hospital mortality, with every 10% FO increase from CRRT initiation to discontinuation conferring a 14% increase in mortality. In the current study when we evaluated those patients that were able to obtain a negative balance (n=99) on CRRT this was associated with a 63% reduction in hospital mortality on adjusted analysis. In the current study we show that those that received early CRRT (CRRT initiation ECMO day 0 or 1) had lower FO at CRRT initiation, lower FO at CRRT discontinuation, and were more likely to have a negative fluid balance on CRRT. While the current study was not intended to identify best practices to improve outcomes we clearly have shown that FO is associated with adverse outcomes and that the utilization of CRRT to manage established FO in children on ECMO is not achieving the stated goal in many cases. With this context, it is important to note that Silversides et al. recently characterized an adult population where fluid balance on day 3 of critical illness was an independent risk factor for 30-day mortality, with a negative fluid balance achieved with de-resuscitative measures - including CRRT - was associated with lower mortality [28]. Furthermore, previous studies and recent work in neonates have clearly shown that early CRRT initiation for those on ECMO may prevent the development of FO [27, 29, 30]. These findings highlight the fact that understanding the impact of the timing of CRRT initiation and the impact of FO at CRRT initiation on outcomes is only a first step. Further work is essential to rigorously develop and evaluate protocols and best practice guidelines for fluid management and FO prevention for all patients on ECMO. This represents a critical area for future research.
The greatest strength of this study is the multicenter approach to capture a sample size large enough to explore the impact of confounders with regard to outcomes. There are, however, several limitations. The retrospective nature of the study design limits our findings to associations as opposed to causality. Our results depend on the local experience and practice patterns in the management of ECMO and CRRT, as no protocol or equipment standardization existed between these centers. However, given center variability in practice patterns in our cohort, it is likely that there are external centers who practice similarly to at least one of our internal centers. We studied a mixed population of neonates, pediatrics, and congenital heart surgery patients. These groups likely behave differently and, practically, often require different access for ECMO. Specifically, we were unable to describe which patients were postoperative cardiac surgery patients and thus be able to evaluate them separately. While efforts were made to adjust for severity of illness, the dataset did not have the information and data points necessary to calculate a variety of severity of illness scores. An additional limitation of this study is that the timing of AKI diagnosis relative to CRRT initiation timing was not available. Furthermore, we acknowledge that the current study is limited by the fact that we did not collect urine output data to include urine output in the AKI definition or details of specific composition of fluids given (nutrition, saline, transfusions, etc.) The current data collection study was performed prior to the seminal publication by the AWARE study group outlining the importance of urine output measurement in defining AKI [31]. Additionally, we were unable to merge approximately 20% of our identified center patients who received ECMO with the ELSO registry. Patients who were excluded after matching had a higher mortality than the study cohort, potentially biasing our results to a lower mortality rate.
Conclusion:
We have demonstrated an association between FO at CRRT initiation and discontinuation and increased mortality and duration of ECMO in a multicenter study of pediatric ECMO patients. Importantly, each 10% increase in FO at CRRT initiation was associated with increased ECMO and hospital mortality. These findings demonstrate an opportunity to improve care by optimizing the use of CRRT in the prevention and management of FO in patients supported by ECMO. While these findings suggest that there is a role for the earlier initiation CRRT in children on ECMO, prior to the development of significant FO, important questions remain and warrant further critical appraisal. This include a better understanding of fluid management strategies prior to CRRT initiation, understanding the impact of urine output, understanding optimal CRRT fluid removal strategies, and the development of specified fluid removal protocols/ guidelines to be utilized in children on ECMO. Only when each of these factors are systematically studied together can we begin to fully understand the impact of the utilization of CRRT in children on ECMO.
Supplementary Material
Acknowledgements:
Elaine Cooley (University of Michigan), Heart Institute Research Core (CCHMC)
Funding Support: RedCap is supported by UL1 TR000445 from NACTS/NIH. Dr. Askenazi receives funding from the NIH (R01 DK13608–01) and the Pediatric and Infant Center for Acute Nephrology (PICAN), which is sponsored by Children’s of Alabama and the University of Alabama at Birmingham (UAB) School of Medicine, as well as by the Department of Pediatrics, and Center for Clinical and Translational Science (CCTS) under award number UL1TR00165. Dr Askenazi and Dr Basu are speakers for Baxter Renal Products. MZ received research salary support from the Fonds de Recherche de Québec-Santé (FRQ-S) during this study.
Conflict of Interest: The authors report no conflict of interest with funding support for RedCap. Dr. Askenazi reports no conflict of interest with funding support as these sources were not used directly in the execution of this study. The results presented in this paper have not been published previously except in abstract form.
Bibliography:
- 1.Weber TR, Connors RH, Tracy TF Jr., Bailey PV, Stephens C, Keenan W (1990) Prognostic determinants in extracorporeal membrane oxygenation for respiratory failure in newborns. Ann Thorac Surg 50:720–723. [DOI] [PubMed] [Google Scholar]
- 2.Heiss KF, Pettit B, Hirschl RB, Cilley RE, Chapman R, Bartlett RH (1987) Renal insufficiency and volume overload in neonatal ECMO managed by continuous ultrafiltration. ASAIO Trans 33:557–560. [PubMed] [Google Scholar]
- 3.Kelly RE Jr, Phillips JD, Foglia RP, Bjerke HS, Barcliff LT, Petrus L, Hall TR (1991) Pulmonary edema and fluid mobilization as determinants of the duration of ECMO support. J Pediatr Surg 26:1016–1022. [DOI] [PubMed] [Google Scholar]
- 4.Smith AH, Hardison DC, Worden CR, Fleming GM, Taylor MB (2009) Acute renal failure during extracorporeal support in the pediatric cardiac patient. ASAIO J 55:412–416. [DOI] [PubMed] [Google Scholar]
- 5.Swaniker F, Kolla S, Moler F, Custer J, Grams R, Barlett R, Hirschl R (2000) Extracorporeal life support outcome for 128 pediatric patients with respiratory failure. J Pediatr Surg 35:197–202. [DOI] [PubMed] [Google Scholar]
- 6.Kolovos NS, Bratton SL, Moler FW, Bove EL, Ohye RG, Bartlett RH, Kulik TJ (2003) Outcome of pediatric patients treated with extracorporeal life support after cardiac surgery. Ann Thorac Surg 76:1435–1441; discussion 1441–1432. [DOI] [PubMed] [Google Scholar]
- 7.Zwiers AJ, de Wildt SN, Hop WC, Dorresteijn EM, Gischler SJ, Tibboel D, Cransberg K (2013) Acute kidney injury is a frequent complication in critically ill neonates receiving extracorporeal membrane oxygenation: a 14-year cohort study. Crit Care 17:R151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gadepalli SK, Selewski DT, Drongowski RA, Mychaliska GB (2011) Acute kidney injury in congenital diaphragmatic hernia requiring extracorporeal life support: an insidious problem. J Pediatr Surg 46:630–635. [DOI] [PubMed] [Google Scholar]
- 9.Fleming GM, Sahay R, Zappitelli M, King E, Askenazi DJ, Bridges BC, Paden ML, Selewski DT, Cooper DS (2016) The Incidence of Acute Kidney Injury and Its Effect on Neonatal and Pediatric Extracorporeal Membrane Oxygenation Outcomes: A Multicenter Report From the Kidney Intervention During Extracorporeal Membrane Oxygenation Study Group. Pediatr Crit Care Med 17:1157–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Selewski DT, Askenazi DJ, Bridges BC, Cooper DS, Fleming GM, Paden ML, Verway M, Sahay R, King E, Zappitelli M (2017) The Impact of Fluid Overload on Outcomes in Children Treated With Extracorporeal Membrane Oxygenation: A Multicenter Retrospective Cohort Study. Pediatr Crit Care Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyer RJ, Brophy PD, Bunchman TE, Annich GM, Maxvold NJ, Mottes TA, Custer JR (2001) Survival and renal function in pediatric patients following extracorporeal life support with hemofiltration. Pediatr Crit Care Med 2:238–242. [DOI] [PubMed] [Google Scholar]
- 12.Paden ML, Warshaw BL, Heard ML, Fortenberry JD (2011) Recovery of renal function and survival after continuous renal replacement therapy during extracorporeal membrane oxygenation. Pediatr Crit Care Med 12:153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleming GM, Askenazi DJ, Bridges BC, Cooper DS, Paden ML, Selewski DT, Zappitelli M (2012) A multicenter international survey of renal supportive therapy during ECMO: the Kidney Intervention During Extracorporeal Membrane Oxygenation (KIDMO) group. ASAIO J 58:407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Selewski DT, Cornell TT, Blatt NB, Han YY, Mottes T, Kommareddi M, Gaies MG, Annich GM, Kershaw DB, Shanley TP, Heung M (2012) Fluid overload and fluid removal in pediatric patients on extracorporeal membrane oxygenation requiring continuous renal replacement therapy. Crit Care Med 40:2694–2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hames DL, Ferguson MA, Salvin JW (2019) Risk Factors for Mortality in Critically Ill Children Requiring Renal Replacement Therapy. Pediatr Crit Care Med 20:1069–1077. [DOI] [PubMed] [Google Scholar]
- 16.Goldstein SL, Currier H, Graf C, Cosio CC, Brewer ED, Sachdeva R (2001) Outcome in children receiving continuous venovenous hemofiltration. Pediatrics 107:1309–1312. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein SL, Somers MJ, Baum MA, Symons JM, Brophy PD, Blowey D, Bunchman TE, Baker C, Mottes T, McAfee N, Barnett J, Morrison G, Rogers K, Fortenberry JD (2005) Pediatric patients with multi-organ dysfunction syndrome receiving continuous renal replacement therapy. Kidney Int 67:653–658. [DOI] [PubMed] [Google Scholar]
- 18.Hayes LW, Oster RA, Tofil NM, Tolwani AJ (2009) Outcomes of critically ill children requiring continuous renal replacement therapy. J Crit Care 24:394–400. [DOI] [PubMed] [Google Scholar]
- 19.Selewski DT, Cornell TT, Lombel RM, Blatt NB, Han YY, Mottes T, Kommareddi M, Kershaw DB, Shanley TP, Heung M (2011) Weight-based determination of fluid overload status and mortality in pediatric intensive care unit patients requiring continuous renal replacement therapy. Intensive Care Med 37:1166–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sutherland SM, Zappitelli M, Alexander SR, Chua AN, Brophy PD, Bunchman TE, Hackbarth R, Somers MJ, Baum M, Symons JM, Flores FX, Benfield M, Askenazi D, Chand D, Fortenberry JD, Mahan JD, McBryde K, Blowey D, Goldstein SL (2010) Fluid overload and mortality in children receiving continuous renal replacement therapy: the prospective pediatric continuous renal replacement therapy registry. Am J Kidney Dis 55:316–325. [DOI] [PubMed] [Google Scholar]
- 21.Vaara ST, Korhonen AM, Kaukonen KM, Nisula S, Inkinen O, Hoppu S, Laurila JJ, Mildh L, Reinikainen M, Lund V, Parviainen I, Pettila V, Group FS (2012) Fluid overload is associated with an increased risk for 90-day mortality in critically ill patients with renal replacement therapy: data from the prospective FINNAKI study. Crit Care 16:R197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silversides JA, Pinto R, Kuint R, Wald R, Hladunewich MA, Lapinsky SE, Adhikari NK (2014) Fluid balance, intradialytic hypotension, and outcomes in critically ill patients undergoing renal replacement therapy: a cohort study. Crit Care 18:624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bouchard J, Soroko SB, Chertow GM, Himmelfarb J, Ikizler TA, Paganini EP, Mehta RL, Program to Improve Care in Acute Renal Disease Study G (2009) Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int 76:422–427. [DOI] [PubMed] [Google Scholar]
- 24.Gillespie RS, Seidel K, Symons JM (2004) Effect of fluid overload and dose of replacement fluid on survival in hemofiltration. Pediatr Nephrol 19:1394–1399. [DOI] [PubMed] [Google Scholar]
- 25.Foland JA, Fortenberry JD, Warshaw BL, Pettignano R, Merritt RK, Heard ML, Rogers K, Reid C, Tanner AJ, Easley KA (2004) Fluid overload before continuous hemofiltration and survival in critically ill children: a retrospective analysis. Crit Care Med 32:1771–1776. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt M, Bailey M, Kelly J, Hodgson C, Cooper DJ, Scheinkestel C, Pellegrino V, Bellomo R, Pilcher D (2014) Impact of fluid balance on outcome of adult patients treated with extracorporeal membrane oxygenation. Intensive Care Med 40:1256–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoover NG, Heard M, Reid C, Wagoner S, Rogers K, Foland J, Paden ML, Fortenberry JD (2008) Enhanced fluid management with continuous venovenous hemofiltration in pediatric respiratory failure patients receiving extracorporeal membrane oxygenation support. Intensive Care Med 34:2241–2247. [DOI] [PubMed] [Google Scholar]
- 28.Silversides JA, Fitzgerald E, Manickavasagam US, Lapinsky SE, Nisenbaum R, Hemmings N, Nutt C, Trinder TJ, Pogson DG, Fan E, Ferguson AJ, McAuley DF, Marshall JC, Role of Active Deresuscitation After Resuscitation I (2018) Deresuscitation of Patients With Iatrogenic Fluid Overload Is Associated With Reduced Mortality in Critical Illness. Crit Care Med 46:1600–1607. [DOI] [PubMed] [Google Scholar]
- 29.Murphy HJ, Cahill JB, Twombley KE, Kiger JR (2018) Early Continuous Renal Replacement Therapy Improves Nutrition Delivery in Neonates During Extracorporeal Life Support. J Ren Nutr 28:64–70. [DOI] [PubMed] [Google Scholar]
- 30.Murphy HJ, Cahill JB, Twombley KE, Annibale DJ, Kiger JR (2018) Implementing a practice change: early initiation of continuous renal replacement therapy during neonatal extracorporeal life support standardizes care and improves short-term outcomes. J Artif Organs 21:76–85. [DOI] [PubMed] [Google Scholar]
- 31.Kaddourah A, Basu RK, Goldstein SL, Sutherland SM, Assessment of Worldwide Acute Kidney Injury RAaEI (2019) Oliguria and Acute Kidney Injury in Critically Ill Children: Implications for Diagnosis and Outcomes. Pediatr Crit Care Med 20:332–339. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.