Supplemental Digital Content is available in the text.
Keywords: Hazardous air pollutants, Air toxics, Telomere length, National Air Toxics Assessment
Abstract
Background:
Telomeres are vital for genomic integrity, and telomere length has been linked to many adverse health outcomes. Some hazardous air pollutants or air toxics increase oxidative stress and inflammation, two possible determinants of shortened telomere length. No studies have examined air toxic–telomere length associations in a nonoccupational setting.
Methods:
This study included 731 Sister Study participants (enrolled 2003–2007) who were randomly selected to assess telomere length in baseline blood samples. Multiplex qPCR was used to determine telomere to single copy gene (T/S) ratios. Census tract concentration estimates of 29 air toxics from the 2005 National Air Toxics Assessment were linked to baseline residential addresses. Air toxics were classified into tertile-based categories of the exposure. Multivariable linear regression was used to estimate β coefficients and 95% confidence intervals (CIs) in single-pollutant models. Multipollutant groups were identified with regression trees.
Results:
The average T/S ratio was 1.24. Benzidine (T3 versus T1 β = −0.08; 95% CI = −0.14, −0.01) and 1,4-dioxane (T3 versus T1 β = −0.06; 95% CI = −0.13, 0.00) in particular, as well as carbon tetrachloride, chloroprene, ethylene dibromide, and propylene dichloride, were associated with shorter relative telomere length. Benzidine (P = 0.02) and 1,4-dioxane (P = 0.06) demonstrated some evidence of a monotonic trend. The regression tree identified age, BMI, physical activity, ethylene oxide, acrylonitrile, ethylidene dichloride, propylene dichloride, and styrene in multipollutant groups related to telomere length.
Conclusions:
In this first study of air toxics and telomere length in a nonoccupational setting, several air toxics, particularly 1,4-dioxane and benzidine, were associated with shorter relative telomere length.
What this study adds
Hazardous air pollutants or air toxics are ubiquitous environmental contaminants, and shorter telomere length is a biomarker associated with a variety of adverse health outcomes. This was the first study to examine specific air toxics in relation to telomere length in a nonoccupational setting and to use a multipollutant approach to explore whether combinations of air toxics were related to telomere length. We found that certain individual air toxics were associated with shorter telomere length. The multipollutant approach did not suggest that high levels of two or more pollutants in combination were associated with shorter telomere length compared with single pollutants.
Introduction
Telomeres cap the ends of deoxyribonucleic acid (DNA) strands and protect chromosomes from degradation.1 Telomere attrition has been linked to a number of adverse health outcomes, including chronic kidney disease,2 heart disease,3–5 diabetes,6 and some cancers,7 that represent a large public health burden in the United States and worldwide.
In addition to age,8 oxidative stress and inflammation are two factors that may lead to telomere shortening.9,10 Therefore, studying environmental exposures, such as hazardous air pollutants, that may operate through oxidative stress and inflammation pathways is of interest.11 Hazardous air pollutants or air toxics include 187 pollutants under the jurisdiction of the Environmental Protection Agency (EPA) that are thought to be carcinogenic or to cause other serious health or environmental effects.12 Millions of tons of air toxics are released annually in the United States from a variety of sources such as large industries, gas stations, power plants, vehicular traffic, and dry cleaners.12–14 Air toxics are a distinct group of air pollutants that differ from the criteria air pollutants (e.g., particulate matter [PM], ozone, and nitrogen dioxide [NO2]). Criteria air pollutants are widely monitored and have human health-based criteria for ambient air set by the EPA that every state must meet.15 In contrast, there are no national ambient air quality standards for air toxics.
The relation between air toxics and telomere length is not established. Studies reported in the past decade have been limited to occupational settings with higher exposures than those experienced by the general population.11 A study of traffic officers reported shorter telomere length with increasing airborne benzene and toluene16; a study of Swedish rubber industry workers reported n-nitrosamines and p-toluidine were associated with shorter telomere length17; and a study of coke oven workers found an inverse association between polycyclic aromatic hydrocarbons and telomere length.18 Other specific air toxics have not been examined in relation to telomere length, and no study has examined air toxics and telomere length in a nonoccupational setting.
Obesity is associated with shorter telomere length,19–21 and physical activity is associated with longer telomere length.22–25 Thus, these factors could modify the air toxic–telomere length associations, particularly through their influence on oxidative stress and inflammation.26–28 Increased physical activity could also lead to greater exposure through increased ventilation and a higher respiratory rate.29 In addition, because individuals breathe mixtures of pollutants, it is important to understand the potentially complex relations between air toxics that may vary by personal characteristics and may be related to telomere length.
The objectives of this study were to (a) evaluate the association between ambient air toxics and relative telomere length; (b) examine whether BMI or physical activity modify air toxic–telomere length associations; and (c) to explore whether there are combinations of air toxics that are particularly related to telomere length.
Methods
Study design and population
The Sister Study cohort consists of 50,884 US women, 35–74 years of age, who had a sister who had been diagnosed with breast cancer, but no prior breast cancer themselves at baseline.30 Enrollment occurred between 2003 and 2009. At the baseline home visit, a 45-ml fasting blood sample was collected by trained examiners from an in-home phlebotomy service. A subcohort of 736 women (from among 29,026 who completed their home visit by 1 June 2007) was randomly selected to assess telomere length using their baseline blood sample.31
All women provided informed written consent, and this study was approved by the Institutional Review Boards of the National Institute of Environmental Health Sciences (NIEHS) and the Copernicus Group. Sister Study data release 6.0 (NIEHS, Research Triangle Park, NC) was used for this study.
Air toxics exposure assessment
The National Air Toxics Assessment (NATA) estimates created by the EPA are the only nationwide data available on air toxic levels in the United States. The NATA database has been previously used to evaluate associations between air toxics and a number of health outcomes.32–40 Versions of NATA have been released for 1996, 1999, 2002, 2005, 2011, and 2014. We used the 2005 version because it was in the middle of the enrollment period for the Sister Study and incorporated modeling improvements over previous versions. The 2005 NATA estimation methods have been described previously.41 Briefly, point (e.g., large factories/waste incinerators/airports), nonpoint (e.g., small manufacturers/gas stations/dry cleaners), and on-road (e.g., cars/buses/trucks) and non–road mobile (e.g., boats/trains) source emissions are compiled in the National Emissions Inventory.42 These emissions are used as inputs in two dispersion models, Human Exposure Model-3 (HEM-3) AMS/EPA Regulatory Model (AERMOD) version (American Meteorological Society (AMS) and EPA) (point and mobile sources) and Assessment System for Population Exposure Nationwide (ASPEN) (EPA, Research Triangle Park, NC) (nonpoint sources), which have previously been described in detail.41,43,44 HEM-3 AERMOD was developed by the American Meteorological Society and the EPA as a steady-state plume model.44 HEM-3 modeling incorporates elevation/terrain data and characteristics of each emission point source, such as stack height and exit gas rate and temperature, and estimated average emission heights and vertical dispersion coefficients for mobile sources and aircraft. Hourly meteorology data used by HEM-3 came from the closest meteorologic station to each source. ASPEN is a steady-state Gaussian model developed by the EPA.43 For nonpoint sources, it incorporates characteristics such as rate and height of gas release, wind speed from the closest meteorologic station, reactive decay, and deposition. The concentrations for a nonpoint source from ASPEN are represented as a polar grid. The concentration within the tract that the source resides is estimated by spatial averaging at all grid receptors in the tract. The concentration from the source that disperse into neighboring tracts is determined from interpolation. Background levels (from long-range transport or persistence from previous years) and secondary formation (from reaction of air toxics in the atmosphere) are also incorporated. The output of the dispersion models is the census tract-level total concentration estimate of each air toxic. The NATA concentration estimates represent an annual average. This information was linked to the Sister Study through the census tract number of the geocoded baseline residential address of participants. Five individuals’ baseline residential addresses were not able to be geocoded at the census tract level, leaving 731 women for inclusion in this analysis.
The 2005 NATA contains information on 177 air toxics. A subset of 37 was chosen for this study because they had been classified as mammary gland carcinogens in a review of animal studies45 and some are suspected to cause oxidative stress46–55 or inflammation,47,48,56 which are biologically relevant for telomere length. Eight of these 37 air toxics had extensive missing information due to incomplete NATA modeling, leaving 29 for analysis in this study. For 1,4-dioxane, 2-chloroacetophenone, and acrylamide, which were among the 29 toxics examined in this study, 14 (1.9%), 2 (0.3%), and 55 (7.5%) women, respectively, were missing air toxic concentrations because NATA modeling was not complete for their baseline residential census tract. Therefore, these women were not included in the analysis of these individual pollutants or in the multipollutant analysis.
Telomere length measurement
Baseline blood samples were stored frozen at −80°C until DNA was extracted using Qiagen Autopure LS (Hilden, Germany) at the National Institute of Environmental Health Sciences (NIEHS) Molecular Genetics Core facility. Extracted DNA was washed with Tris-EDTA (TE) Buffer, quantified with Quant-iT PicoGreen dsDNA reagent (a fluorescent nucleic acid stain) (Invitrogen, Carlsbad, CA), and stored at −20°C.31 Finally, DNA was plated robotically in duplicate using 10-ng aliquots onto four replicate 384-well plates.
Telomere length was quantified from the extracted DNA using monochrome multiplex quantitative polymerase chain reaction (qPCR)57 which measures the factor by which the participant’s sample differs from reference DNA in telomere repeat copy number (T) and single copy gene copy number (S). The relative ratio of these two measures, the T/S ratio, is proportional to the average telomere length. Detailed laboratory parameters and reagent components for the multiplex qPCR used in the Sister Study have been described previously.31,58 The polymerase chain reaction (PCR) cycling for each plate was conducted on the BioRad CFX384 (Hercules, CA) machine. For each assay plate, a five-point standard curve ranging from 1.9 to 75 ng was determined in a 2.5-fold dilution run from a pooled sample of normal subjects and was used to determine the T and S values of the subject samples. Each subject’s sample was run in duplicate on four plates, so the final value was the average of up to eight replicate T/S ratio values.31,58 The average coefficient of variation was 11%, and the intraclass correlation coefficient of a single T/S ratio was 0.85.31
Covariates
Confounders were determined using a directed acyclic graph.59,60 The minimally sufficient adjustment set included age (continuous), race (non-Hispanic white/non-Hispanic black/Hispanic/other), residence type (urban/suburban/small town/rural), highest level of education attained (<high school/high school/some college/≥4-year degree), and cigarette smoking (never/former/current). These confounders were assessed by self-report during a Computer-Assisted Telephone Interview (CATI) at study enrollment.
Body mass index (BMI) and physical activity were considered as potential effect–measure modifiers of the air toxic–telomere length associations. Height and weight measurements taken by trained examiners at the baseline home visit were used to calculate BMI (kg/m2). At the baseline interview, women were asked to report all recreational sport/exercise activities they regularly participated in during the previous 12 months and how many months, days/week, and time/day they did each activity. Average hours/week of physical activity was calculated from this information. To maximize statistical power for the stratified analyses, BMI was dichotomized as <25.0 and ≥25 kg/m2 and physical activity as ≤median (≤2.2 hours/week) and >median (>2.2 hours/week).
Statistical analysis
Single-pollutant analysis
To estimate associations between individual air toxics and telomere length, telomere length was considered as a continuous variable, each air toxic was categorized based on tertiles of the exposure among the population with telomere length measurements, and models were considered separately for each air toxic. We considered air toxic exposures continuously, but associations did not seem linear for most air toxics, so we only reported the categorized results. Due to the minimal amount of missing data (three of 731 women were missing information on a covariate), a complete case analysis was used. Multivariable linear regression was used to estimate regression coefficients (β) and 95% confidence intervals (CIs).61
Effect-measure modification by BMI and physical activity, separately, was assessed using an interaction term between the modifier and air toxic (≥median versus <median). This is presented as a stratified analysis with a β coefficient (95% CI) for each stratum of the modifier and a β coefficient (95% CI) for the interaction term.
In sensitivity analyses, we examined whether the single-pollutant associations with telomere length changed when the study samples were (a) restricted to non-Hispanic whites; (b) restricted to those who had lived in their baseline address >10 years; (c) restricted to those who enrolled in 2005 or later; or when (d) models were additionally adjusted for residence region (Northeast/Midwest/South/West). All analyses were completed in SAS 9.4 (Cary, NC).
Multipollutant analysis
We used Classification and Regression Tree (CART) methods to explore whether there are combinations of air toxics that are particularly related to telomere length.62,63 CART is a supervised forward-selection recursive partitioning technique. At each step, the variable with the strongest association with the outcome is selected to create a binary split. Further splits occur within the previous splits to form “branches.” The branches characterize the expected value of the outcome (average telomere length) within the combinations of the variables in that branch. The tree was allowed to split on the 29 air toxics, age at baseline, BMI, and physical activity. Age, BMI, and physical activity were included in the CART analysis to parallel the single-pollutant analyses. The splitting criterion used for regression trees is the sum of the squared deviations about the mean.64 For the stopping criteria, we explored combinations of maximum depth of the tree, minimum number of observations in a node, and total number of terminal nodes to find a tree that did not grow too large to lose interpretability, but still identified relevant groups for telomere length. As a result, we specified the tree grow to a maximum depth of five levels and that the minimum number of total observations in a node be 10. Cost-complexity pruning62 was used to create a tree with a total of 10 terminal nodes. CART was conducted using SAS function PROC HPSPLIT.
Results
Population characteristics
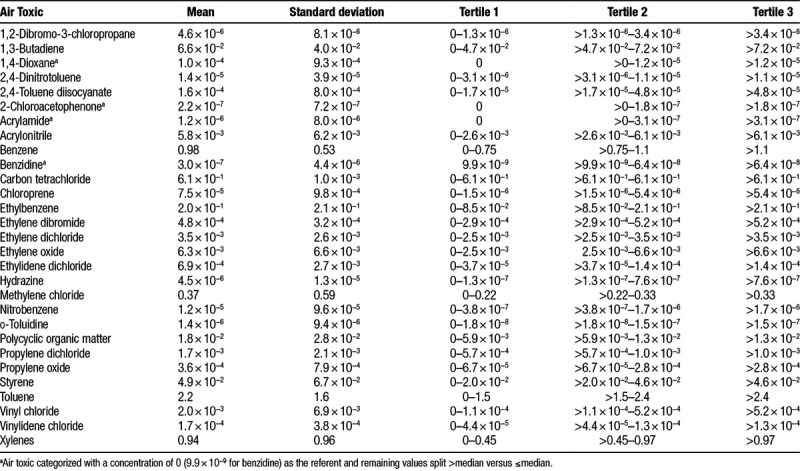
The average T/S ratio among the 731 women in this study was 1.24 (standard deviation [SD] = 0.35). At enrollment, participants were on average 55 years of age, and mostly non-Hispanic white (92%), well educated (55% with a college degree or higher), and never smokers (55%) (Table 1). Mean concentrations of the hazardous air pollutants ranged from 3.0 × 10−7 μg/m3 for benzidine to 2.2 μg/m3 for toluene (Table 2). Tertile cut-points for each air toxic are shown in Table 2.
Table 1.
Baseline characteristics of 731 randomly selected women with measured telomere length: The Sister Study

Table 2.
Estimated concentrations and tertile cut-points of the 29 selected hazardous air pollutants (μg/m3) among the 731 women with telomere length measurements: The Sister Study
Results from single-pollutant models
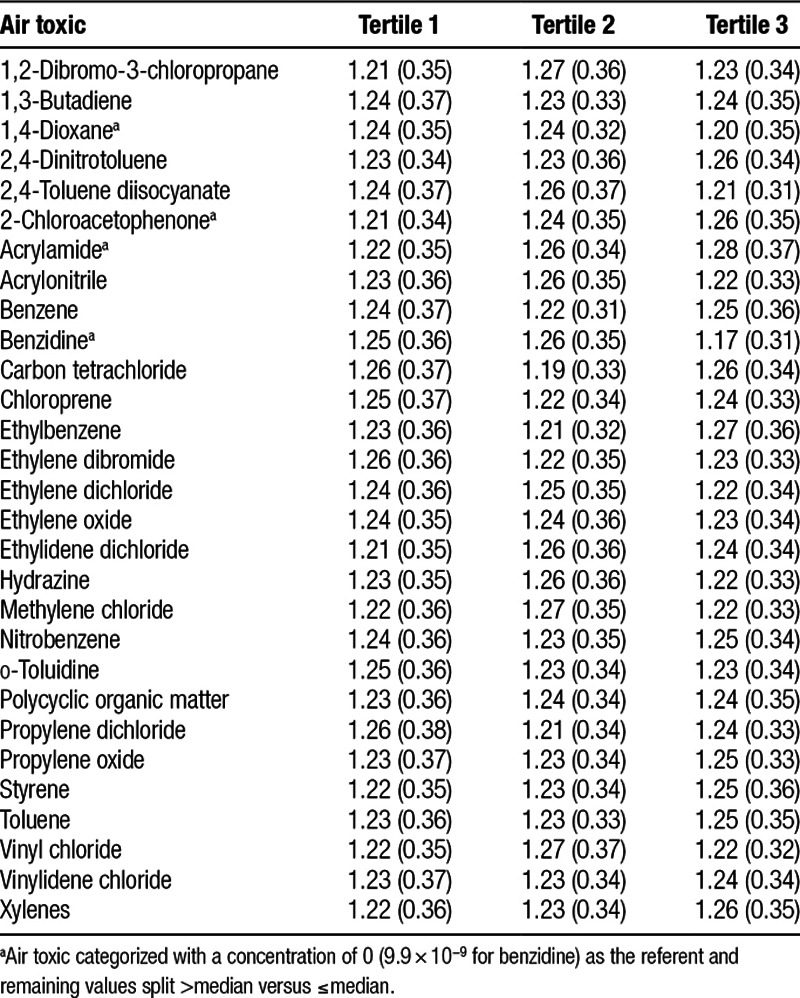
Across tertile-based categories of the air toxics, the shortest mean relative telomere length of 1.17 (SD = 0.31) was observed among participants in the third category of benzidine, which was markedly shorter compared with the first category (1.25; SD = 0.36) (Table 3). Scatterplots of T/S ratio values against 1,4-dioxane and benzidine concentrations are shown in Supplemental Digital Content, eFigure 1; http://links.lww.com/EE/A47.
Table 3.
Mean (SD) telomere length across tertiles of hazardous air pollutant concentration estimates: The Sister Study
In the multivariable linear regression analysis, the third versus first category of benzidine (β = −0.08; 95% CI = −0.14, −0.01) and 1,4-dioxane (β = −0.06; 95% CI = −0.13, 0.00) were associated with shorter relative telomere length, with some evidence of an exposure-response trend (P = 0.02 and P = 0.06, respectively) (Table 4). Carbon tetrachloride, chloroprene, ethylene dibromide, and propylene dichloride were also inversely associated with telomere length.
Table 4.
Regression β coefficients (95% CIs) for the associationsa between hazardous air pollutants and telomere length
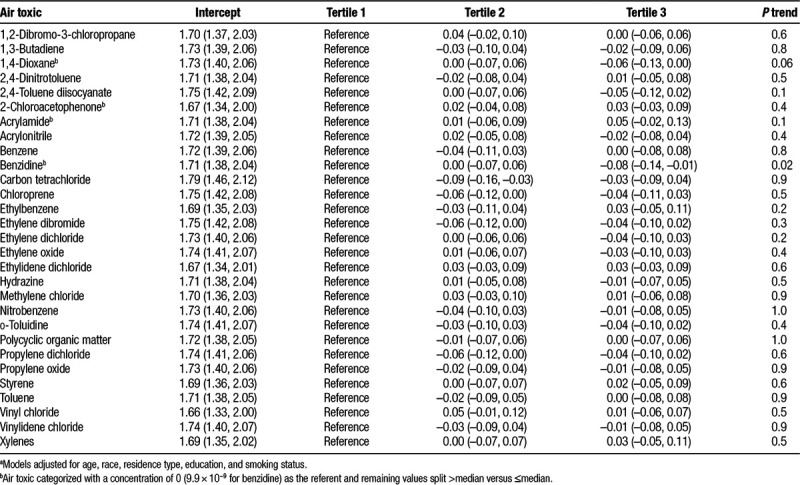
Air toxic–telomere length associations did not differ meaningfully across levels of BMI (Supplemental Digital Content, eTable 1; http://links.lww.com/EE/A47) or physical activity (Supplemental Digital Content, eTable 2; http://links.lww.com/EE/A47). In sensitivity analyses, results were similar when restricted to non-Hispanic whites or when additionally adjusted for geographic region (data not shown). Results for a few air toxics were slightly attenuated, although acrylamide demonstrated a positive association (third category β = 0.12; 95% CI = 0.02, 0.22), when restricted to women who had lived in their baseline address >10 years (data not shown). Among those with their baseline blood draw in 2005 or later, results were similar for most air toxics, apart from acrylamide which demonstrated a positive association (third category β = 0.08; 95% CI = 0.00, 0.16) (data not shown).
Results from the multipollutant regression tree
In the regression tree, the primary split was on age ≥64.3 vs. <64.3 years (Figure 1). Relative telomere length was shorter among those who were ≥64.3 compared with <64.3 years of age. The subgroup with the shortest relative telomere length consisted of those ≥64.3 years of age and with higher (≥0.007 μg/m3) ethylene oxide concentration. The longest relative telomere length was observed in the subset younger than 64.3 years of age and with a BMI <19.3 kg/m2. Other patterns included the following: (a) shorter telomere length with higher (≥0.001 μg/m3) propylene dichloride among those <45.8 years of age, with a BMI ≥19.3 kg/m2, and who participated in ≥0.9 hours/week of physical activity and (b) shorter telomere length with higher (≥0.004 μg/m3) ethylidene dichloride concentration among those 45.8–64.3 years of age with a BMI ≥19.3 kg/m2. However, among those with lower ethylidene dichloride, a longer average telomere length was observed with higher (≥0.064 μg/m3) styrene concentration. Based on the tree, high levels of two or more chemicals in combination did not seem worse for telomere length compared with single exposures.
Figure 1.
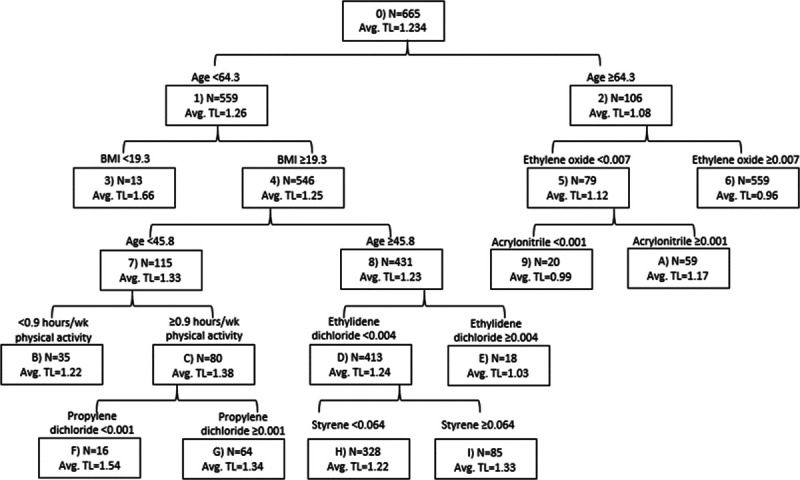
Regression tree for hazardous air pollutants and telomere length (TL): The Sister Study.
Discussion
In this study of 731 US women, higher ambient 1,4-dioxane and benzidine were associated with shorter relative telomere length in single-pollutant models. Additionally, there was evidence that carbon tetrachloride, chloroprene, ethylene dibromide, and propylene dichloride were also associated with shorter telomere length, but a monotonic exposure-response trend was not observed. Body mass index and physical activity did not modify the air toxic–telomere length associations. The regression tree identified those ≥64.3 years of age and with higher (≥ 0.007μg/m3) ethylene oxide exposure as the subgroup with the shortest telomere length.
To our knowledge, ours is the first study to examine the association between specific hazardous air pollutants and relative telomere length in a nonoccupational setting. Therefore, direct comparison with other investigations is difficult. In an occupational study of traffic officers and office workers, telomere length was shorter in traffic officers compared with office workers.16 Further, an interquartile range increase in benzene and toluene measured from personal air monitors in that population was associated with shorter relative telomere length (−6.4%; 95% CI = −2.1, −10.4; and −6.2%; 95% CI = −1.7, −10.4, respectively).16 Air toxics found in vehicle exhaust include 1,3-butadiene, benzene, polycyclic organic matter, ethylbenzene, styrene, toluene, and xylene.65,66 In our study, these air toxics were not associated with relative telomere length. In a study of rubber manufacturers in Sweden, biomarkers of o-toluidines and polycyclic aromatic hydrocarbons (a component of polycyclic organic matter) were not associated with telomere length17; similarly, neither was associated with telomere length in our study.
In the present study, the top category of benzidine was associated with shorter relative telomere length, which also demonstrated a monotonic trend. Benzidine, which in the past was used in the manufacture of dyes and is persistent in the environment, has been shown to be mutagenic and is metabolized through an oxidation pathway that forms reactive oxygen species.67,68 Given that oxidative stress has been shown to lead to telomere shortening,10,69 the association between benzidine and telomere length is biologically plausible. 1,4-Dioxane is a solvent used in the manufacture of certain chemicals and a variety of products. Little is known about the toxicology of 1,4-dioxane70; therefore, the biologic mechanisms for its association with telomere length in our study are unclear. Finally, carbon tetrachloride and propylene dichloride, which demonstrated some association with shorter telomere length, have been shown to induce oxidative stress in animal studies.47,71,72
We originally hypothesized BMI and physical activity to be effect-measure modifiers of the air toxic–telomere length associations. Overweight/obesity increases oxidative stress and impairs the oxidant defense system, which could act synergistically with the oxidative stress induced by some air toxics.26,28 Higher levels of physical activity are associated with longer telomere length,22–25 and it is possible that the benefits of physical activity on lowering oxidative damage and inflammation could counteract that of air toxics.22,27 On the other hand, physical activity may increase exposure to air toxics.29 However, neither BMI nor physical activity modified observed associations, perhaps because of opposing mechanisms (e.g., for physical activity) or of limited power to detect a small magnitude of modification.
A regression tree was used in our study to explore relevant subgroups based on complex joint relations across air toxics and covariates of interest. As expected, due to its established association with telomere length,8 age was the top split in the tree. Among those ≥64.3 years of age, those with ethylene oxide >0.007μg/m3 had the shortest telomere length on the tree. The tree did not identify high levels of two or more chemicals in combination that were associated with shorter relative telomere length. Of the air toxics identified on the tree, only propylene dichloride was associated with telomere length in the single-pollutant models. Although results from a tree and from single-pollutant models may identify similar factors as important, differences between the two approaches should also be expected. The tree allows for nonlinear and nonadditive associations involving multiple air toxics and other covariates that are assumed not to exist for traditional regression methods of single pollutants. Additionally, the tree identifies the best cut-point at which to split a continuous variable based on the sum of squared deviations about the mean, but sometimes that cut-point was well into the third tertile used in the single-pollutant models. However, we used CART in an exploratory manner because it does not provide measures of statistical precision and the size of the tree is set by investigator-specified parameters to enhance interpretability.
NATA air toxic estimates represent an annual average, so for most women in our study, the 2005 NATA estimates corresponded to the year of or 1–2 years before collection of the blood samples used for assessing telomere length. In the context of an outcome like telomere length, which is susceptible to changes from exposures on the day or week before measurement,73,74 this can be considered a long-term exposure. Although not specific to air toxics, two studies of particulate matter (PM) (a criteria air pollutant regulated through national ambient air quality standards) found that acute exposure on the day of or 1–3 days before telomere length measurement was associated with longer telomere length,73,74 whereas a measure of PM representing longer-term exposure over 2 weeks was associated with shorter telomere length.74 This supports our finding of generally inverse associations using a longer-term annual exposure window.
A limitation of our study is the possibility of bias from exposure measurement error using the NATA estimates. Concentrations at the census tract level do not account for variability within that census tract or in an individual’s daily activities. Also, although outdoor ambient sources are a major contributor to air toxic exposure, indoor sources, cigarette smoke, and diet are not included in NATA estimates. NATA estimates may be less precise than estimates derived from methods such as land use regression that rely on monitored data.35 However, air monitors for air toxics are sparse across the United States and do not measure most air toxics. Therefore, monitored concentrations are not reliable estimates for air toxics on a nationwide scale, which makes NATA a unique and valuable resource. Further, we used the 2005 NATA version which included modeling improvements and is more complete than previous versions.41 California has a small number of air monitors that measure select air toxics. A study examined the agreement between monitored and NATA-modeled annual concentrations of 12 air toxics.75 For the 2002 and 2005 NATA estimates, the median monitored concentration fell between the 10th and 90th percentile of modeled concentrations for every air toxic, except for three air toxics (chloroform, styrene, and 1,3-butadiene), and the 2005 NATA had the highest correlation coefficients and less over- or underestimation of concentrations compared with previous versions. Therefore, although there was some suggestion of underestimation of concentrations in the modeled estimates of exposure, the results suggested that the 2002 and 2005 NATA versions would be useful for exposure assessment in study populations with a wide geographic spread.75
In our study, relative telomere length was measured using multiplex qPCR which is considered an improvement over the previous singleplex qPCR method because it reduces variation in the amount of DNA pipetted for measuring the T and S components.57,76 Multiplex qPCR also has a stronger correlation with the gold standard Southern Blot technique than does singleplex qPCR.57 However, qPCR-based methods typically result in a higher coefficient of variation and lower reproducibility compared with Southern Blot,77 which is time and resource intensive and often impractical in large epidemiologic studies.78 Additionally, although a study using a subset of samples from women in the Sister Study found that a telomere length measurement with multiplex qPCR from a single time point had good short-term reliability of an individual’s telomere length,76 changes in telomeres are dynamic. Given that telomere length in our study was measured at one time point, we were not able to assess whether air toxics were associated with changes in telomere length over time.
The women with measured telomere length included in this study came from the 48 continental US states, which allowed us to examine the associations using a larger range of exposure than if a smaller area had been included. Further, ours is the first study of telomere length to examine the role of air toxics at ambient levels of exposure in the general population, rather than occupational levels. We explored complex joint associations between air toxics and other covariates. This is an important consideration because individuals are not exposed to air toxics in isolation and complex patterns are not captured in standard single-pollutant modeling techniques.
Conclusions
In summary, using ambient hazardous air pollutant concentration estimates, 1,4-dioxane and benzidine were associated with shorter relative telomere length. Although the associations were nonmonotonic, carbon tetrachloride, chloroprene, ethylene dibromide, and propylene dichloride also showed some evidence of associations with shorter relative telomere length. Our results support a role for some air toxics in telomere length, an important biologic marker associated with adverse disease outcomes.2–7
Conflicts of interest statement
The authors declare that they have no conflicts of interest with regard to the content of this report.
This work was supported by grants T32-ES007018 from the National Institute of Environmental Health Sciences (NIEHS)-funded training grant to the University of North Carolina (UNC), T32-CA057726 from the National Cancer Institute-funded UNC Lineberger Comprehensive Cancer Center Cancer Control Education Program, and Z01-ES044005 from the Intramural Research Program of the National Institutes of Health (NIH), National Institutes of Environmental Health Science.
Access to data: Data from the Sister Study cannot be shared publically because of a data sharing agreement. NATA data for the exposure are publically available (https://www.epa.gov/national-air-toxics-assessment).
Acknowledgments
We appreciate the helpful comments of Jason Sacks at the Environmental Protection Agency and Christine Parks and Jacob Kresovich at the National Institute of Environmental Health Sciences.
Supplementary Material
Footnotes
Published online 28 June 2019
Sponsorships or competing interests that may be relevant to content are disclosed at the end of the article.
Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.epidem.com).
References
- 1.Aubert G, Lansdorp PM. Telomeres and aging. Physiol Rev 200888557–579 [DOI] [PubMed] [Google Scholar]
- 2.Raschenberger J, Kollerits B, Ritchie J, et al. Association of relative telomere length with progression of chronic kidney disease in two cohorts: effect modification by smoking and diabetes. Sci Rep 2015511887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haycock PC, Heydon EE, Kaptoge S, Butterworth AS, Thompson A, Willeit P. Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. BMJ 2014349g4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carty CL, Kooperberg C, Liu J, et al. Leukocyte telomere length and risks of incident coronary heart disease and mortality in a racially diverse population of postmenopausal women. Arterioscler Thromb Vasc Biol 2015352225–2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scheller Madrid A, Rode L, Nordestgaard BG, Bojesen SE. Short telomere length and ischemic heart disease: observational and genetic studies in 290 022 individuals. Clin Chem 2016621140–1149 [DOI] [PubMed] [Google Scholar]
- 6.Willeit P, Raschenberger J, Heydon EE, et al. Leucocyte telomere length and risk of type 2 diabetes mellitus: new prospective cohort study and literature-based meta-analysis. PLoS One 20149e112483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wentzensen IM, Mirabello L, Pfeiffer RM, Savage SA. The association of telomere length and cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev 2011201238–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Müezzinler A, Zaineddin AK, Brenner H. A systematic review of leukocyte telomere length and age in adults. Ageing Res Rev 201312509–519 [DOI] [PubMed] [Google Scholar]
- 9.Hou L, Zhang X, Gawron AJ, Liu J. Surrogate tissue telomere length and cancer risk: shorter or longer? Cancer Lett 2012319130–135 [DOI] [PubMed] [Google Scholar]
- 10.von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci 200227339–344 [DOI] [PubMed] [Google Scholar]
- 11.Zhang X, Lin S, Funk WE, Hou L. Environmental and occupational exposure to chemicals and telomere length in human studies. Occup Environ Med 201370743–749 [DOI] [PubMed] [Google Scholar]
- 12.Environmental Protection Agency. What are Hazardous Air Pollutants? Available at: https://www.epa.gov/haps/what-are-hazardous-air-pollutants. Accessed 15 October, 2018.
- 13.Environmental Protection Agency. Hazardous Air Pollutants: Sources and Exposure. Available at: https://www.epa.gov/haps/hazardous-air-pollutants-sources-and-exposure. Accessed 15 October, 2018.
- 14.Environmental Protection Agency. Report on the environment: Air toxics emissions. Environmental Protection Agency; 2015. Available at: https://cfpub.epa.gov/roe/indicator.cfm?i=2#1. Accessed 15 October, 2018. [Google Scholar]
- 15.Environmental Protection Agency. Managing Air Quality- Air Pollution Types. Available at: https://www.epa.gov/air-quality-management-process/managing-air-quality-air-pollutant-types. Accessed 15 October, 2018.
- 16.Hoxha M, Dioni L, Bonzini M, et al. Association between leukocyte telomere shortening and exposure to traffic pollution: a cross-sectional study on traffic officers and indoor office workers. Environ Health 2009841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li H, Jönsson BA, Lindh CH, Albin M, Broberg K. N-nitrosamines are associated with shorter telomere length. Scand J Work Environ Health 201137316–324 [DOI] [PubMed] [Google Scholar]
- 18.Pavanello S, Pesatori AC, Dioni L, et al. Shorter telomere length in peripheral blood lymphocytes of workers exposed to polycyclic aromatic hydrocarbons. Carcinogenesis 201031216–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mundstock E, Sarria EE, Zatti H, et al. Effect of obesity on telomere length: Systematic review and meta-analysis. Obesity (Silver Spring) 2015232165–2174 [DOI] [PubMed] [Google Scholar]
- 20.Rode L, Nordestgaard BG, Weischer M, Bojesen SE. Increased body mass index, elevated C-reactive protein, and short telomere length. J Clin Endocrinol Metab 201499E1671–E1675 [DOI] [PubMed] [Google Scholar]
- 21.Valdes AM, Andrew T, Gardner JP, et al. Obesity, cigarette smoking, and telomere length in women. Lancet 2005366662–664 [DOI] [PubMed] [Google Scholar]
- 22.Cherkas LF, Hunkin JL, Kato BS, et al. The association between physical activity in leisure time and leukocyte telomere length. Arch Intern Med 2008168154–158 [DOI] [PubMed] [Google Scholar]
- 23.Du M, Prescott J, Kraft P, et al. Physical activity, sedentary behavior, and leukocyte telomere length in women. Am J Epidemiol 2012175414–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Latifovic L, Peacock SD, Massey TE, King WD. The influence of alcohol consumption, cigarette smoking, and physical activity on leukocyte telomere length. Cancer Epidemiol Biomarkers Prev 201625374–380 [DOI] [PubMed] [Google Scholar]
- 25.Shadyab AH, LaMonte MJ, Kooperberg C, et al. Leisure-time physical activity and leukocyte telomere length among older women. Exp Gerontol 201795141–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manna P, Jain SK. Obesity, oxidative stress, adipose tissue dysfunction, and the Associated Health Risks: causes and therapeutic strategies. Metab Syndr Relat Disord 201513423–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radak Z, Chung HY, Goto S. Systemic adaptation to oxidative challenge induced by regular exercise. Free Radic Biol Med 200844153–159 [DOI] [PubMed] [Google Scholar]
- 28.Weichenthal S, Hoppin JA, Reeves F. Obesity and the cardiovascular health effects of fine particulate air pollution. Obesity (Silver Spring) 2014221580–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giles LV, Koehle MS. The health effects of exercising in air pollution. Sports Med 201444223–249 [DOI] [PubMed] [Google Scholar]
- 30.Sandler DP, Hodgson ME, Deming-Halverson SL, et al. ; Sister Study Research Team The Sister Study Cohort: baseline methods and participant characteristics. Environ Health Perspect 2017125127003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim S, Sandler DP, Carswell G, et al. Telomere length in peripheral blood and breast cancer risk in a prospective case-cohort analysis: results from the Sister Study. Cancer Causes Control 2011221061–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garcia E, Hurley S, Nelson DO, Hertz A, Reynolds P. Hazardous air pollutants and breast cancer risk in California teachers: a cohort study. Environ Health 20151414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hart JE, Bertrand KA, DuPre N, et al. Exposure to hazardous air pollutants and risk of incident breast cancer in the nurses’ health study II. Environ Health 20181728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu R, Nelson DO, Hurley S, Hertz A, Reynolds P. Residential exposure to estrogen disrupting hazardous air pollutants and breast cancer risk: the California Teachers Study. Epidemiology 201526365–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White AJ, O’Brien KM, Niehoff NM, Carroll R, Sandler DP. Metallic air pollutants and breast cancer risk in a nationwide cohort study. Epidemiology 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kalkbrenner AE, Daniels JL, Chen JC, Poole C, Emch M, Morrissey J. Perinatal exposure to hazardous air pollutants and autism spectrum disorders at age 8. Epidemiology 201021631–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malek AM, Barchowsky A, Bowser R, et al. Exposure to hazardous air pollutants and the risk of amyotrophic lateral sclerosis. Environ Pollut 2015197181–186 [DOI] [PubMed] [Google Scholar]
- 38.Roberts AL, Lyall K, Hart JE, et al. Perinatal air pollutant exposures and autism spectrum disorder in the children of Nurses’ Health Study II participants. Environ Health Perspect 2013121978–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cicalese L, Curcuru G, Montalbano M, Shirafkan A, Georgiadis J, Rastellini C. Hazardous air pollutants and primary liver cancer in Texas. PLoS One 201712e0185610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stingone JA, Pandey OP, Claudio L, Pandey G. Using machine learning to identify air pollution exposure profiles associated with early cognitive skills among U.S. children. Environ Pollut 2017230730–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Environmental; Protection Agency. An overview of methods for EPA’s National-Scale Air Toxics Assessment. Durham, NC: Office of Air Quality, Planning, and Standards; 2011. [Google Scholar]
- 42.Environmental Protection Agency. National Emissions Inventory (NEI). https://www.epa.gov/air-emissions-inventories/national-emissions-inventory-nei. Accessed 18 October, 2018.
- 43.Environmental; Protection Agency. User’s Guide for the Assessment System for Population Exposure Nationwide (ASPEN, Version 1.1) Model. Research Triangle Park, NC: Environmental Protection Agency; 2000. Office of Air Quality Planning and Standards. [Google Scholar]
- 44.Environmental Protection Agency. The HEM-3 User’s Guide, HEM-3 Human Exposure Model Version 1.1.0 (AERMOD version) Research Triangle Park, NC: U.S. Environmental Protection Agency; 2007. Office of Air Quality Planning and Standards. [Google Scholar]
- 45.Rudel RA, Attfield KR, Schifano JN, Brody JG. Chemicals causing mammary gland tumors in animals signal new directions for epidemiology, chemicals testing, and risk assessment for breast cancer prevention. Cancer 200710912 suppl2635–2666 [DOI] [PubMed] [Google Scholar]
- 46.Chang FK, Mao IF, Chen ML, Cheng SF. Urinary 8-hydroxydeoxyguanosine as a biomarker of oxidative DNA damage in workers exposed to ethylbenzene. Ann Occup Hyg 201155519–525 [DOI] [PubMed] [Google Scholar]
- 47.Jia R, Cao LP, Du JL, et al. Effects of carbon tetrachloride on oxidative stress, inflammatory response and hepatocyte apoptosis in common carp (Cyprinus carpio). Aquat Toxicol 201415211–19 [DOI] [PubMed] [Google Scholar]
- 48.Ju R, Jia Q, Meng T, et al. Effect of occupational exposure to toluene diisocyanate on workers’ health. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 20163423–26 [DOI] [PubMed] [Google Scholar]
- 49.Kim JH, Moon JY, Park EY, Lee KH, Hong YC. Changes in oxidative stress biomarker and gene expression levels in workers exposed to volatile organic compounds. Ind Health 2011498–14 [DOI] [PubMed] [Google Scholar]
- 50.Lai CH, Liou SH, Lin HC, et al. Exposure to traffic exhausts and oxidative DNA damage. Occup Environ Med 200562216–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mo GW, Cai SX, Zhao HJ, Li WJ, Tong WC, Liu LY. Effect of toluene diisocyanate on reactive oxygen species production and permeability of human bronchial epithelial cells in vitro. Nan Fang Yi Ke Da Xue Xue Bao 201131239–243 [PubMed] [Google Scholar]
- 52.Pu X, Kamendulis LM, Klaunig JE. Acrylonitrile-induced oxidative stress and oxidative DNA damage in male Sprague-Dawley rats. Toxicol Sci 200911164–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sati PC, Khaliq F, Vaney N, Ahmed T, Tripathi AK, Banerjee BD. Pulmonary function and oxidative stress in workers exposed to styrene in plastic factory: occupational hazards in styrene-exposed plastic factory workers. Hum Exp Toxicol 2011301743–1750 [DOI] [PubMed] [Google Scholar]
- 54.Sisto R, Botti T, Cerini L, et al. Oxidative stress biomarkers and otoacoustic emissions in humans exposed to styrene and noise. Int J Audiol 201655523–531 [DOI] [PubMed] [Google Scholar]
- 55.Uzma N, Kumar BS, Hazari MA. Exposure to benzene induces oxidative stress, alters the immune response and expression of p53 in gasoline filling workers. Am J Ind Med 2010531264–1270 [DOI] [PubMed] [Google Scholar]
- 56.Strafella E, Bracci M, Staffolani S, et al. Occupational styrene exposure induces stress-responsive genes involved in cytoprotective and cytotoxic activities. PLoS One 20138e75401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cawthon RM. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res 200937e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim S, Parks CG, Xu Z, et al. Association between genetic variants in DNA and histone methylation and telomere length. PLoS One 20127e40504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology 19991037–48 [PubMed] [Google Scholar]
- 60.Shrier I, Platt RW. Reducing bias through directed acyclic graphs. BMC Med Res Methodol 2008870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Slinker BK, Glantz SA. Multiple linear regression: accounting for multiple simultaneous determinants of a continuous dependent variable. Circulation 20081171732–1737 [DOI] [PubMed] [Google Scholar]
- 62.Breiman L, Friedman J, Olshen R, Stone C. Classification and Regression Trees 19842nd edPacific Grove, CA: Wadsworth [Google Scholar]
- 63.Lemon SC, Roy J, Clark MA, Friedmann PD, Rakowski W. Classification and regression tree analysis in public health: methodological review and comparison with logistic regression. Ann Behav Med 200326172–181 [DOI] [PubMed] [Google Scholar]
- 64.Loh W-Y. Classification and regression trees. Wiley Interdiscip Rev Data Min Knowl Discov 2011114–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fujita EM, Campbell DE, Zielinska B, Arnott WP, Chow JC. Concentrations of air toxics in motor vehicle-dominated environments. Res Rep Health Eff Inst 20111563–77 [PubMed] [Google Scholar]
- 66.Environmental Protection Agency. Air Toxic Emissions from On-road Vehicles in MOVES2014. Washington, DC: Office of Transportation and Air Quality; 2016. [Google Scholar]
- 67.Chen SC, Kao CM, Huang MH, et al. Assessment of genotoxicity of benzidine and its structural analogues to human lymphocytes using comet assay. Toxicol Sci 200372283–288 [DOI] [PubMed] [Google Scholar]
- 68.Makena P, Chung KT. Evidence that 4-aminobiphenyl, benzidine, and benzidine congeners produce genotoxicity through reactive oxygen species. Environ Mol Mutagen 200748404–413 [DOI] [PubMed] [Google Scholar]
- 69.Coluzzi E, Colamartino M, Cozzi R, et al. Oxidative stress induces persistent telomeric DNA damage responsible for nuclear morphology change in mammalian cells. PLoS One 20149e110963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wilbur S, Jones D, Risher J, et al. Toxicological Profile for 1,4-Dioxane 2012Atlanta, GA: Agency for Toxic Substances and Disease Registry; [PubMed] [Google Scholar]
- 71.Faroon O, Taylor J, Roney N, Fransen M, Bogaczyk S, Diamond G. Toxicological Profile for Carbon Tetrachloride 2005Atlanta, GA: Agency for Toxic Substances and Disease Registry [Google Scholar]
- 72.Toyooka T, Yanagiba Y, Suda M, Ibuki Y, Wang RS. 1,2-Dichloropropane generates phosphorylated histone H2AX via cytochrome P450 2E1-mediated metabolism. Toxicol Lett 201727260–67 [DOI] [PubMed] [Google Scholar]
- 73.Dioni L, Hoxha M, Nordio F, et al. Effects of short-term exposure to inhalable particulate matter on telomere length, telomerase expression, and telomerase methylation in steel workers. Environ Health Perspect 2011119622–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hou L, Wang S, Dou C, et al. Air pollution exposure and telomere length in highly exposed subjects in Beijing, China: a repeated-measure study. Environ Int 20124871–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Garcia E, Hurley S, Nelson DO, Gunier RB, Hertz A, Reynolds P. Evaluation of the agreement between modeled and monitored ambient hazardous air pollutants in California. Int J Environ Health Res 201424363–377 [DOI] [PubMed] [Google Scholar]
- 76.Kim S, Sandler DP, Carswell G, Weinberg CR, Taylor JA. Reliability and short-term intra-individual variability of telomere length measurement using monochrome multiplexing quantitative PCR. PLoS One 20116e25774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tarik M, Ramakrishnan L, Sachdev HS, et al. Validation of quantitative polymerase chain reaction with Southern blot method for telomere length analysis. Future Sci OA 20184FSO282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Montpetit AJ, Alhareeri AA, Montpetit M, et al. Telomere length: a review of methods for measurement. Nurs Res 201463289–299 [DOI] [PMC free article] [PubMed] [Google Scholar]


