Abstract
Objective
Nerve growth factor (NGF) has potential in spinal cord injury (SCI) therapy, but limited by the poor physicochemical stability and low ability to cross the blood spinal cord barrier. Novel heparin-poloxamer (HP) thermo-sensitive hydrogel was constructed to enhance the NGF regeneration on SCI.
Method
NGF-HP thermo-sensitive hydrogel was prepared and related characteristics including gelation temperature, rheological behavior and micromorphology were measured. Local NGF delivery to the injured spinal cord was achieved by in situ injection in the injured space. The cellular uptake of NGF-HP hydrogel was evaluated with PC12 cells in vitro. Pathologic characteristics and neuron regeneration effects on the SCI rats were studied to evaluate the enhanced therapy of NGF-HP hydrogel. Endoplasmic reticulum (ER) stress-induced apoptosis was analyzed to explore the related mechanism in SCI regeneration.
Results
NGF-HP hydrogel showed good morphology and stable bioactivity of NGF in vitro. NGF-HP hydrogel combined treatment significantly enhanced the efficiency of NGF cellular uptake (P<0.05) without obvious cytotoxicity. Significant improvements in both neuron functions and tissue morphology on the SCI rats were observed in NGF-HP hydrogel group. Compared with free HP hydrogel and NGF treatment groups, NGF-HP hydrogel group showed significant inhibition on the formation of glial scars in the extreme crushed rat SCI model. The neuroprotective effects of NGF-HP were related to the inhibition of chronic ER stress-induced apoptosis.
Conclusions
HP hydrogel combined with orthotopic injection technique might be an effective method to deliver NGF into the injured site, which will provide an effective strategy for SCI regeneration.
Keywords: spinal cord injury, nerve growth factor (NGF), thermosensitive hydrogel, nerve regeneration, controlled release
1. Introduction
Spinal cord injury (SCI) is a devastating condition that is characterized by extensive tissue degeneration and severe loss of function. As one of the most devastating traumas, SCI leads to sudden loss of sensory, motor, and autonomic function distal to the level of injury. Worldwidely, 15–40 new SCI cases occur per million people annually [1]. The primary mechanical injury causes immediate hemorrhage and ischemia, in turn causing hypoxia, inflammation, edema and ultimately widespread cell death. After spinal cord crushed injury, the initial traumatic injury to spinal cord tissues is followed by a long period of secondary damages including oxidative stress, inflammation, necrosis and apoptosis [2,3].
Current treatment includes decompression surgery, injury stabilization, secondary complications prevention and rehabilitation. However, neurological recovery is limited, and substantial neurological dysfunction and lifelong disability still afflicts most SCI patients [4]. Although there is no cure for SCI, advances in molecular medicine based on improved understanding of the pathophysiology of injury have yielded promising results in animal models [5]. These molecules are aimed at either protecting surviving tissue from degeneration or restoring function already lost. The reactive astrocytes are the main obstacle to neuron axon regeneration after SCI. In normal spinal cord, astrocytes can provide material support neuron metabolism, maintain intracellular environment and pH value of the dynamic balance. Astrocytes can also release neurotropic factor to adjust the nerve cells of a variety of functions such as growth, differentiation and metastasis [6–8]. After SCI, astrocytes are activated to become reactive astrocytes [9]. Compared with normal astrocytes, activated reactive astrocytes mainly express in morphology and molecular changes. Activated reactive astrocytes can also express some iconic proteins (such as acid protein, immature collagen glial fiber cells vimentin and nestin) near the injury, and raise some proteins which inhibit the growth of axons expression. All these effects alter the microenvironment of the injury and its surrounding, inhibit the growth of nerve cells and regeneration of axons [10,11].
In the case of SCI, the failure of axonal regeneration is partly resulted from the lack of neurotrophic factors [12,13], in addition to expression of axonal growth-inhibiting molecules and/or inflammatory reactions [14]. In particular, neurotrophic factors such as brain derived neurotrophic factor (BDNF) and nerve growth factor (NGF) have been reported to be beneficial for axonal regeneration when applied to the injury site of the spinal cord [11,15]. NGF is an important member of the neurotrophin family. NGF supports the survival and maintenance of peripheral sensory and autonomic neurons, during development and adult stages [16,17]. Exogenous NGF-administration in developing animals prevents or reduces peripheral neuropathies induced by chemical and surgical insult [18–20]. However, neurons axon regeneration is a slow process. As a macromolecular protein, NGF can not penetrate the blood spinal cord barrier (BSCB). Therefore, oral and intravenous administration are inaccessible for NGF delivery in SCI. Local epidural or intrathecal delivery by either bolus injection or indwelling catheter/minipump were reported for NGF delivery [21,22].Therefore, novel preparations of NGF are needed to effectively deliver NGF into the spinal cord and maintain sustained release for axon regeneration.
Hydrogels have good affinity and compatibility to biological tissue. With proper formula, hydrogels can load biological macromoleculars to realize controlled release in situ [23–25]. In order to overcome the technical bottlenecks in the treatment of SCI, we developed a novel copolymer---Heparin-Poloxamer (HP)[26], which not only has a good affinity to NGF but also prevents the degradation of protease. In addition, HP has a controlled phase transition according to the variation of temperature. In this study, novel heparin-poloxamer (HP) thermo-sensitive hydrogel was prepared to enhance the spinal cord regeneration of NGF.
2. Materials and methods
All experimental rats were raised in the same environment and randomly selected for each group. The neurobehavioral observation and neuroanatomical evaluation were performed by reviewers who were “blinded” to experimental conditions.
2.1. Materials
Dulbecco’ s modified Eagle’ s medium (DMEM) and fetal bovine serum (FBS) were purchased from Invitrogen (Carlsbad, CA, USA). Nerve growth factor (NGF) was ordered from Gelusite Biology Technology Company, Zhejiang, China. Poloxamer 407–grafted heparin copolymer was synthesized by our laboratory as previously reported [26]. All other chemicals were used as received without further purification. Glial fibrillary acidic protein (GFAP), Caspase-3 and CD31 antibodies were purchased from abcam. Goat anti-rabbit and anti-mouse IgG-HRP were purchased from abcam. All of the other reagents were purchased from Beyotime institute of Biotechnology (shanghai, China) unless otherwise specified.
2.2. Preparation of NGF-HP hydrogels
Heparin-poloxamer (HP) was prepared according to EDC/NHS method as described previously [27]. NGF-HP hydrogels containing different amounts of HP and NGF were prepared using the cold method[28]. In brief, lyophilized HP powder was mixed with NGF solution (phosphate buffered saline, pH=7.6) at 4 °C under gentle stirring. The mixture was kept in a refrigerator at 4℃ overnight until a clear solution was formed.
2.3. Micromorphology of NGF-HP hydrogel
Gelation temperature measurement and rheological behavior of NGF-HP hydrogels were measured in triplicate and the average value of each point was reported [29]. The micromorphology of the dehydrated NGF-HP hydrogels was observed by scanning electron microscope (SEM). The NGF-HP hydrogels were wiped on a copper sheet, and immersed into liquid nitrogen immediately. Then NGF-HP hydrogels were critical point dried by vacuum freeze dryer for 24h. The dehydrated specimens were cross-sectioned and sputter-coated with gold, and their surface morphology was observed in a scanning electron microscope (Hitachi, H-7500, Japan) [30,31].
2.4. Cell Viability Assay and axonal growth
PC12 cells were purchased from the Cell Storage Center of Wuhan University(Wuhan, China). PC12 Cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM, Invitrogen, Carlsbad, CA) supplemented with heat-inactivated 10% fetal bovine serum (FBS, Invitrogen, Carlsbad, CA), 5% horse serum, and antibiotics (100 units/ml penicillin, 100 μg/ml streptomycin), incubated in a humidified atmosphere containing 5% CO2 at 37°C. PC12 cells were seeded on 96-well plates(5×103 cells/well) for 24h and treated with different doses of Hydrogen peroxide solution (H2O2, 0, 50, 100, 200, 300, 400, 600 μmol/L). From the cells survival state, 400 μmol/L H2O2 concentration was selected for the subsequent experiments [Fig. S3].
For determining the protection effect of NGF-HP hydrogels on PC12 cells, various doses of NGF was added 12 h after the addition of H2O2 . After 12h different NGF-administration, the viability and proliferation of PC12 cells were measured with Cell Counting KIT-8 (CCK-8, Dojindo Laboratories Inc., Kumamoto, Japan). Then the cells were incubated in CCK-8 solution in a 5% CO2 incubator at 37 °C for 2 h. The absorbance was measured at 450 nm with a reference wavelength of 650 nm. Cell number was correlated with optical density [32]. The axonal growth of each well was observed and photographed under fluorescent microscopy (Nikon ECLPSE 80i, Nikon, Japan). Based on our previous experiment and a related study[33], 1.0mmol/L NGF (13×106 ng/ml NGF) solution was diluted to 2000ng/ml solution and then used for the preparation of NGF solution and NGF-HP hydrogels. After the treatment of H2O2 for 12h, the medium was replaced with fresh medium. Then NGF solution and NGF-HP hydrogels were added in PC12 wells respectively. According to Fig. S4, 10ul 2000ng/ml NGF/NGF-HP hydrogels were added into the medium (190ul) to achieve a final concentration of NGF at 100 ng /ml.
2.5. The uptake of FITC-HP hydrogel in vitro and in vivo
In order to evaluate the feasibility of in situ NGF-HP hydrogels injection into the spinal cord, FITC-dextran (will be simplified as “FITC” in the remaining text) with similar molecular weight to NGF was used as model drug in cell experiments. The preparation of FITC-HP hydrogels were as the same as “2.2 Preparation of NGF-HP hydrogels”. Two concentrations of FITC (100ng/ml and 200ng/ml) were used for free FITC group and FITC-HP group to evaluate the cellular uptake in vitro. PC12 cells were seeded on 6-well plates for 24h and treated with different FITC-administrated groups. At 12h after FITC-administration, the cellular uptake of FITC was observed by inverted fluorescence microscope.
In addition, the inhibition of NGF-HP hydrogels on cell apoptosis was evaluated. The cell apoptotic rates of SCI model cells in different NGF treatment groups were measured using a PI/Annexin V-FITC kit (Invitrogen, Carlsbad, CA, USA), then analyzed by FACScan flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA) .
To further detect the sustained release behavior of HP hydrogel, the fluorescence signals of FITC-HP hydrogel was also evaluated in vivo. The 100ng/ml FITC solution and FITC-HP hydrogel were injected in situ in normal rat spinal cords. The sustained fluorescence signals were observed under the ultraviolet lamp at 15min, 1h, 3h, 8h and 24h, respectively.
2.6. Animal model of SCI and NGF-HP hydrogels administration
Experiments were performed in accordance with Guide for the Care and Use of Laboratory Animals from National Institutes of Health and approved by the Animal Care and Use Committee of Wenzhou Medical University. Young adult female Sprague–Dawley rats about eight weeks (220–250 g) were purchased from the Animal Center of Chinese Academy of Sciences, Shanghai, China. Animals were maintained for at least 7d before the experiment in a temperature-regulated room (23°C-25°C) on a 12h light/dark cycle and free to water and food.
All the animals were anaesthetized by an intraperitoneal injection of 10% chloralic hydras (3.5ml/kg). After that rats were positioned on a cork platform. The skin was incised along the midline of back, and the vertebral column was exposed and a laminectomy was Roled in T9 segmental level vertebrae and moderate contusion injuries were performed using a vascular clip for 2 minutes (30g forces, Oscar, China)[34]. Control group animals received the same surgical procedures, but impaction was no effect on the spinal cord by the vascular clip.
Table 1 shows the designed groups for different pharmacodynamics study in rats. HP hydrogels (OI) /NGF solution (OI) /NGF-HP hydrogels (OI) were in a micro syringe by single dose orthotopic injection when the clip after 2 minutes and administered at a dose of 6μl (3μg/μl) through the micro syringe after SCI. Postoperative nursing involved the artificial emptying of the bladder, twice a day, until they restore bladder function and management of cefazolin sodium (50 mg/kg, i.p.). All experimental animals were received daily rehabilitation procedures, including passive mobilization of hind legs three times a day. Subsequently, the rats were executed at 1d, 3 d, 7d, 14d and 28d.
Table 1.
Designed groups for different pharmacodynamics study in rats
| Experimental Group (n=5 for each group) | Rat Model | Preparation | Route of Administration |
|---|---|---|---|
| a | Control | / | / |
| b | SCI | / | / |
| c | SCI | HP hydrogels | orthotopic injection(OI) |
| d | SCI | NGF solution | orthotopic injection(OI) |
| e | SCI | NGF-HP hydrogels | orthotopic injection(OI) |
Note: SCI stand for SCI rats; HP hydrogels(OI) 6μl; NGF solution(OI), 3 mg/mL, 6μl; NGF-HP hydrogels, 3 mg/mL, 6μl, respectively
2.7. Functional analysis
Using the Basso, Beattie, and Bresnahan (BBB) scoring method to assess open field locomotor function[35] at 0d, 3 d, 7d, 14d, 21d and 28d. Each hind limb was ranked by two blinded observers and concurrently videotaped. BBB scores range from 0 (no hind limb movement) to 21 (normal gait) and are used to assess functional improvement after injury and treatment. BBB was performed in uninjured animals to determine if the materials had any early detriment to normal motor function.
Via a testing apparatus, the inclined plane test was performed [36] at 0d, 3 d, 7d, 14d, 21d and 28d. The maximum angle at which a rat could retain its position for 5 sec without falling was recorded for each position, and averaged to obtain a single score for each animal.
2.8. Histology evaluation
After 28 days, the animals were anesthetized by an intraperitoneal injection of sodium pentobarbital (4 mg/100 g body weight), and perfused with 0.9% NaCl, followed by 4% paraformaldehyde in 0.01M phosphate buffered saline (PBS, PH=7.4). And the spinal cords from T8–T10 were retrieved, and stored in 4% paraformaldehyde, embedded in paraffin. The lesion epicenter stained with hematoxylin and eosin (HE) for HE staining and examined under a light microscope. One stained with Cresyl Violet for Nissl staining following the instruction. Unbiased stereological approaches were used to undertake all tissue recovery histology evaluation. Counting frames positioned in a systematic random manner throughout the entire counting area ensured that all locations had an equal probability of being sampled within the field regardless of shape, size or orientation. The area of the unbiased counting frame was predetermined to allow only 10–15 axon profiles to be sampled per counting frame. It was more accurate to sample more frames and count fewer profiles per frame, than sample fewer frames and count more profiles per frame. In each of the sections sampled, the counting frame moved across the section at regular predetermined x and y intervals. All measurements were made under×40 lens to reduce errors.
2.9. Anterograde tracer
Two weeks post-SCI, rats were anesthetized with chloral hydrate, and the procedures were performed as described [37]. In brief, after placing the animal in a stereotaxic apparatus (ZS-B\S, ZS Dichuang technique development Ltd., Beijing) the skin was incised in the midline to expose the skull. About 1μL of a mixture of dextran amine conjugated with Texas Red (10% BDA; MW 10,000; Invitrogen) was injected through a micro-syringe (10 μL) at 8 positions on the left hemisphere, approximately spanning the rostro-caudal extent of the hindlimb sensorimotor cortex. BDA delivery took 5min at each site. The micropipette tip remained in place for 20 s before withdrawal.
2.10. Immunohistochemistry
Immunohistochemistry staining was applied to detect the protein expression of GFAP in each experimental group. The transverse paraffin sections were incubated in 3% H2O2 for 15min and 80% carbinol for 30 min and then in blocking solution for 1 h at room temperature. Subsequently, the sections were incubated at 4°C overnight with the following primary antibodies: anti-GFAP (abcam; 1:5000), anti-Caspase-3 (abcam; 1:400), anti-CD31(abcam; 1:300). Then PBS washed three times. The paraffin sections were incubated with horseradish peroxidase-conjugated secondary antibodies for 2 h at 37°C. The reaction was stopped with 3, 3-diaminobenzidine (DAB). The results were imaged at a magnification of 400 using a Nikon ECLPSE 80i (Nikon, Japan). The optical densities and positive neuron numbers of GFAP was counted at least 6 randomly selected fields per sample. The density of GFAP-positive nerve fibers was the quantification of GFAP-positive area/the total area using the Image-Pro Plus software.
2.11. Western blot analysis
For the in vivo protein analysis, a spinal cord segment (0.5 cm length) at the contusion epicenter was dissected at 1d, 3d, 7d and 14d and soon stored at −80°C for western blotting. For protein extraction, the tissue was homogenized in modified RIPA buffer containing protease inhibitor cocktail (10μl/ml, GE Healthcare Biosciences, PA, USA). The complex was then centrifuged at 12,000 rpm and the supernatant obtained for protein assay. For endoplasmic reticulum (ER) stress model in vitro, PC12 cells were lysed in RIPA buffer with protease and phosphatase inhibitors. The extracts above were quantified with bicinchoninic acid (BCA) reagents (Thermo, Rockford, IL, USA). We separated proteins (50 μg) on a 11.5% gel and transferred them onto PVDF membrane (Bio-Rad, Hercules, CA, USA). The membrane was blocked with 5 % milk (Bio-Rad) in TBS with 0.05 % Tween 20 for 1h and incubated with the antibodies: C/EBP homology protein (CHOP) (1:300, Santa Cruz Biotechnology, CA, USA), GRP78 (1:300, Santa Cruz Biotechnology), caspase-12 (1:1000, Santa Cruz Biotechnology), in 5 % milk in TBS with 0.05% Tween 20 overnight. The membranes were washed with TBS for 3 times and treated with horseradish peroxidase-conjugated secondary antibodies for 1h at room temperature. Signals were visualized by ChemiDicTM XRS+ Imaging System (Bio-Rad), and band densities were quantified with Multi Gauge Software of Science Lab 2006 (FUJIFILM Corporation, Tokyo, Japan). We analyzed relative densities of the bands with Quantity One (version 4.5.2; Bio-Rad). Quantities of band densities were normalized using GAPDH.
2.12. Statistical analysis
The data were expressed as the mean ± SEM. One-way ANOVA and Student’s t-test or Kruskal-Wallis test were adopted for statistical comparison using the SAS 8.01 (1999–2000, SAS Institute Inc., Cary, NC, USA). The data difference was considered to be statistically significant when the P-value was less than 0.05.
3. Results
3.1. Micromorphology of NGF-HP hydrogel
NGF-HP hydrogel has a suitable gelation temperature for human body (≧ 37 °C, Fig. S1). Under SEM, NGF-HP hydrogel showed a web-like structure similar to the porous sponge (Fig. 1A), in which the inner pores of the hydrogel are interconnected. From characteristics observation, NGF-HP hydrogel maintains the thermosensitive nature as well as sponge-like scaffold structure, which are favorable for in situ administration, maintaining long time contact with injured spinal cord, accommodating NGF and controlling its release rate.
Fig. 1. Evaluation of HP hydrogels containing NGF or FITC in vitro, and behavior evaluation of NGF-HP hydrogel after in situ administration on SCI model rats.
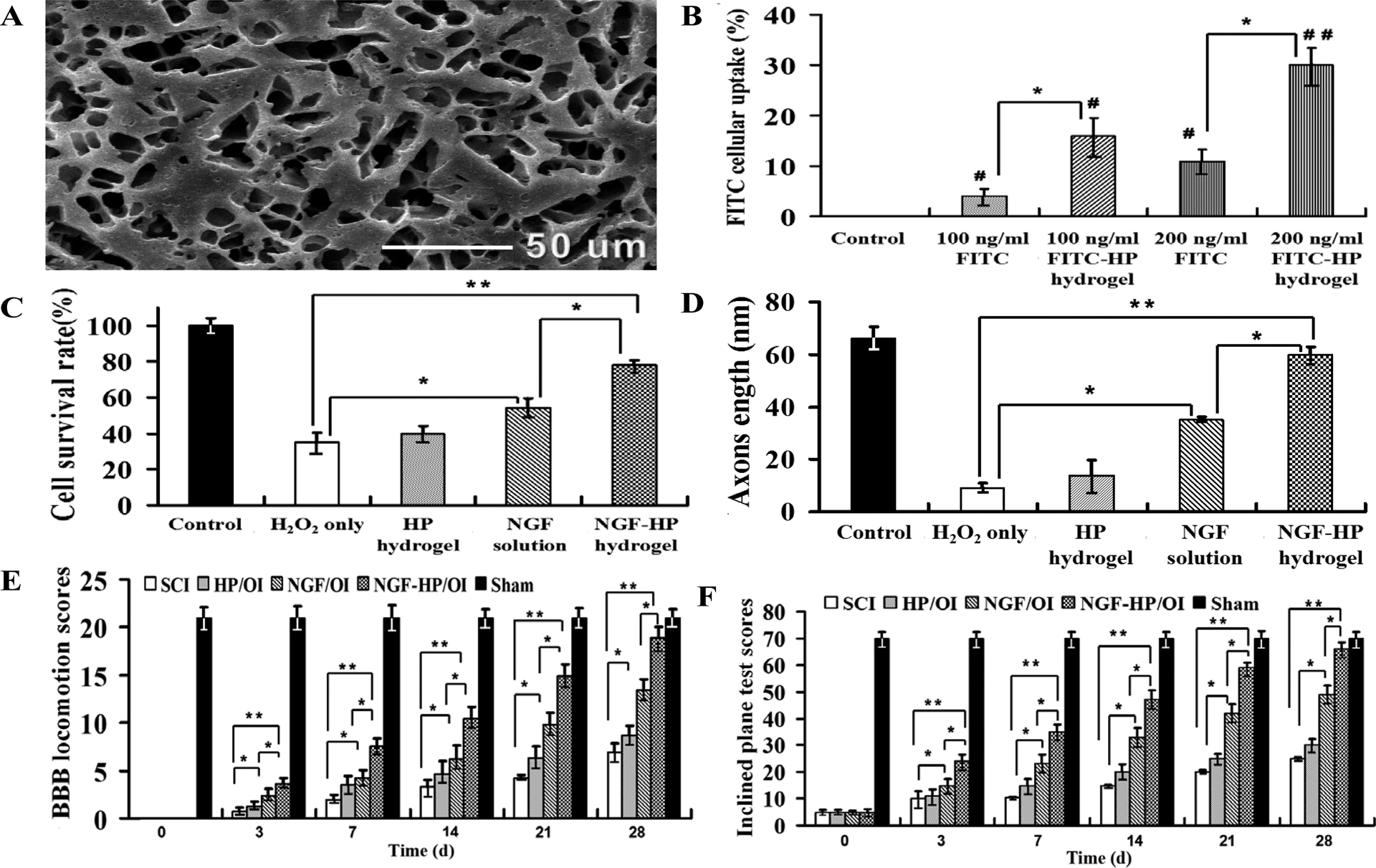
(A: SEM images of the lyophilized NGF-HP hydrogel. B: The efficiency of FITC cellular uptake in vitro. C: Cells viability of various groups in vitro. PC12 cells were induced by H2O2 before the treatments. *P<0.05, **P<0.01 vs. H2O2 group. D: Quantitative analysis the neurite length of H2O2 induced-PC12 cells after different treatments in vitro. E: BBB locomotion assessments of SCI rats at different time points in different groups. The score of control group was 21 points, means normal locomotion SCI group. HP hydrogel, NGF solution and NGF-HP hydrogel were injected in situ (OI). F: The inclined plane test scores of SCI rats in control group, SCI group, HP hydrogel (OI) group, NGF (OI) group and NGF-HP hydrogel (OI) group at different time points. All data are presented as Mean ± SD, n = 5; *P < 0.05 and **P < 0.01, determined by two-tailed student’s t-test.)
3.2. Cellular uptake
The cellular uptake of FITC by PC12 cell in vitro is evaluated by green fluorescence (Fig. S2). Only weak FITC signals were noted in the cells treated with 100 ng/ml FITC. It proved the poor cell permeability of FITC without HP hydrogel as the support carrier. In comparison, the signals were significantly increased in the 100 ng/ml FITC-HP hydrogel group (Fig. 1B, P<0.05). Further significantly increased FITC uptake in 200ng/ml FITC-HP hydrogel group was observed (Fig. 1B, P<0.05).
From the loaded fluorescence images in vivo (Fig. S5), FITC-HP hydrogel maintained much longer time than FITC after in situ administration. Even at 24h, there was a weak fluorescence signal in FITC-HP hydrogel administrated spinal cord. Both FITC-HP hydrogel images in vitro and in vivo proved the controlled-release behaviour of HP hydrogel for loaded macromolecules, which provided the basis for the application of NGF-HP hydrogel in SCI treatment.
3.3. PC12 cell survival rate and axons length
From the OD values of CCK-8 analysis, there was a downward trend of cell survival rate with the increasing concentration of H2O2 (Fig. S3A). From the result, 400 μmol/L H2O2 was selected to establish the cell model. The cell survival rates in NGF-administrated groups increased significantly, compared with H2O2 group (P < 0.05). Among the NGF-administrated groups, NGF-HP hydrogel group reached the highest value (78 ± 2.9 %), followed by HP hydrogel group (40 ± 4.1 %) and NGF group (54 ± 5.4 %) (Fig. 1C).
The neurite length of PC12 cells of various groups was shown in Fig. 1D and Fig. S3B. Compared with the control group, the neurite length of PC12 cells were decreased in 400 μmol/L H2O2 group (9±2.1 nm) (P < 0.05), but increased by treatment of HP hydrogel group (14 ± 5.6 nm) (P > 0.05). NGF-administrated groups showed positive effects on the neurite length of PC12 cells. NGF-HP hydrogel group showed the best neurite length of PC12 cells (60 ± 3.0 nm). The order of PC12 cell neurite length among NGF-administrated groups was the same as that of cell survival rate.
3.4. Motor function
BBB rating scale and inclined plane test in 28d after SCI were used to investigate functional recovery [38]. Hind legs were paralyzed immediately after SCI. From the observation of SCI group, the locomotor function of hind legs restored gradually with the experiment time increasing. There were significant differences in the locomotor function of hind legs among experimental groups (P>0.05, Fig.1E and 1F). HP hydrogel(OI) group showed little improvement on the recovery of hind leg function. NGF-HP hydrogel(OI) group showed the best recovery effect, followed with NGF(OI) group. The locomotor function of NGF-HP hydrogel(OI) group reached about the level of the control group at 28 d (P>0.05).
NGF-HP hydrogel(OI) group after injury significantly increased the hindlimb locomotor function, as assessed by BBB scores. The angle of incline was also significantly higher in NGF-HP hydrogel(OI) group, compared with the NGF(OI) group (P<0.05). Based on the BBB scores (Fig. 1E) and the inclined plane test scores (Fig. 1F), NGF-HP hydrogel (OI) group showed significantly higher values than NGF (OI) group (P<0.05), SCI group (P<0.01) and HP hydrogel(OI) group (P<0.01). However, NGF-HP hydrogel(OI) group and the control group showed little difference in these scores (P>0.05). From these results, NGF-HP hydrogel combined with in situ injection could effectively enhance the motor function recovery, neural protection and repairment after SCI.
3.5. HE staining and Nissl staining
HE staining results of the spinal cord samples from the experimental groups after 28d contusion were shown in Fig. 2A. The lesion center was characterized by the destruction of gray and white matter. The spinal cord neurons in the control group (Fig. 2A) had normal morphology (clear cell outline and cytoplasm with uniform nuclei). Compared with the control group, gray matter in SCI group (Fig. 2A) showed a large area of haemorrhage. Meanwhile, the neurons in the anterior horn of SCI group were shrunken or had pale homogenous cytoplasm, which were consistent with ischemic change.
Fig. 2. Results of pathological evaluation and staining of NGF-HP hydrogel in vitro.
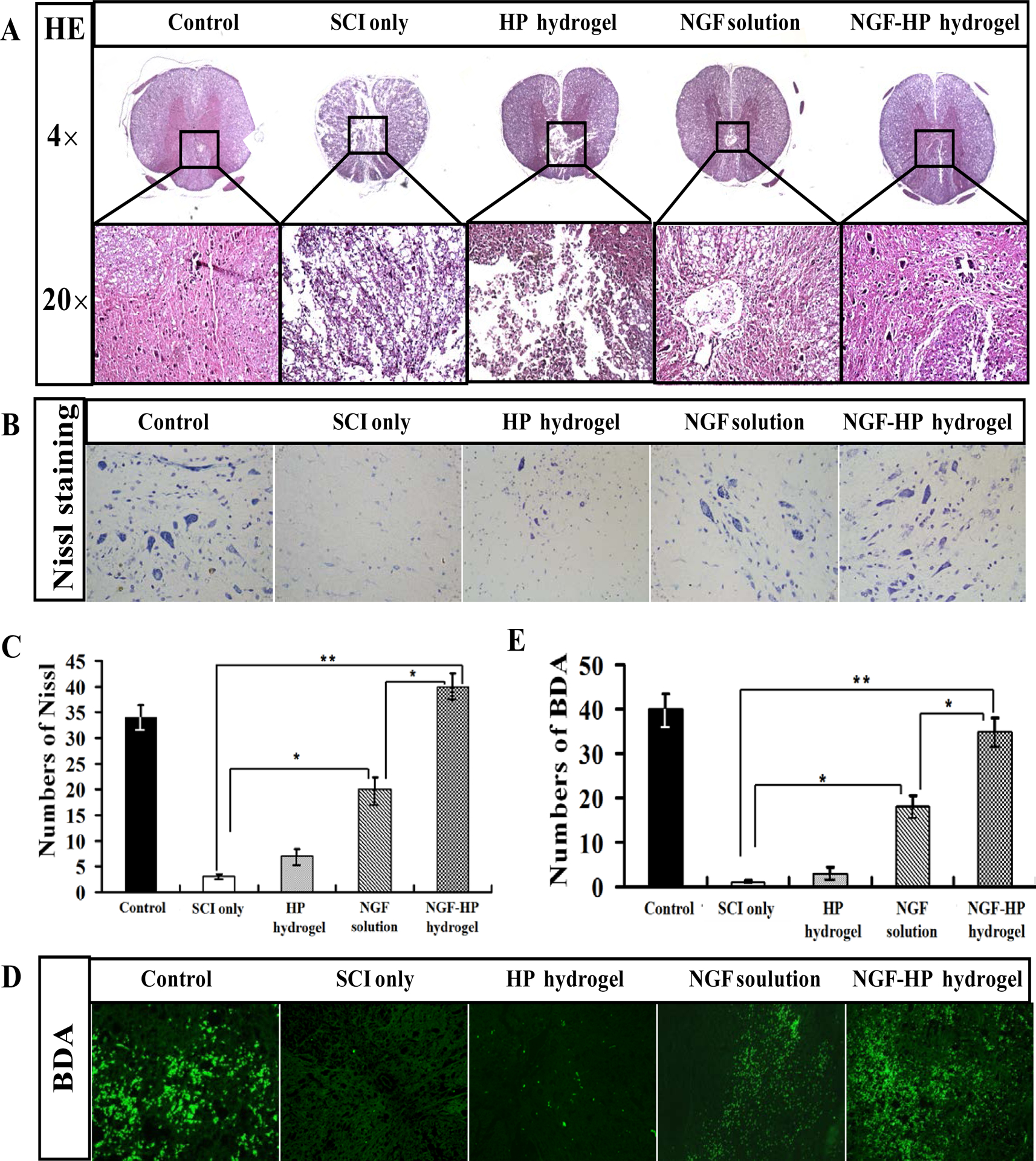
(A: HE staining of crosscutting results (40× and 200×) at 28days after SCI; B: Nissl staining of crosscutting results (400×) at 28days after SCI; C: Quantitative analysis of Nissl staining results at 28days after SCI; D: The expression of BDA protein in the immunohistochemical staining of crosscutting results (400X) on the 28th days by NGF-HP hydrogel treatment after SCI; E: Quantitative analysis of BDA results. HP hydrogel, NGF solution and NGF-HP hydrogel were injected in situ (OI). Data are presented as Mean ± SD, n = 5; *P < 0.05 and **P < 0.01, determined by two-tailed student’s t-test.)
Little improvement on tissue repairment were observed in both HP hydrogel (OI) group and SCI group (Fig. 2A ). In NGF (OI) group, however, tissue repairment in spinal gray matter was observed, including the recovery of nuclei and morphology, reduction of organization air conditioning. NGF-HP hydrogel (OI) group showed good effects on tissue repairment, similar to the control group and better than NGF(OI) group. These results further strengthen the neuroprotective efficacy of NGF under HP hydrogel form for SCI treatment.
Nissl staining showed similar results as HE staining. The Nissl bodies were decreased significantly at 28d after SCI, and increased by NGF treatment (P<0.05). Compared with the control group, SCI group showed significant changes in pathology. For example, the intumescence spinal cord anterior horn of the lateral decreased in the number of large and medium-sized neurons. The neurons in SCI rats lost normal morphology (cell outline was not clear ) and their survival rate decreased (Fig. 2B). From Nissl staining, the order of the enhanced number for Nissl bodies was: NGF-HP hydrogel(OI) group > NGF(OI) group > HP hydrogel(OI) group. Nissl staining further verified the results of HE staining. As shown in Fig. 2C, NGF-HP hydrogel group exerted much better restoration on spinal cord function than NGF(OI) group (P<0.05). All of these data indicated that NGF-HP hydrogel could maximize NGF’s effects on neuron protection and SCI recovery.
3.6. Anterograde tracer of BDA
As an effective anterograde and retrograde tracing neural tracer, biotinylated dextran amine (BDA) becomes the focus of SCI evaluation in recent years[39]. BDA positive cells were found in T12-L1 plane (showed green fluorescence, under the injured site) of the spinal cord in control group. However, BDA positive cells were not observed in the SCI group. All the NGF-administrated groups showed enhanced green fluorescence, compared with the SCI group (Fig. 2D, P < 0.05). The order of integral optical density (IOD) was: NGF-HP hydrogel (OI) group > NGF (OI) group > HP hydrogel (OI) group (Fig. 2E). Based on the results of BDA anterograde tracer, NGF-HP hydrogel significantly enhanced NGF’s therapeutic actions in the process of SCI treatment and regeneration of pathway.
3.7. Regeneration of neurons and axon
3.7.1. Immunohistochemical staining of GFAP and CD31
According to previous studies, GFAP is the hallmark intermediate filament protein in astrocytes, a main type of glial cells in the central nervous system. Therefore, GFAP marker was used to distinguish astrocytes from other glial cells during nerve development in our study [40] . As an indicator of astrocytes, GFAP was investigated to further evaluate the protective effects of NGF-HP hydrogel on neuron survival. The groups treated with NGF showed significant decrease in the density of GFAP staining surrounding the lesion site, compared with the SCI group (Fig. 3A). From analysis of the area of GFAP staining (Fig. 3B), there was little difference in astrogliosis between NGF-HP hydrogel group and the control group (P>0.05). The numbers of GFAP positive cells at 28d in the NGF-HP hydrogel group were much lower than those of other groups (Fig. 3B, P<0.01). NGF-HP hydrogel treatment, therefore, prevented the accumulation of GFAP-positive astrocytes at the lesion site. NGF-HP hydrogel group showed the minimum value among NGF-treated groups (P<0.05), indicating that NGF-HP hydrogel could decrease the area of tissue occupied by reactive astrocytes following injury.
Fig. 3. Immunohistochemical staining of GFAP and CD31.
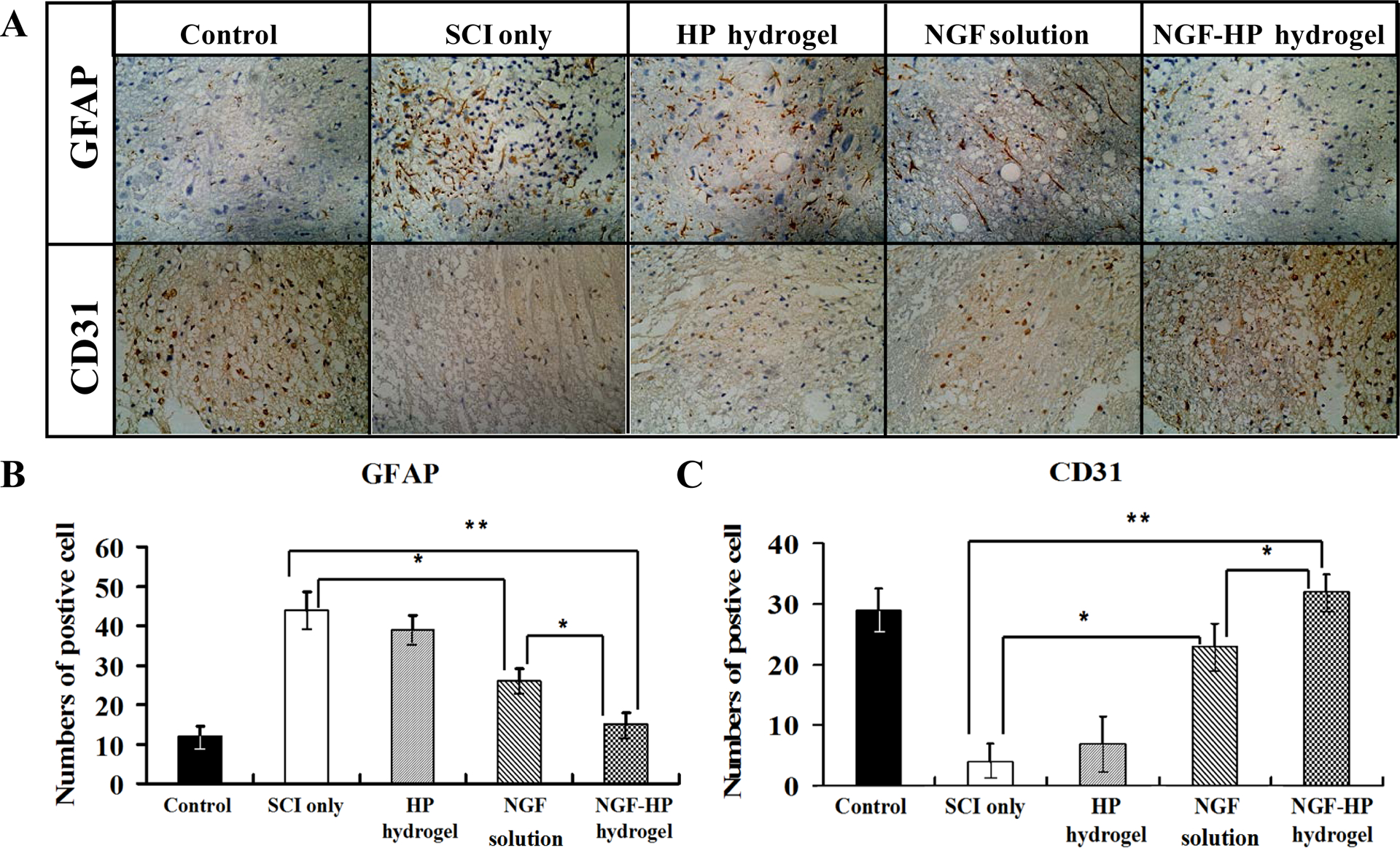
(A: The expression of GFAP and CD31 in the immunohistochemical staining of crosscutting results (400X) on the 28th days by NGF-HP hydrogel treatment after SCI; B: Quantitative analysis of GFAP positive cells of the immunohistochemistry results; C: Quantitative analysis of CD31 positive cells of the immunohistochemistry results. HP hydrogel, NGF solution and NGF-HP hydrogel were injected in situ (OI). Data are presented as Mean ± SD, n = 5; *P < 0.05 and **P < 0.01, determined by two-tailed student’s t-test.)
CD31 is considered as a crucial component of the effective regenerative response in the nervous system. As shown in Fig. 3A, the numbers of CD31 positive cells were obviously increased in the NGF administration groups compared with the SCI group (Fig. 3C, P<0.05). Furthermore, NGF-HP hydrogel group showed significantly larger density area and much more numbers of CD31 positive cells than the NGF(OI) group (P<0.05).
From immunohistochemistry staining of GFAP and CD31, all groups treated by NGF showed somewhat improvement on the regeneration of neurons and axon. Among NGF administrated groups, NGF-HP hydrogel group exhibited the best therapeutic action, which was consistent with the results of motor function, BDA anterograde tracer and results of pathology.
3.7.2. Apoptosis in vitro and in vivo
Cell apoptosis in vitro was analyzed by Flow cytometry with PI/Annexin V-FITC staining (Fig. 4A). Caspase-3 activation is a classic indicator of apoptosis. The Caspase-3 immunohistochemistry was used to evaluate the tissue apoptosis in vivo (Fig. 4B). As shown in Fig. 4A, NGF inhibited the apoptosis induced by H2O2 in PC-12 cells. As shown in Fig. 4B, Caspase-3 positive cells in the SCI group increased significantly at 28d after SCI. The numbers of positive cells at 28d in the NGF-HP hydrogel group were significantly lower than those of other NGF-administrated groups (P< 0.01). As shown in Fig. 4D, the order of immunohistochemistry results on Caspase-3 was consistent with the results of PC12 cell apoptosis analysis in vitro (Fig. 4C). NGF-HP hydrogel group showed the lowest values in apoptosis in vitro and in vivo, which were similar to those of the control group (P > 0.05).
Fig. 4. Apoptosis results of PC12 cells in vitro and SCI rats in vivo after NGF-HP hydrogel treatment.
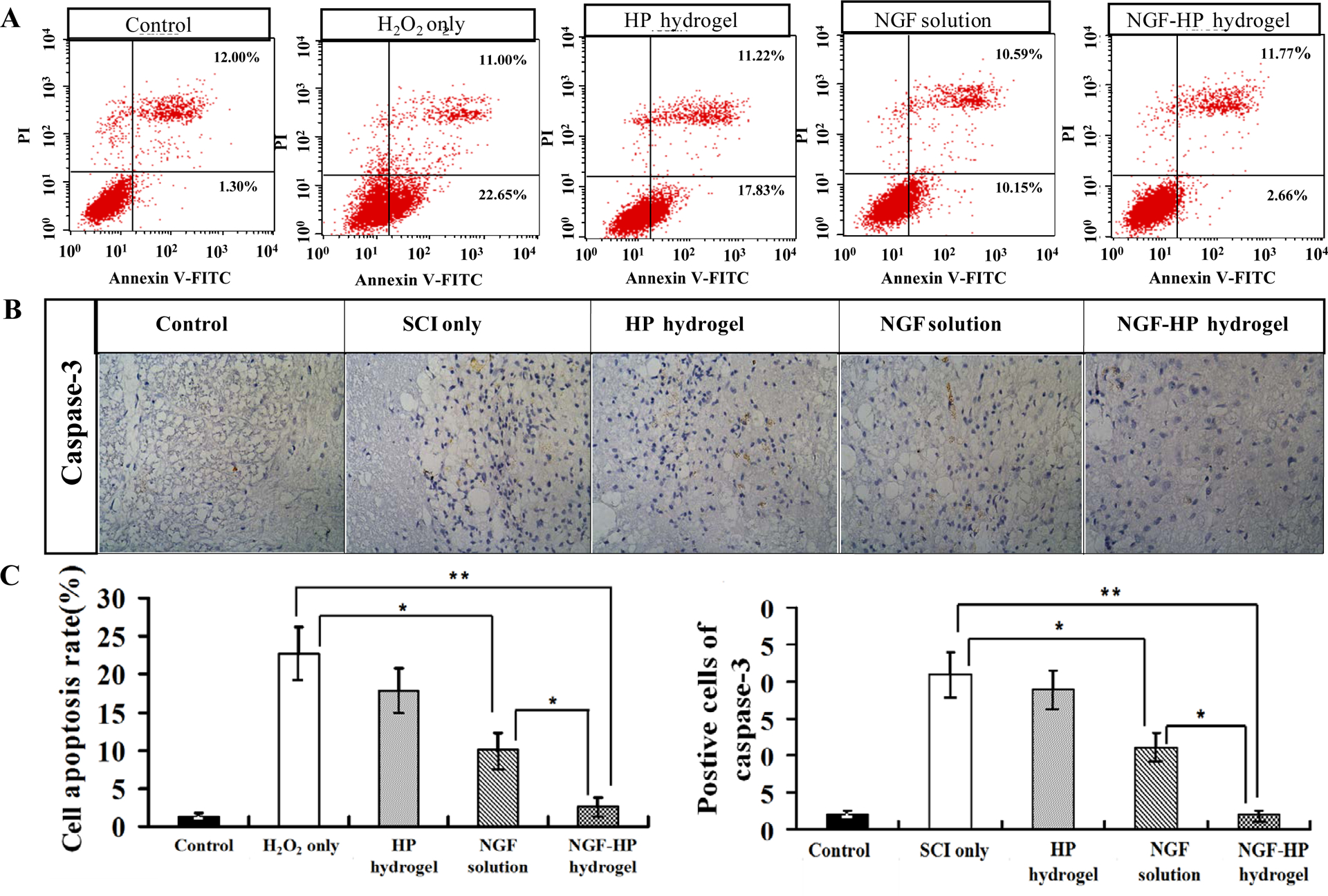
(A: FACScan result of PI/Annexin V-FITC staining for cell apoptosis analysis; B: The expression of Caspase-3 in the immunohistochemical staining of crosscutting results (400×) on the 28th days by NGF-HP hydrogel treatment after SCI; C: Statistical flow cytometry result of apoptosis rate in PC12 cells treated with H2O2 and NGF-HP hydrogel; D: Quantitative analysis of caspase-3 positive cells of the immunohistochemistry. HP hydrogel, NGF solution and NGF-HP hydrogel were injected in situ (OI). Data are presented as Mean ± SD, n = 5; *P < 0.05 and **P < 0.01, determined by two-tailed student’s t-test.)
3.7.3. NGF-HP inhibits ER stress signaling pathway
To illustrate whether the molecular mechanism of NGF-HP is related to the regulation of ER stress, the protein expression of ER stress-induced apoptosis were detected by Western blot. As shown in Fig. 5A, ER stress-induced apoptosis proteins (CHOP, GRP78 and caspase-12) reduced by NGF-HP administration compared with the SCI group after 28d contusion. The protein expression was also detected by Western blot in H2O2 induced PC-12 cells, it was found that the levels of CHOP, GRP78 and caspase-12 protein decreased by the treatment of NGF-HP, compared with the SCI group after 7d contusion (Fig. 5 B). All of these data demonstrated the protective role of NGF-HP in the ER stress-induced apoptosis in vitro and in vivo.
Fig. 5. NGF-HP inhibits the expressions of ER stress-induced apoptosis response proteins, GRP78, CHOP and caspase-12 in SCI rats (A) and H2O2 induced PC-12(B).
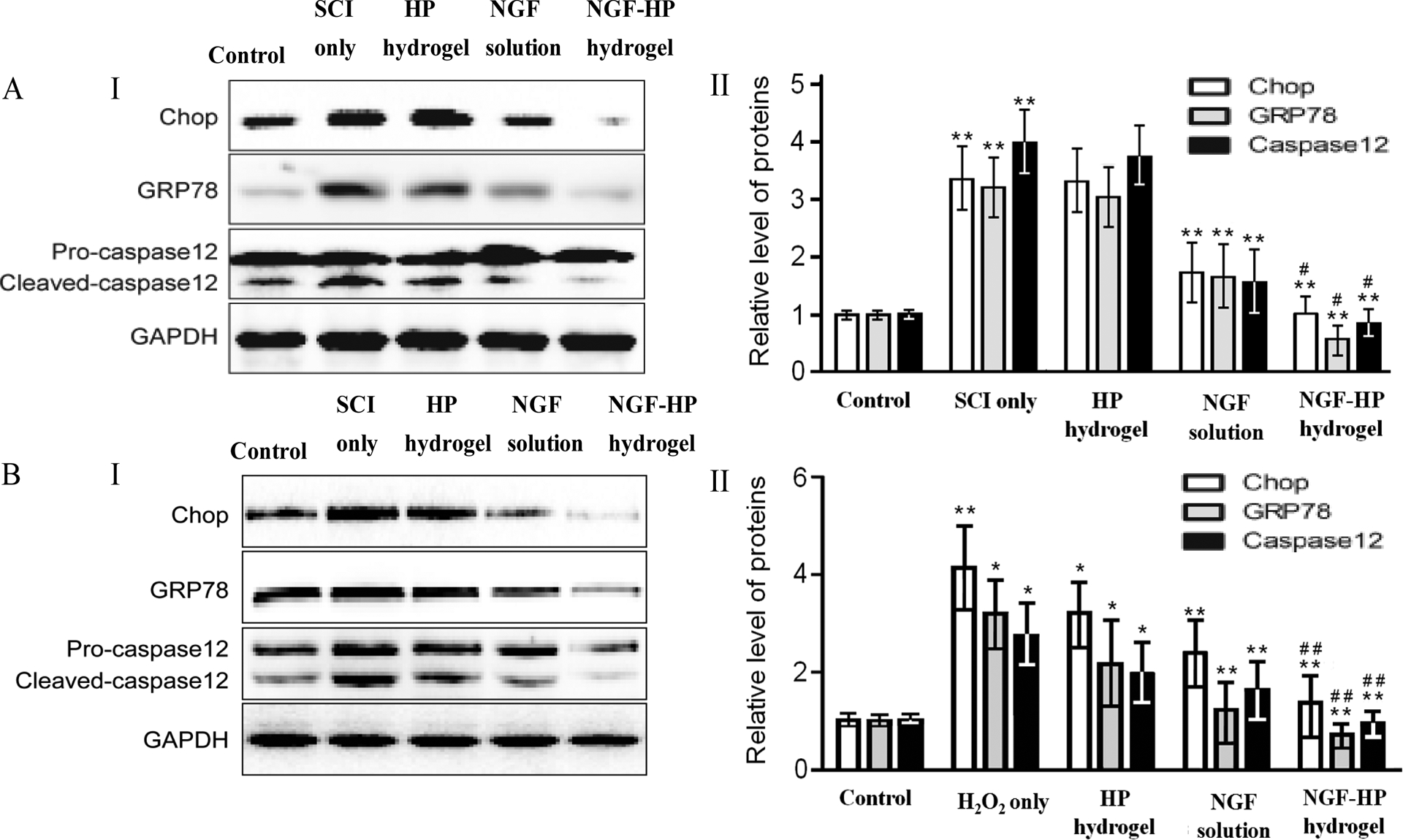
(I: Protein expressions of GRP78, CHOP and caspase-12 for NGF-HP treatment in vivo. GAPDH was used as the loading control and for band density normalization. II: The optical density analysis of GRP78, CHOP, and caspase-12 protein in vitro. HP hydrogel, NGF solution and NGF-HP hydrogel were injected in situ (OI). Data are presented as Mean ± SD, n = 5; **P < 0.01 versus a or b group, # P < 0.05 versus d group, determined by two-tailed student’s t-test.)
4. Discussion
It is well known that SCI results in a loss of neural tissues and formation of cystic cavities that inhibit regenerating axons from crossing the lesion area. The hostile microenvironment negatively influences the preservation and regeneration of neural tissues [41]. In order to enhance therapeutic efficacy, many studies have reported benefits from remodeling the extracellular matrix to provide a suitable scaffold for regeneration of cells and axons [42]. Conventional hydrogels (such as: chitosan, gelation, poloxamer 407, hydroxyethyl methacrylate, etc) are not suitable for wrapping NGF for the treatment of SCI because of the poor affinity to NGF and insufficiency in satisfying the particular needs of SCI. In addition, conventional hydrogels lack suitable mechanical strength at normal body temperature, which may cause spinal cord compression injury to the wound again after administration. In order to overcome the technical bottlenecks in SCI treatment, we prepared a novel Heparin-poloxamer (HP) hydrogel which had a high affinity with NGF and a controlled phase transition temperature suitable for in situ administration [43]. In characteristics experiment, HP hydrogel showed a liquid state in room temperature. Under normal body temperature, HP hydrogel can be transited into a three-dimensional network structure. From in vitro and in vivo study, NGF-HP hydrogel reduced the formation of glial scar by inhibiting the generation of reactive astrocytes after SCI. NGF-HP hydrogel also promoted axon regeneration by reducing reactive astrocytes secretion and inhibit axon regeneration protein (such as chondroitin sulfate proteoglycan and collagen fiber acidic protein). Furthermore, NGF-HP hydrogel increased newly formed blood capillaries for SCI regeneration. Therefore, the potential of NGF on neuron axon regeneration for SCI can be realized under in situ administration of NGF-HP hydrogel [44, 45].
Certain studies have suggested that the ER stress-signal may have a direct role in promoting cell death in neuronal injury diseases. CHOP plays a critical role in ER stress-induced apoptosis, and it is believed to play a central role in ER stress-induced cell death. Following spinal cord injury, rats deficient in CHOP signaling show increased spared white matter and enhanced locomotor recovery by 6 weeks. In this study, we demonstrated that ER stress-induced apoptosis was involved in the responses of SCI, the levels of related proteins including CHOP, GRP78 and caspase-12 protein increased obviously and decreased by treatment of NGF-HP after 28d contusion in vivo, which also been detected in PC12 cell injury models induced by H2O2, suggested the neuroprotective effect of NGF-HP was related to the inhibition of chronic ER stress-induced apoptosis.
Given the exciting results obtained in the present study, there are limitations that need to be addressed. First, the low number of samples limited the optimization dose of experimental parameters for NGF. We also noticed that the improved function and regeneration changes in NGF-HP hydrogel group still did not reach the levels of the control group, which may be related to the short intervention (only 4 weeks after treatment). In addition, the complex mechanism of axon regeneration in SCI model remains to be explored before in situ injection technique can be brought from bench to bedside. Therefore, increasing dosing regimens and intervention time for NGF-HP hydrogel administration should be considered in the future studies. Molecular mechanisms and signaling pathways of NGF in SCI therapy and other potential adverse effects of NGF needs further investigation. While the goal of this study was to demonstrate safety, pathophysiology and short-term efficacy of this treatment, further studies for the long-term effect of single versus repeated treatments using this approach are warranted. Furthermore, alternative approaches, such as to deliver NGF genes or NGF combine cell transplantation to the injury to achieve long term expression of NGF, are also promising strategies.
Supplementary Material
Acknowledgements
This research was supported by the National Natural Science Funds of China (Grant No. 81360195, 81301982 and 81302726). Zhejiang Provincial Foundation for Health Department (Grant No. 2015ZDA023). Science and Technology Program of Guangzhou (201508020001).
Abbreviations:
- SCI
Spinal cord injury
- NGF
Nerve growth factor
- ER
endoplasmic reticulum
- HP
Heparin-poloxamer
- NGF-HP
Nerve growth factor-heparin poloxamer
- BBB
Basso, Beattie, and Bresnahan
- BDA
Biotinylated dextran amine
- GFAP
Glial fibrillary acidic protein
Footnotes
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
References:
- [1].Ackery A, Tator C, Krassioukov A. A global perspective on spinal cord injury epidemiology. J Neurotrauma. 2004; 21(10): 1355–1370. [DOI] [PubMed] [Google Scholar]
- [2].Yong CS, Choi JS, Quan QZ, Rhee JD, Kim CK, Lim SJ, Kim KM, Oh PS, Choi HG. Effect of sodium chloride on the gelation temperature, gel strength and bioadhesive force of poloxamer gels containing diclofenac sodium. Int J Pharm. 2001;226(1–2):195–205. [DOI] [PubMed] [Google Scholar]
- [3].Moon YJ, Lee JY, Oh MS, Pak YK, Park KS, Oh TH, Yune TY. Inhibition of inflammation and oxidative stress by Angelica dahuricae radix extract decreases apoptotic cell death and improves functional recovery after spinal cord injury. J Neurosci Res. 2012;90(1):243–256. [DOI] [PubMed] [Google Scholar]
- [4].Tator CH. Strategies for recovery and regeneration after brain and spinal cord injury. Inj Prev. 2002;8(Suppl. 4):V33–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Girard C, Bemelmans AP, Dufour N, Mallet J, Bachelin C, Nait-Oumesmar B, Baron-Van Evercooren A, Lachapelle F. Grafts of brain-derived neurotrophic factor and neurotrophin 3-transduced primate Schwann cells lead to functional recovery of the demyelinated mouse spinal cord. J Neurosci. 2005;25(35):7924–7933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bozoyan L, Khlghatyan J, Saghatelyan A. Astrocytes control the development of the migration-promoting vasculature scaffold in the postnatal brain via VEGF signaling. J Neurosci. 2012;32:1687–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Powell EM, Geller HM. Dissection of astrocyte-mediated cues in neuronal guidance and process extension. Glia.1999;26:73–83. [DOI] [PubMed] [Google Scholar]
- [8].Ullian EM, Sapperstein SK, Christopherson KS, Barres BA. Control of synapse number by glia. Science, 2001;291:657–661. [DOI] [PubMed] [Google Scholar]
- [9].McKeon RJ, Hoke A, Silver J. Injury-induced proteoglycans inhibit the potential for laminin-mediated axon growth on astrocytic scars. Exp Neurol. 1995;136:32–43. [DOI] [PubMed] [Google Scholar]
- [10].Profyris C1, Cheema SS, Zang D, Azari MF, Boyle K, Petratos S. Degenerative and regenerative mechanisms governing spinal cord injury. Neurobiol Dis. 2004;15(3):415–436. [DOI] [PubMed] [Google Scholar]
- [11].Reier PJ, Houle JD. The glial scar: its bearing on axonal elongation and transplantation approaches to CNS repair. Adv Neurol. 1988;47:87–138. [PubMed] [Google Scholar]
- [12].Widenfalk J1, Lundströmer K, Jubran M, Brene S, Olson L. Neurotrophic factors and receptors in the immature and adult spinal cord after mechanical injury or kainic acid. J Neurosci. 2001;21(10):3457–3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hashimoto M1, Nitta A, Fukumitsu H, Nomoto H, Shen L, Furukawa S. Inflammation-induced GDNF improves locomotor function after spinal cord injury. Neuroreport. 2005;16(2):99–102. [DOI] [PubMed] [Google Scholar]
- [14].Franzen R1, Schoenen J, Leprince P, Joosten E, Moonen G, Martin D. Effects of macrophage transplantation in the injured adult rat spinal cord: a combined immunocytochemical and biochemical study. J Neurosci Res. 1998;51(3):316–327. [DOI] [PubMed] [Google Scholar]
- [15].Menei P1, Montero-Menei C, Whittemore SR, Bunge RP, Bunge MB. Schwann cells genetically modified to secrete human BDNF promote enhanced axonal regrowth across transected adult rat spinal cord. Eur J Neurosci. 1998;10(2):607–621. [DOI] [PubMed] [Google Scholar]
- [16].Barde YA. The nerve growth factor family. Prog Growth Factor Res. 1990; 2: 237–248. [DOI] [PubMed] [Google Scholar]
- [17].Levi-Montalcini R The nerve growth factor 35 years later. Science. 1987; 237: 1154–1162. [DOI] [PubMed] [Google Scholar]
- [18].Abe T, Morgan DA, Gutterman DD. Protective role of nerve growth factor against postischemic dysfunction of sympathetic coronary innervation. Circulation. 1997; 95: 213–220 [DOI] [PubMed] [Google Scholar]
- [19].Aloe L, Levi-Montalcini R. Nerve growth factor induced overgrowth of axotomized superior cervical ganglia in neonatal rats. Similarities and differences with NGF effects in chemically axotomized sympathetic ganglia. Arch Ital Biol. 1979; 117: 287–307 [PubMed] [Google Scholar]
- [20].Apfel SC, Arezzo JC, Lipson L, Kessler JA. Nerve growth factor prevents experimental cisplatin neuropathy. Ann Neurol. 1992; 31: 76–80 [DOI] [PubMed] [Google Scholar]
- [21].Rabchevsky AG, Fugaccia I, Fletcher-Turner A, Blades DA, Mattson MP, Scheff SW. Basic fibroblast growth factor (bFGF) enhances tissue sparing and functional recovery following moderate spinal cord injury. J Neurotrauma. 1999;16(9):817–830. [DOI] [PubMed] [Google Scholar]
- [22].Schwab ME. Nogo and axon regeneration. Curr Opin Neurobiol. 2004;14 (1):118–124. [DOI] [PubMed] [Google Scholar]
- [23].Brandl F, Hammer N, Blunk T, Tessmar J, Goepferich A. Biodegradable hydrogels for time-controlledrelease of tethered peptides or proteins. Biomacromolecules. 2010;11(2):496–504. [DOI] [PubMed] [Google Scholar]
- [24].Yamamoto M, Ikada Y, Tabata Y. Controlled release of growth factors based on biodegradation of gelatin hydrogel. J Biomater Sci. 2001;12:77–88. [DOI] [PubMed] [Google Scholar]
- [25].Meyvis T, De Smedt S, Stubbe B, Hennink W, Demeester J. On the release of proteins from degrading dextran methacrylate hydrogels and the correlation with the rheologic properties of the hydrogels. Pharm Res. 2001; 18(11):1593–1599. [DOI] [PubMed] [Google Scholar]
- [26].Zhao YZ, Lv HF, Lu CT, Chen LJ, Lin M, Zhang M, Jiang X, Shen XT, Jin RR, Cai J, Tian XQ, Wong HL. Evaluation of a novel thermosensitive heparin-poloxamer hydrogel for improving vascular anastomosis quality and safety in a rabbit model. PLoS One. 2013;8(8):e73178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Yoo MK, Cho KY, Song HH, Choi YJ, Kwon JW, Kim MK, Lee JH, Wee WR, Cho CS. Release of ciprofloxacin from chondroitin 6-sulfate-graft-poloxamer hydrogel in vitro for ophthalmic drug delivery. Drug Dev Ind Pharm. 2005;31(4–5):455–463. [DOI] [PubMed] [Google Scholar]
- [28].Yong CS, Choi JS, Quan QZ, Rhee JD, Kim CK, Lim SJ, Kim KM, Oh PS, Choi HG. Effect of sodium chloride on the gelation temperature, gel strength and bioadhesive force of poloxamer gels containing diclofenac sodium. Int J Pharm. 2001;226(1–2):195–205. [DOI] [PubMed] [Google Scholar]
- [29].Niu G, Zhang H, Song L, Cui X, Cao H, Zheng Y, Zhu S, Yang Z, Yang H. Thiol/acrylate-modified PEO-PPO-PEO triblocks used as reactive and thermosensitive copolymers. Biomacromolecules. 2008;9(10):2621–2628. [DOI] [PubMed] [Google Scholar]
- [30].Park KM, Lee SY, Joung YK, Na JS, Lee MC, Park KD. Thermosensitive chitosan-Pluronic hydrogel as an injectable cell delivery carrier for cartilage regeneration. Acta Biomater. 2009;5(6):1956–1965. [DOI] [PubMed] [Google Scholar]
- [31].Tan H1, Ramirez CM, Miljkovic N, Li H, Rubin JP, Marra KG. Thermosensitive injectable hyaluronic acid hydrogel for adipose tissue engineering. Biomaterials. 2009;30(36):6844–6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Yin Z, Chen X, Chen JL, Shen WL, Hieu NT, Gao L, et al. The regulation of tendon stem cell differentiation by the alignment of nanofibers. Biomaterials 2010;31(8):2163–2175. [DOI] [PubMed] [Google Scholar]
- [33].Zhang H, Wu F, Kong X, Yang J, Chen H, Deng L, Cheng Y, Ye L, Zhu S, Zhang X, Wang Z, Shi H, Fu X, Li X, Xu H, Lin L1, Xiao J. Nerve growth factor improves functional recovery by inhibiting endoplasmic reticulum stress-induced neuronal apoptosis in rats with spinal cord injury. J Transl Med. 2014;12:130–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zhang HY, Wang ZG, Wu FZ, Kong XX, Yang J, Lin BB, Zhu SP, Lin L, Gan CS, Fu XB, Li XK, Xu HZ, Xiao J. Regulation of autophagy and ubiquitinated protein accumulation by bFGF promotes functional recovery and neural protection in a rat model of spinal cord injury. Mol Neurobiol. 2013;48(3):452–464. [DOI] [PubMed] [Google Scholar]
- [35].Dinh P, Hazel A, Palispis W, Suryadevara S, Gupta R, Diaz-Ruiz A. Functional assessment after sciatic nerve injury in a rat model. Neurosci Lett. 1999; 266:61–64. [DOI] [PubMed] [Google Scholar]
- [36].Rivlin AS, Tator CH. Objective clinical assessment of motor function after experimental spinal cord injury in the rat. J Neurosurg.1977;47: 577–581. [DOI] [PubMed] [Google Scholar]
- [37].Weidner N, Grill RJ, Tuszynski MH. Elimination of basal lamina and the collagen “scar” after spinal cord injury fails to augment corticospinal tract regeneration. Exp Neurol. 1999;160(1):40–50. [DOI] [PubMed] [Google Scholar]
- [38].Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12: 1–21. [DOI] [PubMed] [Google Scholar]
- [39].Hambly MF, Wiltse LL, Raghavan N, Schneiderman G, Koenig C. The transition zone above a lumbosacral fusion. Spine (Phila Pa 1976). 1998;23(16):1785–1792. [DOI] [PubMed] [Google Scholar]
- [40].Hol EM, Pekny M. Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system. Curr Opin Cell Biol. 2015;32:121–130. [DOI] [PubMed] [Google Scholar]
- [41].Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. [DOI] [PubMed] [Google Scholar]
- [42].Teng YD, Lavik EB, Qu X, Park KI, Ourednik J, Zurakowski D, et al. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc Nat Acad Sci USA. 2002;99:3024–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Tian JL, Zhao YZ, Jin Z, Lu CT, Tang QQ, Xiang Q, Sun CZ, Zhang L, Xu YY, Gao HS, Zhou ZC, Li XK, Zhang Y. Synthesis and characterization of Poloxamer 188-grafted heparin copolymer. Drug Dev Ind Pharm. 2010;36(7):832–838. [DOI] [PubMed] [Google Scholar]
- [44].Deng LX, Hu JG, Liu NK, Wang XF, George MS, Wen XJ, Xu XM. GDNF reverses the inhibitory properties of reactive astrocytes allowing robust axonal regeneration through Schwann Cell-seeded guidance channels after spinal cord injury. Exp Neurol. 2011; 6, 229(2):238–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zhang L, Ma Z, Smith GM, Wen X, Pressman Y, Wood PM, Xu XM. GDNF-enhanced axonal regeneration and myelination following spinal cord injury is mediated by primary effects on neurons. Glia. 2009;57(11):1178–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


