Abstract
Aims
The most frequent indication for revision surgery in total hip arthroplasty (THA) is aseptic loosening. Aseptic loosening is associated with polyethylene liner wear, and wear may be reduced by using vitamin E-doped liners. The primary objective of this study was to compare proximal femoral head penetration into the liner between a) two cross-linked polyethylene (XLPE) liners (vitamin E-doped (vE-PE)) versus standard XLPE liners, and b) two modular femoral head diameters (32 mm and 36 mm).
Methods
Patients scheduled for a THA were randomized to receive a vE-PE or XLPE liner with a 32 mm or 36 mm metal head (four intervention groups in a 2 × 2 factorial design). Head penetration and acetabular component migration were measured using radiostereometric analysis at baseline, three, 12, 24, and 60 months postoperatively. The Harris Hip Score, University of California, Los Angeles (UCLA) Activity Score, EuroQol five-dimension questionnaire (EQ-5D), and 36-Item Short-Form Health Survey questionnaire (SF-36) were assessed at baseline, three, 12, 36, and 60 months.
Results
Of 220 screened patients, 127 were included in this study. In all, 116 received the allocated intervention, and 94 had their results analyzed at five years. Head penetration was similar between liner materials and head sizes at five years, vE-PE versus XLPE was -0.084 mm (95% confidence interval (CI) -0.173 to 0.005; p = 0.064), and 32 mm versus 36 mm was -0.020 mm (95% CI -0.110 to 0.071; p = 0.671), respectively. No differences were found in acetabular component migration or in the patient-reported outcome measures.
Conclusion
No significant difference in head penetration was found at five years between vE-PE and XLPE liners, nor between 32 mm and 36 mm heads.
Cite this article: Bone Joint J 2020;102-B(10):1303–1310.
Keywords: Vitamin E, RSA, Hip
Introduction
The long-term survival of total hip arthroplasties (THAs) is around 80% to 90% after 20 years depending on diagnosis and age.1-4 Implant failure is primarily caused by aseptic loosening, which is associated with wear debris from polyethylene liners.1,5,6 A significant reduction in wear and risk of osteolysis can be achieved by using cross-linked polyethylene (XLPE) liners instead of ultra high molecular weight polyethylene (UHMWPE) liners for metal-on-polyethylene THAs.7-9
To achieve cross-linking, the polyethylene is subjected to radiation. This process produces unwanted free radicals. These are then reduced using either annealing or remelting, but a compromise must be made between the remaining free radicals and reduced wear resistance.10-12 Alternatively, the polyethylene is first cross-linked and then doped with vitamin E (α-tocopherol), an antioxidant that can scavenge free radicals, rendering annealing or remelting redundant.13 This material has shown increased resistance to oxidation and fatigue crack propagation, as well as a low wear rate in experimental settings and has been investigated in vivo.14-20
One advantage of lower wear rates may be to allow the use of larger heads to reduce the risk of dislocation.21 Over the past decade we have seen a decreased use of 28 mm diameter modular heads and increased use of 36 mm.22 Larger heads have shown increased wear in UHMWPE liners,23 but little evidence is available on the effect of head size on vitamin E-doped highly cross-linked polyethylene (vE-PE) liner wear.1,2,4
The clinically relevant endpoint is revision rate, but due to low overall revision rate and high longevity, this is often not a feasible outcome measure. As revision rates and polyethylene wear have been associated, polyethylene wear can be measured using radio stereometric analysis (RSA) and be used as a proxy for measuring revision rate.6,24
The primary aim of this multi-arm randomized controlled trial (RCT) was to compare femoral head penetration into the liner between a) vE-PE liners and conventional XLPE liners and b) 32 mm and 36 mm femoral heads. The secondary aims were to compare acetabular component migration between a) vE-PE and XLPE liners and b) 32 mm and 36 mm heads, and to compare patient-reported outcome measures (PROMs) between 32 mm and 36 mm heads. We hypothesized that vE-PE liners and 32 mm heads would show lower rates of head penetration.
Methods
Trial design
This was a 2 × 2 factorial randomized, single-blinded, controlled trial reported in accordance with the CONSORT statement for multi-arm trials.25
Participants
Patients aged 40 to 70 years who were eligible for an uncemented THA due to idiopathic osteoarthritis and could fit at least a 54 mm component were included at Odense University Hospital and Middelfart Hospital between May 2009 and April 2011. Exclusion criteria were severe anteversion of the femoral neck that required non-standard components, acetabular dysplasia (centre edge angle < 20°), malignancy, previous radiotherapy, inability to participate in the rehabilitation programme, or received screws inserted into the acetabular shell screws or femoral cerclage during surgery. A summary of baseline data is available in Table I.
Table I.
Baseline demographics for allocated patients.
| Characteristic | Individual groups | |||
|---|---|---|---|---|
| vE-PE, 32 mm | vE-PE, 36 mm | XLPE, 32 mm | XLPE, 36 mm | |
| Total, n | 24 | 29 | 30 | 33 |
| Male sex, n (%) | 19 (79) | 20 (69) | 19 (63) | 19 (58) |
| Median age, yrs (IQR) | 65 (57 to 67) | 63 (56 to 66) | 64 (58 to 66) | 61 (54 to 66) |
| Mean BMI, kg/m2 (range) | 28 (22 to 40) | 29 (22 to 36) | 28 (20 to 41) | 27 (21 to 38) |
| ASA class, n (%) | ||||
| 1 | 7 (29) | 9 (31) | 8 (27) | 13 (39) |
| 2 | 13 (54) | 14 (48) | 15 (50) | 8 (24) |
| 3 | 1 (4) | 1 (3) | 1 (3) | 1 (3) |
| Data not available | 3 (13) | 5 (17) | 6 (20) | 11 (33) |
| Median blood loss, ml (IQR) | 450 (225 to 550) | 300 (200 to 600) | 350 (300 to 400) | 350 (225 to 400) |
| Median duration of anaesthesia, mins (IQR) | 90 (75 to 105) | 80 (66 to 95) | 75 (65 to 84) | 83 (71 to 99) |
| Median days hospitalized (IQR) | 3 (3 to 3) | 3 (2 to 3) | 3 (2 to 4) | 3 (3 to 5) |
| Mean Harris Hip Score (range) | 47 (29 to 80) | 49 (18 to 81) | 50 (13 to 74) | 44 (21 to 74) |
| Median UCLA Activity Score (IQR) | 6 (4 to 7) | 6 (4 to 8) | 6 (5 to 7) | 5 (4 to 7) |
| Median EQ-5D Health State (IQR) | 0.72 (0.66 to 0.72) | 0.66 (0.59 to 0.72) | 0.72 (0.57 to 0.72) | 0.72 (0.59 to 0.72) |
| Median EQ-5D VAS (IQR) | 65 (46 to 83) | 60 (40 to 70) | 70 (50 to 79) | 75 (60 to 90) |
| Mean SF-36 PCS (range) | 37 (30 to 48) | 37 (32 to 42) | 37 (26 to 44) | 36 (27 to 45) |
| Mean SF-36 MCS (range) | 41 (34 to 53) | 41 (36 to 47) | 42 (32 to 51) | 41 (31 to 54) |
ASA, American Society of Anesthesiologists Physical Status classification system; BMI, body mass index; EQ-5D Health State, EuroQoL five-dimension three-level questionnaire; EQ-5D VAS, EuroQoL visual analogue scale; IQR, interquartile range; MCS, mental component score; PCS, physical component score; SF-36, 36-Item, Health Survey questionnaire; UCLA, University of California, Los Angeles; vE-PE, vitamin E-doped cross-linked polyethylene liners; XLPE, cross-linked polyethylene liners.
Intervention
The patients were allocated to one of four intervention groups: vE-PE liner (E1, Biomet, Warsaw, Indiana, USA) with a 32 mm head (vE-PE, 32 mm); vE-PE liner with a 36 mm head (vE-PE, 36 mm); XLPE liner (ArComXL, Biomet, Warsaw, Indiana, USA) with 32 mm head, (XLPE, 32 mm); or XLPE with a 36 mm head (XLPE, 36 mm).
All patients received plasma-sprayed porous-coated acetabular shells (Exceed ABT, Biomet, Warsaw, Indiana, USA) and uncemented porous-coated components (Bi-Metric, Biomet, Warsaw, Indiana, USA) with cobalt-chromium (CoCr) alloy modular femoral heads (Biomet) according to manufacturer’s instructions using the posterior approach. Ten tantalum beads (diameter 0.8 mm) were inserted in the periacetabular bone. Only shell sizes from 54 mm and upward were used to ensure at least 5 mm liner-thickness. The patients received tranexamic acid and antibiotics during surgery. Rehabilitation, pain management, and discharge were standardized.
Outcomes
The primary outcome was proximal femoral head penetration into the liner measured using RSA. The secondary outcomes were proximal acetabular component migration measured using RSA and PROMs.
RSA
Patients were placed in a supine position above a uniplanar calibration cage 43 (RSA Biomedical AB, Umeå, Sweden). A ceiling-mounted and a mobile radiography tube exposed two digital radiographs analyzed using UmRSA 7.0 (RSA Biomedical, Umeå, Sweden). The tantalum beads formed the periacetabular bone segment, and the centre of the acetabular component and head were formed using spherical modelling.
RSA was performed at baseline (seven days after surgery), three months, and one, two, and five years. Head penetration is reported as the total proximal head penetration and as penetration rate at each follow-up. Double examinations were performed at the five-year follow-up, and 95% confidence intervals (CIs) for repeatability in the x- (transverse), y- (vertical), and z-axes (anteroposterior) were 0.246 mm, 0.226 mm, and 0.371 mm, respectively.24,26 Analyses where rigid body fitting error > 0.350 mm or condition number > 135 were rejected.24
PROMs
The Harris Hip Score is a scale from 0 to 100 and measures pain, function, deformity, and movement related to the hip.27 The University of California, Los Angeles (UCLA) Activity Score is a ten-point Likert-like scale measuring physical activity and is recommended and used in similar populations.28,29 The EuroQoL five-dimension questionnaire (EQ-5D) is a utility index in health economics based on five descriptive domains-mobility, self-care, usual activities, pain/discomfort, and anxiety/depression-used with the Danish Time Trade-Off (TTO) value set.30,31 The 36-Item Short-Form Health Survey questionnaire (SF-36) is a generic health questionnaire in eight dimensions that can be summarized to a physical and a mental health score.32 PROMs were assessed at baseline (two weeks prior to surgery), three months, one, three, and five years. Function was evaluated with the HHS and UCLA score, and general health with the EQ-5D and SF-36.
Adverse events
We queried the Danish Hip Arthroplasty Register (DHR) for revision surgery on participating patients.1 The DHR is a national registry of surgeon-reported parameters including indication for primary and revision hip arthroplasty and has been shown to have high levels of data capture.1,33
Sample size
This study was powered as a parallel-group trial to show that vE-PE liners had lower wear than XLPE liners. Power was set to 80%, and the risk of type-I error was set to 5%. Wear was expected to drop from 0.05 mm/year in XLPE to 0.0005 mm/year in vE-PE.34 The minimal clinically relevant difference was set to 0.05 mm/year. This resulted in a requirement for 15 hips in each group.35 To account for dropouts and secondary exclusion, study recruitment was planned at 25 patients for each arm.
Randomization and blinding
The allocation was computer-generated in two blocks (one block with 100 lots of 25 per group, another block with 28 lots of seven per group) in random order and lots were placed in sealed envelopes. The envelope was opened just before the liner was inserted. The study was blinded for the patients.
Registration and ethics
This trial was approved by The Regional Committees on Health Research Ethics for Southern Denmark (S-20080151) and The Danish Data Protection Agency (14/35949 and 18/31287) and complied with the Declaration of Helsinki. The trial was registered at ClinicalTrials.gov (NCT02196792).
Statistical analysis
A statistical analysis plan was previously published at ClinicalTrials.gov (NCT02196792). Patients allocated to an intervention group were included in the intention-to-treat (ITT) analysis, and patients who were in the ITT population and present at all RSA follow-ups were included in the per-protocol (PP) analysis. Descriptive statistics were reported using mean and SD or median and interquartile range (IQR) as appropriate.
Data were analyzed using mixed-effect analysis using the restricted maximum likelihood approach.36 Patients were considered as random effects and time, liner material, and head size as fixed effects. Interactions between time and liner material, and time and head size were included. PROM analyses were adjusted for baseline values. Data were analyzed using R v. 1.5.2 (R Foundation, Vienna, Austria) and the following packages: lme v. 4 1.1-19, car v. 3.0-2.37-39 Significance was set at a p-value < 0.05. Significance was tested using Wald tests of the mixed effect estimates unless stated otherwise.39
Results
Recruitment
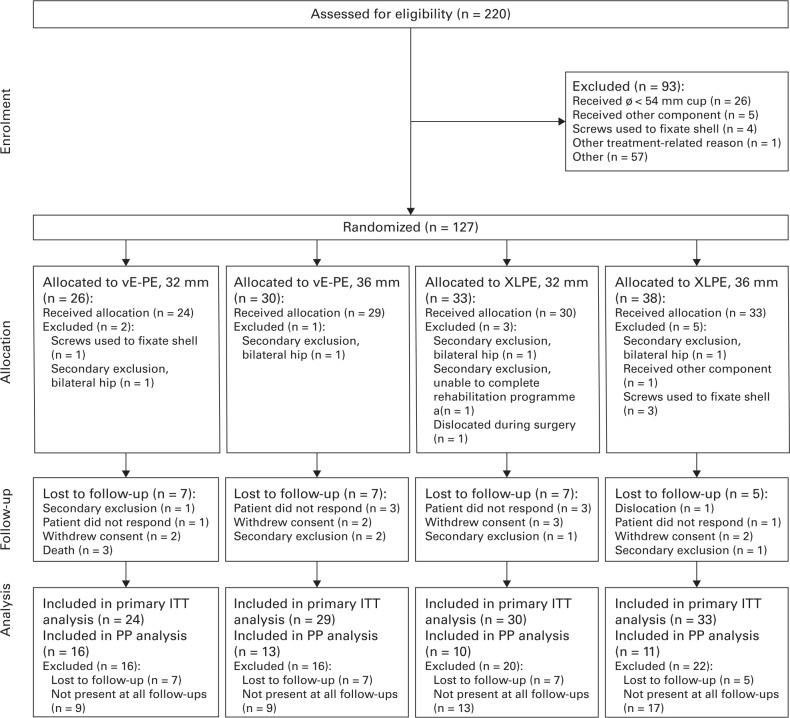
In total, 220 patients were assessed for eligibility (Figure 1), and 93 were excluded: 26 patients received an acetabular component size smaller than 54 mm, five patients received other components, four patients received screws, one was excluded due to other surgical complications, and 57 patients due to other reasons. The remaining 127 patients were randomized, and 116 patients received the allocated intervention, with a total of 11 patients being excluded post-randomization: four patients received shell screws, one received other components, one suffered a dislocation during surgery and received other components and was excluded, and five were excluded due to screening failure. A total of 22 patients were lost to follow-up, and 94 patients were still enrolled after five years.
Fig. 1.
CONSORT flow diagram of total hip arthroplasty candidate patients screened and randomized to either vitamin E-doped polyethylene liner (vE-PE) or cross-linked polyethylene liner (XLPE) with a 32 mm or 36 mm head. Note that 57 patients were recorded as excluded due to other reasons because no screening data were available for these patients. ITT, intention-to-treat; PP, per protocol.
Head penetration
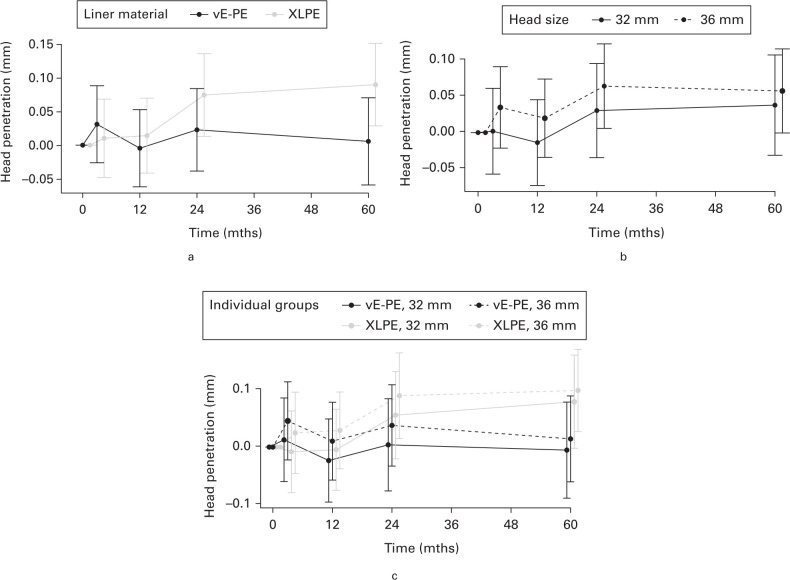
We found no between-group difference in mean total head penetration for liner materials or head sizes at five years (Figure 2, Table II): vE-PE versus XLPE was -0.084 mm (95% CI -0.173 to 0.005; p = 0.064, mixed-effect analysis), and 32 mm versus 36 mm was -0.020 mm (95% CI -0.110 to 0.071; p = 0.671, mixed-effect analysis). The PP analysis of total head penetration (Supplementary Table i) gave similar results except for liner materials where vE-PE had significantly lower penetration (vE-PE vs XLPE was -0.117 (95% CI -0.231 to -0.002; p = 0.046)).
Fig. 2.
Primary intention-to-treat outcomes shown with 95% confidence interval (CI) by grouped and individual intervention group: mean femoral head penetration with 95% CI into the polyethylene liner. a) Head penetration by liner material. b) Head penetration by head size. c) Head penetration according to individual intervention groups. vE-PE, vitamin E-doped cross-linked polyethylene liners; XLPE, cross-linked polyethylene liners; 32 mm, 32 mm CoCr femoral head; 36, 36 mm CoCr femoral head.
Table II.
Mean intention-to-treat outcomes with 95% confidence intervals (CI) according to the mixed-effect analysis for within-group change from baseline to five years and between-group difference at five years. Performed according to prespecified analysis plan.
| Outcome | vE-PE (95% CI) | XLPE (95% CI) | vE-PE vs XLPE (95% CI) | p-value |
|---|---|---|---|---|
| Comparison between vE-PE and XLPE | ||||
| Total head penetration (mm) | 0.006 (-0.059 to 0.070) | 0.090 (0.029 to 0.151) | -0.084 (-0.173 to 0.005) | 0.064 |
| Head penetration rate | ||||
| 0 to 3 months (mm/yr) | 0.124 (-0.105 to 0.353) | 0.041 (-0.192 to 0.274) | 0.083 (-0.243 to 0.410) | 0.617 |
| 3 months to 1 year (mm/yr) | -0.047 (-0.141 to 0.046) | 0.005 (-0.087 to 0.097) | -0.053 (-0.184 to 0.078) | 0.429 |
| 1 to 2 years (mm/yr) | 0.027 (-0.045 to 0.100) | 0.060 (-0.011 to 0.132) | -0.033 (-0.135 to 0.069) | 0.525 |
| 2 to 5 years (mm/yr) | -0.006 (-0.032 to 0.020) | 0.005 (-0.020 to 0.030) | -0.011 (-0.047 to 0.025) | 0.554 |
| Secondary outcomes | ||||
| Cup migration (mm) | 0.293 (0.159 to 0.427) | 0.262 (0.133 to 0.391) | 0.031 (-0.155 to 0.217) | 0.743 |
| Comparison between 32 and 36 mm | 32 mm | 36 mm | 32 mm vs 36 mm | |
| Total head penetration (mm) | 0.038 (-0.031 to 0.107) | 0.058 (-0.001 to 0.116) | -0.020 (-0.110 to 0.071) | 0.671 |
| Head penetration rate | ||||
| 0 to 3 months | 0.008 (-0.229 to 0.245) | 0.139 (-0.086 to 0.364) | -0.132 (-0.458 to 0.195) | 0.430 |
| 3 months to 1 year (mm/yr) | -0.012 (-0.066 to 0.043) | -0.011 (-0.061 to 0.039) | -0.001 (-0.132 to 0.130) | 0.987 |
| 1 to 2 years (mm/yr) | 0.044 (-0.033 to 0.121) | 0.044 (-0.023 to 0.112) | -0.000 (-0.103 to 0.102) | 0.999 |
| 2 to 5 years (mm/yr) | 0.003 (-0.025 to 0.030) | -0.002 (-0.026 to 0.021) | 0.005 (-0.032 to 0.041) | 0.800 |
| Secondary outcomes | ||||
| Cup migration (mm) | 0.199 (0.059 to 0.340) | 0.338 (0.214 to 0.462) | -0.139 (-0.327 to 0.049) | 0.147 |
| Harris Hip Score | 32 (26 to 38) | 33 (27 to 40) | -1 (-8 to 6) | 0.704 |
| UCLA Activity Score | 0.8 (0.1 to 1.5) | 0.9 (-2.3 to 4.1) | -0.2 (-3.3 to 3.0) | 0.172 |
| EQ-5D Health State | 0.20 (0.15 to 0.26) | 0.07 (-0.20 to 0.34) | 0.13 (-0.13 to 0.40) | 0.752 |
| EQ-5D VAS | 17 (11 to 23) | 19 (-11 to 48) | -2 (-30 to 27) | 0.583 |
| SF-36 PCS | 2.7 (1.3 to 4.0) | -0.5 (-6.9 to 5.8) | 3.2 (-3.0 to 9.4) | 0.873 |
| SF-36 MCS | -1.4 (-2.8 to 0.0) | 3.7 (-2.8 to 10.1) | -5.1 (-11.3 to 1.2) | 0.321 |
No interaction was found between the two randomization factors for total head penetration (p = 0.318), steady-state wear (p = 0.285), or cup migration (p = 0.496) using mixed-effect analysis of variance.
Wald test.
CI, confidence interval; EQ-5D, EuroQol five-dimension questionnaire; MCS, mental component score; PCS, physical component score; SE, standard error of the mean SF-36, 36-Item Short-Form Health Survey questionnaire; UCLA, University of California, Los Angeles; VAS, visual analogue scale; vE-PE, vitamin E-doped cross-linked polyethylene liners; XLPE, cross-linked polyethylene liners
The between-group wear rates were similar for liner materials and head sizes at all follow-ups. The effect of liner material and head size did not interact in these analyses.
Acetabular migration
We found no between-group difference in acetabular component migration (Table II), vE-PE versus XLPE was 0.031 mm (95% CI -0.155 to 0.217; p = 0.743), and 32 mm versus 36 mm -0.139 mm (95% CI -0.327 to 0.049; p = 0.147). All components showed significant within-group migration from baseline to last follow-up, and the components stabilized after one year (Supplementary Figure a).
PROMs
No between-group differences were found for any of the PROMs (Table II). Within-group improvements were found for Harris Hip Score (HHS) for both groups, while only 32 mm improved in UCLA, EQ-5D Health State, EQ-5D Visual Analogue Scale (VAS), and SF-36 Physical Component Score (PCS).
Adverse events
One patient allocated to XLPE, 36 mm was revised two days after index surgery due to dislocation and was excluded from the trial (Supplementary Table ii).
Discussion
This study compared THA head penetration into the liner between two types of liners (vE-PE and XLPE) and between two head sizes (32 mm and 36 mm). It showed that both total head penetration and wear rates were independent of liner type and femoral head size. No patients experienced aseptic loosening but given the low wear rate, small sample size, and short duration of the study, this would have been unlikely. These results do not provide compelling evidence to phase out XLPE liners in favour of vE-PE liners, or to choose 32 mm over 36 mm heads.
The well-known creep and wear phases40 were not found, and there are a few possible explanations for this. The repeatability of our RSA setup was slightly higher than that of similar trials.16-18 The accuracy of the model-based approach used in this study is lower than the marker-based approach, though both methods have the necessary accuracy to detect migration at the osteolysis threshold.6,41 Reduced accuracy does not affect the ability to determine true migration, but it increases variance and the necessary sample size for a given power.26 Lastly, the in-hospital location where RSA was performed changed during this trial. The same calibration box and software version was used for all RSA measurements, but this location change could have imposed a systematic error, although comparison of mean square error (MSE) and condition number (CN) between each follow-up does not suggest this (one-way analysis of variance (ANOVA); p = 0.095 for MSE; p = 0.758 for CN). These reasons may, however, explain the lack of distinct creep and wear phases.
Five other RCT shave compared vE-PE and XLPE liner wear.16-20 Two trials report no difference at two or seven years, respectively, while three trials report lower wear in vE-PE liners at five, five, and seven years, respectively. All these studies use the same RSA setup as this study. The disagreement between studies may arise from some of the reasons explained above, differences in sample size, or simply because the true difference in head penetration is low relative to the repeatability.
We have reported linear wear, but volumetric wear may be a more suitable metric for different head sizes. A 32 mm head with a linear wear of 0.100 mm/year has the same volumetric wear as a 36 mm head with a linear wear of 0.079 mm/year, and both head sizes in this study were well below these rates. A recent study by Lindalen et al42 supports the finding that 32 mm heads are not superior to 36 mm in terms of wear.
We did not find a difference in PROMs between 32 mm and 36 mm heads, and this trial was not powered to detect differences in PROMs. The RSA and PROM measurements do not align, as frequent RSA is important in the beginning of follow-up.
A large number of patients (n = 57) were excluded for other reasons. Screening data on these patients were not available at the time of writing, and the number was derived from the total number of THAs performed during the period where participating patients were operated.
Despite being designed to include 100 patients, 127 patients were randomized. Due to decentralized and insufficiently strict trial management, excess patients were screened and scheduled for randomization, and the second randomization block was made. This was unintentional, and this cohort has similar baseline demographics to a more recent cohort in a double-blinded RCT from the same department.43
The sample size calculation was based on optimistic expectations of the treatment effect, but sample size calculations are inherently subjective and similar RCTs have equal or lower sample sizes.16-18,44
We conducted both an ITT and a PP analysis to mitigate the risk of bias due to differential dropouts. The ITT and PP analyses showed very similar effect sizes for head penetration. Both were in favour of vE-PE, but the PP was statistically significant while the ITT was not. With repeatability and model-based approach of the RSA setup and the significance discrepancy between ITT and PP for head penetration in mind, this trial may suffer from a Type II error. However, we wonder if the true head penetration of vE-PE and XLPE groups will diverge and become detectably different at a later follow-up.
The intervention groups only differ in terms of liner material and head size, all other parameters were similar between intervention groups, and the primary outcome was measured using a standardized protocol.24 Among THA patients, 80% are generally diagnosed with osteoarthritis, and we believe the patients in this trial are representative for the majority of THA patients.1
In conclusion, in our investigation we identified no differences in head penetration and acetabular component migration between vE-PE and XLPE liners, or between 32 mm and 36 mm heads. In addition, we found no difference in PROMs between 32 mm and 36 mm heads.
Take home message
- Vitamin E-doped cross-linked total hip arthroplasty liners do not show lower wear rates than conventional cross-linked liners.
- Both liners show very low wear rates overall.
Author contributions
K. Kjærgaard: Obtained the funding, Prepared, analyzed, and interpreted the data, Provided project administration, Wrote, reviewed, and edited the manuscript.
M. Ding: Prepared the study concept and protocol,Reviewed and edited the manuscript.
C. Jensen: Prepared the study protocol,Interpreted the data, Reviewed and edited the manuscript.
C. Bragdon: Preparedthe study concept,Reviewed and edited the manuscript.
H. Malchau: Prepared the study concept and protocol, Reviewed and edited the manuscript.
C. M. Andreasen: Prepared the data, Reviewed and edited the manuscript.
O. Ovesen: Prepared the study concept and protocol,Performed the surgeries,Reviewed and edited the manuscript.
C. Hofbauer: Prepared the study concept and protocol, Performed the surgeries, Reviewed and edited the manuscript.
S. Overgaard: Prepared the study concept and protocol, Obtained the funding, Performed the surgeries, Interpreted the data, Reviewed and edited the paper.
Funding statement
The author or one or more of the authors have received or will receive benefits for personal or professional use from a commercial party related directly or indirectly to the subject of this article.
ICMJE COI statement
K. Kjærgaard, S. Overgaard, and H. Malchau declare institutional payments from Zimmer Biomet, related to this study. K. Kjærgaard also declares institutional payments from the Danish Rheumatisk Society and Toyota-Fund Denmark, related to this study.
Ethical review statement
This trial was approved by The Regional Committees on Health Research Ethics for Southern Denmark (S-20080151) and The Danish Data Protection Agency (14/35949 and 18/31287) and complied with the Declaration of Helsinki.
Trial registration number
The trial was registered at ClinicalTrials.gov (NCT02196792).
Supplementary material
Graphs and estimates from the per-protocol analysis as well as a summary table of dropouts.
Open access statement
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/.
This article was primary edited by J. Hutt and first proof edited by G. Scott.
References
- 1.No authors listed . National annual report 2019. Danish hip arthroplasty register. 2020. http://danskhoftealloplastikregister.dk/wp-content/uploads/2019/09/DHR-%C3%A5rsrapport-2019_til-offentligg%C3%B8relse-1.pdf (date last accessed 24 June 2020).
- 2.Kärrholm J, Mohaddes M, Odin D, et al. Swedish Hip arthroplasty register annual report 2017. Swedish hip arthroplasty register. 2020. https://registercentrum.blob.core.windows.net/shpr/r/Eng_Arsrapport_2017_Hoftprotes_final-Syx2fJPhMN.pdf (date last accessed 24 June 2020).
- 3.No authors listed . Hip, Knee & Shoulder Arthroplasty Annual Report 2018. Australian Orthopaedic Association National Joint Replacement Registry. 2020. https://aoanjrr.sahmri.com/documents/10180/576950/Hip%2C%20Knee%20%26%20Shoulder%20Arthroplasty (date last accessed 24 June 2020).
- 4.No authors listed . The New Zealand joint registry nineteen year report January 1999 to December 2017. New Zealand joint registry. 2018. https://nzoa.org.nz/system/files/DH8152_NZJR_2018_Report_v6_4Decv18.pdf (date last accessed 24 June 2020).
- 5.Harris WH. The problem is osteolysis. Clin Orthop Relat Res. 1995;1(311):46–53. [PubMed] [Google Scholar]
- 6.Dumbleton JH, Manley MT, Edidin AA. A literature review of the association between wear rate and osteolysis in total hip arthroplasty. J Arthroplasty. 2002;17(5):649–661. [DOI] [PubMed] [Google Scholar]
- 7.Broomfield JAJ, Malak TT, Thomas GER, et al. The relationship between polyethylene wear and periprosthetic osteolysis in total hip arthroplasty at 12 years in a randomized controlled trial cohort. J Arthroplasty. 2017;32(4):1186–1191. [DOI] [PubMed] [Google Scholar]
- 8.Engh CA, Hopper RH, Huynh C, et al. A prospective, randomized study of cross-linked and non-cross-linked polyethylene for total hip arthroplasty at 10-year follow-up. J Arthroplasty. 2012;27(8 Suppl):2–7. [DOI] [PubMed] [Google Scholar]
- 9.Hopper RH, Ho H, Sritulanondha S, Williams AC, Engh CA. Otto Aufranc Award: crosslinking reduces THA wear, osteolysis, and revision rates at 15-year followup compared with noncrosslinked polyethylene. Clin Orthop Relat Res. 2018;476(2):279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muratoglu OK, Bragdon CR, O’Connor DO, Jasty M, Harris WH. A novel method of cross-linking ultra-high-molecular-weight polyethylene to improve wear, reduce oxidation, and retain mechanical properties. J Arthroplasty. 2001;16(2):149–160. [DOI] [PubMed] [Google Scholar]
- 11.Kurtz SM, Manley M, Wang A, Taylor S, Dumbleton J. Comparison of the properties of annealed crosslinked (crossfire) and conventional polyethylene as hip bearing materials. Bull Hosp Jt Dis. 2003;61(1-2):17–26. [PubMed] [Google Scholar]
- 12.Atwood SA, Van Citters DW, Patten EW, et al. Tradeoffs amongst fatigue, wear, and oxidation resistance of cross-linked ultra-high molecular weight polyethylene. J Mech Behav Biomed Mater. 2011;4(7):1033–1045. [DOI] [PubMed] [Google Scholar]
- 13.Bracco P, Oral E. Vitamin E-stabilized UHMWPE for total joint implants: a review. Clin Orthop Relat Res. 2011;469(8):2286–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oral E, Rowell SL, Muratoglu OK. The effect of α-tocopherol on the oxidation and free radical decay in irradiated UHMWPE. Biomaterials. 2006;27(32):5580–5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oral E, Wannomae KK, Hawkins N, Harris WH, Muratoglu OK. Alpha-tocopherol-doped irradiated UHMWPE for high fatigue resistance and low wear. Biomaterials. 2004;25(24):5515–5522. [DOI] [PubMed] [Google Scholar]
- 16.Salemyr M, Muren O, Ahl T, et al. Vitamin-E diffused highly cross-linked polyethylene liner compared to standard liners in total hip arthroplasty. A randomized, controlled trial. Int Orthop. 2015;39(8):1499–1505. [DOI] [PubMed] [Google Scholar]
- 17.Nebergall AK, Greene ME, Laursen MB, et al. Vitamin E diffused highly cross-linked polyethylene in total hip arthroplasty at five years: a randomised controlled trial using radiostereometric analysis. Bone Joint J. 2017;99-B(5):577–584. [DOI] [PubMed] [Google Scholar]
- 18.Shareghi B, Johanson P-E, Kärrholm J. Wear of vitamin E-Infused highly cross-linked polyethylene at five years. J Bone Joint Surg. 2017;99(17):1447–1452. [DOI] [PubMed] [Google Scholar]
- 19.Galea VP, Rojanasopondist P, Laursen M, et al. Evaluation of vitamin E-diffused highly crosslinked polyethylene wear and porous titanium-coated shell stability: a seven-year randomized control trial using radiostereometric analysis. Bone Joint J. 2019;101-B(7):760–767. [DOI] [PubMed] [Google Scholar]
- 20.Sköldenberg OG, Rysinska AD, Chammout G, et al. A randomized double-blind noninferiority trial, evaluating migration of a cemented vitamin E-stabilized highly crosslinked component compared with a standard polyethylene component in reverse hybrid total hip arthroplasty. Bone Joint J. 2019;101-B(10):1192–1198. [DOI] [PubMed] [Google Scholar]
- 21.Burroughs BR, Hallstrom B, Golladay GJ, Hoeffel D, Harris WH. Range of motion and stability in total hip arthroplasty with 28-, 32-, 38-, and 44-mm femoral head sizes. J Arthroplasty. 2005;20(1):11–19. [DOI] [PubMed] [Google Scholar]
- 22.Tsikandylakis G, Mohaddes M, Cnudde P, et al. Head size in primary total hip arthroplasty. EFORT Open Reviews. 2018;3(5):225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tarasevicius S, Robertsson O, Kesteris U, Kalesinskas RJ, Wingstrand H. Effect of femoral head size on polyethylene wear and synovitis after total hip arthroplasty: a sonographic and radiographic study of 39 patients. Acta Orthop. 2008;79(4):489–493. [DOI] [PubMed] [Google Scholar]
- 24.Valstar ER, Gill R, Ryd L, et al. Guidelines for standardization of radiostereometry (RSA) of implants. Acta Orthop. 2005;76(4):563–572. [DOI] [PubMed] [Google Scholar]
- 25.Juszczak E, Altman DG, Hopewell S, Schulz K. Reporting of Multi-Arm parallel-group randomized trials: extension of the CONSORT 2010 statement. JAMA. 2019;321(16):1610–1620. [DOI] [PubMed] [Google Scholar]
- 26.Ranstam J, Ryd L, Onsten I. Accurate accuracy assessment: review of basic principles. Acta Orthop Scand. 2000;71(1):106–108. [DOI] [PubMed] [Google Scholar]
- 27.Söderman P, Malchau H. Is the Harris hip score system useful to study the outcome of total hip replacement? Clin Orthop Relat Res. 2001;384:189–197. [DOI] [PubMed] [Google Scholar]
- 28.Terwee CB, Bouwmeester W, van Elsland SL, de Vet HCW, Dekker J. Instruments to assess physical activity in patients with osteoarthritis of the hip or knee: a systematic review of measurement properties. Osteoarthritis Cartilage. 2011;19(6):620–633. [DOI] [PubMed] [Google Scholar]
- 29.Zahiri CA, Schmalzried TP, Szuszczewicz ES, Amstutz HC. Assessing activity in joint replacement patients. J Arthroplasty. 1998;13(8):890–895. [DOI] [PubMed] [Google Scholar]
- 30.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wittrup-Jensen KU, Lauridsen J, Gudex C, Pedersen KM. Generation of a Danish TTO value set for EQ-5D health states. Scand J Public Health. 2009;37(5):459–466. [DOI] [PubMed] [Google Scholar]
- 32.Ware JE. SF-36: Physical and mental health summary scales: a user’s manual. 1. Boston: Health Institute, 1994. [Google Scholar]
- 33.Pedersen A, Johnsen S, Overgaard S, et al. Registration in the Danish hip arthroplasty RegistryCompleteness of total hip arthroplasties and positive predictive value of registered diagnosis and postoperative complications. Acta Orthop Scand. 2004;75(4):434–441. [DOI] [PubMed] [Google Scholar]
- 34.Digas G, Kärrholm J, Thanner J, Malchau H, Herberts P. The Otto Aufranc award. highly cross-linked polyethylene in total hip arthroplasty: randomized evaluation of penetration rate in cemented and uncemented sockets using radiostereometric analysis. Clin Orthop Relat Res. 2004;429:6–16. [PubMed] [Google Scholar]
- 35.Rosner B. Fundamentals of biostatistics. 7th ed. Boston: Cengage Learning, 2010. [Google Scholar]
- 36.Kirkwood BR, Sterne JAC. Essential medical statistics. 2nd ed. Hoboken: Wiley-Blackwell, 2003. [Google Scholar]
- 37.R Core Team . R: a language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria. 2018. https://www.gbif.org/tool/81287/r-a-language-and-environment-for-statistical-computing#citation (date last accessed 24 June 2020).
- 38.Bates D, Mächler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Using lme4. J Stat Softw. 2015;67(1):1–48. [Google Scholar]
- 39.Fox J, Weisberg S. An R companion to applied regression. 2nd ed. Thousand Oaks: Sage Publications, 2011. [Google Scholar]
- 40.Glyn-Jones S, McLardy-Smith P, Gill HS, Murray DW. The creep and wear of highly cross-linked polyethylene: a three-year randomised, controlled trial using radiostereometric analysis. J Bone Joint Surg Br. 2008;90-B(5):556–561. [DOI] [PubMed] [Google Scholar]
- 41.Nebergall AK, Rader K, Palm H, Malchau H, Greene ME. Precision of radiostereometric analysis (RSA) of acetabular cup stability and polyethylene wear improved by adding tantalum beads to the liner. Acta Orthop. 2015;86(5):563–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lindalen E, Thoen PS, Nordsletten L, Høvik Øystein, Röhrl SM. Low wear rate at 6-year follow-up of vitamin E-infused cross-linked polyethylene: a randomised trial using 32- and 36-mm heads. HIP International. 2019;29(4):355–362. [DOI] [PubMed] [Google Scholar]
- 43.Rosenlund S, Broeng L, Holsgaard-Larsen A, Jensen C, Overgaard S. Patient-Reported outcome after total hip arthroplasty: com- parison between lateral and posterior approach a randomized controlled trial in 80 patients with 12-month follow-up. Acta Orthop. 2017;88(3):239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schulz KF, Grimes DA. Sample size calculations in randomised trials: mandatory and mystical. The Lancet. 2005;365(9467):1348–1353. [DOI] [PubMed] [Google Scholar]