Abstract
Aims
Stiffness is a common complication after total knee arthroplasty (TKA). Pathogenesis is not understood, treatment options are limited, and diagnosis is challenging. The aim of this study was to investigate if MRI can be used to visualize intra-articular scarring in patients with stiff, painful knee arthroplasties.
Methods
Well-functioning primary TKAs (n = 11), failed non-fibrotic TKAs (n = 5), and patients with a clinical diagnosis of fibrosis1 (n = 8) underwent an MRI scan with advanced metal suppression (Slice Encoding for Metal Artefact Correction, SEMAC) with gadolinium contrast. Fibrotic tissue (low intensity on T1 and T2, low-moderate post-contrast enhancement) was quantified (presence and tissue thickness) in six compartments: supra/infrapatella, medial/lateral gutters, and posterior medial/lateral.
Results
Fibrotic tissue was identified in all patients studied. However, tissue was significantly thicker in fibrotic patients (4.4 mm ± 0.2 mm) versus non-fibrotic (2.5 mm ± 0.4 mm) and normal TKAs (1.9 mm ± 0.2 mm, p = < 0.05). Significant (> 4 mm thick) tissue was seen in 26/48 (54%) of compartments examined in the fibrotic group, compared with 17/30 (57%) non-fibrotic, and 10/66 (15%) normal TKAs. Although revision surgery did improve range of movement (ROM) in all fibrotic patients, clinically significant restriction remained post-surgery.
Conclusion
Stiff TKAs contain intra-articular fibrotic tissue that is identifiable by MRI. Studies should evaluate whether MRI is useful for surgical planning of debridement, and as a non-invasive measurement tool following interventions for stiffness caused by fibrosis. Revision for stiffness can improve ROM, but outcomes are sub-optimal and new treatments are required.
Cite this article: Bone Joint J 2020;102-B(10):1331–1340.
Keywords: knee replacement, knee arthroplasty, fibrosis, revision surgery, arthritis, collagen
Introduction
Stiffness is a common cause of dissatisfaction following total knee arthroplasty (TKA), affecting between 10% and 20% of patients.1,2 The underlying pathology driving this stiffness may be the development of intra-articular fibrous scar tissue (fibrosis). Treatment is limited to conservative measures (physical therapy), manipulation under anaesthetic to restore range of movement (ROM), or surgical debridement.3 Our understanding of the pathogenesis of fibrosis is incomplete and the outcome of treatment for persistent stiffness is variable.4
Currently, the diagnosis of fibrosis is made clinically, where there is restriction of ROM in the absence of an identifiable cause, such as surgical error in implant positioning.1 Confirmation of clinical diagnosis is provided at time of surgery (either open or arthroscopic) by visualization of intra-articular scar tissue. There is no available non-invasive test to aid the clinician investigating the patient presenting with stiffness that has failed non-surgical measures and in whom an implant-related cause cannot be found.5,6
Fibrosis may occur within the joint or the capsule. Mapping and quantification of areas of fibrosis would facilitate surgical planning and allow monitoring of disease progression and response to medical and surgical therapies.
MRI has been used to describe soft tissue changes around hip arthroplasty implants, in particular the appearance of bone loss7 aseptic lymphocytic vasculitis-associated lesions and synovitis.8,9 However, the use of MRI to investigate problematic TKA is less well studied.5,10 A meta-analysis of MRI investigation of the problematic TKA showed that studies investigating fibrosis are ‘limited and inconclusive.5
We have reported histological11 and cytokine12,13 analysis of patients with fibrosis following TKA undergoing revision surgery, and have not robustly identified differences between fibrotic patients and those undergoing aseptic revision for failed TKA for other causes. Tissue from patients in these two groups show similar inflammatory cytokine profiles and are histologically indistinct from one another, with the presence of fibrous collagenous tissue populated with aSMA staining fibroblast cells, although clinically and intraoperatively the differences are marked and their gene expression profile is significantly different.14
In this study, we investigated whether intra-articular scar tissue can be detected and quantified in patients with a clinical diagnosis of fibrosis following TKA, using MRI scanning as a non-invasive diagnostic technique in the presence of metal artefact.15,16 Here we compared patients with and without fibrotic conditions after knee arthroplasty and attempted to relate the MRI findings with the clinical picture. Identification of fibrotic tissue using MRI would provide clinicians with valuable information about pathology, and provide researchers with a tool to diagnose fibrosis, stratify disease severity, and investigate the effect of interventions in future clinical trials.
Methods
Patient recruitment
This study was performed following approval from the Health Research Authority (HRA) and Research Ethics Committee (16/NW/0414), and informed written consent was obtained from all patients. Demographics of the study population are presented in Table I. Imaging was performed at the host institution, and image analysis was performed in Leeds at the NIHR Biomedical Musculoskeletal Research Centre.
Table I.
Patient demographics.
| Variable | M:F ratio | Age in years, median (range) | BMI, median (range) | Time in months from primary to revision TKA, median (range) | Primary indication for surgery |
|---|---|---|---|---|---|
| Primary TKA (n = 11) | 6:5 | 63 (54 to 79) | 29 (24 to 38) | N/A | Osteoarthritis (n = 11) |
| Revision fibrotic group (n = 8) | 4:4 | 65 (47 to 72) | 30 (23 to 35) | 17.5 (10 to 47) | Joint fibrosis after knee arthroplasty |
| Revision non-fibrotic group (n = 5) | 2:3 | 80 (58 to 80) | 29 (23 to 40) | 170 (33 to 204) | Instability (n = 1) Osteolysis with loose worn components (n = 3 Valgus deformity post-fracture (n = 1) |
BMI, body mass index; N/A, not applicable; TKA, total knee arthroplasty.
Primary TKA cohort
After defining the scan protocol, 11 patients who had undergone TKA for osteoarthritis less than 18 months previously were included. All primary TKA patients had full active extension, with no lag, and flexion beyond 120°.
Revision cohort
All patients undergoing revision surgery over a two-year period were eligible for inclusion in the study. All patients with a clinical diagnosis of fibrosis who were revised during that time were approached and included. The clinical diagnosis of fibrosis was made according to published clinical diagnostic criteria1 with all having limitation of ROM in flexion and/or extension, with no specific diagnosed cause (osseous or prosthetic block to movement from malaligned, malpositioned or incorrectly sized components; infection, pain, complex regional pain syndrome (CRPS), or other specific causes). Malalignment was excluded as a cause for stiffness using CT rotational profile as previously described.17,18 Five of the eight patients in this group had undergone at least one manipulation under anaesthesia (MUA) prior to revision surgery. Clinical details of the fibrotic revision cohort are presented in Table II.
Table II.
Fibrotic patient group clinical details and intraoperative findings.
| Patient | Age (at time of revision) | Comorbidities | Time from primary to revision (years and months) | Clinical history | Rotational profile (CT) | Preoperative ROM (extension-flexion in degrees) | Intraoperative findings | Intraoperative ROM achieved (degrees) | Review date (Years and months post-revision) | Post-op ROM (value add ROM) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 47 | Mild COPD | 1 Y 9 M | Primary KR 2015, MUA 2016 for restricted ROM, failed, revision 2017 | FCA 0.5°, 2° TCA, 2.5°CR | 0 to 80 | Massive intra-articular fibrosis resected | 0 to 120 | 2 Y | 0 to 90 (10°) |
| 2 | 65 | Nil | 1 Y 1 M | Primary 2016, gradual deterioration in ROM from 0 to 110 post-primary, revision 2017 | FCA 3°, 1° TCA, 3°CR | 30 to 80 | Massive intra-articular fibrosis and synovitis | 0 to 120 | 2 Y | 0 to 105 (55°) |
| 3 | 70 | Type 2 diabetes, asthma, COPD | 3 Y 8 M | Primary 2013, MUA 2014 | FCA 1°, 3° TCA, 4°CR | 30 to 90 | Fibrosis resected particularly from supra- and infrapatella pouch | 0 to 120 | 1 Y 6 M | 10 to 90 (20°) |
| 4 | 65 | Gout | 1 Y 4 M | Primary 2016, MUA 2017, failed to restore ROM | FCA 4°, -1° TCA, 3°CR | 30 to 80 | Massive fibrosis throughout joint | 0 to 120 | 1 Y 9 M | 5 to 85 (30°) |
| 5 | 68 | Nil | 2 Y | Primary 2016, progressive ROM restriction | FCA 0°, 0° TCA, 0°CR | 30 to 60 | Massive resection of scar tissue from intercondylar notch, posteriorly after removal of the PCL and both gutters and from retropatellar tendon | 0 to 130 | 2 Y 1 M | 0 to 80 (50°) |
| 6 | 61 | Hypertension | 1 Y 2 M | Primary 2017, ROM restriction, failed MUA | FCA 0°, 2° TCA, 2°CR | 20 to 80 | Intra-articular fibrosis resected | 0 to 120 | 1 Y 6 M | 5 to 90 (25°) |
| 7 | 65 | Ovarian cancer, asthma, TIA | 1 Y 1 M | Primary 2017, MUA failed 2017, revision 2018 | FCA 4°, -2° TCA,2 °CR | 0 to 40 | dense scar tissue excised from behind the patellar tendon, suprapatellar pouch, both gutters, intercondylar notch, above patella, fat pad and either side of the femoral and tibial components, | 0 to 120 | 2 Y 0 M | 0 to 75 (35°) |
| 8 | 72 | Hypertension, dupuytren’s disease | 1 Y 1 M | Primary 2017 | FCA 0.5°, 1° TCA,1.5 °CR | 20 to 80 | Massive intra-articular fibrosis and scarring, resected | 0 to 120 | 1 Y 9 M | 5 to 95 (30°), has extensor lag |
COPD, chronic obstructive pulmonary disease; CR, combined rotation; FCA, femoral component axis; MUA, manipulation under anaesthesia; ROM, range of movement; TCA, tibial component axis.
Positive values denote external rotation, negative values denote internal rotation.
Five patients undergoing revision surgery for causes other than fibrosis were also recruited (Table III). Their indications for surgery are described in Table III (wear and osteolysis in three patients, instability in two). Fibrotic tissue debrided from revision patients was used for histological analysis.
Table III.
Clinical information on aseptic revision surgery patients.
| Patient | Age (at time of revision) | Comorbidities | Time from primary to revision (years and months) | Diagnosis/reason for revision | Clinical history | Intraoperative findings | Review date (Years and months post-revision) | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | 80 | Hypertension, ischaemic heart disease, asthma | 15 Y 0 M | Osteolysis, loose worn components | Primary 2003, ROM 0 to 110 1007, identified failing R TKA 2015 with loosening of tibial and femoral components | Loose tibial base-plate | 2 Y 2 M | Stable knee, 0 to 100 ROM, no extensor lag |
| 2 | 80 | Hypothyroidism | 4 Y 10 M | Instability | Failing, incompetent MCL | Nil significant | 1 Y 6 M | 0 to 110, stable, no extensor lag |
| 3 | 71 | Hypertension | 17 Y 0 M | Loose worn components | Instability and pain developed ∼ 16 years post primary | Significant bearing surface wear and loose components very easy to remove | 1 Y 9 M | 0 to 110, no extensor lag |
| 4 | 80 | Type 2 diabetes, ischaemic heart disease, hypertension | 2 Y 9 M | Instability | Failing, incompetent MCL | Nil significant | 2 Y 1 M | 0 to 100, 5° extensor lag |
| 5 | 58 | Gout, DVT | 12 Y 2 M | Instability, loose worn components | Primary 2007, presented with instability in 2017, revised 2017 | Worn poly, loose components | 1 Y 6 M | 0 to 105, no extensor lag |
DVT, deep vein thrombosis; MCL, medial collateral ligament; ROM, range of movement; TKA, total knee arthroplasty.
Exclusion criteria
Patients with any indication other than osteoarthritis for the primary TKA (including rheumatoid arthritis, septic arthritis, or trauma), obese patients (body mass index (BMI) > 40) and those unable to undergo MRI (either due to renal impairment or inability to tolerate the scan) were exclued.
Implant type
In the primary group, nine patients had a PFC Sigma (DePuy Synthes, West Chester, Pennsylvania, USA) and two had a Triathlon (Stryker, Kalamazoo, Michigan, USA). In the fibrosis cohort, the primary knee arthroplasties were PFC Sigma in four cases, Triathlon in two, and Kinemax (Stryker Howmedica Osteonics, Mahwah, New Jersey, USA) in two. Four of the eight cases in this group had had the patella resurfaced during the primary procedure. In the non-fibrotic revision cohort the primary knee arthroplasties were PFC Sigma in two, Triathlon in one, and Kinemax in two. Revisions were performed using the NexGen rotating hinge (ZimmerBiomet, Warsaw, Indiana, USA; five cases), LCCK (ZimmerBiomet; five cases), and TC3 (DePuy Synthes; three cases).
In all primary and revision TKA patients, local anaesthetic was administered intraoperatively under direct vision (to a maximum dose of 100 ml of 0.2% ropivacaine). All patients were given routine postoperative analgesia, including regular paracetamol and a patient-controlled analgesia device. Patients in the fibrotic revision group were routinely started on continuous passive movement for 48 to 72 hours postoperatively.
Exclusion of infection
Preoperative workup included C-reactive protein (CRP) in all patients and joint aspiration where infection was considered to be clinically plausible (8/8 fibrotic patients and 3/5 non-fibrotic revision patients). CRP was < 10 mg/l in all revision patients, and all preoperative joint aspiration samples were negative after extended culture. Infection was definitively ruled out in all revision patients by microbiological analysis of synovial fluid and of multiple tissue samples taken at revision surgery. All revision patients had negative extended culture of both intraoperative synovial fluid and tissue samples.
Histological analysis
Formal histology reports were available for 7/8 fibrosis revision patients. Reports state that dense, hypocellular, heavily collagenized fibrous tissue foreign body giant cells containing small transparent strongly birefringent foreign particles were identified. The appearances are those of extensive fibrosis together with a focal giant cell reaction to foreign material, a reaction to polyethylene debris from the prosthetic joint implant. There was no evidence of infection reported in any cases.
Infrapatellar fat pad excision
Routine practice in our unit includes excision of the infrapatellar fat pad. All primary patients in the study had their infrapatellar fat pad removed. In the revision cohort, ten had primary surgery performed in our unit and, therefore, had their infrapatellar fat pad removed in the primary procedure. In the remaining three patients, we do not know whether the fat pad was removed or retained.
MRI scanning
The scan protocol was developed at the Leeds Biomedical Musculoskeletal Research Centre. Imaging was performed on a 1.5 Tesla Siemens Aera MRI scanner. SEMAC has been shown to effectively reduce the metal artefact from TKA prostheses.16 Scan optimization was performed on four healthy TKA patients who underwent an extended protocol of nine image sequences, utilizing advanced metal reduction suppression sequences SEMAC where appropriate: Proton density (PD) axial and coronal, PD sagittal SEMAC, T1 coronal (with and without SEMAC), T2 STIR sagittal (with and without SEMAC), and T1 sagittal and coronal SEMAC, post-gadolinium contrast administration. Review of initial images demonstrated the importance of contrast administration and the effect of SEMAC metal reduction. Therefore, non-SEMAC sequences were removed from the final protocol, which included six image sequences pre-contrast (T1 axial SEMAC, T2 STIR axial SEMAC, T1 sagittal SEMAC and T2 STIR sagittal SEMAC) and two sequences post-contrast (T1 axial SEMAC and T1 sagittal SEMAC). This significantly reduced the scan time. The full scan protocol used is attached as a supplementary file available online (online supplementary figure 1).
MRI scan analysis
MR image analysis was performed by two experienced musculoskeletal radiologists who were blind to the clinical condition of the patient. When there was disagreement between radiologists the images were re-reviewed. Re-review was required in 6/24 patients (26/144 measurements (18%)). Fibrotic tissue was defined as low signal intensity tissue on T1 and TIRM sequences and with low enhancement post-contrast. To avoid measurement of synovitic tissue as areas of fibrosis an internationally agreed and widely used tissue definition of synovitis was employed. The Outcome Measures in Rheumatology (OMERACT) definition of synovitis as ‘above-normal post gadolinium enhancement (signal intensity increase)’ was used and requires T1 gadolinium enhancement imaging. The use of gadolinium contrast in this research study is in line with International Society of Magnetic Resonance in Medicine (ISMRM) Safety Committee guidelines,19 which recommend its use where there is a potential benefit to patient care when GBMA could ‘advance scientific discovery’. Full ethical and HRA approval for use of contrast was obtained. Patients with reduced eGFR were excluded from the study (a contra-indication to use of gadolinium contrast). Six anatomical compartments were studied for each knee; suprapatella pouch, infrapatella fat pad, medial and lateral gutters and medial and lateral posterior compartments. Any tissue that met the defined criteria of fibrotic tissue in each compartment was measured across its maximum diameter. Measurements were made on axial T1 post-contrast images, while viewing T1 pre-contrast and fat-sat post-contrast images concurrently.
Statistical analysis
Differences between groups were analyzed using paired t-test (Graphpad Prism 6; GraphPad Software Inc, La Jolla, California, USA). Significance was set at p = < 0.05.
Results
No difference in BMI or age was found comparing the three groups (p = > 0.05, Table I). Time from primary to revision was significantly shorter in fibrotic revision patients (median 17.5 months (interquartile range (IQR) 13 to 24)) compared with 170 months (IQR 44 to 192) for non-fibrotic revisions (p = 0.003).
Protocol optimization resulted in significant improvement in image quality, enabling visualization of soft tissue structures around the implant. Fibrotic tissue was identified in at least one compartment in all patients studied (Table IV). Overall, 46 out of 48 (96%) of compartments studied in the fibrotic group contained fibrotic tissue, compared to 19/30 (63%) in the non-fibrotic revision cohort and 40/66 (61%) in the primary cohort (Tables IV and V). This is consistent with our histological analysis of primary and revision TKA tissue, which shows dense collagenous tissue in all revision TKA patients regardless of indication for revision.11
Table IV.
Tissue thickness (mm) and presence/absence of fibrotic tissue.
| Anatomical region | Patient group | Tissue thickness (mm) | Fibrotic tissue present | Fibrotic tissue > 4 mm thick present | ||
|---|---|---|---|---|---|---|
| Mean | SEM | p-value* | Patients, n (%) | Patients, n (%) | ||
| Suprapatellar | Fibrosis (8) | 5.1 | 1.0 | N/A | 8 (100) | 5 (63) |
| Revision (5) | 2.8 | 1.7 | 0.022 | 2 (40) | 2 (40) | |
| Primary (11) | 2.2 | 0.8 | 0.011 | 5 (45) | 4 (36) | |
| Infrapatellar | Fibrosis | 5.0 | 0.9 | N/A | 7 (88) | 7 (88) |
| Revision | 4.2 | 0.7 | 0.193 | 5 (100) | 2 (40) | |
| Primary | 2.3 | 0.6 | 0.011 | 7 (64) | 3 (27) | |
| Medial gutter | Fibrosis | 4.0 | 0.8 | N/A | 8 (100) | 5 (63) |
| Revision | 1.5 | 0.9 | 0.110 | 2 (40) | 2 (40) | |
| Primary | 2.0 | 0.4 | 0.032 | 8 (73) | 1 (9) | |
| Lateral gutter | Fibrosis | 4.2 | 0.5 | N/A | 8 (100) | 5 (63) |
| Revision | 3.0 | 0.9 | 0.041 | 4 (80) | 2 (40) | |
| Primary | 1.8 | 0.5 | 0.012 | 7 (64) | 1 (9) | |
| Posterior medial | Fibrosis | 4.3 | 0.6 | N/A | 8 (100) | 3 (38) |
| Revision | 1.3 | 0.7 | 0.030 | 3 (60) | 2 (40) | |
| Primary | 1.6 | 0.5 | 0.010 | 6 (55) | 0 (0) | |
| Posterior lateral | Fibrosis | 3.8 | 1.0 | N/A | 8 (100) | 2 (25) |
| Revision | 2.0 | 1.2 | 0.071 | 3 (60) | 2 (40) | |
| Primary | 1.6 | 0.4 | 0.014 | 7 (64) | 1 (9) | |
| Grouped measurements | Fibrosis | 4.4 | 0.2 | N/A | N/A | N/A |
| Revision | 2.5 | 0.4 | 0.021 | N/A | N/A | |
| Primary | 1.9 | 0.2 | 0.013 | N/A | N/A | |
N/A, non applicable; SEM, standard error of the mean.
t-test.
Table V.
Tissue thickness measurements (mm) for each patient.
| Patient | Suprapatellar | Infrapatellar | Medial gutter | Lateral gutter | Posterior medial | Posterior lateral |
|---|---|---|---|---|---|---|
| Fibrosis 1 | 3.7 | 5.0 | 1.5 | 2.8 | 3.4 | 2.8 |
| Fibrosis 2 | 9.2 | 8.6 | 8.8 | 4.3 | 7.3 | 9.8 |
| Fibrosis 3 | 4.2 | 5.0 | 2.4 | 4.4 | 3.5 | 3.5 |
| Fibrosis 4 | 1.5 | 6.2 | 5.0 | 6.9 | 3.9 | 1.0 |
| Fibrosis 5 | 5.5 | 4.0 | 1.5 | 2.8 | 2.0 | 2.0 |
| Fibrosis 6 | 5.5 | 4.0 | 4.2 | 4.1 | 4.8 | 4.9 |
| Fibrosis 7 | 9.3 | Not detected | 4.0 | 3.1 | 4.0 | 2.0 |
| Fibrosis 8 | 2.0 | 6 | 5.0 | 5.0 | 6.0 | 5.0 |
| Non-fibrosis revision 1 | 7.8 | 3.5 | 4.2 | 5.0 | 3.6 | 6.3 |
| Non-fibrosis revision 2 | Not detected | 6.2 | 3.1 | 4.6 | 2.0 | Not detected |
| Non-fibrosis revision 3 | 6 | 2.7 | Not detected | 2.8 | Not detected | 1.0 |
| Non-fibrosis revision 4 | Not detected | 2.9 | Not detected | Not detected | 1.0 | Not detected |
| Non-fibrosis revision 5 | Not detected | 5.5 | Not detected | 2.8 | Not detected | 2.5 |
| Primary 1 | 4.9 | 4.3 | Not detected | 2.9 | 3.4 | 2.1 |
| Primary 2 | 7.0 | 3.0 | 2.0 | 2.0 | Not detected | Not detected |
| Primary 3 | Not detected | Not detected | Not detected | Not detected | Not detected | 2.2 |
| Primary 4 | 2.1 | 2.5 | 2.2 | Not detected | Not detected | Not detected |
| Primary 5 | 6.0 | 4.0 | 4.0 | 2.0 | 3.0 | 2.0 |
| Primary 6 | Not detected | 5.0 | 3.7 | 3.5 | Not detected | Not detected |
| Primary 7 | Not detected | Not detected | Not detected | Not detected | 2.5 | 2.6 |
| Primary 8 | 4.3 | 3.9 | 3.0 | 4.0 | 3.4 | 2.8 |
| Primary 9 | Not detected | Not detected | 3.5 | 2.5 | 2.5 | 4.0 |
| Primary 10 | Not detected | Not detected | Not detected | 3.4 | 3.0 | 2.0 |
| Primary 11 | Not detected | 2.8 | 2.2 | Not detected | Not detected | Not detected |
Although fibrotic tissue was found in all knees, tissue was significantly thicker in fibrotic patients (4.4 mm ± 0.2 mm) versus non-fibrotic (2.5 mm ± 0.4 mm) and normal TKAs (1.9 mm ± 0.2 mm; p =< 0.05). Three MRI sequences are shown for a fibrotic patient in Figure 1. Thick fibrotic tissue is seen throughout the infrapatellar compartment and in the medial and lateral femoral compartments. This tissue shows low enhancement on T1 pre- and post-contrast and low enhancement on fat-saturated imaging. In contrast, axial T1 and fat-saturated images post-contrast of a healthy normal TKA are shown in Figure 2. A small amount of fibrotic tissue is highlighted in the infrapatellar compartment, but no tissue is seen in the posterior medial or lateral compartments, in contrast to Figure 1.
Fig. 1.
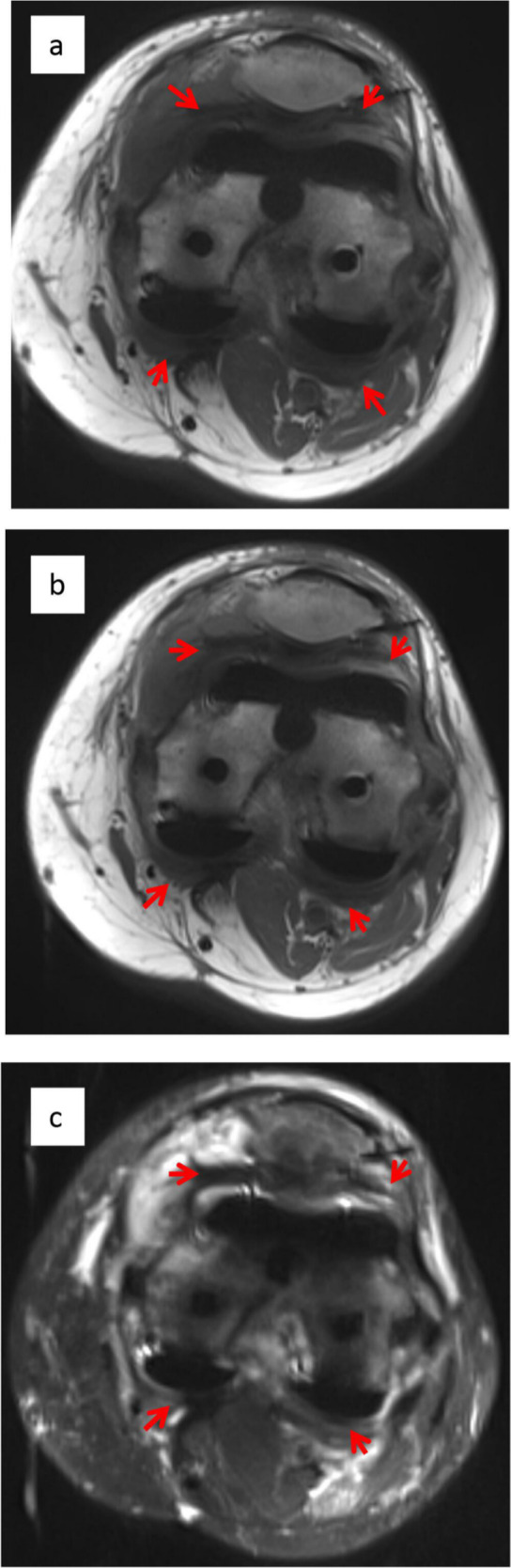
Intra-articular scarring in a patient with a clinical diagnosis of fibrosis. SEMAC images of a fibrotic post-total knee arthroplasty patient demonstrate significant fibrotic tissue in the posterior and anterior compartments (arrows): (a) Axial T1 pre-contrast, (b) axial T1 post-contrast, and (c) axial fat-saturated images.
Fig. 2.
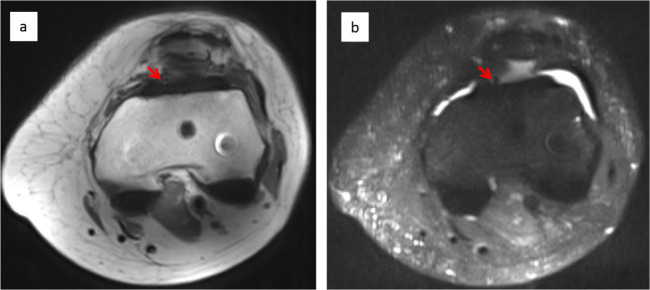
No fibrotic tissue in a healthy TKA. Axial T1 post-contrast (a) and fat-saturated (b) images of a healthy total knee arthroplasty, showing minimal fibrotic tissue in anterior and posterior compartments. A small area of fibrotic tissue detected in the anterior-medial suprapatella compartment is identified (arrows).
We set a threshold at 4 mm to define clinically significant fibrotic tissue and analyzed all anatomical compartments (six per knee) for all patients looking for presence or absence of > 4 mm thick tissue. This threshold was chosen because fibrotic tissue < 4 mm thick was considered unlikely to be mechanically substantial enough to cause ROM restriction, and intraoperative findings in patients with a clinical diagnosis of joint fibrosis consistently showed thick, abundant fibrotic tissue, which was not present in non-fibrotic revision TKAs. All fibrotic revision patients had > 4 mm thick tissue identified in at least one compartment, compared with 4/5 in the non-fibrotic group and 5/11 in the healthy TKA group.
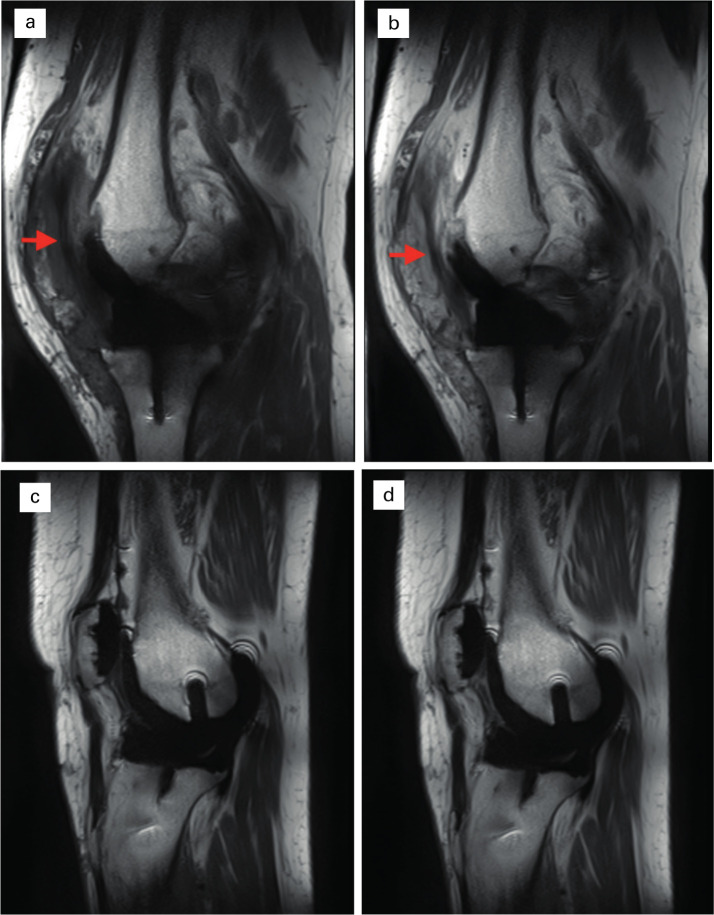
In fibrotic revision patients, 26/48 (54%) of compartments examined contained > 4 mm thick tissue, compared with 8/30 (27%, non-fibrotic) and 10/66 (15%, normal TKA). Examples of sagittal images of fibrotic and healthy TKAs are presented in Figure 3, demonstrating fibrotic tissue in the infrapatella compartment, with no tissue identified in the healthy TKA. These findings are consistent with surgical evaluation of the joint during revision surgery (Table II), which demonstrated thick intra-articular fibrosis in all patients.
Fig. 3.
Fibrotic tissue in the infrapatella region. Sagittal pre- and post-contrast T1 images comparing fibrotic patient (a) and (b) with non-fibrotic (c and d). Fibrotic tissue (arrows) is identified in the infrapatella region in a and b, extending underneath the patella between the infra- and suprapatella pouches. No such band of tissue is seen in a healthy TKA (c and d).
Outcomes following revision surgery for fibrosis
Revision patients were followed up to at least 18 months (18 to 25). All patients had severe pre-revision ROM restriction according to a consensus definition (< 70° flexion and/or > 20° extension deficit).1 Preoperative ROM was 53° ± 5° (30°-60° to 0°-80°). Although there was improvement of ROM post-revision, all patients had residual clinically significant restriction of ROM. Postoperative ROM was 86° ± 3° ( 0°-75° to 0°-105°). Value added ROM (the difference between preoperative and postoperative ROM, was 32° ± 8° (10° to 55°). This change moved four patients into the mild fibrosis severity group (90° to 100° flexion and/or < 10° extension deficit), three to the moderate group (80° to 90° flexion and/or 10° to 20° extension deficit), and one remained with a severe ROM restriction.
Discussion
The main finding of this study – the presence of MRI-measurable abundant thick fibrotic tissue in patients with a clinical diagnosis of knee fibrosis – is of benefit to knee surgeons faced with patients with stiff TKA and can facilitate the decision to debride the knee, restore ROM, and revise the implant. This scan sequence is commercially available and may be easily implemented by units using Siemens 1.5 T scanners. Fibrotic tissue appears to be present in all knees following TKA regardless of their indication for revision surgery.11 However, the amount, thickness, and location of this tissue will have a significant influence on the clinical condition of the patient. This is apparent intraoperatively, when intra-articular fibrous tissue may be found. However, the clinician currently has no diagnostic tools available to investigate intra-articular fibrous tissue, the presence of which is only confirmed arthroscopically or at open surgery.5 Therefore, the decision to undertake debridement can be challenging.
Ultrasound scanning may also be used to investigate fibrosis,20 but MRI was chosen for this study as it is less dependent on user-interpretation, re-analysis of the scan data is possible, and MR images are easily transferred from the surgeon to the radiology research team.
Confirmation that significant intra-articular scarring is present could support the surgeon in the decision-making process. Individual scan results were not available to be analyzed prior to revision surgery; therefore, tissue debridement and sampling was performed as routine in our clinical practice (at least six tissue samples, sent for histology and microbiology analysis). In future, MRI could be used to guide targeted sampling.
Here we have used a metal reducing MRI sequence, optimised to analyze periprosthetic soft tissues, to identify and quantify intra-articular fibrosis in patients with a clinical diagnosis of knee fibrosis. Fibrotic ‘scar’ tissue forms as part of the usual healing process post-TKA surgery, and fibrotic tissue was indeed identified in healthy TKAs more than one year following primary surgery. However, tissue measurements showed consistently thicker tissue in the fibrotic patient group. Setting a threshold at 4 mm for clinically significant fibrotic tissue showed this tissue to be present in > 50% of compartments in fibrotic patients, compared with 15% of compartments in healthy TKAs. At least one compartment contained > 4 mm thick tissue in all fibrotic patients but in only 5/11 normal primary TKAs. We set a threshold at 4 mm for ‘clinically significant fibrotic tissue’. This was arbitrary and based on the assumption that tissue < 4 mm thick is not likely to cause significant movement restriction, the primary problem in joint fibrosis. However, this requires further testing and evaluation in a larger cohort.
CT was used to determine rotational profile, as is routine investigation in our unit for problematic TKAs. However, assessment of implant rotation by MRI has been demonstrated to be accurate and reproducible21 and could be combined with investigation of fibrotic tissue in the assessment of stiff TKAs using MRI.
All samples underwent extended enrichment culture for ten days. However, a role for organisms, such as Propionibacterium acnes, that are challenging to culture, and may be an under-recognized cause of knee prosthetic joint infection, cannot be ruled out absolutely.
The appearance on MRI of synovial tissue has been carefully described in the setting of TKA,22 when polymeric-induced synovitis was differentiated from infection or scarring in a series of > 100 TKAs using a non-contrast enhanced intermediate-weighted Multi-Acquisition Variable Resonance Image Combination (MAVRIC) scan protocol. The scanning protocol used in the present study used the OMERACT definition of synovitic tissue as enhancing on T1 post-contrast imaging.23,24 This definition and the required imaging was selected to minimize the risk of measuring enhancing, synovial tissue as fibrotic (as defined by the OMERACT guidelines),23 but future MRI studies of the stiff TKA could characterise this tissue, which likely plays a significant role in the pathogenesis of scar formation.
There is a significant pro-inflammatory environment in all failed TKAs that we have so far studied12 and recent reports suggest that patients that develop stiffness post-TKA have a distinct raised postoperative pro-inflammatory response.25 In our research of the stiff TKA with significant movement restriction, we have been unable to robustly describe differences in the histological appearance11 or the cytokine profile of tissue and synovial fluid from patients undergoing revision surgery for stiffness compared against non-fibrotic revision patients.12 However, the clinical differences between these two patients groups are stark and the RNA expression profile of fibrotic tissue is significantly different from non-fibrotic tissue from revision TKAs.14 We therefore also studied five patients being revised for reasons other than fibrosis. Thick fibrotic tissue was more often seen in this group than the healthy TKA group, consistent with previous findings that scar tissue forms in all patients with failed TKAs. However, mean tissue thickness was significantly lower in the non-fibrotic revision group than the fibrotic group.
The primary limitation of this study is the small number of patients studied, particularly in the non-fibrotic revision group. These findings need replicating in a larger, cohort. We blinded the radiologists who interpreted the scan data, who were working remotely at a second institution, to the clinical condition of the patient, to reduce the risk of reporting bias.
Validated outcome measures should satisfy key filters (e.g. truth, discrimination, and feasibility).26,27 Here we show initial data that MRI can demonstrate fibrotic tissue around TKA (truth), demonstrated by intraoperative confirmation of massive fibrosis seen on MRI scan. However, we have not undertaken analysis of discrimination, such as reliability (intra- or interobserver)21,22 or sensitivity to change. These factors should be investigated before MRI could be used as a validated outcome measure for intra-articular fibrosis.
We report the outcomes at under 18 months post-revision for patients treated for stiffness. ROM was improved in all patients, with a mean value added ROM of ∼30°. These results are similar to those previously reported.28 It is important to note that all patients still had a clinically significant movement restriction, and it is possible that their ROM will deteriorate further over time.28,29 Outcomes for these patients are unpredictable and sub-optimal, and new treatments are required that target the biological basis of the disease.13
Conclusion
Here we report that a metal-artefact reduction MRI scan sequence may be used to demonstrate intra-articular fibrotic tissue in patients following TKA. The amount of this tissue was significantly increased in patients with restricted ROM in the absence of an identifiable cause, such as infection or implant mal-position. This scan sequence may be easily implemented by clinicians to investigate the stiff TKA and information gained could guide revision surgery. Future studies should evaluate the reproducibility and sensitivity to change of MRI detection of fibrotic tissue in a larger patient cohort. The clinical outcomes for debridement and revision for fibrosis are unpredictable and sub-optimal, although improvement of ROM was achieved in this cohort, and new treatments are required. This non-invasive tool can be used in future studies of fibrotic TKA patients to assess the efficacy of novel therapeutic interventions.
Author contributions
V. Attard: Collected and analyzed the data.
C. Y. Li: Collected and analyzed the data.
A. Self: Designed the study, Scan protocol development, Acquired and analyzed the data.
D. A. Mann: Conceptualized and designed the study, Analyzed the data.
L. A. Borthwick: Conceptualized and designed the study, Analyzed the data.
P. O’Connor: Designed the study, Scan protocol development, Acquired and analyzed the data.
D. J. Deehan: Conceptualized and designed the study, Analyzed the data, Provided patients, some of which were referred from surrounding units.
N. S. Kalson: Conceptualized and designed the study, Performed the study, Analyzed the data.
Funding statement
Funding was kindly provided (to N. Kalson) by the MRC (Confidence in Concept Grant BH153413). N. Kalson is funded by an NIHR Clinical Lectureship. D. A. Mann and L. A. Borthwick are supported in part by grants from the Medical Research Council (MRC) (MR/K001949/1 and MR/R023026/1), and the Wellcome Trust (204787/Z/16/Z). P. O’Connor is funded in part by the NIHR Leeds Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
ICMJE COI statement
L. A. Borthwick and D. A. Mann report employment, board membership and stock/stock options by FibroFind, which are unrelated to this article. D. J. Deehan reports grants/grants pending from WM Leech, Wellcome, MRC, and Dunhill, which are unrelated to this article. D. A. Mann and N. S. Kalson report an MRC grant, which is related to this article.
Acknowledgements
Thanks to Louise Fox and Sarah Branfoot (MR department), and Karen Smith and Yael Barron (orthopaedic research team), Newcastle Trust, for their contribution to MR imaging and patient recruitment.
Open access statement
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/
References
- 1.Kalson NS, Deehan DJ, Mann DA, Borthwick LAetal. International consensus definition of post-surgical knee joint fibrosis. Bone Joint J. 2016;98-B(11):1479–1488. [DOI] [PubMed] [Google Scholar]
- 2.Baker PN, Van der Meulen JH, Lewsey J, Gregg PJ. The role of pain and function in determining patient satisfaction after total knee replacement. J Bone Joint Surg Br. 2007;89-B(7):893–900. [DOI] [PubMed] [Google Scholar]
- 3.Thompson R, Novikov D, Cizmic Z, et al. . Arthrofibrosis After Total Knee Arthroplasty. Orthopedic Clinics of North America Elsevier. 2019;50(3):269–279. [DOI] [PubMed] [Google Scholar]
- 4.Vanlommel L, Luyckx T, Vercruysse G, Bellemans J, Vandenneucker H. Predictors of outcome after manipulation under anaesthesia in patients with a stiff total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc Springer Berlin Heidelberg. 2017;25(11):3637–3643. [DOI] [PubMed] [Google Scholar]
- 5.Schröder FF, Post CE, Wagenaar F-CBM, Verdonschot N, Huis In’t Veld RMHA. Mri as diagnostic modality for analyzing the problematic knee arthroplasty: a systematic review. J Magn Reson Imaging. 2020;51(2):446–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sneag DB, Bogner EA, Potter HG. Magnetic resonance imaging evaluation of the painful total knee arthroplasty. Semin Musculoskelet Radiol Thieme Medical Publishers. 2015;19(1):40–48. [DOI] [PubMed] [Google Scholar]
- 7.Zochowski KC, Miranda MA, Cheung J, et al. . MRI of Hip Arthroplasties: Comparison of Isotropic Multiacquisition Variable-Resonance Image Combination Selective (MAVRIC SL) Acquisitions With a Conventional MAVRIC SL Acquisition. American Journal of Roentgenology American Roentgen Ray Society. 2019;213(6):W277–W286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayter CL, Gold SL, Koff MF, et al. . MRI Findings in Painful Metal-on-Metal Hip Arthroplasty. American Journal of Roentgenology American Roentgen Ray Society. 2012;199(4):884–893. [DOI] [PubMed] [Google Scholar]
- 9.Koff MF, Burge AJ, Potter HG. Clinical magnetic resonance imaging of arthroplasty at 1.5 T. J Orthop Res John Wiley & Sons, Ltd. 2020;38(7):1455–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fritz J, Lurie B, Potter HG. MR Imaging of Knee Arthroplasty Implants. RadioGraphics Radiological Society of North America. 2015;35(5):1483–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdul N, Dixon D, Walker A, et al. . Fibrosis is a common outcome following total knee arthroplasty. Sci Rep Nature Publishing Group. 2015;5(1):16469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paish HL, Baldock TE, Gillespie CS, et al. . Chronic, Active Inflammation in Patients With Failed Total Knee Replacements Undergoing Revision Surgery. J Orthop Res John Wiley & Sons. 2019;373(11):1597–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paish HL, Kalson NS, Smith GR, et al. . Fibroblasts Promote Inflammation and Pain via Interleukin-1α-Induction of the Monocyte Chemoattractant CCL2. Am J Pathol. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bayram B, Limberg AK, Salib CG, et al. . Molecular pathology of human knee arthrofibrosis defined by RNA sequencing. Genomics. 2020;112(4):2703–2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu W, Pauly KB, Gold GE, Pauly JM, Hargreaves BA. SEMAC: slice encoding for metal artifact correction in MRI. Magn Reson Med. 2009;62(1):66–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jawhar A, Reichert M, Kostrzewa M, et al. . Usefulness of slice encoding for metal artifact correction (SEMAC) technique for reducing metal artifacts after total knee arthroplasty. Eur J Orthop Surg Traumatol. 2019;29(3):659–666. [DOI] [PubMed] [Google Scholar]
- 17.Berger RA, Crossett LS, Jacobs JJ, Rubash HE. Malrotation causing patellofemoral complications after total knee arthroplasty. Clinical Orthopaedics and Related Research. 1998;356(356):144–153. [DOI] [PubMed] [Google Scholar]
- 18.Chauhan SK, Clark GW, Lloyd S, et al. . Computer-assisted total knee replacement. A controlled cadaver study using a multi-parameter quantitative CT assessment of alignment (the Perth CT Protocol). J Bone Joint Surg Br. 2004;86(6):818–823. [DOI] [PubMed] [Google Scholar]
- 19.Gulani V, Calamante F, Shellock FG, Kanal E, Reeder SB. International Society for Magnetic Resonance in Medicine. Gadolinium deposition in the brain: summary of evidence and recommendations. Lancet Neurol. 2017;16(7):564–570. [DOI] [PubMed] [Google Scholar]
- 20.Boldt JG, Munzinger UK, Zanetti M, Hodler J. Arthrofibrosis associated with total knee arthroplasty: gray-scale and power Doppler sonographic findings. AJR Am J Roentgenol. 2004;182(2):337–340. [DOI] [PubMed] [Google Scholar]
- 21.Murakami AM, Hash TW, Hepinstall MS, et al. . MRI evaluation of rotational alignment and synovitis in patients with pain after total knee replacement. J Bone Joint Surg Br. 2012;94(9):1209–1215. [DOI] [PubMed] [Google Scholar]
- 22.Li AE, Sneag DB, Greditzer HG, et al. . Total Knee Arthroplasty: Diagnostic Accuracy of Patterns of Synovitis at MR Imaging. Radiology. 2016;281(2):499–506. [DOI] [PubMed] [Google Scholar]
- 23.Østergaard M, Peterfy CG, Bird P, et al. . The OMERACT Rheumatoid Arthritis Magnetic Resonance Imaging (MRI) Scoring System: Updated Recommendations by the OMERACT MRI in Arthritis Working Group. J Rheumatol. 2017;44(11):1706–1712. [DOI] [PubMed] [Google Scholar]
- 24.Østergaard M, Peterfy C, Conaghan P, et al. . OMERACT Rheumatoid Arthritis Magnetic Resonance Imaging Studies. Core set of MRI acquisitions, joint pathology definitions, and the OMERACT RA-MRI scoring system. J Rheumatol. 2003;30(6):1385–1386. [PubMed] [Google Scholar]
- 25.Malahias M-A, Birch GA, Zhong H, et al. . Postoperative Serum Cytokine Levels Are Associated With Early Stiffness After Total Knee Arthroplasty: A Prospective Cohort Study. J Arthroplasty. 2020;35(6S):S336–S347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boers M, Brooks P, Strand CV, Tugwell P. The OMERACT filter for Outcome Measures in Rheumatology. J Rheumatol. 1998;25(2):198–199. [PubMed] [Google Scholar]
- 27.Boers M, Kirwan JR, Wells G, et al. . Developing core outcome measurement sets for clinical trials: OMERACT filter 2.0. J Clin Epidemiol. 2014;67(7):745–753. [DOI] [PubMed] [Google Scholar]
- 28.Millett PJ, Williams RJ, Wickiewicz TL. Open Debridement and Soft Tissue Release as a Salvage Procedure for the Severely Arthrofibrotic Knee. Am J Sports Med. 2016;27(5):552–561 [DOI] [PubMed] [Google Scholar]
- 29.Yercan HS, Sugun TS, Bussiere C, et al. . Stiffness after total knee arthroplasty: prevalence, management and outcomes. Knee. 2006;13(2):111–117. [DOI] [PubMed] [Google Scholar]