Abstract
Background
Selenium or alpha‐tocopherol deficiency can cause neuromuscular disease. Beta‐carotene has limited documentation in horses.
Objective
To evaluate the effect of owner practices on plasma beta‐carotene concentration and risk of selenium and alpha‐tocopherol deficiencies.
Animals
Three‐hundred and forty‐nine adult (≥1 year), university and privately owned horses and mules.
Methods
Cross‐sectional study. Whole blood selenium, plasma alpha‐tocopherol, and plasma beta‐carotene concentrations were measured once. Estimates of daily selenium and vitamin E intake, pasture access, and exercise load were determined by owner questionnaire. Data were analyzed using t tests, Mann‐Whitney tests, parametric or nonparametric analysis of variance (ANOVA), Kruskal‐Wallis test, Spearman's correlation and contingency tables (P < .05).
Results
Nearly 88% of the horses received supplemental selenium; 71.3% received ≥1 mg/d. Low blood selenium concentration (<80 ng/mL) was identified in 3.3% of horses, and 13.6% had marginal concentrations (80‐159 ng/mL). Non‐supplemented horses were much more likely to have low blood selenium (odds ratio [OR], 20.2; 95% confidence interval [CI], 9.26‐42.7; P < .001). Supplemental vitamin E was provided to 87.3% of horses; 57.7% received ≥500 IU/d. Deficient (<1.5 μg/mL) and marginal (1.5‐2.0 μg/mL) plasma (alpha‐tocopherol) occurred in 15.4% and 19.9% of horses, respectively. Pasture access (>6 h/d) and daily provision of ≥500 IU of vitamin E was associated (P < .001) with higher plasma alpha‐tocopherol concentrations. Plasma beta‐carotene concentration was higher in horses with pasture access (0.26 ± 0.43 versus 0.12 ± 0.13 μg/mL, P = .003).
Conclusions and Clinical Importance
Suboptimal blood selenium and plasma alpha‐tocopherol concentrations occurred in 16.7% and 35.5% of horses, respectively, despite most owners providing supplementation. Inadequate pasture access was associated with alpha‐tocopherol deficiency, and reliance on selenium‐containing salt blocks was associated with selenium deficiency.
Keywords: alpha‐tocopherol, dietary supplements, equine, sodium selenite
Abbreviations
- NRC
National Research Council
- OSU
Oregon State University
1. INTRODUCTION
Deficiencies of the antioxidants selenium and alpha‐tocopherol can result in severe and frequently irreversible neuromuscular disease in horses. Clinical signs can include weakness, atrophy, rhabdomyolysis, abnormal gait or limb position, and fasciculations. Selenium deficiency consistently has been associated with skeletal and cardiac muscle damage, and inadequate alpha‐tocopherol status has been linked to degenerative myeloencephalopathy and neuroaxonal dystrophy, motor neuron disease, and vitamin E‐deficient myopathy in horses. 1 , 2 , 3 In addition, supplementation of horses with these antioxidants has been correlated with attenuation of oxidative stress markers and positive effects on fertility and immune function. 4 , 5 , 6 , 7
Soils of volcanic origin and acidic pH contain minimal selenium, and the risk of selenium deficiency is increased in nonsupplemented animals subsisting on grass or hay grown in selenium‐deficient soils. 8 , 9 Grasses and plants produce alpha‐tocopherol via photosynthesis, but content degrades substantially after harvest and storage as hay. 10 , 11 Horses predominantly fed cured hay rations are therefore at risk of alpha‐tocopherol deficiency. Additional factors including poor gastrointestinal absorption and genetics also have been hypothesized to contribute to alpha‐tocopherol deficiency. 12 , 13 Beta‐carotene is also predominantly found in fresh green forage and is subject to storage degradation. 14 Increased intake of beta‐carotene, a vitamin A precursor, has been associated with improved fertility and immune function in several species. 15 , 16 , 17
The National Research Council (NRC) recommends 1 mg of selenium daily for 400 kg horses in heavy or very heavy work, and to increase blood selenium concentrations in 1 to 6‐year‐old horses, with evidence that up to 3 mg daily might improve immune function. 15 The recommended daily intake of vitamin E is 1 IU/kg body weight, with 2 IU/kg body weight recommended for foals and exercising horses. 15 Although studies have identified an impact of both formulation (natural versus synthetic) and dose on alpha‐tocopherol status of horses, 18 current NRC guidelines do not specify a recommended formulation. Many owners actively provide these nutrients in fortified feeds or directed supplements to prevent deficiency. However, the many available products have variable composition and efficacy, and current labeling guidelines do not require specification of vitamin E formulation. 19 , 20 , 21 Currently, no dietary recommendations are available for beta‐carotene, and deliberate supplementation is uncommon.
Our main objective was to determine the impact of owner supplementation and management practices on whole blood selenium and plasma alpha‐tocopherol concentrations in horses located in a region where clinical disease from both deficiencies occurs. Plasma beta‐carotene concentration also was evaluated because it is influenced by similar management practices that affect alpha‐tocopherol, and currently has limited documentation in horses. It was hypothesized that owners often supplement horses inadequately, which, in combination with other specific management factors, could lead to a moderate prevalence of deficiency of these critical nutrients.
2. MATERIALS AND METHODS
2.1. Experimental animals and geographical region
For this cross‐sectional study, subject recruitment was accomplished via internet postings, an informational brochure, and regional veterinarians. Enrolled horses were located at 49 different properties in Oregon and were contained within an area of approximately 31 400 square miles. Soil selenium concentration within this area ranged from 0.12 ± 0.03 to 0.27 ± 0.16 ppm (average, 0.17 ppm); soils containing <0.6 ppm are considered selenium deficient. 22 , 23 Terrain in this region varied from valley with a cool summer Mediterranean climate (Köppen climate classification Csb) to high desert with a semiarid climate (Köppen climate classification BSk). Yearly, average precipitation across the sampled region ranged from 15 to 70 inches. 24 A total of 349 horses and mules were enrolled, but data from 11 horses subsequently were excluded as a result of young age (<1 year old; n = 6) or small body mass (miniature horses and ponies; n = 5) so as to achieve greater homogeneity of body mass and estimated nutrient requirements for adult horses weighing approximately 400 to 500 kg. Two horses with recent history of injection with a vitamin E/selenium‐containing product and 5 horses with incomplete dietary information also were excluded, leaving 331 horses. Study horses were affiliated with Oregon State University (OSU, n = 16) or were privately owned (n = 315). Animal use protocols were approved by the Institutional Animal Care and Use Committee of OSU.
2.2. Questionnaire
Owners, caretakers, or both completed a questionnaire regarding the management and feeding of each horse (Supplementary Figure S1). The questionnaire recorded signalment, use, frequency and duration of exercise, ration composition (including access to green pasture and source, amount and type of hay), and supplement provision. Exercise load was partitioned into (1) no forced exercise, (2) low exercise (<180 minutes per week), and (3) high exercise (>180 minutes per week) based on NRC definition. 15 Horses receiving oil or feed supplements containing ≥10% of calories from fat were defined as receiving dietary fat supplementation. Adequate green pasture access was defined as access to fresh, green grazing for >6 hours per day, which was estimated to provide a minimum 500 IU of alpha‐tocopherol based on the average alpha‐tocopherol content of fresh grass and estimated hourly pasture intake of a 500 kg horse. 10 , 25 Follow‐up telephone conversations with all owners to review completed questionnaires further clarified management history, including pasture quality, frequency, amount, and specific supplements and feeds provided over the preceding 6 months, including any changes in ration or exercise during that time period.
Estimates of the total amount and form of selenium (mg of selenomethionine versus sodium selenite) and vitamin E (IU of natural versus synthetic forms) in each horse's daily ration were collated from information provided in the questionnaire and independent evaluation of commercial feed and supplement labels by 2 of the authors (M.O. Pitel and E.C. McKenzie). Dietary selenium contributions were grouped into the selenomethionine category if specified on the label as “selenomethionine,” “selenium yeast,” or “organic selenium” and into the sodium selenite category if specified on the label as “sodium selenite,” “inorganic selenium,” or “selenium.” Similarly, dietary vitamin E was grouped into the natural category if specified on the label as “natural vitamin E,” “d‐alpha‐tocopherol,” “d‐alpha‐tocopheryl acetate,” “d‐alpha‐tocopherol acetate,” or “d‐alpha‐tocopheryl succinate” and grouped into the synthetic category if specified on the label as “synthetic vitamin E,” “dl‐alpha‐tocopherol,” “dl‐alpha‐tocopheryl acetate,” or “vitamin E supplement.” Any provided hay was assumed to contribute minimal selenium and alpha‐tocopherol to each ration, because all hay was Oregon sourced. 8 , 9 , 10 , 11 Access to a selenium‐containing salt block was documented but no estimates were made in regard to salt's contribution to total selenium content of each ration, because consumption could not be reliably confirmed or quantified.
2.3. Blood collection
A total of 15 mL of blood was collected into EDTA from the jugular vein of each horse, at a single time point. Sample acquisition from all horses was accomplished over 3 weeks in August 2017 to limit seasonal effects on studied variables. Plasma samples for alpha‐tocopherol and beta‐carotene analysis were protected from light after collection, centrifuged within 30 minutes, placed into cryovials, and frozen in a liquid nitrogen Dewar before being stored in a −80°C freezer until analysis at the Linus Pauling Institute of OSU using previously described methods. 26 , 27 Plasma alpha‐tocopherol concentrations were defined as deficient for concentrations <1.5 μg/mL, marginal for concentrations 1.5 to 2.0 μg/mL and adequate for concentrations >2.0 μg/mL3. Whole blood samples were refrigerated before selenium analysis and submitted weekly to the California Animal Health & Food Safety Laboratory in Davis, California. Whole blood selenium concentrations <80 ng/mL were defined as deficient, concentrations 80 to 159 ng/mL as marginal, concentrations 160 to 275 ng/mL as adequate and concentrations >275 ng/mL were defined as high. 13 , 28
2.4. Statistical analysis
To analyze the effects of selenium supplementation, horses receiving adequate supplementation, defined as ≥1 mg of selenium daily, were divided into 3 groups based on the predominant selenium form provided: (1) >90% of selenium as selenomethionine (SeM); (2) >90% as sodium selenite (NaSe); and (3) a combination of both forms with each representing <90% of daily intake (SeM+NaSe). Horses classified as inadequately supplemented with selenium (InSe) were receiving some selenium, but a total amount <1 mg daily. Horses with no directed selenium supplementation were considered nonsupplemented (NonSe), including horses with access to a selenium‐containing salt block.
To analyze the effects of vitamin E supplementation, horses were divided into those with adequate (P, >6 h/d grazing) and inadequate (NP, ≤6 h/d grazing) green pasture access. Horses receiving ≥500 IU of vitamin E daily were further subdivided into groups based on the predominant form of vitamin E provided: (1) >90% as natural (NatE‐P and NatE‐NP); (2) >90% as synthetic (SynE‐P and SynE‐NP); and (3) a combination of both forms with each representing <90% of daily intake (NatE+SynE‐P and NatE+SynE‐NP). Horses estimated to be receiving <500 IU of vitamin E daily were categorized as having inadequate or no supplementation (InE/NonE‐P and InE/NonE‐NP).
Descriptive information collated from the questionnaire included age, sex, breed, and athletic discipline of sampled horses. Statistical calculations were performed using Microsoft Excel (Microsoft Corp, Redmond, Washington), GraphPad Prism version 8 (GraphPad Software, San Diego, California), and SPSS Statistics V26 (Microsoft Corp). Normality testing of distribution was performed by D'Agostino and Pearson analysis.
Analysis of the association between exercise load and whole blood selenium concentration and the association between selenium supplementation group and whole blood selenium were performed using ordinary 1‐way analysis of variance (ANOVA) and Tukey's multiple comparison test. Comparison of the average total selenium intake in each of the selenium supplementation groups for horses receiving ≥1 mg of selenium daily was performed using a Kruskal‐Wallis test with Dunn's multiple comparisons test. The association between exercise load and plasma alpha‐tocopherol concentration was performed using a Kruskal‐Wallis test with Dunn's multiple comparisons test. The association between fat supplementation and plasma alpha‐tocopherol and between green pasture access of >6 h/d and plasma alpha‐tocopherol were performed using a Mann‐Whitney test. The association between vitamin E supplementation groups in horses grazing ≤6 h/d and plasma alpha‐tocopherol was performed using Brown‐Forsythe and Welch ANOVA testing and Dunnett's multiple comparison test. Comparison of the average total vitamin E intake in each of the vitamin E supplementation groups for horses receiving ≥500 IU/d was performed using a Kruskal‐Wallis test with Dunn's multiple comparisons test. The association between green pasture access and plasma beta‐carotene concentration was performed using a Mann‐Whitney test.
Contingency tables were used to generate odds ratios (OR) for: (1) selenium supplementation categories versus whole blood selenium concentration categories and (2) vitamin E supplementation categories versus plasma alpha‐tocopherol concentration categories. Contingency analysis was performed using Fisher's exact test to generate OR for 2 × 2 tables. Cross‐tabulation analysis was used to generate column proportion comparisons in contingency tables by Chi‐square and z‐test using Bonferroni P‐value adjustment. This analysis was applied to: (1) selenium supplementation categories versus whole blood selenium concentration categories and (2) vitamin E supplementation categories and green pasture access categories versus plasma alpha‐tocopherol concentration categories.
3. RESULTS
3.1. Population demographics
Horses included in the final data analysis (n = 331) consisted of 142 mares, 178 geldings, 9 stallions, 1 ovariectomized mare, and 1 horse of unknown sex. Represented breeds included Warmbloods (n = 99), Quarter Horses and Quarter Horse types (including Paints and Appaloosas, n = 70), Arabians (n = 58), Thoroughbreds (n = 27), Morgans (n = 13), Gypsy Vanners (n = 7), 54 horses of mixed or unknown breed (eg, mustangs), and 3 mules. Average age was 12.7 ± 7.2 years (range, 1‐32 years). In regard to athletic disciplines, horses participated in English pleasure (dressage and show jumping, n = 111), endurance (n = 32), trail riding (n = 30), Western pleasure (n = 26), and 3‐day eventing (n = 21). Remaining animals were used in breeding (n = 14), were retired or unbroken (n = 62) or participated in miscellaneous (eg, research, driving) or unknown activities (n = 35). In regard to exercise load, 114 horses were receiving no forced exercise, 116 horses received a low level of exercise, and 100 horses received a high level of exercise. The exercise load of 1 horse was unknown. The majority of horses (n = 215, 65.0%) were fed locally sourced grass hay, 101 (30.5%) were fed a mixture of locally sourced alfalfa and grass hay, 12 (3.6%) were fed local alfalfa, and 3 (0.9%) were fed no hay, receiving a pelleted feed product instead. Most horses (n = 294, 88.9%) were provided feed products in addition to hay or pasture, including fortified feed or grains, complete feeds, nonfortified pelleted feed or grains and top‐dressing supplements. Most commonly, horses were provided top‐dressing products in combination with fortified feed or grains (n = 81), with nonfortified pelleted feed or grains (n = 74), with both fortified and nonfortified feed or grains (n = 50), with complete feeds (n = 19), with both complete feeds and nonfortified feed or grains (n = 3), with all 3 products (n = 2) or with no additional products (n = 10). The remainder of the horses received fortified feed or grains only (n = 42), nonfortified feed or grains only (n = 7), both fortified and nonfortified feed or grains (n = 5) or complete feed only (n = 1). Owners provided a large variety of products (n = 127 different products), of which 75 contained selenium and 88 contained vitamin E. These products were a combination of fortified feed or grains (n = 23), complete feeds (n = 7), nonfortified pelleted feed or grains (n = 7) and top‐dressing supplements (n = 90); 16 of these products provided only vitamin E, selenium or both. A total of 174 (52.6%) horses had adequate (>6 h/d) pasture access and 157 (47.4%) had inadequate pasture access (≤6 h/d). Fat supplementation was provided to 94 (28.4%) horses. Eighty‐one percent of owners (109/134) reported utilizing their veterinarian for advice on nutritional information for their horses.
3.2. Selenium
Whole blood selenium concentration was normally distributed in the study population. Average whole blood selenium concentration for all sampled horses was 210 ± 66 ng/mL (n = 331). Deficient whole blood selenium concentrations (<80 ng/mL) were identified in 11 (3.3%) horses and marginal concentrations (80‐159 ng/mL) in 45 (13.6%). Adequate blood selenium concentrations (160‐275 ng/mL) were identified in the majority of horses (n = 226, 68.3%) with high concentrations (>275 ng/mL) present in the remaining 49 (14.8%, Table 1).
TABLE 1.
Estimated daily selenium intake (mean ± SD, mg/d) of all horses (n = 331) separated into groups based on selenium form and amount, and contingencies (column totals) of selenium supplementation groups and whole blood selenium concentration groups
| Selenium Supplementation Groups | ||||
|---|---|---|---|---|
| NonSe & InSe (n = 41 & 54) | NaSe (n = 46) | SeM (n = 143) | SeM+NaSe (n = 47) | |
| Se intake (mg/d) | None & 0.67 ± 0.24 | 3.04 ± 1.89 | 2.86 ± 1.29 | 2.84 ± 1.49 |
| Whole blood (Se) | ||||
| Deficient <80 ng/mL | 11 (11.6%)a | 0a,b | 0a | 0a, b |
| Marginal 80‐159 ng/mL | 30 (31.6%)a | 3 (6.50%)a,b | 11 (7.70%)a,b | 1 (2.10%)b |
| Optimal 160‐275 ng/mL | 47 (49.5%)b | 42 (91.3%)b | 102 (71.3%)b,c | 35 (74.5%)a,b |
| High >275 ng/mL | 7 (7.40%)b | 1 (2.20%)a | 30 (21.0%)c | 11 (23.4%)a |
Notes: Supplementation groups are horses receiving <1 mg selenium daily (NonSe & InSe) and those receiving ≥1 mg of selenium daily as either >90% sodium selenite (NaSe); >90% selenomethionine (SeM); or a combination of both at <90% each (SeM+NaSe). No significant differences were found among total daily dietary selenium intake in horses supplemented with ≥1 mg of selenium (P = 1 for all comparisons). Superscript letters a,b,c denote statistical significance (P < .05) within a supplementation group (ie, down a column).
Total daily selenium intake was not normally distributed. A total of 290/331 horses (87.6%) were receiving some additional selenium in their ration, of which 236 (71.3%) were considered adequately supplemented, receiving ≥1 mg/d (2.89 ± 1.46 mg/d) in various formulations, with no significant difference in daily selenium intake among supplementation groups (Table 1). Inadequately supplemented horses received <1 mg of daily selenium (InSe, n = 54) or no directed supplementation (NonSe, n = 41), but 26 of these horses had access to a selenium‐containing salt block (Table 1).
Horses in the NonSe group, including the 26 horses with access to selenium‐containing salt blocks, had significantly (P < .001) lower blood selenium concentrations compared to all other supplementation groups, and were 20 times (OR, 20.2; 95% confidence interval [CI], 9.26‐42.7; P < .001) more likely to have deficient blood selenium concentrations than horses receiving any amount of feed selenium supplementation (Figure 1). A higher proportion of inadequately supplemented horses had marginal blood selenium concentrations compared to adequately supplemented horses (24.1 versus 6.4%, P < .001). Of adequately supplemented horses, blood selenium concentration was significantly higher in the NaSe+SeM compared to the NaSe group (P = .02, Figure 1), despite no significant difference in daily selenium intake between the 2 groups (P = 1). No other differences in blood selenium concentration were noted among adequately supplemented horses. Regardless of supplement type provided, the risk of deficiency was significantly lower in horses supplemented with ≥1 mg of selenium daily (P < .001, Table 1). Whole blood selenium concentration was significantly higher in horses in the low (219 ± 66 ng/mL, n = 116) and high (219 ± 57 ng/mL, n = 100) exercise groups compared to horses with no forced exercise (194 ± 70 ng/mL, n = 114; P = .01 and P = .02, respectively).
FIGURE 1.
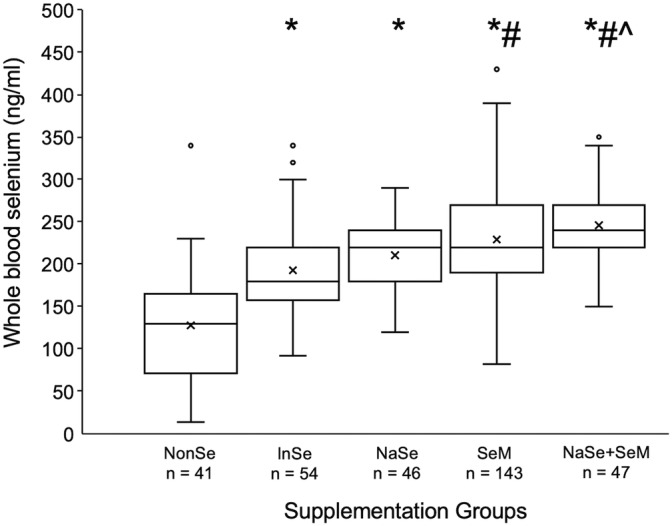
Box and whisker plot of whole blood selenium concentration versus supplementation groups; horses with no directed selenium supplementation, including 26 horses with access to a selenium‐containing salt block (NonSe), horses receiving some selenium but <1 mg daily (InSe), and horses receiving ≥1 mg of selenium per day, as either >90% sodium selenite (NaSe); >90% selenomethionine (SeM); or a combination of both at <90% each (SeM+NaSe). The box describes first, second (median) and third quartiles, “x” signifies the mean, whisker length is 1.5*IQR and dots are outliers. Number of horses included in each supplementation group is provided below the graph. Superscripts *, # and ^ indicate significant difference in whole blood selenium concentration from NonSe (P < .001), InSe (P < .001), and NaSe (P = .02) groups, respectively, via ordinary 1‐way ANOVA and Tukey's multiple comparison test
3.3. Alpha‐tocopherol
Plasma alpha‐tocopherol concentration was not normally distributed in the study population. Average plasma alpha‐tocopherol concentration from all sampled horses was 2.56 ± 1.13 μg/mL (n = 331; Table 2). Plasma alpha‐tocopherol concentrations considered as deficient (<1.5 μg/mL) were identified in 51 (15.4%) and as marginal (1.5‐2 μg/mL) in 66 (19.9%) horses (Table 3). Adequate plasma alpha‐tocopherol concentrations (>2 μg/mL) were identified in the remaining 214 horses (64.7%; Table 3).
TABLE 2.
Plasma alpha‐tocopherol (mean ± SD, μg/mL) of all horses (n = 331) separated into groups based on supplementation form and amount
| Supplementation group | Alpha‐tocopherol (μg/mL) | n (331) | % of horses |
|---|---|---|---|
| InE/NonE | 2.23 ± 0.91 | 140 | 42.3 |
| SynE | 2.73 ± 1.21 | 116 | 35.0 |
| NatE | 2.72 ± 1.13 | 54 | 16.3 |
| NatE+SynE | 3.36 ± 1.35 | 21 | 6.40 |
Notes: Supplementation groups are horses receiving <500 IU of vitamin E daily (excluding pasture access, InE/NonE) and those receiving ≥500 IU as either >90% natural source (NatE); >90% synthetic source (SynE); or a combination of both at <90% each (NatE+SynE).
TABLE 3.
Estimated daily vitamin E intake (mean ± SD, IU/d) of all horses (n = 331) separated into groups based on fresh, green pasture access (P, >6 h/d and NP, ≤6 h/d) and vitamin E form and amount, with contingencies (column totals) of vitamin E supplementation groups versus plasma alpha‐tocopherol concentration groups
| Vitamin E supplementation groups | ||||||||
|---|---|---|---|---|---|---|---|---|
| P | NP | |||||||
| InE/NonE | SynE | NatE | NatE+SynE | InE/NonE | SynE | NatE | NatE+SynE | |
| E intake (IU/d) | 106 ± 122 | 1129 ± 1291 | 2317 ± 3518 | 2121 ± 794^ | 205 ± 154 | 1020 ± 93 | 3460 ± 3362#,^ | 1895 ± 1317^ |
| Plasma (E) | ||||||||
| Deficient <1.5 μg/mL | 13 (16.1%)a | 4 (6.20%)a | 1 (5.30%)a | 0a | 19 (32.2%)a | 10 (19.6%)a | 3 (8.60%)a | 1 (8.30%)a |
| Marginal 1.5‐2 μg/mL | 19 (27.2%)a | 9 (13.9%)a,b | 3 (15.8%)a | 1 (11.1%)a | 14 (23.7%)a,b | 10 (19.6%)a | 10 (28.6%)a | 0a |
| Adequate >2 μg/mL | 49 (56.8%)a | 52 (80.0%)b | 15 (79.0%)a | 8 (88.9%)a | 26 (44.1%)b | 31 (60.8%)a | 22 (62.9%)a | 11 (91.7%)a |
Notes: Supplementation groups are horses receiving <500 IU of vitamin E daily (InE/NonE) and those receiving ≥500 IU as either >90% synthetic source (SynE); >90% natural source (NatE); or a combination of both at <90% each (NatE+SynE). Superscript symbols #, ^ indicate statistically significant differences in daily dietary vitamin E intake between supplementation groups for horses receiving ≥500 IU/d with (P) or without (NP) adequate pasture access. Superscript # indicates a significant difference from the SynE‐P group (P = .01) and ^ indicates a significant difference from the SynE‐NP group (P = .02 for NatE+SynE‐P, P = .04 for NatE+SynE‐NP and P = .003 for NatE‐NP). Superscript letters a, b denote statistical significance (P < .05) between supplementation groups (down a column).
Green pasture access (>6 h/d) was strongly associated with adequate plasma alpha‐tocopherol concentrations (P < .001). Under‐supplemented horses receiving <500 IU of supplemental vitamin E but with adequate pasture access (n = 81) were 2.76 times (OR, 2.76; 95% CI, 1.18‐6.10; P = .02) less likely to have deficient plasma alpha‐tocopherol concentrations compared with under‐supplemented horses with inadequate pasture access (n = 59, Figure 2).
FIGURE 2.
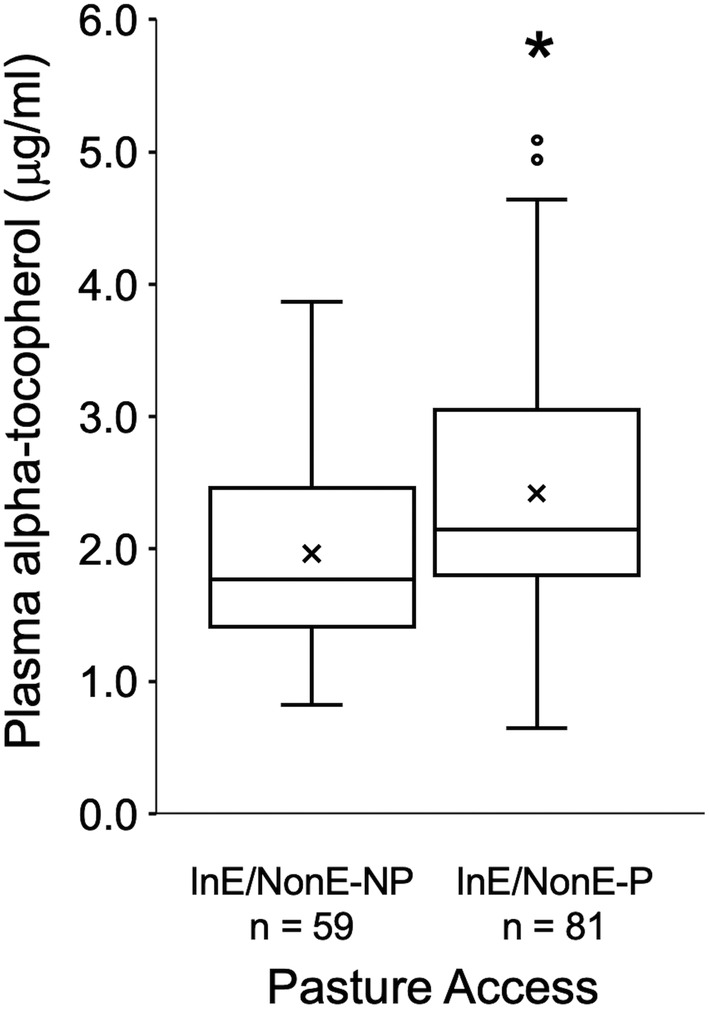
Box and whisker plot of plasma alpha‐tocopherol concentration versus pasture access for horses receiving <500 IU of daily vitamin E (InE/NonE groups). The 2 groups represent horses with (P, >6 h/d) and without (NP, ≤6 h/d) adequate fresh, green pasture access. The box describes first, second (median) and third quartiles, “x” signifies the mean, whisker length is 1.5*IQR and dots are outliers. Superscript * indicates a significant difference between groups (P = .004) via Mann‐Whitney test
Total daily dietary vitamin E intake was not normally distributed in the study population. A total of 191/331 (57.7%) horses were considered adequately supplemented, receiving ≥500 IU of vitamin E per day in feed or supplements, independent of pasture access. In horses with inadequate pasture access (≤6 h/d), vitamin E supplementation at ≥500 IU/d was significantly (P < .001) associated with higher plasma alpha‐tocopherol concentrations compared to horses with inadequate supplementation. This finding was consistent regardless of the form of vitamin E provided (Figure 3). Total daily vitamin E intake in horses receiving ≥500 IU/d, regardless of pasture access, was significantly higher in the NatE+SynE‐P, NatE+SynE‐NP, and NatE‐NP groups compared to the SynE‐NP group (P = .02, P = .04 and P = .003, respectively; Table 3) and also was significantly higher in the NatE‐NP compared to the SynE‐P group (Table 3; P = .01).
FIGURE 3.
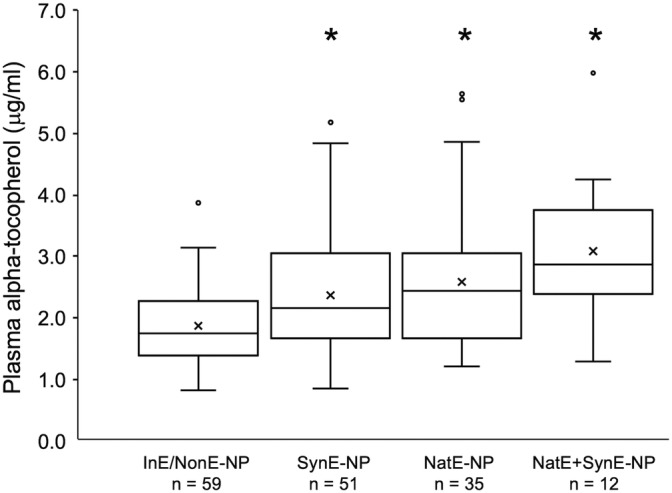
Box and whisker plot of plasma alpha‐tocopherol concentration versus supplement types for horses with inadequate pasture access (NP, grazing ≤6 h/d). Supplementation groups are horses receiving <500 IU of vitamin E daily (InE/NonE) and those receiving ≥500 IU as either >90% natural source (NatE); >90% synthetic source (SynE); or a combination of both at <90% each (NatE+SynE). The box describes first, second (median) and third quartiles, “x” signifies the mean, whisker length is 1.5*IQR and dots are outliers. Number of horses included in each supplementation group is provided below the graph. Superscript * indicates significant difference in plasma alpha‐tocopherol concentration from the InE/NonE‐NP group (P = .04, P = .03, and P = .004 for SynE‐NP, NatE‐NP, and SynE+NatE‐NP, respectively) via Brown‐Forsythe and Welch ANOVA testing and Dunnett's multiple comparison test
A very weak, positive correlation was found between plasma alpha‐tocopherol concentration and estimated daily vitamin E intake for horses with both adequate (n = 174) and inadequate (n = 157) pasture access (Spearman's R = 0.250, P = .004 and R = 0.314, P < .001, respectively; Figure 4). A weak, positive, correlation (Spearman's R = 0.316, P < .001) also was found between plasma alpha‐tocopherol and whole blood selenium concentrations (n = 331, Figure 5). No significant association of plasma alpha‐tocopherol concentration with exercise load or fat supplementation was identified.
FIGURE 4.
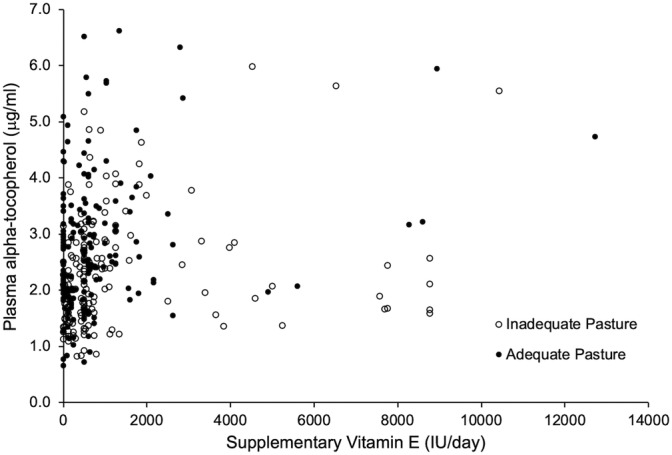
Correlation of plasma alpha‐tocopherol concentration and total IU of daily oral vitamin E supplementation (regardless of supplement type) for horses with (P, >6 h/d, n = 174) and without (NP, ≤6 h/d, n = 157) adequate fresh, green pasture access. Spearman's correlation for P: R = 0.250, P = .004 and for NP: R = 0.314, P < .001
FIGURE 5.
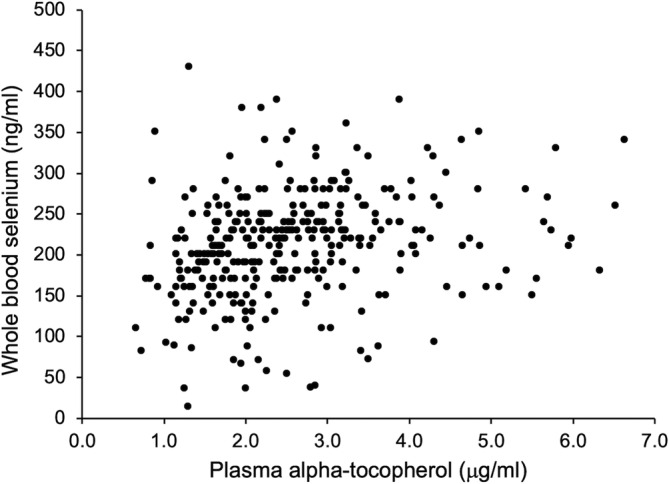
Correlation of plasma alpha‐tocopherol and whole blood selenium concentration for all horses (n = 331). Significant Spearman's correlation R = 0.316, P < .001
3.4. Beta‐carotene
Plasma beta‐carotene concentration was not normally distributed in the study population. Mean plasma beta‐carotene in all sampled horses was 0.19 ± 0.33 μg/mL (range, 0.009‐4.135; n = 331). Plasma beta‐carotene was significantly higher in horses with adequate pasture access (0.26 ± 0.43 μg/mL) than in those with inadequate access (0.12 ± 0.13 μg/mL; P = .003; Figure 6). A moderate, significant correlation was identified between plasma alpha‐tocopherol and beta‐carotene concentrations in inadequately supplemented horses, with a stronger correlation observed in horses with adequate (n = 174) versus inadequate (n = 157) pasture access (Spearman's R = 0.538, P < .001 and R = 0.457, P < .001, respectively).
FIGURE 6.
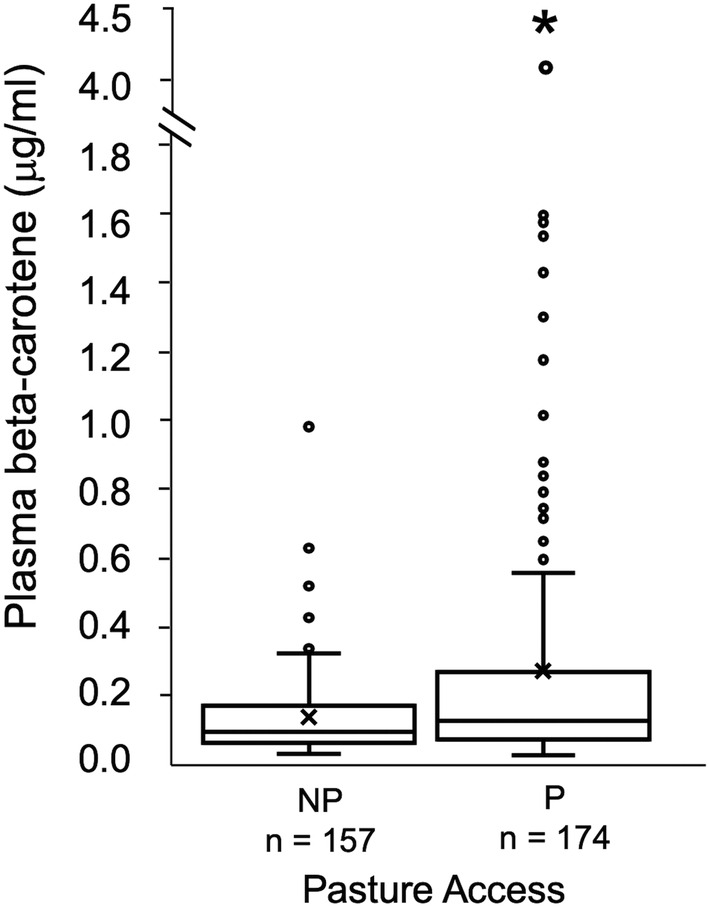
Box and whisker plot of plasma beta‐carotene concentration for horses with (P, >6 h/d) and without (NP, ≤6 h/d) adequate fresh, green pasture access. The box describes first, second (median) and third quartiles, “x” signifies the mean, whisker length is 1.5*IQR and dots are outliers. Superscript * indicates significant difference in plasma (beta‐carotene) based on pasture access (P = .003) via Mann‐Whitney test
4. DISCUSSION
In the population of our study, subclinical deficiency of 2 critical nutrients was prevalent, with 16.7% and 35.5% of all studied horses exhibiting suboptimal selenium and alpha‐tocopherol concentrations, respectively. These deficiencies occurred despite owners providing a very large range of feed and supplement products containing selenium and vitamin E to the majority of horses.
All horses in our study were considered at risk of selenium deficiency because they were located in a region with low soil selenium content and fed locally sourced hay. 8 , 9 , 23 Although previous work has identified improved absorption of selenomethionine compared to sodium selenite in horses, 29 in our study, form of delivered selenium had minimal influence on deficiency and total amount of delivered selenium was more important. Nearly 90% of horses in our study were receiving some directed amount of selenium daily, and no horses receiving ≥1 mg daily selenium had deficient blood concentrations (<80 ng/mL), regardless of form provided. Suboptimal blood selenium concentrations were most frequent in non‐ or inadequately supplemented horses, including a substantial proportion of horses with access to a selenium‐containing salt block. These findings corroborate previous reports that daily intake of at least 1 mg of selenium prevents deficiency in horses. 30 , 31 However, supplementation by selenium‐containing salt block, although popular, was inadequate, and should be discouraged as a sole form of supplementation. Supplemented salt blocks range in selenium content from between 1 ppm to a maximum of 120 ppm. The voluntary daily salt intake of horses is highly variable, but an average intake of 1 to 2 oz per day has been reported. 15 , 32 Therefore, although it is possible for a horse with adequate intake of an appropriate salt block product to ingest at least 1 mg of selenium per day, our results clearly indicate that this mode of supplementation failed in most horses when used alone. It is not clear if this outcome reflected the products utilized, inadequate intake, or a combination of these factors.
Higher blood selenium concentrations were documented in exercised versus nonexercised horses. Although a weak correlation, this difference may reflect more consistent supplementation practices by owners of exercised horses. However, no relationship was identified between alpha‐tocopherol and exercise load in our study to support this hypothesis for both nutrients.
Suboptimal plasma alpha‐tocopherol concentrations were identified in >33% of horses. Horses with inadequate pasture access (≤6 h/d) were more likely to have deficient or marginal alpha‐tocopherol status, in agreement with previous work indicating that fresh grass is a critical source of dietary vitamin E in horses, 33 and that horses with restricted pasture access are at increased risk of deficiency. 2 , 34 Nearly half of the horses in our study had inadequate pasture access, and nearly 40% of those horses were concurrently under‐supplemented, receiving <500 IU of vitamin E daily. These findings indicate that a large proportion of horses are potentially at risk of alpha‐tocopherol deficiency, even though nearly 90% of owners provide some amount of vitamin E in the ration. Furthermore, >20% of horses with apparently adequate vitamin E supplementation and pasture access were found to have deficient or marginal plasma alpha‐tocopherol concentrations. Specific factors contributing to suboptimal status in these horses were not readily apparent, but vitamin E amount, source, form, or quality, 4 , 20 failure to ingest the provided supplement or individual gastrointestinal absorption 12 are possible factors. In our study, horses were classified as having adequate pasture access if they had consistent access to fresh, green grazing over the previous 6 months for >6 h/d. An increase in serum alpha‐tocopherol concentration has been reported after summer grazing, with a decrease over the winter months. 35 Therefore, some of the horses identified to have adequate plasma alpha‐tocopherol concentrations in our study potentially could become deficient during the winter months. In addition, green pasture access may only be available for short periods in some regions, and adjustments to the ration may be required on a seasonal basis to mitigate dietary vitamin E shortfall. Dietary fat supplementation also reportedly can influence vitamin E requirement and absorption, 36 but no effect of fat supplementation on plasma alpha‐tocopherol concentration was identified in our study. Daily fluctuation in plasma alpha‐tocopherol concentration is also possible, particularly in deficient horses, and may have resulted in underestimation of the prevalence of alpha‐tocopherol deficiency in our study because only a single sample was obtained. 3 , 37 Age has been found to influence alpha‐tocopherol status in horses as has age and sex in humans, 3 , 38 but the demographics of our study group, confounded by management practices, precluded evaluation of these factors. Furthermore, 3 mules were included in the study, and their selenium and vitamin E requirements were presumed to be similar to the horses because of similar body size. Although the specific nutritional requirements and normal values for selenium and vitamin E have not been reported in mules, 1 study of a small, varied population of donkeys identified similar plasma vitamin E concentrations and substantially lower plasma selenium concentrations compared to horses. 39 It is unclear, however, if whole blood selenium concentrations in appropriately supplemented donkeys differ from those of horses, and reference intervals for both nutrients are unknown for mules.
Previous studies have indicated differences in absorption between natural and synthetic source vitamin E, with greater cerebrospinal fluid penetration and higher serum and muscle concentrations of alpha‐tocopherol in horses receiving supplementation as d‐alpha‐tocopherol. 4 , 18 , 20 , 40 In our study, no substantial difference was found in plasma alpha‐tocopherol concentrations between horses receiving NatE, SynE, or a combination of the 2 forms when evaluating either the total study population or only those horses with ≤6 h/d of pasture access. However, an important consideration is the disparity in total vitamin E provided in the rations, which were not standardized in this field study of owner practices. In addition, the wide range of supplements used likely varied in individual quality or bioavailability or both, which also could contribute to suboptimal alpha‐tocopherol status in apparently adequately supplemented horses. Subgrouping of natural vitamin E sources provided into esterified and nonesterified formulations (d‐alpha‐tocopherol and d‐alpha‐tocopheryl acetate) might have identified additional differences in plasma alpha‐tocopherol concentration among groups. However, this partition was not feasible because of concurrent use of multiple supplements as well as inconsistent and occasionally incomplete reporting of vitamin E form on product labels. Such challenges frequently are encountered when choosing a product and make selection of effective products difficult for owners, as indicated by our findings. As such, based on the finding of prevalent deficiency despite supplementation, it is recommended that alpha‐tocopherol status be determined by periodic testing, and veterinarians should work with clients to critically evaluate supplementation programs to determine the appropriate amount and type of supplementation for individual horses.
A small but significant correlation was found between whole blood selenium and plasma alpha‐tocopherol concentrations, likely reflecting the close relationship between these 2 antioxidants. When deficiency in 1 is suspected, testing for both is likely prudent, particularly for horses residing in areas of selenium‐deficient soil. A seasonal effect on both whole blood selenium and plasma alpha‐tocopherol concentrations has been reported previously, 28 , 41 and in our study samples were collected over a brief period to limit seasonal influences. A strong, positive correlation also was identified between plasma alpha‐tocopherol and beta‐carotene concentrations. This correlation was likely the result of a comparable influence of dietary and management practices on these nutrients, particularly pasture access. Plasma beta‐carotene concentrations were highly variable among horses, but generally were significantly higher with adequate pasture access, consistent with prior work. 42 Because beta‐carotene is not typically supplemented, high plasma concentrations usually indicate that horses have access to fresh green forage.
Our study had some limitations. Clinical or subclinical evidence of disease or dysfunction associated with nutrient deficiencies was not evaluated and because we relied on owner‐reported practices, results may have been affected by recall bias and incomplete reporting. To mitigate possible inaccuracies associated with owner reporting, all owners were contacted by telephone after submission of questionnaires to verify and complete survey responses, and daily intake of selenium and vitamin E was independently estimated. Analysis of selenium and vitamin E content was not performed on hay samples in our study to confirm the minimal contribution of forage to daily intake of these nutrients, instead minimal contribution was assumed based on existing literature relevant to the geographic region and the reported impact of processing on alpha‐tocopherol content of hay. 8 , 9 , 10 , 11
In conclusion, suboptimal selenium and alpha‐tocopherol status was identified in a large proportion of horses in our study, including horses with apparently adequate supplementation according to current NRC recommendations. Furthermore, common methods of supplementation, such as providing selenium in a salt block, clearly were inadequate for preventing deficiency. This finding indicates that supplementation plans must take into account a range of influencing factors, and that periodic testing should be utilized to confirm successful maintenance of selenium and alpha‐tocopherol status, even when apparently adequate supplementation regimens are employed. Because diseases of deficiency typically represent readily preventable disorders of management, our results indicate that improved management practices, guided by veterinarians, would benefit a large number of horses kept in circumstances similar to those of the study population.
CONFLICT OF INTEREST DECLARATION
Dr. Robert Stuart is the director and owner of Stuart Products, 112 Bedford Road, Bedford, Texas, which produces a vitamin E supplement and other nutritional products.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by the Oregon State University IACUC.
HUMAN ETHICS APPROVAL DECLARATION
The project did not meet the definition of “human subject” under the Common Rule (45 CFR 46) and therefore did not require review by the ethics committee at OSU that oversees research on humans.
Supporting information
Figure S1
ACKNOWLEDGMENTS
Scott Leonard and Maret Traber for assistance with vitamin E analysis.
Pitel MO, McKenzie EC, Johns JL, Stuart RL. Influence of specific management practices on blood selenium, vitamin E, and beta‐carotene concentrations in horses and risk of nutritional deficiency. J Vet Intern Med. 2020;34:2132–2141. 10.1111/jvim.15862
Funding information Oregon State University Department of Clinical Sciences; Stuart Products
REFERENCES
- 1. Bedford HE, Valberg SJ, Firshman AM, Lucio M, Boyce MK, Trumble TN. Histopathologic findings in the sacrocaudalis dorsalis medialis muscle of horses with vitamin E‐responsive muscle atrophy and weakness. J Am Vet Med Assoc. 2013;242:1127‐1137. [DOI] [PubMed] [Google Scholar]
- 2. Mohammed HO, Divers TJ, Summers BA, de Lahunta A. Vitamin E deficiency and risk of equine motor neuron disease. Acta Vet Scand. 2007;49:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Finno CJ, Valberg SJ. A comparative review of vitamin E and associated equine disorders. J Vet Intern Med. 2012;26:1251‐1266. [DOI] [PubMed] [Google Scholar]
- 4. Duberstein KJ, Pazdro R, Lee KC, Abrams A, Kane E, Stuart RL. Effect of supplemental vitamin E form on serum α‐tocopherol levels and blood oxidative stress parameters in response to a novel exercise challenge. J Equine Vet Sci. 2017;57:61‐66. [Google Scholar]
- 5. Youssef MA, El‐khodery SA, Ibrahim HMM. Effect of selenium and vitamin C on clinical outcomes, trace element status, and antioxidant enzyme activity in horses with acute and chronic lower airway disease. A randomized clinical trial. Biol Trace Elem Res. 2013;152:333‐342. [DOI] [PubMed] [Google Scholar]
- 6. Brummer M, Hayes S, Adams AA, Horohov DW, Dawson KA, Lawrence LM. The effect of selenium supplementation on vaccination response and immune function in adult horses. J Anim Sci. 2013;91:3702‐3715. [DOI] [PubMed] [Google Scholar]
- 7. Contri A, De Amicis I, Molinari A, et al. Effect of dietary antioxidant supplementation on fresh semen quality in stallion. Theriogenology. 2011;75:1319‐1326. [DOI] [PubMed] [Google Scholar]
- 8. Carter DL, Brown MJ, Allaway WH, Cary EE. Selenium content of forage and hay crops in the Pacific Northwest. Agron J. 1968;60:532‐534. [Google Scholar]
- 9. Jones GD, Droz B, Greve P, et al. Selenium deficiency risk predicted to increase under future climate change. Proc Natl Acad Sci USA. 2017;114:2848‐2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ballet N, Robert JC, Williams PEV. Vitamins in forages. In: Givens DI, Owen E, Axford RFE, Omed HM, editors. Forage Evaluation in Ruminant Nutrition Wallingford, UK: CABI; 2000:399–431. [Google Scholar]
- 11. Thafvelin B, Oksanen HE. Vitamin E and linolenic acid content of hay as related to different drying conditions. J Dairy Sci. 1966;49:282‐286. [DOI] [PubMed] [Google Scholar]
- 12. Díez de Castro E, Zafra R, Acevedo LM, et al. Eosinophilic enteritis in horses with motor neuron disease. J Vet Intern Med. 2016;30:873‐879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Finno CJ, Estell KE, Katzman S, et al. Blood and cerebrospinal fluid α‐tocopherol and selenium concentrations in neonatal foals with neuroaxonal dystrophy. J Vet Intern Med. 2015;29:1667‐1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pickworth CL, Loerch SC, Kopec RE, Schwartz SJ, Fluharty FL. Concentration of pro‐vitamin A carotenoids in common beef cattle feedstuffs. J Anim Sci. 2012;90:1553‐1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. NRC . Nutrient Requirements of Horses. 6th ed. Washington, DC: National Academies Press; 2007. [Google Scholar]
- 16. Brief S, Chew BP. Effects of vitamin A and beta‐carotene on reproductive performance in gilts. J Anim Sci. 1985;60:998‐1004. [DOI] [PubMed] [Google Scholar]
- 17. Ishida M, Nishijima Y, Ikeda S, et al. Effects of supplemental β‐caroteneon colostral immunoglobulin and plasma β‐carotene and immunoglobulin in Japanese black cows. Anim Sci J. 2018;89:1102‐1106. [DOI] [PubMed] [Google Scholar]
- 18. Ranard KM, Erdman JW. Effects of dietary RRR α‐tocopherol vs all‐racemic α‐tocopherol on health outcomes. Nutr Rev. 2018;76:141‐153. [DOI] [PubMed] [Google Scholar]
- 19. Montgomery JB, Wichtel JJ, Wichtel MG, et al. Effects of selenium source on measures of selenium status and immune function in horses. Can J Vet Res. 2012;76:281‐291. [PMC free article] [PubMed] [Google Scholar]
- 20. Fiorellino NM, Lamprecht ED, Williams CA. Absorption of different oral formulations of natural vitamin E in horses. J Equine Vet Sci. 2009;29:100‐104. [Google Scholar]
- 21. Association of American Feed Control Officials . Official Publication ‐ Association of American Feed Control Officials. 2019. th ed.
- 22. Selenium in Counties of the Northwestern US [Internet] . Reston (VA): U.S. Geological Survey. https://mrdata.usgs.gov/geochem/doc/averages/se/northwestern.html. Accessed June 4, 2020.
- 23. Gupta UC, Gupta SC. Selenium in soils and crops, its deficiencies in livestock and humans: implications for management. Commun Soil Sci Plant Anal. 2000;31:1791‐1807. [Google Scholar]
- 24. PRISM Precipitation & Dew Point Climatology Maps [Internet] . Reno (NV): Western Regional Climate Center. c2016‐2020. https://wrcc.dri.edu/Climate/prism_precip_maps.php. Accessed June 4, 2020.
- 25. Glunk EC, Pratt‐Phillips SE, Siciliano PD. Effect of restricted pasture access on pasture dry matter intake rate, dietary energy intake, and fecal pH in horses. J Equine Vet Sci. 2013;33:421‐426. [Google Scholar]
- 26. Podda M, Weber C, Traber MG, Packer L. Simultaneous determination of tissue tocopherols, tocotrienols, ubiquinols, and ubiquinones. J Lipid Res. 1996;37:893‐901. [PubMed] [Google Scholar]
- 27. Finckh B, Kontush A, Commentz J, Hübner C, Burdelski M, Kohlschütter A. Monitoring of ubiquinol‐10, ubiquinone‐10, carotenoids, and tocopherols in neonatal plasma microsamples using high‐performance liquid chromatography with coulometric electrochemical detection. Anal Biochem. 1995;232:210‐216. [DOI] [PubMed] [Google Scholar]
- 28. Muirhead TL, Wichtel JJ, Stryhn H, McClure J. The selenium and vitamin E status of horses in Prince Edward Island. Can Vet J. 2010;51:979‐985. [PMC free article] [PubMed] [Google Scholar]
- 29. Calamari L, Ferrari A, Bertin G. Effect of selenium source and dose on selenium status of mature horses. J Anim Sci. 2009;87:167‐178. [DOI] [PubMed] [Google Scholar]
- 30. Podoll KL, Bernard JB, Ullrey DE, DeBar SR, Ku PK, Magee WT. Dietary selenate versus selenite for cattle, sheep, and horses. J Anim Sci. 1992;70:1965‐1970. [DOI] [PubMed] [Google Scholar]
- 31. Maylin GA, Rubin DS, Lein DH. Selenium and vitamin E in horses. Cornell Vet. 1980;70:272‐289. [PubMed] [Google Scholar]
- 32. Schryver HF, Parker MT, Daniluk PD, et al. Salt consumption and the effect of salt on mineral metabolism in horses. Cornell Vet. 1987;77:122‐131. [PubMed] [Google Scholar]
- 33. Blakley BR, Bell RJ. The vitamin A and vitamin E status of horses raised in Alberta and Saskatchewan. Can Vet J. 1994;35:297‐300. [PMC free article] [PubMed] [Google Scholar]
- 34. de la Rúa‐Domènech R, Mohammed HO, Cummings JF, et al. Intrinsic, management, and nutritional factors associated with equine motor neuron disease. J Am Vet Med Assoc. 1997;211:1261‐1267. [PubMed] [Google Scholar]
- 35. Mäenpää PH, Koskinen T, Koskinen E. Serum profiles of vitamins A, E and D in mares and foals during different seasons. J Anim Sci. 1988;66:1418‐1423. [DOI] [PubMed] [Google Scholar]
- 36. Harris PL, Embree ND. Quantitative consideration of the effect of polyunsaturated fatty acid content of the diet upon the requirements for vitamin E. Am J Clin Nutr. 1963;13:385‐392. [DOI] [PubMed] [Google Scholar]
- 37. Vanschandevijl K, Nollet H, Deprez P, et al. Variation in deficient serum vitamin E levels and impact on assessment of the vitamin E status in horses. Vlaams Diergeneeskd Tijdschr. 2008;78:28‐33. [Google Scholar]
- 38. Ford ES, Schleicher RL, Mokdad AH, Ajani UA, Liu S. Distribution of serum concentrations of α‐tocopherol and γ‐tocopherol in the US population. Am J Clin Nutr. 2006;84:375‐383. [DOI] [PubMed] [Google Scholar]
- 39. Bazzano M, McLean A, Tesei B, Gallina E, Laus F. Selenium and vitamin E concentrations in a healthy donkey population in Central Italy. J Equine Vet Sci. 2019;78:112‐116. [DOI] [PubMed] [Google Scholar]
- 40. Pusterla N, Puschner B, Steidl S, Collier J, Kane E, Stuart RL. Alpha‐tocopherol concentrations in equine serum and cerebrospinal fluid after vitamin E supplementation. Vet Rec. 2010;166:366‐368. [DOI] [PubMed] [Google Scholar]
- 41. Stowe HD, Herdt TH. Clinical assessment of selenium status of livestock. J Anim Sci. 1992;70:3928‐3933. [DOI] [PubMed] [Google Scholar]
- 42. Dierenfeld ES, Hoppe PP, Woodford MH, Krilov NP, Klimov VV, Yasinetskaya NI. Plasma alpha‐tocopherol, beta‐carotene, and lipid levels in semi‐free‐ranging Przewalski horses (Equus przewalskii). J Zoo Wildl Med. 1997;28:144‐147. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1


