Abstract
Background
Torasemide is a potent loop diuretic with potential to treat congestive heart failure (CHF) in dogs.
Objective
Evaluate the efficacy and safety of torasemide compared to furosemide in dogs with first occurrence of CHF caused by degenerative mitral valve disease (DMVD).
Animals
Three hundred and nineteen dogs with new onset CHF attributable to DMVD.
Methods
Double‐blinded randomized noninferiority study of PO torasemide vs furosemide in addition to standard CHF treatment. The primary efficacy criterion was decreased pulmonary edema and cough and no worsening of dyspnea or exercise tolerance at day 14. Secondary endpoints included clinical response at day 84 and time to death, euthanasia, or premature study withdrawal for cardiac reasons.
Results
Torasemide q24h (n = 161) was noninferior to furosemide q12h (n = 158); percentage of dogs meeting primary efficacy criterion at day 14 was similar between groups (torasemide, 74.4% [95% confidence interval (CI), 66.8%‐81.0%] vs. furosemide, 73.5% [95% CI, 65.7%‐80.4%]; risk ratio [RR], 1.01; 95% CI, 0.89‐1.15; P = .87). Efficacy at day 84 showed similar results (RR, 1.05; 95% CI, 0.88‐1.25; P = .6). Dogs receiving torasemide had a longer time to endpoint and were less than half as likely to experience death, euthanasia, or premature study withdrawal (hazard ratio, 0.36; 95% CI, 0.19‐0.65; P = .001) than dogs receiving furosemide at any time during the study.
Conclusion and Clinical importance
Torasemide was noninferior to furosemide as first line PO treatment for new onset CHF caused by DMVD. Torasemide significantly decreased risk of cardiac‐related death or premature study withdrawal for cardiac reasons compared to furosemide.
Keywords: diuretics, heart disease, myxomatous mitral valve disease, torsemide
Abbreviations
- ACEIs
angiotensin‐converting enzyme inhibitors
- AEs
adverse events
- BUN
blood urea nitrogen
- CHF
congestive heart failure
- CI
confidence interval
- DMVD
degenerative mitral valve disease
- FAS
full analysis set
- FETCH
functional evaluation of cardiac health
- IQR
interquartile range
- LA : Ao
left atrium to aortic root diameter ratio
- LVIDdN
normalized left ventricular dimension at end‐diastole
- LVIDsN
normalized left ventricular dimension at end‐systole
- MMVD, myxomatous mitral valve disease; NT‐proBNP
N‐terminal pro‐B‐type natriuretic peptide
- PP
per protocol
- RR
risk ratio
- SAEs
serious adverse events
- VeDDRA
Veterinary Dictionary for Drug Related Affairs
- VHS
vertebral heart size
1. INTRODUCTION
Diuretics are a cornerstone treatment for congestive heart failure (CHF) caused by degenerative mitral valve disease (DMVD) in the dog. 1 Diuretics, such as furosemide, alleviate congestion by increasing urine and sodium excretion. In humans, and increasingly in dogs, loop diuretics with different pharmacokinetic and pharmacodynamic properties are of interest. One such loop diuretic is torasemide (also spelled as torsemide). Torasemide is a pyridine‐sulfonylurea diuretic with a chemical structure different than furosemide 2 and possesses a longer half‐life, higher PO bioavailability, and greater potency and duration of diuretic action as compared to furosemide. 3 , 4 , 5 , 6 , 7 In humans with chronic CHF, comparative benefits of long‐term treatment with torasemide vs furosemide include lower rates of hospitalization and severity of heart failure, 8 , 9 and in 1 study, 10 a 59% decrease in cardiac mortality.
Many studies 11 , 12 , 13 of torasemide in dogs are observational and utilize torasemide as either a rescue agent in dogs refractory to high doses of PO furosemide or as a replacement for furosemide in dogs with less severe CHF. In a randomized controlled European study, 14 torasemide was noninferior to furosemide with respect to alleviation of clinical signs of dyspnea, cough, and exercise intolerance, as well as evidence of ascites and radiographic signs of pulmonary edema, but most dogs already were receiving furosemide to treat previous or current signs of CHF. Thus, important questions regarding the efficacy of torasemide remain, especially in dogs experiencing their first episode of CHF without previous receipt of diuretics. Our hypothesis was that clinical efficacy of once daily PO torasemide for treatment of dyspnea, cough, exercise tolerance, and radiographic signs of pulmonary edema would be noninferior to twice daily PO furosemide after 14 days of treatment in dogs with first‐time CHF caused by DMVD. Secondary objectives included clinical efficacy after 84 days of treatment, frequency of adverse effects, owner treatment compliance, and time to cardiac‐related death or euthanasia or need to adjust cardiac treatment during the first 14 days.
2. MATERIALS AND METHODS
The CAnine Relief of Pulmonary Oedema by a DIuretic Easy Management (CARPODIEM) study was a pivotal prospective, multicenter, randomized, double‐blind, positive‐controlled, noninferiority, clinical field study. The study hypothesis and objectives were conceived by all authors. The study protocol was designed and executed by the sponsor, and the study results were interpreted and reported by all authors with full access to the study data. The study was conducted at 46 private veterinary practices in 7 European countries including Belgium, France, Germany, Italy, the Netherlands, Portugal, and Spain between February 2013 and December 2015. The study was approved by the national authorities in the participating countries and was conducted according to good clinical practice as part of the marketing authorization process required by the European Medicines Agency (Amsterdam, the Netherlands). Informed owner consent was obtained.
2.1. Study population
Inclusion criteria included left‐sided systolic heart murmur, body weight between 2.5 and 60 kg, echocardiographic diagnosis of DMVD based on characteristic thickening, prolapse, or elongation of the mitral valve leaflets, left atrial‐to‐aortic root ratio (LA : Ao) ≥ 1.5, and radiographic vertebral heart size (VHS) > 10.5. Inclusion criteria also included clinical and radiographic signs of left‐sided CHF that were scored using a predefined system (Table 1). A global clinical assessment of severity of CHF used a modification of previously reported clinical classification system (Table 2). 15 The study only included dogs with first‐time CHF caused by DMVD, defined as those naïve to loop and thiazide diuretic treatment, including furosemide, torasemide, and hydrochlorothiazide, with the following exception: pretrial furosemide was permitted if limited to PO administration for ≤48 hours before enrollment and at a dosage <4 mg/(kg d). Dogs were allowed pimobendan, spironolactone, or angiotensin‐converting enzyme inhibitors (ACEIs) before enrollment and were permitted to receive these medications as long as the dose had not changed within 4 weeks before enrollment. Exclusion criteria involved pregnant or lactating dogs, presence of congenital heart disease or acquired heart disease other than DMVD, known allergy to test medications, CHF of sufficient clinical severity to require hospitalization or parenteral use of diuretics, kidney disease defined as blood urea nitrogen (BUN) > 47 mg/dL (17 mM/L) or serum creatinine concentration > 1.6 mg/dL (141.6 μM/L), presence of ascites or pleural effusion, receipt of nonallowed medications including sildenafil, non‐ACEI vasodilators, digoxin, beta‐blockers, or calcium channel blockers, or known clinically important systemic or noncardiac organ‐related diseases expected to limit the dog's ability to tolerate medication or cause death during the course of the study.
TABLE 1.
Description of the ordinal clinical and radiographic scoring system
| Variable | Score | Clinical description |
|---|---|---|
| Cough | 0 | None |
| 1 | Occasional, few times a week | |
| 2 | Frequent, a few times a day | |
| 3 | Persistent, hourly, or more frequently | |
| Dyspnea | 0 | None |
| 1 | Breathing somewhat labored, deeper than usual | |
| 2 | Breathing marked and labored, dog able to lie in lateral recumbency | |
| 3 | Respiratory distress, dog tends to remain in sternal recumbency | |
| Exercise intolerance | 0 | None |
| 1 | Mild, dogs walks short distances without difficulty, but fatigue evident | |
| 2 | Severe, dog walks short distance with difficulty and severe fatigue | |
| 3 | Exercise is not possible | |
| Demeanor | 0 | Dog is alert and responsive, no depression |
| 1 | Mildly lethargic | |
| 2 | Minimally responsive | |
| 3 | Unresponsive | |
| Appetite | 0 | Normal |
| 1 | Slight decrease compared to normal | |
| 2 | Marked decreased compared to normal | |
| 3 | Anorexic | |
| Syncope | 0 | None |
| 1 | Four or fewer episodes per month | |
| 2 | More than 4 episodes per month | |
| Pulmonary edema | 0 | Findings within normal limits |
| 1 | Mild, interstitial lung pattern, alveolar pattern absent, on lateral view the border of the left atrium is well defined or only moderately obscured | |
| 2 | Moderate, alveolar pattern mainly perihilar with possible extension into caudal lobes | |
| 3 | Severe, alveolar pattern, perihilar and caudodorsal, cardiac silhouette and pulmonary vessels obscured | |
| Heart rhythm | 0 | Normal |
| 1 | Abnormal, arrhythmias of any type present |
TABLE 2.
Clinical classification system
| Class | Clinical definition |
|---|---|
| II | Clinical signs of heart failure are evident at rest or with mild exercise, and adversely affect the quality of life. Typical signs of heart failure include exercise intolerance, cough, tachypnoea, and mild respiratory distress (dyspnea). Hypoperfusion at rest is generally not present. |
| IIIa | Clinical signs of advanced congestive heart failure are immediately obvious. These clinical signs could include respiratory distress (dyspnea), profound exercise intolerance, or hypoperfusion at rest. Alveolar edema compatible with home treatment. |
| IIIb | Hospitalization is mandatory (cardiogenic shock, life‐threatening edema is present). |
2.2. Randomization, allocation, and tested treatments
Eligible dogs were centrally randomized using an adaptive randomized dynamic allocation scheme based on the following stratification factors: country, investigation site, initial severity of pulmonary edema, and the presence and nature of cardiac treatments during the month before the study. The allocation ratio was 1 : 1 to torasemide (ISEMID, Ceva Santé Animale, Libourne, France), the tested treatment, or furosemide (DIMAZON, MSD Santé Animale, Beaucouzé, France), the control treatment. On day 0 of enrollment (D0), dogs were given initial PO doses of study diuretic based on the severity of their pulmonary edema as follows: dogs in class II were given a target dose of once daily torasemide of 0.13 mg/kg (allowable range, 0.13‐0.25 mg/[kg d]) or furosemide 1.3 mg/(kg d) (allowable range, 1.3‐2.5 mg/[kg d]) divided into a twice daily dose; dogs with more severe heart failure in class IIIa were given a target PO dose of once daily torasemide of 0.26 mg/kg (allowable range, 0.26‐0.50 mg/kg) or PO furosemide 3.5 mg/(kg d) (allowable range, 3.5‐7.5 mg/[kg d]) divided into a twice daily dose. For dogs <4 kg, randomized to furosemide, and receiving ≤2.5 mg/(kg d), the daily PO dose of furosemide was given as a single daily dose. The specific torasemide and furosemide doses employed in our study were based on the production of equivalent degrees of diuresis, 3 , 16 , 17 and were in general agreement with a previous clinical trial. 14 For dogs initially in class IIIa, as signs of CHF improved, veterinarians were instructed to attempt to decrease the dose of study medications into the lower dose range. Subsequent up or down titration of doses was permitted as deemed necessary by the veterinarian. Specifically, dogs in class II were permitted to receive higher doses for a period of up to 5 days if the initial doses were not sufficient to alleviate clinical signs and dogs in class III were permitted to receive higher doses if lowering of dose after initial resolution of CHF was associated with reoccurrence of clinical signs. Thus, the study sought to achieve the lowest possible effective dose of either study drug for alleviation of clinical signs of CHF. Owners were instructed to record administration of daily study drug using a supplied diary.
2.3. Blinding
Blinding of treatment allocation involved a second participant on site, the treatment dispenser. The study site's veterinary investigator did the physical and diagnostic examinations and the treatment dispenser was responsible for allocating treatment, treatment dispensing and return, and assessing treatment compliance by the owner. Dog owners were blinded as to which study drug was given once vs twice daily. Predefined procedures were established to permit unblinding in the case of emergencies.
2.4. Evaluation schedule
Dogs underwent a variety of examinations on 5 different occasions including D0, day 4 ± 1 day (D4), day 14 ± 2 days (D14), day 42 ± 3 days (D42), and day 84 ± 3 days (D84) (Table 3). Additional visits were performed 4 days (±1 day) after any prescribed change in study drug. At each visit, dogs underwent a complete physical examination, thoracic radiography, blood sampling, and scoring of clinical signs as previously described. Owners completed a previously validated questionnaire involving functional evaluation of their dog's cardiac health (FETCH) 18 on D0, D14, D42, and D84. The treatment dispenser reviewed owner dose diaries and counted remaining tablets of study drug. Lateral and dorsoventral thoracic radiographs were performed to assess presence and severity of pulmonary edema and to measure VHS. 19 Transthoracic 2‐dimensional and M‐mode echocardiography was performed by the site veterinarian at D0 to make a diagnosis of DMVD, measure LA : Ao, and to measure and subsequently calculate 20 normalized left ventricular internal diameter at end‐diastole (LVIDdN) and end‐systole (LVIDsN). Blood samples were collected for analyses of urea, creatinine, sodium, potassium, phosphorus and chloride, PCV, and N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP). Urine samples were collected for measurement of urine specific gravity. All blood and urine assays were performed at a central commercial laboratory (IDEXX Laboratories, Ludwigsburg, Germany).
TABLE 3.
Diagnostic procedures performed during various study time points
| Procedure | D0 | D4 | D14 | D42 | D84 | Additional visit |
|---|---|---|---|---|---|---|
| Clinical examination | X | X | X | X | X | X |
| Electrocardiogram | X | |||||
| Thoracic radiograph | X | X | X | X | X | X |
| Echocardiography | X | X | X | X | ||
| Biochemical panel | X | X | X | X | X | X |
| NT‐proBNP | X | X | X | X | ||
| Quality of life | X | X | X | X |
Note: Additional visits were conducted in the event of dosage changes to the study drug or in response to adverse events.
Abbreviation: NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
2.5. Postinclusion removal from study
Postinclusion removal from the study occurred for any of the following reasons: need for a nonauthorized diuretic, study drug dose adjustment outside the allowable range, development of systemic or organ‐related disease that might have interfered with ability to continue the study protocol or interfered with ability to assess endpoints, major protocol deviations, owner noncompliance or owner decision to withdraw the dog from the study. Dogs also were removed from the study if pimobendan, ACEIs, or spironolactone was newly introduced during the 14‐day primary efficacy period of study, but beyond D14 investigators were allowed to modify or initiate ACEIs, pimobendan, or spironolactone treatment as needed.
2.6. Clinical efficacy endpoints
The primary efficacy criterion was treatment success at D14, defined as lowering of both radiographic pulmonary edema and clinical cough scores compared to D0, and no worsening of both dyspnea and exercise intolerance scores compared to D0. Secondary efficacy criteria involving treatment response at D14 and D84 were defined as decrease or maintenance at a score of 1 for radiographic pulmonary edema score accompanied by decrease in cough score and no worsening of dyspnea or exercise intolerance scores as compared to D0. Additional variables of study included satisfactory owner compliance in administering the study drug, which was arbitrarily defined as >80% of scheduled doses administered and the veterinarian's overall assessment of study treatment efficacy rated as very poor, poor, medium, good, or excellent at study end or date of last inclusion in the study.
2.7. Safety endpoints
Adverse events (AEs), defined as any unfavorable observation occurring after use of the study drug, whether or not considered related to the study drug, were tabulated. Serious AEs (SAEs) were defined AEs involving death or severe debilitation. Veterinarians identified the organ systems affected by AEs and SAEs using codes established by the Veterinary Dictionary for Drug Related Affairs (Combined VeDDRA list of clinical terms for reporting suspected adverse reactions in animals and humans to veterinary medicinal products; European Medicine Agency. EMA/CVMP/PhVWP/10418/2009).
2.8. Time to clinical outcome endpoint
The effect of study drug on time to a composite clinical outcome was studied. The composite outcome was defined as cardiac‐related death or euthanasia or worsening CHF that necessitated study drug doses exceeding the allowable dose or need for nonallowed cardiac drugs. Dogs that died spontaneously, suddenly, and unexpectedly without evidence of a noncardiac cause were assumed to have experienced cardiac‐related sudden death. The effect of study drug on time to all causes of death also was examined.
2.9. Statistical analysis
Double data entry was performed using a commercial study database (Clinsight v6.2/7; Ennov Clinical, Paris, France). Blinded review of data completeness and consistency was performed by the sponsor before the database being locked and undergoing statistical analysis by an independent company (Atlanstat, Rezé, France). The study was based on an expected D14 success rate for the reference treatment (ie, furosemide) and for the investigational product (ie, torasemide) of 65% with a noninferiority absolute difference margin of 15%. Therefore, the lowest confidence interval (CI) bound of the success rate would remain ≥50%. The margin of 15% corresponded to a noninferiority risk ratio (RR) margin of ρ = (pI − Δ)/pR = 50%/65% = 0.77. Thus, noninferiority would be demonstrated if the lower 95% CI of the RR was >0.77. With a power of 80%, a type I error of α = 2.5% and a 1‐sided test for a ratio of binomial proportions, 250 dogs (ie, 125 dogs per group) were estimated to be needed (East 5.4, Cytel Corporation, Cambridge, Massachusetts). In order to account for drop out, the number of dogs was increased 320 (ie, 160 dogs per group). Patient populations for the purposes of statistical analysis were defined as follows: a safety population that included all dogs that were randomized and received at least 1 dose of study drug, a full analysis set (FAS) consisting of the safety population except for any dogs in violation of inclusion criteria, a per protocol (PP) set that consisted of the FAS except for dogs with a major protocol violation affecting ability to assess treatment success at D14 (PP1), and per protocol set 2 (PP2) that consisted of PP1 dogs except those with major protocol violations affecting ability to assess treatment success at D84. The efficacy endpoints were assessed in the FAS and PP populations. Generally, analysis of the FAS population is considered the most conservative, but the opposite is true for noninferiority trials for which issues such as poor protocol adherence or randomization or treatment errors bias toward a result favoring noninferiority. Thus, demonstration of noninferiority in PP in addition to FAS populations considerably strengthens findings. 21 Success rates regarding treatment efficacy were analyzed using a Zou's modified Poisson approach. A Poisson distribution was assumed, and a log link was used with the GENMOD procedure in commercial software (SAS/STAT 9.4 and R 3.4.4. SAS Institute, Inc, Cary, North Carolina). Treatment, baseline pulmonary edema score, country, and cardiac treatments at inclusion were included in the statistical model as fixed effects. The estimate of the RR, the corresponding 95% CI, and P value were calculated. A mixed model with repeated measures was used to analyze change in the log‐transformed FETCH score between groups. Safety criteria, such as rate of AEs and SAEs, biochemical and hematologic variables, and assessment of owner treatment compliance, as well as time to cardiac death or euthanasia or premature study withdrawal, were assessed in the safety population. Patient demographics and diagnostic findings were tested for normality, summarized, and displayed as mean (SD), median (interquartile range [IQR]), or count (percentage). Comparisons between groups were made using unpaired t tests, Wilcoxon rank sum tests, Chi‐square, or Fisher's exact tests, as appropriate. Time to event analysis was performed using the Kaplan‐Meier method. The log‐rank test was used to compare survival functions between the 2 treatment groups. A shared gamma frailty regression model using a parametrical approach with study site as a random effect was used to detect effects of covariates on time to endpoint, first by univariable analysis, followed by multivariable analysis of variables with P < .2 using backward selection. Sensitivity testing was performed on the final model by including treatment and then performing forward selection starting with the variables with the lowest P values. Goodness‐of‐fit and proportional hazard assumption were assessed using Martingale residuals. P < .05 was considered significant.
3. RESULTS
3.1. Study populations
Three‐hundred and twenty‐one dogs were enrolled. Composition of the various study populations are presented in Figure 1. Three‐hundred and nineteen dogs that were randomized and received at least 1 dose of study drug comprised the safety population. Four dogs were found not to have fully adhered to the inclusion or exclusion criteria and were removed. The remaining 315 dogs, including 159 dogs randomized to torasemide and 156 dogs randomized to furosemide, comprised the FAS population. Twenty dogs in either treatment group were excluded between D0 and D14, resulting in 275 dogs in the PP1 analysis set. An additional 32 dogs in the torasemide group and 26 dogs in the furosemide group were removed between D14 and D84 resulting in 217 dogs in the PP2 analysis set. The baseline characteristics of the dogs in the FAS population are shown in Table 4. The study groups were well balanced at baseline with respect to demographic and diagnostic findings. The severity of clinical CHF signs such as cough, dyspnea, and exercise intolerance was similar between groups. The distribution of dogs between the 2 allowed clinical classes of CHF in each treatment group was also similar. The median daily doses of torasemide and furosemide administered to the FAS population at D0 were 0.15 mg/kg (IQR, 0.13‐0.17) and 1.58 mg/kg (IQR, 1.45‐1.74) in class II and 0.29 mg/kg (IQR, 0.26‐0.32) and 4.29 mg/kg (IQR, 4.29‐5.19) in class IIIa, respectively. The median daily doses of torasemide and furosemide administered to the FAS population at D14 was 0.16 mg/kg (IQR, 0.14‐0.18) and 1.60 mg/kg (IQR, 1.47‐1.77) in class II and 0.29 mg/kg (IQR, 0.27‐0.33) and 4.70 mg/kg (IQR, 4.36‐5.19) in class IIIa, respectively.
FIGURE 1.

Flowchart of 321 dogs enrolled in the study
TABLE 4.
Baseline characteristics of the full analysis set study population
| Variable | Torasemide (n = 159) | Furosemide (n = 156) | P value |
|---|---|---|---|
| Age (y) | 11.0 (3.1) | 11.3 (2.6) | .26 |
| Sex (M/F) | 90/69 | 89/67 | .94 |
| Body weight (kg) | 8.6 (5.4‐11.3) | 8.8 (6.4‐13.4) | .43 |
| Country | .99 | ||
| France | 64 (40.3%) | 59 (37.8%) | |
| Germany | 40 (25.2%) | 40 (25.6%) | |
| Spain | 18 (11.3%) | 20 (12.8%) | |
| The Netherlands | 17 (10.7%) | 16 (10.3%) | |
| Belgium | 14 (8.8%) | 16 (10.3%) | |
| Portugal | 4 (2.5%) | 5 (3.2%) | |
| Italy | 2 (1.3%) | 0 (0.0%) | |
| Heart murmur (1/2/3/4/5/6) | 7/6/37/74/35/0 | 8/7/33/82/26/0 | .72 |
| Pulmonary edema score (0/1/2/3) | 0/113/41/5 | 0/113/34/9 | .41 |
| VHS | 11.9 (1.0) | 11.7 (1.0) | .07 |
| LVIDdN | 1.79 (0.42) | 1.78 (0.41) | .81 |
| LVIDsN | 0.91 (0.24) | 0.92 (0.29) | .74 |
| LA : Ao | 2.04 (0.36) | 2.01 (0.40) | .54 |
| Heart rhythm (0/1) | 148/11 | 141/15 | .38 |
| Heart failure class (II/IIIa) | 113/46 | 113/43 | .79 |
| Cough score (0/1/2/3) | 0/26/110/23 | 0/37/97/22 | .26 |
| Dyspnea score (0/1/2/3) | 0/106/49/4 | 0/112/42/2 | .51 |
| Respiratory rate (breaths/min) | 42 (17) | 40 (18) | .43 |
| Demeanor score (0/1/2/3) | 55/94/10/0 | 56/94/6/0 | .61 |
| Exercise intolerance score (0/1/2/3) | 0/109/49/1 | 0/110/44/2 | .75 |
| Appetite score (0/1/2/3) | 106/40/12/1 | 111/25/14/6 | .06 |
| Syncope (0/1/2) | 138/15/6 | 136/16/4 | .81 |
| Duration of heart disease (d) | 128 (1‐544) | 101 (2‐489) | .56 |
| Pretrial cardiac treatment (Y/N) a | 83/76 | 87/69 | .53 |
| Pimobendan | 16 (19%) | 18 (21%) | |
| ACEIs | 35 (42%) | 40 (46%) | |
| ACEIs + pimobendan | 6 (7%) | 5 (6%) | |
| ACEIs + pimobendan + spironolactone | 5 (6%) | 4 (5%) | |
| ACEIs + spironolactone | 18 (22%) | 16 (18%) | |
| Pimobendan + spironolactone | 3 (4%) | 4 (5%) | |
| Pretrial furosemide treatment b (Y/N) | 17/142 | 18/138 | .81 |
| PCV (%) | 50 (6) | 51 (6) | .67 |
| BUN (mg/dL) | 21 (9) | 22 (11) | .85 |
| Creatinine (mg/dL) | 0.75 (0.25) | 0.79 (0.28) | .16 |
| Sodium (mM/L) | 148 (3) | 149 (3) | .18 |
| Chloride (mM/L) | 110 (5) | 111 (4) | .19 |
| Phosphorus (mM/L) | 1.36 (0.35) | 1.29 (0.33) | .06 |
| USG | 1.030 (0.013) | 1.030 (0.013) | .94 |
| NT‐proBNP (pg/mL) | 1632 (675‐3345) | 1379 (571‐3116) | .33 |
| CKCS/mixed breed/pure breed | 28/35/96 | 24/41/91 | .64 |
| FETCH score | 23 (13‐32) | 21 (13‐30) | .28 |
Note: Data listed as mean (SD), median (interquartile range), or count (percentage).
Abbreviations: ACEIs, angiotensin‐converting enzyme inhibitors; BUN, blood urea nitrogen; CKCS, Cavalier King Charles Spaniel; FETCH, functional evaluation of cardiac health; LA : Ao, left atrium to aortic root diameter ratio; LVIDdN, normalized left ventricular dimension at end‐diastole; LVIDsN, normalized left ventricular dimension at end‐systole; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; USG, urine specific gravity; VHS, vertebral heart size.
Excluding diuretics.
Received PO during previous month for no more than 2 days and at dose <4 mg/(kg d).
3.2. Treatment efficacy and compliance
The percentages of dogs that met the primary efficacy criterion, namely treatment success at D14, were not significantly different (torasemide, 116/156 [74.4%] vs furosemide, 111/151 [73.5%]; RR, 1.01; 95% CI, 0.89‐1.15; P = .87). The lower 95% CI value of the RR was >0.77, which fulfilled the prespecified noninferiority criterion between the 2 treatment groups. Evaluation of primary and secondary clinical endpoints in the PP1 and PP2 populations yielded similar results (Table 5). Thus, torasemide was noninferior to furosemide at both D14 and D84 in all analyzed populations. The effect of treatment on each of the individual efficacy criteria was similar between groups (see Figures S1‐S4). A total of 52/156 (33.3%) and 56/159 (35.2%) of dogs that received furosemide and torasemide had nondiuretic cardiac drugs adjusted during the study period (P = .81). Treatment compliance from D0 and D14 was achieved by a higher percentage of owners administering torasemide (138/141 [97.9%]) vs owners administering furosemide (124/135 [91.9%]; P = .02). Treatment compliance from D0 to D84 was >98% in both groups and not significantly different (P = .53). At study end, study veterinarians rated the efficacy of treatment as either good or excellent in 126/159 (79.2%) and 112/156 (71.8%) of dogs receiving torasemide and furosemide, respectively (P = .12). The differences in the change in log FETCH score from baseline to D14 and D84 were not significant between study groups (D14, 0.07; 95% CI, −0.16 to 0.30; P = .53; D84, 0.05; 95% CI, −0.20 to 0.31; P = .69).
TABLE 5.
Treatment success as count, percentage, and 95%CI, and RR of the torasemide group as compared to the furosemide group in the study's FAS, PP1, and PP2
| Torasemide | Furosemide | ||
|---|---|---|---|
| Primary efficacy criteria | |||
| Day 14 | FAS | 116/156 (3) | 111/151 (5) |
| 74.4% (66.8%‐81.0%) | 73.5% (65.7%‐80.4%) | ||
| RR | 1.01 (0.89‐1.15) | ||
| P = .87 | |||
| PP1 | 107/137 | 101/134 | |
| 78.1% (70.2%‐84.7%) | 75.4% (67.2%‐82.4%) | ||
| Risk ratio | 1.04 (0.92‐1.19) | ||
| P = .51 | |||
| Secondary efficacy criteria | |||
| Day 14 | FAS | 128/156 (3) | 121/151 (5) |
| 82.1% (75.1%‐87.7%) | 80.1% (72.9%‐86.2%) | ||
| Risk ratio | 1.02 (.92‐1.13) | ||
| P = .72 | |||
| PP1 | 116/137 | 108/134 | |
| 84.7% (77.5%‐90.3%) | 80.6% (72.9%‐86.9%) | ||
| Risk ratio | 1.06 (0.95‐1.17) | ||
| P = .29 | |||
| Day 84 | FAS | 93/153 (6) | 86/146 (10) |
| 60.8% (52.6%‐68.6%) | 58.9% (50.5%‐67.0%) | ||
| Risk ratio | 1.05 (0.88‐1.25) | ||
| P = .6 | |||
| PP2 | 67/104 | 69/105 | |
| 64.4% (54.4%‐73.6%) | 65.7% (55.8%‐74.7%) | ||
| Risk ratio | 1.01 (0.83‐1.22) | ||
| P = .93 | |||
Note: Number of FAS cases with missing data are noted in the parentheses. Noninferiority of a torasemide compared to furosemide was established if the lower 95% CI of the RR associated with the primary efficacy criteria on day 14 was >0.77.
Abbreviations: CI, confidence interval; FAS, full analysis set; PP1, per protocol population 1; PP2, per protocol population 2; RR, risk ratio.
3.3. Time to cardiac death or euthanasia or premature study withdrawal
Fifty‐eight dogs died or were euthanized from cardiac‐related causes or were withdrawn for worsening CHF (Table 6). Death or euthanasia occurred in 12/161 (7.5%) dogs receiving torasemide and in 11/158 (7.0%) dogs receiving furosemide (P = .87). Death or euthanasia for cardiac reasons occurred in 10/12 (83.3%) dogs receiving torasemide and in 11/11 (100%) dogs receiving furosemide. Noncardiac reasons for death in the remaining 2 dogs in the torasemide group included pyometra in 1 dog and sudden onset of stupor and ataxia presumed secondary to neurological disease in 1 dog. A significantly higher proportion of dogs receiving furosemide were prematurely withdrawn from the study as compared to those receiving torasemide (P = .02; Table 6). A significant difference was found in the time to endpoint between treatment groups (P = .04; Figure 2). The time to event endpoint primarily was driven by withdrawal from the study for worsening CHF (hazard ratio [HR], 0.25; 95% CI, 0.11‐0.56; P < .001) as opposed to cardiac‐related death (HR, 0.65; 95% CI, 0.26‐1.59; P = .34). Median time to event could not be calculated for either treatment group because >50% of dogs remained event‐free by the end of the study. Results of univariable regression analysis are shown in Table 7. The final multivariable model included 5 variables, consisting of study treatment, dyspnea score, LA : Ao, heart rate, and NT‐proBNP concentration (Table 8). After adjusting for dyspnea, heart rate, NT‐proBNP, and LA : Ao, treatment with torasemide was associated with a 64% reduction in risk of cardiac death or euthanasia or premature study withdrawal because of worsening CHF compared with furosemide at any time during the study period (HR, 0.36; 95% CI, 0.19‐0.65; P = .001). No difference was found in the survival function of dogs receiving torasemide vs those receiving furosemide with respect to all‐cause mortality (P = .65; Figure 3).
TABLE 6.
Numbers of dogs and percentage that reached the various components of the time to event outcome
| Outcome | Number of dogs | |
|---|---|---|
| Torasemide, 161 | Furosemide, 158 | |
| Cardiac death/euthanasia | 10 (6.2%) | 11 (7.0%) |
| Forbidden treatment or dose adjustment outside allowable range | 12 (7.5%) | 25 (15.8%) |
| Total | 22 (13.7%) | 36 (22.8%) |
FIGURE 2.

Kaplan‐Meier survival curves displaying the probability of cardiac death or euthanasia or premature study withdrawal because of cardiac reasons in the torasemide group (green solid line) vs the furosemide group (yellow dotted line). Cross marks represent censored observations
TABLE 7.
Shared gamma frailty univariable regression analysis of baseline variables displaying the HR and 95% CI
| Variable | HR | 95% CI | P value |
|---|---|---|---|
| Treatment (torasemide) | 0.51 | 0.30‐0.89 | .01 |
| Sex (male) | 1.31 | 0.76‐2.28 | .33 |
| Neuter (yes) | 0.83 | 0.47‐1.46 | .52 |
| Heart rhythm (normal) | 0.40 | 0.15‐1.08 | .07 |
| Dyspnea score (2 or 3) | 4.14 | 2.37‐7.24 | <.001 |
| Exercise intolerance score (2 or 3) | 2.27 | 1.30‐3.95 | .004 |
| Demeanor score (0 or 2) | 0.59 | 0.32‐1.07 | .08 |
| Heart failure stage (IIIa) | 3.93 | 2.27‐6.79 | <.001 |
| Appetite score (1, 2, or 3) | 2.03 | 1.18‐3.49 | .01 |
| Duration of heart disease (>270 d) | 0.81 | 0.45‐1.46 | .48 |
| Pretrial cardiac medications (yes) | 1.28 | 0.70‐2.36 | .43 |
| Age (>9 y) | 1.31 | 0.70‐2.46 | .40 |
| Weight (>15 kg) | 1.05 | 0.46‐2.42 | .91 |
| Heart rate (>150 bpm) | 2.37 | 1.35‐4.15 | .003 |
| Chloride (<106 mM/L) | 0.99 | 0.38‐2.54 | .98 |
| Creatinine (≥1.4 mg/dL) | 0.76 | 0.10‐5.71 | .79 |
| Phosphorus (>1.7 mM/L) | 1.40 | 0.66‐2.96 | .38 |
| NT‐proBNP (>900 pM/L) | 6.32 | 2.44‐16.38 | <.001 |
| Sodium (<142 mM/L) | 2.19 | 0.49‐9.75 | .3 |
| Potassium (>3.9 mM/L) | 0.45 | 0.13‐1.57 | .21 |
| BUN (>29 mg/dL) | 1.57 | 0.84‐2.94 | .16 |
| FETCH score (>21) | 3.27 | 1.74‐6.16 | <.001 |
| VHS (≥11.5) | 2.48 | 1.28‐4.78 | .007 |
| LA : Ao (≥1.9) | 3.37 | 1.65‐6.89 | .001 |
| LVIDdN (≥1.8) | 3.28 | 1.73‐6.22 | <.001 |
| LVIDsN (≥0.9) | 1.22 | 0.70‐2.13 | .48 |
Abbreviations: BUN, blood urea nitrogen; CI, confidence interval; FETCH, functional evaluation of cardiac health; HR, hazard ratio; LA : Ao, left atrium to aortic root diameter ratio; LVIDdN, normalized left ventricular dimension at end‐diastole; LVIDsN, normalized left ventricular dimension at end‐systole; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; VHS, vertebral heart size.
TABLE 8.
Shared gamma frailty multivariable regression model displaying the HR and 95% CI
| Variable | HR | 95% CI | P value |
|---|---|---|---|
| Treatment (torasemide) | 0.36 | 0.19‐0.65 | .001 |
| Dyspnea (score 2 or 3 vs 1) | 4.09 | 2.22‐7.51 | <.001 |
| NT‐proBNP (>900 pM/L) | 3.85 | 1.42‐10.43 | .008 |
| Heart rate (>150 bpm) | 1.79 | 1.00‐3.18 | .05 |
| LA : Ao (≥1.9) | 3.00 | 1.39‐6.47 | .005 |
Note: Variance of the study site, 0.74 (SE = 0.43, P = .04).
Abbreviations: CI, confidence interval; HR, hazard ratio; LA : Ao, left atrium to aortic root diameter ratio; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
FIGURE 3.
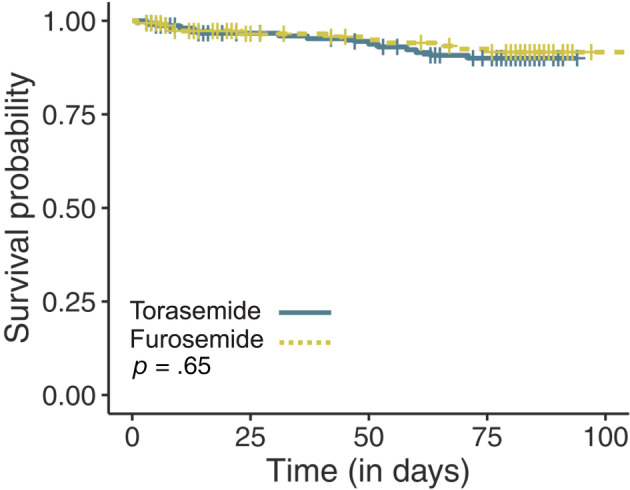
Kaplan‐Meier survival curves displaying the probability of all‐cause mortality in the torasemide group (green solid line) vs the furosemide group (yellow dotted line). Cross marks represent censored observations
3.4. Safety assessment
Over the 84‐day study duration, 377 non‐SAEs were recorded in 173/319 (54.2%) dogs, including 229 events in 97/161 (60.2%) dogs receiving torasemide and 148 events in 76/158 (48.1%) dogs receiving furosemide (P = .03; Table 9). The most common non‐SAEs in each treatment group involved renal disorders, including 107 AEs in 67/161 (41.6%) dogs receiving torasemide and 46 AEs in 30/158 (19.0%) dogs receiving furosemide (P < .001). Renal insufficiency, primarily reported as BUN or serum creatinine concentrations above the normal reference range, comprised the majority of renal or urinary disorders and was more commonly reported in the torasemide group (Table 10). Most instances of renal insufficiency (124/133 [93.2%]) were not specifically treated and the proportion that either improved or remained unchanged without treatment was similar between groups (torasemide, 86/94 [91.5%]; furosemide, 37/39 [94.9%]; P = .72).
TABLE 9.
Adverse events and serious AEs reported by the clinical investigators according to the Veterinary Dictionary for Drug Related Affairs (VeDDRA) code
| Torasemide, 161 | Furosemide, 158 | P value | |||
|---|---|---|---|---|---|
| Events | Dogs | Events | Dogs | ||
| Non‐SAEs | 229 | 97 (60.2) | 148 | 76 (48.1) | .03 |
| Behavioral disorders | 0 (0) | 0 | 2 (1.4) | 1 (0.6) | .5 |
| Blood and lymphatic | 1 (0.4) | 1 (0.6) | 9 (6.1) | 6 (3.8) | .07 |
| Cardiovascular | 7 (3.1) | 6 (3.7) | 5 (3.3) | 5 (3.2) | 1.0 |
| Digestive tract | 31 (13.5) | 24 (14.9) | 35 | 27 (17.1) | .6 |
| Ear and labyrinth | 1 (.4) | 1 (0.62) | 2 (1.4) | 2 (1.3) | .62 |
| Eye | 4 (1.7) | 4 (2.5) | 6 (4.1) | 6 (3.8) | .54 |
| Heptaobillary | 3 (1.3) | 3 (1.9) | 2 (1.4) | 1 (0.6) | .62 |
| Metabolism and nutrition | 5 (2.2) | 4 (2.5) | 1 (0.7) | 1 (0.6) | .37 |
| Musculoskeletal | 10 (4.4) | 8 (5.0) | 3 (2.0) | 3 (1.9) | .22 |
| Neurological | 6 (2.6) | 6 (3.7) | 1 (0.7) | 1 (0.6) | .12 |
| Renal and urinary | 107 (46.7) | 67 (41.6) | 46 (31.1) | 30 (19.0) | <.001 |
| Cystitis | 4 (1.7) | 4 (2.5) | 1 (0.7) | 1 (0.6) | .37 |
| Polyuria | 2 (0.9) | 2 (1.2) | 1 (0.7) | 1 (0.6) | 1.0 |
| Renal insufficiency | 94 (41.0) | 63 (39.1) | 39 (26.4) | 26 (16.5) | <.001 |
| ‐Increased BUN | 43 (18.8) | 36 (22.4) | 24 (16.2) | 19 (12.0) | .02 |
| ‐Increased creatinine | 10 (4.4) | 9 (5.6) | 10 (6.8) | 10 (6.3) | .78 |
| ‐Increased renal markers | 27 (11.8) | 26 (16.1) | 1 (0.7) | 1 (0.6) | <0.001 |
| ‐Renal failure | 1 (0.4) | 1 (0.6) | 1 (0.7) | 1 (0.6) | 1.0 |
| ‐Renal insufficiency | 10 (4.4) | 8 (5.0) | 2 (1.4) | 2 (1.3) | .1 |
| ‐Uremia | 3 (1.3) | 2 (1.2) | 1 (0.7) | 1 (0.6) | 1.0 |
| Urinary incontinence | 4 (1.7) | 4 (2.5) | 2 (1.4) | 2 (1.3) | .68 |
| Urinary tract disorder | 2 (0.9) | 2 (1.2) | 0 (0) | 0 (0) | .5 |
| Urine abnormalities | 1 (0.4) | 1 (0.6) | 2 (1.4) | 1 (0.6) | 1.0 |
| Urolithiasis | 0 (0) | 0 (0) | 1 (0.7) | 1 (0.6) | .5 |
| Reproductive | 3 (1.3) | 3 (1.9) | 1 (0.7) | 1 (0.6) | .62 |
| Respiratory | 13 (5.7) | 12 (7.5) | 12 (8.1) | 12 (7.6) | .96 |
| Skin and appendages | 1 (0.4) | 1 (0.6) | 6 (4.1) | 6 (3.8) | .07 |
| Systemic | 36 (15.7) | 23 (14.3) | 16 (10.8) | 15 (9.5) | .19 |
| Uncoded | 1 (0.4) | 1 (0.6) | 1 (0.7) | 1 (0.63) | 1.0 |
| SAEs | 49 | 28 (17.4) | 21 | 16 (10.1) | .06 |
| Behavioral | 0 (0) | 0 (0) | 1 (5) | 1 (.63) | .5 |
| Cardiovascular | 4 (8) | 4 (2.5) | 5 (24) | 3 (1.9) | 1.0 |
| Digestive tract | 5 (10) | 4 (2.5) | 0 (0) | 0 (0) | .05 |
| Metabolism and nutrition | 1 (2) | 1 (0.6) | 0 (0) | 0 (0) | 1.0 |
| Musculoskeletal | 0 (0) | 0 (0) | 2 (10) | 2 (1.3) | .24 |
| Neurological | 3 (6) | 3 (1.9) | 0 (0) | 0 (0) | .28 |
| Renal and urinary | 10 (20) | 9 (5.6) | 1 (5) | 1 (0.6) | .01 |
| Polyuria | 1 (2) | 1 (0.6) | 0 (0) | 0 (0) | 1.0 |
| Renal insufficiency | 9 (12) | 8 (5.0) | 1 (4.8) | 1 (0.6) | .04 |
| ‐Acute renal failure | 1 (2) | 1 (0.6) | 1 (4.8) | 1 (0.6) | 1.0 |
| ‐Chronic renal failure | 1 (2) | 1 (0.6) | 0 (0) | 0 (0) | 1.0 |
| ‐Increased BUN | 2 (4) | 2 (1.2) | 0 (0) | 0 (0) | .5 |
| ‐Increased renal markers | 4 (8) | 4 (2.5) | 0 (0) | 0 (0) | .13 |
| ‐Renal failure | 1 (2) | 1 (0.6) | 0 (0) | 0 (0) | 1.0 |
| Reproductive | 4 (8) | 4 (2.5) | 0 (0) | 0 (0) | .12 |
| Respiratory | 4 (8) | 4 (2.5) | 6 (29) | 6 (3.8) | .5 |
| Systemic | 18 (37) | 12 (7.5) | 6 (29) | 6 (3.8) | .16 |
Note: Data are listed as number of events (%), number of dogs (%), and comparison of number of affected dogs between study groups. Coding subcategories are listed in italics for any system organ class that demonstrated significant differences between study groups. Some dogs had AEs in >1 VeDDRA code.
Abbreviations: AEs, adverse events; BUN, blood urea nitrogen; SAEs, serious adverse events; VeDDRA, Veterinary Dictionary for Drug Related Affairs.
No significant difference was found in the percentage of dogs experiencing SAE between groups (P = .06). Seventy SAEs were recorded in 44 dogs, including 49 SAEs in 28/161 (17.4%) dogs receiving torasemide and 21 SAEs in 16/158 (10.1%) dogs receiving furosemide (Table 10). Renal and urinary system SAEs comprised 10/49 (20.4%) events involving 9/161 (5.6%) dogs in the torasemide group and 1/21 (4.8%) events involving 1/158 (0.6%) dogs in the furosemide group (P = .01). Seven dogs in the torasemide group and 1 dog in the furosemide group subsequently were withdrawn from the study for renal SAEs or AEs by the investigator or at the request of the owner. One of 158 dogs (0.6%) in the torsemide group and 4/161 (2.5%) dogs in the furosemide dose were unblinded as to treatment allocation so that the attending veterinarian could better diagnose and treat an AE.
4. DISCUSSION
Our study achieved its aim in demonstrating that clinical PO treatment of first‐onset CHF caused by DMVD using torasemide was noninferior (ie, no worse) than using furosemide. Results of secondary endpoints indicated that torasemide was safe, achieved higher owner treatment compliance in the initial treatment phase because of once daily dosing, and was associated with decreased risk for the combined outcomes of death or euthanasia for cardiac causes or worsening CHF that required medications or doses outside of prespecified criteria. Quality of life improved to a similar extent in each study group. These results are in general agreement with a previous clinical trial, 14 but a unique finding of our study is the specific demonstration of the efficacy and safety of torasemide as the first PO loop diuretic prescribed to dogs with first time CHF caused by DMVD. These results support use of torasemide in clinical instances other than as a replacement for existing furosemide or as a rescue diuretic used only when clinical response to furosemide is deemed no longer adequate. Our study expands existing knowledge to a wider spectrum of CHF patients and provides 2 safe and effective dosing guidelines based on severity of new clinical signs in dogs with first‐onset CHF.
Torasemide was significantly associated with less than half the risk of cardiac death or euthanasia or worsening CHF as compared to furosemide. Results were primarily driven by a lower number of instances of worsening CHF that required doses above the prespecified range in the torasemide group. As previously stated, the specific torasemide and furosemide doses employed in our study were based on the production of equivalent degrees of diuresis 3 , 16 , 17 and were in general agreement with a previous clinical trial. 14 The comparative effect of torasemide vs furosemide on survival time in humans is a subject of interest, with 1 study 10 in favor of torasemide and others 8 , 9 , 22 , 23 reporting a neutral effect. The effects of torasemide on other important patient‐centered endpoints such as quality of life, heart failure class, and hospitalization in human patients with CHF generally favor torasemide. 8 , 9 , 24 , 25 , 26 , 27 One problem common to these studies is that patients with more severe disease were more likely to be prescribed torasemide. 25 At the time of writing, the US National Heart, Lung, and Blood Institute is conducting a large prospective randomized pragmatic clinical effectiveness study involving over 6000 human patients to answer whether or not torasemide improves overall mortality at 1 year of follow‐up (TRANSFORM‐HF: Torsemide comparison with furosemide for management of heart failure. https://clinicaltrials.gov/ct2/show/NCT03296813. Accessed on 11 June 2019).
Veterinary experience with torasemide is relatively new and it is informative to consider medical treatment of CHF in humans, 28 in whom torasemide has been used for at least 2 decades. Torasemide is 1 of 3 loop diuretics, along with furosemide and bumetanide, recommended to treat CHF in humans. 29 Although the majority of human patients receive furosemide, some respond better to torasemide or bumetanide because of greater bioavailability. 30 The overall proportion of CHF patients receiving torasemide is unknown, but at 1 large tertiary medical center, 31 torasemide usage steadily increased from 2000 to 2010, such that by 2010 approximately one‐third of human CHF patients were receiving torasemide as their primary loop diuretic. At that center, overall usage of torasemide was more common in patients with pre‐existing renal dysfunction. 31 In fact, in each of the 3 largest nonrandomized studies to date, 22 , 25 , 31 human patients with pre‐existing increases in renal markers were more likely to be prescribed torasemide by their physician. The driving reason for this practice is the perceived need to prescribe a more potent diuretic to overcome inherent resistance and decreased diuresis in response to loss of tubular function. 32
Our study indicated that use of torasemide was safe, but as with all diuretics, not without potential AEs. In our study, torasemide was associated with a significantly higher percentage of dogs experiencing nonserious renal and urinary AEs, primarily involving asymptomatic increases in BUN and serum creatinine concentrations that were self‐limiting and did not warrant specific treatment. Dogs in the torasemide group also experienced a higher number of renal SAEs, but no dog in either study group died of renal or urinary causes. The higher frequency of increased concentrations of renal markers in our study and previous studies 14 is likely a consequence of prerenal azotemia in response to the greater potency of torasemide. In most instances of asymptomatic or mild increases in renal marker concentrations, either the diuretic dose is decreased, titration slowed, or no specific changes to treatment are instituted. In cases of moderate to severe increases in concentrations of renal markers, and especially in patients with pre‐existing renal disease, the concern for potential renal injury increases.
Efficacy of torasemide was achieved using once daily dosing, and owner compliance was significantly improved during the primary treatment period. Previous guidelines regarding treatment of CHF caused by myxomatous mitral valve disease (MMVD) in dogs 1 specifically point to owner noncompliance as a cause of so‐called diuretic resistance, and improving compliance ostensibly will lead to better resolution of CHF during the initial treatment period. Moreover, the need to frequently administer medications to a sick pet has the potential to negatively affect both the pet and pet owner's quality of life. 18 , 33 , 34
Our study had some limitations. The follow‐up time was relatively short, and studies demonstrating clinical effectiveness of torasemide over longer periods of time are warranted. Few cardiac‐related deaths occurred during our study, and studies primarily conducted to determine the potential benefit on survival in dogs with CHF also are of interest. Study subjects were recruited and examined by several different study veterinarians, and diagnostic results, such as radiographs or echocardiograms, were not subject to review by a central adjudicating committee. As such, it is possible that dogs with non‐DMVD conditions causing signs of respiratory disease were enrolled, but randomization should have distributed these cases equally, and baseline clinicopathological and diagnostic characteristics were similar between groups. The equipotency of study drug dose was based on data from healthy dogs, and studies comparing diuretic response in dogs with spontaneous DMVD are lacking. In our study, a relative greater potency of torasemide as compared to furosemide might help explain the higher incidence of renal AEs, the majority of which were self‐limiting, while simultaneously decreasing the number of dogs prematurely withdrawn from the study because of need for higher diuretic dose. Frequency of dosing was different between study drugs, and dog owners could have attempted to use this information to break the blinding but we have no objective evidence to determine whether or not this occurred.
In conclusion, torasemide was noninferior (ie, no worse) than furosemide in treating clinical and radiographic signs of first‐onset CHF in dogs with DMVD using 2 different initial dosing guidelines based on the severity of clinical signs. Torasemide had the advantage of once‐daily dosing, increased owner compliance during the first 14 days of treatment, and less than half the risk of cardiac death or euthanasia or worsening CHF vs furosemide. Monitoring of clinical response, hydration status, renal function, and serum electrolyte concentrations should be performed when using torasemide or any diuretic. Future studies specifically addressing the long‐term efficacy of torasemide, effect on survival, and relevance of renal markers as a monitoring tool during diuretic treatment are of particular interest.
CONFLICT OF INTEREST DECLARATION
Beatrice Besche, Thomas Blondel, Emilie Guillot, and Cathering Garelli‐Paar are employees of Ceva Santé Animale. Mark A. Oyama has received research funding, reimbursement for travel, honoraria for speaking and preparation of educational materials, and consulting fees from Ceva Santé Animale within the past 5 years.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Supplemental Figure 1 Distribution of dogs in the full analysis set according to radiographic pulmonary edema score at each study visit.
Supplemental Figure 2. Distribution of dogs in the full analysis set according to cough score at each study visit.
Supplemental Figure 3. Distribution of dogs in the full analysis set according to exercise intolerance score at each study visit.
Supplemental Figure 4. Distribution of dogs in the full analysis set according to dyspnea score at each study visit.
ACKNOWLEDGMENTS
The authors thank the veterinary practices, veterinarians, and dog owners who contributed to the study.
Besche B, Blondel T, Guillot E, Garelli‐Paar C, Oyama MA. Efficacy of oral torasemide in dogs with degenerative mitral valve disease and new onset congestive heart failure: The CARPODIEM study. J Vet Intern Med. 2020;34:1746–1758. 10.1111/jvim.15864
Funding information Ceva Sante Animale
REFERENCES
- 1. Keene BW, Atkins CE, Bonagura JD, et al. ACVIM consensus guidelines for the diagnosis and treatment of myxomatous mitral valve disease in dogs. J Vet Intern Med. 2019;33:1127‐1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Delarge J. Chemistry and pharmacological properties of the pyridine‐3‐sulfonylurea derivative torasemide. Arzneimittelforschung. 1988;38:144‐150. [PubMed] [Google Scholar]
- 3. Uechi M, Matsuoka M, Kuwajima E, et al. The effects of the loop diuretics furosemide and torasemide on diuresis in dogs and cats. J Vet Med Sci. 2003;65:1057‐1061. [DOI] [PubMed] [Google Scholar]
- 4. Sogame Y, Okano K, Hayashi K, et al. Urinary excretion profile of torasemide and its diuretic action in dogs. J Pharm Pharmacol. 1996;48:375‐379. [DOI] [PubMed] [Google Scholar]
- 5. Uchida T, Ohtaki Y, Kido H, et al. Diuretic profile of a novel loop diuretic torasemide in rats and dogs. Drugs Exp Clin Res. 1991;17:293‐298. [PubMed] [Google Scholar]
- 6. Ghys A, Denef J, de Suray JM, et al. Pharmacological properties of the new potent diuretic torasemide in rats and dogs. Arzneimittelforschung. 1985;35:1520‐1526. [PubMed] [Google Scholar]
- 7. Paulin A, Schneider M, Dron F, Woehrlé F. A pharmacokinetic/pharmacodynamic model capturing the time course of torasemide‐induced diuresis in the dog. J Vet Pharmacol Ther. 2016;39:547‐559. [DOI] [PubMed] [Google Scholar]
- 8. Miles JA, Hanumanthu BK, Patel K, Chen M, Siegel RM, Kokkinidis DG. Torsemide versus furosemide and intermediate‐term outcomes in patients with heart failure: an updated meta‐analysis. J Cardiovasc Med. 2019;20:379‐388. [DOI] [PubMed] [Google Scholar]
- 9. Shah P, Patel H, Mithawala P, Doshi R. Torsemide versus furosemide in heart failure patients: a meta‐analysis of randomized controlled trials. Eur J Intern Med. 2018;57:e38‐e340. [DOI] [PubMed] [Google Scholar]
- 10. Cosin J, Diez J. Torasemide in chronic heart failure: results of the TORIC study. Eur J Heart Fail. 2002;4:507‐513. [DOI] [PubMed] [Google Scholar]
- 11. Peddle GD, Singletary GE, Reynolds CA, Trafny DJ, Machen MC, Oyama MA. Effect of torsemide and furosemide on clinical, laboratory, radiographic and quality of life variables in dogs with heart failure secondary to mitral valve disease. J Vet Cardiol. 2012;14:253‐259. [DOI] [PubMed] [Google Scholar]
- 12. Oyama MA, Peddle GD, Reynolds CA, Singletary GE. Use of the loop diuretic torsemide in three dogs with advanced heart failure. J Vet Cardiol. 2011;13:287‐292. [DOI] [PubMed] [Google Scholar]
- 13. Caro‐Vadillo A, Ynaraja‐Ramirez E, Montoya‐Alonso JA. Effect of torsemide on serum and urine electrolyte levels in dogs with congestive heart failure. Vet Rec. 2007;160:847‐848. [DOI] [PubMed] [Google Scholar]
- 14. Chetboul V, Pouchelon JL, Menard J, et al. Short‐term efficacy and safety of torasemide and furosemide in 366 dogs with degenerative mitral valve disease: the TEST study. J Vet Intern Med. 2017;31:1629‐1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Council ISACH . Appendix A: recommendations for diagnosis of heart disease and treatment of heart failure in small animals In: Fox PR, Sisson DD, Moise NS, eds. Textbook of Canine and Feline Cardiology. 2nd ed. Philadelphia, PA: WB Saunders; 1999:883‐901. [Google Scholar]
- 16. European Medicines Agency‐Committee for Medicinal Products for Veterinary Use (CVMP) . CVMP Assessment Report for ISEMID (EMEA/V/C/004345/000). European Medicines Agency; 2018. https://www.ema.europa.eu/en/documents/assessment-report/isemid-epar-public-assessment-report_en.pdf. Accessed November 11, 2020.
- 17. Pelligand L, Guillot E, Geneteau A, et al. Population pharmacokinetics and pharmacodynamics modeling of torasemide and furosemide after oral repeated administratin in healthy dogs. Front Vet Sci. 2020;7:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Freeman LM, Rush JE, Farabaugh AE, Must A. Development and evaluation of a questionnaire for assessing health‐related quality of life in dogs with cardiac disease. J Am Vet Med Assoc. 2005;226:1864‐1868. [DOI] [PubMed] [Google Scholar]
- 19. Buchanan JW, Bucheler J. Vertebral scale system to measure canine heart size in radiographs. J Am Vet Med Assoc. 1995;206:194‐199. [PubMed] [Google Scholar]
- 20. Cornell CC, Kittleson MD, Della TP, et al. Allometric scaling of M‐mode cardiac measurements in normal adult dogs. J Vet Intern Med. 2004;18:311‐321. [DOI] [PubMed] [Google Scholar]
- 21. Schumi J, Wittes JT. Through the looking glass: understanding non‐inferiority. Trials. 2011;12:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mentz RJ, Hasselblad V, DeVore AD, et al. Torsemide versus furosemide in patients with acute heart failure (from the ASCEND‐HF trial). Am J Cardiol. 2016;117:404‐411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tager T, Frohlich H, Grundtvig M, et al. Comparative effectiveness of loop diuretics on mortality in the treatment of patients with chronic heart failure ‐ a multicenter propensity score matched analysis. Int J Cardiol. 2019;289:83‐90. [DOI] [PubMed] [Google Scholar]
- 24. Ozieranski K, Balsam P, Kaplon‐Cieslicka A, et al. Comparative analysis of long‐term outcomes of torasemide and furosemide in heart failure patients in heart failure registries of the European Society of Cardiology. Cardiovasc Drugs Ther. 2019;33:77‐86. [DOI] [PubMed] [Google Scholar]
- 25. Mentz RJ, Velazquez EJ, Metra M, et al. Comparative effectiveness of torsemide versus furosemide in heart failure patients: insights from the PROTECT trial. Future Cardiol. 2015;11:585‐595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kido K, Shimizu M, Hashiguchi M. Comparing torsemide versus furosemide in patients with heart failure: a meta‐analysis. J Am Pharm Assoc. 2019;59(3):432‐438. [DOI] [PubMed] [Google Scholar]
- 27. Muller K, Gamba G, Jaquet F, et al. Torasemide vs. furosemide in primary care patients with chronic heart failure NYHA II to IV–efficacy and quality of life. Eur J Heart Fail. 2003;5:793‐801. [DOI] [PubMed] [Google Scholar]
- 28. Buggey J, Mentz RJ, Pitt B, et al. A reappraisal of loop diuretic choice in heart failure patients. Am Heart J. 2015;169:323‐333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:1810‐1852. [DOI] [PubMed] [Google Scholar]
- 30. Vargo DL, Kramer WG, Black PK, Smith WB, Serpas T, Brater DC. Bioavailability, pharmacokinetics, and pharmacodynamics of torsemide and furosemide in patients with congestive heart failure. Clin Pharmacol Ther. 1995;57:601‐609. [DOI] [PubMed] [Google Scholar]
- 31. Mentz RJ, Buggey J, Fiuzat M, et al. Torsemide versus furosemide in heart failure patients: insights from Duke University Hospital. J Cardiovasc Pharmacol. 2015;65:438‐443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ter Maaten JM, Rao VS, Hanberg JS, et al. Renal tubular resistance is the primary driver for loop diuretic resistance in acute heart failure. Eur J Heart Fail. 2017;19:1014‐1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yeates JW, Mullan S, Stone M, Main DCJ. Promoting discussions and decisions about dogs’ quality‐of‐life. J Small Anim Pract. 2011;52:459‐463. [DOI] [PubMed] [Google Scholar]
- 34. Belshaw Z, Asher L, Harvey ND, Dean RS. Quality of life assessment in domestic dogs: an evidence‐based rapid review. Vet J. 2015;206:203‐212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 Distribution of dogs in the full analysis set according to radiographic pulmonary edema score at each study visit.
Supplemental Figure 2. Distribution of dogs in the full analysis set according to cough score at each study visit.
Supplemental Figure 3. Distribution of dogs in the full analysis set according to exercise intolerance score at each study visit.
Supplemental Figure 4. Distribution of dogs in the full analysis set according to dyspnea score at each study visit.


