Abstract
Background
Non‐Hodgkin lymphoma in humans is associated with environmental chemical exposures, and risk is enhanced by genetic variants in glutathione S‐transferases (GST) enzymes.
Objective
We hypothesized that boxer dogs, a breed at risk for lymphoma, would have a higher prevalence of GST variants with predicted low activity, and greater accumulated DNA damage, compared to other breeds. We also hypothesized that lymphoma in boxers would be associated with specific environmental exposures and a higher prevalence of canine GST variants.
Animals
Fifty‐four healthy boxers and 56 age‐matched nonboxer controls; 63 boxers with lymphoma and 89 unaffected boxers ≥10 years old.
Methods
We resequenced variant loci in canine GSTT1, GSTT5, GSTM1, and GSTP1 and compared endogenous DNA damage in peripheral leukocytes of boxers and nonboxers using the comet assay. We also compared GST variants and questionnaire‐based environmental exposures in boxers with and without lymphoma.
Results
Endogenous DNA damage did not differ between boxers and nonboxers. Boxers with lymphoma were more likely to live within 10 miles of a nuclear power plant and within 2 miles of a chemical supplier or crematorium. Lymphoma risk was not modulated by known canine GST variants.
Conclusions and Clinical Importance
Proximity to nuclear power plants, chemical suppliers, and crematoria were significant risk factors for lymphoma in this population of boxers. These results support the hypothesis that aggregate exposures to environmental chemicals and industrial waste may contribute to lymphoma risk in dogs.
Keywords: canine, detoxification, exposure, lymphosarcoma
Abbreviations
- CI
confidence interval
- GST
glutathione S‐transferase
1. INTRODUCTION
Lymphoma is a common cancer in dogs, but its underlying causes are not well understood. Several breeds have a higher risk of lymphoma, including boxers, golden retrievers, bulldogs, bull mastiffs, Bernese mountain dogs, Rottweilers, German shepherd dogs, Cocker spaniels, Briards, Dogue de Bordeaux, and standard schnauzers. 1 , 2 , 3 , 4 Boxers are particularly predisposed to T‐cell lymphoma, with a median age of onset of 7 and 10 years for high and low grade tumors, respectively. 4 Lymphoma typically is not cured in dogs even with multimodal chemotherapy, with median survival times of only 8 to 9 months for T‐cell lymphoma. 5 , 6
Molecular genetic research in dogs with lymphoma has identified somatic tumor mutations that may be prognostic markers or targets for chemotherapy. 3 , 7 , 8 , 9 , 10 Acquired tumor mutations in lymphomas of dogs can target several gene pathways, 3 but relatively little is known about the risks for accumulation of these mutations. Understanding factors that contribute to the risk of cancer before it develops may lead to evidence‐based cancer prevention strategies for owners of high‐risk dogs.
Lymphoma in dogs resembles non‐Hodgkin lymphoma (NHL) in humans, which is more common in people in industrialized nations than in developing countries (www.wcrf.org). Specific environmental chemicals associated with NHL include benzene (found in vehicle exhaust, second‐hand tobacco smoke, and petrochemical solvents), 11 , 12 chlorinated hydrocarbons, 13 and various pesticides, 14 , 15 herbicides, and fungicides. 16 In dogs, environmental risks for lymphoma also have been documented, but few studies are available. Demonstrated risk factors include exposure to commercially applied pesticides, 17 herbicides, 18 , 19 , 20 and household chemicals, 21 as well as living in industrial areas or in proximity to polluted sites. 2 , 21 One study in France found a correlation between the geographical distributions of both lymphoma in dogs and NHL in humans, suggesting shared environmental risk factors for both species. 2
Lymphoma risk in humans is further modified by enzymatic pathways that biotransform environmental carcinogens. The most common enzymes in humans implicated in lymphoma risk are glutathione S‐transferases (GSTs). The GSTs conjugate reactive chemicals to glutathione, which typically leads to detoxification of xenobiotics that otherwise could damage DNA. These GSTs also detoxify reactive endogenous molecules, such as genotoxic hydroperoxides that are generated during oxidative stress. 22 Multiple classes of GSTs have been identified in humans, and the most studied are GST‐theta (GSTT), GST‐pi (GSTP), and GST‐mu (GSTM). Low activity variants in the genes encoding these enzymes have been associated with higher levels of in vivo DNA damage in humans, 23 , 24 as well as a wide variety of cancers, including leukemia and lymphoma. 12 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32
Our overall hypothesis was that lymphoma risk in boxers would be modulated by a higher prevalence of low activity GST alleles, a breed‐related increase in DNA damage, and higher reported exposures to environmental chemicals. Our aims were: (a) to determine whether functionally important genetic variants in canine GSTT1, GSTT5, GSTM1, or GSTP1 were overrepresented in the boxer dog compared to nonboxer breeds; (b) to evaluate whether boxers have more endogenous DNA damage, as measured by the comet assay in peripheral leukocytes, than age‐matched nonboxers, and whether DNA damage is associated with specific GST alleles; and (c) to assess whether lymphoma in boxers is associated with GSTT1, GSTT5, GSTM1, or GSTP1 variants or with exposures to potential environmental carcinogens, compared to unaffected geriatric boxers.
2. MATERIALS AND METHODS
2.1. Healthy dog recruitment
We recruited clinically healthy purebred boxer dogs of any age and without clinical evidence of systemic cancer, from the University of Wisconsin‐Madison veterinary teaching hospital, from public dog events, and through outreach to boxer rescue organizations. For the purposes of the study, clinical health status was based on a targeted history obtained from the owner. Boxer breed was determined from veterinary or owner records along with observed clinical phenotype. Readily discernible boxer mixed breed dogs were not included.
For controls, a group of nonboxer dogs, excluding breeds also considered at higher risk for lymphoma (ie, excluding bulldogs, bullmastiffs, Bernese mountain dogs, Rottweilers, golden retrievers, German shepherd dogs, Cocker spaniels, Briards, dogue de Bordeaux, and standard schnauzers) 1 , 2 , 3 , 4 were recruited from the University of Wisconsin‐Madison veterinary teaching hospital and from public dog events, and matched by age to the healthy boxers. Both healthy boxers and nonboxer controls were evaluated for GST genotypes and endogenous DNA damage.
2.2. Recruitment of dogs with lymphoma
Boxer dogs with a confirmed cytologic or histologic diagnosis of lymphoma were prospectively recruited from the UW Veterinary Care Oncology service and from oncologists at Colorado State University, the University of Georgia, Seattle Veterinary Specialists, BluePearl Pet Hospital in Franklin TN, the Wisconsin Veterinary Referral Center, and the VCA network of specialty oncologists. Immunophenotyping and cytologic grading were not required for enrollment, and dogs could be at any stage of treatment when evaluated.
Controls for the boxer dogs with lymphoma were clinically unaffected boxer dogs ≥10 years of age, which is the median age of onset for low grade T‐cell lymphoma in boxers. 4 Because many older boxer dogs have concurrent disease, those with cardiac, respiratory, gastrointestinal, hepatic, endocrine, urinary, dermatologic, or neurologic disorders still were included in the control group as long as an underlying non‐neoplastic etiology had been established and clinical signs were stable. Because dogs could still develop lymphoma after 10 years of age, owner follow‐up was obtained for older control boxers until the end of the 2‐year recruitment period, as available, to confirm that lymphoma was not subsequently diagnosed. If this occurred, these dogs were moved to the lymphoma group for GST genotyping analyses. Owners provided written informed consent using a form included in a study kit. Home addresses were voluntarily provided by owners in the questionnaire, and questionnaire responses were deidentified on data entry using a unique study ID for each dog. The study protocol was approved by the Institutional Animal Care and Use Committee at the University of Wisconsin‐Madison School of Veterinary Medicine.
2.3. GST genotyping
Genomic DNA was obtained from buccal brush swabs from all groups of dogs: healthy boxer dogs, healthy nonboxer dogs, boxers with lymphoma, and geriatric boxer controls. Variants in canine GSTT1, GSTT5, GSTM1, and GSTP1 were identified from several sources: screening results from healthy dogs of various breeds in previous studies, 33 , 34 , 35 canine variants, formerly listed for nonhuman species, in the National Center for Biotechnology Information (NCBI) dbSNP database, and evaluation of whole genome sequencing data from 281 domestic dogs through the National Human Genome Research Institute Dog Genome Project (data files kindly provided by Drs Brian Davis and Elaine Ostrander). Variants were evaluated for possible functional significance using multiple in silico prediction programs, including Human Splicing Finder, Polyphen‐2, Align‐GVDG, ProVean, PANTHER, ESE Finder, PMut, Lasagne, PredictSNP, SIFT, MAPP, PhD‐SNP, SNAP, Vertebrate Transfac matrices, M‐fold, and miRBase/BLASTN.
Thirty‐two alleles with a minor allele frequency of ≥3% in 1 or more dog populations and that were predicted to be deleterious in silico were chosen for screening in the study (Supplemental Table S1). The reference allele at each locus was defined as the most prevalent allele in previously screened dogs of various breeds.
Allelic variants were identified by PCR of genomic DNA and direct sequencing, as previously described. 35 , 36 Primers were designed using the canine genome assembly (CanFam3.1) as a template. Sequence alignment and polymorphism screening were carried out using SerialCloner v2.6 (SerialBasics) and FinchTV chromatogram reader software (Geospiza Inc).
2.4. DNA damage in boxers vs other breeds
The classical alkaline comet assay, which detects damaged DNA as “tails” when cells are embedded in agarose and subject to electrophoresis, was used to compare DNA damage in boxer vs nonboxer dogs. Whole blood in EDTA (0.25 mL) was mixed with 0.1× volume of dimethyl sulfoxide, aliquoted, and frozen at −80°C; samples are stable for up to 1 week at −20°C or up to 1 month at −80°C under these conditions. 37 The comet assay was performed using standard techniques, 37 with a Nikon Eclipse E600 fluorescent microscope and Comet Assay IV software (Instem, Stone, Staffordshire, UK). Samples were run in batched assays during recruitment and included both boxers and nonboxers in each run. Etoposide‐treated cells (Alkaline Control Cells, Trevigen, Gaithersburg, Maryland) were used as positive controls. For each dog, DNA damage was quantified as tail moment normalized to negative control cells (sample CC0 from the Alkaline Control Cell set) within each assay. Because DNA damage can accrue with age, 38 nonboxer control dogs were aged‐matched (±1 year) to healthy boxers for these analyses. In addition, dogs with a history of mutagenic drug administration within the previous month (eg, metronidazole) 39 were ineligible for comet assay analyses.
2.5. Environmental exposures in dogs with lymphoma
Owners of dogs enrolled in the case‐control part of the study were asked to complete a questionnaire about their boxer dog's environment over the year before the date of lymphoma diagnosis, or over the year before enrollment for controls. The 1‐year period was chosen to capture all 4 seasons without requiring extended recall. 40 Questionnaires surveyed urbanicity, drive‐by traffic, insecticide and herbicide treatments, drinking water sources, and second‐hand smoke (Supplemental Figure S1). Proximity of the home to potential sources of pollution was evaluated using the household address and the “nearby” function on Google Maps (www.googlemaps.com). The following sites were searched for within 2 miles of the home: manufacturer, chemical plant or supplier, incinerator, crematorium, bus depot, landfill, farm, and golf course. In addition, active nuclear power plants, coal plants or coal mines were identified within 10 miles of the home.
2.6. Statistical analyses
The GST allele and genotype frequencies were compared between healthy boxers and control nonboxer dogs, and between boxers with lymphoma and unaffected geriatric boxers, using Chi square or Fisher's exact test, as appropriate. The DNA damage, as measured by normalized tail moments from the comet assay in peripheral leukocytes, was compared between boxers and age‐matched nonboxer controls using a Mann Whitney U test, with P < .05 used to ascribe statistical significance. The DNA damage also was correlated with age across both groups using a Spearman's rank correlation test. The association between GST variants and DNA damage was examined in 2 ways: linear regression for increasing variant dose and increasing DNA damage, and Fisher's exact test comparing GST allele frequencies in dogs in the lowest 25th and highest 75th percentiles for DNA damage. A Bonferroni correction was performed to control for concerns related to multiple comparisons.
Environmental exposures were encoded as collapsed categorical variables and were compared between boxers with lymphoma and geriatric controls, using Chi square or Fisher's exact tests, with unadjusted P values in this exploratory analysis. Interactions between GST alleles and environmental exposures with lymphoma outcome were assessed using Multifactor Dimensionality Reduction, which is designed to detect complex interactions in the presence or absence of main effects in case‐control studies. 41 , 42 , 43 All possible main effects of variables, 2‐way combination of variables, and 3‐way combination of variables were evaluated, with permutation testing used for ascribing significance.
3. RESULTS
3.1. GST genotypes in boxers vs nonboxers
Fifty‐four clinically healthy boxers, with a median age of 6.1 years (range, 0.5‐11.0) and 56 clinically healthy nonboxers (median age 6.5, range 1.0‐12.0) were recruited for GST genotyping and DNA damage assays. Demographic data for both groups are summarized in Table 1. Variants at 9 previously documented loci were absent in these 110 dogs, including GSTM1 c.422, c.497, c.530, c.609, and 2 variants at *6_7; and GSTP1 variants at −872, −656, and −37. Allele frequencies for the detected GST variants are shown in Table 2. Three complex indel loci in the GSTM1 promoter could not be resolved with confidence in all dogs.
TABLE 1.
Demographic data for 54 healthy boxer dogs and 56 healthy nonboxer dogs (excluding breeds with reported increased risk for lymphoma) assayed for DNA damage in peripheral leukocytes using the comet assay
| Boxers | Nonboxers | |
|---|---|---|
| Number | 54 | 56 |
| Age (median and range) | 6.1 years (0.5–11.0) | 6.5 years (1.0–12.0) |
| Sex |
FS 21 FI 4 MN 24 MI 5 |
FS 20 FI 5 MN 21 MI 10 |
| Breeds represented more than once (n) | Boxers (54) |
Labrador retriever (6) Australian shepherd (3) Dachshund (3) Boston terrier (2) Cavalier King Charles (2) Doberman pinscher (2) Greyhound (2) Pit bull terrier (2) Samoyed (2) Springer spaniel (2) West Highland white terrier (2) |
TABLE 2.
Minor allele frequencies (MAF) for 21 canine GST variants that were detected in boxer dogs and nonboxer breed dogs, screened for DNA damage using the comet assay
| GST variant | MAF in boxer dogs | MAF in nonboxer dogs | P value | AdjustedP value a |
|---|---|---|---|---|
| GSTT1 I2+28G>A | 0.000 | 0.143 | .0003 | .06 |
| GSTT1 I2+68T>A | 0.000 | 0.048 | NS | .02 |
| GSTT1 I2+69A>T | 0.000 | 0.048 | NS | <.002 |
| GSTT1 I2+72T>A | 0.000 | 0.107 | .0009 | |
| GSTT1 I2+168T>C | 0.000 | 0.258 | <.0001 | |
| GSTT1 241C>T | 0.016 | 0.013 | NS | |
| GSTT1 I4+70T>C | 0.065 | 0.351 | <.0001 | <.002 |
| GSTT1 674C>T | 0.000 | 0.159 | <.0001 | <.002 |
| GSTT1 *3T>C | 0.033 | 0.171 | .0038 | .08 |
| GSTT1 *101_102insT, *190C>A, *203T>C | 0.011 | 0.171 | .0002 | .004 |
| GSTT5 c. 387_392delGGACCA delAsp129_Gln130 | 0.010 | 0.102 | .0044 | .09 |
| GSTP1–350C>A | 0.020 | 0.461 | <.0001 | <.002 |
| GSTP1–228C>A | 0.010 | 0.390 | <.0001 | <.002 |
| GSTP1–185delT | 0.000 | 0.080 | .0068 | .1 |
| GSTP1–68C>T | 0.010 | 0.206 | <.0001 | <.002 |
|
GSTP1–66 to −16(GCC)n = 10‐22 Allele frequency listed for n = 17 unit repeat |
0.980 | 0.367 | <.0001 | <.002 |
| GSTP1–46T>C | 0.000 | 0.408 | <.0001 | <.002 |
| GSTP1–43C>T | 0.010 | 0.190 | <.0001 | <.002 |
| GSTP1–27G>A | 0.000 | 0.120 | .0003 | .006 |
| GSTP1–21A>G | 0.010 | 0.440 | <.0001 | <.002 |
| GSTP1 c.336T>C | 0.000 | 0.500 | <.0001 | <.002 |
P values listed in bold are statistically significant.
Adjusted for 21 comparisons.
There was generally less allelic heterogeneity in the boxer group compared to the control nonboxer group, as expected when comparing a single breed to a group of various breeds. Many GST variants were significantly less common, or absent, in boxers (Table 2). The 1 variant that was more prevalent in boxers was a 17‐unit microsatellite repeat in the GSTP1 promoter (allele frequency 0.980 vs 0.367 in nonboxers; adjusted P < .002). The next most common variant in nonboxers was a 16‐unit repeat (16*1), 44 with an allele frequency of .306. The 17‐unit variant was predicted to decrease the number of WT1‐KTS transcription factor binding from 25 sites (for 16*1) to 18 sites (for 17‐units).
Two functionally characterized GST variants, the GSTT1 3′UTR haplotype and the GSTT5 coding variant (c. 387_392delGGACCA; delAsp129_Gln130), were not overrepresented in boxers. The deleterious GSTT5 variant was found in 10.0% of boxers and 10.2% of nonboxers overall. Another deleterious coding variant, GSTT1 674 C>T (Pro225Leu), was not found in boxers but was detected in 15.9% of nonboxers in the current study.
3.2. DNA damage in boxers vs nonboxers
Peripheral leukocytes from the 54 healthy boxers and 56 healthy nonboxers were assayed for DNA damage using the alkaline comet assay. Contrary to our hypothesis, no significant difference was found in DNA damage between healthy boxers and those breeds at lower risk for lymphoma (P = .65; Figure 1). Furthermore, DNA damage was not correlated with age across boxer dogs (r = −.12; P = .4). We also examined DNA damage for associations with GST variant alleles. No association was found between increasing GST variants and increasing DNA damage at any GST locus. In addition, when dogs with ≥ the highest 75th percentile of DNA damage (normalized tail moment >3.8, n = 28, including 12 boxers and 16 nonboxers) were compared to dogs with ≤ the lowest 25th percentile of DNA damage (normalized tail moment <1.19, n = 27, including 16 boxers and 11 nonboxers), none of the variants were overrepresented in the higher DNA damage subset (data not shown).
FIGURE 1.
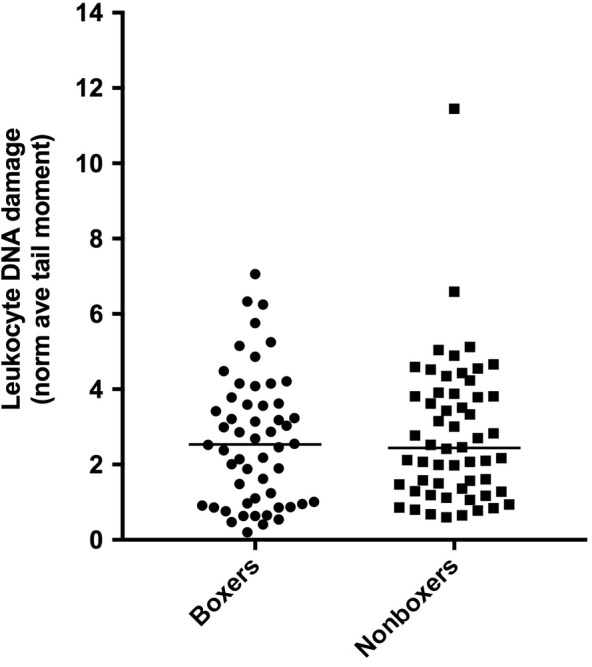
DNA damage in peripheral leukocytes of boxers (n = 54) vs nonboxer dogs (n = 56) matched for age, as measured by normalized tail moment in the comet assay (P = .65 between groups)
3.3. Boxers with lymphoma and unaffected geriatric boxers
Sixty‐eight boxers with lymphoma were recruited; 5 were not eligible because the diagnosis of lymphoma was not confirmed by cytology or histopathology. Overall, 63 dogs with lymphoma were enrolled, with a median age of 8 years (range, 3.5‐15 years; Table 3). The control group consisted of 89 clinically healthy geriatric boxers with a median age of 10.5 years (range, 10‐15 years). Most dogs with lymphoma were recruited from veterinary referral hospital populations, whereas most control dogs were recruited by outreach to boxer owners through dog events and Facebook (Table 3).
TABLE 3.
Demographic and owner‐reported household data for boxer dogs with lymphoma and unaffected boxer dogs ≥10 years of age
| Boxers with lymphoma n = 63 | Unaffected control boxers n = 89 | |
|---|---|---|
| Age (median and range) | 8 years (3.5‐15) | 10.5 years (10‐15) |
| Sex |
FS 30 FI 0 MN 30 MI 3 |
FS 48 FI 0 MN 37 MI 4 |
| Recruitment sites a (dogs per site) |
UW Veterinary Care (9) Other referral hospitals (39) Outreach to boxer owners (15) 2 |
UW Veterinary Care (14) Other referral hospitals (3) Outreach to boxer owners b (72) |
| Dog's home environment |
59 respondents Urban 7 dogs (11.9%) Suburban 38 dogs (64.4%) Rural/Farm 9 dogs (15.3%) Mixed 5 dogs (8.4%) |
87 unique households Urban 11 dogs (12.6%) Suburban 46 dogs (52.9%) Rural/Farm 25 dogs (28.7%) Mixed 5 dogs (5.7%) |
| Heavy traffic by home |
58 respondents 2 dogs (3.4%) |
86 respondents 3 dogs (3.5%) |
| Home use of pesticides or insecticides c |
59 respondents 40 dogs (67.8%) |
79 respondents 58 dogs (73.4%) |
| Home use of weed killer or commercial lawn treatment c |
54 respondents 23 dogs (39.0%) |
86 respondents 45 dogs (52.3%) |
| Predominantly municipal (chlorinated) drinking water |
54 respondents 43 dogs (79.6%) |
86 respondents 61 dogs (70.9%) |
| Smokers in the home |
58 respondents 8 dogs (13.8%) |
87 respondents 9 dogs (10.3%) |
Referral hospitals included Colorado State University, the University of Georgia, and specialty practices in Seattle, Wisconsin, Tennessee, and throughout the VCA national network.
Outreach to boxer owners was at local Wisconsin dog events, and nationally through boxer rescues and Facebook.
Within the past year.
Fifty‐nine of 63 owners of boxers with lymphoma completed environmental questionnaires, and 58 provided a full home address for proximity searching. Owners of 87 of the 89 control boxers also completed questionnaires; 2 of these dogs were censored from environmental analyses because they were from the same household as another control dog. Of these 85 control dogs, 84 provided a full household address.
According to the owner questionnaires (Table 3), boxers with lymphoma did not differ from geriatric control boxers in the percentage of households in an urban area (11.9% vs. 12.6%, P = .99); with heavy drive‐by traffic (P > .99); that used insecticides (P = .57) or weed killer (P = .13) in the past year; had a chlorinated municipal drinking water source (P = .43); or reported smokers on the property (P = .6). When data were analyzed by households reported to be in a “rural area,” 15.3% of dogs with lymphoma were from a rural household compared to 28.7% of control dogs (odds ratio [OR], 0.76; 95% confidence interval [CI], 0.33‐1.8), but this difference did not reach significance (P = .07).
According to Google maps data, boxers with lymphoma were significantly more likely to live within 10 miles of a nuclear power plant (17.2%) compared to unaffected geriatric boxers (3.5%; OR, 5.76; 95% CI, 1.54‐20.06; P = .007; Table 4). Boxers with lymphoma were also more likely to live within 2 miles of a chemical manufacturer or supplier (OR, 2.28; 95% CI, 1.15‐4.63; P = .02) or a crematorium (OR, 2.17; 95% CI, 1.02‐4.38; P = .04).
TABLE 4.
Household proximity data for boxer dogs with lymphoma and clinically healthy boxer dogs ≥10 years of age, using the owner's home address and the “nearby” function on Google Maps
| Household proximity | Boxers with lymphoma 58 unique households providing full address | Unaffected control boxers 86 unique households with full address | Odds ratio (OR) (95% confidence interval) |
|---|---|---|---|
| Manufacturing a | 81.0% (47 dogs) | 83.7% (72 dogs) |
.83 (.34‐1.94) P = .8 |
| Chemical industry b (manufacturer or supplier) | 48.3% (28 dogs) | 29.1% (25 dogs) |
2.28 (1.15‐4.63) P = .02 |
| Incinerator | 8.6% (5 dogs) | 5.8% (5 dogs) |
1.53 (.47‐4.98) P = .5 |
| Crematorium | 39.7% (23 dogs) | 23.3% (20 dogs) |
2.17 (1.02–4.38) P = .04 |
| Bus depot | 43.1% (25 dogs) | 43.0% (37 dogs) |
1.00 (.52‐1.91) P = 1.0 |
| Landfill | 43.1% (25 dogs) | 32.6% (28 dogs) |
1.57 (.79‐3.11) P = .2 |
| Farm | 81.0% (47 dogs) | 84.9% (73 dogs) |
0.76 (.32‐1.80) P = .7 |
| Golf course | 81.0% (47 dogs) | 68.6% (59 dogs) |
1.96 (.90‐4.17) P = .1 |
| Nuclear power plant c | 17.2% (10 dogs) | 3.5% (3 dogs) |
5.76 (1.54‐20.06) P = .007 |
| Coal plant or coal mine c | 8.6% (5 dogs) | 3.5% (3 dogs) |
2.61 (.65‐10.12) P = .3 |
Note: All proximities were tested within 2 miles of the household, unless otherwise noted. Bolded P values are statistically significant between groups.
Observed manufacturing sites included electronics, plastics, machinery, instrumentation, semiconductors, boats, motorcycles, film, rubber, textiles, and flooring.
Observed chemical industries included petroleum products, agrochemical, pharmaceutical, medical, and industrial chemicals.
Within 10 miles of household.
In univariate analyses, lymphoma in boxers was not associated with any of the GST variants tested (Table 5). Furthermore, we did not detect any interactions between these GST variants and proximity to a nuclear power plant, chemical manufacturer, or crematorium.
TABLE 5.
Minor allele frequencies (MAF) for canine GST variants detected in boxer dogs with lymphoma (n = 63) compared to geriatric unaffected boxer dogs (n = 89)
| GSTT1 | MAF boxers with lymphoma a | MAF unaffected boxer dogs a |
|---|---|---|
| GSTT1 I2+168T>C | 0.014 | 0.013 |
| GSTT1 I4+70T>C | 0.026 | 0.000 |
|
GSTM1–399 to −400 insCGGAGCCGAGGGGGCG |
0.120 | 0.152 |
|
GSTM1–260 to −273 delCGGAGCCGAGGGGG |
0.677 | 0.763 |
|
GSTM1–231 to −244 delGGAGCCGAGGGGGC |
0.033 | 0.010 |
| GSTM1 c.422T>C | 0.022 | 0.007 |
| GSTP1–350C>A | 0.011 | 0.026 |
| GSTP1–228C>A | 0.000 | 0.038 |
| GSTP1–185 delT | 0.000 | 0.026 |
| GSTP1–68C>T | 0.021 | 0.013 |
|
GSTP1–66 to −16(GCC)n = 10‐22 Allele frequency listed for n = 17 unit repeats |
0.975 | 0.959 |
| GSTP1‐52 C>T | 0.011 | 0.013 |
| GSTP1‐48G>A | 0.023 | 0.006 |
| GSTP1‐46T>C | 0.011 | 0.019 |
| GSTP1‐43C>T | 0.043 | 0.019 |
| GSTP1‐37C>T | 0.011 | 0.006 |
| GSTP1‐27G>A | 0.064 | 0.026 |
| GSTP1‐21A>G | 0.032 | 0.013 |
| GSTP1 c.336T>C | 0.000 | 0.032 |
| GSTP1 c.548G>A | 0.000 | 0.008 |
Note: Allele frequencies were not significantly different between groups at any tested loci.
Allele frequencies could not be determined in all dogs at all loci.
4. DISCUSSION
Allele frequencies for most GST variants in boxers were quite low (≤6.5%) and were not significantly higher than in nonboxers. An exception was a high prevalence 17‐unit microsatellite repeat in the GSTP1 promoter. This allele is predicted to decrease transcription factor binding sites for WT1‐KTS, 44 but this effect has not been evaluated experimentally. The specific effects of this microsatellite region on GSTP1 expression deserve characterization, particularly in comparison to the common 16*1 variant found in nonboxers in this population (MAF.306) and in another study of 278 dogs of various breeds (MAF.302). 44
Multiple GST variants were significantly less common in boxers compared to a heterogeneous group of nonboxers, which is expected given the low levels of heterozygosity in boxers. 45 Two functionally characterized GST variants were not overrepresented in boxers: a GSTT1 3′UTR haplotype that decreases expression by 50%, 36 and a GSTT5 coding deletion variant (c. 387_392delGGACCA; delAsp129_Gln130) that decreases enzyme activity by >90%. 33 However, this GSTT5 coding deletion was found in approximately 10% of dogs overall in the current study, as well as in 14.4% of canine livers from various breeds. 33 Another deleterious coding variant, GSTT1 674 C>T (Pro225Leu), also was found in 15.9% of nonboxers. Further work is needed to characterize the substrate range of canine GSTT1 and GSTT5 for potentially carcinogenic environmental chemicals, in order to understand the clinical and toxicological impact of these dysfunctional coding variants.
We found no difference in leukocyte DNA damage, as measured by the comet assay, between boxers and age‐matched nonboxer dogs, nor did we see an association with DNA damage and advancing age within the boxer breed. These findings do not support the hypothesis that the risk of lymphoma in boxers is related to breed‐specific accumulated DNA damage. However, we did not assess response to induced DNA damage ex vivo in boxers vs nonboxers, which could uncover DNA repair defects that are masked in a population with heterogeneous exposures. Lymphocytes from golden retrievers with lymphoma show increased susceptibility to DNA damage ex vivo, but golden retrievers as a breed do not share this defect. 46 We did not compare DNA damage in boxers with lymphoma to geriatric boxers because of the confounding factors of already‐transformed lymphocytes and the effects of ongoing chemotherapy. However, comparing pretreatment DNA damage in boxers with lymphoma to that in age‐matched nonboxers with lymphoma may be informative in future studies.
Lymphoma in boxers was not associated with any of the canine GST variants screened in our study. We initially had found an association between GSTT1 I2+28A and lymphoma in dogs of various breeds, 35 but did not find the same association in Golden retrievers 35 , 36 or in the boxers in the present study. Furthermore, the 2 low functioning canine GST alleles that have been characterized to date, the GSTT1 3′UTR haplotype 36 and the GSTT5 6 bp coding deletion 33 were not overrepresented in boxers with lymphoma in our study. Overall, we found a very low prevalence of GST coding variants of predicted functional relevance among all of the boxers in this population.
In humans, 2 major human GST variants, GSTM1 null (found in 28%‐58% of subjects) 29 and GSTT1 null, (found in 8%‐54% of subjects), 29 lead to a complete lack of gene expression. Defective coding variants in GSTP1, notably Ile105Val, also are found in one‐third of populations. 29 Non‐Hodgkin lymphoma primarily has been associated with the GSTT1 null allele, 25 , 30 , 31 , 32 , 47 , 48 with some associations found with GSTP1 Ile105Val. 12 , 30 The difference between these human associations and our negative findings in boxers with lymphoma could be a result of several factors. One factor is our focus on a single breed, because different human racial populations show different GST allele frequencies and risk profiles. 29 The second is the presence of GST null variants with no gene expression in humans, which we have not yet recognized in dogs. However, canine GSTT5 delAsp129_Gln130 encodes an enzyme that virtually lacks activity, 33 and further studies of the substrate range of this polymorphic canine enzyme will be helpful in ongoing molecular epidemiologic studies.
For environmental exposures, we found that boxers with lymphoma were 5‐fold more likely to live within 10 miles of a nuclear power plant than older boxers without a diagnosis of lymphoma. We also found that living within 2 miles of a crematorium or a chemical manufacturer or supplier were significant risk factors for lymphoma. These apparent risk factors could result from increased carcinogen exposures through air, soil, or water, and may be individually important or may be surrogates of greater industrial activity in aggregate. Studies in other countries have associated lymphoma in dogs with environmental pollutants, including industrial areas and waste dumping sites in Italy 21 , 49 and areas with incinerators, polluted sites and radioactive waste in France. 2 We observed fewer dogs with lymphoma living in a rural household (15.3%) compared to controls (28.7%), but this observed difference did not reach significance (P = .07) A post hoc sample size calculation indicated that 161 cases and 161 controls would be needed to show this difference, if real, to be statistically significant (P < .05, 80% power; biostats.info).
We did not find an increased incidence of owner‐reported insecticide or herbicide use in the dogs with lymphoma. A previous case‐control study of lymphoma in dogs did find a positive association with professionally applied pesticides (OR, 1.7; 95% CI, 1.1‐2.7). 17 The previous study included more dogs (263 cases) and a longer owner questionnaire, and thus may have had greater sensitivity to detect associations. An older study found an association with application of the phenoxyherbicide 2,4‐D and lymphoma in dogs, 18 , 19 but others have disputed these data. 50 , 51 We observed that owners filling out our questionnaires often could not identify or recall what chemical products were used on their properties, and incorporating product labels in future questionnaires might improve sensitivity.
Our findings were limited somewhat by group sizes and by constraints inherent in questionnaire‐based epidemiologic studies. Our questionnaire was developed for the present study and another study on cancer risk in dogs, 52 and was not independently validated. Some owners seemed unsure about what chemicals and water treatments were used in their homes, and some of the questionnaire assessments were subjective, such as rural vs suburban or urban. We did not specifically ask how long dogs had been in the current home, but we did ask owners to answer questions based on the previous year. This time frame may not have captured early exposures of relevance but was chosen to reflect seasonal pesticide use without requiring too much recall. In addition, most of our lymphoma cases were recruited from veterinary specialty hospitals and most of our unaffected geriatric boxers were recruited through direct outreach to owners, which could contribute to bias in our population structure. Therefore, our findings of risk related to nuclear power plants, chemical industries, and crematoriums need further exploration.
Overall, our data do not support involvement of known canine GST variants in lymphoma risk in the boxer dog. Further work is underway to understand the substrate range of polymorphic canine GSTs toward specific chemical carcinogens that are relevant to the risk of lymphoma and other cancers in dogs. Direct measurement of chemical exposures in blood, along with more sensitive measures of early DNA damage in lymphocytes, may further refine our understanding of breed‐related lymphoma risk in dogs.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Data S1 Questionnaire administered to owners of boxer dogs with lymphoma and unaffected control boxers to assess possible environmental exposures.
Table S1 Variants (n = 32) in canine GSTT1, GSTT5, GSTM1, and GSTP1 targeted for resequencing in boxer and nonboxer dogs. Criteria for inclusion were a minor allele frequency (MAF) >3% previously documented in healthy dog populations, along with characterized low expression/activity or predicted functional effects in silico. Several additional variants were included from dbSNP and from de novo observations from the current study. DGP: Whole genome sequencing data from up to 281 domestic dogs from the NHGRI Dog Genome Project. dbSNP: formerly found on the NCBI dbSNP website, which no longer includes nonhuman SNP data.
ACKNOWLEDGMENTS
This study was supported by an Oak Grant 02318 from the AKC Canine Health Foundation. We thank Green Aces Boxer Rescue in Mequon WI, and boxer owners across the United States, as well as our oncologist collaborators Drs Ruthanne Chun, Doug Thamm, Corey Saba, Phil Bergman, Nicholas Szigetvari and Laura Goodman, and Ms. Rachel McNally, for generous assistance with boxer recruitment. We also acknowledge the contributions of Kyle Granger, Nate Latus, Brianna Lynch and Mia Roccaro to DNA resequencing.
Craun K, Ekena J, Sacco J, Jiang T, Motsinger‐Reif A, Trepanier LA. Genetic and environmental risk for lymphoma in boxer dogs. J Vet Intern Med. 2020;34:2068–2077. 10.1111/jvim.15849
Kaitlyn Craun and Joanne Ekena contributed equally to this study.
[Correction added on August 3, 2020 after first online publication: funding information updated.]
Funding information AKC Canine Health Foundation, Oak, Grant/Award Number: 02318
REFERENCES
- 1. Edwards DS, Henley WE, Harding EF, Dobson JM, Wood JLN. Breed incidence of lymphoma in a UK population of insured dogs. Vet Comp Oncol. 2003;1:200‐206. [DOI] [PubMed] [Google Scholar]
- 2. Pastor M, Chalvet‐Monfray K, Marchal T, et al. Genetic and environmental risk indicators in canine non‐Hodgkin's lymphomas: breed associations and geographic distribution of 608 cases diagnosed throughout France over 1 year. J Vet Intern Med. 2009;23:301‐310. [DOI] [PubMed] [Google Scholar]
- 3. Elvers I, Turner‐Maier J, Swofford R, et al. Exome sequencing of lymphomas from three dog breeds reveals somatic mutation patterns reflecting genetic background. Genome Res. 2015;25:1634‐1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jankowska U, Jagielski D, Czopowicz M, et al. The animal‐dependent risk factors in canine T‐cell lymphomas. Vet Comp Oncol. 2015. [DOI] [PubMed] [Google Scholar]
- 5. Brodsky EM, Maudlin GN, Lachowicz JL, Post GS. Asparaginase and MOPP treatment of dogs with lymphoma. J Vet Intern Med. 2009;23:578‐584. [DOI] [PubMed] [Google Scholar]
- 6. Rebhun RB, Kent MS, Borrofka SA, et al. CHOP chemotherapy for the treatment of canine multicentric T‐cell lymphoma. Vet Comp Oncol. 2011;9:38‐44. [DOI] [PubMed] [Google Scholar]
- 7. Richards KL, Motsinger‐Reif AA, Chen HW, et al. Gene profiling of canine B‐cell lymphoma reveals germinal center and postgerminal center subtypes with different survival times, modeling human DLBCL. Cancer Res. 2013;73:5029‐5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bushell KR, Kim Y, Chan FC, et al. Genetic inactivation of TRAF3 in canine and human B‐cell lymphoma. Blood. 2015;125:999‐1005. [DOI] [PubMed] [Google Scholar]
- 9. Gramer I, Kessler M, Geyer J. Detection of novel polymorphisms in the ckit gene of canine patients with lymphoma, melanoma, haemangiosarcoma, and osteosarcoma. Vet Res Commun. 2016;40:89‐95. [DOI] [PubMed] [Google Scholar]
- 10. Koshino A, Goto‐Koshino Y, Setoguchi A, Ohno K, Tsujimoto H. Mutation of p53 gene and its correlation with the clinical outcome in dogs with lymphoma. J Vet Intern Med. 2016;30:223‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. O'Connor SR, Farmer PB, Lauder I. Benzene and non‐Hodgkin's lymphoma. J Pathol. 1999;189:448‐453. [DOI] [PubMed] [Google Scholar]
- 12. Sarmanova J, Benesova K, Gut I, et al. Genetic polymorphisms of biotransformation enzymes in patients with Hodgkin's and non‐Hodgkin's lymphomas. Hum Mol Genet. 2001;10:1265‐1273. [DOI] [PubMed] [Google Scholar]
- 13. Seidler A, Mohner M, Berger J, et al. Solvent exposure and malignant lymphoma: a population‐based case‐control study in Germany. J Occup Med Toxicol. 2007;2:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fritschi L, Benke G, Hughes AM, et al. Occupational exposure to pesticides and risk of non‐Hodgkin's lymphoma. Am J Epidemiol. 2005;162:849‐857. [DOI] [PubMed] [Google Scholar]
- 15. Karunanayake CP, Spinelli JJ, McLaughlin JR, et al. Hodgkin lymphoma and pesticides exposure in men: a Canadian case‐control study. J Agromedicine. 2012;17:30‐39. [DOI] [PubMed] [Google Scholar]
- 16. Orsi L, Delabre L, Monnereau A, et al. Occupational exposure to pesticides and lymphoid neoplasms among men: results of a French case‐control study. Occup Environ Med. 2009;66:291‐298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Takashima‐Uebelhoer BB, Barber LG, Zagarins SE, et al. Household chemical exposures and the risk of canine malignant lymphoma, a model for human non‐Hodgkin's lymphoma. Environ Res. 2012;112:171‐176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hayes HM, Tarone RE, Cantor KP, Jessen CR, McCurnin DM, Richardson RC. Case‐control study of canine malignant lymphoma: positive association with dog owner's use of 2,4‐dichlorophenoxyacetic acid herbicides. J Natl Cancer Inst. 1991;83:1226‐1231. [DOI] [PubMed] [Google Scholar]
- 19. Hayes HM, Tarone RE, Cantor KP. On the association between canine malignant lymphoma and opportunity for exposure to 2,4‐dichlorophenoxyacetic acid. Environ Res. 1995;70:119‐125. [DOI] [PubMed] [Google Scholar]
- 20. Schofield I, Stevens KB, Pittaway C, et al. Geographic distribution and environmental risk factors of lymphoma in dogs under primary‐care in the UK. J Small Anim Pract. 2019;60:746‐754. [DOI] [PubMed] [Google Scholar]
- 21. Gavazza A, Presciuttini S, Barale R, Lubas G, Gugliucci B. Association between canine malignant lymphoma, living in industrial areas, and use of chemicals by dog owners. J Vet Intern Med. 2001;15:190‐195. [PubMed] [Google Scholar]
- 22. Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol. 2005;45:51‐88. [DOI] [PubMed] [Google Scholar]
- 23. Laffon B, Teixeira JP, Silva S, et al. Assessment of occupational genotoxic risk in the production of rubber tyres. Ann Occup Hyg. 2006;50:583‐592. [DOI] [PubMed] [Google Scholar]
- 24. Wlodarczyk M, Nowicka G. Common polymorphisms in CYP1A1, GSTM1, GSTT1, GSTP1 and XPD genes and endogenous DNA damage. Mol Biol Rep. 2012;39:5699‐5704. [DOI] [PubMed] [Google Scholar]
- 25. Kerridge I, Lincz L, Scorgie F, Hickey D, Granter N, Spencer A. Association between xenobiotic gene polymorphisms and non‐Hodgkin's lymphoma risk. Br J Haematol. 2002;118:477‐481. [DOI] [PubMed] [Google Scholar]
- 26. Soucek P, Sarmanova J, Kristensen VN, et al. Genetic polymorphisms of biotransformation enzymes in patients with Hodgkin's and non‐Hodgkin's lymphomas. Int Arch Occup Environ Health. 2002;75(Suppl):S86‐S92. [DOI] [PubMed] [Google Scholar]
- 27. Krajinovic M, Labuda D, Sinnett D. Glutathione S‐transferase P1 genetic polymorphisms and susceptibility to childhood acute lymphoblastic leukaemia. Pharmacogenetics. 2002;12:655‐658. [DOI] [PubMed] [Google Scholar]
- 28. Hohaus S, Massini G, D'Alo F, et al. Association between glutathione S‐transferase genotypes and Hodgkin's lymphoma risk and prognosis. Clin Cancer Res. 2003;9:3435‐3440. [PubMed] [Google Scholar]
- 29. Ye Z, Song H. Glutathione S‐transferase polymorphisms (GSTM1, GSTP1 and GSTT1) and the risk of acute leukaemia: a systematic review and meta‐analysis. Eur J Cancer. 2005;41:980‐989. [DOI] [PubMed] [Google Scholar]
- 30. Al‐Dayel F, Al‐Rasheed M, Ibrahim M, et al. Polymorphisms of drug‐metabolizing enzymes CYP1A1, GSTT and GSTP contribute to the development of diffuse large B‐cell lymphoma risk in the Saudi Arabian population. Leuk Lymphoma. 2008;49:122‐129. [DOI] [PubMed] [Google Scholar]
- 31. Ruiz‐Cosano J, Conesa‐Zamora P, Gonzalez‐Conejero R, et al. Role of GSTT1 and M1 null genotypes as risk factors for B‐cell lymphoma: influence of geographical factors and occupational exposure. Mol Carcinog. 2012;51:508‐513. [DOI] [PubMed] [Google Scholar]
- 32. Abdel Rahman HA, Khorshied MM, Elazzamy HH, Khorshid OM. The link between genetic polymorphism of glutathione‐S‐transferases, GSTM1, and GSTT1 and diffuse large B‐cell lymphoma in Egypt. J Cancer Res Clin Oncol. 2012;138:1363‐1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Craft S, Ekena J, Sacco J, Luethcke K, Trepanier L. A 6‐bp deletion variant in a novel canine glutathione‐S‐transferase gene (GSTT5) leads to loss of enzyme function. J Vet Intern Med. 2017;31:1833‐1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ekena J, Wood E, Manchester A, Chun R, Trepanier LA. Glutathione‐S‐transferase‐theta genotypes and the risk of cyclophosphamide toxicity in dogs. Vet Comp Oncol Epub. 2018;16:529‐534. [DOI] [PubMed] [Google Scholar]
- 35. Ginn J, Sacco J, Wong YY, Motsinger‐Reif A, Chun R, Trepanier LA. Positive association between a glutathione‐S‐transferase polymorphism and lymphoma in dogs. Vet Comp Oncol. 2014;12:227‐236. [DOI] [PubMed] [Google Scholar]
- 36. Craft S, Ekena J, Mayer B, et al. Characterization of a low expression haplotype in canine glutathione S‐transferase (GSTT1) and its prevalence in golden retrievers. Vet Comp Oncol. 2018;16:E61‐E67. [DOI] [PubMed] [Google Scholar]
- 37. Al‐Salmani K, Abbas HH, Schulpen S, et al. Simplified method for the collection, storage, and comet assay analysis of DNA damage in whole blood. Free Radic Biol Med. 2011;51:719‐725. [DOI] [PubMed] [Google Scholar]
- 38. Shen S, Cooley DM, Glickman LT, Glickman N, Waters DJ. Reduction in DNA damage in brain and peripheral blood lymphocytes of elderly dogs after treatment with dehydroepiandrosterone (DHEA). Mutat Res. 2001;480‐481:153‐162. [DOI] [PubMed] [Google Scholar]
- 39. Sekis I, Ramstead K, Rishniw M, et al. Single‐dose pharmacokinetics and genotoxicity of metronidazole in cats. J Feline Med Surg. 2009;11:60‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nieuwenhuijsen MJ. Design of exposure questionnaires for epidemiological studies. Occup Environ Med. 2005;62:272‐280. 212‐274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ritchie MD, Hahn LW, Roodi N, et al. Multifactor‐dimensionality reduction reveals high‐order interactions among estrogen‐metabolism genes in sporadic breast cancer. Am J Hum Genet. 2001;69:138‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Motsinger AA, Ritchie MD. Multifactor dimensionality reduction: an analysis strategy for modelling and detecting gene‐gene interactions in human genetics and pharmacogenomics studies. Hum Genomics. 2006;2:318‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Motsinger AA, Brassat D, Caillier SJ, et al. Complex gene‐gene interactions in multiple sclerosis: a multifactorial approach reveals associations with inflammatory genes. Neurogenetics. 2007;8:11‐20. [DOI] [PubMed] [Google Scholar]
- 44. Sacco J, Mann S, Toral K. Single nucleotide polymorphisms and microsatellites in the canine glutathione S‐transferase pi 1 (GSTP1) gene promoter. Canine Genet Epidemiol. 2017;4:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Parker HG, Kim LV, Sutter NB, et al. Genetic structure of the purebred domestic dog. Science. 2004;304:1160‐1164. [DOI] [PubMed] [Google Scholar]
- 46. Thamm DH, Grunerud KK, Rose BJ, Vail DM, Bailey SM. DNA repair deficiency as a susceptibility marker for spontaneous lymphoma in golden retriever dogs: a case‐control study. PLoS One. 2013;8:e69192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yri OE, Ekstrom PO, Hilden V, et al. Polymorphisms in genes encoding interleukin‐10 and drug metabolizing enzymes GSTP1, GSTT1, GSTA1 and UGT1A1 influence risk and outcome in Hodgkin lymphoma. Leuk Lymphoma. 2012;53:1934‐1944. [DOI] [PubMed] [Google Scholar]
- 48. Bin Q, Luo J. Role of polymorphisms of GSTM1, GSTT1 and GSTP1 Ile105Val in Hodgkin and non‐Hodgkin lymphoma risk: a human genome epidemiology (HuGE) review. Leuk Lymphoma. 2013;54:14‐20. [DOI] [PubMed] [Google Scholar]
- 49. Marconato L, Leo C, Girelli R, et al. Association between waste management and cancer in companion animals. J Vet Intern Med. 2009;23:564‐569. [DOI] [PubMed] [Google Scholar]
- 50. Carlo GL, Cole P, Miller AB, Munro IC, Solomon KR, Squire RA. Review of a study reporting an association between 2,4‐dichlorophenoxyacetic acid and canine malignant lymphoma: report of an expert panel. Regul Toxicol Pharmacol. 1992;16:245‐252. [DOI] [PubMed] [Google Scholar]
- 51. Kaneene JB, Miller R. Re‐analysis of 2,4‐D use and the occurrence of canine malignant lymphoma. Vet Hum Toxicol. 1999;41:164‐170. [PubMed] [Google Scholar]
- 52. Luethcke KR, Ekena J, Chun R, Trepanier LA. Glutathione S‐transferase theta genotypes and environmental exposures in the risk of canine transitional cell carcinoma. J Vet Intern Med. 2019;33:1414‐1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1 Questionnaire administered to owners of boxer dogs with lymphoma and unaffected control boxers to assess possible environmental exposures.
Table S1 Variants (n = 32) in canine GSTT1, GSTT5, GSTM1, and GSTP1 targeted for resequencing in boxer and nonboxer dogs. Criteria for inclusion were a minor allele frequency (MAF) >3% previously documented in healthy dog populations, along with characterized low expression/activity or predicted functional effects in silico. Several additional variants were included from dbSNP and from de novo observations from the current study. DGP: Whole genome sequencing data from up to 281 domestic dogs from the NHGRI Dog Genome Project. dbSNP: formerly found on the NCBI dbSNP website, which no longer includes nonhuman SNP data.


