Abstract
Myasthenia, a syndrome of impaired neuromuscular transmission, occurs as either an acquired or congenital condition. Myasthenia gravis (MG) is an acquired autoimmune disorder with autoantibodies against the neuromuscular junction (NMJ) of skeletal muscle whereas congenital myasthenic syndromes (CMSs) are a clinically heterogeneous group of genetic disorders affecting the NMJ with a young age of onset. Both conditions are diseases for which recognition is important with regard to treatment and outcome. We review the published literature on MG and CMSs in dogs and cats, and by comparison with published classification used in humans, propose a classification system for MG and CMSs in dogs and cats. Myasthenia gravis is first classified based on focal, generalized, or acute fulminating presentation. It then is subclassified according to the autoimmune disease mechanism or seronegativity. Autoimmune disease mechanism relates to the presence or absence of a thymoma, or administration of thiourylene medication in cats. Congenital myasthenic syndromes are classified according to the affected NMJ component, the mechanism of the defect of neuromuscular transmission, the affected protein, and ultimately the mutated gene responsible. In proposing this categorization of MG and CMSs, we hope to aid recognition of the disease groups for both conditions, as well as guide treatment, refine prognosis, and provide a framework for additional studies of these conditions.
Keywords: canine, feline, megaesophagus, thymoma, weakness
Abbreviations
- 3,4‐DAP
3,4‐diaminopyridine
- ACh
acetylcholine
- AChE
acetylcholinesterase
- AChE‐I
acetylcholinesterase inhibitor
- AChE‐Is
acetylcholinesterase inhibitors
- AChR
acetylcholine receptor
- AChRs
acetylcholine receptors
- CHAT
choline acetyltransferase
- CHRNE
cholinergic receptor nicotinic ε subunit
- CMS
congenital myasthenic syndrome
- CMSs
congenital myasthenic syndromes
- COLQ
collagen‐like tail subunit of asymmetric acetylcholinesterase
- EMG
electromyography
- LRP4
low‐density lipoprotein receptor‐related protein 4
- MG
myasthenia gravis
- MUSK
muscle specific kinase
- NMJ
neuromuscular junction
- RNS
repetitive nerve stimulation
1. INTRODUCTION
Myasthenia gravis (MG) is a disorder of neuromuscular transmission affecting dogs and cats that has historically encompassed an acquired or autoimmune form, characterized by autoantibodies against the neuromuscular junction (NMJ), 1 , 2 with a reported onset from 6 months of age onwards, 3 , 4 and a congenital form in which no autoimmunity against the NMJ is present, 1 , 2 , 3 with reported onset in the first weeks to months of life. 1 , 2 , 3 , 5
Historically, the congenital form of MG originally was thought to solely be the result of a postsynaptic deficiency of acetylcholine receptors (AChRs) in the absence of autoantibodies directed against them. 2 , 6 , 7 However, it is now clear that it is only 1 of several clinically heterogeneous congenital syndromes affecting the NMJ, resulting in skeletal muscle weakness and fatigability. 5 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 These syndromes now collectively are referred to as congenital myasthenic syndromes (CMSs), and considered as a separate disease entity, meaning that the term MG now solely refers to autoimmunity against the NMJ. 5 , 17 There is currently no established classification system for CMSs in dogs and cats.
Myasthenia gravis currently is classified in dogs and cats according to generalization, progression, and severity of skeletal muscle weakness and fatigability. 1 , 2 , 18 , 19 However, factors such as the presence or absence of a thymoma, or administration of thiourylene medication in cats, can influence treatment, outcome, or both, frequently leading to the division of affected dogs and cats into separate disease groups based on these factors. 1 , 2 , 4 , 18 , 20 , 21 , 22 , 23 , 24 , 25 , 26
Our goal was to review the published literature on MG and CMSs in dogs and cats, and by comparison with published classifications for humans of these 2 conditions, propose a classification system for MG and CMSs in dogs and cats to facilitate their recognition, as well as guide treatment, refine prognosis, and provide a framework for additional studies of these conditions (Figure 1).
FIGURE 1.
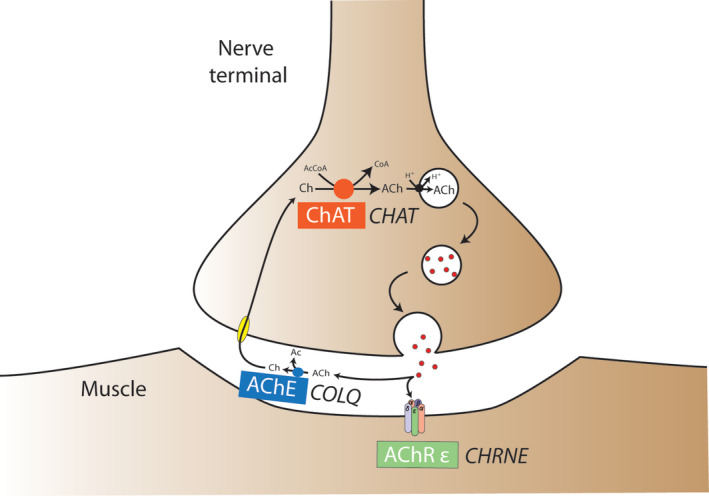
Schematic illustration of currently reported CMSs in dogs and cats including the affected neuromuscular compartment, the mechanism of failure of neuromuscular transmission, the affected protein, and the underlying mutated gene. Ac, acetyl; AcCoa, acetyl‐coenzyme A; ACh, acetylcholine; AChE, acetylcholinesterase; AChR ε, acetylcholine receptor ε subunit; Ch, choline; ChAT, choline acetyltransferase protein; CHAT, choline acetyltransferase gene; CHRNE, cholinergic receptor nicotinic ε subunit gene; CoA, coenzyme A; COLQ, collagen‐like tail subunit of asymmetric acetylcholinesterase gene; CMS, congenital myasthenic syndrome; H+, hydrogen ion
2. MYASTHENIA GRAVIS
Myasthenia gravis is an autoimmune disorder that impairs neuromuscular transmission by the production of autoantibodies against the NMJ. 5 In dogs, these autoantibodies most frequently target the acetylcholine receptor (AChR) 1 , 2 , 5 , 18 but autoantibodies targeting a protein called muscle specific kinase (MUSK) also are reported. 27 In cats, however, autoantibodies are exclusively reported against the AChR, with no reports of autoantibodies against other components of the NMJ. 2 , 24 Myasthenia gravis can present focally, in a generalized fashion, or in an acute fulminating manner. 19 Myasthenia gravis also can occur with or without a thymoma in dogs and cats, 1 , 2 , 4 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 or after administration of thiourylene medication in cats, 1 , 2 , 20 , 21 , 22 , 23 and the autoimmune disease mechanism differs based on these factors, 1 , 2 , 18 , 20 , 22 , 23 as can treatment, outcome, or both. 1 , 2 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26
2.1. Terminology and definition used in humans
Myasthenia gravis is defined in humans as skeletal muscle weakness and fatigability caused by autoantibodies against components of the NMJ of skeletal muscle. 28 , 29 , 30 This definition is used because autoantibodies are not limited to the AChR, but instead can be directed against MUSK, or low‐density lipoprotein receptor‐related protein 4 (LRP4). 28 , 29 , 30
2.2. Classification used in humans
Because clinical presentation, diagnosis, optimal treatment, and outcome vary among human MG patients, subgrouping is necessary. 28 , 29 , 30 It is performed according to the autoimmune disease mechanism, protein or proteins targeted, status of the thymus gland, genetic characteristics, response to treatment, and whether skeletal muscle involvement is focal or generalized. 28 , 29 , 30 Subgroups include AChR autoantibody positive ocular, early onset, late onset, or thymoma‐associated MG, AChR autoantibody negative MUSK or LRP4 autoantibody positive MG, and seronegative MG (see Table 1). 28 , 29 , 30
TABLE 1.
Classification of myasthenia gravis subgroups in humans as previously described 29
| MG subgroups | Autoantibody target | Age of onset | Sex | Haplotype associations | Thymic status |
|---|---|---|---|---|---|
| Early onset | AChR | <50 years | Female predisposition | Various | Hyperplasia common |
| Late onset | AChR (titin, ryanodine receptor) | >50 years | Male predisposition | Various | Atrophy common |
| Thymoma | AChR (titin, ryanodine receptor) | Any age | … | … | Lymphoepithelioma |
| MUSK | MUSK | Any age | Female predisposition | DRB1:14, DRB1:16, DQB1:5 | Normal |
| LRP4 | LRP4 | Any age | … | … | Normal |
| Ocular | Variable | Any age | … | … | Normal |
| Seronegative | Unknown | Any age | … | … | Normal or hyperplasia |
Abbreviations: AChR, acetylcholine receptor; LRP4, low‐density lipoprotein receptor‐related protein 4; MG, myasthenia gravis; MUSK, muscle specific kinase.
2.3. Terminology and definition in dogs and cats
Myasthenia gravis classification historically has included both acquired and congenital forms of the disease. 1 , 2 In recent years, the term CMS has replaced the term congenital MG in dogs and cats, and the term MG now refers only to autoimmunity against the NMJ. 5 , 17
2.4. Classification in dogs and cats
Current classification of MG includes focal, generalized and acute fulminating presentations based on a previous classification system used in humans. 19 , 31 Focal MG is defined as weakness in ≥1 focal skeletal muscle group that does not involve the appendicular skeletal muscles. 1 , 2 , 19 , 31 , 32 These focal skeletal muscle groups are the facial, esophageal, pharyngeal, and laryngeal skeletal muscles. 1 , 2 , 19 , 31 , 32 Generalized MG is defined as appendicular skeletal muscle weakness, which can range from mild to severe, with or without facial, esophageal, pharyngeal, or laryngeal skeletal muscle involvement. 1 , 2 , 18 , 19 , 31 Acute fulminant MG is defined as an acute, rapidly progressive and very severe form of generalized MG frequently but not necessarily causing respiratory failure and death. 1 , 2 , 18 , 19 , 31 , 33 , 34 This classification is widely accepted, and will therefore be retained. However we believe that differentiation between generalized and fulminant presentations can be difficult because of lack of objective criteria to differentiate a fulminant presentation from a severe generalized presentation, and that they might represent a continuous spectrum of disease. A separately classified acute fulminant MG form no longer exists in people, rather it is accepted that the disease spectrum of generalized MG ranges from mild to extreme weakness with some patients initially presenting in a myasthenic crisis, which is objectively defined as onset or exacerbation of skeletal muscle weakness to the point that intubation and mechanical ventilation are required. 28 , 29 , 30 , 35 In people, a myasthenic crisis can occur at any time point in the disease including as the initial presentation of the disease. 35
As in humans, the autoimmune disease mechanism can affect treatment, outcome, or both, and relates to the presence or absence of a thymoma, or administration of thiourylene medication in cats. 1 , 2 , 18 , 20 , 21 , 22 , 23 , 24 , 25 , 26 We therefore introduce subgroup classification according to the autoimmune disease mechanism. Lastly, as in humans, NMJ autoantibody testing can be negative in some dogs with generalized MG. 1 , 27 Such cases must fulfill specific criteria to be referred to as seronegative, including serum AChR autoantibody testing to have been negative at least twice. 1 , 27 A seronegative subgroup was not included in the previous classification of MG in dogs because of a lack of defining criteria. 19 Such criteria are now available, and classification based on seronegativity is therefore possible in dogs. 1 , 27 A summary of our proposed classification of MG in dogs and cats is presented in Table 2.
TABLE 2.
Classification of myasthenia gravis in dogs and cats
| Focal myasthenia gravis | Nonthymoma associated subgroup |
| Thymoma associated subgroup | |
| Generalized myasthenia gravis | Nonthymoma associated subgroup |
| Thymoma associated subgroup | |
| Thiourylene medication associated subgroup (cats only) | |
| Seronegative subgroup (dogs only) | |
| Acute fulminant myasthenia gravis | Nonthymoma associated subgroup |
| Thymoma associated subgroup |
Additional characteristics of the disease that are used in humans to further classify patients, such as protein or proteins targeted, thymus gland status, and genetic characteristics cannot be used at this time for classification of MG in dogs and cats. The underlying reasons are discussed below.
Regardless of the subgroup, certain dogs and cats can present with associated coexisting diseases. 1 , 31 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 The implications of the presence of these coexisting diseases on patients with MG are currently unknown. 1 Although such affected dogs and cats are classified into specific subgroups, we recognize that treatment, outcome, or both might differ from the remainder of their respective subgroup depending on the concurrent condition or conditions. However the decision to include such cases into a specific subgroup appears reasonable given that all other aspects of their MG are consistent with the subgroup in which they are categorized.
2.4.1. Non–thymoma‐associated myasthenia gravis
Dogs and cats in the focal, generalized, and acute fulminating categories that do not have a thymoma are referred to by this designation. The targeted protein is the AChR, 1 , 2 , 18 , 19 , 20 , 21 , 24 although striational autoantibodies against titin and the ryanodine receptor are reported in some dogs. 1 , 44 These striational autoantibodies are typically accompanied by AChR autoantibodies when clinical signs of skeletal muscle weakness and fatigability are present, and are thought not to be pathogenic, hence they are not classified separately, although they might serve as markers of disease severity. 29 , 30 , 44 Attempts at categorizing some dogs and cats into early or late onset non–thymoma‐associated MG subgroups, as in humans, are reported. 41 , 44 , 45 These range from being based on severity of clinical signs alone, 41 to using the bimodal age of onset alongside either demonstration of an association with the DLA‐DQB1 haplotype, 45 itself frequently observed in humans with early onset MG, or by identification of striational autoantibodies, 44 which are predominantly but not exclusively reported in late onset MG in humans. 30 However, the frequency of thymic hyperplasia or atrophy is unknown in dog and cats, 19 , 21 , 46 as is the benefit of removing the thymus in those cases. 19 , 21 The presence of an age‐related sex predisposition as seen in humans is not clear in dogs and cats, 19 , 31 and despite a bimodal age of onset, no cut‐off value permits separation into early and late onset MG subgroups. 19 , 31 Because of this lack of necessary information, it is prudent not to subgroup dogs and cats into early and late onset non–thymoma‐associated MG subgroups at this time.
2.4.2. Thymoma‐associated myasthenia gravis
Dogs and cats in the focal, generalized, and acute fulminating categories that have a thymoma are referred to by this designation. In these dogs and cats, MG is the result of a paraneoplastic syndrome. 1 , 2 , 18 , 19 , 21 The targeted protein is the AChR, 1 , 2 , 19 , 21 and striational autoantibodies against titin and the ryanodine receptor also are reported in some dogs. 1 , 44 In creating a thymoma‐associated MG subgroup, we recognize that classification of dogs or cats with any concurrent neoplasia, but specifically those with cranial mediastinal neoplasia, might be difficult because it could be argued that such tumors also might induce MG as part of a paraneoplastic syndrome. 1 However, aside from individual reports, no neoplasia is repeatedly identified in conjunction with MG in dogs, 1 , 47 , 48 , 49 cats, 21 or even humans, 30 suggesting that the presence of concurrent neoplasia that is not a thymoma might be coincidental.
2.4.3. Thiourylene medication‐associated myasthenia gravis
Myasthenia gravis occurs secondarily to the administration of thiourylene medication in some cats as a consequence of a reversible break in tolerance to self‐AChRs. 1 , 2 , 20 , 22 , 23 Myasthenia gravis appears drug‐induced and reversible in this subgroup because clinical signs often resolve after discontinuation of the medication. 2 This subgroup is only present in the generalized category of MG, because all cats with thiourylene medication‐associated MG to date have been reported to have generalized clinical signs. 1 , 2 , 20 , 22 , 23
2.4.4. Seronegative myasthenia gravis
Acetylcholine receptor autoantibody testing by radioimmunoassay is negative in approximately 2% of dogs with generalized MG and they are referred to as seronegative. 1 To be classified as such, dogs are required to have general and neurological examination, pharmacologic, and electrophysiologic findings consistent with MG, as well as normalization of clinical signs after acetylcholinesterase inhibitor (AChE‐I) treatment and for serum AChR autoantibody testing by radioimmunoassay to have been negative at least twice. 1 Because AChR autoantibody testing can be negative early in the course of the disease and because many patients subsequently can seroconvert, measurement of serum AChR should be repeated 1 to 2 months after a negative AChR test. 1 Prior treatment with immunosuppressive medication can result in a negative result, 1 , 37 and should therefore be ruled out. Possible explanations as to why AChR autoantibody radioimmunoassay testing can be negative in dogs with MG include damage to the antigenic epitopes during the process of solubilization thereby preventing recognition of autoreactive AChR autoantibodies, the majority of autoantibodies being bound in the skeletal muscle thereby causing circulating AChR autoantibody concentration to be within normal limits, or autoantibodies being directed against the toxin binding site. 1 Additionally, autoantibodies can be directed against other components of the postsynaptic NMJ. 27 This has been demonstrated in 1 dog seronegative for the AChR in which autoantibodies were detected against MUSK. 27 Availability of MUSK autoantibody testing is limited and no established reference range is available in dogs. 27 The percentage of dogs with focal MG, and cats with focal or generalized MG, that are seronegative is unknown. 1 , 37 Such cases might exist, 19 however because they have not been reported and no criteria are available to define them, such subgroups are not included in our classification.
2.5. DIAGNOSIS
The gold standard for the diagnosis of MG in dogs and cats is positive NMJ autoantibody testing by measurement of AChR autoantibody concentration using radioimmunoassay. 1 , 2 , 18 The presence of skeletal muscle weakness and fatigability supports the diagnosis. 1 , 2 , 18 Ancillary tests such as pharmacological testing and electrophysiology continue to play a key role in the diagnostic approach to MG in dogs and cats. 1 , 2 , 18 They can support the clinical suspicion of MG while NMJ autoantibody testing is pending, but they lack the combined sensitivity and specificity required to permit a definitive diagnosis. 1 , 2 , 18 For instance, although dramatic improvement in skeletal muscle weakness and fatigability usually is expected after administration of acetylcholinesterase inhibitors (AChE‐Is) in dogs and cats with MG, subjective improvement also can be observed in other myopathic or neuropathic disorders, hence mimicking MG. 1 Furthermore, pharmacological testing can be negative in dogs and cats with MG and primarily is observed in those with focal MG as opposed to generalized MG. 1 Evaluation for the presence or absence of a cranial mediastinal mass, which in most cases is a thymoma, is important in dogs and cats. 1 , 2 , 4 , 19 , 21 This evaluation should be done using thoracic imaging, either thoracic radiography or ideally computed tomography. 24 The incidence of a concurrent cranial mediastinal mass in dogs and cats is 3.4% and 52%, respectively. 4 , 21 , 37 In addition to evaluation for the presence or absence of a thymoma, inquiring about the administration of any thiourylene medication in cats is necessary to permit adequate subgrouping. Lastly, given that MG can be associated with certain coexisting diseases in dogs and cats, investigations should evaluate for their presence or absence. 1 , 18 , 37
2.6. Treatment and outcome
Currently, limited evidence is available to guide treatment, and hence controlled studies are needed. 1 Treatment of MG can be divided into that which is beneficial for all subgroups and that which is specific for a given subgroup or subgroups in dogs and cats. 1 , 2 , 18 Symptomatic treatment using AChE‐Is, immunosuppression, and supportive treatment are all successfully reported in the management of all subgroups in dogs and cats. 1 , 2 , 18
Supportive care is an important aspect of management. 1 Unlike the situation in people, megaesophagus and associated aspiration pneumonia require particular attention in dogs and cats. 1 Aspiration pneumonia is the most frequent cause of death, and its management therefore represents a cornerstone of treatment. 1
Recommendations in humans are to start symptomatic treatment, usually with pyridostigmine and then add immunosuppression, usually with prednisolone if clinical remission is not achieved with symptomatic treatment alone. 29 , 30 A similar approach is suggested in dogs given the high incidence of megaesophagus and therefore risk of aspiration pneumonia, although AChE‐Is can themselves cause serious adverse effects manifested as cholinergic crises. 1 No recommendations currently exist in cats. 1 , 2 Although the incidence of megaesophagus, and therefore risk of aspiration pneumonia is also high, cats are suggested to be more prone to the adverse effects of AChE‐Is. 20 , 50 Furthermore, immunosuppression with corticosteroids is thought to be more beneficial, and cats appear more tolerant to the adverse effects of corticosteroids, unlike dogs, which often experience exacerbation of their skeletal muscle weakness. 1 , 2
Dogs and cats with acute fulminant MG, with deterioration of their skeletal muscle weakness because of surgery or general anesthesia, or with aspiration pneumonia causing respiratory distress, often require intensive care including intubation and mechanical ventilation. 1 , 2 , 37 Plasmapheresis has been reported as a treatment in addition to prednisolone in a dog with MG, resulting in clinical remission. 51 Additionally, human IV immunoglobulin also has been reported as a treatment in 2 dogs with MG resulting in transient clinical remission. 37 In humans, plasmapheresis and IV immunoglobulins are used for the management of myasthenic crises for all subgroups. 29 , 30 Perhaps all MG subgroups in dogs and cats might similarly benefit from these treatments in an emergency situation. Most dogs and cats with acute fulminating MG are reported to die either because of respiratory failure or severe aspiration pneumonia. 1 , 2 , 19 , 31
Although some aspects of treatment are common to all subgroups, the presence or absence of a thymoma or the administration of thiourylene medication in cats can affect treatment, outcome, or both. 1 , 2 , 18 , 20 , 21 , 22 , 23 , 24 , 25 , 26
Clinical remission is defined by the resolution of clinical signs of MG, whereas immune remission is defined by the resolution of clinical signs in conjunction with discontinuation of treatment and normalization of serum AChR autoantibody concentration. 24 A peculiarity of generalized non–thymoma‐associated MG in cats is that spontaneous immune remission, as defined as resolution of the clinical signs and normalization of serum AChR autoantibody concentration without any treatment, can occur, suggesting that treatment is not always necessary. 24 Furthermore, immune remission is frequent in dogs with focal and generalized non–thymoma‐associated MG, and hence treatment often can be discontinued, and long‐term outcome is usually excellent if the dog does not succumb to aspiration pneumonia or respiratory failure before achieving immune remission. 26 This is not as clear in cats with focal and generalized non–thymoma‐associated MG, although in 1 study immune remission was achieved with excellent long‐term outcome in 8/8 cats without the need for continued treatment. 24 In the absence of immunosuppression, serial measurement of AChR autoantibody concentration is useful to monitor the course of the disease in affected dogs and cats. 1 , 2
In focal and generalized thymoma‐associated MG in dogs, anecdotal evidence suggests that a complete thymectomy is necessary for these patients to achieve immune remission. 1 However the percentage of dogs that achieve immune remission after a complete thymectomy is unknown. 1 In cats with focal and generalized thymoma‐associated MG, no difference in outcome was reported whether thymectomy was performed or not in 1 study, but the completeness of the resection of the thymomas was not known. 21 To date, the only reported cat with thymoma‐associated MG to have achieved immune remission underwent thymectomy. 21 Aside from these dogs and cat reported to have achieved immune remission after thymectomy, the long‐term outcome of dogs and cats with focal and generalized thymoma‐associated MG does not appear as favorable as that of dogs and cats in the focal or generalized non–thymoma subgroups because immune remission is not reported and therefore continued treatment with medication is required if they do not succumb to aspiration pneumonia or respiratory failure, but clinical remission can be achieved. 1 , 2 , 21 , 24 , 52 , 53 Furthermore, thymomas can occupy a large amount of space in the thorax, invade local tissues, metastasize to other organs, or induce hemothorax which might result in death. 1 , 54 , 55 , 56 , 57 Hence for this reason alone, thymectomy generally is recommended. 1 , 37 A major caveat to thymectomy however is the requirement for general anesthesia which is a substantial risk for dogs and cats with MG, and the surgery itself can cause a clinically relevant postoperative exacerbation of skeletal muscle weakness and fatigability, which is thought to be the result of surgical stress. 1 , 37
Given that MG is caused by administration of thiourylene medication in cats with thiourylene medication‐associated MG, this medication should be discontinued if possible. 1 , 2 Furthermore, immune remission has only been reported with discontinuation of the thiourylene medication being administered. 20 , 23 In these cats, treatment eventually was discontinued, and long‐term outcome was excellent. 20 , 23 Comparatively, although anecdotally, the outcome of cases in which thiourylene medication administration was not discontinued does not appear to be as favorable, because immune remission is not reported and therefore continued treatment with medication is required although clinical remission can be achieved. 20 , 21 , 22 , 23
Aspiration pneumonia and respiratory failure remain frequent causes of death from MG in dogs and cats, regardless of the subgroup. 1 , 2 , 18 , 58 The 1‐year mortality rate for the disease including all subgroups together is 40% to 60% in dogs 4 , 18 , 19 , 37 and 15% in cats. 31 A high rate of euthanasia (58%) also was reported in cats with MG, but it is not known whether their disease was the reason for euthanasia. 21 The mortality rate for each subgroup currently is undetermined other than for cats with generalized non–thymoma‐associated MG in the absence of megaesophagus, in which no fatalities occurred in 1 study, although sample size was small. 24
3. CONGENTIAL MYASTHENIC SYNDROMES
Many syndromes resembling but distinct from MG have been recognized in dogs and cats since 1974. 1 , 2 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 Affected dogs and cats also manifest skeletal muscle weakness and fatigability, but NMJ autoantibody testing for AChRs is negative. 1 , 2 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 A notable feature of these syndromes is that onset is no older than a few weeks to months of age and a genetic basis is generally identified or suspected. 5 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 59 , 60 , 61 , 62 , 63 , 64 , 66 , 67 , 68 , 69 , 70 , 71 When determined, the pathogenesis of these syndromes invariably involves deficient neuromuscular transmission although the location and mechanism of this failure vary, as do their clinical features including response to a given treatment. 5 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 17 , 68
3.1. Terminology and definition used in humans
In humans, the term CMSs is used to refer to a clinically heterogeneous group of genetic disorders causing aberrant neuromuscular transmission. 72 , 73 , 74 , 75
3.2. Classification used in humans
Although CMSs share the common features of skeletal muscle weakness and fatigability associated with inadequate neuromuscular transmission, their clinical presentation (ie, age of onset, presenting signs, distribution of skeletal muscle weakness and fatigability, and response to treatment) varies according to the mutation. 72 , 73 , 74 , 75 , 76 , 77 Over 30 mutations have been identified in humans, and affect proteins involved in NMJ structure, function, or repair. 70 , 71 , 72 , 73 , 74 , 75 Numerous criteria can be used to classify these CMSs although the most frequently used classification system is based on the affected NMJ component or components, thereby organizing them into presynaptic, synaptic, postsynaptic, and concurrent presynaptic‐ and postsynaptic CMSs (see Table 3). 72 , 74 , 75 These CMSs then are categorized further according to the mechanism of the defect of neuromuscular transmission, the affected protein, and ultimately the mutated gene (see Table 3). 72 , 73 , 74 , 75 For some mutations, however, the location of the affected protein or proteins is unknown, meaning that these CMSs cannot presently be classified, and are referred to as “other”. 72 , 74
TABLE 3.
Classification of congenital myasthenic syndromes in humans as previously described 75
| Site of defect | Mechanism | Gene | Protein |
|---|---|---|---|
| Presynaptic | Defect in ACh recycling | SLC5A7 (solute carrier family 5 member 7) | ChT (choline transporter) |
| Defect in ACh synthesis | CHAT | ChAT (choline acetyltransferase) | |
| Defects in loading of ACh in synaptic vesicles | SLC18A3 (solute carrier family 18 member A3) | VAchT (vesicular acetylcholine transporter) | |
| Defect in synaptic vesicle docking, priming, fusing and exocytosis | SNAP25B (synaptosomal nerve‐associated protein 25) | Soluble N‐ethylmeleimide‐sensitive factor attachment protein receptor 25 | |
| UNC13A (unc‐13 Homolog A) | Munc 13 (mammalian uncoordinated‐13) | ||
| SYB1 (Synaptobrevin‐1)/VAMP1 (vesicle associated membrane Protein 1) | SYB1/VAMP1 | ||
| SYT2 (synaptotagmin 2) | SYT2 | ||
| PREPL (prolyl‐endopeptidase Like) | PREPL | ||
| Defect in axonal transport of proteins | MYO9A (myosin IXA) | MYO9A | |
| Synaptic | Acetylcholinesterase deficiency | COLQ | COLQ |
| Synaptic basement membrane defects | COL13A1 (collagen type 13 α 1) | COL13A1 | |
| LAMA5 (laminin α5) | LAMA5 | ||
| LAMB2 (laminin β2) | LAMB2 | ||
| Defects in AChR clustering pathway | AGRN (agrin) | AGRN | |
| Postsynaptic | Reduced numbers of AChR (AChR deficiency) | CHRNE, CHRNA1 (cholinergic receptor nicotinic α1 subunit), CHRNB1 (cholinergic receptor nicotinic β1 subunit), CHRND (cholinergic receptor nicotinic δ subunit) | AChR subunits |
| Kinetic changes in AChR function (slow channel syndromes) | CHRNA1, CHRNB1, CHRND, CHRNE | AChR subunits | |
| Kinetic changes in AChR function (fast channel syndromes) | CHRNA1, CHRND, CHRNB1, CHRNE | AChR subunits | |
| Defect in AChR clustering pathway | LRP4 | LRP4 | |
| MUSK | MUSK | ||
| DOK7 (downstream of kinase 7) | DOK7 | ||
| RAPSN (rapsyn) | RAPSN | ||
| Defect in skeletal muscle voltage‐gated sodium channel | SCN4A (sodium voltage gated channel α4) | SCN4A | |
| Plectin deficiency | PLEC (plectin) | PLEC | |
| Pre + post synaptic | Defective glycosylation | ALG2 (A‐1,3,Mannosyltransferase) | ALG2 |
| ALG14 (ALG14 UDP‐N‐Acetylglucosaminyltransferase Subunit) | ALG14 | ||
| DPAGT1 (dolichyl‐phosphate N‐acetyl‐glucosaminephosphotransferase 1) | DPAGT1 | ||
| GFPT1 (glutamine‐fructose‐6‐phosphate transaminase 1) | GFPT1 | ||
| GMPPB (GDP‐mannose pyrophosphorylase) | GMPPB |
Abbreviations: ACh, acetylcholine; AChR, acetylcholine receptor; CHAT, choline acetyltransferase; CHRNE, cholinergic receptor nicotinic ε subunit; COLQ, collagen‐like tail subunit of asymmetric acetylcholinesterase; LRP4, low‐density lipoprotein receptor‐related protein 4; MUSK, muscle specific kinase.
3.3. Terminology and definition in dogs and cats
Previous terminology frequently has referred to these syndromes as congenital MG, but more recent publications follow the terminology used in humans and use the term CMS 5 , 8 , 17 because it more appropriately characterizes the genetic and clinical heterogeneity of these syndromes. Similar to humans, CMSs also are defined in dogs and cats as a clinically heterogeneous group of genetic disorders causing aberrant neuromuscular transmission. 5 , 17
3.4. Classification in dogs and cats
Given the shared genetic basis of CMSs in humans, dogs, and cats, a similar classification system of CMSs in dogs and cats to that used in humans appears appropriate. Following the classification system used in humans, we propose to classify CMSs in dogs and cats according to the affected NMJ component, the mechanism of the defect of neuromuscular transmission, the affected protein, and ultimately the mutated gene (see Table 4). 75 A concurrent presynaptic and postsynaptic category is not included in this classification of CMSs in dogs and cats because at the present time such CMSs are not reported in these species. 5 , 10 This situation might may change as new CMSs are discovered. Despite efforts to classify CMSs in dogs and cats, many suspected CMSs presently cannot be classified because of a lack of identification of an underlying genetic mutation 7 , 15 , 16 , 60 , 61 , 64 , 65 , 67 Among these suspected CMSs, many are suspected to be postsynaptic because of a lack of postsynaptic AChRs, 7 , 15 , 16 , 64 but these suspected CMSs still cannot be classified, because concurrent presynaptic involvement cannot be excluded because of a lack of knowledge of the suspected underlying genetic mutation, 7 , 15 , 16 , 64 and as such a deficiency although marginal also is reported in some synaptic CMSs in dogs. 8 , 10
TABLE 4.
Classification of congenital myasthenic syndromes in dogs and cats
| Affected neuromuscular junction component | Mechanism of the defect of neuromuscular transmission | Protein | Gene | Species, breed |
|---|---|---|---|---|
| Presynaptic | Defect in ACh synthesis | ChAT (choline acetyltransferase) | CHAT | Dogs: Old Danish Pointing dog |
| Synaptic | AChE deficiency | COLQ | COLQ |
Dogs: Labrador Retriever, Golden Retriever Cats: Sphynx, Devon Rex |
| Postsynaptic | AChR deficiency | AChR ε subunit | CHRNE |
Dogs: Jack Russell Terrier, Heideterrier |
Abbreviations: ACh, acetylcholine; AChE, acetylcholinesterase; AChR, acetylcholine receptor; CHAT, choline acetyltransferase; CHRNE, cholinergic receptor nicotinic ε subunit; COLQ, collagen‐like tail subunit of asymmetric acetylcholinesterase.
3.4.1. Presynaptic CMSs
Defect in the synthesis of acetylcholine
A presynaptic CMS is reported in Old Danish Pointing dogs. 11 , 63 , 66 , 71 It involves a missense mutation in exon 6 of the choline acetyltransferase (CHAT) gene the normal function of which is the synthesis of acetylcholine (ACh), and is inherited in an autosomal recessive manner. 11 Reported onset is 12 to 16 weeks of age, and a period of exercise is required before observation of skeletal muscle weakness and fatigability, at which time palpation of the affected skeletal muscles can identify hypertonicity. 11 , 66 , 71 A decremental response is observed upon repetitive nerve stimulation (RNS), but a preceding high frequency conditioning train is required, and postsynaptic AChR concentration is normal. 11 , 66 , 71 Neuromuscular junction autoantibody testing for the AChR is negative. 11 , 66 Administration of AChE‐Is has no clinical or electrophysiologic effect, although guanidine can result in transient electrophysiologic improvement. 11 , 71 Genetic testing for this mutation is available for this breed. 11
3.4.2. Synaptic CMSs
Acetylcholinesterase deficiency
A synaptic CMS is identified in Labrador Retrievers, 8 Golden Retrievers, 10 Sphynxes and Devon Rexes. 12 , 13 These CMSs involve a mutation in the collagen‐like tail subunit of asymmetric acetylcholinesterase (COLQ) gene, which anchors acetylcholinesterase (AChE) to the basal lamina of the NMJ. 8 , 10 , 12 , 13 In Labrador Retrievers, it is caused by a nonsynonymous mutation in exon 14, and is inherited in an autosomal recessive manner. 8 Reported onset is 2 to 3 weeks of age, electromyography (EMG) is normal, and a decremental response is observed upon RNS. 8 Skeletal muscle and nerve biopsy results are normal, but postsynaptic AChR concentration is marginally decreased. 8 Neuromuscular junction autoantibody testing for the AChR is negative. 8 Administration of pyridostigmine bromide results in worsening of skeletal muscle weakness and fatigability. 8 Genetic testing for this mutation is available for this breed. 8 In Golden Retrievers, it is caused by a nonconserved missense mutation in exon 13 that is inherited in an autosomal recessive manner. 10 Reported onset is 6 to 8 weeks of age, EMG is normal, but motor nerve conduction studies can identify a mild decrease in the amplitude of the M wave, and RNS identifies a decremental response. 10 Skeletal muscle and nerve biopsy results are normal, but postsynaptic AChR concentration also is marginally decreased. 10 Neuromuscular junction autoantibody testing for the AChR is negative. 10 Administration of edrophonium chloride either results in worsening of skeletal muscle weakness and fatigability or has no effect, whereas administration of albuterol results in temporary improvement of skeletal muscle weakness and fatigability. 10 Genetic testing for this mutation is available for this breed. 10 In Sphynx and Devon Rex cats, the mutation is shared, and is a missense mutation in exon 15, which is inherited in an autosomal recessive manner. 12 , 13 Reported onset is 3 to 23 weeks of age, affected cats often have decreased skeletal muscle mass and dorsal protrusion of the scapulae. 12 , 13 , 14 Electromyography can disclose positive sharp waves and complex repetitive discharges. 12 , 13 A decremental response can be observed upon RNS, but in some cats RNS is within normal limits. 12 , 13 Skeletal muscle biopsy samples frequently have dystrophic changes whereas nerve biopsy results are normal, and postsynaptic AChR concentration is normal. 12 , 13 , 14 Neuromuscular junction autoantibody testing for the AChR is negative. 12 , 13 Administration of edrophonium chloride can result in exacerbation of skeletal muscle weakness and fatigability. 13 Genetic testing for this mutation is available for these breeds. 12 , 13
3.4.3. Postsynaptic CMSs
Primary deficiency of the acetylcholine receptor
A postsynaptic CMS is described in Jack Russell Terriers 9 and in a Heideterrier. 68 Both of these CMSs are associated with a mutation in the cholinergic receptor nicotinic ε subunit (CHRNE) gene, which codes for the ε subunit of the AChR. 9 , 68 In Jack Russell Terriers it is caused by a deletion mutation in exon 7, and is inherited in an autosomal recessive manner. 9 Reported onset is usually 6 to 8 weeks of age. 9 Electromyography is normal, but a decremental response is observed upon RNS. 9 Skeletal muscle and nerve biopsy results are normal, but postsynaptic nicotinic AChR concentration is markedly decreased. 9 Neuromuscular junction autoantibody testing for the AChR is negative. 9 Administration of AChE‐Is results in electrophysiologic as well as temporary clinical improvement. 9 Genetic testing for this mutation is available for this breed. 9 In the Heideterrier, a nonsynonymous mutation in exon 31 is reported in 1 dog with skeletal muscle weakness and fatigability. 68 Reported onset was <1 week of age, and skeletal muscle weakness and fatigability were described to initially affect the thoracic limbs. 68
4. DIAGNOSIS
The combination of skeletal muscle weakness and fatigability, onset from birth or a few weeks or months of age, decremental response upon RNS, presence of affected related individuals, absence of skeletal muscle or nerve pathology, and negative NMJ autoantibody testing for the AChR is highly suggestive of a CMS. 1 , 5 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 16 , 17 Additionally, ultrastructural or in vitro electrophysiologic evidence of a junctionopathy along with having ruled out all other causes can further increase the index of suspicion of a CMS. 1 , 16 However the definitive diagnosis requires identification of a causative genetic mutation, along with clinical signs of skeletal muscle weakness and fatigability. 10 , 16 Administration of edrophonium chloride as part of a challenge test can result in transient marked improvement of skeletal muscle weakness and fatigability, but can otherwise result in exacerbation of skeletal muscle weakness and fatigability, or might have no effect depending on the CMS. 1 , 8 , 9 , 10 , 11 , 13 Hence the result of the edrophonium chloride challenge test should be interpreted in light of what is reported for the specific CMS being investigated. 1 , 8 , 9 , 10 , 11 , 13 Although a decremental response frequently is observed upon RNS in most CMSs, 8 , 9 , 10 there is a CMS in cats in which RNS often can be normal, 12 , 13 and a CMS in dogs in which a preceding high frequency conditioning train usually is required before observation of a decremental response. 11 Ultrastructural and in vitro electrophysiologic testing is available at a few specialized laboratories. 1 Genetic testing is available for known mutations, but when negative, whole genome sequencing analysis should be considered and might permit discovery of previously unknown mutations. 10 , 16
4.1. Treatment and outcome
At present, no treatment targeting the genetic etiology of CMSs in dogs or cats is available, meaning that treatment is restricted to symptomatic management of clinical signs. 8 , 9 , 10 , 11 , 12 , 13 With the recent identification of the genetic mutations responsible for some CMSs in dogs and cats, it is now clear that the causative gene plays a pivotal role as to which symptomatic treatment benefits a given CMS. 8 , 9 , 10 , 11 , 13 , 71 Efforts must therefore be made to obtain a genetic diagnosis, permitting institution of appropriate symptomatic treatment because inappropriate symptomatic treatment can result in exacerbation of clinical signs. 8 , 10 , 13
In the CHAT‐associated CMS of Old Danish Pointing dogs, administration of AChE‐Is had no clinical effect. 11 , 71 Treatment for CHAT‐associated CMSs in humans includes medications that increase the amount of ACh in the synaptic cleft, such as AChE‐Is or 3,4‐diaminopyridine (3,4‐DAP). 76 , 77
Administration of AChE‐Is either has no effect or results in exacerbation of clinical signs in the COLQ‐associated CMSs reported in dogs and cats. 8 , 10 , 13 Administration of albuterol, however, can result in a temporary improvement of clinical signs in the COLQ‐associated CMS reported in Golden Retrievers. 10 Similarly, COLQ‐associated CMSs in humans respond positively to β2‐adrenergic receptor agonists such as albuterol and ephedrine, which are thought to stabilize the NMJ and decrease dispersion of AChRs. 76 , 77 Acetylcholinesterase inhibitors or 3,4‐DAP however are contraindicated in COLQ‐associated CMSs in humans because they generally result in exacerbation of clinical signs. 76 , 77
Jack Russell terriers with CHRNE‐associated CMS generally benefit from treatment with AChE‐Is, but the response can be transient because drug resistance occurs. 9 Similarly, CHRNE‐associated CMSs in humans benefit from AChE‐Is, but also from 3,4‐DAP. 76 , 77 Additionally, drug resistance to AChE‐Is also is observed in CHRNE‐associated CMSs in humans, and addition of a β2‐adrenergic receptor agonist to their treatment is recommended because it counteracts the adverse effects of long‐term AChE‐I treatment on the NMJ. 78
Given the shared pathogenesis, and similarities in the response to a given symptomatic treatment among CMSs in humans, dogs, and cats with shared causative mutations, it is logical to consider similar treatments in dogs and cats as those used in humans for a given CMS, which emphasizes the importance of correctly classifying CMSs in dogs and cats.
Most reported CMSs in dogs and cats have an unfavorable outcome and are fatal. 8 , 9 , 10 , 11 , 12 , 13 However certain Jack Russell terriers and Devon Rexes respectively affected by CHRNE‐ and COLQ‐associated CMS can survive for years, and a suspected CMS reported in Smooth Haired Miniature Dachshunds appears to resolve spontaneously. 9 , 14 , 15
5. CONCLUSION
Myasthenia gravis is an autoimmune disorder that impairs neuromuscular transmission by the production of autoantibodies against the NMJ of skeletal muscle. Congenital myasthenic syndromes are a clinically heterogeneous group of genetic disorders causing aberrant neuromuscular transmission. Both conditions encompass disease groups, the recognition of which is important with regard to treatment, outcome, or both. We have provided a classification system of MG and CMSs in dogs and cats to aid recognition of the disease groups for both conditions, as well as guide treatment, refine prognosis, and provide a framework for additional studies of these conditions. This classification system has been purposefully designed to accommodate possible novel disease groups, but revisions eventually will be needed.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Mignan T, Targett M, Lowrie M. Classification of myasthenia gravis and congenital myasthenic syndromes in dogs and cats. J Vet Intern Med. 2020;34:1707–1717. 10.1111/jvim.15855
REFERENCES
- 1. Shelton GD. Myasthenia gravis and disorders of neuromuscular transmission. Vet Clin North Am Small Anim Pract. 2002;32(1):189‐206. [DOI] [PubMed] [Google Scholar]
- 2. Dickinson PJ, LeCouteur RA. Feline neuromuscular disorder. Vet Clin North Am Small Anim Pract. 2004;34(6):1307‐1359. [DOI] [PubMed] [Google Scholar]
- 3. Palmer AC, Lennon VA, Beadle C, Goodyear JV. Autoimmune form of myasthenia gravis in a juvenile Yorkshire Terrier x Jack Russell Terrier hydrid contrasted with congenital (non‐autoimmmune) myasthenia gravis of the Jack Russell. J Small Anim Pract. 1980;21:359‐364. [DOI] [PubMed] [Google Scholar]
- 4. Shelton GD, Schule A, Kass PH. Risk factors for acquired myasthenia gravis in dogs: 1154 cases (1991‐1995). J Am Vet Med Assoc. 1997;211(1):1428‐1431. [PubMed] [Google Scholar]
- 5. Shelton GD. Myasthenia gravis and congenital myasthenic syndromes in dogs and cats: a history and mini‐review. Neuromuscul Disord. 2016;26(6):331‐334. [DOI] [PubMed] [Google Scholar]
- 6. Lennon VA, Lambert EH, Palmer AC, Cunningham JG, Christie TR. Acquired and congenital myasthenia gravis in dogs: a study of 20 cases In: Satoyoshi E, ed. Myasthenia Gravis‐Pathogenesis and Treatment. Tokyo, Japan: Tokyo University Press; 1980:41‐54. [Google Scholar]
- 7. Indieri RJ, Creighton SR, Lambert EH, Lennon VA. Myasthenia gravis in two cats. J Am Vet Med Assoc. 1983;182:57‐60. [PubMed] [Google Scholar]
- 8. Rinz CJ, Levine J, Minor KM, et al. A COLQ missense mutation in Labrador retrievers having congenital myasthenic syndrome. PLoS One. 2014;9(8):e106425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rinz CJ, Lennon VA, James F, et al. A CHRNE frameshift mutation causes congenital myasthenic syndrome in young Jack Russell terriers. Neuromuscul Disord. 2015;25:921‐927. [DOI] [PubMed] [Google Scholar]
- 10. Tsai KL, Vernau KM, Winger K, et al. Congenital myasthenic syndrome in Golden retrievers is associated with a novel COLQ mutation. J Vet Intern Med. 2019;34:1‐8. 10.1111/jvim.15667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Proschowsky HT, Flagstad A, Cirera S, Joergensen CB, Fredholm M. Identification of a mutation in the CHAT gene of Old Danish Pointing dogs affected with congenital myasthenic syndrome. J Hered. 2007;98:539‐543. [DOI] [PubMed] [Google Scholar]
- 12. Abitbol M, Hitte C, Bossé P, et al. A COLQ missense mutation in Sphynx and Devon Rex cats with congenital myasthenic syndrome. PLos One. 2015;10(9):e0137019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gandolfi B, Grahn RA, Creighton EK, et al. A COLQ variant associated with Devon Rex and Sphynx feline hereditary myopathy. Anim Genet. 2015;46:711‐715. 10.11111/age.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Malik R, Mepstead K, Yang F, Harper C. Hereditary myopathy of Devon Rex cats. J Small Anim Pract. 1993;34:539‐545. [Google Scholar]
- 15. Dickinson PJ, Sturges BK, Shelton GD, LeCouteur RA. Congenital myasthenia gravis in Smooth‐Haired Miniature Dachshund dogs. J Vet Intern Med. 2005;19(6):920‐923. [DOI] [PubMed] [Google Scholar]
- 16. Blakey TJ, Michaels JR, Guo LT, Hodshon AJ, Shelton GD. Congenital myasthenic syndrome in a mixed breed dog. Front Vet Sci. 2017;4:173 10.3389/fvets.2017.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shelton GD. Muscular disorders In: Ettinger SJ, Feldman EC, Côté E, eds. Textbook of Veterinary Internal Medicine: Diseases of the Dog and the Cat. Vol 2 8th ed. St. Louis, MO: Elsevier; 2017:2149. [Google Scholar]
- 18. Khorzad R, Whelan M, Sisson A, Shelton GD. Myasthenia gravis in dogs with an emphasis on treatment and critical care management. J Vet Emerg Crit Care. 2011;21(3):193‐208. [DOI] [PubMed] [Google Scholar]
- 19. Dewey CW, Bailey CS, Shelton GD, Kass PH, Cardinet GH III. Clinical forms of acquired myasthenia gravis in dogs: 25 cases (1988‐1995). J Vet Intern Med. 1997;11(2):50‐57. [DOI] [PubMed] [Google Scholar]
- 20. Shelton GD, Ho M, Kass PH. Risk factors for acquired myasthenia gravis in cats: 105 cases (1986‐1998). J Am Vet Med Assoc. 2000;216(1):55‐57. [DOI] [PubMed] [Google Scholar]
- 21. Hague DW, Humphries HD, Mitchell MA, Shelton GD. Risk factors and outcome in cats with acquired myasthenia gravis (2001‐2012). J Vet Intern Med. 2015;29(5):1307‐1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bell ET, Mansfield CS, James FE. Immune‐mediated myasthenia gravis in a methimazole‐treated cat. J Small Anim Pract. 2012;53(11):661‐663. [DOI] [PubMed] [Google Scholar]
- 23. Ellis J, Tappin S. Management and resolution of acquired myasthenia gravis in a carbimazole‐treated hyperthyroid domestic shorthair cat. Vet Rec Case Rep. 2019;7:e000806 10.1136/vetreccr-2018-000806. [DOI] [Google Scholar]
- 24. Mignan T, Garosi L, Targett M, Lowrie M. Long‐term outcome of cats with acquired myasthenia gravis without evidence of a cranial mediastinal mass. J Vet Intern Med. 2019;34:1‐6. 10.1111/jvim.15655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lainesse MF, Taylor SM, Myers SL, Haines D, Fowler JD. Focal myasthenia gravis as a paraneoplastic syndrome of canine thymoma: improvement following thymectomy. J Am Anim Hosp Assoc. 1996;32(2):111‐117. [DOI] [PubMed] [Google Scholar]
- 26. Shelton GD, Lindstrom JM. Spontaneous remission in canine myasthenia gravis: implications for assessing human MG therapies. Neurology. 2001;57(11):2139‐2141. [DOI] [PubMed] [Google Scholar]
- 27. Shelton GD. Routine and specialized laboratory testing for the diagnosis of neuromuscular diseases in dogs and cats. Vet Clin Pathol. 2010;39:278‐295. [DOI] [PubMed] [Google Scholar]
- 28. Berrih‐Aknin S, Frenkian‐Cuvelier M, Eymard B. Diagnostic and clinical classification of autoimmune myasthenia gravis. J Autoimmun. 2014;48‐49:143‐148. [DOI] [PubMed] [Google Scholar]
- 29. Gilhus NE, Verschuuren JJ. Myasthenia gravis: subgroup classification and therapeutic strategies. Lancet Neurol. 2015;14(10):1023‐1036. [DOI] [PubMed] [Google Scholar]
- 30. Gilhus NE. Myasthenia gravis. N Engl J Med. 2016;375(26):2570‐2581. [DOI] [PubMed] [Google Scholar]
- 31. Ducoté JM, Dewey CW, Coates JR. Clinical forms of acquired myasthenia gravis in cats. Compend Contin Educ Pract Vet. 1999;21(5):440‐448. [Google Scholar]
- 32. Shelton GD, Willard MD, Cardinet GH III, Lindstrom J. Acquired myasthenia gravis: selective involvement of esophageal, and facial muscles. J Vet Intern Med. 1990;4:281‐284. [DOI] [PubMed] [Google Scholar]
- 33. King LG, Vite CH. Acute fulminating myasthenia gravis in five dogs. J Am Vet Med Assoc. 1998;212(6):830‐834. [PubMed] [Google Scholar]
- 34. Richardson D. Acquired myasthenia gravis in a poodle. Can Vet J. 2011;52(2):169‐172. [PMC free article] [PubMed] [Google Scholar]
- 35. Wendell LC, Levine JM. Myasthenic crisis. Neurohospitalist. 2011;1(1):16‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shelton GD. Myasthenia gravis: lessons from the past 10 years. J Small Anim Pract. 1998;39(8):368‐372. [DOI] [PubMed] [Google Scholar]
- 37. Penderis J, Martin‐Vaquero P. Junctionopathies: disorders of the neuromuscular junction In: Dewey CW, Da Costa RC, eds. A Practical Guide to Canine and Feline Neurology. 3rd ed. Ames, IA: Wiley‐Blackwell; 2016:521‐557. [Google Scholar]
- 38. Dewey CW, Shelton GD, Bailey CS, et al. Neuromuscular dysfunction in five dogs with acquired myasthenia gravis and presumptive hypothyroidism. Prog Vet Neurol. 1995;6:117‐123. [Google Scholar]
- 39. Hackett TB, Van Pelt DR, Willard MD, et al. Third degree atrioventricular block and acquired myasthenia gravis in four dogs. J Am Vet Med Assoc. 1995;206:1173‐1176. [PubMed] [Google Scholar]
- 40. Levine JM, Bergman RL, Coates JR, Shelton GD. Myasthenia gravis and hypothyroidism in a dog with meningomyelitis. J Am Anim Hosp Assoc. 2005;41(4):247‐251. [DOI] [PubMed] [Google Scholar]
- 41. Mayousse V, Jeandel A, Blanchard‐Gutton N, Escriou C, Shelton GD, Blot S. Evaluation of coexisting polymyositis in feline myasthenia gravis: a case series. Neuromuscul Disord. 2017;27(9):804‐815. [DOI] [PubMed] [Google Scholar]
- 42. Hill PB, Collins D, fearnside S, Olivry T. Putative paraneoplastic pemphigus and myasthenia gravis in a cat with a lymphocytic thymoma. Vet Dermatol. 2013;24(6):646‐649. [DOI] [PubMed] [Google Scholar]
- 43. Singh A, Boston S, Poma R. Thymoma‐associated exfoliative dermatitis with post‐thymectomy myasthenia gravis in a cat. Can Vet J. 2010;51(7):757‐760. [PMC free article] [PubMed] [Google Scholar]
- 44. Shelton GD, Skeie GO, Kass PH, Aarli JA. Titin and ryanodine receptor autoantibodies in dogs with thymoma and late‐onset myasthenia gravis. Vet Immunol Immunopathol. 2001;78(1):97‐105. [DOI] [PubMed] [Google Scholar]
- 45. Wolf Z, Vernau K, Safra N, et al. Association of early onset myasthenia gravis in Newfoundland dogs with the canine major histocompatibility complex I. Neuromuscul Disord. 2017;27(5):409‐416. [DOI] [PubMed] [Google Scholar]
- 46. Day MJ. Review of thymic pathology in 30 cats and 36 dogs. J Small Anim Pract. 1997;38(9):393‐403. [DOI] [PubMed] [Google Scholar]
- 47. Krotje LJ, Fix AS, Potthoff AD. Acquired myasthenia gravis and cholangiocellular carcinoma in a dog. J Am Vet Med Assoc. 1990;197:488‐490. [PubMed] [Google Scholar]
- 48. Moore AS, Madewell BR, Cardinet GH III, et al. Osteogenic sarcoma and myasthenia gravis in a dog. J Am Vet Med Assoc. 1990;197:226‐227. [PubMed] [Google Scholar]
- 49. Ridyard AE, Rhind SM, French AT, Munro EAC, Hill PB. Myasthenia gravis associated with cutaneous lymphoma in a dog. J Small Anim Pract. 2000;41:348‐351. [DOI] [PubMed] [Google Scholar]
- 50. Wismer T, Means C. Toxicology of newer insecticides in small animals. Vet Clin North Am Small Anim Pract. 2012;42(2):335‐347. [DOI] [PubMed] [Google Scholar]
- 51. Bartges JW, Klausner JS, Bostwick EF, Hakala JE, Lennon VA. Clinical remission following plasmapheresis and corticosteroid treatment in a dog with acquired myasthenia gravis. J Am Vet Med Assoc. 1990;196(8):1276‐1278. [PubMed] [Google Scholar]
- 52. Meeking SA, Prittie J, Barton L. Myasthenia gravis associated with thymic neoplasia in a cat. J Vet Emerg Crit Care. 2008;18(2):177‐183. [Google Scholar]
- 53. Nagata N, Miyoshi T, Otake Y, et al. Temporal deterioration of neurological symptoms and increase of serum acetylcholine receptor antibody levels after thymectomy: a case report of a cat with myasthenia gravis. J Vet Med Sci. 2017;78(12):1893‐1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Moller CA, Bender H. Pathology in practice. J Am Vet Med Assoc. 2017;250(4):387‐390. [DOI] [PubMed] [Google Scholar]
- 55. Robat CS, Cesario L, Gaeta R, Miller M, Schrempp D, Chun R. Clinical features, treatment options and outcome in dogs with thymoma: 116 cases (1999‐2010). J Am Vet Med Assoc. 2013;243(10):1448‐1454. [DOI] [PubMed] [Google Scholar]
- 56. Serfilippi LM, Quance JL. Pathology in practice. J Am Vet Med Assoc. 2018;253(2):173‐176. [DOI] [PubMed] [Google Scholar]
- 57. Bisesi MA, Hammond TN, Oura TJ. What is your diagnosis? J Am Vet Med Assoc. 2016;248(6):609‐611. [DOI] [PubMed] [Google Scholar]
- 58. Dewey CW. Acquired myasthenia gravis in dogs part 1. Compend Contin Educ Pract Vet. 1997;19(12):1340‐1353. [Google Scholar]
- 59. Palmer AC, Barker J. Myasthenia in the dog. Vet Rec. 1974;95(20):452‐454. [DOI] [PubMed] [Google Scholar]
- 60. Johnson RP, Watson ADJ, Smith J, et al. Myasthenia in springer spaniel littermates. J Small Anim Pract. 1975;16:641‐647. [DOI] [PubMed] [Google Scholar]
- 61. Jenkins WL, van Dyk E, McDonald CB. Myasthenia gravis in a fox terrier litter. J S Afr Vet Assoc. 1976;47:59‐62. [PubMed] [Google Scholar]
- 62. Palmer AC, Goodyear JV. Congenital myasthenia in the Jack Russell terrier. Vet Rec. 1978;103(19):433‐434. [DOI] [PubMed] [Google Scholar]
- 63. Flagstad A. A new hereditary neuromuscular disease in the dog breed “Gammel Dansk Honsehund”. Genetic investigations. Hereditas. 1982;96(2):211‐214. [DOI] [PubMed] [Google Scholar]
- 64. Miller LM, Lennon VA, Lambert EH, et al. Congenital myasthenia gravis in 13 smooth fox terriers. J Am Vet Med Assoc. 1983;182:694‐697. [PubMed] [Google Scholar]
- 65. Joseph RJ, Carrillo JM, Lennon VA. Myasthenia gravis in the cat. J Vet Intern Med. 1988;2(2):75‐79. [DOI] [PubMed] [Google Scholar]
- 66. Flagstad A, Trojaborg W, Gammeltoft S. Congenital myasthenic syndrome in the dog breed Gammel Dansk Honsehund: clinical, electrophysiological, pharmacological and immunological comparison with acquired myasthenia gravis. Acta Vet Scand. 1989;30:89‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Van Ham LML. A myasthenic‐like syndrome in young related mongrel dogs. Prog Vet Neurol. 1992;3:135‐139. [Google Scholar]
- 68. Herder V, Ciurkiewicz M, Baumgärtner M, Jagnnathan V, Leeb T. Frame‐shift variant in the CHRNE gene in a juvenile dog with suspected myasthenia gravis‐like disease. Anim Genet. 2017;48:625 10.1111/age.12558. [DOI] [PubMed] [Google Scholar]
- 69. Miller LM, Hegreberg GA, Prieur DJ, Hamilton MMJ. Inheritance of congenital myasthenia gravis in smooth fox terrier dogs. J Hered. 1984;75(3):163‐166. [DOI] [PubMed] [Google Scholar]
- 70. Wallace ME, Palmer AC. Recessive mode of inheritance in myasthenia gravis in the Jack Russell terrier. Vet Rec. 1984;114(14):350. [DOI] [PubMed] [Google Scholar]
- 71. Trojaborg W, Flagstad A. A hereditary neuromuscular disorder in dogs. Muscle Nerve. 1982;5(9):30‐38. [PubMed] [Google Scholar]
- 72. Engel AG, Shen XM, Selcen D, Sine SM. Congenital myasthenic syndromes: pathogenesis, diagnosis, and treatment. Lancet Neurol. 2015;14(5):420‐434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Engel AG. Congenital myasthenic syndromes in 2018. Curr Neurol Neurosci Rep. 2018;18(8):46 10.1007/s11910-018-0852-4. [DOI] [PubMed] [Google Scholar]
- 74. Farmakidis C, Pasnoor M, Barohn RJ, Dimachkie MM. Congenital myasthenic syndromes: a clinical and treatment approach. Curr Treat Options Neurol. 2018;20:36 10.1007/s11940-018-0520-7. [DOI] [PubMed] [Google Scholar]
- 75. Vanhaesebrouck AE, Beeson D. The congenital myasthenic syndromes: expanding genetic and phenotypic spectrums and refining treatment strategies. Curr Opin Neurol. 2019;32(5):696‐703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lee M, Beeson D, Palace J. Therapeutic strategies for congenital myasthenic syndromes. Ann NY Acad Sci. 2018;1412(1):129‐136. [DOI] [PubMed] [Google Scholar]
- 77. Finsterer J. Congenital myasthenic syndrome. Orphanet J Rare Dis. 2019;14(1):57 10.1186/s13023-019-1025-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Vanhaesebrouck AE, Webster R, Maxwell S, et al. β2‐Adrenergic receptor agonists ameliorate the adverse effect of long‐term pyridostigmine on neuromuscular junction structure. Brain. 2019;142(12):3713‐3727. [DOI] [PMC free article] [PubMed] [Google Scholar]


