Abstract
Introduction
Rhabdomyolysis in general trauma patients is a cause of acute kidney injury. However, there are limited data on prevalence, risk factors and the dynamics of creatine kinase (CK) as a surrogate marker for rhabdomyolysis.
Aim
This study aimed to examine the characteristics of CK elevation in general trauma patients and identify the risk factors of acute kidney injury (AKI).
Method
A retrospective study of trauma patients at Songklanagarind Hospital from January 2009 to August 2014 using CK, clinical characteristics and trauma severity as predictors and AKI as defined by the Acute Kidney Injury Network, 2005 criteria as the outcomes.
Results
Of the 372 patients included in the study, the prevalence of rhabdomyolysis was 40.3%, the mean injury severity score (ISS) was 19.5 and 7% had AKI. The CK level peaked at 40 hours after admission with a rate change of 50.98 U/L/h. An initial mean arterial pressure < 65 mmHg, ISS ≥ 25 and CK elevation > 1,000 U/L/h were independently associated with AKI.
Conclusion
Traumatic rhabdomyolysis resulting in AKI occurs in patients with a high peak CK with an increasing rate of change and a high ISS. These parameters could identify patients who needed close monitoring of CK and renal function.
Keywords: acute kidney injury, creatine kinase, rhabdomyolysis, trauma
Introduction
Traumatic rhabdomyolysis is found in 13-16% of all cases of rhabdomyolysis.[1,2] Also, acute kidney injury (AKI) can occur in 12-15% of patients with traumatic rhabdomyolysis. The mechanism is related to the formation of myoglobin casts released from the injured myocytes and the direct cytotoxic effect of myoglobin on renal epithelial cells in conjugation with volume depletion.[3,4]
The serum creatine kinase (CK) level is a convenient test to detect rhabdomyolysis; however, it has a low specificity. Furthermore, there is no definite cutoff point for diagnosis, although the cut-off point of 5,000 U/L is commonly used.[5] The initial and peak levels of CK are associated with AKI and this was found to have a dose response relationship.[6,7] The initial CK does not predict AKI or deaths among patients with a CK > 2,000 U/L.[8] However, a peak CK of 1,000 U/L increases the risk of AKI by three-fold.[1] Patients with a combination of factors, including age ≥ 55 years, injury severity score (ISS) ≥ 16 and CK ≥ 5,000 U/L were found to have a 41% probability of developing AKI.[6]
Previous retrospective studies and case series in rhabdomyolysis resulting from major disasters found that fluid administration to achieve urine output of 100-200 mL/h in the first 24 to 72 hours decreased the rate of AKI.[9-11] Early fluid administration within four hours after injury was also found to lower the need for hemodialysis.[11] CK usually peaked at 72 hours after muscle injury and myoglobinuria was usually not present.[12] Identifying other risk factors and features of patients who will have elevated CK are beneficial for guiding rapid fluid administration. Thus, we conducted a retrospective study to examine the characteristics of CK elevation in general trauma patients and identify the risk factors of acute kidney injury in this population.
Materials and Methods
Study Population
This study protocol was reviewed and approved by the ethics committee for research in human beings of the Faculty of Medicine, Prince of Songkla University. Patient records were retrieved from the trauma registry of Songklanagarind Hospital, which is a large university hospital in southern Thailand. All patients aged 15 years or older who were admitted to the surgical intensive care unit and general trauma ward with traumatic injuries between January 2009 and August 2014 and had at least one record of CK were included into the study. Those who had a history of chronic kidney disease defined as a persistent glomerular filtration rate < 60 mL/min/1.73m2 for at least three months were excluded.[13]
Data Retrieval and Study Design
Data extracted from the electronic patient records included the initial vital signs at the emergency department, fluid intake, urine output and laboratory values for CK and creatinine (Cr) within the first 72 hours after admission. Mechanism of injury and the severity of injury based on the ISS were obtained from the trauma registry.
Rhabdomyolysis was defined as CK > 5,000 U/L at any time point. Vascular injury was defined as injury to peripheral vessels confirmed by computed tomographic angiogram or conventional angiogram. Traumatic amputation or mangled limbs were also included if hard signs of vascular injury were present. Injuries to the extremities included long bone fractures and mangled extremities or traumatic amputation of limbs. Penetrating injuries included gunshot and stab injury while blunt injuries included falls from height and motor vehicle crash. Acute kidney injury was defined according to the Acute Kidney Injury Network (AKIN), 2005 criteria as serum creatinine elevation ≥ 0.3 mg/dL within 48 hours or urine output < 0.5 mL/kg/h for a continuous duration of 6 hours or a condition when the patient needed emergency dialysis during hospitalisation.[14]
Statistical Analysis
Patient and injury characteristics were described using mean and standard deviation or percentage as appropriate. The median CK levels of each individual at each interval from the time of admission were compared between the AKI and non-AKI patients. Multiple logistic regression modeling with dichotomous backward stepwise regression method was performed to identify the associated factors of AKI among patients with elevated CK. AKI was the dependent variable and clinical characteristics that had a p value < 0.2 in the univariate analysis were selected as independent variables. The level of significance was set at 0.05. The initial CK, mean CK and rate of change of CK per hour were tested for the power to predict AKI using the ROC curve and partial ROC analysis. The best cut-off point was selected from the point on the ROC curve closest to the left upper corner. Data were analyzed using the R program[15] with the Epicalc,[16] OptimalCutpoints[17] and pROC[18] package.
Results
Sample Characteristics
Between January 2009 and August 2014, 8,462 adult trauma patients were admitted in Songklanagarind Hospital. Among these, 713 patients had at least one record of CK during admission and 327 patients had at least two records of CK in the course of hospitalization. The majority of the patients were male with a mean age of 34.5 years. Blunt injuries were the most common type, accounting for 62.7% of the patients with motorcycle collision as the most common cause (38.5%). Among those with penetrating injuries (22.9%), gunshot wound was reported as the major cause. Of all patients, about half had proven vascular injuries while 75.2% had extremity injuries. The mean ISS was 19.54 (range, 1-75), which indicated moderate severity. This corresponded to the revised trauma score and trauma and injury severity score. Overall, 23 patients (7%) were found to have AKI based on the AKIN 2005 criteria (Table 1). Most patients received a median of 3-4 L of fluid per day and had a net positive fluid balance within the first 72 hours (Table 2).
Table 1. Demographic data, mechanisms and severity of injury.
ISS, injury severity score; RTS, revised trauma score; TRISS, trauma and injury severity score.
|
N |
327 |
|
Age, years (SD) |
34.5 (14.76) |
|
Male Gender, n (%) |
261 (79.8) |
|
Mechanism of injury |
Number (%) |
|
1. Blunt injury |
205 (62.7) |
|
1.1 Automobile versus pedestrian |
9 (2.8) |
|
1.2 Motor vehicle crash |
42 (12.8) |
|
1.3 Motor cycle collision |
126 (38.5) |
|
1.4 Crush injury |
11 (3.4) |
|
1.5 Human assault |
1 (0.3) |
|
1.6 Fall from height |
14 (4.3) |
|
2. Penetrating injury |
75 (22.9) |
|
2.1 Gunshot wound |
56 (17.1) |
|
2.2 Stab wound |
18 (5.5) |
|
3. Blast injury |
26 (8.0) |
|
4. Electrical injury |
12 (3.7) |
|
5. Other (burn, animal attack) |
8 (2.4) |
|
Proven vascular injury |
175 (53.5) |
|
Extremity injury |
246 (75.2) |
|
Trauma severity scores |
|
|
Mean ISS (SD) |
19.54 (12.74) |
|
ISS 25-75 (%) |
80 (24.5) |
|
ISS ≤ 25 (%) |
247 (75.5) |
|
Mean RTS (SD) |
7.21 (1.24) |
|
Mean TRISS (SD) |
0.913 (0.2) |
|
Acute kidney injury |
23 (7.0) |
Table 2. Fluid intake and fluid balance, median (inter-quartile range, IQR).
mL, milliliter.
|
|
Fluid Intake (mL/24 hours) |
Fluid Balance (mL/24 hours) |
|
Day 1 |
4,139 (2,385, 114,000) |
1,186 (137, 2,883) |
|
Day 2 |
3,829 (3,020, 4,950) |
1,410 (265, 2,493) |
|
Day 3 |
3,174 (2,320, 4,062) |
513 (-410, 1,551) |
Creatine Kinase Elevation and Diagnosis of Rhabdomyolysis
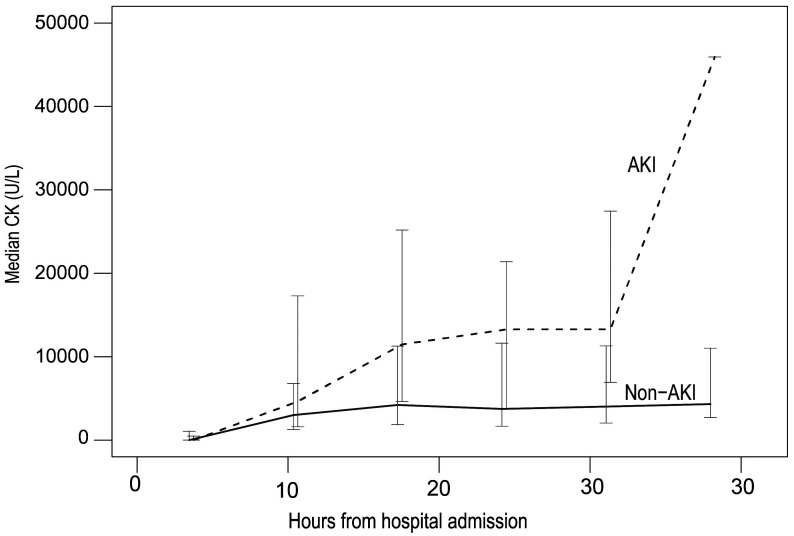
From the cohort of patients with at least one CK record, there were 436 (61.2%) and 155 (21.7%) cases with mean CK cut-off points of 1,000 U/L and 5,000 U/L, respectively, defined as rhabdomyolysis. Among those with at least two measurements of CK, 40.3% had at least one CK measurement ≥ 5,000 U/L (Table 3). The CK measurements were carried out during a maximum of 41.2 hours after hospitalization. The frequency of CK measurements depended on the preference of the attending physician, with a range of 1 to 9 times and a median of every 8 hours after admission. The AKI group had a significantly higher CK and a more rapid rate of elevation per hour, compared to the non-AKI group (Table 4). Figure 1 shows the changes of the mean CK levels over time. The CK level peaked and then plateaued at 40 hours after admission with a median rate change of 50.98 U/L/h. However, a quarter of the patients had a reduction of CK after admission.
Table 3. Distributions of mean CK in AKI and non-AKI, n (%).
AKI, acute kidney injury; CK, creatine kinase.
|
Mean CK (U/L) |
< 195 |
195-999 |
1,000-4,999 |
≥ 5,000 |
|
Non-AKI |
8 (2.6) |
75 (27) |
142 (46.7) |
79 (26.0) |
|
AKI |
0 |
4 (17.4) |
8 (34.8) |
11 (47.8) |
|
All |
8 (2.4) |
79 (24.2) |
150 (45.9) |
90 (27.5) |
Table 4. Pattern of CK elevation in AKI and non-AKI groups, median (inter-quartile range, IQR).
AKI, acute kidney injury; CK, creatine kinase.
|
|
Non-AKI |
AKI |
p value |
All |
|
Initial CK (U/L) |
1,872.5 (841.8, 4,743.8) |
1,641 (439.5, 7,023.5) |
0.589 |
1,859 (804, 4,764) |
|
Peak CK (U/L) |
3,594.5 (1,671.8, 9,309.5) |
9,986 (2,999, 33,303.5) |
0.016 |
3,842 (1,768, 10,080) |
|
Mean CK within 72 h (U/L) |
2,042.1 (914.6, 5,369) |
4,590.2 (1,226, 14,054.5) |
0.072 |
2,078 (932.5, 5,826) |
|
Rate change (U/L/h) |
46 (-6.6,185) |
147.1 (25,1,014.4) |
0.023 |
50.990 (-3.098, 200.400) |
Figure 1. Mean creatine kinase (U/L) changes vs time (hours).
Factors Associated with Renal Failure
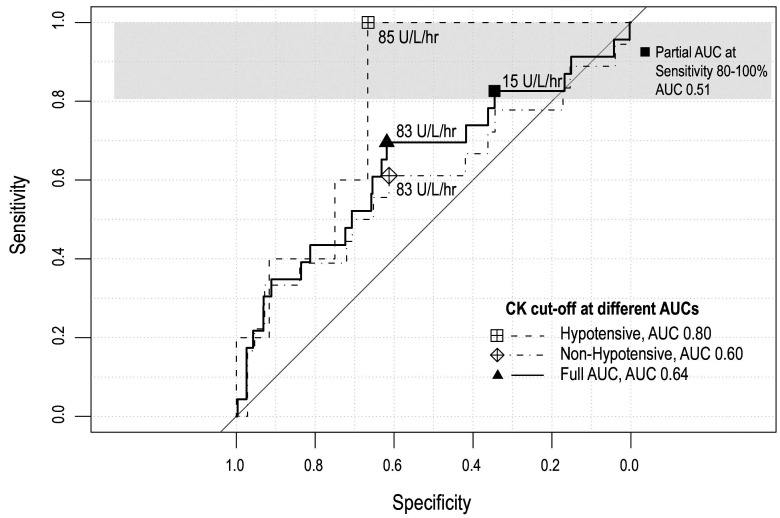
There were 23 patients with AKI. The mortality rate in the AKI group was 26% and 17.3% needed acute hemodialysis. These patients had a significantly higher ISS but no differences in age or mechanism of injury were found. Univariate analysis showed that factors significantly associated with the risk of AKI included systolic blood pressure < 90 mmHg, initial mean arterial pressure (MAP) < 65 mmHg, pulse rate > 100/min, ISS > 25, initial CK < 900 U/L, mean CK > 5,000 U/L, an increasing rate change of CK > 1,000 U/L/h, extremity injury and fluid balance on day 2 after admission. These variables were included in the multivariate modeling. Factors found to be independently associated with AKI were ISS ≥ 25, initial MAP < 65 mmHg, mean CK > 5,000 U/L and extremity injury (Table 5). The mean CK showed a doseresponse relationship with the development of AKI and the rate of change in the CK level of ≥ 1,000 U/L/h also showed an independent association with AKI, if the mean CK was omitted. The rate change had an odds ratio of 7.52 (95% CI 1.8, 31.49) and a p value of 0.006. The mean and initial CK had poor specificity and sensitivity in predicting AKI. By selecting the cut-off point with the highest sensitivity we found that a CK rate of change of 15 U/L/hr predicted AKI with a sensitivity of 82.60% and specificity of 34.53%. Further analysis in the subset of patients with MAP < 65 mmHg found that a CK rate of change of 84.87 U/L/hr predicted AKI with a sensitivity of 100% and specificity of 66% (Table 6) (Figure 2).
Table 5. Multivariate analysis for factors associated with acute kidney injury.
OR, odds ratio; ISS, injury severity score; CK, creatine kinase; MAP, mean arterial pressure.
|
|
Crude OR (95% CI) |
Adjusted OR (95% CI) |
p value |
|
ISS ≥ 25 |
6.87 (2.63, 17.94) |
5.32 (1.82, 15.59) |
0.002 |
|
ISS < 25 |
1 |
1 |
|
|
Initial MAP < 65 mmHg |
4.65 (1.64, 13.15) |
4.25 (1.24, 14.61) |
0.029 |
|
Initial MAP ≥ 65 mmHg |
1 |
1 |
|
|
Mean CK ≥ 5,000 U/L |
2.37 (0.95, 5.94) |
4.59 (1.39, 15.2) |
0.011 |
|
Mean CK < 5,000 U/L |
1 |
1 |
|
|
Extremity injury |
0.23 (0.09, 0.58) |
0.29 (0.09, 0.89) |
0.03 |
|
No extremity injury |
1 |
1 |
1 |
Table 6. Best cut-off values of rate of change of CK/hr to predict AKI.
a AUC, area under the curve.
|
|
AUCa |
95% CI |
Cut-Off Value (U/L/hr) |
Sensitivity |
Specificity |
|
All |
0.64 |
0.5073-0.7779 |
83.04 |
0.61 |
0.70 |
|
Partial AUC |
0.51 |
0.4564-0.6679 |
15.01 |
0.83 |
0.35 |
|
Non Shock |
0.60 |
0.4347-0.7596 |
83.04 |
0.61 |
0.61 |
|
Shock |
0.8 |
0.6209-0.9791 |
84.87 |
1.00 |
0.67 |
Figure 2. ROC curve and best cut-off points of CK elevation to predict AKI.
Discussion
We found that 40.3% of our trauma cases had CK ≥ 5,000 U/L at some point during the first 72 hours after admission which is defined as rhabdomy-olysis. From the 327 cases included in the study, 7% had AKI and the CK gradually rose and peaked at 40 hours after admission. The peak and mean CK values were significantly higher in the groups of patients with AKI and the mean CK correlated with the development of AKI in a dose-response relationship. However, the predictive values of the mean CK or initial CK were not as strong as the rate change of CK per hour. Other factors that were independently associated with AKI were initial MAP < 65 mmHg, ISS ≥ 25, mean CK ≥ 5,000 U/L, extremity injury and increasing CK ≥1,000 U/L/h.
Using CK levels of 1,000 U/L and 5,000 U/L as the cut-off points, the prevalences of rhabdomyolysis in our study were 61.2% and 21.7%, respectively. These results were similar to previous studies in trauma and ICU patients.[1,6,7] Our cohort had a mean ISS score of 19.54 which was comparable to that found in the only study done in trauma patients by Brown et al.[6] The majority of our patients had CK elevations between 1,000-5,000 U/L with blunt injury from motor vehicle crash as the predominant mechanism of injury, which is similar to the subset of trauma patients in a previous study.[1,7]
The prevalence of AKI in our study was 7% and the proportion of AKI in patients with CK > 5,000 U/L was 12.2%, which is concordant with 15% and 9.5% found in previous studies. A much higher proportion of AKI was found by El-Abdellati and colleagues1 which may be due to the inclusion of other ICU patients such as post-cardiac surgery patients and patients with severe sepsis or drug abuse who possibly had more severe baseline renal dysfunction.
Early clinical and laboratory predictors of AKI from rhabdomyolysis could guide a physician in the fluid management of these patients. CK has been most often investigated. The mean CK was found to be linearly correlated to serum Cr.[15] Peak and mean CK levels were extensively studied and the reported ranges of CK were from 1,000 to 5,000 U/L.[6,7,16-18] However, the cause of rhabdomyolysis in these studies was mostly due to ischemia, drugs/toxins and infection which could result in more extensive muscular injuries and the baseline characteristics of the patients could be different from trauma patients.
We found that an increased rate of > 1,000 U/L/h in the CK level was significantly associated with AKI and if CK increased at a rate of 15 U/L/h, it could predict AKI with a sensitivity of 82%. Rapid elevation of CK could be due to the extensive muscle damage causing the formation of myoglobin casts and fluid sequestration into the damaged muscle augmenting the AKI process. Similar to the study by Brown et al.,[6] we found an independent association of peak CK level of > 5,000 U/L to the development of AKI.
We also found other clinical predictors such as high ISS score and low initial MAP as independent predictors of AKI which were in agreement with previous studies that found a combination of age > 55, ISS > 16 and CK > 5,000 U/L had a 41% probability of developing AKI.[6] However, extremity injury was found to be a protective factor and vascular injury was also not significantly associated with AKI. This relationship was not confounded by the amount of fluid intake or fluid balance as hypothesized. Thus, other factors such as the extent of injuries, duration of shock or blood loss need to be further investigated.
As concluded by a historical cohort and previous studies,[19,20] other factors such as sepsis, hyper/hypothermia, acidosis and hypoalbuminemia usually precipitate AKI in patients with rhabdomyolysis. As we did not collect data regarding hemodynamic parameters and concurrent infection during the course of admission, the outcome could be confounded by these factors, which is the nature of a retrospective study design. Another limitation is that we collected data only within the first 72 hours after admission, which may not include the true peak CK in some patients. The main strength of our study is that we were able to demonstrate that a change in the rate of CK was associated with AKI, which was not found in other studies. We were also able to gather data from a comprehensive trauma registry and a digitalized hospital record system, which provided accurate laboratory data during the course of hospital admission.
Conclusion
Traumatic rhabdomyolysis resulting in acute kidney injury occurs in patients with a mean CK of ≥ 5,000 U/L, high ISS > 25 indicating multi-system trauma and a high rate of change in the CK level of > 1,000 U/L/hr. These parameters could identify patients who needed close monitoring of CK and renal function.
Conflicts of Interest
None to declare.
Acknowledgments
We thank the staff in the division of information technology of Songklanagarind Hospital for their assistance in accessing the electronic hospital records. We also express our appreciation to Ms. Nannapat Pruphetkaew and Mr. Edward McNeil, for their guidance on the data analysis. We would like to also acknowledge Mr. Glenn Shingledecker at the International Affairs Office of the Faculty of Medicine, Prince of Songkla University for his English language editing service.
References
- 1.El-Abdellati E, Eyselbergs M, Sirimsi H, et al An observational study on rhabdomyolysis in the intensive care unit. Exploring its risk factors and main complication: acute kidney injury. Ann Intensive Care. 2013;3:8. doi: 10.1186/2110-5820-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McMahon G M, Zeng X, Waikar S S. A risk prediction score for kidney failure or mortality in rhabdomyolysis. JAMA Intern Med. 2013;173:1821–1828. doi: 10.1001/jamainternmed.2013.9774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chatzizisis Y S, Misirli G, Hatzitolios A I, Giannoglou G D. The syndrome of rhabdomyolysis: complications and treatment. Eur J Intern Med. 2008;19:568–574. doi: 10.1016/j.ejim.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 4.Bosch X, Poch E, Grau J M. Rhabdomyolysis and acute kidney injury. N Engl J Med. 2009;361:62–72. doi: 10.1056/NEJMra0801327. [DOI] [PubMed] [Google Scholar]
- 5.Huerta-Alardín A L, Varon J, Marik P E. Bench-to-bedside review: rhabdomyolysis -- an overview for clinicians. Crit Care. 2005;9:158–169. doi: 10.1186/cc2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown C V R, Rhee P, Chan L, Evans K, Demetriades D, Velmahos G C. Preventing renal failure in patients with rhabdomyolysis: do bicarbonate and mannitol make a difference? doi: 10.1097/01.ta.0000130761.78627.10. [DOI] [PubMed] [Google Scholar]
- 7.Sharp L S, Rozycki G S, Feliciano D V. Rhabdomyolysis and secondary renal failure in critically ill surgical patients. Am J Surg. 2004;188:801–806. doi: 10.1016/j.amjsurg.2004.08.050. [DOI] [PubMed] [Google Scholar]
- 8.Baeza-Trinidad R, Brea-Hernando A, Morera-Rodriguez S, et al Creatinine as predictor value of mortality and acute kidney injury in rhabdomyolysis. Intern Med J. 2015;45:1173–1178. doi: 10.1111/imj.12815. [DOI] [PubMed] [Google Scholar]
- 9.Oda J, Tanaka H, Yoshioka T. Analysis of 372 patients with crush syndrome caused by the Hanshin-Awaji earthquake. J Trauma. 1997;42:470–476. doi: 10.1097/00005373-199703000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Shimazu T, Yoshioka T, Nakata Y, et al Fluid resuscitation and systemic complications in crush syndrome: 14 Hanshin-Awaji earthquake patients. J Trauma. 1997;42:641–646. doi: 10.1097/00005373-199704000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Gunal A I, Celiker H, Dogukan A, et al Early and vigorous fluid resuscitation prevents acute renal failure in the crush victims of catastrophic earthquakes. J Am Soc Nephrol. 2004;15:1862–1867. doi: 10.1097/01.asn.0000129336.09976.73. [DOI] [PubMed] [Google Scholar]
- 12.Brancaccio P, Lippi G, Maffulli N. Biochemical markers of muscular damage. Clin Chem Lab Med. 2010;48:757–767. doi: 10.1515/CCLM.2010.179. [DOI] [PubMed] [Google Scholar]
- 13.Chapter 1: definition and classification of CKD. Int Suppl (2011) 2013;3:19–62. doi: 10.1038/kisup.2012.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopes J A, Jorge S. The RIFLE and AKIN classifications for acute kidney injury: a critical and comprehensive review. Clin Kidney J. 2013;6:8–14. doi: 10.1093/ckj/sfs160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melli G, Chaudhry V, Cornblath D R. Rhabdomyolysis: an evaluation of 475 hospitalized patients. Medicine (Baltimore) 2005;84:377–385. doi: 10.1097/01.md.0000188565.48918.41. [DOI] [PubMed] [Google Scholar]
- 16.Ward M M. Factors predictive of acute renal failure in rhabdomyolysis. Arch Intern Med. 1988;148:1553–1557. [PubMed] [Google Scholar]
- 17.Veenstra J, Smit W M, Krediet R T, Arisz L. Relationship between elevated creatine phosphokinase and the clinical spectrum of rhabdomyolysis. Nephrol Dial Transplant. 1994;9:637–641. doi: 10.1093/ndt/9.6.637. [DOI] [PubMed] [Google Scholar]
- 18.de Meijer A R, Fikkers B G, de Keijzer M H, van Engelen B G, Drenth J P. Serum creatine kinase as predictor of clinical course in rhabdomyolysis: a 5-year intensive care survey. Intensive Care Med. 2003;29:1121–1125. doi: 10.1007/s00134-003-1800-5. [DOI] [PubMed] [Google Scholar]
- 19.Ward M M. Factors predictive of acute renal failure in rhabdomyolysis. Arch Intern Med. 1988;148:1553–1557. [PubMed] [Google Scholar]
- 20.Rodríguez E, Soler M J, Rap O, Barrios C, Orfila M A, Pascual J. Risk factors for acute kidney injury in severe rhabdomyolysis. PLoS One. 2013;8:e82992. doi: 10.1371/journal.pone.0082992. [DOI] [PMC free article] [PubMed] [Google Scholar]