Abstract
Introduction
Acute aortic dissection (AAD) is a life-threatening emergency. A small portion of AAD patients presents as an acute stroke without chest pain. A missed or delayed diagnosis of AAD often brings catastrophic outcome. We aimed to identify clinical markers suggestive of the presence of painless AAD in acute stroke patients.
Methods
From January 2007 through December 2014, painless AAD patients were retrospectively collected from our stroke registry. We expanded the search by reviewing Medline and the Science Citation Index Expanded from 1981 until March 2015. We enrolled 200 consecutive cases of acute ischemic stroke without AAD as the control. Univariate analyses were performed to compare clinical markers, followed by logistic regression to analyze the markers with signifi cant differences.
Results
The AAD group had more female, younger patients and fewer co-morbidities. They more frequently had consciousness disturbances (p < 0.001), were brought to the hospital sooner (p < 0.001), arrived more frequently with impaired consciousness (p = 0.001), hypotension and bradycardia (p < 0.001) and left-sided weakness (70.2%; p < 0.001). In the risk factor analysis, hypotension (OR 48.86, 95% CI 5.70-420.28), bradycardia (OR 8.11, 95% CI 2.71-24.24), initial loss of consciousness (OR 5.27, 95% CI 1.88-14.76), andleft-sided weakness (OR 3.31, 95% CI 1.17-9.40) were observed more frequently in the AAD group.
Conclusions
Consider to rule out a painless AAD in stroke patients presenting with hypotension, bradycardia, initial loss of consciousness, or left-sided weakness.
Keywords: painless, acute aortic dissection, stroke, thrombolytic therapy
Introduction
The incidence of stroke is declining in many developed countries; however, the absolute number of strokes continues to increase due to aging populations. [1] To improve stroke care and reduce stroke burdens, stroke chains of survival have been constructed with an emphasis on delivering patient care in a coordinated and timely fashion. [2,3] Intravenous thrombolytic therapy for acute ischemic stroke has become a standard treatment for patients without contraindications. [4] The sooner the intravenous thrombolytic therapy, the greater the benefit. [5,6] Both in-hospital and pre-hospital medical professionals are encouraged to work together to shorten the time from symptom onset to the commencement of thrombolytic therapy in every eligible stroke patient. The American Heart Association and related authorities have developed and implemented a goal-directed and well organized algorithm to deliver timely management in patients with suspected stroke. [2]
Acute aortic dissection (AAD) is a life-threatening emergency. Approximately 17% to 40% of patients with AAD present with neurological symptoms at onset, including stroke, transient ischemic attack (TIA), hypoxic encephalopathy, spinal cord ischemia, and ischemic neuropathy. [7-10] If a patient presents with acute thoracic or back pain with accompanying cardiovascular symptoms, the diagnosis of an AAD is straight-forward as most modern emergency departments (EDs) are equipped with helical multidetector computed tomography (CT) scanners, which can rapidly detect AADs with a reported sensitivity of 100% and a specificity of 98% to 99%. [11] However, 5% to 17% of AAD patients did not report chest or back pain. [12-14] A diagnosis of aortic dissection might be delayed or missed in patients without pain.
Although sporadic cases of favorable outcomes in patients with stroke secondary to AADs who received intravenous thrombolytic therapy have been reported, the rapid diagnosis and treatment of AADs were considered the main factors in patient survival. [15,16] Thrombolytic therapy remains contraindicated because severe complications and poor outcomes are likely to occur if a patient with an AAD is inadvertently treated with thrombolytic agents. While aortic dissections, with or without extension to the carotid or vertebral arteries, are readily recognized by CT-angiography, it is not practical to scan every stroke patient for AAD because these imaging examinations may delay thrombolytic therapy, add to the cost of their care, and increase the patient exposure to ionizing radiation. We investigated the clinical characteristics of painless AAD patients with stroke to identify markers suggestive of a concurrent AAD.
Methods
After approved by the hospital’s institutional review board, we searched the stroke registry of our hospital from January 2007 through December 2014 to find patients with painless AAD who were admitted due to acute ischemic stroke. Because of the rarity of such patients, we electronically searched Medline and the Science Citation Index Expanded from 1981 until March 2015 using the terms “stroke,” “infarction,” “intracranial thrombosis,” “brain embolism,” “dissecting aneurysm,” and “aortic dissection”. Reported cases that were initially treated as an acute stroke, and with sufficient clinical information, including demographics, records of co-morbidity, onset time, vital signs on hospital arrival, clinical manifestation, type of AAD, course of treatment, and outcomes, were included to improve the generalizability of the findings.
For comparison we chose 200 consecutive patients presenting to our hospital in 2013 whose principal discharge diagnose was acute ischemic stroke without AAD as the control group. In these two groups of patients, we collected their clinical parameters including demographics, co-morbidities, symptoms, times from symptom onset to hospital arrival, and vital signs on arrival. Results of physical examination, use of intravenous thrombolytic therapy, the type of AAD, and outcomes were also reviewed.
Two stages of analysis were conducted. First, we compared each clinical marker between the two groups of patients. Student’s t-tests or Mann-Whitney U tests were used for continuous variables based on their normality. Chi-square tests were used for categorical variables. A two-sided p value of less than 0.05 was considered statistically significant. Second, we used logistic regression to analyze the clinical markers that displayed significant differences between the two groups of patients. Odds ratios (ORs) with 95% confidence intervals (CIs) were reported. Because not every patient had previous blood pressure records and symptoms of hypotension might not be evident, we adopted the common definition of a systolic blood pressure of less than 90 mmHg as hypotension, and a heart rate of less than 60 beats/min as bradycardia.
Results
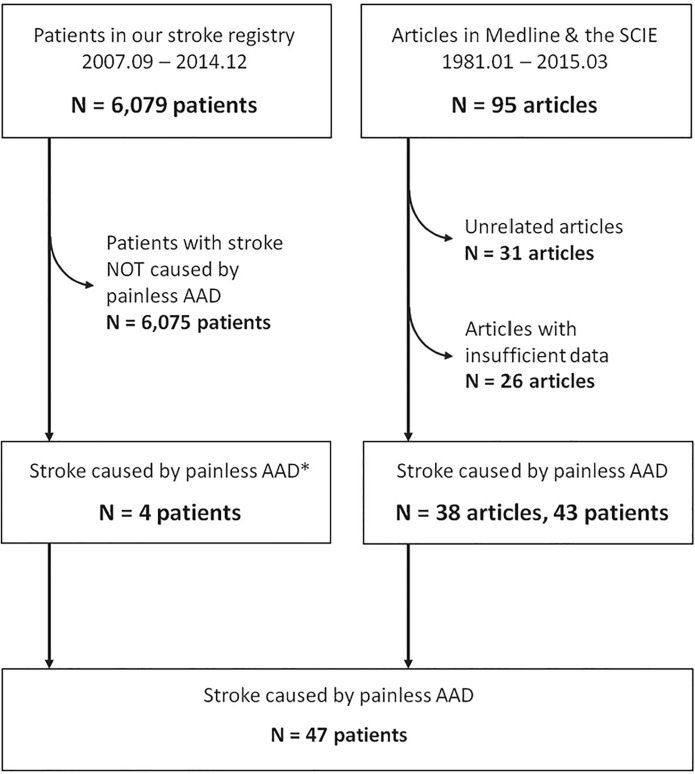
Using our research strategy, 75 articles were obtained. Forty-three patients with sufficient clinical information were collected from 35 articles. Together with 4 cases of our own, we gathered a total of 47 patients with painless AAD presenting as acute ischemic stroke (AAD group) (Figure 1). Another 200 patients with ischemic stroke without concurrent AAD were collected as the control group.
Figure 1. Patient accrual.
SCIE, Science Citation Index Expanded; AAD, Acute aortic dissection.
A comparison of the two groups of patients revealed that the AAD group had more female patients (29/47; 61.7% vs. 78/200; 39.0%; p = 0.005), included younger patients (64.1 ± 12.3 years old vs. 72.4 ± 12.4 years old; p < 0.001), and had fewer comorbidities (57.4% vs. 97.0%; p < 0.001) (Table 1). A higher percentage of the AAD group patients experienced sudden onset of consciousness disturbance (52.2% vs. 13.0%; p < 0.001) and remained in a state of impaired consciousness on hospital arrival (41.0% vs. 17.5%; p = 0.001). There was no difference in the percentage who experienced aphasia or dysarthria (55.6% vs. 52.5%; p = 0.711). On arrival, the AAD patients had significantly lower blood pressures and slower heart rates (p < 0.001); however, there was no significant difference in respiratory rates and temperatures (Table 2). AAD patients had a disproportionately high percentage of left-sided weakness (70.2%) while the control group had an almost equal distribution between right- and left-sided weakness (p < 0.001) (Table 2). Peripheral pulses were evaluated in 19 of the AAD patients and nine of them were reported to have symmetrical pulses.
Table 1. Demographics and co-morbidities of the painless acute aortic dissection (AAD) stroke patients and the non-AAD stroke patients.
1 CAD/IHD: Coronary artery diseases/ischemic heart diseases. 2 CVD/TIA: Cerebrovascular diseases/transient ischemic attack. 3 PAD: Peripheral artery diseases. 4 CKD/ESRD: Chronic kidney diseases/end-stage renal disease.
|
|
AAD Strokes (n = 47) |
Non-AAD Strokes (n = 200) |
p value |
|
Sex [M/F (ratio)] |
18/29 (0.62) |
122/78 (1.56) |
0.005 |
|
Age (years old) |
64.1 ± 12.3 |
72.4 ± 12.4 |
< 0.001 |
|
Co-morbidity |
57.4 % |
97.0 % |
< 0.001 |
|
Hypertension |
23/47 (48.9%) |
159/200 (79.5%) |
|
|
Diabetes mellitus |
2/47 (4.3%) |
81/200 (40.5%) |
|
|
Hyperlipidemia |
2/47 (4.3%) |
76/200 (38.0%) |
|
|
CAD/IHD1 |
2/47 (4.3%) |
30/200 (15.0%) |
|
|
Atrial fibrillation |
1/47 (2.1%) |
25/200 (12.5%) |
|
|
Heart failure |
1/47 (2.1%) |
6/200 (3.0%) |
|
|
Old CVD/TIA2 |
3/47 (6.4%) |
61/200 (30.5%) |
|
|
PAD3 |
1/47 (2.1%) |
1/200 (0.5%) |
|
|
CKD/ESRD4 |
2/47 (4.3%) |
28/200 (14.0%) |
|
|
Others |
6/47 (12.8%) |
75/200 (37.5%) |
|
Table 2. Presentations and clinical courses of the painless acute aortic dissection (AAD) stroke patients and the non-AAD stroke patients.
|
|
AAD Strokes (n = 47) |
Non-AAD Strokes (n = 200) |
p value |
|
Clinical Manifestations |
|
|
|
|
Sudden unconsciousness |
52.2 % |
13.0 % |
< 0.001 |
|
Unconscious on arrival |
41.0 % |
17.5 % |
0.001 |
|
Aphasia or dysarthria on arrival |
55.6 % |
52.5 % |
0.711 |
|
Left-sided weakness |
70.2 % |
40.5 % |
< 0.001 |
|
Time from onset to Hospital |
|
|
< 0.001 |
|
< 3 hours |
90.0 % |
25.0 % |
|
|
3 to 4.5 hours |
2.5 % |
4.5 % |
|
|
> 4.5 hours |
7.5 % |
70.5 % |
|
|
Vital Signs on Arrival |
|
|
|
|
Systolic blood pressure (mmHg) |
122.4 ± 38.7 |
159.3 ± 32.4 |
< 0.001 |
|
Diastolic blood pressure (mmHg) |
73.8 ± 28.1 |
91.1 ± 19.0 |
< 0.001 |
|
Heart rate (beats/min) |
65.3 ± 23.1 |
82.7 ± 18.0 |
< 0.001 |
|
Respiratory rate (breaths/min) |
19.2 ± 7.0 |
19.4 ± 1.4 |
0.901 |
|
Temperature (°C) |
36.0 ± 1.3 |
36.3 ± 2.1 |
0.576 |
|
Treatment & Outcome |
|
|
|
|
Fibrinolytic therapy |
25.5 % |
11.0 % |
0.009 |
|
In-hospital mortality |
39.1 % |
1.0 % |
< 0.001 |
A higher percentage of AAD patients were brought to the hospital sooner after their attacks (p < 0.001), and received intravenous thrombolytic therapy (25.5% vs. 11.0%; p = 0.009). AAD patients had a significantly higher mortality rate (39.1% vs. 1.0%; p < 0.001) (Table 2). All of the AAD patients had Stanford type A aortic dissections.
In the risk factor analysis, hypotension (OR 48.86, 95% CI 5.70-420.28), bradycardia (OR 8.11, 95% CI 2.71-24.24), initial loss of consciousness (OR 5.27, 95% CI 1.88-14.76), and left-sided weakness (OR 3.31, 95% CI 1.17-9.40) were observed more frequently in the AAD group. However, female gender (OR 1.58, 95% CI 0.59-4.20) and disturbance of consciousness on hospital arrival (OR 1.47, 95% CI 0.49-4.36) were not associated with a higher risk of AAD.
Discussion
In our study, we found that painless AAD stroke patients were younger, had less comorbidity, more frequently experienced a sudden onset of unconsciousness, and were brought to the hospital earlier than their non-AAD stroke counterparts. A significantly higher percentage of them were unconscious, hypotensive, and had bradycardia and left-sided weakness on arrival to the ED. With the current protocol of initial evaluation and management of a suspected stroke, it is difficult to detect patients whose stroke is caused by a painless AAD. The painless AAD stroke patients were brought to the hospital sooner. They usually had negative results on non-contrast CT of the brain, and a lack of contraindications of thrombolytic therapy could be identified in the short time frame for thrombolytic therapy. A significantly higher percentage of painless AAD stroke patients received recombinant tissue-type plasminogen activator (tPA), and a significantly higher mortality rate was also observed among them.
In this study, all of the AAD stroke patients had Stanford type a dissection. This type of dissection involves the ascending aorta and is most common in the 6th and 7th decades of life, while Stanford type B dissections involve the descending aorta only and are more common in older individuals. [17] Although most AAD patients are hypertensive, many patients with type A dissection are normotensive or even hypotensive on presentation. [18] Hypotension in AAD usually results from complications that may include aortic rupture, cardiac tamponade, heart failure secondary to severe aortic regurgitation or myocardial infarction. Acute stroke patients often have high initial blood pressure due to physiological compensation for brain ischemia. [19,20] Although persistent or severe hypertension is associated with poor outcomes, low blood pressure in acute stroke is associated with increased mortality. [21,22] Severe stroke cases, such as total anterior circulation syndrome and cardiovascular diseases that compromise cardiac output were found to contribute to hypotension in acute stroke, and their poor outcomes resulted from cerebral hypoperfusion, larger infarcts, and shock. [22] Clinicians should thereafter be cautious with hypotensive patients who present as either acute chest pain or acute stroke.
Neurological complications, including ischemic stroke, TIA, hypoxic encephalopathy, spinal cord ischemia, and ischemic neuropathy, are common in AAD, especially in type A dissections. [7-10,17,18] Neurological manifestations usually appear at or soon after the onset of dissection. These disabilities are often evanescent, fluctuating, and may fully remitted because of transient arterial occlusion at the moment of propagation of the dissection. [23] Syncope was reported to occur in varying percentages of AAD patients and may be caused by low cardiac output secondary to aortic rupture and/or cardiac tamponade, carotid artery occlusion, or the activation of vascular baroreceptors. [7,14,17] Reflex bradycardia can occur in AAD patients with carotid occlusion. [24] Up to 85% of AAD patients complicated with stroke and TIA were reported to be due to carotid occlusion, predominantly on the right side. [23,25] This dominance of right hemispheric stroke despite mostly bilateral carotid dissection was attributed to the different mechanical dynamics in the progression of the dissecting hematoma which most frequently affects the innominate and carotid arteries because of their proximity to the aortic arch. [23]
In addition to prevention, timely restoration of blood flow is the key to decrease the burden imposed by acute ischemic stroke. To increase the efficiency and effectiveness of stroke care, the “D’s of Stroke Care” have been emphasized as benchmarks for reducing unwanted delays in the diagnosis and treatment of stroke. [2] Besides every effort in the prehospital cares, when the patient arrives at the hospital, rapid triage, evaluation, and management, including decisions regarding reperfusion therapy by experts and rapid admission to a stroke unit should meet predetermined time goals. [2] Although there have been increasing reports of endovascular treatment for acute ischemic stroke, the rapid administration of intravenous tPA in eligible patients remains the mainstay of early treatment for acute ischemic stroke, regardless of whether endovascular treatment is considered. [3,26] In current stroke guidelines, emergent brain imaging is recommended to differentiate ischemic from hemorrhagic strokes before any specific treatment is begun. [3,26] In most instances, nonenhanced brain CT will provide the necessary information for emergent management. A noninvasive intracranial vascular study, either through CT angiography (CTA) or magnetic resonance angiography (MRA), is strongly recommended during the initial imaging evaluation of the acute stroke patient, but this should not delay intravenous thrombolytic therapy. If endovascular therapy is contemplated but CTA or MRA is not done in the initial imaging assessment, the initiation of intravenous tPA before CTA or MRA is recommended. [26] The narrow time window for thrombolytic therapy in the current protocol makes it difficult to justify the use of other examinations without a good reason because any additional examination will prolong the door-todrug interval.
Helical multi-detector CT scanners can detect AAD well with sensitivities of up to 100% and specificities of 98% to 99%. [27] Magnetic resonance imaging (MRI) and transesophageal echocardiography (TEE) have been shown to have equivalent or better sensitivities and specificities in the diagnosis of AAD. [28-30] The stroke in AAD patients usually comes from the occlusion of carotid or vertebral arteries from either dissection extension or obstruction by flaps. Both CTA and MRA are highly diagnostic of carotid and vertebral occlusion or dissection. [31-33] However, the prolonged duration of imaging acquisition required, contraindications in patients with metallic implants, pacemakers or claustrophobia, and lack of widespread availability in EDs limit the use of MRI on an emergency basis. [28] TEE is effective in identifying aortic dissections and intramural hematomas in the ascending aorta; however, it has some blind spots in the aortic arch and descending aorta. A mobile linear artefact can frequently be seen in TEE imagings in the aortic lumen. These artifacts, which arise from a mirror image or reverberation, may be mistakenly identified by inexperienced echocardiographers. [27,34] In patients with cervical artery dissection, an intimal flap floating in the lumen, false lumen, thrombus, or absence of flow can be identified by ultrasound in some cases. However, operator dependence and challenges from a short muscular neck, tortuous vessels, inability to lie flat or to rotate the head, calcification of previous atherosclerosis, and other causes of increased or decreased flow make ultrasound a less than ideal choice in an emergency. [35-38]
Because a minimized door-to-drug interval is pursued, it is impractical to scan the chest and neck in every case of acute stroke. However, a high suspicion should be maintained in patients presenting with initial loss of consciousness, hypotension, bradycardia on arrival, or left-sided weakness. Additional CTA imaging of the chest and neck should be considered to detect the possible but occult painless AAD. Bedside ultrasound of the aorta and cervical arteries can be performed, but this should not delay the use of highly diagnostic CTA examination.
Limitations
There are some limitations to this study. First, as we enriched the collection of painless AAD stroke patients from an extensive literature search, only a limited number of studies, most of which are case reports, fulfilled the inclusion criteria. Considering the incidence of AAD, the reported ratios of painless stroke presentation, and cases not reported or diagnosed, the cases we found might not be fully representative of this pathology. Second, the cases of painless AAD with stroke in our study occurred in many countries; however, the comparison group patients were all Asians. Although previous reviews have revealed some racial and ethnic diversity of ages and prevalence of atrial fibrillation in stroke patients, similar prevalence of hypertension, diabetes, smoking and alcoholic intake was reported. [39,40] Furthermore, no evidence was found to support ethnic differences in stroke severity, after adjusting for socioeconomic factors. [41-44] Third, our AAD cases were collected from 1981 to 2015; however, patients in the control group were collected in 2013. Because painless AAD stroke patients are uncommon or easily missed, we could not find enough cases in a short time interval. Although time-matched selection of stroke patients not related to AAD might reduce some bias in one hand, the increase in other confounding could be inevitable. Fourth, this is a retrospective, observational study. Although some high-risk, warning signs were identified, validation studies performed in a prospective manner across nationalities and ethnicities are needed.
References
- 1.WHO publishes definitive atlas on global heart disease and stroke epidemic. Indian J Med Sci. 2004;58 [PubMed] [Google Scholar]
- 2.Jauch EC, Cucchiara B, Adeoye O, et al Part 11: adult stroke: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122 doi: 10.1161/CIRCULATIONAHA.110.971044. [DOI] [PubMed] [Google Scholar]
- 3.Jauch Edward C., Saver Jeffrey L., Adams Harold P., Bruno Askiel, Connors J.J. (Buddy), Demaerschalk Bart M., Khatri Pooja, McMullan Paul W., Qureshi Adnan I., Rosenfield Kenneth, Scott Phillip A., Summers Debbie R., Wang David Z., Wintermark Max, Yonas Howard. Guidelines for the Early Management of Patients With Acute Ischemic Stroke. Stroke. 2013 Mar;44(3) doi: 10.1161/str.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- 4.National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333 doi: 10.1056/NEJM199906103402302. [DOI] [PubMed] [Google Scholar]
- 5.Hacke W, Donnan G, Fieschi C, et al Association of out come with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363 doi: 10.1016/S0140-6736(04)15692-4. [DOI] [PubMed] [Google Scholar]
- 6.Wardlaw JM, Murray V, Berge E, del Zoppo GJ. Thrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev. 2014;7 doi: 10.1002/14651858.CD000213.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang DJ, Fan FD, Wang Q, et al Preliminary characterization of acute aortic dissection in the mainland of China. Chin Med J. 2011;124 [PubMed] [Google Scholar]
- 8.Bossone E, Corteville DC, Harris KM, et al Stroke and outcomes in patients with acute type A aortic dissection. Circulation. 2013;128 doi: 10.1161/CIRCULATIONAHA.112.000327. [DOI] [PubMed] [Google Scholar]
- 9.Di Eusanio M, Patel HJ, Nienaber CA, et al Patients with type a acute aortic dissection presenting with major brain injury: should we operate on them? Thorac Cardiovasc Surg. 2013;145 doi: 10.1016/j.jtcvs.2012.11.054. [DOI] [PubMed] [Google Scholar]
- 10.Lee SJ, Kim JH, Na CY, et al Eleven years of experience with the neurologic complications in Korean patients with acute aortic dissection: a retrospective study. BMC Neurol. 2013;13 doi: 10.1186/1471-2377-13-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abbas A, Brown IW, Peebles CR, Harden SP, Shambrook JS. The role of multidetector-row CT in the diagnosis, classification and management of acute aortic syndrome. Br J Radiol. 2014;87 doi: 10.1259/bjr.20140354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park SW, Hutchison S, Mehta RH. Association of painless acute aortic dissection with increased mortality. Mayo Clin Proc. 2004;79 doi: 10.4065/79.10.1252. [DOI] [PubMed] [Google Scholar]
- 13.Tsai TY, Seow VK, Shen TC, et al Painless aortic dissection masquerading as brainstem stroke with catastrophic anticoagulant use. Am J Emerg Med. 2008;26 doi: 10.1016/j.ajem.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 14.Imamura H, Sekiguchi Y, Iwashita T, et al Painless acute aortic dissection -- Diagnostic, prognostic and clinical implications. Circ J. 2011;75 doi: 10.1253/circj.cj-10-0183. [DOI] [PubMed] [Google Scholar]
- 15.Noel M, Short J, Farooq MU. Thrombolytic therapy in a patient with acute ischemic stroke caused by aortic dissection. Clin Neurol Neurosurg. 2010;112 doi: 10.1016/j.clineuro.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 16.Mendes A, Mendonca T, Sousa A, Moreira G, Carvalho M. Stroke secondary to aortic dissection treated with a thrombolytic: a successful case. Neurol Sci. 2012;33 doi: 10.1007/s10072-011-0616-2. [DOI] [PubMed] [Google Scholar]
- 17.Braverman Alan C. Acute Aortic Dissection. Circulation. 2010 Jul 13;122(2) doi: 10.1161/circulationaha.110.958975. [DOI] [PubMed] [Google Scholar]
- 18.Hagan Peter G., Nienaber Christoph A., Isselbacher Eric M., Bruckman David, Karavite Dean J., Russman Pamela L., Evangelista Arturo, Fattori Rossella, Suzuki Toru, Oh Jae K., Moore Andrew G., Malouf Joseph F., Pape Linda A., Gaca Charlene, Sechtem Udo, Lenferink Suzanne, Deutsch Hans Josef, Diedrichs Holger, Marcos y Robles Jose, Llovet Alfredo, Gilon Dan, Das Sugata K., Armstrong William F., Deeb G. Michael, Eagle Kim A. The International Registry of Acute Aortic Dissection (IRAD) JAMA. 2000 Feb 16;283(7) doi: 10.1001/jama.283.7.897. [DOI] [PubMed] [Google Scholar]
- 19.Wallace JD, Levy LL. Blood pressure after stroke. JAMA. 1981;246 [PubMed] [Google Scholar]
- 20.Mattle Heinrich P., Kappeler Liliane, Arnold Marcel, Fischer Urs, Nedeltchev Krassen, Remonda Luca, Jakob Stephan M., Schroth Gerhard. Blood Pressure and Vessel Recanalization in the First Hours After Ischemic Stroke. Stroke. 2005 Feb;36(2) doi: 10.1161/01.str.0000153052.59113.89. [DOI] [PubMed] [Google Scholar]
- 21.Leonardi-Bee Jo, Bath Philip M.W., Phillips Stephen J., Sandercock Peter A.G. Blood Pressure and Clinical Outcomes in the International Stroke Trial. Stroke. 2002 May;33(5) doi: 10.1161/01.str.0000014509.11540.66. [DOI] [PubMed] [Google Scholar]
- 22.Okumura Koichiro, Ohya Yusuke, Maehara Aiwa, Wakugami Kiyoshi, Iseki Kunitoshi, Takishita Shuichi. Effects of blood pressure levels on case fatality after acute stroke. Journal of Hypertension. 2005 Jun;23(6) doi: 10.1097/01.hjh.0000170385.76826.4a. [DOI] [PubMed] [Google Scholar]
- 23.Gaul Charly, Dietrich Wenke, Friedrich Ivar, Sirch Joachim, Erbguth Frank J. Neurological Symptoms in Type A Aortic Dissections. Stroke. 2007 Feb;38(2) doi: 10.1161/01.str.0000254594.33408.b1. [DOI] [PubMed] [Google Scholar]
- 24.Ho Cheng-Hsuan, Chen Yu-long, Lin Yen-Yue, Liao Wen-I, Lin Chih-Yuan, Hsu Chin-Wang, Tsai Shih-Hung. Acute aortic dissection complicated by acute ischemic stroke: diagnostic challenges. The American Journal of Emergency Medicine. 2012 Nov;30(9) doi: 10.1016/j.ajem.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 25.Cambria RP, Brewster DC, Gertler J, et al Vascular complications associated with spontaneous aortic dissection. J Vasc Surg. 1988;7 [PubMed] [Google Scholar]
- 26.Powers William J., Derdeyn Colin P., Biller José, Coffey Christopher S., Hoh Brian L., Jauch Edward C., Johnston Karen C., Johnston S. Claiborne, Khalessi Alexander A., Kidwell Chelsea S., Meschia James F., Ovbiagele Bruce, Yavagal Dileep R. 2015 American Heart Association/American Stroke Association Focused Update of the 2013 Guidelines for the Early Management of Patients With Acute Ischemic Stroke Regarding Endovascular Treatment. Stroke. 2015 Oct;46(10) doi: 10.1161/str.0000000000000074. [DOI] [PubMed] [Google Scholar]
- 27.Hiratzka Loren F., Bakris George L., Beckman Joshua A., Bersin Robert M., Carr Vincent F., Casey Donald E., Eagle Kim A., Hermann Luke K., Isselbacher Eric M., Kazerooni Ella A., Kouchoukos Nicholas T., Lytle Bruce W., Milewicz Dianna M., Reich David L., Sen Souvik, Shinn Julie A., Svensson Lars G., Williams David M. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the Diagnosis and Management of Patients With Thoracic Aortic Disease Circulation. 2010 Apr 06;121(13) doi: 10.1161/cir.0b013e3181d4739e. [DOI] [Google Scholar]
- 28.Moore Andrew G, Eagle Kim A, Bruckman David, Moon Brenda S, Malouf Joseph F, Fattori Rossella, Evangelista Arturo, Isselbacher Eric M, Suzuki Toru, Nienaber Christoph A, Gilon Dan, Oh Jae K. Choice of computed tomography, transesophageal echocardiography, magnetic resonance imaging, and aortography in acute aortic dissection: International Registry of Acute Aortic Dissection (IRAD) The American Journal of Cardiology. 2002 May;89(10) doi: 10.1016/s0002-9149(02)02316-0. [DOI] [PubMed] [Google Scholar]
- 29.Shiga Toshiya, Wajima Zen’ichiro, Apfel Christian C., Inoue Tetsuo, Ohe Yoko. Diagnostic Accuracy of Transesophageal Echocardiography, Helical Computed Tomography, and Magnetic Resonance Imaging for Suspected Thoracic Aortic Dissection. Archives of Internal Medicine. 2006 Jul 10;166(13) doi: 10.1001/archinte.166.13.1350. [DOI] [PubMed] [Google Scholar]
- 30.Kapustin Andrew J., Litt Harold I. Diagnostic Imaging for Aortic Dissection. Seminars in Thoracic and Cardiovascular Surgery. 2005 Sep;17(3) doi: 10.1053/j.semtcvs.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 31.Flis Christine M., Jäger H. Rolf, Sidhu Paul S. Carotid and vertebral artery dissections: clinical aspects, imaging features and endovascular treatment. European Radiology. 2006 Jul 27;17(3) doi: 10.1007/s00330-006-0346-7. [DOI] [PubMed] [Google Scholar]
- 32.Vertinsky A.T., Schwartz N.E., Fischbein N.J., Rosenberg J., Albers G.W., Zaharchuk G. Comparison of Multidetector CT Angiography and MR Imaging of Cervical Artery Dissection. American Journal of Neuroradiology. 2008 Jul 17;29(9) doi: 10.3174/ajnr.a1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Provenzale James M., Sarikaya Basar. Comparison of Test Performance Characteristics of MRI, MR Angiography, and CT Angiography in the Diagnosis of Carotid and Vertebral Artery Dissection: A Review of the Medical Literature. American Journal of Roentgenology. 2009 Oct;193(4) doi: 10.2214/ajr.08.1688. [DOI] [PubMed] [Google Scholar]
- 34.Sechtem U., Achenbach S., Friedrich M., Wackers F., Zamorano J. L. Non-invasive imaging in acute chest pain syndromes. European Heart Journal - Cardiovascular Imaging. 2011 Nov 17;13(1) doi: 10.1093/ejechocard/jer250. [DOI] [PubMed] [Google Scholar]
- 35.Patel Raj Ramabhai, Adam Richard, Maldjian Catherine, Lincoln Christie M., Yuen Annie, Arneja Amrita. Cervical Carotid Artery Dissection. Cardiology in Review. 2012;20(3) doi: 10.1097/crd.0b013e318247cd15. [DOI] [PubMed] [Google Scholar]
- 36.Tahmasebpour Hamid R., Buckley Anne R., Cooperberg Peter L., Fix Cathy H. Sonographic Examination of the Carotid Arteries. RadioGraphics. 2005 Nov;25(6) doi: 10.1148/rg.256045013. [DOI] [PubMed] [Google Scholar]
- 37.Romero Javier M., Lev Michael H., Chan Suk-Tak, Connelly Molly M., Curiel Ryan C., Jackson Anna E., Gonzalez R. Gilberto, Ackerman Robert H. US of Neurovascular Occlusive Disease: Interpretive Pearls and Pitfalls. RadioGraphics. 2002 Sep;22(5) doi: 10.1148/radiographics.22.5.g02se141165. [DOI] [PubMed] [Google Scholar]
- 38.Flis Christine M., Jäger H. Rolf, Sidhu Paul S. Carotid and vertebral artery dissections: clinical aspects, imaging features and endovascular treatment. European Radiology. 2006 Jul 27;17(3) doi: 10.1007/s00330-006-0346-7. [DOI] [PubMed] [Google Scholar]
- 39.Tsai Chung-Fen, Anderson Niall, Thomas Brenda, Sudlow Cathie L. M. Risk Factors for Ischemic Stroke and its Subtypes in Chinese vs. Caucasians: Systematic Review and Meta-Analysis. International Journal of Stroke. 2015 Apr 23;10(4) doi: 10.1111/ijs.12508. [DOI] [PubMed] [Google Scholar]
- 40.Tsai Chung-Fen, Anderson Niall, Thomas Brenda, Sudlow Cathie L. M. Quinn Terence J., editor. Comparing Risk Factor Profiles between Intracerebral Hemorrhage and Ischemic Stroke in Chinese and White Populations: Systematic Review and Meta-Analysis. PLOS ONE. 2016 Mar 18;11(3) doi: 10.1371/journal.pone.0151743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones Michael R., Horner Ronnie D., Edwards Lloyd J., Hoff Jennifer, Armstrong S. Beth, Smith-Hammond Carol A., Matchar David B., Oddone Eugene Z. Racial Variation in Initial Stroke Severity. Stroke. 2000 Mar;31(3) doi: 10.1161/01.str.31.3.563. [DOI] [PubMed] [Google Scholar]
- 42.Connor Myles D., Modi Girish, Warlow Charles P. Differences in the Nature of Stroke in a Multiethnic Urban South African Population. Stroke. 2009 Feb;40(2) doi: 10.1161/strokeaha.108.521609. [DOI] [PubMed] [Google Scholar]
- 43.Fletcher Jeffrey J., Morgenstern Lewis B., Lisabeth Lynda D., Sánchez Brisa N., Skolarus Lesli E., Smith Melinda A., Garcia Nelda M., Zahuranec Darin B. A Population-Based Analysis of Ethnic Differences in Admission to the Intensive Care Unit after Stroke. Neurocritical Care. 2012 Aug 15;17(3) doi: 10.1007/s12028-012-9770-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wing Jeffrey J., Baek Jonggyu, Sánchez Brisa N., Lisabeth Lynda D., Smith Melinda A., Morgenstern Lewis B., Zahuranec Darin B. Differences in Initial Stroke Severity Between Mexican Americans and Non-Hispanic Whites Vary by Age: The Brain Attack Surveillance in Corpus Christi (BASIC) Project. Cerebrovascular Diseases. 2014;38(5) doi: 10.1159/000366468. [DOI] [PMC free article] [PubMed] [Google Scholar]