Abstract
Objectives:
To compare survival and progression outcomes between 2 nodal assessment approaches in patients with nonbulky stage IIIC endometrial cancer (EC).
Methods:
Patients with stage IIIC EC treated at 2 institutions were retrospectively identified. At 1 institution, a historical series (2004–2008) was treated with systematic pelvic and para-aortic lymphadenectomy (LND cohort). At the other institution, more contemporary patients (2006–2013) were treated using a sentinel lymph node algorithm (SLN cohort). Outcomes (hazard ratios [HRs]) within the first 5 years after surgery were compared between cohorts using Cox models adjusted for type of adjuvant therapy.
Results:
The study included 104 patients (48 LND, 56 SLN). The use of chemoradiotherapy was similar in the 2 cohorts (46% LND vs 50% SLN), but the use of chemotherapy alone (19% vs 36%) or radiotherapy alone (15% vs 2%) differed. Although there was evidence of higher risk of cause-specific death (HR, 2.10; 95% CI, 0.79–5.58; P=.14) and lower risk of para-aortic progression (HR, 0.27; 95% CI, 0.05–1.42; P=.12) for the LND group, the associations did not meet statistical significance. The risk of progression was not significantly different between the groups (HR, 1.27; 95% CI, 0.60–2.67; P=.53). In parsimonious multivariable models, high-risk tumor characteristics and nonendometrioid type were independently associated with lower cause-specific survival and progression-free survival.
Conclusions:
In EC patients with nonbulky positive lymph nodes, use of the SLN algorithm with limited nodal dissection does not compromise survival compared with LND. Aggressive pathologic features of the primary tumor are the strongest determinants of prognosis.
Keywords: comprehensive surgical staging, endometrial cancer, positive lymph node, sentinel lymph node algorithm, stage IIIC
Introduction
Endometrial cancer (EC) is the most frequent gynecologic cancer, with 61,880 new cases and 12,160 deaths estimated to occur in the United States in 2019 [1]. Current guidelines for the treatment of EC recommend comprehensive surgical staging including both pelvic and para-aortic lymphadenectomy (LND) [2], yet the role of surgical staging is still controversial among gynecologic oncologists, and staging strategies vary from no LND to systematic pelvic plus para-aortic LND up to the renal vessels [3]. The controversy is mainly due to the results of 2 randomized controlled trials (RCTs) demonstrating that LND in patients with EC is not associated with survival benefits [4, 5] but is associated with higher rates of intraoperative and postoperative complications compared with hysterectomy plus bilateral salpingo-oophorectomy alone [5]. These RCTs were highly criticized, however, because of multiple inherent problems in their design that affect the confidence in the estimate [6]. In contrast, several retrospective studies have shown longer survival in patients with EC who underwent pelvic and para-aortic LND, especially those at high risk for recurrence [7–10].
With the intent of finding a balance between risks and harms of no nodal staging vs systematic pelvic and para-aortic LND, sentinel lymph node (SLN) mapping [11, 12] is gaining wide acceptance among gynecologists and has been included in the National Comprehensive Cancer Network (NCCN) guidelines as a valuable option in the management of EC [13]. However, a few areas of uncertainty regarding SLN mapping are still present [14], including the role of completing the LND in the presence of positive SLNs. To evaluate the safety of the SLN algorithm and preservation of oncologic outcomes among patients at high risk for recurrence, we compared survival and progression outcomes between systematic LND and the SLN algorithm in patients with nonbulky stage IIIC EC.
Methods
Patients with stage IIIC EC according to the International Federation of Gynecology and Obstetrics 2009 classification [15] treated at 2 collaborating institutions were retrospectively identified. At Mayo Clinic, Rochester, Minnesota, a historical series (2004–2008) was treated according to the Mayo Clinic surgical algorithm (LND cohort) [16]. On the basis of that algorithm, patients at risk for lymph node (LN) metastasis on the basis of frozen section histologic evaluation of the uterus (tumor diameter >2 cm, myometrial invasion >50%, grade 3, and nonendometrioid), which accounts for approximately 70% of all patients with EC, were treated with comprehensive surgical staging including hysterectomy, bilateral salpingo-oophorectomy, peritoneal cytologic evaluation, and pelvic plus para-aortic LND up to the renal vessel. Systematic pelvic and para-aortic LNDs were performed predominantly by laparotomy. In the remaining patients who were not at risk, both pelvic and para-aortic LND were omitted because of the negligible risk of LN involvement (<1%) [17].
At Memorial Sloan Kettering Cancer Center (MSKCC), New York, New York, a more contemporary series was treated using the SLN algorithm from 2006 through 2013 (SLN cohort) [18, 19]. According to the SLN algorithm used at MSKCC, which is included in the NCCN guidelines [13] and has been adopted by several institutions worldwide [3], for all cases of newly diagnosed EC, the cervical stroma was injected with either methylene blue or indocyanine green. Any positive SLNs were removed and analyzed by a pathologist following an institutional protocol that includes ultrastaging with hematoxylin-eosin and immunohistochemical staining with anticytokeratin AE1/AE3 antibody (Ventana Medical Systems, Inc) [20]. Since the priority for SLN mapping remains the identification of all LN metastases, if there was no detection, bilateral pelvic LND was performed; if there was unilateral detection, side-specific LND was performed. Any suspicious nodes were removed. Para-aortic LND was performed at the surgeon’s discretion.
For the purpose of this analysis, we defined “high-risk” patients as those with a) grade 3 endometrioid disease with 50% or greater myometrial invasion or b) nonendometrioid disease. All other patients were considered to be at “low or intermediate risk”.
For the current study, only LN metastases larger than 0.2 mm were considered node-positive. To avoid introducing bias in the comparison between the 2 cohorts by including patients with isolated tumor cells detected by ultrastaging only in the SLN cohort, and which are most likely associated with better survival outcomes [21], we excluded these patients. In the SLN cohort, all patients with preoperative imaging suspicious for LN metastasis (presence of enlarged LNs) were also excluded from our comparison. Thus, to avoid introducing bias, we also excluded from the LND cohort all patients with enlarged and suspicious LNs at preoperative imaging based on the RECIST criteria version 1.1 (Normal: short axis <10 mm; Measurable (Target): short axis ≥15 mm; Nonmeasurable: short axis 10 to <15 mm) [22] and patients without preoperative imaging who had bulky LNs at the time of surgery. Patients with stage IV disease or synchronous cancer or who underwent neoadjuvant therapy also were excluded from both cohorts. Adjuvant therapy was administered per recommended institutional guidelines and according to each patient’s values and preferences [23]. The study was approved by the institutional review boards of Mayo Clinic and MSKCC.
Data Analysis
Patient and tumor variables were summarized as number (percentage), mean (SD), or median (interquartile range [IQR]), as appropriate. Baseline characteristics including age, body mass index (BMI), histologic findings, grade, myometrial invasion, lymphovascular space invasion, cervical stroma invasion, peritoneal cytologic findings, presence of positive pelvic LNs, presence of positive para-aortic LNs, stage, and adjuvant therapy (chemotherapy vs all others) were compared between the LND and SLN cohorts using the 2-sample t test for age and BMI, the Wilcoxon rank sum test for the number of LNs, and the χ2 test or Fisher exact test for all other categorical baseline characteristics. The outcomes of interest were cause-specific survival (CSS), overall progression-free survival (PFS), and route-specific PFS and were estimated using the Kaplan-Meier method. Because of the different time frames of the 2 cohorts, follow-up was restricted to the first 5 years after surgery for the analysis of each time-to-event outcome. Baseline characteristics were each evaluated univariately for an association with CSS, overall PFS, and lymphatic PFS, based on fitting univariate Cox proportional hazards regression models stratified by cohort (LND vs SLN) to allow for a different baseline hazard for each cohort. Given that the total number of events ranged from 19 to 34 for the 3 outcomes of interest, parsimonious multivariable models with a combination of variables that accounted for no more than 2 to 3 degrees of freedom were selected on the basis of the global score χ2 statistic. Given that the adjuvant treatment approach was not balanced between the LND and SLN cohorts, the outcomes of interest were compared between the 2 cohorts by fitting Cox models with and without adjusting for adjuvant therapy. To evaluate whether the number of LNs removed in the SLN cohort affected survival outcomes, we compared CSS, overall PFS, and lymphatic PFS between patients with 10 or fewer nodes removed vs more than 10 nodes removed using the log-rank test. Associations were reported using the hazard ratio (HR) and corresponding 95% CI derived from the Cox models. All calculated P values were 2-sided, and P<.05 was considered statistically significant. Statistical analyses were performed using the SAS version 9.4 software package (SAS Institute Inc).
Results
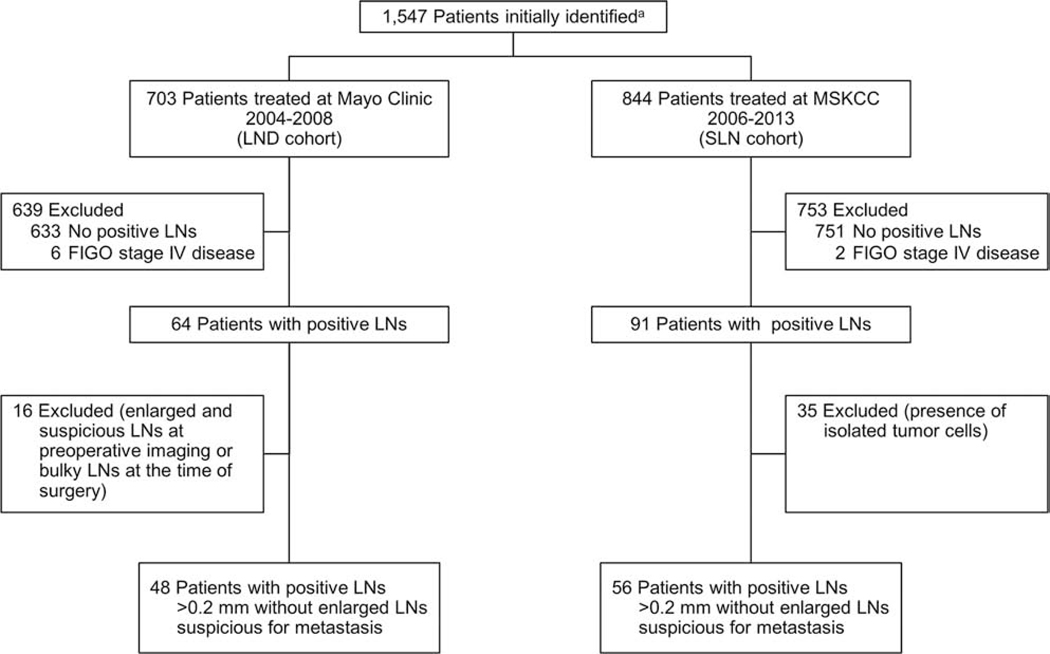
A total of 1,547 patients with EC were initially identified: 703 in the Mayo Clinic LND cohort and 844 in the MSKCC SLN cohort. Of these, 104 had positive LNs and met the specified inclusion criteria (48 [46%] in the LND cohort, 56 [54%] in the SLN cohort) (Figure 1). Patients with nonbulky positive LNs (with metastases >0.2 mm) accounted for 6.8% of the ECs in the initial LND cohort and 6.6% of the initial SLN cohort.
Figure 1.
Flow Chart of the Study Population. a Initial patients identified with surgical management for primary endometrial cancer at Mayo Clinic and Memorial Sloan Kettering Cancer Center (MSKCC), without synchronous invasive cancer or neoadjuvant therapy and, in the MSKCC cohort, without gross intra-abdominal disease or bulky nodes. FIGO indicates International Federation of Gynecology and Obstetrics; LN, lymph node; LND, lymphadenectomy; SLN, sentinel lymph node.
Patient and tumor characteristics, extent of surgical staging, and adjuvant therapy are shown in Table 1. Between the 2 cohorts, there were no differences in age, histologic type, International Federation of Gynecology and Obstetrics grade, and myometrial invasion. In the LND cohort, BMI was significantly higher (mean, 33.5 vs 29.5 kg/m2; P=.02) and the percentage of patients with lymphovascular space invasion was significantly lower (33% vs 84%; P<.001) (possibly a difference in pathologic interpretation between the institutions) [24]. As expected, there were significant differences in the extent of surgical staging between the 2 cohorts. Pelvic LNs were removed in 98% and 100% of patients in the LND and SLN cohorts, respectively. However, the median number of pelvic LNs removed was significantly higher in the LND cohort (33 vs 12 in the SLN cohort; P<.001). Assessment of the para-aortic area was performed in 100% of the LND cohort and 48% of the SLN cohort (P<.001), with significantly more para-aortic nodes excised in the LND cohort (median, 18 vs 4 in the SLN cohort; P<.001). The type of adjuvant therapy used was not significantly different overall (P=.05), but the use of external-beam radiotherapy only was significantly higher in the LND group (15% vs 2%; P=.02). A total of 7 patients in the LND cohort (15%) received no adjuvant treatment, compared with 5 (9%) in the SLN cohort. Reasons for not receiving adjuvant treatment were surgical complications, patient’s decision, or more advanced disseminated disease (brain metastases) discovered after surgery.
Table 1.
Comparison of Patient and Tumor Characteristics, Extent of Surgical Staging, and Adjuvant Therapya
| Characteristic | LND Cohort (n=48) | SLN Cohort (n=56) | Pb |
|---|---|---|---|
| Age, y | 65.6 (12.1) | 64.9 (11.2) | .77 |
| BMI, kg/m2 | 33.5 (10.0) | 29.5 (6.4) | .02 |
| Nonendometrioid histologic type | 17 (35) | 21 (38) | .83 |
| FIGO grade | .62 | ||
| 1 | 9 (19) | 15 (27) | |
| 2 | 15 (31) | 15 (27) | |
| 3 | 24 (50) | 26 (46) | |
| Myometrial invasion | .99 | ||
| None | 4 (8) | 5 (9) | |
| <50% | 17 (35) | 20 (36) | |
| ≥50% | 27 (56) | 31 (55) | |
| High riskc | 23 (48) | 25 (45) | .74 |
| Lymphovascular space invasion | 16 (33) | 47 (84) | <.001 |
| Cervical stroma invasion | 5 (10) | 9 (16) | .40 |
| Positive peritoneal cytologic findings | 16 (35) | 17 (30) | .63 |
| (n=46) | |||
| Pelvic lymphadenectomy | 47 (98) | 56 (100) | .28 |
| No. of pelvic nodes removed | |||
| Right | 18 (13–22) | 5 (3–9) | <.001 |
| Left | 17 (13–20) | 5 (2–8) | <.001 |
| Total | 33 (26–40) | 12 (5–16) | <.001 |
| Positive pelvic nodes | 37 (77) | 52 (93) | .02 |
| No. of positive pelvic nodes among those with positive nodes | |||
| Right | 1 (0–2) | 1 (0–1) | .61 |
| Left | 1 (0–1) | 1 (0–2) | .51 |
| Total | 1 (1–2) | 1 (1–2) | .82 |
| Para-aortic lymphadenectomy | 48 (100) | 27 (48) | <.001 |
| No. of para-aortic nodes removed | 18 (10–23) | 4 (2–9) | <.001 |
| Positive para-aortic nodes | 25 (52) | 14 (25) | .004 |
| No. of positive para-aortic nodes among those with positive nodes | 2 (1–3) | 1 (1–2) | .18 |
| Adjuvant therapy | .05 | ||
| None | 7 (15) | 5 (9) | |
| EBRT ± brachytherapy | 7 (15) | 1 (2) | |
| Chemotherapy ± brachytherapy | 9 (19) | 20 (36) | |
| Chemotherapy and EBRT ± brachytherapy | 22 (46) | 28 (50) | |
| Unknown | 3 (6) | 2 (4) | |
Abbreviations: ±, with or without; BMI, body mass index; EBRT, external-beam radiotherapy; FIGO, International Federation of Gynecology and Obstetrics; LND, lymphadenectomy; SLN, sentinel lymph node.
Values are mean (SD), No. of patients (%), or median (interquartile range).
Comparisons between cohorts were evaluated using the 2-sample t test for age and BMI, the Wilcoxon rank sum test for the No. of nodes, and the χ2 test or Fisher exact test for all other categorical baseline characteristics.
High risk was defined as grade 3 endometrioid type with myometrial invasion ≥50% or nonendometrioid type.
Within the first 5 years after surgery, 28 of 104 patients died (20 LND and 8 SLN), 20 of whom died of disease (13 LND and 7 SLN). Among the 76 living patients, the median duration of follow-up was 3.2 (IQR, 2.3–4.7) years in the LND cohort and 2.4 (IQR, 1.5–3.6) years in the SLN cohort. A total of 34 patients had disease progression within 5 years after surgery, with the first progression being isolated lymph nodal in 10 patients, vaginal only in 3, hematogenous or peritoneal, with or without vaginal, in 10, both lymph nodal and hematogenous or peritoneal, with or without vaginal, in 9, and an undocumented route in 2.
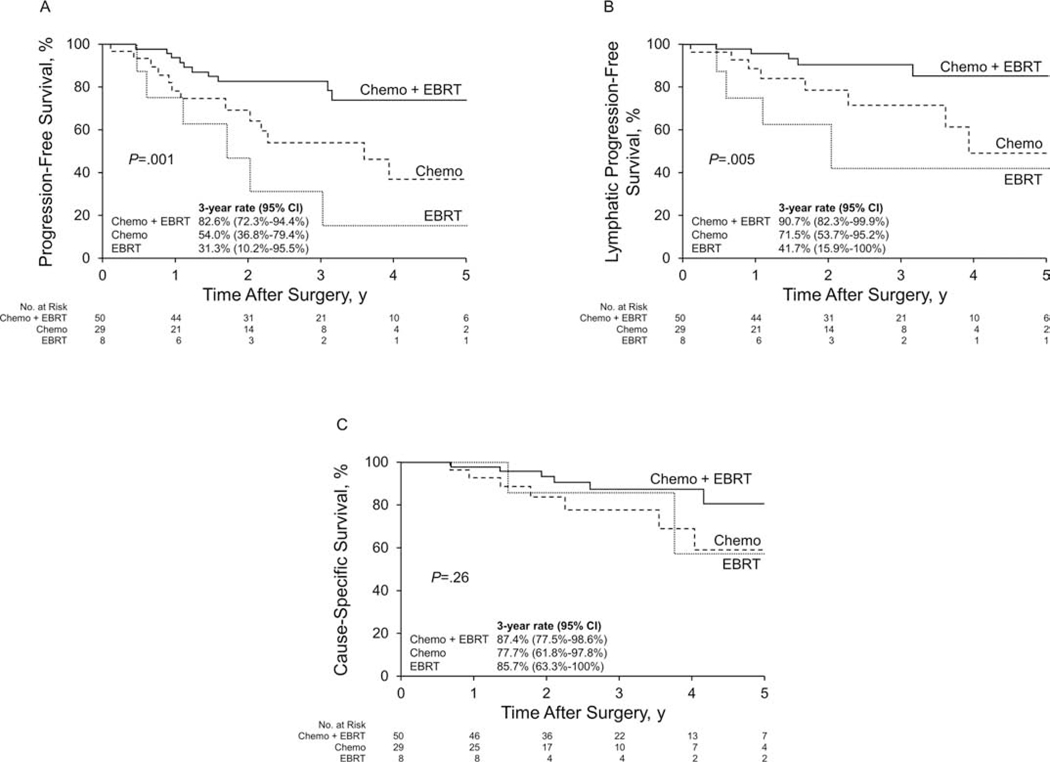
On univariate analysis in the entire cohort (N=104), grade 3 cancer and “high-risk” disease (grade 3 endometrioid with myometrial invasion ≥50% or nonendometrioid) were each significantly associated with an increased risk of death due to disease, disease progression, and lymphatic progression (Table 2). The risk of disease progression was significantly higher for patients who received chemotherapy only (HR, 2.77; 95% CI, 1.20–6.37) or radiotherapy only (HR, 4.50; 95% CI, 1.54–13.16) compared with those who received chemoradiotherapy (Table 2). Associated PFS is shown in Figure 2 A. A similar pattern was observed for the risk of lymphatic progression (Table 2, Figure 2 B). Associated CSS is shown in Figure 2 C.
Table 2.
Univariate Analysis for Factors Associated With Cause-Specific Death, Disease Progression, and Lymphatic Progression Within 5 Years
| Characteristic | Cause-Specific Deatha |
Disease Progressiona |
Lymphatic Progressiona |
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Age, yb | 1.42 (0.95–2.12) | .08 | 1.26 (0.93–1.71) | .13 | NA | |
| BMI, kg/m2,b | 0.84 (0.62–1.13) | .24 | 0.98 (0.80–1.21) | .87 | NA | |
| Histologic type | .14 | .11 | .02 | |||
| Endometrioid (n=66) | Ref | Ref | Ref | |||
| Nonendometrioid (n=38) | 1.96 (0.81–4.76) | 1.75 (0.89–3.44) | 3.16 (1.24–8.09) | |||
| FIGO grade | .005 | .006 | .008 | |||
| 1 or 2 (n=54) | Ref | Ref | Ref | |||
| 3 (n=50) | 4.93 (1.63–14.90) | 2.79 (1.35–5.78) | 4.04 (1.43–11.36) | |||
| Myometrial invasion | .15 | .60 | .30 | |||
| None (n=9) | Ref | Ref | Ref | |||
| <50% (n=37) | 0.24 (0.05–1.09) | 0.59 (0.20–1.74) | 0.58 (0.17–1.95) | |||
| ≥50% (n=58) | 0.74 (0.24–2.28) | 0.79 (0.29–2.16) | 0.37 (0.11–1.31) | |||
| High riskc | .003 | .006 | .005 | |||
| No (n=56) | Ref | Ref | Ref | |||
| Yes (n=48) | 5.38 (1.78–16.22) | 2.69 (1.32–5.47) | 4.38 (1.56–12.29) | |||
| Cervical stroma invasion | .04 | .50 | NA | |||
| No (n=90) | Ref | Ref | ||||
| Yes (n=14) | 3.42 (1.06–10.97) | 1.45 (0.50–4.21) | ||||
| Peritoneal cytologic findings | .02 | .27 | NA | |||
| Negative or not sampled (n=71) | Ref | Ref | ||||
| Positive (n=33) | 3.01 (1.24–7.30) | 1.48 (0.74–2.96) | ||||
| No. of pelvic nodes removed | .86 | .66 | .65 | |||
| 0–12 (n=36) | Ref | Ref | Ref | |||
| 13–30 (n=33) | 1.31 (0.35–4.87) | 1.30 (0.51–3.30) | 0.95 (0.29–3.05) | |||
| ≥31 (n=35) | 1.52 (0.35–6.61) | 1.70 (0.54–5.42) | 1.82 (0.38–8.65) | |||
| Positive pelvic nodes | .65 | .16 | .21 | |||
| No, or pelvic LND not done (n=15) | Ref | Ref | Ref | |||
| Yes (n=89) | 1.34 (0.38–4.73) | 2.38 (0.71–7.96) | 3.70 (0.48–28.37) | |||
| Para-aortic LND | .72 | .90 | .33 | |||
| No (n=29) | Ref | Ref | Ref | |||
| Yes (n=75) | 1.32 (0.29–5.94) | 0.94 (0.35–2.51) | 0.54 (0.16–1.86) | |||
| No. of para-aortic nodes removed | .98 | .68 | .21 | |||
| 0–1 (n=34) | Ref | Ref | Ref | |||
| 2–11 (n=34) | 1.15 (0.30–4.49) | 0.68 (0.27–1.73) | 0.60 (0.19–1.85) | |||
| ≥12 (n=36) | 1.17 (0.22–6.24) | 0.63 (0.19–2.11) | 0.23 (0.05–1.19) | |||
| Positive para-aortic nodes | .38 | .09 | .52 | |||
| No, or para-aortic LND not done (n=65) | Ref | Ref | Ref | |||
| Yes (n=39) | 0.66 (0.26–1.68) | 0.53 (0.25–1.11) | 0.73 (0.27–1.92) | |||
| Adjuvant therapy | .07 | .02 | .04 | |||
| Chemotherapy (n=29) | 2.54 (0.85–7.65) | 2.77 (1.20–6.37) | 3.04 (0.99–9.38) | |||
| Chemotherapy and EBRT (n=50) | Ref | Ref | Ref | |||
| EBRT (n=8) | 1.61 (0.31–8.38) | 4.50 (1.54–13.16) | 7.81 (1.81–33.63) | |||
| None or unknown (n=17) | 4.98 (1.45–17.05) | 2.93 (0.98–8.75) | 2.59 (0.49–13.79) | |||
Abbreviations: BMI, body mass index; EBRT, external-beam radiotherapy; FIGO, International Federation of Gynecology and Obstetrics; HR, hazard ratio; LND, lymphadenectomy; NA, not analyzed; Ref, reference.
Each factor was evaluated in a separate univariate Cox proportional hazards regression model, stratified by cohort (LND vs sentinel lymph node).
HR per 10-year increase in age and per 5-unit increase in BMI.
High risk defined as grade 3 endometrioid type with myometrial invasion ≥50% or nonendometrioid type.
Figure 2.
Kaplan-Meier Survival Analysis for Patients Who Received Adjuvant Therapy, According to Type of Adjuvant Therapy. A, Progression-free survival. B, Lymphatic progression-free survival. C, Cause-specific survival. Chemo indicates chemotherapy; EBRT, external-beam radiotherapy.
In parsimonious multivariable models that included variables for histologic type and “high risk”, both factors were independently associated with CSS and PFS. Given the interdependency between these 2 factors, patients were classified into 3 subgroups: a) “low/intermediate-risk” endometrioid, b) “high-risk” endometrioid, and c) nonendometrioid. Compared with the low/intermediate-risk endometrioid group, the risk of death due to disease was significantly higher for patients with high-risk endometrioid disease (HR, 18.88; 95% CI, 4.57–77.90; P<.001) or with nonendometrioid disease (HR, 4.14; 95% CI; 1.31–13.14; P=.02). Likewise, the risk of disease progression was significantly higher for patients with high-risk endometrioid disease (HR, 6.12; 95% CI, 2.06–18.14; P=.001) or nonendometrioid disease (HR, 2.33; 95% CI, 1.11–4.91; P=.03).
In the Cox models comparing outcomes between the LND and SLN cohorts, both the unadjusted and adjusted analyses showed no statistically significant differences in any of the outcomes measured (all P>.05) within the first 5 years after surgery (Table 3). Although not statistically significant, the risk of death due to disease was 2-fold higher in the LND cohort than the SLN cohort (adjusted HR, 2.10; 95% CI, 0.79–5.58; P=.14). The relative risk of any progression was not as high (HR, 1.27; 95% CI, 0.60–2.67; P=.53). In contrast, the risk of para-aortic progression was 3.7 times higher in the SLN cohort than the LND cohort (HR, 3.66; 95% CI, 0.70–19.03; P=.12). Ten women overall (4/48 LND vs 6/56 SLN) had a para-aortic recurrence. Of the 4 in the LND group, 2 were isolated para-aortic, 1 was para-aortic and hematogenous, and 1 was para-aortic, hematogenous, and peritoneal. Of the 6 in the SLN group, 4 were isolated para-aortic, 1 was para-aortic and peritoneal, and 1 was para-aortic, hematogenous, and peritoneal.
Table 3.
Comparison of Outcomes Between the 2 Cohorts
| No. of Events Within 5 Years | Unadjusted Analysis |
Adjusted Analysisa |
||||
|---|---|---|---|---|---|---|
| Outcome | Cohort | HR (95% CI) | P | HR (95% CI) | P | |
| Death due to disease | SLN (n=56) | 7 | Ref | Ref | ||
| LND (n=48) | 13 | 2.18 (0.87–5.47) | .10 | 2.10 (0.79–5.58) | .14 | |
| Any disease progression | SLN (n=56) | 16 | Ref | Ref | ||
| LND (n=48) | 18 | 1.45 (0.74–2.85) | .28 | 1.27 (0.60–2.67) | .53 | |
| Nonvaginal progression | SLN (n=56) | 15 | Ref | Ref | ||
| LND (n=48) | 15 | 1.28 (0.62–2.62) | .50 | 1.13 (0.51–2.50) | .76 | |
| Lymphatic progression | SLN (n=56) | 11 | Ref | Ref | ||
| LND (n=48) | 8 | 0.91 (0.37–2.27) | .84 | 0.68 (0.24–1.97) | .48 | |
| Para-aortic progression | SLN (n=56) | 6 | Ref | Ref | ||
| LND (n=48) | 4 | 0.84 (0.24–3.00) | .79 | 0.27 (0.05–1.42) | .12 | |
| Isolated lymphatic progression | SLN (n=56) | 6 | Ref | Ref | ||
| LND (n=48) | 4 | 0.80 (0.23–2.85) | .73 | 0.74 (0.16–3.35) | .69 | |
| HP progression | SLN (n=56) | 9 | Ref | Ref | ||
| LND (n=48) | 10 | 1.46 (0.59–3.59) | .41 | 1.27 (0.48–3.37) | .64 | |
Abbreviations: HP, hematogenous or peritoneal; HR, hazard ratio; LND, lymphadenectomy; Ref, reference; SLN, sentinel lymph node.
Adjusted results based on fitting Cox proportional hazards models that included variables for cohort (SLN vs LND) and adjuvant therapy (none/unknown, chemotherapy only, radiation only, or chemotherapy and radiotherapy).
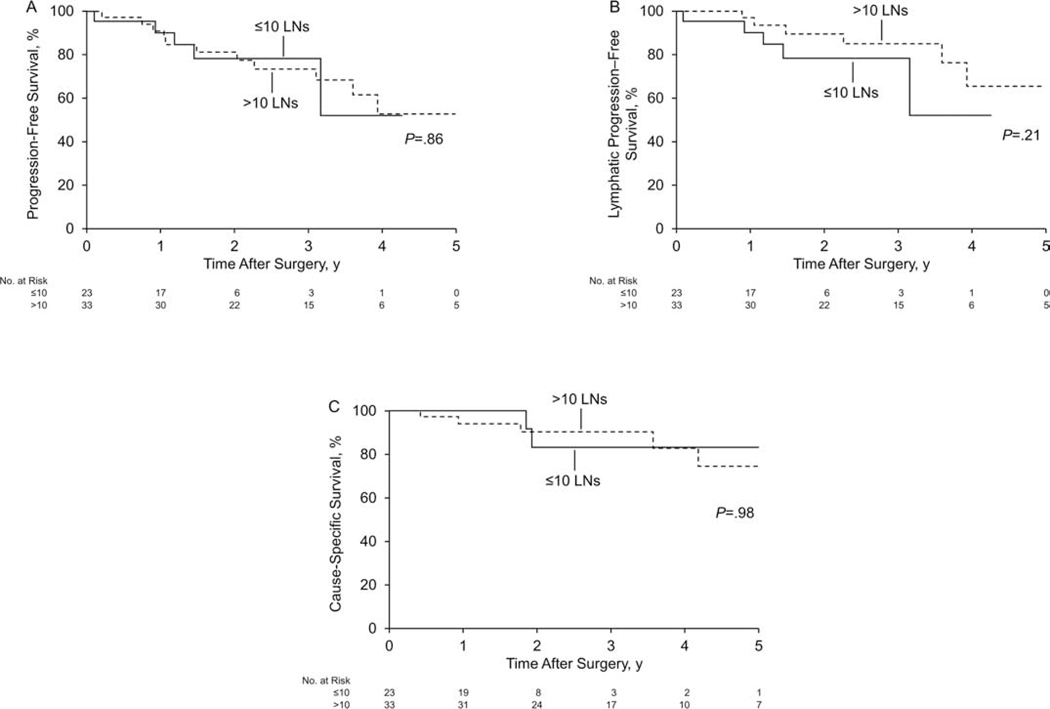
Evaluation of PFS, lymphatic PFS, and CSS in the SLN cohort showed no significant differences between patients with 10 or fewer LNs removed (n=23) vs more than 10 LNs removed (n=33) (all log-rank P>.05) (Figure 3).
Figure 3.
Kaplan-Meier Survival Analysis for the Sentinel Lymph Node Cohort According to Number of Lymph Nodes (LNs) Removed (≤10 [n=23] vs >10 [n=33]). A, Progression-free survival. B, Lymphatic progression-free survival. C, Cause-specific survival.
Discussion
The safety of the SLN algorithm with regard to preserving survival outcomes of patients with early-stage EC has been previously reported [24, 25]. To our knowledge, however, this is the first comparative study suggesting that the extent of LND does not significantly affect survival in patients with nonbulky positive LNs, despite a possible increase in para-aortic recurrences. The biology of the tumor (ie, histologic grade and subtype) and the type of postoperative treatment (ie, combined vs single therapy) are stronger predictors of overall prognosis. In fact, we observed no evidence of benefit, in terms of CSS and PFS, in patients who had extensive pelvic and para-aortic LN dissection compared with the SLN algorithm. In agreement with our results, a classification and regression tree analysis designed to identify factors influencing overall survival in patients with EC showed that the number of LNs removed and the assessment of the para-aortic area are not associated with overall survival [26]. Similar to our observations in EC, a recent international RCT comparing completion of LN dissection vs observation in patients with node-positive melanoma reported no increase in melanoma-specific survival for patients receiving complete LN dissection [27]. Furthermore, after 2 RCTs [28, 29] showed similar survival outcomes for patients with SLN biopsy compared with complete axillary LN dissection in node-positive breast cancer, the American Society of Clinical Oncology guidelines changed from recommending complete axillary dissection for all patients with positive LNs to recommending against complete axillary dissection in patients with fewer than 3 positive LNs [30].
In our study, the LND and SLN cohorts had no differences in overall lymphatic progression; this suggests, as observed in a previous report [31], that comprehensive LND may not be sufficient treatment for lymphatic dissemination. Also, our findings suggest that postoperative adjuvant therapy is likely to at least partially treat any residual lymphatic metastases that may have remained after a more limited surgery. This therapeutic effect should mitigate any potential differences in lymphatic progression possibly associated with variations in the extent of LND.
Although the risk of a para-aortic progression was 3.7 times higher in the SLN cohort, the rates of isolated para-aortic progression were similar (3.6% SLN vs 4.1% LND). This finding emphasizes that many para-aortic progression are associated with concomitant distant dissemination. Consistent with our study, Aloisi et al [32] recently observed that isolated para-aortic recurrences in patients with stage IIIC1 disease who did not undergo para-aortic LND are uncommon (3.9%). Yoon et al [33] also observed that, although extensive para-aortic LND improved overall survival in stage IIIC patients who received only limited postoperative treatment (ie, radiotherapy only), the extent of para-aortic LND did not affect survival in patients who received combined adjuvant therapy (ie, both chemotherapy and radiotherapy).
The optimal adjuvant treatment modality for stage IIIC EC remains controversial. Despite the relatively high incidence of both local and distant progression, stage IIIC ECs are not sufficiently common to allow adequately powered clinical trials comparing treatment modalities in this subgroup alone. These patients are usually enrolled in clinical trials in combination with patients at other stages of disease [34–36]. However, on the basis of data from national registries [37] and from the recently completed PORTEC-3 trial [36], it is reasonable to treat stage IIIC patients with combined chemotherapy and radiotherapy. In accordance with the data in the literature, we found that the administration of chemoradiotherapy (vs chemotherapy only) was associated with improved PFS, which supports the importance of the type of adjuvant therapy (more than the extent of LN dissection) in patients with nonbulky LN dissemination.
Our median of 12 pelvic LNs removed in the SLN cohort was higher than the number reported by other authors. However, according to the SLN algorithm proposed at MSKCC and published in the NCCN guidelines, all enlarged suspicious lymph nodes should be removed regardless of mapping, and bilateral or side-specific lymphadenectomy should be performed in the case of no mapping or unilateral mapping, respectively. In addition, in the time period of the SLN cohort, many surgeons were still on learning curves, which may be partly responsible for the increased number of LNs removed. However, because intraoperative frozen section is not used at MSKCC, none of the patients in the SLN cohort received backup LND if positive LNs were detected at frozen section.
It is important to emphasize that the algorithm used in the LND cohort is historical and the surgical staging approach at Mayo Clinic has changed since early 2009. First, the minimally invasive approach replaced laparotomy, with 90% of patients undergoing minimally invasive surgery in 2013–2014 [38]. Moreover, starting in 2013, surgical treatment of EC has started to incorporate the use of SLN mapping, with most current patients being treated with the SLN algorithm [39].
It is not clear why the current study did not show the same survival benefit of LND observed in previous retrospective studies [7, 9, 10], but those studies mainly compared no or minimal LND vs full LND, and it is likely that the group of patients who had no or minimal LND actually included some patients with occult undetected nodal disease [40] and who did not receive adjuvant therapy. Moreover, our study excluded patients with bulky nodes. It is possible that, in at least some of the patients with bulky nodes, there may be a therapeutic role for surgical removal. In addition, compared with the older studies, our series included a higher proportion of patients who received combined adjuvant chemotherapy and external-beam radiotherapy, which seems to be an effective treatment in patients with LN dissemination.
This study has several limitations. First, its retrospective and nonrandomized design may have introduced bias inherent with such design. Also, although we included all consecutive stage IIIC ECs treated during a long period at 2 high-volume institutions, our study was only powered to detect large effect sizes for the outcomes of interest (ie, minimum detectable HRs of 2.62, 3.52, and 5.91 with 80% power and 34, 20, and 10 events, respectively, based on a 2-sided log-rank test with type I error of 5%). However, it is unlikely that this question will be addressed by an RCT because of the large sample size required: Assuming that the 5-year CSS for stage IIIC EC is 70% [41], a hypothetical noninferiority RCT with a noninferiority margin of 5% for the CSS estimate would require enrollment of 2,868 patients with stage IIIC disease (1,434 per arm). This calculation was based on a 1-sided log-rank test with 80% power and a type I error of .05, assuming a 10% exponential dropout rate and that all patients would be followed up for 5 years regardless of when they were enrolled. Considering that stage IIIC accounts for approximately 11% of all ECs, a total of 26,073 patients would need to be screened before surgery. An additional limitation is the low number of patients with nonendometrioid type in our study, which did not allow us to stratify the results by histologic type.
In conclusion, our results suggest that, in patients with nonbulky stage IIIC EC who receive appropriate adjuvant therapy (ie, combined chemotherapy and radiotherapy), systematic LND may be omitted without compromising oncologic outcomes. Of note, this suggestion does not apply to patients with bulky and “suspicious” nodes, in which surgical removal may have therapeutic value. We also observed that, in stage IIIC EC, the type of adjuvant therapy (chemoradiotherapy seems to provide the best outcomes), histologic grade, and “high-risk” clinicopathologic variables (“high risk” being considered as grade 3 endometrioid type with myometrial invasion ≥50% or nonendometrioid type on final pathologic analysis) are the strongest predictors of oncologic outcomes. These results further support the use of the SLN algorithm to identify patients with stage IIIC EC who may benefit from adjuvant treatment or enrollment in clinical trials. With recognition of the limitations of a nonrandomized retrospective approach, more studies are warranted to confirm our findings.
Acknowledgments
Funding/Support: Drs Leitao and Abu-Rustum are funded in part by the NIH/NCI Memorial Sloan Kettering Cancer Center Support Grant P30 CA008748.
This publication was made possible by CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Role of the funder/sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Abbreviations
- BMI
body mass index
- CSS
cause-specific survival
- EC
endometrial cancer
- HR
hazard ratio
- IQR
interquartile range
- LN
lymph node
- LND
lymphadenectomy
- MSKCC
Memorial Sloan Kettering Cancer Center
- NCCN
National Comprehensive Cancer Network
- PFS
progression-free survival
- RCT
randomized controlled trial
- SLN
sentinel lymph node
Footnotes
Conflict of Interest Disclosures: None reported.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Francesco Multinu, Department of Obstetrics and Gynecology, Mayo Clinic, Rochester, Minnesota; European Institute of Oncology (IEO), Instituto di Ricovero e Cura a Corattere Scientifico, Milan, Italy..
Jennifer A. Ducie, Gynecology Service, Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, New York; The Gynecologic Oncology Center, Mercy Medical Center, Baltimore, Maryland.
Ane Gerda Zahl Eriksson, Gynecology Service, Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, New York; Department of Gynecologic Oncology, The Norwegian Radium Hospital, Oslo, Norway.
Brooke A. Schlappe, Gynecology Service, Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, New York; Aurora Gynecologic Oncology, Aurora Health Care, Milwaukee, Wisconsin.
William A. Cliby, Department of Obstetrics and Gynecology, Mayo Clinic, Rochester, Minnesota.
Gretchen E. Glaser, Department of Obstetrics and Gynecology, Mayo Clinic, Rochester, Minnesota.
Tommaso Grassi, Department of Obstetrics and Gynecology, Mayo Clinic, Rochester, Minnesota.
Gary L. Keeney, Division of Anatomic Pathology, Mayo Clinic, Rochester, Minnesota.
Amy L. Weaver, Division of Biomedical Statistics and Informatics, Mayo Clinic, Rochester, Minnesota.
Nadeem R. Abu-Rustum, Gynecology Service, Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, New York; Department of Obstetrics and Gynecology, Weill Cornell Medical College, New York, New York.
Mario M. Leitao, Jr, Gynecology Service, Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, New York; Department of Obstetrics and Gynecology, Weill Cornell Medical College, New York, New York.
Andrea Mariani, Department of Obstetrics and Gynecology, Mayo Clinic, Rochester, Minnesota.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- [2].Practice Bulletin No. 149: Endometrial cancer. Obstet Gynecol. 2015;125:1006–26. [DOI] [PubMed] [Google Scholar]
- [3].Casarin J, Multinu F, Abu-Rustum N, Cibula D, Cliby WA, Ghezzi F, et al. Factors influencing the adoption of the sentinel lymph node technique for endometrial cancer staging: an international survey of gynecologic oncologists. Int J Gynecol Cancer. 2019;29:60–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].group As, Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009;373:125–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Benedetti Panici P, Basile S, Maneschi F, Alberto Lissoni A, Signorelli M, Scambia G, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100:1707–16. [DOI] [PubMed] [Google Scholar]
- [6].Dowdy SC, Mariani A. Lymphadenectomy in endometrial cancer: when, not if. Lancet. 2010;375:1138–40. [DOI] [PubMed] [Google Scholar]
- [7].Todo Y, Kato H, Kaneuchi M, Watari H, Takeda M, Sakuragi N. Survival effect of para-aortic lymphadenectomy in endometrial cancer (SEPAL study): a retrospective cohort analysis. Lancet. 2010;375:1165–72. [DOI] [PubMed] [Google Scholar]
- [8].Chan JK, Cheung MK, Huh WK, Osann K, Husain A, Teng NN, et al. Therapeutic role of lymph node resection in endometrioid corpus cancer: a study of 12,333 patients. Cancer. 2006;107:1823–30. [DOI] [PubMed] [Google Scholar]
- [9].Abu-Rustum NR, Iasonos A, Zhou Q, Oke E, Soslow RA, Alektiar KM, et al. Is there a therapeutic impact to regional lymphadenectomy in the surgical treatment of endometrial carcinoma? Am J Obstet Gynecol. 2008;198:457 e1–5; discussion e5–6. [DOI] [PubMed] [Google Scholar]
- [10].Mariani A, Webb MJ, Galli L, Podratz KC. Potential therapeutic role of para-aortic lymphadenectomy in node-positive endometrial cancer. Gynecol Oncol. 2000;76:348–56. [DOI] [PubMed] [Google Scholar]
- [11].Rossi EC, Kowalski LD, Scalici J, Cantrell L, Schuler K, Hanna RK, et al. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study. Lancet Oncol. 2017;18:384–92. [DOI] [PubMed] [Google Scholar]
- [12].Bodurtha Smith AJ, Fader AN, Tanner EJ. Sentinel lymph node assessment in endometrial cancer: a systematic review and meta-analysis. Am J Obstet Gynecol. 2017;216:459–76. [DOI] [PubMed] [Google Scholar]
- [13].Koh WJ, Abu-Rustum NR, Bean S, Bradley K, Campos SM, Cho KR, et al. Uterine Neoplasms, Version 1.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:170–99. [DOI] [PubMed] [Google Scholar]
- [14].Multinu F, Casarin J, Mariani A. Point/Counterpoint: Is Lymphadenectomy Required in Endometrial Cancer for Adequate Surgical Staging? Oncology (Williston Park). 2017;31:390-1, 401. [PubMed] [Google Scholar]
- [15].Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105:103–4. [DOI] [PubMed] [Google Scholar]
- [16].Mariani A, Dowdy SC, Cliby WA, Gostout BS, Jones MB, Wilson TO, et al. Prospective assessment of lymphatic dissemination in endometrial cancer: a paradigm shift in surgical staging. Gynecol Oncol. 2008;109:11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mariani A, Webb MJ, Keeney GL, Haddock MG, Calori G, Podratz KC. Low-risk corpus cancer: is lymphadenectomy or radiotherapy necessary? Am J Obstet Gynecol. 2000;182:1506–19. [DOI] [PubMed] [Google Scholar]
- [18].Abu-Rustum NR. Update on sentinel node mapping in uterine cancer: 10-year experience at Memorial Sloan-Kettering Cancer Center. J Obstet Gynaecol Res. 2014;40:327–34. [DOI] [PubMed] [Google Scholar]
- [19].Barlin JN, Khoury-Collado F, Kim CH, Leitao MM Jr., Chi DS, Sonoda Y, et al. The importance of applying a sentinel lymph node mapping algorithm in endometrial cancer staging: beyond removal of blue nodes. Gynecol Oncol. 2012;125:531–5. [DOI] [PubMed] [Google Scholar]
- [20].Kim CH, Soslow RA, Park KJ, Barber EL, Khoury-Collado F, Barlin JN, et al. Pathologic ultrastaging improves micrometastasis detection in sentinel lymph nodes during endometrial cancer staging. Int J Gynecol Cancer. 2013;23:964–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Plante M, Stanleigh J, Renaud MC, Sebastianelli A, Grondin K, Gregoire J. Isolated tumor cells identified by sentinel lymph node mapping in endometrial cancer: Does adjuvant treatment matter? Gynecol Oncol. 2017;146:240–6. [DOI] [PubMed] [Google Scholar]
- [22].Schwartz LH, Bogaerts J, Ford R, Shankar L, Therasse P, Gwyther S, et al. Evaluation of lymph nodes with RECIST 1.1. Eur J Cancer. 2009;45:261–7. [DOI] [PubMed] [Google Scholar]
- [23].Montori VM. Decision Making and the Patient In: Guyatt G, Meade MO, Rennie D, Cook DJ, editors. Users’ Guides to the Medical Literature: A Manual for Evidence-Based Clinical Practice; 3 ed2015. [Google Scholar]
- [24].Schlappe BA, Weaver AL, Ducie JA, Eriksson AGZ, Dowdy SC, Cliby WA, et al. Multicenter study comparing oncologic outcomes between two nodal assessment methods in patients with deeply invasive endometrioid endometrial carcinoma: A sentinel lymph node algorithm versus a comprehensive pelvic and para-aortic lymphadenectomy. Gynecol Oncol. 2018;151:235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zahl Eriksson AG, Ducie JA, Ali N, McGree ME, Weaver AL, Bogani G, et al. Comparison of a sentinel lymph node and a selective lymphadenectomy algorithm in patients with endometrioid endometrial carcinoma and limited myometrial invasion. Gynecol Oncol. 2016;140:394–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Barlin JN, Zhou Q, St Clair CM, Iasonos A, Soslow RA, Alektiar KM, et al. Classification and regression tree (CART) analysis of endometrial carcinoma: Seeing the forest for the trees. Gynecol Oncol. 2013;130:452–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Faries MB, Thompson JF, Cochran AJ, Andtbacka RH, Mozzillo N, Zager JS, et al. Completion Dissection or Observation for Sentinel-Node Metastasis in Melanoma. N Engl J Med. 2017;376:2211–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Giuliano AE, Hunt KK, Ballman KV, Beitsch PD, Whitworth PW, Blumencranz PW, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305:569–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Galimberti V, Cole BF, Zurrida S, Viale G, Luini A, Veronesi P, et al. Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23–01): a phase 3 randomised controlled trial. Lancet Oncol. 2013;14:297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lyman GH, Temin S, Edge SB, Newman LA, Turner RR, Weaver DL, et al. Sentinel lymph node biopsy for patients with early-stage breast cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2014;32:1365–83. [DOI] [PubMed] [Google Scholar]
- [31].Mariani A, Dowdy SC, Cliby WA, Haddock MG, Keeney GL, Lesnick TG, et al. Efficacy of systematic lymphadenectomy and adjuvant radiotherapy in node-positive endometrial cancer patients. Gynecol Oncol. 2006;101:200–8. [DOI] [PubMed] [Google Scholar]
- [32].Aloisi A, Casanova JM, Tseng JH, Seader KA, Nguyen NT, Alektiar KM, et al. Patterns of FIRST recurrence of stage IIIC1 endometrial cancer with no PARA-AORTIC nodal assessment. Gynecol Oncol. 2018;151:395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yoon MS, Park W, Huh SJ, Kim HJ, Kim YS, Kim YB, et al. Impact of para-aortic lymphadenectomy for endometrial cancer with positive pelvic lymph nodes: A Korean Radiation Oncology Group study (KROG 13–17). Eur J Surg Oncol. 2016;42:1497–505. [DOI] [PubMed] [Google Scholar]
- [34].Randall ME, Filiaci VL, Muss H, Spirtos NM, Mannel RS, Fowler J, et al. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2006;24:36–44. [DOI] [PubMed] [Google Scholar]
- [35].Maggi R, Lissoni A, Spina F, Melpignano M, Zola P, Favalli G, et al. Adjuvant chemotherapy vs radiotherapy in high-risk endometrial carcinoma: results of a randomised trial. Br J Cancer. 2006;95:266–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].de Boer SM, Powell ME, Mileshkin L, Katsaros D, Bessette P, HaieMeder C, et al. Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): final results of an international, open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19:295–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wong AT, Rineer J, Lee YC, Schwartz D, Safdieh J, Weiner J, et al. Utilization of adjuvant therapies and their impact on survival for women with stage IIIC endometrial adenocarcinoma. Gynecol Oncol. 2016;142:514–9. [DOI] [PubMed] [Google Scholar]
- [38].Bergstrom J, Aloisi A, Armbruster S, Yen TT, Casarin J, Leitao MM Jr., et al. Minimally invasive hysterectomy surgery rates for endometrial cancer performed at National Comprehensive Cancer Network (NCCN) Centers. Gynecol Oncol. 2018;148:480–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Casarin J, Multinu F, Pasupathy K, Weaver A, McGree M, Tortorella L, et al. Frozen Section for Detection of Lymph Nodes After Cervical Injection with Indocyanine Green (ICG) for Sentinel Lymph Node Technique in Endometrial Cancer Staging. Ann Surg Oncol. 2018;25:3692–8. [DOI] [PubMed] [Google Scholar]
- [40].How J, Boldeanu I, Lau S, Salvador S, How E, Gotlieb R, et al. Unexpected locations of sentinel lymph nodes in endometrial cancer. Gynecol Oncol. 2017;147:18–23. [DOI] [PubMed] [Google Scholar]
- [41].Rauh-Hain JA, Vargas RJ, Clemmer J, Clark RM, Bradford LS, Growdon WB, et al. Mucinous Adenocarcinoma of the Endometrium Compared With Endometrioid Endometrial Cancer: A SEER Analysis. Am J Clin Oncol. 2016;39:43–8. [DOI] [PubMed] [Google Scholar]