Abstract
Background:
Triple-negative breast cancer (TNBC) represents an aggressive breast cancer subtype with historically poor overall outcomes, due primarily to a lack of effective targeted agents. Chemotherapy has been the primary treatment approach, although immune checkpoint inhibitors (ICIs) are currently being investigated to improve patient outcomes. This review examines the clinical implications of current evidence on the use of ICIs for the treatment of metastatic TNBC.
Methods:
Our systematic search identified two phase III and five phase I/II trials reporting on the efficacy of ICIs used as monotherapy or combined with chemotherapy for the treatment of metastatic TNBC.
Results:
The phase III IMpassion 130 trial showed a significant improvement in median progression-free survival in the intent-to-treat (net 1.7 months, p = 0.002) and PD-L1-positive populations (net 2.5 months, p < 0.001) for the addition of first-line atezolizumab versus placebo to nab-paclitaxel in metastatic TNBC. Although median overall survival was not significantly improved in patients receiving atezolizumab overall [net 2.3 months, hazard ratio (HR) 0.86, 95% confidence interval (CI) 0.72–1.02, p = 0.078], numerical improvements in the PD-L1-positive population were compelling (net 7.0 months, HR 0.71; 95% CI 0.54–0.93). Toxicity profiles were as expected, and no new safety signals were observed. Pembrolizumab monotherapy did not significantly improve overall survival in similar patients that had received prior treatment in KEYNOTE-119.
Conclusions:
Atezolizumab plus nab-paclitaxel represents a potential new first-line standard of care for patients with metastatic PD-L1-positive TNBC. Other ICIs used as monotherapy, or combined with chemotherapy for advanced TNBC, as well as their use for earlier stage disease, are areas of ongoing investigation.
Keywords: Anti-PD-1, Anti-PD-L1, atezolizumab, checkpoint inhibitors, immunotherapy, TNBC, triple-negative breast cancer
Introduction
Breast cancer is the most common cancer diagnosis, and the leading cause of cancer related death in females, with nearly 2.1 million new cases resulting in over 620,000 deaths worldwide in 2018, and approximately 6% of patients presenting with metastatic disease.1,2 Triple-negative breast cancer (TNBC) is a subtype of breast cancer characterized by the lack of expression of the human epidermal growth factor receptor 2, estrogen receptor, and progesterone receptor.3 TNBC accounts for 13–20% of all breast cancers and represents an aggressive breast cancer subtype.2–4 Outcomes for patients who develop metastatic TNBC are poor, owing in part due to a lack of effective targeted therapeutic agents, and the mainstay of treatment has therefore been traditional chemotherapy.4–7
Although the classification of TNBC is useful from a clinical standpoint, it does not provide much insight into the heterogeneity of the disease.3,4 There are multiple approaches to further characterize TNBC, including classic clinical pathology techniques, gene expression profiling, and the assessment of genomic alterations.3,8 Immunohistochemistry studies indicate a range of TNBC tumors, which can be androgen receptor positive or negative, show varying degrees of proliferation estimated by the expression of Ki-67, and contain tumor-infiltrating lymphocytes (TILs).3,9–11 Gene expression profiling shows a broad spectrum of TNBC subtypes, ranging from basal-like to luminal, with most, but not all, of TNBCs expressing basal-like attributes.3,12 Other subtypes of interest include immunomodulatory TNBC, which is enriched for gene signatures involved in immune cell processes;3,8 and basal-like immune activated TNBC, which have basal-like features characterized by STAT transcription factors and high cytokine levels.3,13 Many genomic alterations have been identified in TNBC, including TP53, PIK3CA, and germline BRCA1 or BRCA2 mutations.3,14 germline BRCA1/2 mutations occur in approximately 11% of unselected TNBC cases,15 and TNBC has the highest frequency of mutations among breast cancer subtypes.3,16 It has become evident that TNBC is indeed highly heterogeneous, adding to the challenge of identifying a consistent therapeutic target for this disease.
The standard of care for metastatic TNBC consists of treatment with multiple lines of chemotherapy, preferably given sequentially without treatment breaks based on progression free survival (PFS) and overall survival (OS) benefit.17,18 International guidelines support the use of single-agent anthracyclines and taxanes for first-line treatment, followed by sequential single agent chemotherapy including eribulin,19–24 until either a rapid decline in performance status or the approach of end of life.25 TNBC patients with germline BRCA1/2 mutations can receive poly ADP ribose polymerase (PARP)-inhibitors based on results from the phase III OlympiAD and EMBRACA trials,21,26,27 or platinum therapy based on results of the phase III TNT study.28 Guidelines also suggest that chemotherapy combinations may play a role in those with high tumor burden, visceral crisis, or rapidly progressing disease.19,21,22 The overall lack of targeted therapy options and targetable mutations for TNBC means that new therapeutic approaches are needed for these aggressive and heterogeneous tumors.
Although breast cancer has historically been considered nonimmunogenic,29 there are a number of biological rationales for the use of immune checkpoint inhibitors (ICIs) in TNBC.30,31 TNBC is often associated with increased numbers of TILs,13,30–32 which are associated with high expression of the programmed cell death ligand-1 (PD-L1),33 indicating an immunogenic environment. PD-L1 expression can decrease T-cell proliferation and increase apoptosis in the tumor microenvironment,31 providing additional rationale for targeting the PD-1 (programmed cell death protein-1)/PD-L1 axis to improve tumor control. Most TNBC patients will receive chemotherapy, either in the adjuvant or metastatic settings. These tumors therefore may be more primed to respond to ICIs, as cytotoxic chemotherapy can also increase the production of tumor cell (TC) antigens,34,35 decrease production of T-cell inhibitory molecules,34,35 and possibly increase PD-L1 expression.36 Furthermore, combining ICIs with standard chemotherapeutic agents has the potential to increase the magnitude and duration of response in patients with TNBC. ICIs, including the PD-1 inhibitor nivolumab and pembrolizumab, and the PD-L1 inhibitors atezolizumab, durvalumab, and avelumab, are currently under investigation, either as monotherapy or in combination with chemotherapy, for the treatment of patients with advanced TNBC.37
The rapidly evolving role of ICIs in TNBC is currently being investigated and early clinical indicators suggest that PD-1/PD-L1 ICIs are active in the treatment of TNBC.30,31 The objective of this clinical review is to discuss phase I-III efficacy and safety data on the use of ICIs alone, or in combination with chemotherapy, for the treatment of patients with advanced TNBC, and to consider future clinical applications.
Methods
A search of published and presented literature was conducted to identify clinical trials (phase I–III) reporting outcomes on the use of ICIs as monotherapy or in combination with chemotherapy for the treatment of metastatic TNBC. PubMed (all time to 9 June 2019), the proceedings of the American Society of Clinical Oncology (ASCO 2019) annual meetings, and the proceedings of the San Antonio Breast Cancer Symposium (SABCS 2018) were searched for clinical trials assessing ICIs in the treatment of TNBC using the key search terms “checkpoint inhibitor” OR “immunotherapy” AND “triple-negative” AND “metastatic” AND “breast cancer” OR respective aliases. A supplemental bibliographic search of review articles and pooled/meta-analyses was also conducted.3,31,37–39 In addition, ClinicalTrials.gov was searched on 3 June 2019 for ongoing phase III trials of ICIs in the treatment in TNBC using the key search terms “checkpoint inhibitor” AND “triple-negative” AND “breast cancer” OR respective aliases AND the “phase III” filter.
English language records were vetted at abstract level and confirmed at full text as needed. Studies were excluded if they were nonoriginal research, preclinical studies only, correlative science, not specific to breast cancer (phase I studies in all solid tumors), outside the metastatic setting, or addressed nonchemotherapy combinations; duplicate or prior reports or studies without reported outcomes were also excluded. Although not formally included in our search, phase I–III studies of combinations with neoadjuvant chemotherapy and ongoing phase III trials were summarized and addressed in the discussion.
Findings
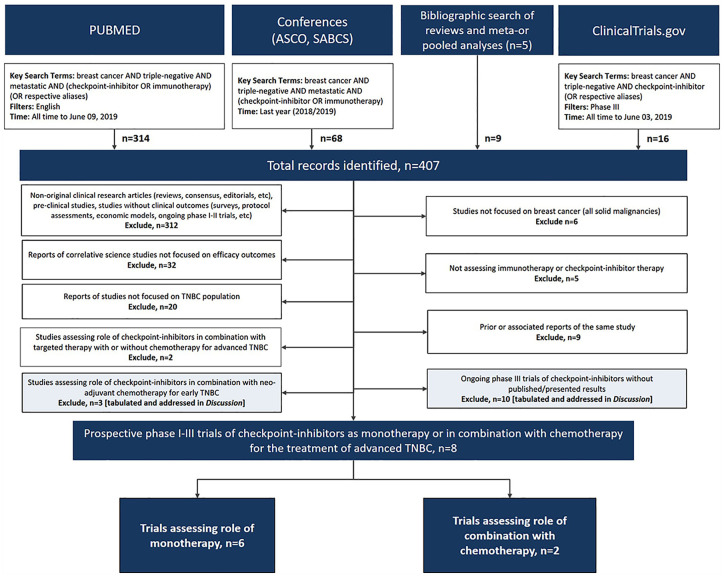
The literature search identified a total of 407 records, which after vetting resulted in a total of eight clinical trials reporting efficacy outcomes on the use of ICIs as monotherapy (n = 6) or in combination with chemotherapy (n = 2) for the treatment of metastatic TNBC (Figure 1).40–48 The phase II TONIC trial was excluded as this trial assessed ICI induction therapy and did not meet the eligibility criteria of being used either as monotherapy or in combination with chemotherapy.49
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram.
ASCO, American Society of Clinical Oncology; SABCS, San Antonio Breast Cancer Symposium; TNBC, triple-negative breast cancer.
ICIs as monotherapy
The activity of ICIs as monotherapy in metastatic TNBC was reported in four phase I/II studies,43–47 and one phase III trial (Table 1).48 Two recent phase I studies evaluated avelumab or atezolizumab in patients with PD-L1 unselected advanced TNBC.45,46 JAVELIN included 58 patients with TNBC receiving avelumab, showing an objective response rate (ORR) of 5.2% [95% confidence interval (CI) 1.1–14.4], with a median duration of response (DOR) that was not estimable,45 and the PCD4989g study evaluated atezolizumab in 115 TNBC patients showing an ORR of 10% (95% CI 4.9–16.5) with a median DOR of 21 months (95% CI 3–38+) (Table 1). Pembrolizumab was also assessed in phase I, II, and III trials in advanced TNBC.43,44,47,48 The small Ib KEYNOTE-012 trial showed a promising ORR of 18.5% (95% CI 6.3–38.1) with a median DOR not yet reached (range 3.4–10.9+ months) among 27 PD-L1-positive patients.47 The larger phase II KEYNOTE-086 study assessed pembrolizumab in two cohorts: the first-line PD-L1-positive cohort (B, n = 84) showed an ORR of 21.4% (95% CI 13.9–31.4) with a median DOR of 10.4 months (range 4.2–19.2+),43 and the second- or later-line PD-L1 unselected cohort (A, n = 170) showed an ORR of 5.3% (95% CI 2.7–9.9) with the median DOR not yet reached (range 1.2+–21.5+).44 Pembrolizumab was also assessed in the phase III KEYNOTE-119 trial, which randomized 622 second- or third-line PD-L1 unselected advanced TNBC patients to receive either pembrolizumab or physicians choice of single agent chemotherapy. The coprimary endpoints of this study were OS in the intent-to-treat (ITT) population and also in patients with PD-L1-positive tumors with a combined positive score {CPS; [total TCs + immune cells (ICs)]/total TCs × 100 ⩾1 and ⩾10}. At a median follow up of 9.9–10.9 months, no significant improvement in median PFS [2.1 versus 3.3 months, hazard ratio (HR) 1.60, 95% CI 1.33–1.92] or OS (9.9 versus 10.8 months, HR 0.97, 95% CI 0.82–1.15) was observed for pembrolizumab versus chemotherapy in ITT patients.48 Nor did PD-L1 subgroup analyses show an improvement in OS among patients with CPS ⩾1 (10.7 versus 10.2 months, stratified HR 0.86, 95% CI 0.69–1.06, p = .073), or CPS ⩾ 10 tumors (12.7 versus 11.6 months, HR 0.78, 95% CI 0.57–1.06, p = .057). In ITT patients, ORRs were 9.6% versus 10.6%, and median DORs were 12.2 versus 8.3 months in the pembrolizumab versus chemotherapy arms.
Table 1.
Efficacy of single agent immune checkpoint inhibitors in advanced TNBC. Efficacy outcomes of immune checkpoint inhibitor trials in metastatic TNBC ordered by line of therapy then size of trial.
| Trial | Setting Line of treatment |
Regimen(s) | n | Overall response rate, % (95% CI) |
Median duration of response, months (95% CI) [range] |
Median progression free survival, months (95% CI) |
Median overall survival, months (95% CI) |
|---|---|---|---|---|---|---|---|
| JAVELIN
Phase Ib Subgroup45 |
PD-L1 unselected 50.0% ⩽second-line |
Avelumab | 58 | 5.2 (1.1–14.4) |
NE | 1.4a
(1.3–1.6) |
9.2 (4.3–NE) |
| PCD4989g
Phase I46 |
PD-L1 unselectedb
18.3% first-line |
Atezolizumab | 115 | 10c
(4.9–16.5) |
21 [3–38+] |
1.4 (1.3–1.6) |
8.9d
(7.0–12.6) |
| KEYNOTE-012
Phase Ib47 |
PD-L1-positive 15.6% first-linee |
Pembrolizumab | 27 | 18.5 (6.3–38.1) |
NYR [3.4–10.9+] |
1.9 (1.7–5.5) |
11.2 (5.3–NYR) |
| KEYNOTE-086
Phase II Cohort B43 |
PD-L1-positive 100% first-line |
Pembrolizumab | 84 | 21.4 (13.9–31.4) |
10.4 [4.2–19.2+] |
2.1 (2.0–2.2) |
18.0 (12.9–23.0) |
| KEYNOTE-086
Phase II Cohort A44 |
PD-L1 unselected second-line+ 31.2% second-line |
Pembrolizumab | 170 | 5.3 (2.7–9.9) |
NYR [1.2+–21.5+] |
2.0 (1.9–2.0) |
9.0 (7.6–11.2) |
| KEYNOTE-119
Phase III41,48 |
PD-L1 Unselected second- or third-line |
Pembrolizumab | 312 | 9.6 | 12.2 [2.2–32.5+] |
2.1 HR 1.60 (1.33–1.92) |
9.9 HR 0.97 (0.82–1.15) |
| Chemotherapyf | 310 | 10.6 | 8.3 [2.1–33.0+] |
3.3 | 10.8 |
Weeks converted to months using 4.35 weeks/month as conversion factor.
First 25 patients selected for PD-L1 ⩾5% ICs; enrollment subsequently extended all patients regardless of PD-L1 status.
ORR for first-line: 24% (95% CI 8.2–47.2), second-line: 6% (95% CI 2.4–13.4).
OS for first-line: 17.6 months (95% CI 10.2-NE), second-line: 7.3 months (95% CI 6.1–10.8).
Of 32 enrolled patients.
Physician’s choice of single agent chemotherapy; capecitabine, eribulin, gemcitabine, or vinorelbine.
CI, confidence interval; HR, hazard ratio; NE, not estimable; NS, not significant; NYR, not yet reported; PD-L1, programmed cell death ligand-1; TNBC, triple-negative breast cancer.
ICIs plus chemotherapy combinations
Efficacy of first-line therapy
The activity of atezolizumab in combination with nab-paclitaxel was reported in a phase I and phase III trial in metastatic TNBC (Table 2).40,42 The phase I GP28328 study investigated this regimen in 33 patients, showing an ORR of 39.4% (95% CI 22.9–57.9) in patients overall and a median DOR of 9.1 months (95% CI 2.0–20.9 months), with a subgroup analysis showing higher ORRs in first-line (53.8%) compared with later lines (30.0%) of therapy.42 These findings formed the basis for a large phase III IMpassion 130 trial, which randomized patients to receive first-line atezolizumab (n = 451) or placebo (n = 451) plus nab-paclitaxel in both arms until progressive disease or intolerable toxicity. The coprimary endpoints of the trial were PFS and OS in both ITT and PD-L1-positive populations. At a median follow up of 12.9 months, a significant improvement in median PFS was observed in the atezolizumab versus placebo arm in both the ITT (7.2 versus 5.5 months, HR 0.80, 95% CI 0.69–0.92, p = 0.002) and in patients with PD-L1-positive disease (n = 369, 7.5 versus 5.0 months, stratified HR 0.62, 95% CI 0.49–0.78, p < 0.001).40 Median OS was not significantly improved in the ITT population for atezolizumab compared with placebo either at a median follow up of 12.9 months (21.3 versus 17.6 months, HR 0.84, 95% CI, 0.69–1.02, p = 0.08) or 18.0 months (21.0 versus 18.7 months, HR 0.86, 95% CI 0.72–1.02, p = 0.078).40,50 In the stratified PD-L1-positive subgroup, median OS was numerically higher in the atezolizumab compared with placebo arm (25.0 versus 18.0 months, HR 0.71, 95% CI 0.54–0.93 with a CI that did not cross unity) at a median follow up of 18.0 months.50 ORRs were 56.0% versus 45.9% in the atezolizumab versus placebo group [odds ratio (OR) 1.52, 95% CI 1.16–1.97, p = 0.002 although nonsignificant based on prespecified criteria of p < 0.001], with a median DOR of 7.4 versus 5.6 months (HR 0.78, 95% CI 0.63–0.98) in the atezolizumab/nab-paclitaxel versus placebo/nab-paclitaxel groups, respectively.40
Table 2.
Efficacy of immune checkpoint inhibitor plus chemotherapy combinations in advanced TNBC. Efficacy outcomes of immune checkpoint inhibitor trials in TNBC ordered by setting then size of trial.
| Trial | Setting Line of treatment |
Regimen(s) | n | Overall response rate, % (95% CI) |
Median duration of response, months (95% CI) |
Median progression free survival, months (95% CI) |
Median overall survival, months (95% CI) |
|---|---|---|---|---|---|---|---|
| GP28328
Phase Ib42 |
PD-L1 unselected 39.4% first-line |
Atezolizumab + nab-paclitaxel | 33 | 39.4 (22.9–57.9) |
9.1 (2.0–20.9) |
5.5 (5.1–7.7) |
14.7 (10.1-NE) |
| IMpassion 130
Phase III40 |
PD-L1 unselected first-line |
Atezolizumab + nab-paclitaxel | 451 | 56.0 (51.3–60.6) |
7.4 (6.9–9.0) |
7.2 HR 0.80 (0.69–0.92) p = 0.002 |
21.3 HR 0.84 (0.69–1.02) p = 0.08 |
| Placebo + nab-paclitaxel |
451 | 45.9 (41.2–50.6) |
5.6 (5.5–6.0) |
5.5 | 17.6 |
CI, confidence interval; HR, hazard ratio; NE, not estimable; PD-L1, programmed cell death ligand-1; TNBC, triple-negative breast cancer.
Safety of immune checkpoint inhibitors
Five trials reported on ICIs as monotherapy in advanced TNBC.41,43–48 The phase I JAVELIN45 and PCD4989g46 trials reported low rates of avelumab and atezolizumab discontinuation due to treatment-related adverse events (TRAEs, 4.8% and 3%, respectively), with similar low rates reported for pembrolizumab in both the first-line (B, 1.2%)43 and previously treated cohort (A, 4.1%) of KEYNOTE-086.44 In KEYNOTE-119, adverse events (AEs) of any cause led to discontinuation of therapy in 4.5% and 5.5% of patients and grade 3/5 immune-mediated AEs or infusion reactions occurred in 3.2% and 1.0% of patients receiving pembrolizumab and chemotherapy, respectively.48 Nine deaths due to AEs of any cause occurred in each arm (2.9% versus 3.1%) in patients receiving pembrolizumab versus chemotherapy.
The large phase III IMpassion 130 trial assessed the safety of atezolizumab in combination with nab-paclitaxel in first-line advanced TNBC. Overall toxicity profiles were as expected based on known AEs of each study drug and no new safety signals were identified.40 Rates of discontinuation of any treatment due to AEs (15.9% versus 8.2%) and grade 3/4 TRAEs (39.6% versus 30.1%) were higher in the atezolizumab compared with placebo arms. Three deaths (0.7%, autoimmune hepatitis, mucosal inflammation, and septic shock) in the atezolizumab arm and one (0.2%, hepatic failure) in the placebo group were considered treatment-related.
Discussion
What is the clinical impact of ICIs for advanced TNBC?
Traditionally, new anti-neoplastic agents are first tested in later lines of advanced disease, and adopted for earlier lines of treatment only if proven effective. The current standard of care for metastatic TNBC without a driver mutation (e.g. germline BRCA1/2 mutation) is sequential chemotherapy.19–22 In the first-line setting, a change in clinical practice is warranted if a new treatment improves OS, PFS, or quality of life (QoL) in a phase III trial compared with single agent anthracycline or taxane therapy in patients overall or compared with platinum or PARP-inhibitor therapy in patients with germline BRCA1/2 mutations. IMpassion 130 was the only phase III trial to evaluate the addition of atezolizumab to nab-paclitaxel in first-line TNBC. Enrolled patients were stratified based on PD-L1 status [PD-L1 expression on tumor-infiltrating ICs, PD-L1-positive ⩾1% and negative (PD-L1–) <1%] with PFS assessed in the ITT population and in patients with PD-L1-positive tumors. The study used a prespecified hierarchical testing approach so that if the coprimary endpoint of OS was significantly improved for the combination in the ITT population, patients with PD-L1-positive disease (41% of patients) would be formally tested. At the first interim analysis with a median follow up of 12.9 months, the study demonstrated a significant improvement in median PFS with the addition of atezolizumab in both ITT (net 1.7 months, HR 0.80, p = 0.002) and PD-L1-positive patients (net 2.5 months, HR 0.62, p < 0.001).40 The study did not show significantly improved OS in the ITT population at either the first (net 3.7 months, HR 0.84, p = 0.08) or second (median follow-up 18.0 months, net 2.3 months, HR 0.86, p = 0.078) interim analyses.50 Although the design of the IMpassion 130 trial did not support an assessment of significantly improved OS in the PD-L1-positive subpopulation, an exploratory PD-L1-positive subgroup analysis showed a numerical OS benefit in favor of atezolizumab compared with placebo at the first (net 9.5 months, HR 0.62, 95% CI 0.45–0.86) and second (net 7.0 months, HR 0.71, 95% CI 0.54–0.93) interim analyses.50 The benefit, although not statistically significant, was clear, with tight confidence intervals that did not cross unity at either analysis suggesting that the benefit was real and clinically meaningful, leading to accelerated approval by the United States Food and Drug Administration (FDA) on 8 March 2019, and the European Commission on 29 August 2019 for patients with PD-L1-positive metastatic TNBC based on PFS benefit in that population.51 Moreover, the National Comprehensive Cancer Network (NCCN) recently updated their guidelines to include atezolizumab plus nab-paclitaxel in PD-L1-positive metastatic TNBC patients.21 The PFS and OS improvements observed in the PD-L1-positive subgroup of this trial support the use of atezolizumab in combination with nab-paclitaxel as the new potential standard first-line therapy for patients with PD-L1-positive metastatic TNBC.
Approximately 11% of unselected TNBC cases have germline BRCA1/2 mutations,15 and BRCA1-mutated TNBC shows a higher mutational load, more TILs, and increased expression of PD-L1.52 Either PARP-inhibitor therapy or platinum-based chemotherapy are standard treatment in these patients.21 As IMpassion 130 assessed the addition of atezolizumab to nab-paclitaxel, it is unclear how this combination compares to treatment with established therapies such as PARP-inhibitors or platinum chemotherapy. Results from the upcoming phase III IMpassion 132 (ClinicalTrials.gov identifier: NCT03371017) study combining atezolizumab with either gemcitabine-carboplatin or capecitabine alone will determine the benefits of adding atezolizumab to platinum therapy in early relapsing patients (progressing <12 months following early chemotherapy) who were excluded from IMpassion 130.40,53 In the 15% of IMpassion 130 patients who had germline BRCA1/2 mutations, treatment with the combination was associated with a significant PFS benefit only in those with PD-L1+ tumors and a trend toward improved OS, although patient numbers were small.54,55 Research into ICI and PARP-inhibitor combinations is ongoing, with results from the phase I/II TOPACIO/KEYNOTE-162 trial evaluating niraparib plus pembrolizumab in patients with TNBC or ovarian cancer showing higher ORRs in patients with TNBC having germline BRCA1/2 mutations compared with the overall population (ORR 60% versus 28%).56
For second- or later-lines of therapy for metastatic TNBC, a change in clinical practice is warranted if a new treatment improves OS and maintains or improves QoL compared with chemotherapy in a phase III trial. The PD-1-inhibitor pembrolizumab was studied in both a large phase II and a phase III trial.44,48 KEYNOTE-086 (Cohort A) demonstrated a modest ORR (5.3%) and PFS (2.0 months) with a promising OS of 9.0 months in 170 previously treated patients.44 The KEYNOTE-119 phase III trial compared second- or third-line pembrolizumab monotherapy to chemotherapy in patients with metastatic TNBC. There was no OS significant benefit for pembrolizumab compared with chemotherapy in the ITT (net –0.9 months, HR 0.97, 95% CI 0.82–1.15), CPS ⩾1 (net 0.5 months, HR 0.86, p = 0.073), or CPS ⩾ 10 (net 1.1 months, HR 0.78, p = 0.057) populations.48 Pembrolizumab monotherapy is therefore not recommended in this setting.
Are ICI plus chemotherapy combinations for treatment of advanced TNBC safe?
ICIs have been combined with chemotherapy in a number of disease sites.57 In IMpassion 130, the addition of atezolizumab to nab-paclitaxel showed good tolerability compared with the placebo plus nab-paclitaxel arm, with comparable rates of any grade TRAEs (96.5% versus 93.6%), grade 3/4 TRAEs (39.6% versus 30.1%), and death due to TRAEs (0.7% versus 0.2%).40 Rates of discontinuation of any treatment were nearly double with the addition of atezolizumab to nab-paclitaxel (15.9%) compared with placebo plus nab-paclitaxel (8.2%), with the majority of patients in the atezolizumab arm discontinuing nab-paclitaxel compared with atezolizumab (15.9% and 6.4%, respectively) despite prespecified dose reductions protocols to manage nab-paclitaxel toxicities. The higher rates of discontinuation due to toxicity for the atezolizumab arm are reasonable, given that patients on that arm were on treatment longer and had a greater opportunity to develop an AE.
No new AEs of special interest (AESIs) were identified with the addition of atezolizumab in IMpassion 130. Higher rates of any AESIs were seen in the atezolizumab versus placebo arms (57.3% versus 41.8%),40 although this difference was due primarily to any grade immune-related hypothyroidism (17.3% versus 4.3%), which can be easily monitored and managed,58 and none of these events were grade 3/4 or led to treatment discontinuation.40 Low rates of grade 3/4 AESIs were reported in both the atezolizumab and placebo arms (7.5% versus 4.3%, respectively). Two AESI-related hepatic-deaths were reported, including one receiving atezolizumab (autoimmune hepatitis) and one in the placebo plus nab-paclitaxel group (hepatic failure). Immune-related hepatitis was the most common grade 3/4 AESI (5.1% versus 3.0%), and other immune-related grade 3/4 AESIs in the atezolizumab arm included rash (0.9%) in addition to hyperthryoidism, pneumonitis, colitis, adrenal insufficiency, pancreatitis, and diabetes mellitus (0.2% for each). Overall, the addition of atezolizumab to nab-paclitaxel was tolerable, and did not appear to greatly increase toxicities. This combination is considered safe, although close monitoring may be required for select AESIs and clinicians should be alerted to possible hepatic toxicities, which have been shown to be effectively managed with a short course of steroids.59
Who will benefit from ICI plus chemotherapy combinations for advanced TNBC?
Given the heterogeneity of TNBC, biomarkers can be particularly powerful tools for selecting patients for therapy, thereby reducing overall cost of treatment and improving overall safety. Immunogenic subtypes of TNBC contain high levels of TILs, which are an important component of the overall ICs in the tumor microenvironment, and are expected to be more responsive to ICIs.3 PD-L1 is an established biomarker used to guide therapy in other disease settings, including advanced non-small cell lung cancer, urothelial carcinoma, head and neck squamous cell, gastric, esophageal, and cervical cancer.60–62 In other tumor types, common immunohistochemical assays used are the Dako Link48 (Dako Colorado, Inc., Fort Collins, CO, USA) and Ventana Benchmark (Ventana Medical Systems, Oro Valley, AZ, USA), which test PD-L1 expression on TCs alone [pembrolizumab (22C3)] or both TCs and ICs [atezolizumab (SP142)]. In TNBC, atezolizumab studies defined PD-L1 positivity based on ICs alone using the Ventana SP142 assay (⩾1% expression on ICs),40,42,46 whereas the phase II/III pembrolizumab studies defined PD-L1 positivity (CPS⩾1) based on tumor cells as well as lymphocytes and macrophages [(total TCs + ICs)/total TCs × 100] using the 22C3 antibody (pharmDx kit, Agilent, Carpinteria, CA, USA).43,44,48,63 A post hoc substudy of the IMpassion 130 evaluated the predictive capacity of various PD-L1 assays. The 22C3 (CPS ⩾1), SP263 (IC ⩾1%), and SP142 (IC ⩾1%) PD-L1 assays showed a PD-L1-positive prevalence of 81%, 75%, and 46%, respectively, with poor concordance for 22C3 and SP263 relative to SP142.64 Moreover, the SP142 assay was associated with lower HRs for median PFS and OS compared with 22C3 and SP263, indicating a greater predictive capacity for atezolizumab in this setting. Given the specificity of the Ventana SP142 assay (⩾1% expression on ICs), and the FDA approval of this diagnostic test,65 it is recommended to guide atezolizumab and nab-paclitaxel treatment in this setting.66
PD-L1 expression is somewhat predictive of ICI benefit in both monotherapy and combination therapy trials, although earlier phase studies assessing ORR were less consistent with regard to predictive benefit. The phase I PCD4989g study assessed atezolizumab, and indicated a potential correlation with an ORR of 12% (95% CI 6–21) in patients with PD-L1-positive disease (81.3% of patients), and no responses in PD-L1-negative disease (18.8% of patients, ORR 0%, 95% CI 0–17),46 while the phase II KEYNOTE-086 trial cohort A evaluating pembrolizumab indicated a lack of correlation with comparable responses among PD-L1-positive (61.8% of patients, ORR 5.7%, 95% CI 2.4–12.2) and PD-L1-negative (37.6% of patients, ORR 4.7%, 95% CI 1.1–13.4) patients.44 However, the larger phase III trials assessing OS were more clearly indicative of a correlation, although significance was not established due to the trial design limitations. KEYNOTE-119 showed greater OS benefit for pembrolizumab compared with placebo, with increasing levels of PD-L1 expression beginning in patients with CPS ⩾1 (net 0.5 months, HR 0.86, p = 0.073) followed by CPS ⩾10 (net 1.1 months, HR 0.78, p = 0.057) and CPS ⩾20 disease (exploratory, net 2.4 month, HR 0.58, 95% CI 0.38–0.88).48 A similar correlation between PD-L1 expression and OS was seen in IMpassion 130 assessing the addition of atezolizumab to chemotherapy. At a median follow up of 18.0 months, PD-L1-positive patients experienced a 7-month net OS benefit (HR 0.71, 95% CI 0.54–0.93) compared with little-to-no benefit seen in PD-L1-negative patients (net 0.1 months, HR 0.97, 95% CI 0.78–1.20).50 Although PD-L1 expression has some predictive capacity, additional biomarkers are needed to tailor treatment. Biomarkers under investigation include TILs, tumor mutational burden, gene signatures, microsatellite instability, and mismatch repair deficiency.66,67
In addition to PD-L1 expression, treatment history and baseline characteristics can also influence study outcomes. An exploratory analysis of key baseline factors affecting median PFS was performed for both ITT and PD-L1-positive subpopulations of IMpassion 130. Median PFS was longer for the atezolizumab combination in the majority of patient subgroups, with select groups showing more or less benefit compared with the overall population in both analyses. In the small number of patients with lymph node only disease, the atezolizumab PFS benefit was pronounced compared with placebo in both ITT (6.2% of patients, HR 0.44, 95% CI 0.24–0.83) and PD-L1-positive (8.4% of patients, HR 0.31, 95% CI 0.13–0.77) populations.40 A similar benefit was seen in PD-L1-positive patients with locally advanced disease (12.7% of patients, HR 0.44, 95% CI 0.22–0.89). In contrast, the PFS benefit was not very pronounced in the small number of patients with central nervous system (CNS) metastases in both ITT (6.8% of patients, HR 0.86, 95% CI 0.50–1.49) and PD-L1-positive (2.9% of patients, HR 1.40, 95% CI 0.57–3.44) populations. Although patients with bone metastases receiving atezolizumab did not have a very pronounced PFS benefit in the ITT population (31.7% of patients, HR 1.02, 95% CI 0.79–1.31), the benefit was sound in patients with PD-L1-positive disease (27.9% of patients, HR 0.62, 95% CI 0.41–0.95). Based on results reported to date, however, caution should be used when considering subgroup analyses to guide therapy as these exploratory analyses are not powered for significance and do not control for the influence of baseline factors. Moreover, caution should be used when considering combination therapy in patients with an Eastern Cooperative Oncology Group Performance Score (ECOG PS) ⩾2, untreated CNS metastases, and who are ineligible for taxanes, as these patients were not included in the IMpassion 130 study. Taken together, clinicians should undertake an individualized approach to treatment considering clinical characteristics, treatment history, the safety and efficacy of available treatment options, and potential prognostic factors.
What are the next steps?
IMpassion 130 combined the PD-L1 inhibitor atezolizumab with nab-paclitaxel for the first-line treatment of TNBC. To date, this is the only recommended ICI plus chemotherapy combination for first-line disease. However, outcomes from the phase III IMpassion 132 (ClinicalTrials.gov identifier: NCT03371017) and IMpassion 131 trials (ClinicalTrials.gov identifier: NCT03125902), are due this year (Table 3). Findings from these trials will assess whether paclitaxel or non-taxane chemotherapy options such as gemcitabine-carboplatin or capecitabine can replace nab-paclitaxel as a chemotherapy companion to atezolizumab. Moreover, results from the phase III KEYNOTE-355 trial (ClinicalTrials.gov identifier: NCT02819518) will determine whether the PD-1 inhibitor, pembrolizumab, is first safe and then active in combination with either carboplatin and gemcitabine, paclitaxel or nab-paclitaxel for advanced TNBC excluding early relapsing patients (<6 months of adjuvant chemotherapy)68
Table 3.
Efficacy of neoadjuvant immune checkpoint inhibitor plus chemotherapy combinations in TNBC. Efficacy outcomes of immune checkpoint inhibitor trials in TNBC ordered by setting then size of trial.
| Trial | Setting Line of treatment |
Regimen(s) | n | pCR, % (95% CI) |
|---|---|---|---|---|
| I-SPY-2
Bayesian Model Adaptively-Rd Phase II Subset69 |
PD-L1 unselected invasive BC ⩾2.5 cm by exam or ⩾2 cm by imaging |
Pembrolizumab plus paclitaxel followed by AC | 29 | 60a,b
(0.43–0.78)c |
| GeparNuevo
Rd Phase II70 |
Uni- or bilateral invasive cT1b to cT4a-d |
Durvalumab plus nab-paclitaxel followed durvalumab plus AC | 88 | 53.4d
(42.5–61.4) OR = 1.45 (0.80–2.63) p = .23 |
| Placebo plus nab-paclitaxel followed by placebo plus AC | 86 | 44.2d
(33.5–55.3) |
||
| KEYNOTE-522
Phase III71 |
Locally advanced T1c, N1-N2 T2, N0-N2 T3, N0-N2 T4a-d, N0-N2 |
Pembrolizumab plus paclitaxel plus carboplatin followed by pembrolizumab plus ACe | 1,174 | 64.8f
p = .0005 |
| Placebo plus paclitaxel plus carboplatin followed by placebo plus ACe | 51.2f |
Probability that pembrolizumab is superior to control: 99%.
ypT0/is and ypN0.
95% PI.
ypT0 and ypN0.
Patients receive adjuvant pembrolizumab or placebo following surgery.
Assessment of pCR was based on the first 602 patients randomized 2:1 to receive pembrolizumab or placebo.
AC, anthracycline (doxorubicin or epirubicin) plus cyclophosphamide; BC, breast cancer; CI, confidence interval; OR, odds ratio; pCR, pathological complete response; PI, probability interval; Rd, randomized; TNBC, triple-negative breast cancer.
The efficacy of ICIs combined with chemotherapy as neoadjuvant therapy has been explored in three randomized clinical trials69–71 (Table 3). The phase II GeparNuevo trial randomized 174 patients with invasive stage cT1b to cT4a-d TNBC to receive durvalumab or placebo combined with chemotherapy, showing a pathological complete response (pCR) of 53.4% (95% CI 42.5–61.4%) for the addition of durvalumab compared with 44.2% (95% CI 33.5–55.3%) in the placebo arm.70 Interestingly, a subgroup analysis assessing durvalumab timing showed that patients pretreated with a single durvalumab dose (n = 117) had a significantly higher pCR rate compared with those starting durvalumab and chemotherapy together (61.0% versus 37.9%, interaction p = 0.048). The phase II I-SPY 2 trial assessed pembrolizumab plus paclitaxel followed by doxorubicin/cyclophosphamide in 29 patients with high-risk invasive TNBC, reporting an estimated pCR rate of 60% [95% probability interval (PI) 43–78%] compared with 20% for controls (95% PI 6–33%), suggesting a >99% probability that the addition of pembrolizumab was superior to chemotherapy alone in this setting.69 The large phase III KEYNOTE-522 trial randomized 1174 patients with locally advanced PD-L1 unselected TNBC to receive neoadjuvant pembrolizumab or placebo in combination with chemotherapy, showing a statistically significant improvement in pCR for the pembrolizumab versus placebo arms (64.8% versus 51.2%, p = .0005), with an early analysis showing a trend toward greater event-free survival at 18 months with pembrolizumab (91.3% versus 85.3%).72
Additional research into the PD-L1 inhibitors, atezolizumab and avelumab, is underway in both the neoadjuvant and adjuvant settings (Table 4), with readouts from the neoadjuvant IMpassion 031 trial (ClinicalTrials.gov identifier: NCT03197935) expected in September 2020,73 and the adjuvant A-Brave trial (ClinicalTrials.gov identifier: NCT02926196) expected in January 2021.74 Pembrolizumab is also under investigation in the adjuvant setting, with results from the S1418 trial (ClinicalTrials.gov identifier: NCT02954874) expected in May 2026.75
Table 4.
Ongoing phase III clinical trials of immune checkpoint inhibitors in TNBC. Ongoing (trials that are actively recruiting for which efficacy outcomes are not yet available) phase III trials of immune checkpoint inhibitors for first-line disease or earlier as listed at CT.gov on 27 July 2018 ordered by line of therapy and estimated primary completion date.
| PD-(L)1 inhibitor (Target) |
Trial ID (NCT#) |
Patient population | Experimental regimen | Comparator | Primary endpoint(s) | Estimated PCD |
|---|---|---|---|---|---|---|
| Early, neoadjuvant | ||||||
| Atezolizumab (PD-L1) |
IMpassion-031 (NCT03197935) | cT2-cT4, cN0-cN3, cM0 | Atezolizumab plus CT → surgery → atezolizumab | Placebo plus chemotherapy → surgery → observation | pCR | Sept 2020 |
| Atezolizumab (PD-L1) |
NeoTRIPaPDL1 (NCT02620280) |
Locally Advanced | Atezolizumab plus chemotherapy → surgery → chemotherapy | chemotherapy → surgery → chemotherapy | EFS | May 2022 |
| Atezolizumab (PD-L1) |
NSABP B-59/GBG 96-GeparDouze (NCT03281954) |
N0, T2-T3 cN1+ or cN2-N3, T1c/T2-T3 |
Atezolizumab plus chemotherapy → surgery → atezolizumab | Placebo plus chemotherapy → surgery → placebo | pCR, EFS | Dec 2023 |
| Early, adjuvant | ||||||
| Avelumab (PD-L1) |
A-Brave (NCT02926196) |
Nonmetastatic | Avelumab | Observation | DFS | June 2021 |
| Atezolizumab (PD-L1) |
IMpassion-030 (NCT03498716) |
Stage II-III | Atezolizumab plus chemotherapy | Chemotherapy | iDFS | Jan 2022 |
| Pembrolizumab (PD-1) |
S1418 (NCT02954874) |
⩾1 cm residual IBC or >pN1mic | Pembrolizumab | Observation | iDFS | May 2026 |
| Advanced, first line | ||||||
| Atezolizumab (PD-L1) | IMpassion-132 (NCT03371017) |
Recurrent | Atezolizumab plus chemotherapy | Placebo plus chemotherapy | OS | July 2019 |
| Pembrolizumab (PD-1) |
KEYNOTE-355 (NCT02819518) |
Unresectable Stage III-IV | Pembrolizumab plus chemotherapy | Chemotherapy | PFS, OS | Dec 2019 |
| JS-001 (PD-1) |
KEYSTONE (NCT03777579) |
Stage IV | JS-001 plus nab-paclitaxel | Placebo plus nab-paclitaxel | PFS | Dec 2019 |
| Atezolizumab (PD-L1) |
IMpassion-131 (NCT03125902) |
Unresectable Stage III-IV | Atezolizumab plus paclitaxel | Placebo plus paclitaxel | PFS | Jan 2020 |
DFS, disease-free survival; EFS, event-free survival; IBC, invasive breast cancer; iDFS, invasive disease-free survival; NCT#, ClinicalTrials.gov identifier; OS, overall survival, pCR, pathological complete response; PCD, primary completion date; PD-1, programmed cell death receptor-1; PD-L1, programmed cell death ligand-1; PFS, progression-free survival; →, followed by; TNBC, triple-negative breast cancer.
Summary
Results from the IMpassion 130 trial support the use of ICIs combined with nab-paclitaxel as an effective therapy for PD-L1-positive TNBC based on significant PFS improvements and a numerical increase in OS, leading to the approval of this regimen in both the United States and Europe. The addition of atezolizumab to chemotherapy was considered safe, with no new safety signals reported. Use should be restricted to PD-L1-positive patients, and used with caution in patients with an ECOG PS⩾2, in those with untreated CNS metastases, or in patients ineligible for taxanes as data in these patients is limited. Research into additional combinations with chemotherapy, the use of other ICI combinations, as well as use in earlier lines of therapy for TNBC is ongoing.
Supplemental Material
Supplemental material, PubMed_Electronic_Search_-_Supplement for Positive progress: current and evolving role of immune checkpoint inhibitors in metastatic triple-negative breast cancer by Christine E. Simmons, Christine Brezden-Masley, Joy McCarthy, Deanna McLeod and Anil Abraham Joy in Therapeutic Advances in Medical Oncology
Acknowledgments
We would like to thank Stacy Grieve, Paul Card, and Emily Grydziuszko of Kaleidoscope Strategic Inc. for their research, editorial, and administrative support, as well as Roche Canada, Merck Canada Inc., Pfizer Canada and Eli Lilly Canada Inc. for their support.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for this review was provided through unrestricted educational grants from Roche Canada, Merck Canada Inc., Pfizer Canada, and Eli Lilly Canada Inc. No discussion or viewing of review content was permitted with sponsors at any stage of review development.
Conflict of interest statement: Christine E. Simmons has received research funding from Roche, Merck, Lilly, Pfizer, and Amgen.
Christine Brezden-Masley has worked in a consultant/advisory role for Amgen, Roche, Pfizer, Eli Lilly, Novartis, and Astra Zeneca, has received Honoraria/Speaker Bureau/Travel funds from Amgen, Astra Zeneca, Pfizer, Roche, Novartis, and Eli Lilly, and has received research funding from Eli Lilly, Amgen, Roche, Pfizer, Novartis, and Astra Zeneca.
Joy McCarthy has received Honoraria/Speaker Bureau/Travel funds from Pfizer, Merck, Genomic Health, and Ipsen, and has received research funding from Astra Zeneca.
Deanna McLeod has nothing to disclose.
Anil A. Joy has worked in a consultant/advisory role for AstraZeneca, BMS, and Roche.
Disclaimer: This review was prepared according to ICMJE standards with editorial assistance from Kaleidoscope Strategic Inc.
ORCID iD: Christine E. Simmons  https://orcid.org/0000-0002-6571-4587
https://orcid.org/0000-0002-6571-4587
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Christine E. Simmons, Division of Medical Oncology, BC Cancer Agency–Vancouver, 600 West 10th Avenue, Vancouver, British Columbia, V5Z 4E6, Canada.
Christine Brezden-Masley, Mount Sinai Hospital, Toronto, ON, Canada.
Joy McCarthy, Dr H. Bliss Murphy Cancer Centre, St. John’s, Newfoundland, Canada.
Deanna McLeod, Kaleidoscope Strategic Inc., Toronto, ON, Canada.
Anil Abraham Joy, Cross Cancer Institute, Edmonton, Alberta, Canada.
References
- 1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 2. National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) program. Cancer stat facts: female breast cancer, https://seer.cancer.gov/statfacts/html/breast.html (accessed 20 June 2019).
- 3. Denkert C, Liedtke C, Tutt A, et al. Molecular alterations in triple-negative breast cancer-the road to new treatment strategies. Lancet 2017; 389: 2430–2442. [DOI] [PubMed] [Google Scholar]
- 4. Metzger-Filho O, Tutt A, de Azambuja E, et al. Dissecting the heterogeneity of triple-negative breast cancer. J Clin Oncol 2012; 30: 1879–1887. [DOI] [PubMed] [Google Scholar]
- 5. Bianchini G, Balko JM, Mayer IA, et al. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol 2016; 13: 674–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fallahpour S, Navaneelan T, De P, et al. Breast cancer survival by molecular subtype: a population-based analysis of cancer registry data. CMAJ Open. 2017; 5: E734–E739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Malorni L, Shetty PB, De Angelis C, et al. Clinical and biologic features of triple-negative breast cancers in a large cohort of patients with long-term follow-up. Breast Cancer Res Treat 2012; 136: 795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lehmann BD, Bauer JA, Chen X, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 2011; 121: 2750–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carey LA, Dees EC, Sawyer L, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007; 13: 2329–2334. [DOI] [PubMed] [Google Scholar]
- 10. Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A 2001; 98: 10869–10874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang RX, Chen S, Jin X, et al. Value of Ki-67 expression in triple-negative breast cancer before and after neoadjuvant chemotherapy with weekly paclitaxel plus carboplatin. Sci Rep 2016; 6: 30091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Prat A, Adamo B, Cheang MC, et al. Molecular characterization of basal-like and non-basal-like triple-negative breast cancer. Oncologist 2013; 18: 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Burstein MD, Tsimelzon A, Poage GM, et al. Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin Cancer Res 2015; 21: 1688–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shah SP, Roth A, Goya R, et al. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature 2012; 486: 395–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Couch FJ, Hart SN, Sharma P, et al. Inherited mutations in 17 breast cancer susceptibility genes among a large triple-negative breast cancer cohort unselected for family history of breast cancer. J Clin Oncol 2015; 33: 304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu Z, Li M, Jiang Z, et al. A comprehensive immunologic portrait of triple-negative breast cancer. Transl Oncol 2018; 11: 311–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gennari A, Stockler M, Puntoni M, et al. Duration of chemotherapy for metastatic breast cancer: a systematic review and meta-analysis of randomized clinical trials. J Clin Oncol 2011; 29: 2144–2149. [DOI] [PubMed] [Google Scholar]
- 18. Erdkamp F, Claessens A, Lopez-Yurda M, et al. 158P_PR Intermittent versus continuous chemotherapy beyond first-line for patients with HER2-negative advanced breast cancer (BOOG 2010-02). Ann Oncol. 2019; 30: mdz100009. [Google Scholar]
- 19. Cardoso F, Senkus E, Costa A, et al. 4th ESO-ESMO International consensus guidelines for advanced breast cancer (ABC 4)†. Ann Oncol. 2018; 29: 1634–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Partridge AH, Rumble RB, Carey LA, et al. Chemotherapy and targeted therapy for women with human epidermal growth factor receptor 2–negative (or unknown) advanced breast cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2014; 32: 3307–3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Breast Cancer. Version 1.2019 — March 14, 2019, https://www.nccn.org/professionals/physician_gls/recently_updated.aspx (accessed 24 June 2019).
- 22. National Institute for Health and Care Excellence (NICE). Advanced breast cancer: diagnosis and treatment, https://www.nice.org.uk/guidance/cg81 (accessed 11 July 2019). [PubMed]
- 23. Mougalian SS, Copher R, Kish JK, et al. Clinical benefit of treatment with eribulin mesylate for metastatic triple-negative breast cancer: Long-term outcomes of patients treated in the US community oncology setting. Cancer Med 2018; 7: 4371–4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Twelves C, Cortes J, Vahdat L, et al. Efficacy of eribulin in women with metastatic breast cancer: a pooled analysis of two phase 3 studies. Breast Cancer Res Treat 2014; 148: 553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. PDQ® Supportive and Palliative Care Editorial Board. Planning the transition to end-of-life care in advanced cancer (PDQ®). PDQ Cancer Information Summaries [Internet]. National Cancer Institute, 2014. [Google Scholar]
- 26. Robson M, Im SA, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med 2017; 377: 523–533. [DOI] [PubMed] [Google Scholar]
- 27. Litton JK, Rugo HS, Ettl J, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med 2018; 379: 753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tutt A, Tovey H, Cheang MCU, et al. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: the TNT Trial. Nat Med 2018; 24: 628–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bense RD, Sotiriou C, Piccart-Gebhart MJ, et al. Relevance of tumor-infiltrating immune cell composition and functionality for disease outcome in breast cancer. J Natl Cancer Inst 2016; 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Katz H, Alsharedi M. Immunotherapy in triple-negative breast cancer. Med Oncol 2017; 35: 13. [DOI] [PubMed] [Google Scholar]
- 31. Li Z, Qiu Y, Lu W, et al. Immunotherapeutic interventions of triple negative breast cancer. J Transl Med 2018; 16: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Harano K, Wang Y, Lim B, et al. Rates of immune cell infiltration in patients with triple-negative breast cancer by molecular subtype. PLoS One 2018; 13: e0204513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kitano A, Ono M, Yoshida M, et al. Tumour-infiltrating lymphocytes are correlated with higher expression levels of PD-1 and PD-L1 in early breast cancer. ESMO Open 2017; 2: e000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fournier C, Rivera Vargas T, Martin T, et al. Immunotherapeutic properties of chemotherapy. Curr Opin Pharmacol 2017; 35: 83–88. [DOI] [PubMed] [Google Scholar]
- 35. Harris SJ, Brown J, Lopez J, et al. Immuno-oncology combinations: raising the tail of the survival curve. Cancer Biol Med 2016; 13: 171–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lin EP-Y, Yang C-Y, Lin C-W, et al. Priming PD-L1 expression by chemotherapeutic agents in non-small cell lung cancers. J Clin Oncol. 2017; 35: e20087. [Google Scholar]
- 37. Adams S, Gatti-Mays ME, Kalinsky K, et al. Current landscape of immunotherapy in breast cancer: a review. JAMA Oncol. Epub ahead of print 11 April 2019. DOI: 10.1001/jamaoncol.2018.7147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Heimes AS, Schmidt M. Atezolizumab for the treatment of triple-negative breast cancer. Expert Opin Investig Drugs 2019; 28: 1–5. [DOI] [PubMed] [Google Scholar]
- 39. Polk A, Svane IM, Andersson M, et al. Checkpoint inhibitors in breast cancer - current status. Cancer Treat Rev 2018; 63: 122–134. [DOI] [PubMed] [Google Scholar]
- 40. Schmid P, Adams S, Rugo HS, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 2018; 379: 2108–2121. [DOI] [PubMed] [Google Scholar]
- 41. Press Release: Merck Provides Update on Phase 3 KEYNOTE-119 Study of KEYTRUDA® (pembrolizumab) monotherapy in previously-treated patients with metastatic triple-negative breast cancer, https://investors.merck.com/news/press-release-details/2019/Merck-Provides-Update-on-Phase-3-KEYNOTE-119-Study-of-KEYTRUDA-pembrolizumab-Monotherapy-in-Previously-Treated-Patients-with-Metastatic-Triple-Negative-Breast-Cancer/default.aspx. (accessed 19 July 2019).
- 42. Adams S, Diamond JR, Hamilton E, et al. Atezolizumab plus nab-paclitaxel in the treatment of metastatic triple-negative breast cancer with 2-year survival follow-up: a phase 1b clinical trial. JAMA Oncol 2019; 5: 334–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Adams S, Loi S, Toppmeyer D, et al. Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: cohort B of the phase II KEYNOTE-086 study. Ann Oncol 2018; 30: 405–411. [DOI] [PubMed] [Google Scholar]
- 44. Adams S, Schmid P, Rugo H, et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Ann Oncol 2018; 30: 397–404. [DOI] [PubMed] [Google Scholar]
- 45. Dirix LY, Takacs I, Jerusalem G, et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN Solid Tumor study. Breast Cancer Res Treat 2018; 167: 671–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Emens LA, Cruz C, Eder JP, et al. Long-term clinical outcomes and biomarker analyses of atezolizumab therapy for patients with metastatic triple-negative breast cancer: a phase 1 study. JAMA Oncol 2019; 5: 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nanda R, Chow LQ, Dees EC, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 study. J Clin Oncol 2016; 34: 2460–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cortés J, Lipatov O, Im S, et al. KEYNOTE-119: Phase III study of pembrolizumab (pembro) versus single-agent chemotherapy (chemo) for metastatic triple negative breast cancer (mTNBC). Presented at the European Society of Medical Oncology (ESMO) Congress, September 27–October 1, 2019, Barcelona, Spain. Abstract LBA21. [Google Scholar]
- 49. ClinicalTrials.gov. Nivolumab after induction treatment in Triple-negative Breast Cancer (TNBC) patients (TONIC), https://clinicaltrials.gov/ct2/show/NCT02499367 (accessed 25 November 2019).
- 50. Schmid P, Adams S, Rugo HS, et al. IMpassion130: updated overall survival (OS) from a global, randomized, double-blind, placebo-controlled, Phase III study of atezolizumab (atezo)+ nab-paclitaxel (nP) in previously untreated locally advanced or metastatic triple-negative breast cancer (mTNBC). J Clin Oncol 2019; 37(Suppl. 15): 1003. [Google Scholar]
- 51. Media Release. European Commission approves Roche’s Tecentriq in combination with Abraxane for people with PD-L1-positive, metastatic triple-negative breast cancer, https://www.roche.com/media/releases/med-cor-2019-08-29.htm (accessed 25 November 2019).
- 52. Nolan E, Savas P, Policheni AN, et al. Combined immune checkpoint blockade as a therapeutic strategy for BRCA1-mutated breast cancer. Sci Transl Med 2017; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. ClinicalTrials.gov. A study of the efficacy and safety of atezolizumab plus chemotherapy for patients with early relapsing recurrent triple-negative breast cancer (IMpassion132), https://clinicaltrials.gov/ct2/show/NCT03371017 (accessed 30 August 2019).
- 54. Emens LA, Loi S, Rugo HS, et al. IMpassion130: efficacy in immune biomarker subgroups from the global, randomized, double-blind, placebo-controlled, phase III study of atezolizumab+ nab-paclitaxel in patients with treatment-naïve, locally advanced or metastatic triple-negative breast cancer. Presented at the San Antonio Breast Cancer Symposium, 5 December 2018. Abstract GS1-04. [Google Scholar]
- 55. The ASCO Post. IMpassion130 substudy: Atezolizumab/nab-paclitaxel survival benefits limited to PD-L1–positive triple-negative breast cancer, https://www.ascopost.com/issues/march-25-2019/impassion130-substudy/ (accessed 30 August 2019).
- 56. Vinayak S, Tolaney S, Schwartzberg L, et al. Abstract PD5-02: Durability of clinical benefit with niraparib+ pembrolizumab in patients with advanced triple-negative breast cancer beyond BRCA: (TOPACIO/Keynote-162). AACR, 2019. [Google Scholar]
- 57. Yan Y, Kumar AB, Finnes H, et al. Combining immune checkpoint inhibitors with conventional cancer therapy. Front Immunol 2018; 9: 1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Illouz F, Briet C, Cloix L, et al. Endocrine toxicity of immune checkpoint inhibitors: essential crosstalk between endocrinologists and oncologists. Cancer Med 2017; 6: 1923–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lleo A, Rimassa L, Colombo M. Hepatotoxicity of immune check point inhibitors: approach and management. Dig Liver Dis 2019; 51: 1074–1078. [DOI] [PubMed] [Google Scholar]
- 60. FDA.gov. TECENTRIQ® (atezolizumab), https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761034s010lbl.pdf (accessed 8 August 2019).
- 61. Yi M, Jiao D, Xu H, et al. Biomarkers for predicting efficacy of PD-1/PD-L1 inhibitors. Mol Cancer 2018; 17: 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. FDA.gov. KEYTRUDA® (pembrolizumab). https://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf (accessed 8 August 2019).
- 63. Kulangara K, Zhang N, Corigliano E, et al. Clinical Utility of the combined positive score for programmed death ligand-1 expression and the approval of pembrolizumab for treatment of gastric cancer. Arch Pathol Lab Med 2019; 143: 330–337. [DOI] [PubMed] [Google Scholar]
- 64. Rugo H, Loi S, Adams S, et al. LBA20 performance of PD-L1 immunohistochemistry (IHC) assays in unresectable locally advanced or metastatic triple-negative breast cancer (mTNBC): post-hoc analysis of IMpassion130. Ann Oncol 2019; 30: mdz394009. [Google Scholar]
- 65. FDA.gov. FDA approves atezolizumab for PD-L1 positive unresectable locally advanced or metastatic triple-negative breast cancer, https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-atezolizumab-pd-l1-positive-unresectable-locally-advanced-or-metastatic-triple-negative (accessed 6 August 2019). [DOI] [PubMed]
- 66. Cyprian FS, Akhtar S, Gatalica Z, et al. Targeted immunotherapy with a checkpoint inhibitor in combination with chemotherapy: a new clinical paradigm in the treatment of triple-negative breast cancer. Bosn J Basic Med Sci 2019; 19: 227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Marra A, Viale G, Curigliano G. Recent advances in triple negative breast cancer: the immunotherapy era. BMC Med 2019; 17: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. ClinicalTrials.gov. Study of pembrolizumab (MK-3475) plus chemotherapy vs. placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple negative breast cancer (MK-3475-355/KEYNOTE-355), https://clinicaltrials.gov/ct2/show/NCT02819518 (accessed 9 August 2019).
- 69. Nanda R, Liu MC, Yau C, et al. Pembrolizumab plus standard neoadjuvant therapy for high-risk breast cancer (BC): results from I-SPY 2. J Clin Oncol 2017; 35(Suppl. 15): abstract 506. [Google Scholar]
- 70. Loibl S, Untch M, Burchardi N, et al. A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple negative breast cancer–clinical results and biomarker analysis of GeparNuevo study. Ann Oncol 2019; 30: 1279–1288. [DOI] [PubMed] [Google Scholar]
- 71. Merck’s KEYTRUDA® (pembrolizumab) in combination with chemotherapy met primary endpoint of pathological complete response (pCR) in pivotal phase 3 KEYNOTE-522 trial in patients with triple-negative breast cancer (TNBC), https://investors.merck.com/news/press-release-details/2019/Mercks-KEYTRUDA-pembrolizumab-in-Combination-with-Chemotherapy-Met-Primary-Endpoint-of-Pathological-Complete-Response-pCR-in-Pivotal-Phase-3-KEYNOTE-522-Trial-in-Patients-with-Triple-Negative-Breast-Cancer-TNBC/default.aspx (accessed 31 July 2019).
- 72. evaluate.com: Esmo 2019 – Keynote-522 stokes adjuvant hopes for Keytruda, https://www.evaluate.com/vantage/articles/events/conferences/esmo-2019-keynote-522-stokes-adjuvant-hopes-keytruda (accessed 7 October 2019).
- 73. ClinicalTrials.gov. A study to investigate atezolizumab and chemotherapy compared with placebo and chemotherapy in the neoadjuvant setting in participants with early stage triple negative breast cancer (IMpassion031). https://clinicaltrials.gov/ct2/show/NCT03197935 (accessed 26 November 2019).
- 74. ClinicalTrials.gov. Adjuvant treatment for high-risk triple negative breast cancer patients with the anti-PD-l1 antibody avelumab (A-Brave), https://clinicaltrials.gov/ct2/show/NCT02926196 (accessed 26 November 2019).
- 75. ClinicalTrials.gov. Pembrolizumab in treating patients with triple-negative breast cancer, https://clinicaltrials.gov/ct2/show/NCT02954874 (accessed 26 November 2019).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, PubMed_Electronic_Search_-_Supplement for Positive progress: current and evolving role of immune checkpoint inhibitors in metastatic triple-negative breast cancer by Christine E. Simmons, Christine Brezden-Masley, Joy McCarthy, Deanna McLeod and Anil Abraham Joy in Therapeutic Advances in Medical Oncology