Abstract
Purpose:
Medullary thyroid carcinoma is a rare endocrine malignancy; 75% of patients with this disease have sporadic medullary thyroid carcinoma. While surgery is the only curative treatment, the benefit of prophylactic lateral neck dissection is unclear. This study aimed to analyze the clinicopathological risk factors associated with lateral lymph node metastases and determine the indication for prophylactic lateral neck dissection in patients with sporadic medullary thyroid carcinoma.
Methods:
The medical records of patients with medullary thyroid carcinoma who were treated at our hospital between January 2002 and January 2020 were retrospectively reviewed; a database of their demographic characteristics, test results, and pathological information was constructed. The relationship between lateral lymph node metastases and clinicopathologic sporadic medullary thyroid carcinoma features were analyzed using univariate and multivariate analyses.
Results:
Overall, 125 patients with sporadic medullary thyroid carcinoma were included; 47.2% and 39.2% had confirmed central and lateral lymph node metastases, respectively. Univariate and multivariate analyses identified 2 independent factors associated with lateral lymph node metastases: positive central lymph node metastases (odds ratio = 9.764, 95% confidence interval: 2.610–36.523; p = 0.001) and positive lateral lymph nodes on ultrasonography (odds ratio = 101.747, 95% confidence interval: 14.666–705.869; p < 0.001).
Conclusion:
Medullary thyroid carcinoma is a rare endocrine malignancy. Lymph node metastases are common in patients with sporadic medullary thyroid carcinoma. Prophylactic lateral neck dissection is recommended for patients who exhibit positive central lymph node metastases and/or positive lateral lymph nodes on ultrasonography.
Keywords: risk factor, central lymph node, lateral lymph node, metastases, sporadic medullary thyroid carcinoma
Introduction
Thyroid carcinoma is the most common endocrinological malignancy, and its prevalence has increased worldwide in the past several decades. In the United States, thyroid carcinoma was the fifth most common carcinoma among women in 2015,1 and in China, more than 90,000 patients were diagnosed with thyroid carcinoma in 2015.2 Medullary thyroid carcinoma (MTC) is rare and only accounts for 1–2% of all thyroid cancers.3 Cancer development originates in the parafollicular C cells and can cause a series of neuroendocrine symptoms such as flushing, diarrhea, and heart palpitations. MTCs can be classified as sporadic (SMTC) and hereditary. The hereditary form includes 3 subtypes: multiple endocrine neoplasia type 2A (MEN 2A), MEN 2B, and familial non-MEN MTC. Although surgery is uniformly recommended to treat various types of MTC, the clinicopathological characteristics of SMTC and hereditary MTC are distinct.4,5
Presently, the American Thyroid Association Guidelines, the United Kingdom National Multidisciplinary Guidelines for the management of thyroid cancer, and several different individual investigators all recommend total thyroidectomy and central lymph node dissection for patients with MTC.3,4,6,7 However, the benefit of prophylactic lateral lymph node dissection remains controversial. Therefore, it is very important to identify the risk factors for lateral lymph node metastases (LNM) in patients with MTC. As SMTCs account for approximately 75% of MTCs,3,8 the aim of this study was to identify the potential clinicopathological risk factors associated with lateral LNM in order to better determine the indication for prophylactic lateral lymph node dissection in patients with SMTC.
Materials and Methods
Ethics
The study was approved by the Peking Union Medical College Hospital Institutional Review Board (S-K1282). All patients or their legal guardians provided written informed consent for the surgical procedures performed. The requirement of informed consent for the publication of data was waived owing to the retrospective nature of the study.
Patients
The medical records of patients with MTC treated at our hospital between January 2002 and January 2020 were retrospectively reviewed. Patients were selected based on the following inclusion criteria: (i) MTC diagnosed via postoperative pathology, (ii) patients who underwent surgery at our hospital, and (iii) complete medical records. The exclusion criteria were (i) patients diagnosed with hereditary MTC and (ii) patients who had undergone primary surgery at other hospitals before they were transferred to our institution for radical repeat-surgery but could not provide detailed pathological information on the primary surgery (those who could provide it were not excluded). Clinical data were compiled from both outpatient and inpatient medical records by 2 independent surgeons; any disagreements between them were resolved by discussion. A retrospective database containing demographic characteristics, laboratory tests, ultrasonography outcomes, and pathological results was constructed for analysis.
Treatment
All patients underwent preoperative ultrasonographic examination performed by least 2 different experienced sonographers. Fine-needle aspiration cytology of the thyroid lesion was recommended but not required. All patients were tested for thyroid function and autoantibodies. The surgical procedures performed included a total thyroidectomy with central lymph node dissection in patients who underwent primary surgery at our hospital, and a residual lobectomy with central lymph node dissection in those who had undergone primary surgery at other hospitals before transferring to ours. Lateral lymph node dissection was performed in patients with suspected central LNM or clinically positive lateral lymph nodes on ultrasonography. The extent of lateral lymph node dissection included levels II-V. Suspected central LNM was defined as positive central lymph nodes on preoperative ultrasonography or swelling and hard lymph nodes identified intraoperatively. In order to improve the intraoperative diagnosis rate of central LNM, intraoperative frozen sections were used for assessment of the central lymph nodes in recent years. Postoperative pathological results were diagnosed and confirmed by at least 2 different experienced pathologists. A tumor was considered multifocal if at least 2 foci were discovered in bilateral or unilateral lobes. The major tumor size was defined as the diameter of the largest lesion. The total tumor size was defined as the sum of the diameters of all lesions. For patients who did not undergo lateral neck dissection, ultrasonography was performed 3 and 6 months after surgery to confirm the lack of positive lateral LNM. Computed tomography was not routinely performed preoperatively but would be used to rule out lung metastases during follow-up.
Sonography
High-resolution ultrasound examinations of the thyroid and cervical lymph nodes were performed in all patients with Phillips IU22 (Philips Healthcare, Eindhoven, Netherlands), GELogiq9 (GE Healthcare, Milwaukee, WI, USA) scanners equipped with a 5 to 12 MHz linear array transducer. Patients were in a supine position during examination. All tests were performed by sonographers with more than 3 years of experience in thyroid imaging. The nodule location, size, number, margin, cystic appearance, echogenicity, calcification, and vascularity were evaluated.
The sonographic features of MTC were defined as ill-defined or spiculated margins, hypoechoic, presence of microcalcifications, and hypervascularity. However, these features could also be seen in other thyroid carcinomas. The sonographic features of metastatic lymph nodes were defined as rounded in shape, internal microcalcifications, atypical inclusions, atypical vascular pattern, and intermediately bright internal echostructure without any normal anatomic components.
Statistical Analysis
The Statistical Package for Social Sciences software (version 25.0, IBM Corp., Armonk, NY, USA) was used for our analyses. Categorical variables are shown as an absolute number or frequency. Continuous variables are presented as means ± standard deviations or medians and 25th to 75th percentiles. Differences between study groups were analyzed using the Chi-square test or Student’s t-test as appropriate. Logistic multivariate regression analyses were performed to identify independent risk factors for lateral LNM. A p-value < 0.05 was considered statistically significant.
Results
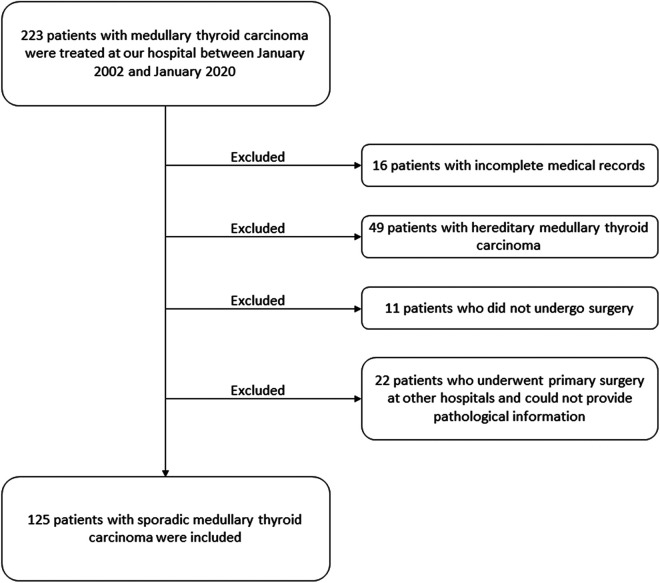
Between January 2002 and January 2020, 223 patients with MTC were treated at our hospital. Ninety-eight patients were excluded because of incomplete medical records (n = 16), presence of hereditary MTC (n = 49), lack of surgical history (n = 11), and because the primary surgery was performed at other hospitals leading to a lack of detailed pathological information (n = 22). One hundred and twenty-five patients were identified (Figure 1) which included 46 men (36.8%) and 79 women (63.2%). Overall, 105 had their primary surgery at our hospital and the other 20 patients were operated on initially at outside hospitals and referred for radical repeat-surgery with detailed pathological reports. The mean age at diagnosis was 48.8 ± 11.2 years (range, 22–76 years). One hundred and eleven patients (88.8%) were asymptomatic and were diagnosed with thyroid nodules during routine health examinations. Only 14 patients (11.2%) had preoperative symptoms such as neck discomfort (n = 7), dysphagia (n = 3), diarrhea (n = 1), heart palpitations (n = 1), dyspnea (n = 1), and hoarseness (n = 1). The clinicopathological characteristics of the 125 patients are listed in Table 1. Fifty-five patients underwent total thyroidectomy with central lymph node dissection, while the remaining 70 underwent total thyroidectomy with central and lateral lymph node dissection. The mean number of lymph nodes removed for the central and lateral dissections were 8.8 ± 6.8 and 25.0 ± 14.4, respectively. The LNM rates in the central and lateral compartments were 47.2% and 39.2%, respectively. Fifty-nine patients had central LNM; the mean number of positive lymph nodes was 3.2 ± 2.6 (range, 1–13), the mean size of positive lymph nodes was 1.5 ± 1.1 cm (range, 0.2–6.0 cm). Forty-nine patients had lateral LNM; the median number of positive lymph nodes was 5.0 (2.0-8.0) and the mean size of positive lymph nodes was 1.6 ± 1.5 cm (range, 0.5–4.5 cm). Lymph node skip metastases (lateral neck metastases without central compartment metastases) were discovered in 10 patients. Of the 59 patients with central LNM, 27, 18, 9, and 5 patients were firstly diagnosed by preoperative ultrasonography, postoperative pathology, frozen sections, and intraoperative clinical assessment, respectively. The characteristic sonographic features of MTC and metastatic lymph nodes are shown in Figure 2. For patients who had negative lateral lymph nodes or did not undergo lateral neck dissection, ultrasonography was performed 3 and 6 months after surgery to exclude subclinical, residual or recurrent lateral LNM. No positive lateral lymph node was found.
Figure 1.
Flow diagram of the selection process of patients included in this study. Forty-two patients had undergone primary surgery at other hospitals before they were transferred to our facility for radical repeat-surgery. Twenty-two of them who lacked detailed pathological information of their primary surgeries were excluded; the remaining 20 could provide their pathological reports and were therefore included.
Table 1.
Clinicopathological Characteristics of the 125 Patients With SMTC.
| Characteristic | Value |
|---|---|
| Sex, male/female (n) | 46/79 |
| Age (years) | 48.8 ± 11.2 |
| BMI (kg/m2) | 23.9 ± 3.3 |
| Major tumor size (cm) | 2.0 ± 1.3 |
| Total tumor size (cm) | 2.2 ± 1.8 |
| Central lymph node metastases (n) | 59 (47.2%) |
| Lateral lymph node metastases (n) | 49 (39.2%) |
| Neutrophil-to-lymphocyte ratio | 2.4 ± 1.4 |
| Platelet-to-lymphocyte ratio | 155.3 ± 87.8 |
SMTC: sporadic medullary thyroid carcinoma; BMI: body mass index.
The continuous variables are presented as the means ± standard deviations.
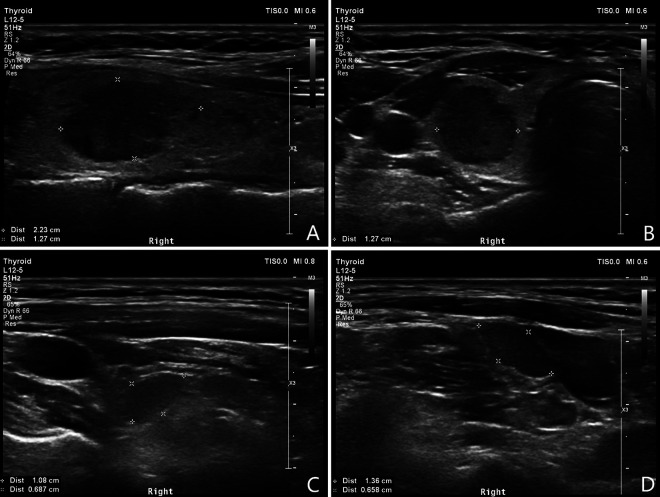
Figure 2.
Characteristic sonographic features of medullary thyroid carcinoma and metastatic lymph nodes. A, Longitudinal ultrasound section of the right thyroid lobe, showing a well-defined hypoechogenic tumor with irregular contours and internal microcalcifications. B, Transverse ultrasound section of the same lesion. C, Central compartment of the same patient, showing ovoid, probably metastatic lymph nodes without hilum. D, Lateral compartment of the same patient, showing ovoid hypoechogenic lesion without echogenic hilum.
Based on postoperative pathology and serial ultrasonography results, patients were divided into 2 groups: lateral LNM positive (n = 49, 39.2%) and lateral LNM negative (n = 76, 60.8%). The demographic data and test results are analyzed and compared between the 2 groups (Table 2). In comparing the lateral LNM positive and negative groups, statistically significant differences were found for the presence of Hashimoto’s thyroiditis, high calcitonin and carcinoembryonic antigen (CEA) levels, and positive lateral lymph nodes on ultrasonography. The pathology results are presented and compared in Table 3. The lateral LNM positive group had significantly higher proportions of patients with capsular invasion, extrathyroidal invasion, and positive central LNM than did the lateral LNM negative groups.
Table 2.
Demographic Data and Test Results of Patients With SMTC.
| Factors | Total (n = 125) | Lateral lymph node metastases | p | |
|---|---|---|---|---|
| (+) (n = 49) | (−) (n = 76) | |||
| Male n(%) | 46 (36.8%) | 23 (46.9%) | 23 (30.1%) | 0.059 |
| Age (years) | 48.8 ± 11.2 | 50.3 ± 9.6 | 47.9 ± 12.2 | 0.210 |
| BMI (kg/m2) | 23.9 ± 3.3 | 23.8 ± 3.4 | 23.9 ± 3.3 | 0.787 |
| Smoking n(%) | 15 (12.0%) | 8 (16.3%) | 7 (9.2%) | 0.232 |
| HT n(%) | 17 (13.6%) | 2 (4.1%) | 15 (19.7%) | 0.013 |
| FT3 (pg/mL) | 3.1 ± 0.4 | 3.0 ± 0.3 | 3.1 ± 0.4 | 0.119 |
| FT4 (ng/dL) | 1.2 ± 0.2 | 1.2 ± 0.2 | 1.2 ± 0.2 | 0.443 |
| TSH (μIU/mL) | 2.3 ± 1.6 | 2.6 ± 1.7 | 2.1 ± 1.5 | 0.100 |
| CT >30 pg/mL n(%) | 36 (28.8%) | 19 (38.8%) | 17 (22.4%) | 0.048 |
| CEA >15 ng/mL n(%) | 27 (21.6%) | 15 (30.6%) | 12 (15.8%) | 0.049 |
| NLR | 2.4 ± 1.4 | 2.2 ± 1.0 | 2.4 ± 1.5 | 0.364 |
| PLR | 155.3 ± 87.8 | 143.9 ± 55.7 | 162.7 ± 103.0 | 0.244 |
| lateral lymph nodes (+) on ultrasonography n(%) | 36 (28.8%) | 34 (69.4%) | 2 (2.6%) | <0.001 |
SMTC: sporadic medullary thyroid carcinoma; BMI: body mass index; HT: Hashimoto’s thyroiditis; FT3: free triiodothyronine; FT4: free thyroxine; TSH: thyroid-stimulating hormone; CT: calcitonin; CEA: carcinoembryonic antigen; NLR: neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio.
The continuous variables are presented as the means ± standard deviations.
Table 3.
Pathology Results of Patients With SMTC.
| Factors | Total (n = 125) | Lateral lymph node metastases | p | |
|---|---|---|---|---|
| (+) (n = 49) | (−) (n = 76) | |||
| Left/right lobe (n) | 65/60 | 25/24 | 40/36 | 0.860 |
| Multifocality n(%) | 20 (16.0%) | 9 (18.4%) | 11 (14.5%) | 0.562 |
| Bilaterality n(%) | 17 (13.6%) | 7 (14.3%) | 10 (13.2%) | 0.857 |
| Major tumor size (cm) | 2.0 ± 1.3 | 2.2 ± 1.6 | 1.8 ± 1.1 | 0.065 |
| Total tumor size (cm) | 2.2 ± 1.8 | 2.5 ± 2.3 | 1.9 ± 1.3 | 0.087 |
| Capsular invasion n(%) | 34 (27.2%) | 22 (44.9%) | 12 (15.8%) | <0.001 |
| Extrathyroidal invasion n(%) | 11 (8.8%) | 8 (16.3%) | 3 (3.9%) | 0.039 |
| Ki67 ≥5% n(%) | 20 (16.0%) | 9 (18.4%) | 11 (14.5%) | 0.562 |
| Positive central LNM n(%) | 59 (47.2%) | 39 (79.6%) | 20 (26.3%) | <0.001 |
SMTC: sporadic medullary thyroid carcinoma; LNM: lymph node metastases.
The continuous variables are presented as the means ± standard deviations.
Multivariate logistic regression analyses were performed to examine associations between select variables and lateral LNM (Table 4). Two variables proved to be independently associated with lateral LNM: positive central LNM (odds ratio [OR] = 9.764, 95% confidence interval [CI]: 2.610–36.523; p = 0.001), and positive lateral lymph nodes on ultrasonography (OR = 101.747, 95% CI: 14.666–705.869; p < 0.001).
Table 4.
Multivariate Analysis of Risk Factors for Lateral LNM Among Patients With SMTC.
| p | OR | 95% CI | |
|---|---|---|---|
| lateral lymph nodes (+) on ultrasonography | <0.001 | 101.747 | 14.666–705.869 |
| positive central LNM | 0.001 | 9.764 | 2.610–36.523 |
| CT >30 pg/mL | 0.120 | ||
| CEA >15 ng/mL | 0.252 | ||
| Hashimoto’s thyroiditis | 0.240 | ||
| Capsular invasion | 0.640 | ||
| Extrathyroidal invasion | 0.873 |
LNM: lymph node metastases; SMTC: sporadic medullary thyroid carcinoma; OR: odds ratio; CI: confidence interval; CT: calcitonin; CEA: carcinoembryonic antigen.
Overall, 36 patients had positive lateral lymph nodes on ultrasonography (Table 2). For the remaining 89 patients with negative lateral lymph nodes on preoperative ultrasonography, select variables were compared between lateral LNM positive and negative groups (Table 5). The lateral LNM positive group had significantly more patients with positive central LNM (p = 0.003).
Table 5.
Clinicopathologic Features of Patients With Negative Lateral Lymph Nodes on Preoperative Ultrasonography.
| Factors | Total (n = 89) | Lateral lymph node metastases | P | |
|---|---|---|---|---|
| (+) (n = 15) | (−) (n = 74) | |||
| Male n(%) | 28 (31.5%) | 7 (46.7%) | 21 (28.4%) | 0.277 |
| Age (years) | 48.5 ± 11.5 | 50.4 ± 7.9 | 48.1 ± 12.1 | 0.371 |
| CT >30 pg/mL n(%) | 18 (20.2%) | 1 (6.7%) | 17 (23.0%) | 0.280 |
| CEA >15 ng/mL n(%) | 15 (16.9%) | 3 (20.0%) | 12 (16.2%) | 1.000 |
| Multifocality n(%) | 14 (15.7%) | 3 (20.0%) | 11 (14.9%) | 0.913 |
| Bilaterality n(%) | 12 (13.5%) | 3 (20.0%) | 9 (12.2%) | 0.692 |
| Total tumor size (cm) | 1.9 ± 1.3 | 1.5 ± 0.9 | 1.9 ± 1.3 | 0.244 |
| Capsular invasion n(%) | 15 (16.9%) | 3 (20.0%) | 12 (16.2%) | 1.000 |
| Extrathyroidal invasion n(%) | 4 (4.5%) | 1 (6.7%) | 3 (4.1%) | 0.529 |
| positive central LNM n(%) | 30 (33.7%) | 10 (66.7%) | 20 (27.0%) | 0.003 |
CT: calcitonin; CEA: carcinoembryonic antigen.
The continuous variables are presented as the means ± standard deviations.
Discussion
The present study found that the rates of central and lateral LNM in SMTC were 47.2% and 39.2%, respectively. Positive central LNM and positive lateral lymph nodes on ultrasonography were found to be independently associated with lateral LNM. As such, we recommend that patients with SMTC who exhibit these risk factors undergo prophylactic lateral compartment dissection during their initial surgery.
The prevalence of MTC as a proportion of all thyroid carcinomas has reportedly decreased in recent years.3 A possible reason is the marked increase in the incidence of papillary thyroid carcinoma as opposed to a decrease in the number of patients with MTC. In contrast, the incidence of MTC in the United States increased by almost 50% between 1983 and 2012.9 MTC has a worse prognosis than papillary thyroid carcinoma and is responsible for approximately 15% of thyroid carcinoma-related deaths.10 The overall effects of chemotherapy and radiotherapy are unsatisfactory, and surgery remains the only curative treatment for MTC.11-13 Cervical LNM have been reported to be significantly associated with local recurrence, distant metastases, and survival.14-16 Therefore, it is critical to determine the appropriate extent of surgery required during the initial operation. Unlike the current consensus on performing total thyroidectomy with central lymph node dissection, the indications for prophylactic lateral lymph node dissection are controversial.
Two predictive factors for lateral LNM in patients with SMTC were discovered in our present study: positive central LNM and positive lateral lymph nodes on ultrasonography. Ultrasonography is the preferred method for examining the thyroid owing to its convenience, reproducibility, and non-intrusiveness. The sensitivities of ultrasonography in terms of identifying MTC in the correct lobe and in diagnosing LNM are 90% and 43%, respectively.17 Compared to thyroid lobes, lymph node scanning can be impeded by gas, bones, and glands18; as such, the sensitivity of ultrasonography is not unsatisfactory. In contrast, the specificity of this method in terms of identifying LNM is as high as 97%,17 which is similar to that of LNM arising from other types of thyroid cancer.19,20 The sonographic features of MTC patients with lateral LNM are significantly different from those without lateral LNM.21 Therefore, the probability of metastases is very high if abnormal lymph nodes are detected on ultrasonography. The presence of central LNM suggests that malignant cells have broken through thyroid gland capsule and entered the lymphatic circulation system. This would increase the possibility of lateral LNM. Tumor cells can be easily transported to the lateral compartment lymph nodes via lymphatic flow along the superior thyroid artery. This may be the reason why positive central LNM becomes an independent predicting factor. Bae et al.22 investigated 93 patients with MTC and revealed that ipsilateral lateral LNM occurred before contralateral central LNM. They recommended ipsilateral lateral lymph node dissection in MTC patients with contralateral central LNM. Machens et al.14 studied 195 patients with MTC and found that the greater the number of positive central lymph nodes, the higher the probability of lateral LNM. Therefore, they recommended prophylactic lateral neck dissection when multiple positive central compartment nodes were present. Our current findings are consistent with those of the previous studies. Accurate preoperative and intraoperative diagnosis of central LNM is very important. Fine-needle aspiration cytology and frozen pathology of central lymph nodes are recommended. The latter was commonly used in our hospital in recent years.
Several other meaningful clinicopathological factors were also investigated in our study, although multivariate analyses found them not to be significantly associated with lateral LNM. These included tumor size, high calcitonin and CEA levels, capsular invasion, neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR). Tumor size is a key characteristic in tumor staging systems; the eighth American Joint Committee on Cancer TNM staging system considers 2 cm and 4 cm as thresholds for staging.23 The United Kingdom National Multidisciplinary Guidelines for the management of thyroid cancer even recommend prophylactic bilateral lateral neck dissection for all patients with T2–T4 tumors.4 Momin et al.24 studied 67 patients with MTC and found that a larger tumor size was a poor prognostic factor; another study also found that larger-sized MTC was a high risk factor for lateral LNM.21 Serum calcitonin is a very sensitive and significant variable that is used for the preoperative diagnosis of MTC as well as postoperative follow-up.25,26 Oh et al.21 reviewed 73 patients with MTC and found that high preoperative calcitonin levels were associated with a higher risk of lateral LNM. Opsahl et al.27 reported that lateral LNM were found in 16%, 50%, and 71% patients at calcitonin levels ≤500, 501–1,000, and >1,000 pmol/L, respectively. In addition to calcitonin, CEA is another powerful biomarker of MTC,28 and several studies found that high CEA levels were suggestive of LNM.6,29 Capsular invasion, which indicates that tumor cells likely penetrated the surrounding tissue, increases the probability of LNM and has been reported to be an independent risk factor for it among patients with MTC.30 Fan et al.30 retrospectively reviewed 65 patients with SMTC and recommended prophylactic lateral compartment dissection in those found to have thyroid capsule invasion or high serum CEA levels. Some researchers also found that capsular invasion is predictive of LNM in other thyroid carcinoma types.31,32 However, our data did not corroborate previous findings, which may be owing to the limited number of patients in our study. Meanwhile, it took approximately a month to test calcitonin in our institution due to the rarity of MTC, and most patients could not wait that long before surgery. So calcitonin level was not well used to plan surgery extent. Many systemic inflammatory markers, including NLR and PLR, have been documented to be prognostic biomarkers in several different cancers.33-35 Jiang et al.36 studied 70 patients with MTC and found that the preoperative PLR was significantly associated with LNM. We also investigated the association between lateral LNM and preoperative NLR and PLR; while we found no significant association, a larger sample size is required to validate our findings.
There were some limitations to this study. First, the registration information, investigated variables, and patient volume could not be planned beforehand owing to the study’s retrospective nature. Second, this was a single-center analysis, and given that the incidence of MTC is very low, the sample size was limited. Third, lateral neck dissection was not performed in all patients, and subclinical lateral LNM may have been present in some of them. Fourth, frozen pathological sections of central lymph nodes were not obtained routinely for assessment. Multicenter and prospective clinical trials should be performed to derive more supporting evidence with greater reliability.
Conclusion
MTC is a rare endocrine malignancy. Most patients were asymptomatic, and only 11.2% had preoperative nonspecific or neuroendocrine-related symptoms. The rates of central and lateral LNM were found to be 47.2% and 39.2%, respectively. Positive central LNM and positive lateral lymph nodes on ultrasonography are independent risk factors for lateral LNM. As such, prophylactic lateral compartment dissection is recommended during the initial surgery for patients with SMTC who exhibit these factors.
Acknowledgments
We wish to thank our colleagues in the Department of Medical Records for their cooperation.
Abbreviations
- CEA
carcinoembryonic antigen
- CI
confidence interval
- LNM
lymph node metastases
- MEN
multiple endocrine neoplasia
- MTC
medullary thyroid carcinoma
- NLR
neutrophil-to-lymphocyte ratio
- OR
odds ratio
- PLR
platelet-to-lymphocyte ratio
- SMTC
sporadic medullary thyroid carcinoma.
Authors’ Note: The study was reviewed and approved by the Peking Union Medical College Hospital Institutional Review Board (S-K1282). The need for informed consent was waived due to the retrospective nature of the study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported by the Fundamental Research Funds for the Central Universities of China 3332019022.
ORCID iD: Xin Wu  https://orcid.org/0000-0002-3839-4768
https://orcid.org/0000-0002-3839-4768
References
- 1. Cabanillas ME, McFadden DG, Durante C. Thyroid cancer. Lancet. 2016;388(10061):2783–2795. [DOI] [PubMed] [Google Scholar]
- 2. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. [DOI] [PubMed] [Google Scholar]
- 3. Wells SA, Jr, Asa SL, Dralle H, et al. Revised American Thyroid Association Guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015;25(6):567–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mitchell AL, Gandhi A, Scott-Coombes D, Perros P. Management of thyroid cancer: United Kingdom national multidisciplinary guidelines. J Laryngol Otol. 2016;130(S2):S150–S160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cherian AJ, Ramakant P, Pai R, et al. Outcome of treatment for medullary thyroid carcinoma—a single centre experience. Indian J Surg Oncol. 2018;9(1):52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maia AL, Siqueira DR, Kulcsar MA, Tincani AJ, Mazeto GM, Maciel LM. Diagnosis, treatment, and follow-up of medullary thyroid carcinoma: recommendations by the thyroid department of the Brazilian Society of Endocrinology and Metabolism. Arq Bras Endocrinol Metabol. 2014;58(7):667–700. [DOI] [PubMed] [Google Scholar]
- 7. Konstantinidis A, Stang M, Roman SA, Sosa JA. Surgical management of medullary thyroid carcinoma. Updates Surg. 2017;69(2):151–160. [DOI] [PubMed] [Google Scholar]
- 8. Rowland KJ, Moley JF. Hereditary thyroid cancer syndromes and genetic testing. J Surg Oncol. 2015;111(1):51–60. [DOI] [PubMed] [Google Scholar]
- 9. Randle RW, Balentine CJ, Leverson GE, et al. Trends in the presentation, treatment, and survival of patients with medullary thyroid cancer over the past 30 years. Surgery. 2017;161(1):137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ahmed SR, Ball DW. Clinical review: incidentally discovered medullary thyroid cancer: diagnostic strategies and treatment. J Clin Endocrinol Metab. 2011;96(5):1237–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ceolin L, Duval MADS, Benini AF, Ferreira CV, Maia AL. Medullary thyroid carcinoma beyond surgery: advances, challenges, and perspectives. Endocr Relat Cancer. 2019;26(9):R499–R518. [DOI] [PubMed] [Google Scholar]
- 12. Call JA, Caudill JS, Mclver B, Foote RL. A role for radiotherapy in the management of advanced medullary thyroid carcinoma: the mayo clinic experience. Rare Tumors. 2013;5(3):e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hadoux J, Schlumberger M. Chemotherapy and tyrosine-kinase inhibitors for medullary thyroid cancer. Best Pract Res Clin Endocrinol Metab. 2017;31(3):335–347. [DOI] [PubMed] [Google Scholar]
- 14. Machens A, Hauptmann S, Dralle H. Prediction of lateral lymph node metastases in medullary thyroid cancer. Br J Surg. 2008;95(5):586–591. [DOI] [PubMed] [Google Scholar]
- 15. Esfandiari NH, Hughes DT, Yin H, Banerjee M, Haymart MR. The effect of extent of surgery and number of lymph node metastases on overall survival in patients with medullary thyroid cancer. J Clin Endocrinol Metab. 2014;99(2):448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jin LX, Moley JF. Surgery for lymph node metastases of medullary thyroid carcinoma: a review. Cancer. 2016;122(3):358–366. [DOI] [PubMed] [Google Scholar]
- 17. Brammen L, Niederle MB, Riss P, et al. Medullary thyroid carcinoma: do ultrasonography and F-DOPA-PET-CT influence the initial surgical strategy? Ann Surg Oncol. 2018;25(13):3919–3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hwang HS, Orloff LA. Efficacy of preoperative neck ultrasound in the detection of cervical lymph node metastasis from thyroid cancer. Laryngoscope. 2011;121(3):487–491. [DOI] [PubMed] [Google Scholar]
- 19. Huang XP, Ye TT, Zhang L, et al. Sonographic features of papillary thyroid microcarcinoma predicting high-volume central neck lymph node metastasis. Surg Oncol. 2018;27(2):172–176. [DOI] [PubMed] [Google Scholar]
- 20. Wu X, Li BL, Zheng CJ, He XD. Predictive factors for central lymph node metastases in papillary thyroid microcarcinoma. World J Clin Cases. 2020;8(8):1350–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Oh HS, Kwon H, Song E, et al. Preoperative clinical and sonographic predictors for lateral cervical lymph node metastases in sporadic medullary thyroid carcinoma. Thyroid. 2018;28(3):362–368. [DOI] [PubMed] [Google Scholar]
- 22. Bae SY, Jung SP, Choe JH, Kim JS, Kim JH. Prediction of lateral neck lymph node metastasis according to preoperative calcitonin level and tumor size for medullary thyroid carcinoma. Kaohsiung J Med Sci. 2019;35(12):772–777. [DOI] [PubMed] [Google Scholar]
- 23. Rosen JE, Lloyd RV, Brierley JD, et al. Thyroid—medullary In: Amin MB, ed. AJCC Cancer Staging Manual. 8th ed Springer; 2017:891–901. [Google Scholar]
- 24. Momin S, Chute D, Burkey B, Scharpf J. Prognostic variables affecting primary treatment outcome for medullary thyroid cancer. Endocr Pract. 2017;23(9):1053–1058. [DOI] [PubMed] [Google Scholar]
- 25. Elisei R, Bottici V, Luchetti F, et al. Impact of routine measurement of serum calcitonin on the diagnosis and outcome of medullary thyroid cancer: experience in 10,864 patients with nodular thyroid disorders. J Clin Endocrinol Metab. 2004;89(1):163–168. [DOI] [PubMed] [Google Scholar]
- 26. Costante G, Meringolo D, Durante C, et al. Predictive value of serum calcitonin levels for preoperative diagnosis of medullary thyroid carcinoma in a cohort of 5817 consecutive patients with thyroid nodules. J Clin Endocrinol Metab. 2007;92(2):450–455. [DOI] [PubMed] [Google Scholar]
- 27. Opsahl EM, Akslen LA, Schlichting E, et al. The role of calcitonin in predicting the extent of surgery in medullary thyroid carcinoma: a nationwide population-based study in Norway. Eur Thyroid J. 2019;8(3):159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Barbet J, Campion L, Kraeber-Bodéré F, Chatal JF; GTE Study Group. Prognostic impact of serum calcitonin and carcinoembryonic antigen doubling-times in patients with medullary thyroid carcinoma. J Clin Endocrinol Metab. 2005;90(11):6077–6084. [DOI] [PubMed] [Google Scholar]
- 29. Machens A, Dralle H. Biomarker-based risk stratification for previously untreated medullary thyroid cancer. J Clin Endocrinol Metab. 2010;95(6):2655–2663. [DOI] [PubMed] [Google Scholar]
- 30. Fan W, Xiao C, Wu F. Analysis of risk factors for cervical lymph node metastases in patients with sporadic medullary thyroid carcinoma. J Int Med Res. 2018;46(5):1982–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gülben K, Berberoğlu U, Celen O, Mersin HH. Incidental papillary microcarcinoma of the thyroid—factors affecting lymph node metastasis. Langenbecks Arch Surg. 2008;393(1):25–29. [DOI] [PubMed] [Google Scholar]
- 32. Wang W, Gu J, Shang J, Wang K. Correlation analysis on central lymph node metastasis in 276 patients with cN0 papillary thyroid carcinoma. Int J Clin Exp Pathol. 2013;6(3):510–515. [PMC free article] [PubMed] [Google Scholar]
- 33. Takahashi Y, Kawamura M, Hato T, Harada M, Matsutani N, Horio H. Neutrophil-lymphocyte ratio as a prognostic marker for lung adenocarcinoma after complete resection. World J Surg. 2016;40(2):365–372. [DOI] [PubMed] [Google Scholar]
- 34. Wang SC, Chou JF, Strong VE, Brennan MF, Capanu M, Coit DG. Pretreatment neutrophil to lymphocyte ratio independently predicts disease-specific survival in resectable gastroesophageal junction and gastric adenocarcinoma. Ann Surg. 2016;263(2):292–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen ZY, Raghav K, Lieu CH, et al. Cytokine profile and prognostic significance of high neutrophil-lymphocyte ratio in colorectal cancer. Br J Cancer. 2015;112(6):1088–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jiang K, Lei J, Chen W, et al. Association of the preoperative neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios with lymph node metastasis and recurrence in patients with medullary thyroid carcinoma. Medicine (Baltimore). 2016;95(40):e5079. [DOI] [PMC free article] [PubMed] [Google Scholar]