Abstract
Myrcene (MC), an organic hydrocarbon, was found to exert anti-inflammatory, analgesic, antimutagenic and antioxidant properties. However, the protective role of MC has not been reported against neonatal asthma. Wistar rats induced with asthma were administered with MC; while asthma control and vehicle control were maintained without MC administration. At the end of the experimental period, lung histology, inflammatory cell counts, cytokine analysis, matrix protein expressions were elucidated. Rats administered with MC exerted significant (P < 0.05) defense in protecting the lung tissue with the evidenced restoration of alveolar thickening of the lung tissues. Also, the present study elicited the anti-asthmatic activity of MC, especially via modulating the extracellular matrix protein expression in the asthma-induced animals, while a significant reduction (P < 0.05) in the fibrotic markers were found in MC treated animals. Moreover, the protective effect of MC was evidenced with reduced leukocyte infiltration in BALF, hypersensitive specific IgE levels with a profound decrease in the inflammatory cytokines such as IL-2, IL-4, IL-18, and IL-21 in MC administered animals compared to the asthma-induced group. To an extent, the markers of asthmatic inflammation such as CD14, MCP-1, and TARC were also found to be attenuated in MC exposed animals. The possible application of MC is a promising drug for the treatment of asthma-mediated complications.
Keywords: anti-asthmatic activity, matrix remodeling, myrcene, neonatal rats
Introduction
Asthma is a common inflammatory disease that affects the lungs and people worldwide are affected in millions due to this obstructive lung disease.1–3 Important advancements have been made in the drugs used for therapy on the care, and its prevalence has not decreased much in recent decades. Although the mortality rates are considerably low, it can be avoided in many cases due to routine care.4 But it has been projected to be the leading cause of death worldwide due to the incidence of industrialization and rapid change in the weather conditions of the earth that the pollutants and asthma causing agents can travel to far off places. It would cause impairment in the quality of life physiologically.
The major symptoms of asthma include bronchial hyper-responsiveness, increased mucus production and narrowing of airways and its remodeling which are due to the infiltration of the immune cells into the lungs and the subsequent consequences causing lung inflammation. Due to this, the patients may exhibit narrowing of the airways and accumulation of the mucus causing shortness of breath, chest discomfort, wheezing, and cough.5,6
Anti-inflammatory medications that are taken orally and inhaled are the usual bronchodilator medications that are recommended for asthma7 and the associated chronic obstructive pulmonary disease (COPD), has been used for decades and are not without any side effects.8 Hence there is a need to consider the alternative medicines that are based on phytochemicals.
Myrcene is an acyclic monoterpene found in the essential oil of lemongrass, hop, mango, verbena, bay, and cannabis. It is volatile and is widely used in the fragrance industry.9 It is a non-mutagenic compound10 and is generally regarded to be safe and is used in traditional medicines as a pain killer. The anti-nociceptive properties of myrcene have been already demonstrated.11,12 The production of anti-inflammatory cytokines is mediated by myrcene and is active against inflammation13 due to external agent induction. It is known to exert its immunoregulation activity by inhibiting the production of NO and IL-4 that are produced during lung inflammation.14,15 The anti-inflammatory property of myrcene has been used in our experimental model in which ovalbumin (OVA) was used to induce asthma and after treatment with myrcene (MC), we have evaluated the effect of MC in controlling the asthma by decreasing the expression of pro-inflammatory mediators and reducing the effect of lung fibrosis.
Materials and methods
Chemicals
Ovalbumin (chicken), ß-myrcene (~90% purity) were obtained from Sigma Aldrich, USA. RNA isolation kits, cDNA synthesis, and SYBR Green/ROX master mix were from Thermo Fischer Scientific, USA. Primer sequences for PCR were obtained from Eurofins MWG (Operon). Assay kits for IgE, histamine, and nitrotyrosine were from Abcam Inc, USA. ELISA assay for IL-2, IL-3, IL-4, IL-18, IL-6, and IL-21 were from Fine test biotech, China. MMP-2, MMP-9, TGF-β1, Collagen-1 assay kits are from Cusabio, USA. Periostin, CXCR2 ELISA kits were from Abcam Inc, USA. All other chemical reagents used were analytical grade.
Asthma experimental animal model
Wistar rats (8–10 g) were used as a model in the study. The animals were maintained acrylic plastic cages in air-conditioned rooms (22°C, humidity 60%, 12 h each day, and night light cycles were maintained). Pellet food and tap water were available ad libitum. All experiments were approved by the Institutional Animal Care and Use Committee, and the procedures were followed strictly as per the guidelines of the committee. Wistar rat pups of 10 days old were used in the current investigation and grouped as follows. Vehicle-treated as control group, Asthmatic group (OVA administered) as an induced group, asthma rats treated with myrcene (MC) (25 mg/kg dissolved in peanut oil (pharma grade), oral administration, and daily, before the OVA exposure) as the treatment group, and MC drug-control group. The vehicle-control group rats received a similar treatment without MC (peanut oil, 2.5 mL/kg body weight alone).
Experimental analysis
For the experimentation, the pups were sensitized with intraperitoneal administration of OVA (20 μg) on day 1 and 7th and from the 14th until 28th days along with 30 min aerosol exposure of 1% OVA from 14th day onwards for 2 weeks.16 At the end of the experimental period, the animals were killed, bronchoalveolar lavage fluid (BALF) was collected to quantify infiltrating inflammatory cells17 and blood was collected by cardiac puncture. Also, the lung tissues were collected for hematoxylin and eosin staining and Masson’s trichrome staining for collagen accumulation and the fibrosis was scored compared to the control group.18 Further, asthma specific IgE levels, histamine, and nitrotyrosine were estimated using commercial assay kits as per the manufacturer’s instructions.
Estimation of inflammatory cytokines
The assessment of pro-inflammatory and anti-inflammatory cytokines such as IL-2, IL-3, IL-4, IL-18, IL-6, and IL-21 in the serum samples and BALF samples was estimated using commercial ELISA kits as per the manufacturer’s instruction. Further, the fibrosis markers such as MMP-2, MMP-9, TGF-β1, collagen-1, periostin, CXCR2 were also estimated using commercial ELISA kits.
Reverse transcription-PCR
For the elucidation of asthma-related genes, the total RNA was isolated from the lung tissues using TRIzol reagent. Briefly, the tissues were homogenized using TRIzol, and the homogenate was mixed with chloroform and centrifuged for 10,000 rpm for 20 min, the upper aqueous layer was collected, and an equal amount of isopropanol was added and centrifuged. To the precipitate, ethanol was added and centrifuged. The pelleted RNA was washed and quantified using a spectrophotometer. The purity of RNA was analysed using the 2% formaldehyde agarose gel electrophoresis. About 20 μL total RNA (quantified and equal amount from all groups) was converted into cDNA using the high-capacity cDNA Reverse Transcription Kit. The real-time RT-PCR was done for specific genes using SYBR® Green PCR Kit, and the gene-specific primers used in the present study were listed in Table 1. The Ct values were used, and the gene expressions were determined by the comparative Ct method (ΔΔCT). The fold increase of the gene of interest was analyzed using GAPDH as a control-house-keeping gene.
Table 1.
List of primers used in the study.
| Gene | Primer | Sequence (5′-3′) | Annealing | Accession number |
|---|---|---|---|---|
| Fibronectin | F | TCCACCTGTACACGCTCAAC | 59 | XM_032901311.1 |
| R | GGGTGTGGAAGGGTAACCAG | |||
| CTGF | F | GGGAGTCAGGTGACACGAAC | 59 | AB023068.1 |
| R | CACACACCCAGCTCTTGCTA | |||
| CD-14 | F | GTTGGGCGAGAAAGGACTGA | 58 | XM_032886019.1 |
| R | GTTATACGCCTCCGACTGGG | |||
| MCP-1 | F | GATCCCAATGAGTCGGCTGG | 57 | M57441.1 |
| R | ACAGAAGTGCTTGAGGTGGT | |||
| TARC | F | TGATGTCACTTCAGATGCTGCT | 58 | NM_057151.1 |
| R | TCTGTGCAGATAAGCCTTCCC | |||
| IFN-γ | F | CAGGCCATCAGCAACAACAT | 59 | XM_032890807.1 |
| R | GGCACACTCTCTACCCCAGA | |||
| GAPDH | F | GCATCTTCTTGTGCAGTGCC | 59 | XM_032902285.1 |
| R | GATGGTGATGGGTTTCCCGT |
Statistical analysis
Statistical significance was assessed using Graph pad prism software. The statistical analysis was performed using a student t-test, and the differences between groups with a P-value of less than 0.05 were considered statistically significant.
Results
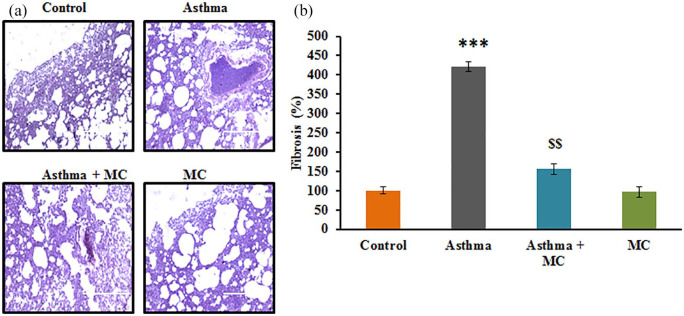
The present study aimed at elucidating the protective role of MC against neonatal. From the in vivo experiments, the results of lung histology demonstrated that asthma induced rats displayed the characteristic hypertrophy with the accumulation of smooth muscle mass in the mucous gland compared to control. Meanwhile, the analysis of collagen accumulation demonstrated a 3-fold increase in collagen accumulation in asthma induced animals. On the other hand, MC treatment displayed a significant reduction in smooth muscle matrix accumulation with reduced collagen accumulation (Figure 1) demonstrating the protective effect of MC against asthma.
Figure 1.
(a) Lung tissue histology stained using hematoxylin and eosin stain. (b) The fibrosis score of the control and experimental group of rats. The details of staining and scoring were given in the methodology section. Values are expressed as mean ± standard error (SE) (n = 10). Statistical significance expressed as ***P < 0.001 compared to vehicle-treated controls; $$ P < 0.01 compared to asthma rats.
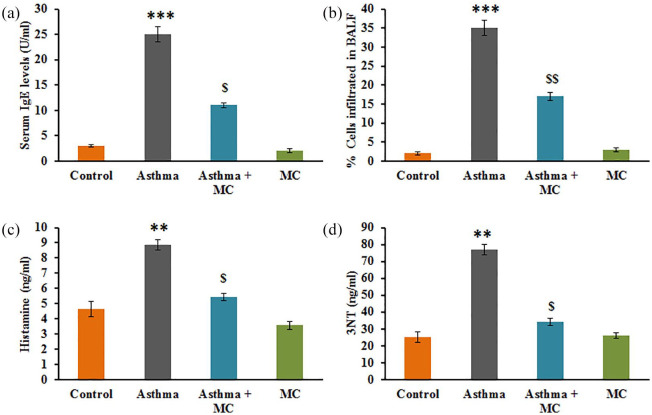
Figure 2 represents the analysis of the infiltration of eosinophils into BALF, and the results demonstrated a significant number of cells were infiltrated into the BALF compared to control. While, the hypersensitivity based IgE levels, histamine, and nitrotyrosine were profoundly increased in asthma-induced rats. However, animals pretreated with MC displayed a significant reduction in the asthmatic metabolites (Figure 2).
Figure 2.
(a–d) IgE, infiltrating cells in BALF, histamine and nitrotyrosine levels of BALF in the control, and experimental group of rats. The experimental details were given in the methodology section. Values are expressed as mean ± SE (n = 10). Statistical significance expressed as **P < 0.01, ***P < 0.001 compared to vehicle-treated controls, $P < 0.05, $$P < 0.01 compared to asthma rats.
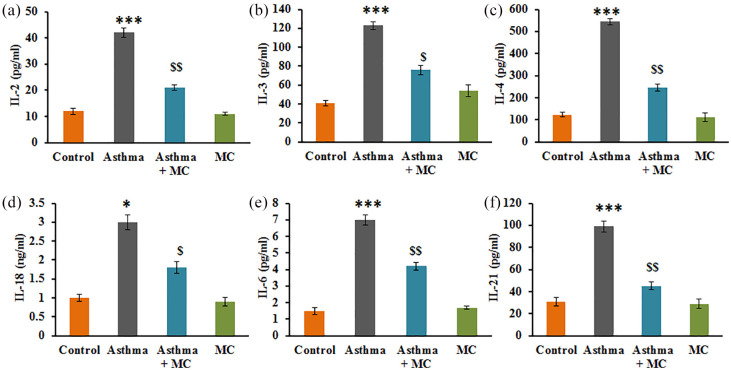
Additionally, the analysis of the cytokines’ levels in control and experimental of rats is presented in Figure 3. The results displayed that the asthma rats established a significant increase in the levels of cytokines such as IL-2 (P < 0.001), IL-3 (P < 0.001), IL-4 (P < 0.001), IL-18 (P < 0.001), IL-6 (P < 0.001), and IL-21 (P < 0.001) compared to control. While these inflammatory cytokines were reduced in MC treatment, demonstrate the protective effect of MC is also thorough, the modulation of inflammatory molecules (Figure 3).
Figure 3.
(a–f) Cytokine expression analysis of IL-2, IL-3, IL-4, IL-18, IL-6, and IL-21 in the control and experimental group of rats. The experimental details were given in the methodology section. Values are expressed as mean ± SE (n = 8). Statistical significance expressed as *P < 0.05, ***P < 0.001 compared to vehicle-treated controls, $P < 0.05 compared to asthma rats.
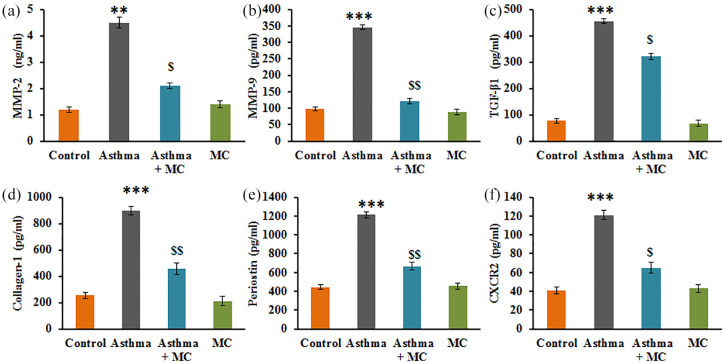
Additionally, the analysis of the level of the fibrotic marker in the control and experimental of rats is presented in Figure 3. The results displayed that the asthma rats established a significant increase in the levels of matrix and fibrotic markers such as MMP-2 (P < 0.01), MMP-9 (P < 0.001), TGF-β1 (P < 0.001), collagen-1 (P < 0.001), periostin (P < 0.01), CXCR2 (P < 0.05) compared to control. While these markers were found reduced in MC treatment, suggest that the protective effect of MC is also thorough, the modulation of fibrosis-related molecules (Figure 4).
Figure 4.
(a–f) The fibrosis marker expression analysis in the lung tissues of the control and experimental group of rats. The experimental details were given in the methodology section. Values are expressed as mean ± SE (n = 8). Statistical significance expressed as **P < 0.01, ***P < 0.001 compared to vehicle-treated controls, $P < 0.05, $$P < 0.01 compared to asthma rats.
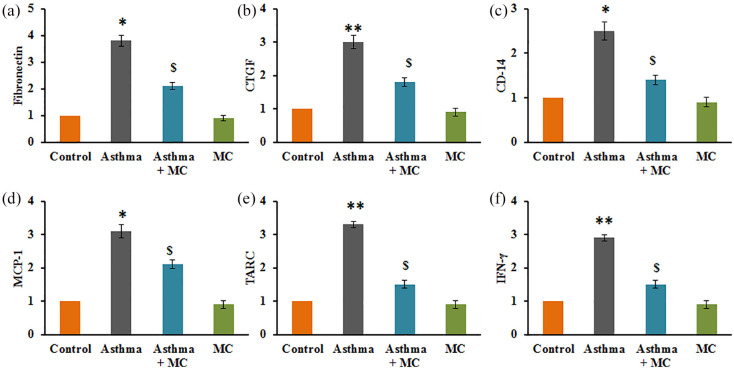
To substantiate the role of MC on the modulation of asthma-related matrix protein markers, the mRNA levels of few genes were elucidated in the control and experimental of rats, and the results were presented in Figure 5. The results demonstrated that a profound increase (P < 0.01) in the mRNA transcript expression of fibronectin (4-fold), CTGF (3.2-fold), CD-14 (2.6-fold), MCP-1 (3.3-fold), TARC (3.2-fold), IFN-γ (2.8-fold) in rats with asthma compared to vehicle-treated controls. However, the increased levels of these marker genes were attenuated in MC treatment indicate the protective effect of MC against neonatal asthma is through the modulation of matrix-associated proteins (Figure 5).
Figure 5.
(a–f) qRT-PCR mRNA expression analysis in the control and experimental group of rats. The qRT-PCR experimental details were given in the methodology section. Values are expressed as mean ± SE (n = 8). Statistical significance expressed as *P < 0.05, **P < 0.01 compared to vehicle-treated controls, $P < 0.05 compared to asthma rats.
Discussion
Asthma is a chronic inflammatory disease characterized by an increased airway hyper-responsiveness to irritants such as dust, smoke, pollen, and other allergens, causing inflammation in the airway.19 This would cause airway tissue destruction that ultimately leads to airway remodeling that is displayed as structural changes in the airway. Symptoms of asthma include chest discomfort, mucus accumulation in the lungs, wheezing, and difficulty in breathing in human beings. However, the scientific research deployed the use of murine models of asthma that can give detailed information on the association between the (Immuno) pathology and various aspects of airway functions similar to human airways20 during the allergen invade. These allergens activate the immune cells, and they cause an increase in the mucus secretion in the lungs and an increased infiltration of eosinophils, mast cells in the airway.21,22
Immune cells secrete various inflammatory cytokines, chemokines, metalloproteases that are the precursors for the onset of structural alteration in the airway that is developed into the remodeling of the airway.22,23 These include the thickening of the basement membrane, loss of epithelial integrity, and tissue fibrosis in the sub-epithelial region. The goblet cells are enlarged and are increased in their numbers with the cartilage integrity being compromised and a large release of matrix proteins is observed asthma.24 The deposition of the collagen in the airway wall of the animals induced with asthma with an allergen was characterized using lung histology analysis indicate the structural changes that occur in the airway wall which is part of the remodeling process.25,26 The amount of collagen deposition indicates the severity of the disease27 and the rigidity and the stiffness of the airway wall28 of the affected animals. When the asthma-induced animals were treated with MC, collagen deposition was reduced and the smooth muscle matrix indicating the amelioration of the symptoms associated with OVA-induced asthma.
The main pathological feature of OVA-induced asthma is the infiltration of the immune cells into the airway, especially the eosinophilic infiltration observed in the BALF of the OVA-induced animals. These cause the subsequent airway inflammation and elicit the hyperimmune reaction to the OVA in the induced animals. This is observed with a profound increase in the OVA-specific IgE,29 and such increases were reversed with the treatment with MC, which has decreased the immune reactivity against OVA and decreased the IgE levels.30 The eosinophilic infiltration has also decreased with MC treatment indicating that MC could reliable to be used against inflammation.14 Histamine secretion is part of the hyperresponsiveness13 and increase in nitrotyrosine due to oxidative stress31 were also reduced with the MC treatment indicates that the effect of the drug is through alleviation of oxidative stress.
In response to the OVA stimulation, the level of IL-2 was increased significantly would have lead to T lymphocyte proliferation and production of other cytokines,32,33 and mediators for eosinophil infiltration33 and bronchoconstriction. Similarly, the levels of IL-4 which were elevated in asthmatic animals got reduced with MC treatment speculates that the proinflammatory cytokine-IL-6 is increased with asthmatic animals has decreased and it is attributed to the anti-inflammatory effect of MC proving its protective effect.14,34,35 The Th2 immunity that is activated with the OVA-induction has led to increasing in IL-336,37 that was countered by MC. Further, IL-18, IL-21 was found increased in the serum of patients also in the asthma-induced animals induces the major role as a cofactor in Th2 cell development and is the main component in the pathogenesis of asthma.38
Fibrosis in asthmatic individuals is majorly contributed by MMP-9 by mediating the matrix reorganization, airway inflammation by enhancing the infiltration of eosinophils, revascularization39 and smooth muscle hyperplasia.40 Our results have shown that MMP-2 and MMP-9 have increased in the OVA-induced asthmatic rats and such increases have contributed to the ECM degradation and thus resulted in vascular remodeling.41,42 Growth factors play significantly in the pathogenesis of matrix remodeling in asthma. CTGF, which plays a coordinative role in MMP-9 to increase its proteolytic role in vascular remodeling, has been found increased in asthmatic animals.43,44 It induces the smooth muscle cells to proliferate faster and release fibronectin and collagen I in asthmatic animals.45–47 Further, it mediates the increase of TGF-β1 to cause angiogenesis in the smooth muscle cells in the airway remodeling in OVA-induced asthmatic animals.48 However, these effects were reduced with MC treatment which is already substantiated in other inflammatory diseases.12
High expression levels of periostin have been observed in in vivo models of asthma49 having high angiogenesis, and TGF-beta1 expression was observed in the present study asthmatic rat model. It is well known for its association with sinus50 and severe inflammatory conditions of fibrotic remodeling.51 Severe exacerbations of asthma occur with the neutrophil recruitment to the lung tissue on the increased expression of CXCR2 that attracts to its ligand CXCL8.52–54 the present study observation of an elevated CD14 in asthma affected animals suggest that the disease severity due to OVA is on the next stage and it involves macrophage activation and hence the observation,55 and it can be reversed with the anti-inflammatory action of MC.56,57 Other inflammatory mediators such as MCP-1,58 TARC and IFN-gamma34 were also reduced with the anti-inflammatory action of MC.
Conclusion
Hence, in our findings, it was clearly stated that the airway remodeling occurs with the eosinophil infiltration into the smooth muscle cells, which was augmented, by growth factors. These effects were high in the OVA-induced asthmatic animals, and these effects were controlled by the administration of MC that reduced the effects of inflammation in the airway and the subsequent structural alterations in the process of airway remodeling. Our candidate molecule could be an effective drug in the fight against asthma-related complications without having any known side effects. We have a disadvantage that our results did not state the pathway or the molecular mechanism that is involved in the drug actions against asthma. Hence, it could be asserted in the later research that would be useful in the development of its drug. Even though the present research demonstrated the protective efficacy of MC in a small animal model, the study has its own limitations with respect to the extent to which they accurate human disease. Hence, an extensive validation by clinical studies in the future is warranted.
Footnotes
Author contributions: Y.D., J.L., R.P.J., J.L. carried out all experiments; Y.M. designed the experiments and wrote the manuscript. All authors read and approved the manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: All procedures involving animals in this study confirmed to the Regulations for the Administration of Affairs Concerning Experimental Animals and were under the approval of the Institutional Animal Ethical Committee of No. 115, Shandong Provincial Third Hospital, Cheeloo College of Medicine, Shandong University, Jinan 250031, Shandong, China. All efforts were made to minimize the suffering of the animals.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Yan Ma  https://orcid.org/0000-0001-9695-4682
https://orcid.org/0000-0001-9695-4682
References
- 1. Bellanti JA, Settipane RA. (2014) Environmental exposure, allergic disease and asthma: The distinguishing hallmark of allergy-immunology. Allergy Asthma Proceedings 35(6): 427–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brusselle GG, Joos GF, Bracke KR. (2011) New insights into the immunology of chronic obstructive pulmonary disease. Lancet 378(9795): 1015–1026. [DOI] [PubMed] [Google Scholar]
- 3. Lopez AD, Mathers CD, Ezzati M, et al. (2006) Measuring the global burden of disease and risk factors, 1990–2001. In: Lopez AD, Mathers CD, Ezzati M, et al. (eds) Global Burden of Disease and Risk Factors. Washington, DC: World Bank and Oxford University Press. [Google Scholar]
- 4. Boulet LP, Reddel HK, Bateman E, et al. (2019) The global initiative for asthma (GINA): 25 years later. European Respiratory Journal 54(2): 1900598. [DOI] [PubMed] [Google Scholar]
- 5. Grenier PA, Kanne JP. (2019) Current approach to acute and chronic airway disease. In: Hodler J, Kubik-Huch RA, von Schulthess GK. (eds) Diseases of the Chest, Breast, Heart and Vessels 2019–2022: Diagnostic and Interventional Imaging. Cham: Springer, pp. 57–64. [Google Scholar]
- 6. Jones TL, Neville DM, Chauhan AJ. (2018) Diagnosis and treatment of severe asthma: A phenotype-based approach. Clinical Medicine Journal 18(Suppl. 2): s36–s40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shen YC, Chen L, Wen FQ. (2018) [Inter [retation of 2019 Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease]. Zhonghua Yi Xue Za Zhi 98(48): 3913–3916. [DOI] [PubMed] [Google Scholar]
- 8. Fan RR, Wen ZH, Wang DW, et al. (2019) Chinese herbal medicine for the treatment of cough variant asthma: A study protocol for a double-blind, randomized controlled trial. Trials 20(1): 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kraft P, Panten J. (2014) Flavors & fragrances 2013. Chemistry & Biodiversity 11(10): 1457–1461. [DOI] [PubMed] [Google Scholar]
- 10. Gomes-Carneiro MR, Viana ME, Felzenszwalb I, et al. (2005) Evaluation of beta-myrcene, alpha-terpinene and (+)- and (-)-alpha-pinene in the Salmonella/microsome assay. Food and Chemical Toxicology 43(2): 247–252. [DOI] [PubMed] [Google Scholar]
- 11. Tavares AC, Goncalves MJ, Cruz MT, et al. (2010) Essential oils from Distichoselinum tenuifolium: Chemical composition, cytotoxicity, antifungal and anti-inflammatory properties. Journal of Ethnophar-macology 130(3): 593–598. [DOI] [PubMed] [Google Scholar]
- 12. Rufino AT, Ribeiro M, Sousa C, et al. (2015) Evaluation of the anti-inflammatory, anti-catabolic and pro-anabolic effects of E-caryophyllene, myrcene and limonene in a cell model of osteoarthritis. European Journal of Pharmacology 750: 141–150. [DOI] [PubMed] [Google Scholar]
- 13. Sousa OV, Silverio MS, Del-Vechio-Vieira G, et al. (2008) Anti-nociceptive and anti-inflammatory effects of the essential oil from Eremanthus erythropappus leaves. Journal of Pharmacy and Pharmacology 60(6): 771–777. [DOI] [PubMed] [Google Scholar]
- 14. Souza MC, Siani AC, Ramos MF, et al. (2003) Evaluation of anti-inflammatory activity of essential oils from two Asteraceae species. Die Pharmazie-An International Journal of Pharmaceutical Sciences 58(8): 582–586. [PubMed] [Google Scholar]
- 15. Gour N, Wills-Karp M. (2015) IL-4 and IL-13 signaling in allergic airway disease. Cytokine 75(1): 68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kodesh E, Zaldivar F, Schwindt C, et al. (2011) A rat model of exercise-induced asthma: A nonspecific response to a specific immunogen. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 300(4): R917–R924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dua K, Shukla SD, Hansbro PM. (2017) Aspiration techniques for bronchoalveolar lavage in translational respiratory research: Paving the way to develop novel therapeutic moieties. Journal of Biological Methods 4(3): e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Umadevi S, Gopi V, Elangovan V. (2014) Regulatory mechanism of gallic acid against advanced glycation end products induced cardiac remodeling in experimental rats. Chemico-Biological Interactions 208: 28–36. [DOI] [PubMed] [Google Scholar]
- 19. Kalita M, Tian B, Gao B, et al. (2013) Systems approaches to modeling chronic mucosal inflammation. BioMed Research International 2013: 505864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kips JC, Anderson GP, Fredberg JJ, et al. (2003) Murine models of asthma. European Respiratory Journal 22(2): 374–382. [DOI] [PubMed] [Google Scholar]
- 21. Sethi S, Mahler DA, Marcus P, et al. (2012) Inflammation in COPD: Implications for management. American Journal of Medicine 125(12): 1162–1170. [DOI] [PubMed] [Google Scholar]
- 22. Yang YG, Tian WM, Zhang H, et al. (2013) Nerve growth factor exacerbates allergic lung inflammation and airway remodeling in a rat model of chronic asthma. Experimental and Therapeutic Medicine 6(5): 1251–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kemi C, Grunewald J, Eklund A, et al. (2006) Differential regulation of neurotrophin expression in human bronchial smooth muscle cells. Respiratory Research 7(1): 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. An SS, Bai TR, Bates JH, et al. (2007) Airway smooth muscle dynamics: A common pathway of airway obstruction in asthma. European Respiratory Journal 29(5): 834–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gosens R, Grainge C. (2015) Bronchoconstriction and airway biology: Potential impact and therapeutic opportunities. Chest 147(3): 798–803. [DOI] [PubMed] [Google Scholar]
- 26. Grainge CL, Lau LC, Ward JA, et al. (2011) Effect of bronchoconstriction on airway remodeling in asthma. New England Journal of Medicine 364(21): 2006–2015. [DOI] [PubMed] [Google Scholar]
- 27. Chu HW, Halliday JL, Martin RJ, et al. (1998) Collagen deposition in large airways may not differentiate severe asthma from milder forms of the disease. American Journal of Respiratory and Critical Care Medicine 158(6): 1936–1944. [DOI] [PubMed] [Google Scholar]
- 28. Palmans E, Pauwels RA, Kips JC. (2002) Repeated allergen exposure changes collagen composition in airways of sensitised Brown Norway rats. European Respiratory Journal 20(2): 280–285. [DOI] [PubMed] [Google Scholar]
- 29. Barnes PJ. (2011) Pathophysiology of allergic inflammation. Immunological Reviews 242(1): 31–50. [DOI] [PubMed] [Google Scholar]
- 30. Guo RH, Park JU, Jo SJ, et al. (2018) Anti-allergic inflammatory effects of the essential oil from fruits of Zanthoxylum coreanum Nakai. Frontiers in Pharmacology 9: 1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gugliandolo A, Pollastro F, Grassi G, et al. (2018) In vitro model of neuroinflammation: Efficacy of cannabigerol, a non-psychoactive cannabinoid. International Journal of Molecular Sciences 19(7): 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nag S, Lamkhioued B, Renzi PM. (2002) Interleukin-2-induced increased airway responsiveness, and lung Th2 cytokine expression occurs after antigen challenge through the leukotriene pathway. American Journal of Respiratory and Critical Care Medicine 165(11): 1540–1545. [DOI] [PubMed] [Google Scholar]
- 33. Renzi PM, Xu L, Yang XX, et al. (1999) IL-2 enhances allergic airway responses in rats by increased inflammation but not through increased synthesis of cysteinyl leukotrienes. Journal of Allergy and Clinical Immunology 104(1): 145–152. [DOI] [PubMed] [Google Scholar]
- 34. de Cassia da Silveira e Sa R, Andrade LN, de Sousa DP. (2013) A review on anti-inflammatory activity of monoterpenes. Molecules 18(1): 1227–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Miguel MG. (2010) Antioxidant and anti-inflammatory activities of essential oils: A short review. Molecules 15(12): 9252–9287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Asquith KL, Ramshaw HS, Hansbro PM, et al. (2008) The IL-3/IL-5/GM-CSF common receptor plays a pivotal role in the regulation of Th2 immunity and allergic airway inflammation. Journal of Immunology 180(2): 1199–1206. [DOI] [PubMed] [Google Scholar]
- 37. Wegmann M. (2011) Targeting eosinophil biology in asthma therapy. American Journal of Respiratory Cell and Molecular Biology 45(4): 667–674. [DOI] [PubMed] [Google Scholar]
- 38. Xu MH, Yuan FL, Wang SJ, et al. (2017) Association of interleukin-18 and asthma. Inflammation 40(1): 324–327. [DOI] [PubMed] [Google Scholar]
- 39. Johnson C, Sung HJ, Lessner SM, et al. (2004) Matrix metalloproteinase-9 is required for adequate angiogenic revascularization of ischemic tissues: Potential role in capillary branching. Circulation Research 94(2): 262–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Johnson C, Galis ZS. (2004) Matrix metalloproteinase-2 and -9 differentially regulate smooth muscle cell migration and cell-mediated collagen organization. Arteriosclerosis, Thrombosis, and Vascular Biology 24(1): 54–60. [DOI] [PubMed] [Google Scholar]
- 41. Lu P, Takai K, Weaver VM, et al. (2011) Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harbor Perspectives in Biology 3(12): a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vitlianova K, Georgieva J, Milanova M, et al. (2015) Blood pressure control predicts plasma matrix metalloproteinase-9 in diabetes mellitus type II. Archives of Medical Science 11(1): 85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vignola AM, Riccobono L, Mirabella A, et al. (1998) Sputum metalloproteinase-9/tissue inhibitor of metalloproteinase-1 ratio correlates with airflow obstruction in asthma and chronic bronchitis. American Journal of Respiratory and Critical Care Medicine 158(6): 1945–1950. [DOI] [PubMed] [Google Scholar]
- 44. Suzuki R, Kato T, Miyazaki Y, et al. (2001) Matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases in sputum from patients with bronchial asthma. Journal of Asthma 38(6): 477–484. [DOI] [PubMed] [Google Scholar]
- 45. Burgess JK, Johnson PR, Ge Q, et al. (2003) Expression of connective tissue growth factor in asthmatic airway smooth muscle cells. American Journal of Respiratory and Critical Care Medicine 167(1): 71–77. [DOI] [PubMed] [Google Scholar]
- 46. Black JL, Burgess JK, Johnson PR. (2003) Airway smooth muscle–its relationship to the extracellular matrix. Respiratory Physiology & Neurobiology 137(2–3): 339–346. [DOI] [PubMed] [Google Scholar]
- 47. Johnson PR, Burgess JK, Ge Q, et al. (2006) Connective tissue growth factor induces extracellular matrix in asthmatic airway smooth muscle. American Journal of Respiratory and Critical Care Medicine 173(1): 32–41. [DOI] [PubMed] [Google Scholar]
- 48. Simcock DE, Kanabar V, Clarke GW, et al. (2008) Induction of angiogenesis by airway smooth muscle from patients with asthma. American Journal of Respiratory and Critical Care Medicine 178(5): 460–468. [DOI] [PubMed] [Google Scholar]
- 49. Li W, Gao P, Zhi Y, et al. (2015) Periostin: Its role in asthma and its potential as a diagnostic or therapeutic target. Respiratory Research 16(1): 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ishida A, Ohta N, Suzuki Y, et al. (2012) Expression of pendrin and periostin in allergic rhinitis and chronic rhinosinusitis. Allergology International 61(4): 589–595. [DOI] [PubMed] [Google Scholar]
- 51. Conway SJ, Izuhara K, Kudo Y, et al. (2014) The role of periostin in tissue remodeling across health and disease. Cellular and Molecular Life Sciences 71(7): 1279–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Qiu Y, Zhu J, Bandi V, et al. (2003) Biopsy neutrophilia, neutrophil chemokine and receptor gene expression in severe exacerbations of chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 168(8): 968–975. [DOI] [PubMed] [Google Scholar]
- 53. Todd CM, Salter BM, Murphy DM, et al. (2016) The effects of a CXCR1/CXCR2 antagonist on neutrophil migration in mild atopic asthmatic subjects. Pulmonary Pharmacology and Therapeutics 41: 34–39. [DOI] [PubMed] [Google Scholar]
- 54. Ha H, Debnath B, Neamati N. (2017) Role of the CXCL8-CXCR1/2 axis in cancer and inflammatory diseases. Theranostics 7(6): 1543–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. He S, Li T, Chen H, et al. (2011) CD14+ cell-derived IL-29 modulates pro-inflammatory cytokine production in patients with allergic airway inflammation. Allergy 66(2): 238–246. [DOI] [PubMed] [Google Scholar]
- 56. Carvalho CE, Sobrinho-Junior EP, Brito LM, et al. (2017) Anti-leishmania activity of essential oil of Myracrodruon urundeuva (Engl.) Fr. All.: Composition, cytotoxicity and possible mechanisms of action. Experimental Parasitology 175: 59–67. [DOI] [PubMed] [Google Scholar]
- 57. Park Y, Yoo SA, Kim WU, et al. (2016) Anti-inflammatory effects of essential oils extracted from Chamaecyparis obtusa on murine models of inflammation and RAW 264.7 cells. Molecular Medicine Reports 13(4): 3335–3341. [DOI] [PubMed] [Google Scholar]
- 58. Cho KS, Lim YR, Lee K, et al. (2017) Terpenes from forests and human health. Toxicological Research 33(2): 97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]