Abstract
Objectives
Normokalemic periodic paralysis (NormoKPP) of skeletal muscle is an autosomal dominant disorder caused by mutations in the gene encoding voltage-gated sodium channel protein type 4 subunit alpha (SCN4A), which leads to ion channel dysfunction. Little is known about the relationship between genotype and the clinical symptoms of NormoKPP. The present study aimed to evaluate the genetic variation in a large Chinese family with NormoKPP. The patients in this pedigree did not respond to saline treatment, but calcium gluconate treatment was effective.
Methods
We performed a series of clinical examinations and genetic analyses, using whole-exome and Sanger sequencing, to examine the mutation status of SCN4A in a Chinese family segregating for NormoKPP.
Results
Whole-exome sequencing revealed a c.2111C>T substitution in SCN4A in most of the affected family members. This mutation results in the amino acid substitution p.T704M.
Conclusions
These results support a causative role of this mutation in SCN4A in NormoKPP, and provide information about the relationship between genotype and atypical clinical symptoms.
Keywords: Normokalemic periodic paralysis, ion channel, mutation, SCN4A, whole-exome sequencing, Sanger sequencing
Introduction
Periodic paralysis (PP) refers to a group of diseases involving ion channel dysfunction1 that are characterized by episodic flaccid weakness. Based on patients’ serum potassium concentrations during the episodes, PP can be classified into hypokalemic PP, hyperkalemic PP (HyperKPP), and normokalemic PP (NormoKPP).
The term NormoKPP was originally used in the 1960s2 to describe a very rare disease characterized by repeated attacks of flaccid muscle weakness or paralysis, despite normal serum potassium levels. The trigger factors for these episodes include rest after acute exercise, cold, hunger, emotional tension, carbohydrate-rich meals, and potassium supplements. Episodes of paralysis are most common in the early morning or during breaks from vigorous activities.3,4 Several familial and sporadic cases of this disease have been reported.5,6
The Nav1.4 channel is a complex of pore-forming α and auxiliary β1 subunits. The α subunit contains four homologous domains (I–IV) with six transmembrane segments (S1–S6), and is encoded by the muscle voltage-gated sodium channel protein type 4 subunit alpha gene (SCN4A).7–9 Pathogenic mutations in exons 12, 13, 18, and 24 of SCN4A, leading to the amino acid changes T704M, R675G, R675W, R675H, R675Q, R1129Q, and M1592V, have been reported to cause NormoKPP in patients from China as well as from other countries.5,6,10,11 Compared with other diseases causing PP, NormoKPP has no specific clinical features; during an attack, patients have normal results of electromyography, electrocardiogram, blood sugar, cerebrospinal fluid, and routine biochemical laboratory and auxiliary examinations.
In the current study, we used the Ion Torrent Proton sequencing platform for whole-exome scanning, which has advantages over Sanger sequencing in terms of read throughput, cost, operation time, and potential for use as a standardized procedure in hospitals. The scope of the investigation was expanded to the whole-exome scale to obtain comprehensive information about the genetics underlying the pathogenic features of NormoKPP. We reported a Chinese family with atypical NormoKPP features, and identified a classical c.2111C>T (p.T704M) mutation in SCN4A that is predicted to be responsible for the disease.
Patients and methods
Patients and families
Detailed records were obtained of the patients’ medical histories, including physical examinations, blood potassium levels, and electromyogram results during attacks. A diagnosis of NormoKPP was made based on symptoms, physical signs, and normal blood potassium levels during attacks. All participants in this study were enrolled and evaluated at the First Affiliated Hospital of Bengbu Medical College. Written informed consent was obtained from participants under the research protocols approved by the Bengbu Medical College ethics review board (BYYFY-2012KY04).
DNA extraction, Ion Torrent Proton library preparation, and sequencing
Peripheral anticoagulated whole blood samples were collected from some family members. Genomic DNA was extracted using a commercial Blood Genomic DNA Miniprep Kit (Axygen, Union City, CA, USA). The Qubit dsDNA HS (High Sensitivity) Assay Kit was used to quantify DNA with the Qubit® Fluorometer (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA), according to the manufacturer’s instructions.
Ion Torrent Proton adapter-ligated libraries were generated for each of the analyzed family members using the Ion AmpliSeq Library Kit 2.0 (Thermo Fisher Scientific, Inc., cat. no. 4476610), following the manufacturer’s protocols. After AMPureXP bead purification (Beckman Coulter Inc., Brea, CA, USA), the library concentrations were determined by quantitative polymerase chain reaction (PCR) using an Ion Library TaqMan Quantitation Kit (Thermo Fisher Scientific, Inc., cat. no. 4468802). Emulsion PCR and sample enrichment were performed using the Ion PI HI-Q Template OT2 200 Kit v2 (Thermo Fisher Scientific, Inc., cat. no. A26434), according to the manufacturer’s instructions. Isolation of the templates on Ion Sphere Particles (ISPs) was performed using the Ion OneTouch ES (Thermo Fisher Scientific, Inc.). Template-positive ISPs were enriched and sequencing was performed using an Ion PI HI-Q Sequencing 200 Kit v2 (Thermo Fisher Scientific, Inc., cat. no. A26433) on the Ion Torrent Proton instrument (Thermo Fisher Scientific, Inc.).
Bioinformatic analyses
Raw data from Ion Torrent Proton runs were processed using the official Ion Torrent platform-specific pipeline software, Torrent Suite v4.6 (Thermo Fisher Scientific, Inc.), to generate and align sequence reads. Initial variant calling was performed using the classic Ion Torrent platform pipeline software with the “variant caller” program. Two annotation steps were used to obtain information about gene mutations associated with the disease. The Torrent Suite Variant Caller (TSVC; v4.6; Thermo Fisher Scientific, Inc.) plug-in-generated files were filtered and annotated using ANNOVAR software,12 and the variant caller format (VCF) files generated by ANNOVAR were then further filtered and annotated using wANNOVAR software.13 LOVD database and PubMed literature queries were performed to determine whether variants had been previously reported as pathogenic, and to identify disease loci. Suspected pathogenic mutations were further verified by Sanger sequencing.
Sanger sequencing
Mutations in the candidate gene SCN4A exon 13 (SCN4A, NM_000334.4) were confirmed by Sanger sequencing. Primers (Table 1) were designed using Primer Premier 5.0 (Premier Biosoft, San Francisco, CA, USA). Amplified fragments were sequenced on an ABI Prism 3730XL DNA analyzer (Applied Biosystems, Foster City, CA, USA) using a Big Dye Terminator Cycle Sequencing Kit v2 (Applied Biosystems). Chromatograms illustrating the Sanger sequencing results were analyzed using the SeqMan Pro application in DNASTAR’s Lasergene Molecular Biology Suite software (http://www.dnastar.com/t-dnastar-lasergene-chinese.aspx).
Table 1.
PCR primers used for SCN4A exon 13 screening.
| Gene | Primer name | Primer sequence |
|---|---|---|
| SCN4A | E13F | TCCTAAGGCTGGGGCTGCCT |
| E13R | GGCCGGGGATCTATGTTTTA |
PCR, polymerase chain reaction.
Results
Clinical presentations
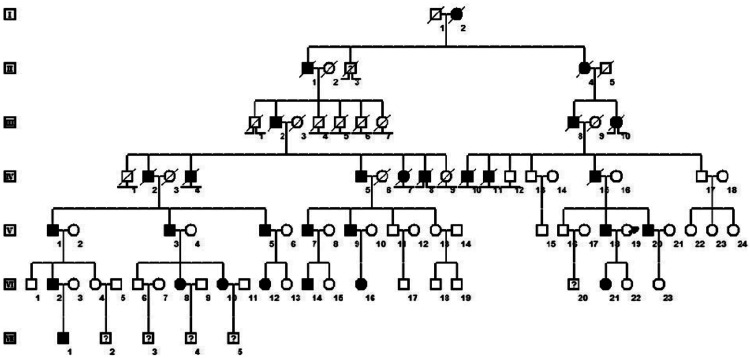
A Chinese family containing 29 members with NormoKPP was recruited from the city of Bengbu, China (Figure 1). The proband was a 32-year-old man with a history of recurrent muscle weakness and gait disturbance, who was hospitalized in 2015. His age of initial onset was 8 years. The common clinical features affecting the 16 living, affected family members were as follows (Table 2): episodic paralysis of the limbs with concomitant normal serum potassium concentrations; onset at 7 to 10 years old; patients could stand but not walk, and could lift their upper limbs but found it difficult to hold them up; and no dyspnea or dysphagia and no episodes of painful cramps or stiffness in limbs. Interestingly, patient VI12 had experienced only one attack of NormoKPP. Electrocardiogram, thyroid function, and muscle enzyme examinations generated normal results, and electromyogram performance showed no myotonic discharge or increased insertion activity. The patients generally experienced paralysis in the morning following vigorous exercise and at cold temperatures. In other Chinese NormoKPP families,6,14 large doses of physiological saline have been reported to be effective, but saline treatment had no effect on this family. Patients who were treated with intravenous 10% calcium gluconate (10 mL twice daily) usually recovered completely within 3 days. In addition, acetazolamide (125 mg three times daily) was able to reduce the number of attacks and alleviate attack symptoms.
Figure 1.
Family pedigree. Black symbols, affected individuals; open symbols, unaffected individuals; square, male; circle, female; question mark, unknown phenotype; ⊥ non-offspring; ⨼⨽ lost to follow-up; slash, deceased individuals; arrow, proband.
Table 2.
Clinical picture of the affected individuals in the normokalemic periodic paralysis pedigree.
| Patient | Sex | Age (years) | Onset (years) | Course of attack (days) | Trigger factor | Attack period |
Acute treatment (calcium gluconate) | Preventive treatment (acetazolamide) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Serum potassium | Muscle enzymes | EMG | ||||||||
| IV5 | M | 61 | 8 | 10 | C/V | N | N | N | E | E |
| V1 | M | 55 | 9 | 12 | C/V | N | N | N | E | E |
| V3 | M | 51 | 10 | 14 | C/V | N | N | N | E | E |
| V5 | M | 46 | 6 | 12 | C/V | N | N | N | E | E |
| V7 | M | 44 | 5 | 9 | C/V | N | N | N | E | E |
| V9 | M | 41 | 7 | 11 | C/V | N | N | N | E | E |
| V18 | M | 35 | 8 | 12 | C/V | N | N | N | E | E |
| V20 | M | 32 | 6 | 13 | C/V | N | N | N | E | E |
| VI2 | M | 38 | 6 | 9 | C/V | N | N | N | E | E |
| VI8 | F | 36 | 8 | 14 | C/V | N | N | N | E | E |
| VI10 | F | 28 | 5 | 10 | C/V | N | N | N | E | E |
| VI12 | F | 20 | 5 | – | – | N | N | N | E | – |
| VI14 | M | 16 | 6 | 12 | C/V | N | N | N | E | E |
| VI16 | F | 14 | 6 | 11 | C/V | N | N | N | E | E |
| VI21 | F | 10 | 8 | 10 | C/V | N | N | N | E | E |
| VII1 | M | 8 | 3 | 9 | C/V | N | N | N | E | E |
EMG, electromyogram; M, male; F, female; C, cold temperatures; V, vigorous exercise; N, normal; E, effective.
Genetic analysis
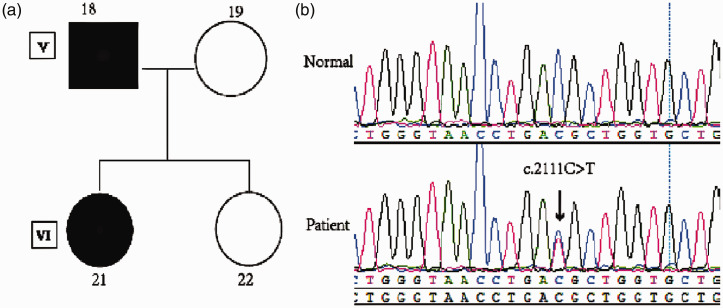
We performed whole-exome sequencing of four family members: a father (V18; affected), mother (V19; unaffected), and two daughters (one affected and one unaffected; VI21 and VI22, respectively). For the sequencing, we used >100× coverage (Figure 2a). On average, 75 million high-quality reads (quality [Q] ≥20) were generated per sample. The average depth of total coverage was 148×, and the minimal coverage was 28×. Candidate variants (n = 94) were extracted after being mapped to the reference genome and were filtered using relevant databases. Finally, one variant was identified in a gene of interest (SCN4A, p.T704M). The results of exome sequencing were verified by Sanger sequencing (Figure 2b) in the affected patients (IV5, V1, V3, V5, V7, V9, V18, V20, VI2, VI8, VI10, VI14, VI16, VI21, and VII1). However, patient VI12 had no SCN4A variant.
Figure 2.
SCN4A mutation detection in a family with normokalemic periodic paralysis (NormoKPP). (a) Pedigree structure of a NormoKPP family carrying the c.2111C>T mutation in SCN4A. (b) SCN4A DNA sequencing results of a patient and a healthy control.
Discussion
The SCN4A gene encodes the α subunit of the voltage-gated sodium channel Nav1.4, whose structure consists of four domains (DI–DIV) of six transmembrane helical segments (S1–S6). Mutations of M1592V, V781I, R675G, R675W, R675H, R675Q, and T704M in SCN4A have been reported to cause NormoKPP.5,14–19 Here, we report a pathological mutation in the SCN4A gene in a family presenting with hereditary NormoKPP. In the present study, whole-exome and Sanger sequencing identified C/T heterozygosity in SCN4A at nucleotide position 2111 of exon 13. All affected members in the family except VI12 carried the p.T704M mutation, whereas the unaffected members did not. The biological function of the known HyperKPP mutation T704M disrupts slow inactivation of Nav1.4.20–22 The T704M mutation also shifts the midpoints of steady-state activation and inactivation along the voltage axis, without affecting the rate or voltage dependence of either process. These functional abnormalities can lead to a persistent sodium current (known as a window current) at voltages where the activation and inactivation curves overlap. This disruption of slow inactivation, in combination with enhanced window currents, is expected to result in sustained currents.21,22 Other gain-of-function features typically associated with HyperKPP may present in patients diagnosed with NormoKPP, but the reason for this association with distinct potassium concentrations remains unknown.
It is interesting that there are no clear correlations between this genetic variant and patients’ clinical presentations. Different families with the p.T704M mutation of SCN4A show varied clinical pictures. Patients of the family in the present study exhibited NormoKPP, and treatment with high doses of physiological saline was ineffective, whereas calcium gluconate treatment was effective. Other patients with the p.T704M mutation have also been reported to exhibit NormoKPP.18,19 However, the p.T704M mutation identified in the present family accounts for the majority of patients with HyperKPP.23,24 Some patients harboring this mutation also exhibit periodica paramyotonia (paramyotonia congenita).25,26 Furthermore, some families diagnosed with NormoKPP have been subsequently found to have the HyperKPP mutations T704M or M1592V, leading to the suggestion that NormoKPP may be a phenotypic variant of HyperKPP.27 In addition, patients with NormoKPP may experience transient high serum potassium levels before attacks. Why might the genotype–phenotype correlations for SCN4A mutations not be straightforward? One example of a possible modifying gene is KCNE3, which encodes a potassium channel; mutations in this gene are a rare cause of hypokalemic or hyperkalemic periodic paralysis.28 Furthermore, it now appears that calcium-activated potassium (BK) channels encoded by one gene (KCNMA1) may be relevant in disorders associated with abnormal potassium homeostasis, including periodic paralysis and myotonia.29,30
Saline solution is the first choice for acute management of NormoKPP.31 The required dose of saline solution usually needs to be more than 3000 mL per day, and patients with NormoKPP regularly recover within 2 to 3 days of treatment. However, in the present report, the patients with NormoKPP showed no response to saline solution; thus, intravenous calcium gluconate was used to treat patients according to the protocol outlined in the Neurology textbook.32 We presume that the mechanism of this treatment was via calcium gluconate enhancing the opening of calcium-activated potassium channels.27,28 Prophylactic therapies with diuretics are also reportedly effective in treating NormoKPP. Acetazolamide is an empirical treatment for NormoKPP, although the mechanism of action is unclear. Moreover, another diuretic, hydrochlorothiazide, has been confirmed as effective in the prophylactic treatment of NormoKPP caused by the p.Thr704Met SCN4A mutation.33
The results of the present study demonstrate new possible clinical features of PP that are associated with the p.T704M mutation in SCN4A. In addition, our findings demonstrate the feasibility of next-generation sequencing for mutation detection in complex monogenic diseases.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work was supported by University Natural Science Research Project of Anhui Province (CN) (KJ2013A189).
ORCID iD
Daoqian Sang https://orcid.org/0000-0003-4794-4909
References
- 1.Lehmann-Horn F, Küther G, Ricker K, et al. Adynamia episodica hereditaria with myotonia: a noninactivating sodium current and the effect of extracellular pH. Muscle Nerve 1987; 10: 363–374. [DOI] [PubMed] [Google Scholar]
- 2.Jurkat-Rott K, Holzherr B, Fauler M, et al. Sodium channelopathies of skeletal muscle result from gain or loss of function. Pflugers Arch 2010; 60: 239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bulman DE, Scoggan KA, Van Oene MD, et al. A novel sodium channel mutation in a family with hypokalemic periodic paralysis. Neurology 1999; 53: 1932–1936. [DOI] [PubMed] [Google Scholar]
- 4.Fontaine B, Vale-Santos J, Jurkat-Rott K, et al. Mapping of the hypokalaemic periodic paralysis (HypoPP) locus to chromosome 1q31-32 in three European families. Nat Genet 1994; 6: 267–272. [DOI] [PubMed] [Google Scholar]
- 5.Vicart S, Sternberg D, Fournier E, et al. New mutations of SCN4A cause a potassium-sensitive normokalemic periodic paralysis. Neurology 2004; 63: 2120–2127. [DOI] [PubMed] [Google Scholar]
- 6.Xiuhai G, Weiping W, Ke Z, et al. Mutations of sodium channel alpha-subunit genes in Chinese patients with normokalemic periodic paralysis. Cell Mol Neurobiol 2008; 28: 653–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Catterall WA, Goldin AL, Waxman SG. International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol Rev 2005; 57: 397–409. [DOI] [PubMed] [Google Scholar]
- 8.Yan Z, Zhou Q, Wang L, et al. Structure of the Nav1.4-beta1 complex from electric eel. Cell 2017; 170: 470–482.e11. [DOI] [PubMed] [Google Scholar]
- 9.Shen H, Zhou Q, Pan X, et al. Structure of a eukaryotic voltage-gated sodium channel at near-atomic resolution. Science 2017; 355: eaal4326. [DOI] [PubMed] [Google Scholar]
- 10.Rüdel R, Ricker K, Lehmann-Horn F. Genotype-phenotype correlations in human skeletal muscle sodium channel diseases. Arch Neurol 1993; 50: 1241–1248. [DOI] [PubMed] [Google Scholar]
- 11.Hong D, Luan X, Chen B, et al. Both hypokalemic and normokalemic periodic paralysis in different members of a single family with novel R1129Q mutation in SCN4A gene. J Neurol Neurosurg Psychiatry 2010; 81: 703–704. [DOI] [PubMed] [Google Scholar]
- 12.Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res 2010; 38: e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang X, Wang K. wANNOVAR: annotating genetic variants for personal genomes via the web. J Med Genet 2012; 49: 433–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo XH, Wu WP, Zhang YH, et al. The mutation V781I in SCN4A gene exists in Chinese patients with normokalemic periodic paralysis. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 2004; 21: 566–569. [PubMed] [Google Scholar]
- 15.Fu C, Wang Z, Wang L, et al. Familial normokalemic periodic paralysis associated with mutation in the SCN4A p.M1592V. Front Neurol 2018; 7: 430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu L, Wu W, Yan G, et al. The construction and preliminary investigation of the cell model of a novel mutation R675Q in the SCN4A gene identified in a Chinese family with normokalemic periodic paralysis. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 2008; 25: 629–632. [PubMed] [Google Scholar]
- 17.Shi J, Qu Q, Liu H, et al. SCN4A p.R675Q mutation leading to normokalemic periodic paralysis: a family report and literature review. Front Neurol 2019; 25: 1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei CJ, Wang D, Wang S, et al. Clinical and molecular genetic analysis of a family with normokalemic periodic paralysis. Zhonghua Er Ke Za Zhi 2013; 51: 47–51. [PubMed] [Google Scholar]
- 19.Ren X, Bu BT, Yao Q, et al. Mutation of Thr704Met in SCN4A causes normoKPP in a Chinese family. Yi Chuan 2006; 28: 923–926. [PubMed] [Google Scholar]
- 20.Hayward LJ, Sandoval GM, Cannon SC. Defective slow inactivation of sodium channels contributes to familial periodic paralysis. Neurology 1999; 52: 1447–1453. [DOI] [PubMed] [Google Scholar]
- 21.Bendahhou S, Cummins TR, Tawil R, et al. Activation and inactivation of the voltage-gated sodium channel: role of segment S5 revealed by a novel hyperkalaemic periodic paralysis mutation. J Neurosci 1999; 19: 4762–4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang N, Ji S, Zhou M, et al. Sodium channel mutations in paramyotonia congenita exhibit similar biophysical phenotypes in vitro. Proc Natl Acad Sci USA 1994; 91: 12785–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller TM, Dias Da Silva MR, Miller HA, et al. Correlating phenotype and genotype in the periodic paralyses. Neurology 2004; 63: 1647–1655. [DOI] [PubMed] [Google Scholar]
- 24.Lee YH, Lee HS, Lee HE, et al. Whole-body muscle MRI in patients with hyperkalemic periodic paralysis carrying the SCN4A mutation T704M: evidence for chronic progressive myopathy with selective muscle involvement. J Clin Neurol 2015; 11: 331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim J, Hahn Y, Sohn EH, et al. Phenotypic variation of a Thr704Met mutation in skeletal sodium channel gene in a family with paralysis periodica paramyotonica. J Neurol Neurosurg Psychiatry 2001; 70: 618–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brancati F, Valente EM, Davies NP, et al. Severe infantile hyperkalaemic periodic paralysis and paramyotonia congenita: broadening the clinical spectrum associated with the T704M mutation in SCN4A. J Neurol Neurosurg Psychiatry 2003; 74: 1339–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chinnery PF, Walls TJ, Hanna MG, et al. Normokalemic periodic paralysis revisited: Does it exist? Ann Neurol 2002; 52: 251–252. [DOI] [PubMed] [Google Scholar]
- 28.Abbott GW, Butler MH, Bendahhou S, et al. MiRP2 forms potassium channels in skeletal muscle with Kv3.4 and is associated with periodic paralysis. Cell 2001; 104: 217–231. [DOI] [PubMed] [Google Scholar]
- 29.Tricarico D, Mele A, Calzolaro S, et al. Emerging role of calcium-activated potassium channel in the regulation of cell viability following potassium ions challenge in HEK293 cells and pharmacological modulation. PLoS One 2013; 8: e69551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dinardo MM, Camerino G, Mele A, et al. Splicing of the rSlo gene affects the molecular composition and drug response of Ca2+activated K+ channels in skeletal muscle. PLoS One 2012; 7: e40235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weizhi W. Neurology. 1st ed China: People's Medical Publishing House, 2001, p.1269. [Google Scholar]
- 32.Jianping J, Shengdi C. Neurology. 8th ed China: People's Medical Publishing House, 2018, p.423. [Google Scholar]
- 33.Akaba Y, Takahashi S, Sasaki Y, et al. Successful treatment of normokalemic periodic paralysis with hydrochlorothiazide. Brain Dev 2018; 40: 833–836. [DOI] [PubMed] [Google Scholar]