Abstract
Objective:
Our aim was to provide practical recommendations on the management of patients with metastatic castration-resistant prostate cancer (mCRPC) who have progressed after docetaxel plus androgen-deprivation therapy (ADT) or abiraterone plus ADT.
Methods:
Systematic literature review (SLR), nominal group meeting, and Delphi process. A panel of 12 experts was established who defined the scope, users, and sections of the document. We performed an SLR in order to assess the efficacy and safety of available drugs in patients with mCRPC. Abstracts from the American Society of Oncology and European Society for Medical Oncology meetings were also examined. The results were discussed during an expert meeting in which 14 recommendations were generated. The level of agreement with the recommendations was also tested by 13 additional experts following the Delphi process. Recommendations were voted by means of scores ranging from 0 (total disagreement) to 10 (total agreement). We defined agreement when at least 70% of the experts voted ⩾7. Next, we assigned a level of evidence and grade to the recommendation using the Oxford Centre for Evidence-based Medicine Levels of Evidence, following which the final document was drafted.
Results:
The literature search did not find any articles meeting the inclusion criteria. Finally, 13 out of 14 recommendations were accepted after two Delphi rounds (two were modified after the first round). They pertain to general and individual case-based treatment recommendations.
Conclusions:
In mCRPC patients who have progressed after docetaxel or abiraterone plus ADT in the metastatic hormone-sensitive prostate cancer setting, these recommendations may support treatment decision-making, due to the lack of evidence or other globally accepted sequencing algorithms.
Keywords: abiraterone acetate, androgen-deprivation therapy, chemotherapy, enzalutamide, metastatic castration-resistant prostate cancer, radium-223
Introduction
Prostate cancer is still a leading cause of death worldwide largely due to metastatic disease.1 For patients with an initial diagnosis of metastatic hormone-sensitive prostate cancer (mHSPC), continuous androgen-deprivation therapy (ADT) represented the standard of care until 2015, when trials such as the Androgen Ablation Therapy with or without Chemotherapy in Treating Patients with Metastatic Prostate Cancer (CHAARTED)2 showed that ADT combined with six courses of docetaxel (DOC) significantly prolonged/extended the overall survival (OS) of patients with high-volume disease. Similarly, in 2017, the LATITUDE trial demonstrated that adding abiraterone acetate (ABI) and prednisone to ADT significantly increased OS and radiographic progression-free survival in mHSPC men with high-risk features.3 These trials contributed to modifying the initial treatment approach of mHSPC.
In the previous years, several agents were approved for the management of men with mCRPC, including ABI,4 enzalutamide (ENZ),5 radium-223,6 cabazitaxel (CAB),7 and sipuleucel-T.8 Nonetheless, little is known about optimal sequencing and combination strategies, or how cross-resistance is likely to affect subsequent treatments in the continuum of patient care.
On the other hand, patients following CHAARTED and LATITUDE strategies will likely progress later on to metastatic castration-resistant prostate cancer (mCRPC). Therefore, in the near future, many patients presently on these new schemes will progress and be seen in daily practice, while the available evidence to define the best next step proves to be scarce.
In 2018, there was no robust evidence to define the best next step for patients progressing to the castration-resistant state after ADT plus DOC (CHAARTED) or ABI (LATITUTE). Therefore, the aim of this document is to provide practical recommendations for the management of patients in this setting. These recommendations are based on the available evidence and experience of a panel of prostate cancer experts, covering common clinical scenarios and the characteristics of patients attended in daily practice. We are confident that these recommendations are likely to support health professionals involved in the treatment of these patients in the decision-making process.
Methods
The nominal group and Delphi techniques were employed to elaborate the consensus. The document was created following the distribution of tasks and comments to the participants with the help of a systematic literature review (SLR) and other comprehensive literature searches across international oncology congresses. All processes were supervised by a methodologist. The expert panel comprised 12 oncologists with recognized experience in the management of prostate cancer. Two were also coordinators of the project.
SLR
A SLR was performed to address the experts’ questions regarding the management of patients with mCRPC who have progressed after on ADT plus DOC or ADT plus ABI. For this purpose, a protocol was defined with the coordinators and the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement was followed.9
Search strategy
Studies were identified by sensitive search strategies in the main bibliographic databases (supplemental data). An expert librarian collaborated with the expert panel and checked the search strategies. The following bibliographic databases were screened: Medline and Embase from 1961 to 11 January 2018, and Cochrane Central register of Controlled Trials (CENTRAL) up to 11 January 2018. We used specific Medical Subject Headings (MeSH) and additional keywords to identify studies. The strategy combined disease and treatment terms as listed above, with a controlled vocabulary to describe any of them. All the retrieved references were managed using Endnote X5 (Thomson Reuters).
The abstracts of the scientific meetings of the American Society of Oncology (ASCO/ASCOGU; 2017, 2018) and European Society for Medical Oncology (ESMO; 2016, 2017) were similarly examined through simple keywords in the organizations’ websites.
Study selection
The studies retrieved by the search strategies were incorporated if they met the following pre-established inclusion criteria: (a) involving mCRPC patients who have progressed after on ADT plus DOC or ADT plus ABI; (b) focusing on those who start a new available antineoplastic treatment, such as DOC, CAB, ABI, ENZ, or radium-223; (c) analyzing outcomes that are usually evaluated in mCRPC trials, such as OS, PFS, prostate-specific antigen (PSA) response or progression, radiographic response or progression, quality of life, and safety; (d) meta-analyses, systematic reviews, randomized clinical trials (RCT) were solely selected, as were English and Spanish articles. We excluded articles on animals, basic science, as well as publications analyzing sipuleucel-T, as this product has been withdrawn from use in the European Union.
Finally, a hand search was conducted by reviewing the references of the included studies, along with all the publications or other information related to the SLR provided by the experts.
Screening of studies, data collection, quality evaluation, and data analysis
These processes were performed by two reviewers. Both reviewers independently screened the titles and abstracts of the retrieved articles for selection criteria. When the reviewers encountered any discrepancy, a consensus was reached by including a third reviewer (LC). After the articles originating from the selection process had been read in detail following the same principles, a list of studies to be included was established. Afterwards, the reviewers collected the articles data independently. As in previous phases, in the event of discrepancies, a consensus was reached by looking at the original article or by including a third reviewer (LC).
To grade the quality, we used the Jadad score10 for RCT and a modification of The Oxford Centre for Evidence-based Medicine Levels of Evidence in its updated version of May 201111 for the remaining study designs. Evidence tables were produced. Meta-analysis was only scheduled when enough homogeneity existed among the included studies.
Nominal group meeting
The expert panel held a nominal group meeting in which objectives, scope, and users were defined. Then, the results of the SLR and of the ASCO/ASCOGU and ESMO meetings were presented and discussed. Taking into account the lack of robust evidence, the experts considered their experience, patients/disease/health system characteristics, indirect data from other RCTs, and preliminary published data (observational studies). Through a guided discussion, different clinical scenarios were addressed and recommendations for optimal treatment proposed. These recommendations were rephrased several times during the meeting in order to achieve the best wording and the maximum level of agreement. For each clinical scenario, specific patient profiles and treatments were similarly considered, in addition alternative treatment possibilities.
Delphi and final document
After several expert reviews, definitive recommendations were generated and subsequently submitted to on-line Delphi voting. Delphi was extended to a group of 24 oncologists with experience in the management of patients with prostate cancer. The participants voted each recommendation using a scale ranging from 0–10 (0 = totally disagree; 10 = totally agree). Agreement was obtained when at least 70% of the participants voted ⩾7. The recommendations with a lower level of agreement (LA) were reassessed and, if appropriate, re-edited and voted in a second Delphi round.
Subsequently, the final document was written. For each recommendation, the level of evidence (LE) and grade of recommendations (GR) were applied according to the Oxford Centre for Evidence Based Medicine Guidelines,11 along with the LA according to the Delphi process, as previously presented. The document was then distributed to the experts for final assessment and comments.
Results
SLR and Delphi results
The SLR retrieved more than 5000 articles. We additionally analyzed 633 abstracts from ASCO/ASCO-GU and ESMO meetings, yet none met the inclusion criteria (see supplemental data). As described in the methods section, due to a lack of robust evidence the experts based their recommendations on other aspects like their experience, patients/disease/health system characteristics, indirect data from other RCTs, and preliminary published data. Finally, we show the preliminary data (from observational studies or phase I–II RCTs) captured from the project.
The expert panel generated 14 recommendations that were voted. The Delphi response rate was 70%. All but one recommendation achieved the pre-established LA in the first round. During this round, however, the Spanish Medicine Agency issued a press release recommending a restriction in the use of radium-223 (Xofigo©) to only patients who have undergone two previous treatments for mCRPC or were not eligible for any other systemic treatment (see recommendations 12 and 13). As two of the recommendations were related to the use of radium-223 (one did not obtain agreement), they were re-formulated and evaluated in a second Delphi round, with only one eventually accepted.
General considerations and other evidence
Despite the lack of robust evidence, there are some observational data concerning patients with mCRPC who have progressed after undergoing DOC plus ADT or ABI plus ADT that we would like to briefly describe.
A recent retrospective study has shown that rechallenging with DOC at castration-resistance was only active in a limited number of patients (14%) treated upfront with ADT plus DOC for metastatic castration-naive prostate cancer.12 Though based on a small number of patients, anticancer activity was observed with ABI or ENZ in this article.12 In line with this finding, another small-sized retrospective observational study seemed to support these data.13
We would like to point out that the decision-making process for these patients must take into account several aspects, especially the time-to-progression, patient’s performance status and fitness for a specific treatment,14 and previous and potential drugs toxicities or cross-resistance between treatments. Yet, there are also patients with other characteristics (high volume, visceral disease, aggressive variants, etc.) for whom an individual case-based strategy appears to be most appropriate, as we will further explain later on.
In addition, the panel considers that treatment decisions should be individualized according to the physicians’ experience and patients’ characteristics and preferences. Therefore, patient information and discussions prove to be crucial.
Of note is that there is evidence supporting drug-sequencing in mCRPC patients who were only treated following progression after ADT, and these data may likewise contribute to the decision making.15,16
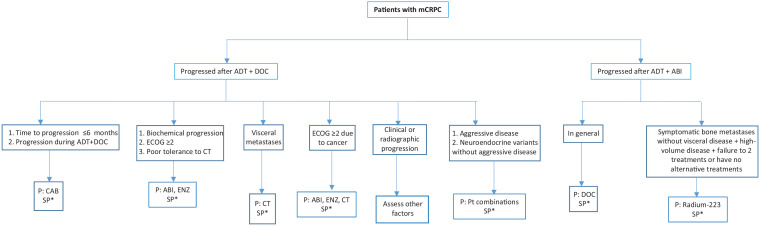
General recommendations are presented below and in Table 1, with a treatment algorithm depicted in Figure 1.
Table 1.
Recommendations and results of the Delphi process.
| # | Recommendation | Mean | SD | Median | P25 | P75 | Min | Max | % ⩾7 | LE | GR |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | In patients with mCRPC who have progressed after ADT+DOC treatment (1st line) and present a time-to-progression up to 6 months from the last DOC cycle, the panel considers CAB to be preferable | 8.04 | 2.3 | 9 | 7 | 9 | 1 | 10 | 90% | 4 | D |
| 2 | In patients with mCRPC who have progressed during treatment with ADT+DOC (1st line), the panel considers CAB to be preferable | 8.72 | 1.3 | 9 | 8 | 10 | 6 | 10 | 90% | 5 | D |
| 3 | In patients with mCRPC who have progressed after ADT+DOC treatment (1st line) and only present biochemical progression, the panel considers either ABI or ENZ to be preferable | 7.4 | 2.7 | 8 | 7 | 9 | 1 | 10 | 75% | 5 | D |
| 4 | In patients with mCRPC who have progressed after ADT+DOC treatment (1st line) and present clinical or radiographic progression, the panel considers it appropriate to analyze other factors before making a final treatment decision | 8.16 | 2.3 | 9 | 8 | 10 | 1 | 10 | 85% | 5 | D |
| 5 | In patients with mCRPC who have progressed after ADT+DOC treatment (1st line) and present visceral metastases (hepatic), the panel considers chemotherapy to be preferable | 8.72 | 1.7 | 9 | 8 | 10 | 3 | 10 | 90% | 5 | D |
| 6 | In patients with mCRPC who have progressed after ADT+DOC treatment (1st line) and present an ECOG score ⩾2, the panel considers either ABI or ENZ to be preferable | 6.6 | 2.6 | 7 | 6 | 8 | 1 | 10 | 70% | 5 | D |
| 7 | In patients with mCRPC who have progressed after ADT+DOC treatment (1st line) and present an ECOG score ⩾2, deemed to be cancer-related, the panel considers chemotherapy to be a potential treatment option | 8.2 | 1.8 | 9 | 7 | 10 | 3 | 10 | 85% | 5 | D |
| 8 | In patients with mCRPC who have progressed after ADT+DOC treatment (1st line) and present aggressive disease, the panel considers platinum-based combinations as treatment option | 8.88 | 1.3 | 9 | 8 | 10 | 5 | 10 | 95% | 5 | D |
| 9 | In patients with mCRPC who have progressed after ADT+DOC treatment (1st line) and present neuroendocrine variants without aggressive disease criteria, the panel considers that platinum-based combinations are preferable | 8.8 | 1.7 | 9 | 8 | 10 | 2 | 10 | 95% | 5 | D |
| 10 | In patients with mCRPC who have progressed after ADT+DOC treatment (1st line) and exhibit poor tolerance to chemotherapy, the panel considers either ABI or ENZ to be preferable | 6.92 | 2.5 | 8 | 7 | 8 | 1 | 10 | 70% | 5 | D |
| 11 | In patients with mCRPC who have progressed after ADT+ABI treatment (1st line), the panel considers DOC to be generally preferable | 8.92 | 1.9 | 10 | 9 | 10 | 1 | 10 | 90% | 5 | D |
| 12 | Taking into account the new EMA restrictions regarding the use of radium-223 in patients with mCRPC who have progressed after ADT+ DTX treatment, the panel considers radium-223 as an treatment option in patients with symptomatic bone metastases without visceral disease, and with high-volume disease, but only in patients who have previously failed two previous treatments for mCRPC or have no other treatment alternatives | 7.41 | 3.2 | 9 | 5 | 10 | 1 | 10 | 71% | 5 | D |
| 13 | In patients with mCRPC who have progressed after ADT+ABI treatment (1st line) and are unfit according to SIOG criteria, the panel considers ENZ to be preferable, taking into account thatradium-223 is restricted to patients who have previously failed in two treatments for mCRPC or have no other cancer treatment alternatives | 4.41 | 2.6 | 4 | 3 | 6 | 1 | 10 | 24% | 5 | D |
| 14 | In any treatment decision-making, the panel considers it crucial to take into account the patient’s preferences | 8.24 | 2.4 | 9 | 7 | 10 | 1 | 10 | 85% | 5 | D |
ABI, abiraterone; ADT, androgen-deprivation therapy; CAB, cabazitaxel; DOC, docetaxel; ECOG, Eastern Cooperative Oncology Group; EMA, European Medicines Agency; GR, grade of recommendation; LE, level of evidence; Max, maximum; mCRPC, metastatic castration-resistant prostate cancer; Min, minimum; p25, percentile 25; p75, percentile 75; SD, standard deviation; SIOG, International Society of Geriatric Oncology.
Figure 1.
Treatment algorithm for patients with mCRPC upon DOC or ABI plus ADT (1st line) in metastatic prostate cancer.
ABI, abiraterone acetate; ADT, androgen-deprivation therapy; CAB, cabazitaxel; CT, Chemotherapy; DOC, docetaxel; ECOG, Eastern Cooperative Oncology Group performance status; mCRPC, metastatic castration-resistant prostate cancer; P, preferable; PT, platinum; SP, special profiles.
*Check within the main test the treatment options according to other patients or disease particular characteristics.
Recommendations (general settings and specific profiles)
Recommendation 1. In patients with mCRPC who have progressed after ADT+DOC treatment (1st line) and present a time-to-progression up to 6 months from the last cycle of DOC, the panel considers CAB to be preferable (LE 4; GR D; LA 90%).
The panel considers it important to highlight that time-to-progression is an essential factor to consider. Although it is not possible to establish what this time should be, 6 months seem to be an acceptable cut-off. A small case series supports the use of CAB if the time-to-progression is less than 6 months following upfront DOC.17 In any case, close monitoring is highly recommended.
As previously exposed, there are factors that must similarly be considered, such as previous toxicity of chemotherapy and the patient′s health status. For example, for patients with poor chemotherapy tolerance, ABI or ENZ should be considered. These drugs have shown benefits in a small-sized case series of patients who progressed after ADT+DOC treatment.18
Similarly, for patients who develop asymptomatic or PSA-only progression, ABI or ENZ are alternative options, whereas in this setting, the panel recommends close monitoring of drug efficacy so as to allow for switching to other treatments in the event of inefficacy or of clinical doubts regarding efficacy.
Recommendation 2. In patients with mCRPC who have progressed during treatment with ADT+DOC (1st line), the panel considers CAB to be preferable (LE 5; GR D; LA 90%).
Considering the rapid progression during ADT, it could be assumed or should be borne in mind that these patients will not respond as expected to second-generation antihormonal drugs. Although more studies are required to confirm this, CAB can be beneficial in patients with rapid progression during DOC.17,19
Recommendation 3. In patients with mCRPC who have progressed after ADT+DOC treatment (1st line) yet only present biochemical progression, the panel considers ABI or ENZ (LE 5; GR D; LA 75%) to be preferable.
The COU-AA-3013,20 and AFFIRM trials21 evaluated the efficacy and safety of ABI and ENZ versus placebo in patients with mCRPC progressing after DOC. Both trials depicted a dramatic PSA-response. Given that there is no direct comparison between ABI and ENZ and because of apparent similar efficacy and acceptable safety profiles, treatment selection must be individualized, taking into account both the patients comorbidities and drug characteristic features.
Recommendation 4. In patients with mCRPC who have progressed after ADT+DOC treatment (1st line) and present clinical or radiographic progression, the panel considers it appropriate to analyze other factors before making a final treatment decision (LE 5; GR D; LA 85%).
Given this clinical scenario, due to the lack of direct or indirect evidence in favor of a specific drug, the panel proposes to base treatment decisions on other variables and outcomes, (such as time-to-progression, the presence of symptoms and symptom intensity, previous therapeutic response and treatment toxicity, location of metastases, comorbidities, etc.)
Recommendation 5. In patients with mCRPC who have progressed after ADT+DOC treatment (1st line) and present hepatic metastases, the panel considers chemotherapy to be preferable (LE 5; GR D; LA 90%).
Owing to the poor cancer prognosis when associated with many visceral metastases (in general), but especially hepatic metastases, the panel agreed on recommending chemotherapy. Yet, there may be patients, such as those with elevated PSA levels or poor health status, in whom ENZ administration could be assessed. If ENZ turns out to be the final treatment decision, close monitoring of ENZ efficacy must be performed. In other cases, such as small number/size of metastases, certain metastasis localizations, or longer time-to-progression, a different treatment to chemotherapy could be considered as well.
Recommendation 6. In patients with mCRPC who have progressed after ADT+DOC treatment (1st line) and present an ECOG score ⩾2, the panel considers ABI or ENZ to be preferable (LE 5; GR D; LA 70%).
Recommendation 7. In patients with mCRPC who have progressed after ADT+DOC treatment (1st line) and present an ECOG score ⩾2, deemed to be cancer-related, the panel considers chemotherapy to be a potential treatment option (LE 5; GR D; LA 85%).
A patient with an impaired performance status may, in general, be ruled out for chemotherapy, as this setting is associated with both poor prognosis and reduced drug tolerance.22 However, if the performance status is deemed to be related to disease progression and when the clinician considers it to be possibly reversible, chemotherapy could be discussed with the patient and, if agreed upon, delivered following appropriate dose and schedule adjustments.
Recommendation 8. In patients with mCRPC who have progressed after ADT+DOC treatment (1st line) and present aggressive disease, the panel considers platinum-based combinations as treatment option (LE 5; GR D; LA 95%).
The sequence of first-line carboplatin plus DOC followed by second-line etoposide plus cisplatin was evaluated in a phase II trial that involved 120 mCRPC patients with at least one anaplastic clinical criterion.23 It was found that, of the seven “anaplastic” criteria, bulky tumor mass was significantly associated with poor outcome, lactic acid dehydrogenase strongly predicted OS (and rapid progression), and serum carcinoembryonic antigen concentration strongly predicted OS (but not rapid progression), whereas neuroendocrine markers were unable to predict outcome or response to therapy. The authors conclude that patients with “anaplastic” prostate cancer are a recognizable subset, characterized by a high response rate of short duration to platinum-containing chemotherapies. More recently, a phase I–II RCT has shown promising activity with carboplatin added to CAB in metastatic castration-resistant prostate cancers.24 Although the results require further confirmation, these findings may support decision-making in patients with mCRPC and aggressive disease.
Recommendation 9. In patients with mCRPC who have progressed after ADT+DOC treatment (1st line) and present neuroendocrine variants without other “anaplastic” disease criteria, the panel considers platinum-based combinations as treatment option (LE 5; GR D; LA 95%).
Different non-randomized, retrospective studies have demonstrated platinum-based chemotherapies to be active in men with neuroendocrine prostate cancer.25–27 Therefore, platinum-based chemotherapy is a treatment option for these patients with poor prognosis.
Recommendation 10. In patients with mCRPC who have progressed after ADT+DOC treatment (1st line) and exhibit poor tolerance to chemotherapy, the panel considers ABI or ENZ to be preferable (LE 5; GR D; LA 70%).
Poor tolerance to previous chemotherapy generally guides the selection of a different drug class for subsequent treatment lines.28,29 Nevertheless, as ABI and ENZ are also associated with undesirable effects, clinicians must be familiar with the diagnosis and management of these undesirable effects.29
Recommendation 11. In patients with mCRPC who have progressed after ADT+ABI treatment (1st line), the panel considers DOC to be generally preferable (LE 5; GR D; LA 90%).
As in other general settings, patients with poor ECOG performance status may benefit from other treatment options. The panel has additionally considered the possibility of cross-resistance between ABI and ENZ.30,31 It should indeed be noted that cross-resistance among different treatments for mCRPC was reported.19,32
Recommendation 12. Considering the new EMA restrictions with respect to radium-223, for patients with mCRPC who have progressed after ADT+DOC treatment (1st line), the panel considers the possibility of using radium-223 in patients with symptomatic bone- and high-volume disease, yet only in those who have failed in two previous treatments for mCRPC or for whom no alternative treatments are available (LE 5; GR D; LA 71%).
We have previously commented that, as a consequence of the EMA restriction press release during the first Delphi round, the original recommendation that stated that radium-223 could be considered in patients with mCRPC who have progressed after ADT+ABI treatment (1st line) and present symptomatic bone metastases without visceral disease was rephrased, thereby clearly limiting the indications for radium-223. Despite the EMA restrictions, this recommendation reached the stated agreement level.
Recommendation 13. In patients with mCRPC who have progressed after ADT+ABI treatment (1st line), yet are unfit for chemotherapy according to the International Society of Geriatric Oncology (SIOG) criteria, the panel considers ENZ to be preferable, taking into account that using radium-223 is restricted to patients who have previously failed in two treatments for mCRPC or have no alternative cancer treatments available (LE 5; GR D; LA 24%).
The original recommendation claimed that, in this patient group, radium-223 could be considered in those with symptomatic bone metastases without visceral disease, or for palliative care. Following the EMA restrictions, this recommendation was, however, rephrased the same way as the previous recommendation, thereby restricting the use of radium-223. Nonetheless, the level of agreement obtained for this case scenario turned out to be poor.
This recommendation will be further discussed in the next section.
Recommendation 14. For any treatment decision, the panel considers it essential that clinicians and patients work together to make healthcare choices that primarily consider the patient’s preferences and values (LE 5; GR D; LA 85%).
To select the optimal treatment for a given patient, the decision-making process must undeniably seek the patient′s participation, evidence-based information regarding treatment options, and experience and knowledge of the treatment provider.
Discussion
The results of CHAARTED and LATITUDE trials,2,3,33 along with the approval of several new drugs,4–8 have raised new questions and issues as for the management of mCRPC. In this setting, the impact of upfront DOC or ABI plus ADT on subsequent therapies has so far been poorly explored.
In this research, we sought to create practical recommendations for different clinical scenarios, which prove to be quite common in daily practice. As there are no randomized controlled trials pertaining to this clinical setting, we performed a comprehensive literature search using not only the main medical databases, but also the proceedings of international congresses and scientific meetings. We decided to deliver a treatment algorithm and formulate recommendations for general clinical scenarios, and provide some guidance for specific situations within these scenarios. Finally, we limited our work so as to define the first-line treatment for mCRPC following progression in the metastatic hormone-naïve state.
Concerning the different clinical scenarios, we would like to comment on several issues that were discussed during the project. Recent observational studies have shown that baseline characteristics of CRPC disease could help identify the best therapy option for patients previously treated with DOC or ABI+ADT in mHSPC.34,35 The same way data from RCTs in mHSPC patients like the ENZAMET and TITAN trial might also contribute.33,36,37 To formulate recommendations, we therefore considered several different scenarios, such as pattern of progression, time to develop mCRPC, presence of clinical aggressive disease, patient health status, prior tolerance to chemotherapy, cross-resistance between drugs, as well as tumor histology.
The lack of evidence in mCRPC following the referred schemes thus resulted in recommendations with a very low evidence level. Nevertheless, we would like to highlight that following the Delphi process, for all except one recommendation, agreement was reached with a high level of agreement for most recommendations. In our view, this markedly increases the validity of the recommendations herein presented.
As described in the results section, two recommendations that included radium-223 were rephrased following the release of EMA restrictions, with one not reaching the pre-established level of agreement. This recommendation proposed ENZ to be the first preference in patients with mCRPC who have progressed after ADT+ABI treatment and are unfit according to SIOG criteria. Given this context, the disagreement may be partly accounted for by the fact that DOC could be a therapeutic option, yet with some reservation as for the patient health status. This could still be an option, bearing in mind the possibility of cross-resistances between ABI and ENZ. Yet, this could be a clear scenario for radium-223 administration in other patients. Nevertheless, radium-223 is now recommended for patients who have either previously failed in two treatments or have no alternative cancer treatments available. Likewise, this may explain the disagreement concerning this recommendation.
In summary, treating metastatic prostate cancer is proving increasingly complex and challenging. While the introduction of the CHAARTED, STAMPEDE, and LATITUDE schemes have clearly improved the prognosis of patients with metastatic hormone-naïve prostate cancer, they have also altered the traditional sequencing when castration-resistance occurs, yet with only scarce evidence available to guide treatment decisions. Therefore, we are now confident that our recommendations for different clinical scenarios will support health professionals involved in the care of these patients.
Supplemental Material
Supplemental material, Supplementary_data for Expert recommendations on the management of patients with metastatic castration-resistant prostate cancer who progress after CHAARTED or LATITUDE by Javier Puente, Urbano Anido, Miguel Ángel Climent, Enrique Gonzalez-Billalabeitia, Nuria Lainez, Julio Lambea, José Pablo Maroto, Maria Jose Mendez-Vidal, Álvaro Montesa, Angel Rodriguez, Curro Zambrana and Aránzazu González-del-Alba in Therapeutic Advances in Medical Oncology
Acknowledgments
We would like to thank Luis León Mateos, Natalia Fernández Nuñez, Martín Lázaro Quintela, Sergio Vázquez Estevez, Ovidio Fernández Calvo, Francisco Javier Afonso Afonso, Rodrigo Lastra, Pablo Gajate Borau, Carlos Aguado, Guillermo de Velasco, Carlos Álvarez Fernández, María Sereno Moyano, and Diego Soto de Prado for their participation in the Delphi process.
Footnotes
Author contributions: All of the authors contributed in the study design, analysis and interpretation of data, drafting the article, and revising it critically. All of them have also approved the final draft.
Conflict of interest statement: Javier Puente has received honoraria as consultant on advisory boards from Pfizer, Astellas, Janssen, MSD, Bayer, Roche, BMS, Boehringer, Astra Zeneca, Ipsen, Novartis, Eusa Pharma, Eisai and Sanofi; and as speaker from Kyowa, Celgene, Lilly and Merck; Aranzazu González del Alba has received honoraria for advisory boards, consultancy, speaker and for travel support from Pierre Fabre, Roche, Bristol-Myers Squibb, MSD, Pfizer, Novartis, Bayer, Janssen, Sanofi, Astellas, EUSA pharma, Ipsen, EISAI and Astra-Zeneca; Pablo Maroto has received honoraria for consulting or advisory role from Sanofi, Janssen, Astellas, and Bayer. Urbano Anido has received honoraria from Pfizer, Novartis, Bayer, Bristol-Myers Squibb, EUSA Pharma, and Eisai; Nuria Lainez has received honoraria for consulting or advisory role from Pfizer, Sanofi, Ipsen, BMS, Roche, and Astra Zéneca. The rest of authors declare no conflicts of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was funded by an unrestricted grant from Sanofi.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Javier Puente, Medical Oncology Department, Hospital Clínico San Carlos, Instituto de Investigación Sanitaria del Hospital Clínico San Carlos (IdISSC), CIBERONC, Madrid, Spain.
Urbano Anido, Oncology Department, Complejo Hospitalario Universitario de Santiago, Santiago de Compostela, Spain.
Miguel Ángel Climent, Medical Oncology Department, Instituto Valenciano de Oncología (IVO), Valencia, Spain.
Enrique Gonzalez-Billalabeitia, Hematology & Medical Oncology Department, Hospital Universitario Morales Meseguer, IMIB-Universidad de Murcia, Murcia, Spain; Universidad Católica San Antonio de Murcia-UCAM, Murcia, Spain.
Nuria Lainez, Medical Oncology Department, Complejo Hospitalario de Navarra, Pamplona, Spain.
Julio Lambea, Medical Oncology Department, Hospital Clínico Universitario Lozano Blesa, Zaragoza, Spain.
José Pablo Maroto, Medical Oncology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain.
Maria Jose Mendez-Vidal, Medical Oncology Department, Hospital Universitario Reina Sofia, Córdoba, Spain.
Álvaro Montesa, Medical Oncology Department, Hospital Regional de Málaga, Málaga, Spain.
Angel Rodriguez, Medical Oncology Department, Hospital de León, León, Spain.
Curro Zambrana, Medical Oncology Department, Hospital Universitario Infanta Sofía, San Sebastián De Los Reyes, Spain.
Aránzazu González-del-Alba, Medical Ongology Department, Hospital Universitario Puerta de Hierro Majadahonda, Calle Joaquin Rodrigo 2, Majadahonda, Madrid 28222, Spain.
References
- 1. Taitt HE. Global trends and prostate cancer: a review of incidence, detection, and mortality as influenced by race, ethnicity, and geographic location. Am J Mens Health 2018; 12: 1807–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med 2015; 373: 737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fizazi K, Scher HI, Molina A, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 2012; 13: 983–992. [DOI] [PubMed] [Google Scholar]
- 4. de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 2011; 364: 1995–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 2014; 371: 424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013; 369: 213–223. [DOI] [PubMed] [Google Scholar]
- 7. de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 2010; 376: 1147–1154. [DOI] [PubMed] [Google Scholar]
- 8. Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010; 363: 411–422. [DOI] [PubMed] [Google Scholar]
- 9. Moher D, Liberati A, Tetzlaff J, et al. ; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17: 1–12. [DOI] [PubMed] [Google Scholar]
- 11. CEBM. Oxford centre for evidence-based medicine – levels of evidence (March 2009). University of Oxford, http://www.cebm.net/index.aspx?o=1025 (2011, accessed 4 November 2018). [Google Scholar]
- 12. Lavaud P, Gravis G, Foulon S, et al. Anticancer activity and tolerance of treatments received beyond progression in men treated upfront with androgen deprivation therapy with or without docetaxel for metastatic castration-naive prostate cancer in the GETUG-AFU 15 phase 3 trial. Eur Urol 2018; 73: 696–703. [DOI] [PubMed] [Google Scholar]
- 13. Francini E, Yip S, Ahmed S, et al. Clinical outcomes of first-line abiraterone acetate or enzalutamide for metastatic castration-resistant prostate cancer after androgen deprivation therapy + docetaxel or ADT alone for metastatic hormone-sensitive prostate cancer. Clin Genitourin Cancer 2018; 16: 130–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982; 5: 649–655. [PubMed] [Google Scholar]
- 15. Lebdai S, Basset V, Branchereau J, et al. What do we know about treatment sequencing of abiraterone, enzalutamide, and chemotherapy in metastatic castration-resistant prostate cancer? World J Urol 2016; 34: 617–624. [DOI] [PubMed] [Google Scholar]
- 16. Maines F, Caffo O, Veccia A, et al. Sequencing new agents after docetaxel in patients with metastatic castration-resistant prostate cancer. Crit Rev Oncol Hematol 2015; 96: 498–506. [DOI] [PubMed] [Google Scholar]
- 17. Rayner L, Challapalli A, Blackmore E, et al. Upfront docetaxel with androgen deprivation therapy in the elderly patient with metastatic hormone-naïve prostate cancer: single institution experience. J Clin Oncol 2018; 36(Suppl. 6): 328. [Google Scholar]
- 18. Chahal J, Bradley TP, Sison C, et al. The clinical outcome of second generation anti-hormonal therapy in patients with metastatic castrate-resistant prostate cancer following CHAARTED regimen. J Clin Oncol 2018; 36(Suppl. 15): e17044. [Google Scholar]
- 19. van Soest RJ, de Morree ES, Kweldam CF, et al. Targeting the androgen receptor confers in vivo cross-resistance between enzalutamide and docetaxel, but not cabazitaxel, in castration-resistant prostate cancer. Eur Urol 2015; 67: 981–985. [DOI] [PubMed] [Google Scholar]
- 20. Harland S, Staffurth J, Molina A, et al. Effect of abiraterone acetate treatment on the quality of life of patients with metastatic castration-resistant prostate cancer after failure of docetaxel chemotherapy. Eur J Cancer 2013; 49: 3648–3657. [DOI] [PubMed] [Google Scholar]
- 21. Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012; 367: 1187–1197. [DOI] [PubMed] [Google Scholar]
- 22. Van Praet C, Rottey S, Van Hende F, et al. Which factors predict overall survival in patients with metastatic castration-resistant prostate cancer treated with abiraterone acetate post-docetaxel? Clin Genitourin Cancer 2017; 15: 502–508. [DOI] [PubMed] [Google Scholar]
- 23. Aparicio AM, Harzstark AL, Corn PG, et al. Platinum-based chemotherapy for variant castrate-resistant prostate cancer. Clin Cancer Res 2013; 19: 3621–3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Corn PG, Heath EI, Zurita A, et al. Cabazitaxel plus carboplatin for the treatment of men with metastatic castration-resistant prostate cancers: a randomised, open-label, phase 1-2 trial. Lancet Oncol 2019; 20: 1432–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nakabayashi M, Sartor O, Jacobus S, et al. Response to docetaxel/carboplatin-based chemotherapy as first- and second-line therapy in patients with metastatic hormone-refractory prostate cancer. BJU Int 2008; 101: 308–312. [DOI] [PubMed] [Google Scholar]
- 26. Ross RW, Beer TM, Jacobus S, et al. A phase 2 study of carboplatin plus docetaxel in men with metastatic hormone-refractory prostate cancer who are refractory to docetaxel. Cancer 2008; 112: 521–526. [DOI] [PubMed] [Google Scholar]
- 27. Humeniuk MS, Gupta RT, Healy P, et al. Platinum sensitivity in metastatic prostate cancer: does histology matter? Prostate Cancer Prostatic Dis 2018; 21: 92–99. [DOI] [PubMed] [Google Scholar]
- 28. Zheng H, Chen J, Qiu W, et al. Safety and efficacy of first-line treatments for chemotherapy-naive metastatic castration-resistant prostate cancer: a systematic review and indirect comparison. Biomed Res Int 2017; 2017: 3941217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Summers N, Vanderpuye-Orgle J, Reinhart M, et al. Efficacy and safety of post-docetaxel therapies in metastatic castration-resistant prostate cancer: a systematic review of the literature. Curr Med Res Opin 2017; 33: 1995–2008. [DOI] [PubMed] [Google Scholar]
- 30. Karantanos T, Evans CP, Tombal B, et al. Understanding the mechanisms of androgen deprivation resistance in prostate cancer at the molecular level. Eur Urol 2015; 67: 470–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Azad AA, Eigl BJ, Murray RN, et al. Efficacy of enzalutamide following abiraterone acetate in chemotherapy-naive metastatic castration-resistant prostate cancer patients. Eur Urol 2015; 67: 23–29. [DOI] [PubMed] [Google Scholar]
- 32. Schweizer MT, Zhou XC, Wang H, et al. The influence of prior abiraterone treatment on the clinical activity of docetaxel in men with metastatic castration-resistant prostate cancer. Eur Urol 2014; 66: 646–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 2016; 387: 1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gajate Borau P, Martin Marino A, Gallegos Sancho I, et al. Efficacy of treatments after progression to up-front Docetaxel (D) in combination with androgen deprivation therapy (ADT) for metastatic hormone-sensitive prostate cancer (mHSPC). Ann Oncol 2018; 29(Suppl. 8): viii271–viii302. [Google Scholar]
- 35. Aguado De La Rosa C, Iciar Garcia Carbonero I, Guillermo De Velasco G, et al. How do patterns of progression influence treatment selection after chemohormonal therapy in patients with metastatic hormone sensitive prostate cancer? J Clin Oncol 2017; 35(Suppl. 15): e16504. [Google Scholar]
- 36. Davis ID, Martin AJ, Stockler MR, et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med 2019; 381: 121–131. [DOI] [PubMed] [Google Scholar]
- 37. Chi KN, Agarwal N, Bjartell A, et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med 2019; 381: 13–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_data for Expert recommendations on the management of patients with metastatic castration-resistant prostate cancer who progress after CHAARTED or LATITUDE by Javier Puente, Urbano Anido, Miguel Ángel Climent, Enrique Gonzalez-Billalabeitia, Nuria Lainez, Julio Lambea, José Pablo Maroto, Maria Jose Mendez-Vidal, Álvaro Montesa, Angel Rodriguez, Curro Zambrana and Aránzazu González-del-Alba in Therapeutic Advances in Medical Oncology