Abstract
Background
Gastric schwannoma is a rarely seen gastric tumor accounting for only 0.2% of all gastric tumors. It is difficult to distinguish a gastric schwannoma from other gastric tumors preoperatively.
Case presentation: A 30-year-old man with no significant medical history or physical examination findings presented with a 1-month history of right upper abdominal discomfort. The preoperative diagnosis was a gastrointestinal stromal tumor, but the postoperative pathologic and immunohistochemical examinations confirmed a gastric schwannoma. The patient underwent laparoscopic wedge resection of the stomach without additional postoperative treatment, and his postoperative recovery was uneventful. No recurrence or metastasis was found at the 2-year follow-up examination.
Conclusion
Although gastric schwannomas are usually not malignant, they are difficult to distinguish from other malignant stromal tumors preoperatively. Surgical resection should be recommended when a schwannoma is malignant or considered to be at risk of becoming malignant.
Keywords: Schwannoma, gastric schwannoma, general surgery, gastroenterology and hepatology, gastrointestinal stromal tumor, laparoscopic wedge resection
Introduction
A gastric schwannoma is a rarely seen gastric tumor that originates from the Schwann cell sheath.1 Representing as little as 0.2% of all types of gastric tumors, gastric schwannomas are usually asymptomatic and non-malignant.2 We herein present a rare case of a gastric schwannoma. A retrospective analysis was performed based on the clinical manifestations, auxiliary examination findings, and treatment of a patient with gastric schwannoma in December 2017. The preoperative diagnosis was a gastrointestinal stromal tumor (GIST), but the pathologic and immunohistochemical examination findings confirmed a gastric schwannoma.
Case report
A 30-year-old man presented with a 1-month history of right upper abdominal discomfort. Physical examination and laboratory tests revealed no abnormities.
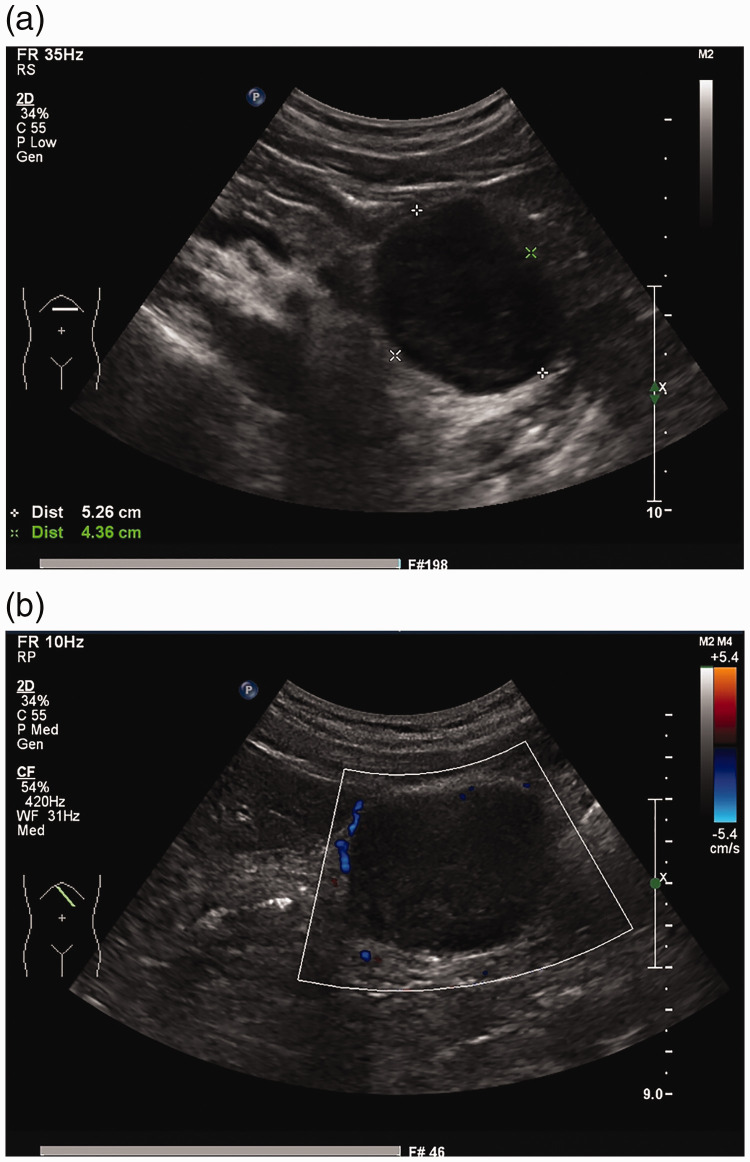
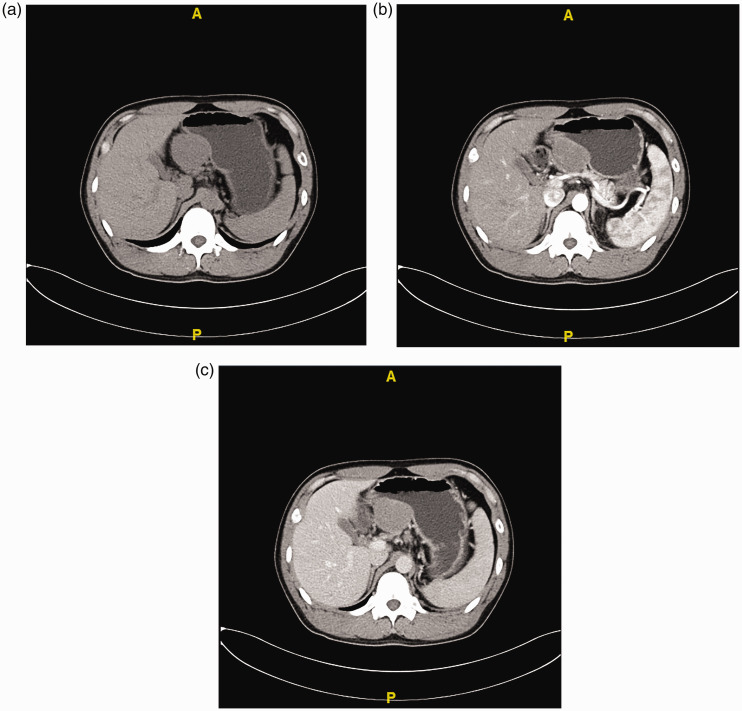
B-Mode ultrasound examination revealed a regularly shaped 5.3- × 4.4-cm mass with low echogenicity and a clear border in the serosal and muscular layers of the gastric body (Figure 1). Enhanced computed tomography (CT) showed that the mass was enhanced, measured 5.1 × 4.4 × 5.1 cm, and was entirely exophytic. The boundary of the mass was less clear, but the local gastric mucosal structure remained clear. Based on these findings, our preoperative diagnosis was a GIST (Figure 2). Because of the CT findings, we did not perform magnetic resonance imaging (MRI) or biopsy.
Figure 1.
B-mode ultrasound findings. (a) A hypoechoic mass measuring 5.3 × 4.4 cm was observed in the posterior wall of the stomach. (b) Color Doppler flow imaging showed that the internal blood flow signal was not abundant.
Figure 2.
Computed tomography findings. (a) An oval-shaped mass measuring 5.1 × 4.4 × 5.1 cm was found in the lesser gastric curvature. On plain computed tomography, it exhibited low density. The mass was (b) enhanced in the arterial phase (51 HU) and (c) slightly enhanced in the venous phase (67 HU).
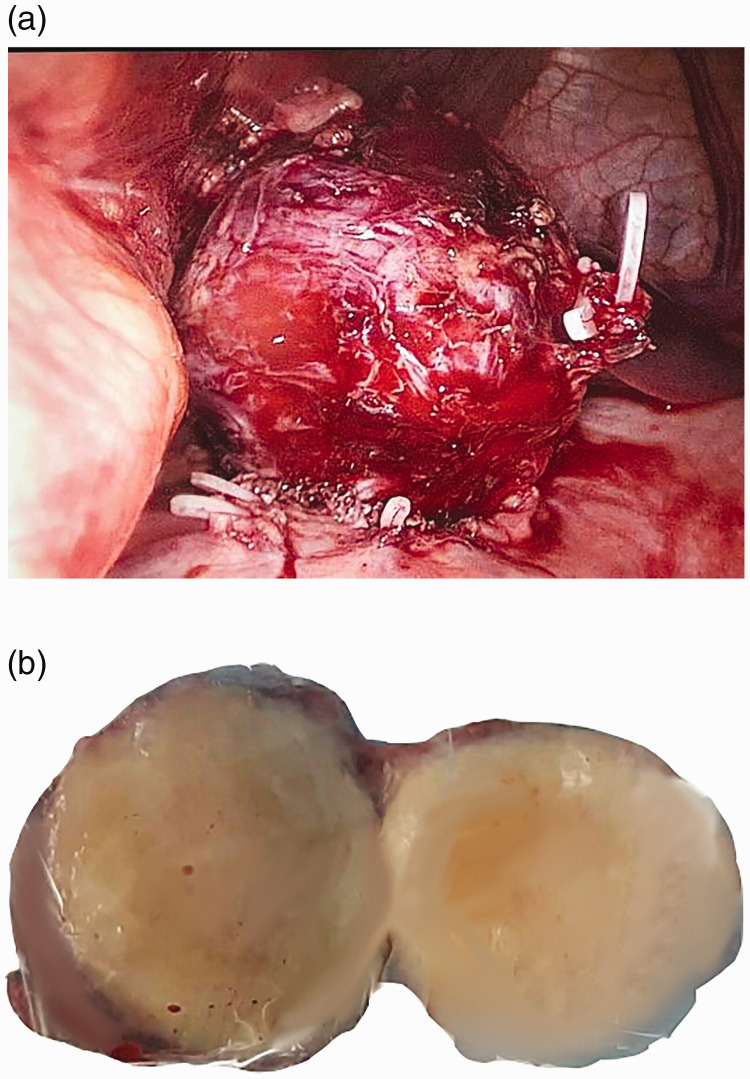
The mass was removed by laparoscopic wedge resection. The tumor measured 5 × 4 cm and was red with a smooth surface (Figure 3).
Figure 3.
(a) Laparoscopic imaging. The tumor measured 5 × 4 cm and was rough with a clear margin. (b) The section appeared gray.
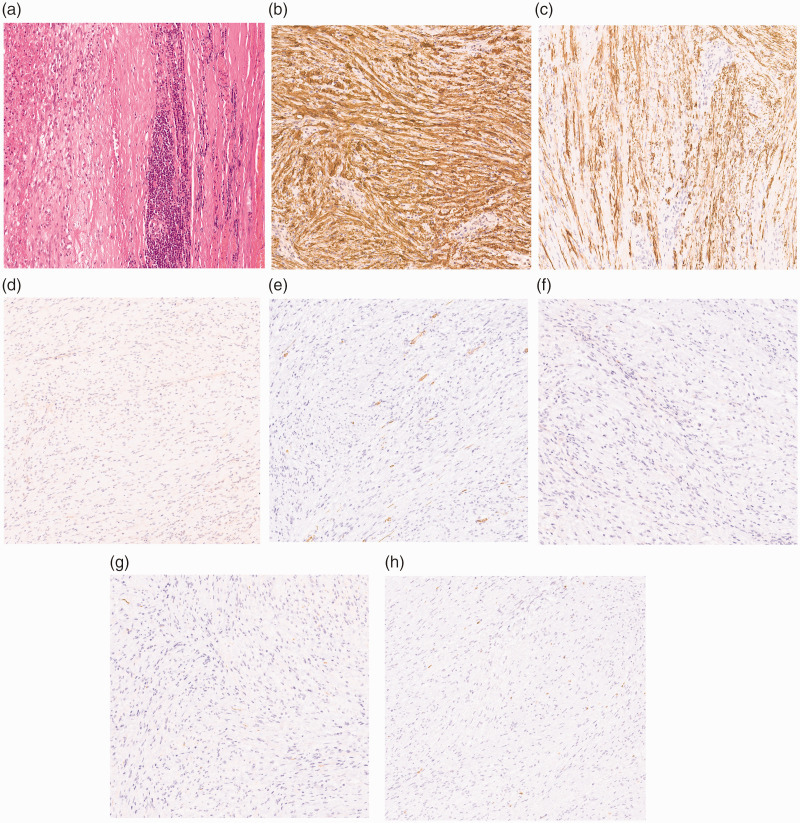
The immunohistochemical staining results led to a diagnosis of gastric schwannoma. The tumor was positive for S-100 and glial fibrillary acidic protein (GFAP) and negative for CD34 and CD117 (Figure 4). The patient was given postoperative anti-inflammatory and nutritional support. Upon recovery of his intestinal function, he began to drink water and gradually reverted to a semi-liquid diet. The recovery process was smooth and uneventful. The 2-year follow-up examination showed no evidence of recurrence.
Figure 4.
Pathological findings. (a) Pathologic examination revealed that the mass was made of spindle cells. Immunohistochemistry revealed positive staining of (b) S-100 and (c) glial fibrillary acidic protein and negative staining of (d) CD117, (e) CD34, (f) DOG1, (g) desmin, and (h) Ki-67 (magnification, ×100).
Discussion
Schwannomas are spindle cell mesenchymal tumors arising from the Schwann cell sheath, and gastric schwannomas originate from the gastrointestinal neural plexus.1 Gastric schwannomas grow slowly and are rarely seen. Previous research indicates that gastric schwannomas account for 0.2% of all gastric tumors, 4.0% of all benign gastric tumors, and 6.3% of all gastric mesenchymal tumors.2 Voltaggio et al.3 reported that the incidence ratio of gastric stromal tumors and gastric schwannomas is 45:1. However, Daimaru et al.4 and Prevot et al.5 reported a higher ratio ranging from 8:1 to 14:1. The difference is suspected to be attributed to the different group specifications and methods of calculation among these studies.
Most gastric schwannomas are benign, and transformation from a benign tumor to a malignant tumor is rare. Malignant schwannomas are rarely seen, and very few cases have been reported in the literature.6–8 These slowly growing tumors are encapsulated. When the tumor expands, the nerve is forced to transfer to the tumor’s periphery but still retains its neural function. A marked female preponderance has been reported (female:male ratio of 4:1), and the tumor is generally found in patients aged 40 to 60 years.3
Most commonly observed in the neck and head, schwannomas are rarely discovered in the gastrointestinal tract.9 Nevertheless, the most common site of gastrointestinal schwannomas is the stomach, where 60% to 70% of these tumors are located.3,10 Gastric schwannomas usually arise from the body of the stomach (50%), with fewer cases arising from the antrum (32%) or fundus (18%).11 In a study involving 33 cases of gastrointestinal schwannomas, 24 were located in the stomach, 4 in the esophagus, 3 in the rectum, and 2 in the colon.12 In another study,3 gastrointestinal schwannomas were mostly located in the stomach (51 cases), with the second most common site being the colon (20 cases).13 Gastrointestinal schwannomas rarely occur in the esophagus and small intestine. Gastric schwannomas can involve the submucosal nerve plexuses, such as Auerbach’s plexus or Meissner’s plexus.14
Although they are frequently asymptomatic, gastric schwannoma can cause abdominal discomfort and a palpable mass,2 and they sometimes result in gastroduodenal intussusception and gastrointestinal bleeding.15 Gastrointestinal hemorrhage may occur when the growing mass compresses the submucosa, potentially leading to ischemia and ulceration.16 Endoscopic biopsies may be falsely negative because the lesion is submucosal. In addition, if the tumor is large in size and exophytic, it may contact or compress nearby tissues or organs.
Pathologic examination is the only way to confirm a diagnosis of gastric schwannoma. However, useful information can also be obtained through an upper gastrointestinal barium study, ultrasonography, gastrointestinal endoscopy, CT, MRI, and endoscopic ultrasonography (EUS). A CT scan is effective in distinguishing benign from malignant tumors. In contrast to GISTs, gastric schwannomas are hypodense, well-demarcated, and homogeneous on CT.9,17 MRI can provide further information about how the tumor relates to its surrounding tissues. On MRI, the tumor is also strongly enhanced with low to medium signal intensity on T1-weighted images and high signal intensity on T2-weighted images.9,18 B-Mode ultrasound shows that compared with the surrounding normal proper muscle layer, gastric schwannomas are heterogeneously hypoechoic lesions with marginal halos, distinct borders, and decreased echogenicity.19 In contrast, >50% of GISTs exhibit either increased or identical echogenicity.20
Endoscopy can facilitate precise localization of the tumor, and endoscopic needle biopsy can assist in confirming the diagnosis of a submucosal tumor by pathology.21,22 EUS is regarded as the most suitable approach to the diagnosis of small lesions, while EUS-guided fine-needle aspiration can also be helpful in achieving a diagnosis.23 For malignant tumors, however, needle biopsy is associated with a risk of rupture and spread. Phosphogluconate dehydrogenase-positron emission tomography/CT (PDG-PET/CT) plays a significant role in diagnosing gastric schwannomas, particularly with respect to positioning rather than distinguishing between benign and malignant tumors. Komatsu et al.24 were the first to report increased accumulation of 18-fluorodeoxyglucose (18-FDG) on PET imaging in a gastric schwannoma. Oh et al.25 also reported a case of 18-FDG accumulation in a gastric schwannoma; in this case, the benign gastric schwannoma was misdiagnosed as malignant. Beaulieu et al.26 reported that the glycometabolism of Schwann cells is suspected to contribute to the high uptake of FDG in schwannomas. In another study, technetium-99m sestamibi single-photon emission CT/CT was also found to be conducive to achieving accurate anatomical localization.27
Immunohistochemistry is of major significance to the differential diagnosis among GISTs, leiomyomas, and gastrointestinal autonomic nerve tumors. Schwannomas are positive for S100 protein and vimentin but negative for CD34 and CD117, which is in stark contrast to GISTs.28 Unlike leiomyomas, schwannomas are negative for smooth muscle actin.29 Gastrointestinal autonomic nerve tumors are mostly positive for CD117 and CD34 and usually negative for S-100 protein and GFAP.30 They may also be positive for GFAP, which is in contrast to peripheral schwannomas.12 These markers are considered the most appropriate diagnostic indicators of gastric schwannomas. S-100 protein, which is derived from cells of the neural crest, is a unique indicator of schwannomas. Molecular research has shown that genetically, gastric schwannomas typically exhibit a lack of c-Kit (CD117), mutation of PDGFRA,30 chromosome 22 monosomy, and polyploidy of chromosomes 2 and 18.31 Particular gene alterations are extremely constructive in the differential diagnosis.
Surgical en bloc resection (e.g., laparoscopic or open local resection) or partial resection may be effective for gastric schwannomas, but the size, location, and surrounding structure of the tumor will lead to different surgical outcomes. These tumors rarely recur.32 However, because of its excellent follow-up outcome, endoscopic surgery is also an effective and safe therapy for gastric schwannomas. Such procedures include endoscopic submucosal dissection, endoscopic full-thickness resection, and ligation-assisted endoscopic enucleation.33,34
Lymph node dissection is not required because gastric schwannomas, which are usually benign, are unlikely to metastasize to the lymph nodes. Almost no metastasis is encountered in enlarged regional lymph nodes of patients with gastric schwannoma; instead, the lymph node enlargement is usually caused by inflammatory hyperplasia. PET/CT can distinguish between benign and malignant tumors, including lymph nodes. Positive lymph nodes may be considered malignant and require surgical resection.35,36 Radiotherapy and chemotherapy are not required after surgery because most tumors are benign.37 Advanced malignant gastric schwannomas metastasize to the liver,8 but not to the lymph nodes.8,38 Because chemotherapy and radiotherapy are ineffective in such cases,8 surgery is the definitive treatment method.
In the present case, the tumor was completely removed by surgery and radical cure was achieved. However, the lack of gastroscopy and biopsy in the preoperative examination affected the preoperative diagnosis and formulation of the surgical plan.
Conclusion
Specific clinical manifestations and imaging findings of gastric schwannomas are rarely observed. Preoperative diagnosis is thus difficult, and a postoperative pathological examination is required to achieve a definitive diagnosis. Although gastric schwannomas are usually not malignant, they are difficult to distinguish from other malignant stromal tumors preoperatively. Therefore, surgical resection is considered the most effective treatment.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Ethical approval
All procedures described in this report were performed in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Funding
This work was supported by Capital’s Funds for Health Improvement and Research (Project number 2020-2-8021) and Peking University International Hospital Key Project (Project number YN2019ZD02).
Informed consent
The patient provided informed consent for publication of his case.
ORCID iD
Changsheng Pu https://orcid.org/0000-0002-5292-5721
References
- 1.Hong X, Wu W, Wang M, et al. Benign gastric schwannoma: how long should we follow up to monitor the recurrence? A case report and comprehensive review of literature of 137 cases. Int Surg 2015; 100: 744–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sreevathsa MR, Pipara G. Gastric schwannoma: a case report and review of literature. Indian J Surg Oncol 2015; 6: 123–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voltaggio L, Murray R, Lasota J, et al. Gastric schwannoma: a clinicopathologic study of 51 cases and critical review of the literature. Hum Pathol 2012; 43: 650–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daimaru Y, Kido H, Hashimoto H, et al. Benign schwannoma of the gastrointestinal tract: a clinicopathologic and immunohistochemical study. Hum Pathol 1988; 19: 257–264. [DOI] [PubMed] [Google Scholar]
- 5.Prevot S, Bienvenu L, Vaillant JC, et al. Benign schwannoma of the digestive tract: a clinicopathologic and immunohistochemical study of five cases, including a case of esophageal tumor. Am J Surg Pathol 1999; 23: 431–436. [DOI] [PubMed] [Google Scholar]
- 6.Loffeld RJ, Balk TG, Oomen JL, et al. Upper gastrointestinal bleeding due to a malignant Schwannoma of the stomach. Eur J Gastroenterol Hepatol 1998; 10: 159–162. [DOI] [PubMed] [Google Scholar]
- 7.Watanabe A, Ojima H, Suzuki S, et al. An individual with gastric schwannoma with pathologically malignant potential surviving two years after laparoscopy-assisted partial gastrectomy. Case Rep Gastroenterol 2011; 5: 502–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takemura M, Yoshida K, Takii M, et al. Gastric malignant schwannoma presenting with upper gastrointestinal bleeding: a case report. J Med Case Rep 2012; 6: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raber MH, Ziedses Des Plantes CM, Vink R, et al. Gastric schwannoma presenting as an incidentaloma on CT-scan and MRI. Gastroenterology Res 2010; 3: 276–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarlomo-Rikala M, Miettinen M. Gastric schwannoma–a clinicopathological analysis of six cases. Histopathology 1995; 27: 355–360. [DOI] [PubMed] [Google Scholar]
- 11.Bruneton JN, Drouillard J, Roux P, et al. Neurogenic tumors of the stomach. Report of 18 cases and review of the literature. Rofo 1983; 139: 192–198. [DOI] [PubMed] [Google Scholar]
- 12.Hou YY, Tan YS, Xu JF, et al. Schwannoma of the gastrointestinal tract: a clinicopathological, immunohistochemical and ultrastructural study of 33 cases. Histopathology 2006; 48: 536–545. [DOI] [PubMed] [Google Scholar]
- 13.Miettinen M, Shekitka KM, Sobin LH. Schwannomas in the colon and rectum: a clinicopathologic and immunohistochemical study of 20 cases. Am J Surg Pathol 2001; 25: 846–855. [DOI] [PubMed] [Google Scholar]
- 14.Lin CS, Hsu HS, Tsai CH, et al. Gastric schwannoma. J Chin Med Assoc 2004; 67: 583–586. [PubMed] [Google Scholar]
- 15.Yang JH, Zhang M, Zhao ZH, et al. Gastroduodenal intussusception due to gastric schwannoma treated by Billroth II distal gastrectomy: one case report. World J Gastroenterol 2015; 21: 2225–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manji M, Ismail A, Komba E. Gastric schwannoma: case report from Tanzania and brief review of literature. Clin Case Rep 2015; 3: 562–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levy AD, Quiles AM, Miettinen M, et al. Gastrointestinal schwannomas: CT features with clinicopathologic correlation. AJR Am J Roentgenol 2005; 184: 797–802. [DOI] [PubMed] [Google Scholar]
- 18.Karabulut N, Martin DR, Yang M. Case report: gastric schwannoma: MRI findings. Br J Radiol 2002; 75: 624–626. [DOI] [PubMed] [Google Scholar]
- 19.Yoon JM, Kim GH, Park DY, et al. Endosonographic features of gastric schwannoma: a single center experience. Clin Endosc 2016; 49: 548–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim GH, Park DY, Kim S, et al. Is it possible to differentiate gastric GISTs from gastric leiomyomas by EUS? World J Gastroenterol 2009; 15: 3376–3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rana SS, Sharma V, Sharma R, et al. Gastric gastrointestinal stromal tumor mimicking cystic tumor of the pancreas: diagnosed by endoscopic ultrasound-fine-needle aspiration. Endosc Ultrasound 2015; 4: 351–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choudhary NS, Puri R, Lipi L, et al. Eosinophilic gastroenteritis mimicking as a malignant gastric ulcer with lymphadenopathy as shown by computed tomography and endoscopic ultrasound. Endosc Ultrasound 2015; 4: 78–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hong SW, Cho WY, Kim JO, et al. Gastric schwannoma diagnosed by endoscopic ultrasonography-guided trucut biopsy. Clin Endosc 2013; 46: 284–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Komatsu D, Koide N, Hiraga R, et al. Gastric schwannoma exhibiting increased fluorodeoxyglucose uptake. Gastric Cancer 2009; 12: 225–228. DOI: 10.1007/s10120-009-0526-7. [DOI] [PubMed] [Google Scholar]
- 25.Oh SJ, Suh BJ, Park JK. Gastric schwannoma mimicking malignant gastrointestinal stromal tumor exhibiting increased fluorodeoxyglucose uptake. Case Rep Oncol 2016; 9: 228–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beaulieu S, Rubin B, Djang D, et al. Positron emission tomography of schwannomas: emphasizing its potential in preoperative planning. AJR Am J Roentgenol 2004; 182: 971–974. [DOI] [PubMed] [Google Scholar]
- 27.Shawgi M, Ali T, Scott M, et al. 99m-Technetium sestamibi uptake in a gastric schwannoma. World J Nucl Med 2018; 17: 49–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miettinen M, Lasota J. Gastrointestinal stromal tumors–definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch 2001; 438: 1–12. [DOI] [PubMed] [Google Scholar]
- 29.Miettinen M, Virolainen M. and Maarit-Sarlomo-Rikala . Gastrointestinal stromal tumors–value of CD34 antigen in their identification and separation from true leiomyomas and schwannomas. Am J Surg Pathol 1995; 19: 207–216. [DOI] [PubMed] [Google Scholar]
- 30.Lasota J, Miettinen M. Clinical significance of oncogenic KIT and PDGFRA mutations in gastrointestinal stromal tumours. Histopathology 2008; 53: 245–266. [DOI] [PubMed] [Google Scholar]
- 31.Diaz De Stahl T, Hansson CM, De Bustos C, et al. High-resolution array-CGH profiling of germline and tumor-specific copy number alterations on chromosome 22 in patients affected with schwannomas. Hum Genet 2005; 118: 35–44. [DOI] [PubMed] [Google Scholar]
- 32.Atmatzidis S, Chatzimavroudis G, Dragoumis D, et al. Gastric schwannoma: a case report and literature review. Hippokratia 2012; 16: 280–282. [PMC free article] [PubMed] [Google Scholar]
- 33.Hu J, Liu X, Ge N, et al. Role of endoscopic ultrasound and endoscopic resection for the treatment of gastric schwannoma. Medicine (Baltimore) 2017; 96: e7175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu BG, Wu FJ, Zhu J, et al. Gastric schwannoma: a tumor must be included in differential diagnoses of gastric submucosal tumors. Case Rep Gastrointest Med 2017; 2017: 9615359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Namikawa T, Kobayashi M, Hanazaki K. Gastric schwannoma with regional lymphadenopathy. Clin Gastroenterol Hepatol 2017; 15: e145–e146. DOI: 10.1016/j.cgh.2017.01.029 [DOI] [PubMed] [Google Scholar]
- 36.Shimizu S, Saito H, Kono Y, Murakami Y, et al. Gastric schwannoma with enlargement of the regional lymph nodes resected using laparoscopic distal gastrectomy: report of a patient. Yonago Acta Med 2017; 60: 59–63. [PMC free article] [PubMed] [Google Scholar]
- 37.Tao K, Chang W, Zhao E, et al. Clinicopathologic features of gastric schwannoma: 8-year experience at a single institution in China. Medicine (Baltimore) 2015; 94: e1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sordillo PP, Helson L, Hajdu SI, et al. Malignant schwannoma–clinical characteristics, survival, and response to therapy. Cancer 1981; 47: 2503–2509. [DOI] [PubMed] [Google Scholar]