Abstract
Background
Diabetic nephropathy (DN), the formation of albuminuria, is one of the most important complications seen in diabetic patients. IL-37, an inhibitor of congenital inflammation and immune response, plays an important role in the occurrence and development of diabetes, but its study in DN has not been previously reported.
Material/Methods
Podocyte transfection techniques were used to overexpress STAT3 and cyclophilin A (CypA). The expression of IL-37, STAT3, and CypA was detected by RT-qPCR and western blot. Cell survival was detected by CCK-8. The expression of inflammatory factors and molecules related to oxidative stress was detected by ELISA and western blot, and cell apoptosis was detected by flow cytometry and western blot.
Results
The expression of IL-37 was significantly decreased in high glucose-treated podocytes. IL-37 improved the survival rate of podocytes suffering from high glucose-induced apoptosis. It inhibited the expression of the inflammation-related factors tumor necrosis factor α (TNF-α), IL-1β, IL-6, malondialdehyde (MDA), and lactate dehydrogenase (LDH), and promoted the expression of superoxide dismutase (SOD) in high glucose-treated podocytes. In addition, IL-37 inhibited the expression of the inflammation-related proteins MCP-1, NLRP3, ASC, and caspase-1. IL-37 also inhibited high glucose-induced apoptosis of podocytes by inhibiting the expression of the apoptosis-related proteins Bax and cleaved caspase-3/6/9, and by promoting the expression of Bcl-2. At the same time, we found that the STAT3/CypA signaling pathway was activated after induction by high glucose, while it was inhibited after treatment with IL-37. Overexpression of STAT3 and CypA inhibited the effects of IL-37 on the alleviation of inflammation and oxidative stress and on the reduction of apoptosis of high glucose-treated podocytes.
Conclusions
IL-37 can significantly reduce podocyte inflammation, oxidative stress, and apoptosis induced by high glucose, and can inhibit the STAT3-CypA signaling pathway. Upregulation of the STAT3-CypA signaling pathway can inhibit the protective effect of IL-37 against podocyte injury induced by high glucose.
MeSH Keywords: Apoptosis; Diabetic Nephropathies; Inflammation; Oxidative Stress; Podocytes; Receptors, Interleukin
Background
Diabetic nephropathy (DN) is one of the most common forms of diabetic microangiopathy. High glucose-induced podocyte inflammation, oxidative stress, and apoptosis play a key role in the occurrence and development of DN [1]. In the early stage of DN, there are no obvious clinical symptoms, so it is easily missed. As the disease progresses, renal insufficiency will appear, and eventually develop into the terminal stage of renal failure [2]. Therefore, novel insights into the pathophysiology and clinical aspects of diabetic nephropathy are urgently needed [2]. Podocytes are highly differentiated epithelial cells located in the glomerular basement membrane (GBM), which, together with the GBM and capillary endothelium, constitute the glomerular filtration membrane barrier. The main clinical feature of DN is a significant increase in proteinuria in patients. The occurrence of proteinuria is closely related to the integrity of the glomerular filtration barrier (GFB), the failure of which is a major cause of end-stage nephropathy. Many diabetic animal model studies and cell experiments have suggested that hyperglycemia can cause the destruction of podocyte structure and function, thus leading to the development of DN and proteinuria [3].
IL-37, a newly discovered member of the IL-1 family, is a natural inhibitor of congenital inflammation and immune response. During acute or chronic inflammation, inflammatory stimulation promotes the stabilization of the expression of IL-37 mRNA, preventing excessive inflammation in cells and thereby exerting anti-inflammatory effects[4,5]. IL-37 can reduce myocardial ischemia reperfusion injury in mice, accompanied by myocardial cell apoptosis and reactive oxygen species (ROS) reduction [6]. In addition, IL-37 expression levels have been significantly correlated with insulin resistance in type 2 diabetes and are highly expressed in insulin-sensitive mice. Subsequently, overexpression of IL-37 was found to inhibit the development of diabetes in mice[7]. However, the effects of IL-37 on high glucose-induced podocyte inflammation, oxidative stress, and apoptosis have not been reported.
Signal transducer and activator of transcription (STAT3) regulates the biological behavior of immune cells by mediating extracellular signals of inflammatory mediators. It is an indispensable key molecule in the process of inflammation. The activation of STAT3 promotes the production of inflammatory cytokines and chemokines as well as apoptosis in cells [8]. Cyclophilin A (CypA) is an inflammatory protein that is highly expressed in atherosclerosis and DN [9,10]. It has been reported that the STAT3-CypA signaling pathway stimulates human umbilical vein endothelial cell apoptosis in vitro [11]. In addition, IL-37 can alleviate allergic inflammation in allergic rhinitis by inhibiting the expression of STAT3 and p-STAT3. We hypothesized that IL-37 could inhibit the STAT3-CypA pathway and reduce hyperglycemia-induced podocyte inflammation, oxidative stress, and apoptosis.
In this paper, we mainly studied the effects of IL-37 on high glucose-induced podocyte inflammation, oxidative stress, and apoptosis, and discussed the mechanism underlying these effects, thus providing potential therapeutic targets and ideas for the treatment of DN.
Material and methods
Cell culture and induction
Podocytes (mouse origin) were obtained from the Shanghai Cell Collection (Shanghai, China) and cultured in Dulbecco’s Modified Eagle’s medium (DMEM) (Gibco; Thermo Fisher Scientific) supplemented with 10% fetal bovine serum (FBS) (Gibco; Thermo Fisher Scientific). The podocytes were incubated at 37°C in 5% CO2. To construct the cell model of DN, podocytes were incubated in medium with 30 mM glucose; this group was designated the high-glucose group (HG) [12]. The normal glucose group (medium with 5.5 mM glucose) was used as the control group (NG), and mannitol-treated podocytes were used as the osmotic pressure control group (MA). High-glycemic podocytes incubated with IL-37 (R&D Systems, Minneapolis, MN, USA) were used as the experimental group. Each set of experiments was repeated three times.
RNA extraction and quantitative real-time polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol Reagent (Invitrogen, Thermo Fisher Scientific, Inc.), and RNA concentration and quantification were assessed using a Nanodrop spectrophotometer (Thermo Fisher Scientific, Inc.). RNA was reverse transcribed using the First-Strand cDNA synthesis kit (Takara Biotechnology Co., Ltd., Dalian, China). Subsequently, cDNA was analyzed using a TaqMan® Universal PCR Master Mix kit (Thermo Fisher Scientific, Inc.), according to the manufacturer’s protocols. Amplification conditions were as follows: 95°C for 10 min, followed by 40 cycles of 95°C for 10 sec and 60°C for 60 sec. Primer sequences used in PCR were obtained from GenScript (Piscataway, NJ, USA). The sequences of all primers, including those to amplify GAPDH, are listed in Table 1. The relative quantification of gene expression level was conducted using the 2−ΔΔCq method, and normalized to GAPDH expression [13].
Table 1.
Primer sequences for qRT-PCR.
| Gene | Sequence (5′-3′) |
|---|---|
| IL-37 | Forward CTCCTGGGGGTCTCTAAAGG |
| Reverse TACAATTGCAGGAGGTGCAG | |
| STAT3 | Forward GAGGACTGAGCATCGAGCA |
| Reverse CATGTGATCTGACACCCTGAA | |
| CYPA | Forward AAAGCATACAGGTCCTGGCATC |
| Reverse GGTCCTCAGTGTAGCCCAAG | |
| GAPDH | Forward TGGGTGTGAACCACGAGAA |
| Reverse GGCATGGACTGTGGTCATGA |
Western blot
Total protein was extracted with a Radio Immunoprecipitation Assay (RIPA) lysis solution. The proteins were denatured and then separated using sodium dodecyl sulfonate (SDS) lysis buffer (Cell Signaling Technology, Inc., Danvers, MA, USA) and then transferred onto polyvinylidene difluoride membranes. Following blocking with 5% nonfat milk at room temperature for 2 h, membranes were incubated with the primary antibodies against IL-37, monocyte chemotactic protein 1 (MCP-1), NOD-like receptor family protein 3 (NLPR3), apoptosis-associated speck-like protein containing CARD (ASC), caspase-1, Bcl-2, Bax, cleaved caspase-3, cleaved caspase-6, cleaved caspase-9, caspase-3, caspase-6, caspase-9, STAT3, CypA (all 1: 1,000; all from Cell Signaling Technology, Inc.) at 4°C overnight. Subsequently, the membranes were incubated with secondary antibodies at room temperature for 2 h. GAPDH (1: 5,000; Cell Signaling Technology, Inc.) was used as the internal control. A semiquantitative analysis was conducted using ImageJ software.
Cell Counting Kit-8 (CCK-8)
Cell viability was measured using a CCK-8 assay kit (Dojindo Molecular Technologies, Inc.). Cells were seeded in 96-well plates at a density of 2,000/well. The cells were treated according to their experimental group, 10 μl CCK-8 solution was added to each well, and the plates were incubated at 37°C for 2 h. The absorbance at 450 nm of each group was measured by a multimode reader (Bio-Rad Model 550).
Enzyme-linked immunosorbent assay (ELISA)
Protein levels of tumor necrosis factor α (TNF-α), IL-1β, IL-6, malondialdehyde (MDA), lactate dehydrogenase (LDH), and superoxide dismutase (SOD) were determined using an ELISA kit (Thermo Scientific, Inc.), which displayed results based on the semiquantitative enzyme immunoassay technique. The absorbance of the resulting yellow product was measured at 450 nm. The test samples multiply the interpolated value obtained from the standard curve by a dilution factor to calculate concentrations of human TNF-α, IL-1β, and IL-6 in the sample. The calculations for MDA, LDH, and SOD were conducted according to the instructions in the kit.
Flow cytometry
The collected podocytes were washed with cold PBS and then were collected and stained with Annexin V/PI double stain (Annexin V-FITC, Vazyme Biotech Co. Lt, Nanjing City, China) for 10 min. Cell apoptotic data were then analyzed using flow cytometry (FACSCalibur; Becton-Dickinson, Franklin Lakes, NJ, USA) and quantified by Flow Jo software (version 7.6.1; Flow Jo LLC, Ashland, OR, USA).
Cell transfection
Total RNA was extracted from podocytes, and cDNA was synthesized by reverse transcription. Full-length genes encoding STAT3 and CypA were amplified with specific primers and cloned into the pBabe vector (Addgene company, USA). The cells were transfected with STAT3 and CypA plasmids or negative control (NC) using the Lipofectamine2000 transfer kit (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer’s instructions. Cells of the blank control group (Control) did not receive any treatment.
Statistical analysis
Statistical analysis was conducted using SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA). Data were expressed as the mean±standard deviation. Comparisons between two groups were analyzed with an independent Student’s t-test. One-way ANOVA followed by the Student-Newman-Keuls post-hoc test was used to analyze the differences among multiple groups. P<0.05 was considered to indicate a statistically significant difference.
Results
IL-37 was decreased in high glucose-treated podocytes and this increased their survival rate
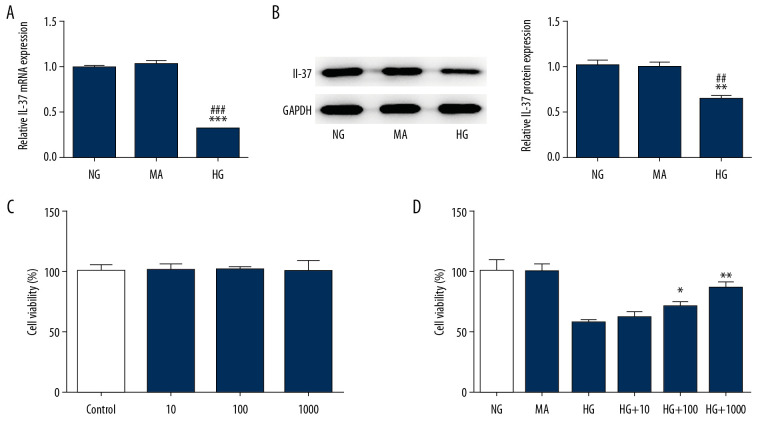
Podocytes were treated with glucose at a concentration of 30 mM (HG group) to establish a model of DN. Glucose treatment at a concentration of 5.5 mM was used as the control group; namely, the NG group. Mannitol treatment was used as the osmotic pressure control group; namely, the MA group. As shown in Figure 1A and 1B, the expression of IL-37 in the HG group decreased significantly compared with the NG group and the MA group. Subsequently, podocytes were treated with IL-37 at concentrations of 0, 10, 100, and 1000 ng/mL for 48 h, and cell survival was detected by CCK-8, with no significant difference found (Figure 1C). However, after treatment of high glucose-treated podocytes with different concentrations of IL-37 for 48 h, we found that, compared with the NG and MA groups, the cell survival rate of HG-group podocytes decreased significantly. Compared with the HG group, the cell survival rate increased significantly with increasing concentrations of IL-37 (Figure 1D). Therefore, we chose 1000 ng/ml IL-37 for follow-up experiments.
Figure 1.
The expression of IL-37 decreased in high glucose-treated podocytes. (A) The expression of IL-37 was detected by RT-qPCR. (B) The expression of IL-37 was detected by western blot. (C, D) The CCK-8 technique was used to detect cell survival rate. ** p<0.01; *** p<0.001 vs. NG; ## p<0.01; ### p<0.001 vs. MA; & p<0.05, && p<0.01 vs. HG. NG – normal glucose group (medium with 5.5 mM glucose); MA – mannitol-treated podocytes used as the osmotic pressure control group; HG – high-glucose group (medium with 30 mM glucose).
IL-37 reduces inflammation and oxidative stress in high glucose-treated podocytes
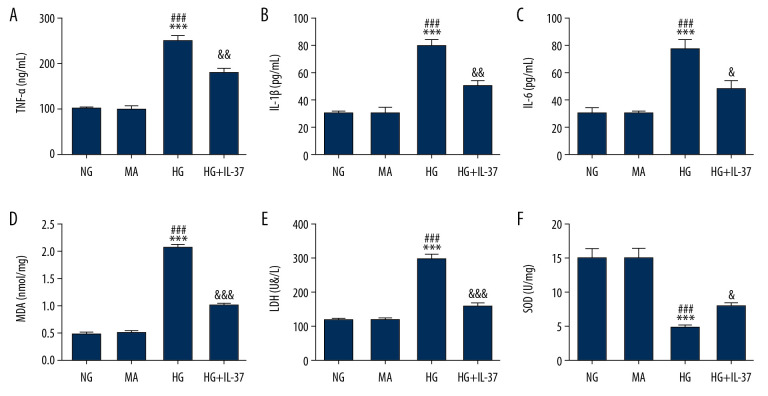
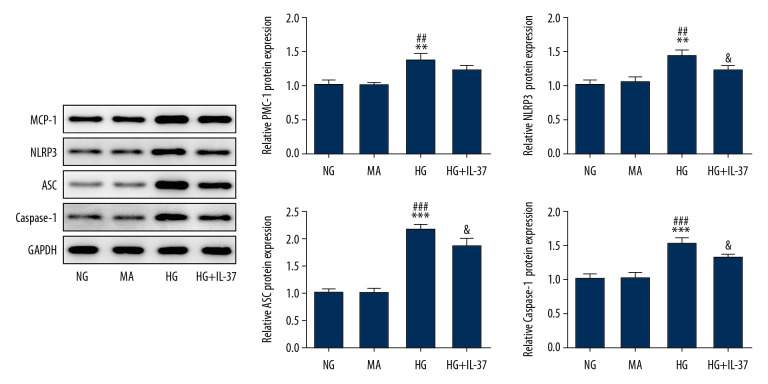
Cells were divided into the NG group, MA group, HG group, and HG+IL-37 group. The expression of inflammatory factors was detected by ELISA. Compared with the NG group and MA group, the expression levels of TNF-α (Figure 2A), IL-1β (Figure 2B), and IL-6 (Figure 2C) in the HG group were significantly increased, indicating that high glucose induced inflammation in podocytes. However, after IL-37 treatment, compared with the HG group, the expression levels of TNF-α, IL-1β, and IL-6 in the HG+IL-37 group were obviously decreased, indicating that IL-37 reversed the expression level of inflammatory cytokines of podocytes treated with high glucose. Subsequently, the oxidative stress level of cells was detected by ELISA. Compared with the NG and MA groups, the expression levels of MDA (Figure 2D) and LDH (Figure 2E) in the HG group were increased and the expression of SOD (Figure 2F) was decreased, indicating that high glucose induced an oxidative stress response in podocytes. However, after IL-37 treatment, compared with the HG group, the expression levels of MDA and LDH decreased, while SOD expression in the HG+IL-37 group increased significantly, indicating that IL-37 reduced the oxidative stress level of podocytes treated with high glucose. Western blot was used to detect the expression level of inflammation-related proteins, such as MCP-1, NLRP3, apoptosis-associated speck-like protein containing a CARD (ASC), and caspase-1. The trend was consistent with the expression level of the inflammatory factors (Figure 3). The results showed that IL-37 can reduce the inflammation and oxidative stress induced in podocytes by high glucose.
Figure 2.
IL-37 reduces inflammation and oxidative stress of high glucose-treated podocytes. The expression of the inflammatory cytokines TNF-α (A), IL-1β (B), and IL-6 (C) as well as the oxidative stress-related molecules MDA (D), LDH (E), and SOD (F) was detected by ELISA. *** p<0.001 vs. NG; ### p<0.001 vs. MA; & p<0.05, && p<0.01, &&& p<0.001 vs. HG. NG – normal glucose group (medium with 5.5 mM glucose); MA – mannitol-treated podocytes used as the osmotic pressure control group; HG – high-glucose group (medium with 30 mM glucose).
Figure 3.
IL-37 reduces inflammation of high glucose-treated podocytes. The expression levels of the inflammatory proteins MCP-1, NLRP3, ASC, and caspase-1 were detected by western blot. ** p<0.01, *** p<0.001 vs. NG; ## p<0.01, ### p<0.001 vs. MA; & p<0.05 vs. HG. NG – normal glucose group (medium with 5.5 mM glucose); MA – mannitol-treated podocytes used as the osmotic pressure control group; HG – high-glucose group (medium with 30 mM glucose).
IL-37 alleviated apoptosis of high glucose-treated podocytes
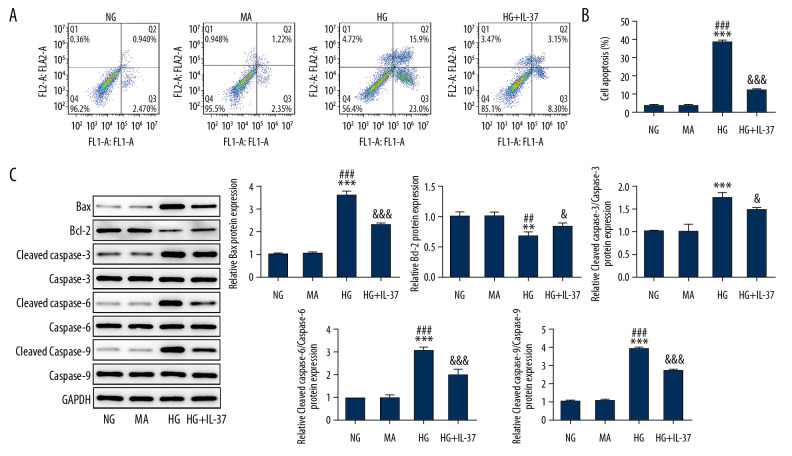
Next, we detected cell apoptosis by flow cytometry and western blot. As shown in Figure 4, we found that, compared with the NG and MA groups, the cell survival rate of the HG group was decreased. This decrease in survival was accompanied by an increase in the apoptotic protein Bax, cleaved-caspase-3, cleaved-caspase-6, and cleaved-caspase-9, and a decrease in bcl-2 protein. Compared with the HG group, the cell survival rate of the HG+IL-37 group increased, accompanied by decreased Bax, cleaved-caspase-3, cleaved-caspase-6, and cleaved-caspase-9 and by increased bcl-2 protein. These results showed that IL-37 can reduce podocyte apoptosis induced by high glucose.
Figure 4.
IL-37 alleviated the apoptosis induced by high-glucose treatment of podocytes. (A) The expression of apoptosis was detected by flow cytometry. (B) Statistical analysis of apoptosis. (C) Western blot was used to detect the expression of apoptosis-related proteins. ** p<0.01, *** p<0.001 vs. NG; ## p<0.01, ### p<0.001 vs. MA; & p<0.05, &&& p<0.001 vs. HG. NG – normal glucose group (medium with 5.5 mM glucose); MA – mannitol-treated podocytes used as the osmotic pressure control group; HG – high-glucose group (medium with 30 mM glucose).
Overexpression of STAT3 and CypA inhibited the IL-37-induced alleviation of inflammation and oxidative stress in high glucose-treated podocytes
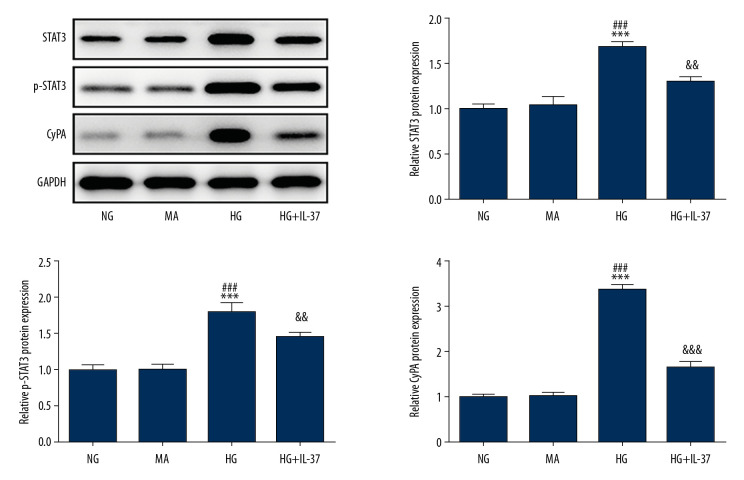
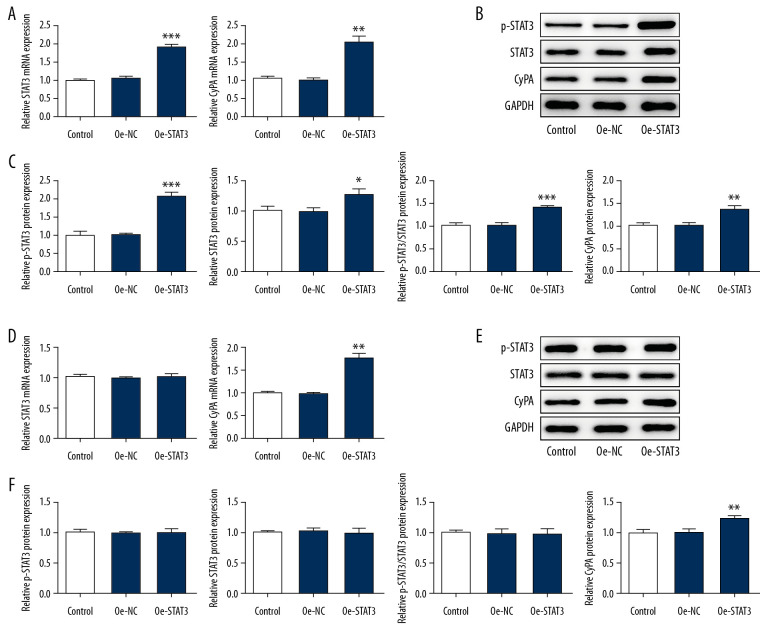
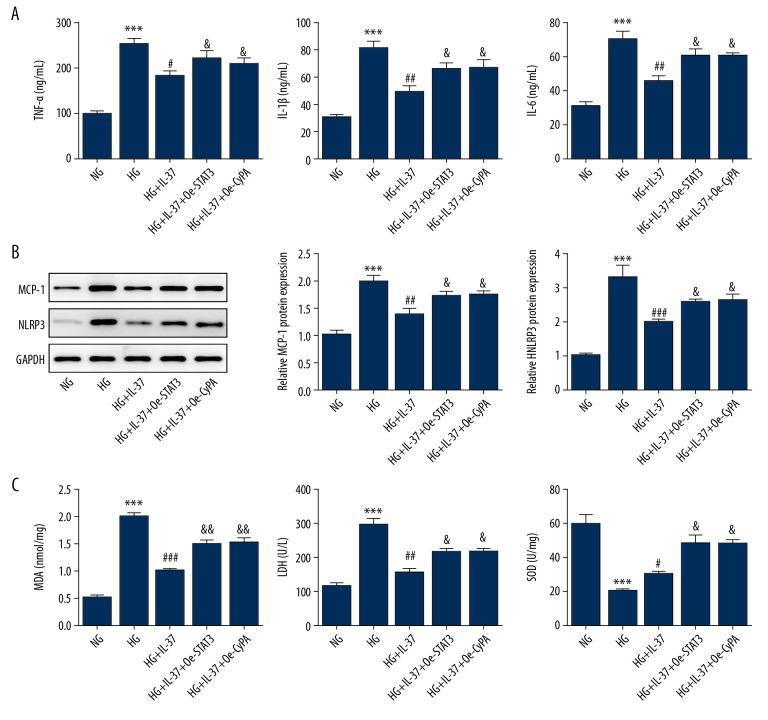
We treated podocyte cells with high glucose and found that the expression of STAT3, p-STAT3, and CypA in the STAT3-CypA signaling pathway increased markedly compared with the NG and MA groups, but decreased significantly after the addition of IL-37 (Figure 5). Therefore, we speculated that IL-37 might play a regulatory role in the inflammation, oxidative stress, and apoptosis of podocytes treated with high glucose via the STAT3-CypA signaling pathway. Therefore, cell transfection technology was used to overexpress STAT3 and CypA, and the expression levels of STAT3 and CypA were detected by western blot and RT-qPCR. Figure 6A shows that, after the detection of overexpression of STAT3 by RT-qPCR, the expression of STAT3 and CypA clearly increased, compared with that of the overexpression negative control (Oe-NC) group. The western blot data showed that, compared with Oe-NC, the expression levels of STAT3, p-STAT3, and CypA were obviously increased after the overexpression of STAT3 alone (Figure 6B, 6C). After CypA overexpression alone, the results of RT-qPCR indicated a clear increase in the expression of CypA, while there was no significant change in the level of STAT3 (Figure 6D), indicating successful transfection and overexpression of CypA. In addition, western blot was used to detect the expression of STAT3, p-STAT3, and CypA, as shown in Figure 6E and 6F. We found that, compared with the Oe-NC group, the expression of CypA in the Oe-CypA group was significantly increased, which was consistent with the results of RT-qPCR, while the expression of STAT3 and p-STAT3 was not changed. Thereafter, we divided the cells into the following groups: NG group, HG group, HG+IL-37 group, HG+IL-37+Oe-STAT3 group, and HG+IL-37+Oe-CypA group. We found that, compared with the NG group, the HG cells showed significantly increased levels of cellular oxidative stress (Figure 7). Specifically, the expression levels of the cytokines TNF-α, IL-1β, and IL-6, and the inflammation-related proteins MCP-1 and NLRP3, were increased. The expression levels of the oxidative stress factors MDA and LDH also increased, while the expression of SOD decreased. However, after the addition of IL-37, the oxidative stress level of the cells decreased significantly. After overexpression of STAT3 and CypA, compared with the HG+IL-37 group, the expression levels of the inflammatory cytokines TNF-α, IL-1β, and IL-6 increased after the overexpression of STAT3 and CypA (Figure 7A), and the expression levels of the inflammation-related proteins MCP-1 and NLRP3 were also elevated (Figure 7B). Furthermore, the expression of oxidative stress parameters such as MDA and LDH increased (Figure 7C), which showed that overexpression of STAT3 and CypA inhibited the effects of IL-37 on the alleviation of inflammation and oxidative stress of podocytes treated with high glucose. However, overexpression of STAT3 and CypA did not decrease the expression of SOD.
Figure 5.
The expression of STAT3/CypA in cells treated with IL-37. Western blot was used to detect the expression of STAT3, p-STAT3, and CypA in the cells. *** p<0.001 vs. NG; ### p<0.001 vs. MA; && p<0.01, &&& p<0.001 vs. HG. NG, normal glucose group (medium with 5.5 mM glucose); MA – mannitol-treated podocytes used as the osmotic pressure control group; HG – high-glucose group (medium with 30 mM glucose).
Figure 6.
Overexpression of STAT3 regulated the expression of downstream CypA in cells treated with IL-37. (A) After overexpression of STAT3, expression levels of STAT3 and CypA were detected by RT-qPCR. (B) After overexpression of STAT3, expression of STAT3 and CypA was detected by western blot. (C) Statistical analysis chart of B. (D) After overexpression of CypA, expression levels of STAT3 and CypA were detected by RT-qPCR. (E) After overexpression of CypA, expression of STAT3 and CypA was detected by western blot. (F) Statistical analysis chart of E. * p<0.05, ** p<0.01, *** p<0.001 vs. Oe-NC. Oe-NC – overexpression-negative control.
Figure 7.
Overexpression of STAT3 and CypA inhibited the IL-37-induced reduction of inflammation and oxidative stress in high glucose-treated podocytes. (A) Expression of the inflammatory cytokines TNF-α, IL-1β, and IL-6 was detected by ELISA. (B) Expression of the inflammatory proteins MCP-1 and NLRP3 was detected by western blot. (C) Expression of the oxidative stress-related factors MDA, LDH, and SOD was detected by ELISA. *** p<0.001 vs. NG; # p<0.05, ## p<0.01, ### p<0.001 vs. HG; & p<0.05 vs. HG+IL-37. NG – normal glucose group (medium with 5.5 mM glucose); HG – high-glucose group (medium with 30 mM glucose); HG+IL-37 – high-glucose group with added IL-37.
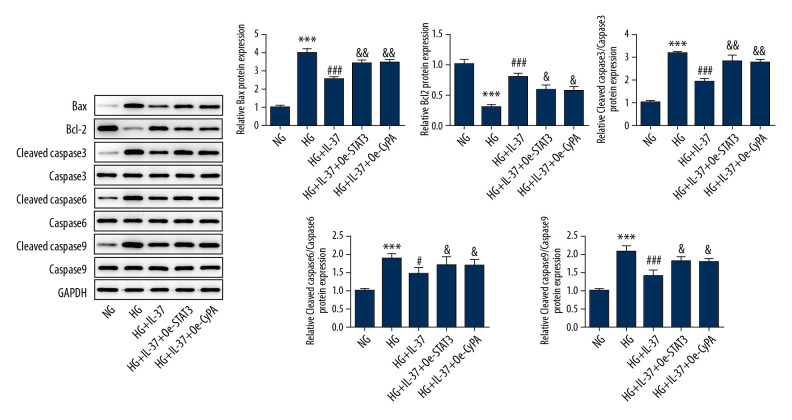
Overexpression of STAT3 and CypA inhibited the anti-apoptotic effects of IL-37 in high glucose-treated podocytes
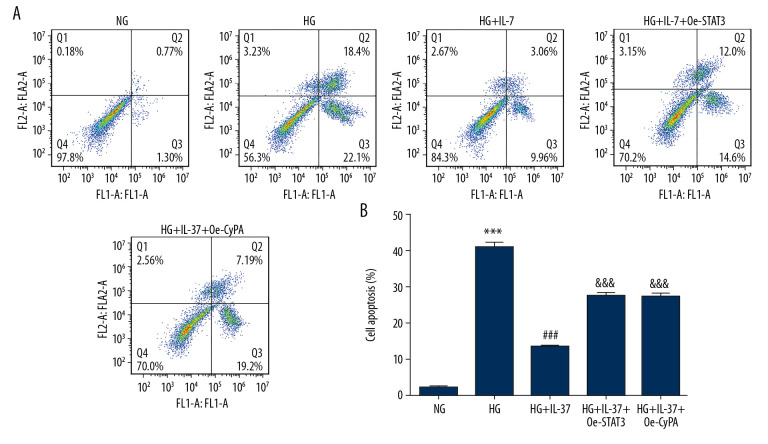
Next, we looked at cell apoptosis. As shown in Figure 8A and 8B, the apoptosis rate of podocytes increased after treatment with high glucose, accompanied by increased expression of Bax, cleaved caspase-3, cleaved caspase-6, and cleaved caspase-9, while there was no significant change in the expression of caspase-3, caspase-6, and caspase-9. Decreased expression of Bcl-2 protein was also found. The apoptosis rate of damaged podocytes decreased substantially after the introduction of IL-37, accompanied by decreased expression of Bax, cleaved caspase-3, cleaved caspase-6, and cleaved caspase-9 and increased expression of Bcl-2 protein. This result is consistent with Figure 4. Compared with the HG+IL-37 group, after the overexpression of STAT3 and CypA, apoptosis increased, accompanied by increased expression of Bax, cleaved caspase-3, cleaved caspase-6, cleaved caspase-9, and decreased expression of Bcl-2 protein (Figure 9), which showed that overexpression of STAT3 and CypA inhibited the reduction in apoptosis by IL-37 in podocytes treated with high glucose.
Figure 8.
Overexpression of STAT3 and CypA inhibited the IL-37-induced reduction of apoptosis in high glucose-treated podocytes. (A) Apoptosis was detected by flow cytometry. B. Statistical analysis of apoptosis. *** p<0.001 vs. NG; ### p<0.001 vs. HG; &&& p<0.001 vs. HG+IL-37. NG – normal glucose group (medium with 5.5 mM glucose); HG – high-glucose group (medium with 30 mM glucose); HG+IL-37 – high-glucose group with added IL-37.
Figure 9.
Overexpression of STAT3 and CypA inhibited the IL-37-induced reduction of apoptosis of high glucose-treated podocytes. Western blot was used to detect the expression of apoptosis-related proteins in cells. *** p<0.001 vs. NG; ### p<0.001 vs. HG; & p<0.05, && p<0.01 vs. HG+IL-37. NG – normal glucose group (medium with 5.5 mM glucose); HG – high-glucose group (medium with 30 mM glucose); HG+IL-37 – high-glucose group with added IL-37.
Discussion
The most common clinical feature of DN is progressive proteinuria due to impairment of the GFB, and the normal morphology and function of podocytes play an important role in proteinuria formation and maintenance of renal function [14,15]. In many studies, podocytes have been treated with high glucose to cause DN in experimental models [16,17]. In this study, we treated podocytes with high glucose to construct a cell model of DN, and found that high glucose induced increased levels of inflammation and cell damage in podocytes. This was consistent with the findings in the literature we reviewed [18].
In this study, we found that the expression of IL-37 was significantly decreased in podocytes treated with high glucose. This finding indicated that the expression of IL-37 was closely related to the development of diabetes. It has been documented that the expression of IL-37 is decreased significantly in type 2 diabetes mellitus and that overexpression of IL-37 inhibits the development of diabetes mellitus [7]. This is consistent with our results. IL-37 inhibits inflammatory responses and thus protects pregnant women from developing gestational diabetes [19]. Moreover, IL-37 reduces the production of pro-inflammatory cytokines in adipose tissue, thereby improving insulin sensitivity in mice with metabolic syndrome [20]. In this study, we treated cells with 1000 ng/mL IL-37 and found that high glucose-induced podocyte inflammation and oxidative stress were clearly reduced. Next, we tested for cell apoptosis and found that IL-37 alleviated apoptosis induced by high-glucose treatment of podocytes. This suggests that IL-37 can reduce DN symptoms by inhibiting inflammation and cell damage.
In the course of the experiment, we found that the expression levels of STAT3, p-STAT3, and CypA were significantly increased in the high-sugar-treated cells, indicating that the STAT3-CypA signaling pathway was activated in these cells upon high glucose stimulation. STAT3 is a transcription factor central to cytokine signaling that regulates the inflammatory response by inducing the expression of cytokines, adhesion molecules, and chemokines. Inhibition of STAT3 can prevent lung inflammation, remodeling, and accumulation of Th2 and Th17 cells in a murine asthma model [21]. CypA is an inflammatory mediator that plays an important role in both atherosclerosis and myocardial inflammation [9,22]. In addition, CypA has been identified as a new biomarker for early diabetic nephropathy [10,23–25]. Moreover, by inhibiting the activation of NF-κB and STAT3, IL-37 can relieve asthma airway inflammation and remodeling [26]. IL-37 also downregulates the expression of phosphorylated STAT6 and phosphorylated STAT3 to reduce inflammation in allergic rhinitis [27].
It has been reported that the STAT3-CypA signaling pathway stimulates apoptosis of human umbilical vein endothelial cells in vitro [11]. Therefore, we speculated that IL-37 could inhibit the STAT3-CypA pathway to alleviate high glucose-induced podocyte inflammation, oxidative stress, and apoptosis. The results of our experiment confirm this speculation. After the addition of IL-37, the expression levels of STAT3, P-STAT3, and CypA decreased, and the levels of cell inflammation, oxidative stress, and apoptosis also decreased significantly, which suggested that IL-37 may inhibit cell inflammation, oxidative stress, and apoptosis by inhibiting the STAT3-CypA signaling pathway.
To further validate our findings, STAT3 and CypA were overexpressed in the high glucose-treated+IL-37-treated podocytes. As a result, the alleviating effects of IL-37 on inflammation and injury of podocytes induced by high glucose were inhibited. This conclusion is also consistent with the experimental results of Wang et al. [27]. However, overexpression of STAT3 and CypA did not decrease the expression of SOD. The reason for this may be that the increase in SOD is pathologic. In the early stage of acute diseases, when the body produces a large amount of free radicals, the body will soon cause oxidative stress, and affect the expression of proteins and other regulatory and response mechanisms. Under conditions of induction of free radicals and the compensatory stress of the body, in order to enhance antioxidant capacity, SOD level will be transiently increased. Therefore, we concluded that upregulation of the STAT3-CypA signaling pathway could inhibit the protective effect of IL-37 against podocyte injury induced by high glucose.
Conclusions
IL-37 can significantly reduce podocyte inflammation, oxidative stress, and apoptosis induced by high glucose, and inhibit the STAT3-CypA signaling pathway. Upregulation of the STAT3-CypA signaling pathway can inhibit the protective effect of IL-37 on podocyte injury induced by high glucose.
Footnotes
Cnflict of interests
None.
Source of support: Departmental sources
References
- 1.Ni WJ, Tang LQ, Wei W. Research progress in signalling pathway in diabetic nephropathy. Diabetes Metab Res Rev. 2015;31(3):221–33. doi: 10.1002/dmrr.2568. [DOI] [PubMed] [Google Scholar]
- 2.Ilyas Z, Chaiban JT, Krikorian A. Novel insights into the pathophysiology and clinical aspects of diabetic nephropathy. Rev Endocr Metab Disord. 2017;18(1):21–28. doi: 10.1007/s11154-017-9422-3. [DOI] [PubMed] [Google Scholar]
- 3.Roselli S, Heidet L, Sich M, et al. Early glomerular filtration defect and severe renal disease in podocin-deficient mice. Mol Cell Biol. 2004;24(2):550–60. doi: 10.1128/MCB.24.2.550-560.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nold-Petry CA, Lo CY, Rudloff I, et al. IL-37 requires the receptors IL-18Ralpha and IL-1R8 (SIGIRR) to carry out its multifaceted anti-inflammatory program upon innate signal transduction. Nat Immunol. 2015;16(4):354–65. doi: 10.1038/ni.3103. [DOI] [PubMed] [Google Scholar]
- 5.Ji Q, Zeng Q, Huang Y, Shi Y, et al. Elevated plasma IL-37, IL-18, and IL-18BP concentrations in patients with acute coronary syndrome. Mediators Inflamm. 2014;2014 doi: 10.1155/2014/165742. 165742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu B, Meng K, Ji Q, et al. Interleukin-37 ameliorates myocardial ischaemia/reperfusion injury in mice. Clin Exp Immunol. 2014;176(3):438–51. doi: 10.1111/cei.12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li T, Li H, Li W, et al. Interleukin-37 sensitize the elderly type 2 diabetic patients to insulin therapy through suppressing the gut microbiota dysbiosis. Mol Immunol. 2019;112:322–29. doi: 10.1016/j.molimm.2019.06.008. [DOI] [PubMed] [Google Scholar]
- 8.Saravanan S, Islam VI, Babu NP, et al. Swertiamarin attenuates inflammation mediators via modulating NF-kappaB/I kappaB and JAK2/STAT3 transcription factors in adjuvant induced arthritis. Eur J Pharm Sci. 2014;56:70–86. doi: 10.1016/j.ejps.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Nigro P, Satoh K, O’Dell MR, et al. Cyclophilin A is an inflammatory mediator that promotes atherosclerosis in apolipoprotein E-deficient mice. J Exp Med. 2011;208(1):53–66. doi: 10.1084/jem.20101174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsai SF, Su CW, Wu MJ, et al. Urinary cyclophilin a as a new marker for diabetic nephropathy: A cross-sectional analysis of diabetes mellitus. Medicine (Baltimore) 2015;94(42):e1802. doi: 10.1097/MD.0000000000001802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie Y, Li X, Ge J. STAT3-CyPA signaling pathway in endothelial cell apoptosis. Cell Signal. 2020;65:109413. doi: 10.1016/j.cellsig.2019.109413. [DOI] [PubMed] [Google Scholar]
- 12.Guo K, Pan P, Wu M, et al. Hyposialylated angiopoietin-like-4 induces apoptosis of podocytes via beta1 Integrin/FAK signaling in diabetic nephropathy. Mol Cell Endocrinol. 2020;505:110730. doi: 10.1016/j.mce.2020.110730. [DOI] [PubMed] [Google Scholar]
- 13.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 14.Kang JS, Lee SJ, Lee JH, et al. Angiotensin II-mediated MYH9 downregulation causes structural and functional podocyte injury in diabetic kidney disease. Sci Rep. 2019;9(1):7679. doi: 10.1038/s41598-019-44194-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Podgorski P, Konieczny A, Lis L, et al. Glomerular podocytes in diabetic renal disease. Adv Clin Exp Med. 2019;28(12):1711–15. doi: 10.17219/acem/104534. [DOI] [PubMed] [Google Scholar]
- 16.Liang X, Wang P, Chen B, et al. Glycogen synthase kinase 3beta hyperactivity in urinary exfoliated cells predicts progression of diabetic kidney disease. Kidney Int. 2020;97(1):175–92. doi: 10.1016/j.kint.2019.08.036. [DOI] [PubMed] [Google Scholar]
- 17.Tu Q, Li Y, Jin J, et al. Curcumin alleviates diabetic nephropathy via inhibiting podocyte mesenchymal transdifferentiation and inducing autophagy in rats and MPC5 cells. Pharm Biol. 2019;57(1):778–86. doi: 10.1080/13880209.2019.1688843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu H, Li Y, Zhang T, et al. Salidroside reduces high-glucose-induced podocyte apoptosis and oxidative stress via upregulating heme oxygenase-1 (HO-1) expression. Med Sci Monit. 2017;23:4067–76. doi: 10.12659/MSM.902806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu Z, Liu J, Zhang R, et al. IL-37 and 38 signalling in gestational diabetes. J Reprod Immunol. 2017;124:8–14. doi: 10.1016/j.jri.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 20.Ballak DB, Li S, Cavalli G, et al. Interleukin-37 treatment of mice with metabolic syndrome improves insulin sensitivity and reduces pro-inflammatory cytokine production in adipose tissue. J Biol Chem. 2018;293(37):14224–36. doi: 10.1074/jbc.RA118.003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gavino AC, Nahmod K, Bharadwaj U, et al. STAT3 inhibition prevents lung inflammation, remodeling, and accumulation of Th2 and Th17 cells in a murine asthma model. Allergy. 2016;71(12):1684–92. doi: 10.1111/all.12937. [DOI] [PubMed] [Google Scholar]
- 22.Heinzmann D, Bangert A, Muller AM, et al. The novel extracellular cyclophilin A (CyPA) – inhibitor MM284 reduces myocardial inflammation and remodeling in a mouse model of troponin I – induced myocarditis. PLoS One. 2015;10(4):e0124606. doi: 10.1371/journal.pone.0124606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdel Ghafar MT, Shalaby KH, Okda HI, et al. Assessment of two novel renal tubular proteins in type 2 diabetic patients with nephropathy. J Investig Med. 2020;68(3):748–55. doi: 10.1136/jim-2019-001135. [DOI] [PubMed] [Google Scholar]
- 24.Chiu PF, Su SL, Tsai CC, et al. Cyclophilin A and CD147 associate with progression of diabetic nephropathy. Free Radic Res. 2018;52(11–12):1456–63. doi: 10.1080/10715762.2018.1523545. [DOI] [PubMed] [Google Scholar]
- 25.Tsai SF, Hsieh CC, Wu MJ, et al. Novel findings of secreted cyclophilin A in diabetic nephropathy and its association with renal protection of dipeptidyl peptidase 4 inhibitor. Clin Chim Acta. 2016;463:181–92. doi: 10.1016/j.cca.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Huang N, Liu K, Liu J, et al. Interleukin-37 alleviates airway inflammation and remodeling in asthma via inhibiting the activation of NF-kappaB and STAT3 signalings. Int Immunopharmacol. 2018;55:198–204. doi: 10.1016/j.intimp.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 27.Wang J, Shen Y, Li C, et al. IL-37 attenuates allergic process via STAT6/STAT3 pathways in murine allergic rhinitis. Int Immunopharmacol. 2019;69:27–33. doi: 10.1016/j.intimp.2019.01.013. [DOI] [PubMed] [Google Scholar]