Abstract
Cystic fibrosis (CF) is a genetic disease caused by mutations in the CFTR gene. Although viral respiratory tract infections are, in general, more severe in patients with CF compared with the general population, a small number of studies indicate that SARS-CoV-2 does not cause a worse infection in CF. This is surprising since comorbidities including preexisting lung disease have been reported to be associated with worse outcomes in SARS-CoV-2 infections. Several recent studies provide insight into why SARS-CoV-2 may not produce more severe outcomes in CF. First, ACE and ACE2, genes that play key roles in SARS-CoV-2 infection, have some variants that are predicted to reduce the severity of SARS-CoV-2 infection. Second, mRNA for ACE2 is elevated and mRNA for TMPRSS2, a serine protease, is decreased in CF airway epithelial cells. Increased ACE2 is predicted to enhance SARS-CoV-2 binding to cells but would increase conversion of angiotensin II, which is proinflammatory, to angiotensin-1–7, which is anti-inflammatory. Thus, increased ACE2 would reduce inflammation and lung damage due to SARS-CoV-2. Moreover, decreased TMPRSS2 would reduce SARS-CoV-2 entry into airway epithelial cells. Second, many CF patients are treated with azithromycin, which suppresses viral infection and lung inflammation and inhibits the activity of furin, a serine protease. Finally, the CF lung contains high levels of serine protease inhibitors including ecotin and SERPINB1, which are predicted to reduce the ability of TMPRSS2 to facilitate SARS-CoV-2 entry into airway epithelial cells. Thus, a variety of factors may mitigate the severity of SARS-CoV-2 in CF.
Keywords: ACE2, COVID-19, cystic fibrosis, SARS-CoV-2, TMPRSS2
INTRODUCTION: CYSTIC FIBROSIS
Cystic fibrosis (CF), the most common genetic disease in Caucasians, is caused by mutations in the CFTR gene (63). Approximately 80,000 people have CF, and most die prematurely (mean age of death in 2018 was 30.8 yr) from respiratory/cardiorespiratory failure due to chronic bacterial infections of the lungs (22, 20a, 61). Mutations in CFTR reduce the abundance of functional CFTR ion channels in the apical cell membrane of airway epithelial cells, leading to a dramatic reduction in chloride and bicarbonate secretion, a decrease in the depth and pH of the airway surface liquid, and hypersecretion of thick, sticky mucus by submucosal glands, which, together, lead to ciliastasis and an inability to clear bacteria and other pathogens from the lungs (8, 71). In addition, a reduction of CFTR in the plasma membrane of airway epithelial cells increases epithelial sodium channel (ENaC)-mediated sodium absorption, which also reduces the volume of airway surface liquid and mucociliary clearance (45, 63, 71). The lungs of people with CF become colonized with bacteria very early in life because of decreased mucociliary clearance, the release of DNA by neutrophils, which increases mucus viscosity, acidification of the periciliary layer that reduces the activity of antimicrobial peptides, and a reduction in the ability of macrophages and neutrophils to eliminate bacteria (7, 61). Although there is a high degree of variability in the lung microbiome in CF, the most common pathogens include Pseudomonas aeruginosa, Staphylococcus aureus, methicillin-resistant S. aureus (MRSA), and Aspergillus species (6, 61). Clinical exacerbations requiring hospitalization caused by bacterial and viral infections are associated with a precipitous decline in lung function, which often does not recover after hospitalization and antibiotic treatment (22, 20a, 61). Moreover, CF is a multiorgan disease that leads to exocrine pancreatic insufficiency resulting in diabetes, gastrointestinal malabsorption that results in malnutrition and impaired growth, and sinusitis (22, 20a, 61). Mutations in CFTR also adversely impact innate and adaptive immunity, dramatically reduce the ability of neutrophils and macrophages in the lungs to eliminate bacteria, and dramatically increase the levels of proinflammatory cytokines in the lungs, even in the absence of infection (7, 14, 61, 69, 74, 83). The most common mutation in CFTR results in a deletion of a phenylalanine (Phe508del), which leads to premature degradation of CFTR in the proteasome and activation of the unfolded protein response that has adverse effects on protein processing and a number of other biological pathways (5, 46). In addition, there is considerable phenotypic variation in CF, even in cohorts with the same mutation in CFTR (61). This is due to modifier genes, including SLC26A9, SLC9A3, SLC6A14, TNF, and TGFb1, as well as secondhand smoke and pollutants (61). Fortunately, significant advances in clinical care, as well as the Food and Drug Administration (FDA) approval of new drugs, including DNase, antibiotics, and so-called potentiator and corrector drugs developed by Vertex Pharmaceuticals, have dramatically improved lung function in individuals with CF and have significantly improved outcomes and life expectancy (13, 20a, 27, 43, 67). As a result of the advances in clinical care and new drugs, the median predicted survival age of those born in 2018 is 47.4 yr according to the 2018 CF Foundation Registry report (20a).
VIRAL LUNG INFECTIONS IN CF
Studies have shown that viral infections cause ~60% of acute pulmonary exacerbations in CF (58, 77). In 2009 the influenza (H1N1) pandemic caused significant morbidity in patients with CF, including a precipitous decline in respiratory function and death (15, 18). In a large study comparing patient data from the CF Patient Registry and viral infection surveillance data from the World Health Organization (WHO), a strong correlation was observed between pulmonary exacerbations and respiratory syncytial virus (RSV) infection in children (70). CF patients have a higher prevalence of RSV and a higher viral load in bronchoalveolar lavage fluid (BALF) than non-CF patients (37). Moreover, ~15–25% of CF patients with a respiratory viral infection also culture positive for a known CF bacterial pathogen (23, 50, 78). Coinfection with RSV and P. aeruginosa is associated with declines in pulmonary function and increased morbidity and mortality (28, 53). Factors that contribute to morbidity during respiratory viral infection in CF include reduced antiviral immunity by airway epithelial cells, resulting in increased viral replication (17, 36, 37, 40, 56, 57, 65, 85). In a recent study, infection of CF airway epithelial cells with P. aeruginosa reduced the interferon response to RSV and increased the RSV load (16). RSV also increased P. aeruginosa biofilm formation by inducing the transcytosis of iron, a critical nutrient, across airway epithelial cells into the airway surface liquid (29).
Given that the severity of many respiratory viral infections is more severe in CF than non-CF individuals, it is surprising, but heartening, that in preliminary reports on 51 individuals with CF, some with severe lung disease, SARS-CoV-2, which causes significant morbidity and mortality especially in those with preexisting medical conditions, did not cause worse outcomes in CF (18, 20, 59). One possible reason for this may be that SARS-CoV-2, influenza, and RSV infect cells by different mechanisms (10, 31). Although in initial reports the percentage of CF individuals infected with SARS-CoV-2 appears lower than that in the general population, this may be because CF patients have always paid close attention to infection control and social distancing (18, 20). Clearly, more data on a larger set of CF patients are needed to determine the true impact of SARS-CoV-2 on CF lung disease.
In this review, we first discuss recent studies on SARS-CoV-2, and then we review the literature on CF to predict how SARS-CoV-2 may impact individuals with CF. In addition, on the basis of our review of the literature, we propose several areas of investigation that might identify targets for drug development to combat SARS-CoV-2 infection.
SARS-CoV-2
SARS-CoV-2, a highly contagious positive-strand RNA virus, is the cause of the COVID-19 global pandemic (51, 73, 86). Compared with other respiratory viruses it is highly infectious and has a high fatality rate (73, 86). Individuals with preexisting medical conditions, notably hypertension, diabetes, cardiovascular disease, cerebrovascular illness, and chronic obstructive pulmonary disease (COPD), have worse clinical outcomes when infected with SARS-CoV-2 than those without preexisting conditions (73, 86). Cell entry of SARS-CoV-2 depends on binding of the spike (S) coat protein to angiotensin-converting enzyme2 (ACE2), which is present on the plasma membrane of airway epithelial cells, goblet secretory cells, and type II pneumocytes, and on S protein priming by the serine protease transmembrane serine protease 2 (TMPRSS2) (30, 88). Mutations in the SARS-CoV-2 S protein increase its affinity for ACE2 compared with SARS-CoV S protein, making it highly infectious (80). Compared with influenza and RSV, SARS-CoV-2 elicits a less robust cytokine response, including the type I and type III interferons, which may result in worse outcomes (12). Severe cases of SARS-CoV-2 infection progress to acute respiratory distress syndrome, a condition that is characterized by the excessive production of proinflammatory cytokines and chemokines, which recruit monocytes, macrophages, and T cells to the lungs (73). This, in turn, leads to the production of additional proinflammatory cytokines, which damages the lungs and leads to respiratory failure, the major cause of death in patients infected with SARS-CoV-2 (73). In addition, 50% of patients with SARS-CoV-2 who died also had a bacterial coinfection (86). As of this writing, numerous clinical trials are underway to develop therapies to reduce the severity of SARS-CoV-2 infection, including, but not limited to, vaccines, antivirals, drugs to repair airway damage, and drugs to reduce SARS-CoV-2 infectivity and reduce inflammation (73).
DO VARIATIONS IN THE EXPRESSION AND FUNCTION OF ACE2, ACE, AND TMPRSS2 AFFECT THE SEVERITY OF SARS-CoV-2 LUNG INFECTIONS?
Studies have identified several variants of the ACE2 gene, leading to the suggestion that ACE2 polymorphisms may alter host susceptibility to SARS-CoV-2 by affecting ACE2-S protein interaction and/or ACE2 abundance (72). ACE2 mediates antiproliferative and antifibrotic effects by downregulating angiotensin II (ANG II) and counterbalances the proinflammatory effects of angiotensin-converting enzyme (ACE) (64) (Fig. 1A). Stawiski et al. (72) examined ACE2 variants that are predicted to alter the virus-host interaction and thereby potentially alter host susceptibility to SARS-CoV-2. Several variants including S19P, I21V, E23K, K26R, T27A, N64K, T92I, Q102P, and H378R are predicted to increase binding to the S protein, and thereby increase susceptibility to infection, whereas other variants including K31R, N33I, H34R, E35K, E37K, D38V, Y50F, N51S, M62V, K68E, F72V, Y83H, G326E, G352V, D355N, Q388L, and D509Y are predicted to decrease binding to the S protein. Although ACE2 variants are rare, they may, at least in part, account for some of the variability in respiratory symptoms with SARS-CoV-2 that have been reported, including in those with CF (73). Given that ACE2 was recently demonstrated to be an interferon (IFN)-stimulated gene in nasal epithelial cells, SARS-CoV-2 may exploit IFN-driven upregulation of ACE2, a key tissue-protective mediator during lung injury, to enhance infection (35, 88). On the other hand, SARS-CoV has been reported to reduce ACE2 expression in lung cells, which is associated with acute lung injury (41, 73). Given that the ACE2 gene is located on the X chromosome, the increased severity of SARS-CoV-2 infection in men may be explained by decreased ACE2 activity, which would increase levels of ANG II and lung damage (66).
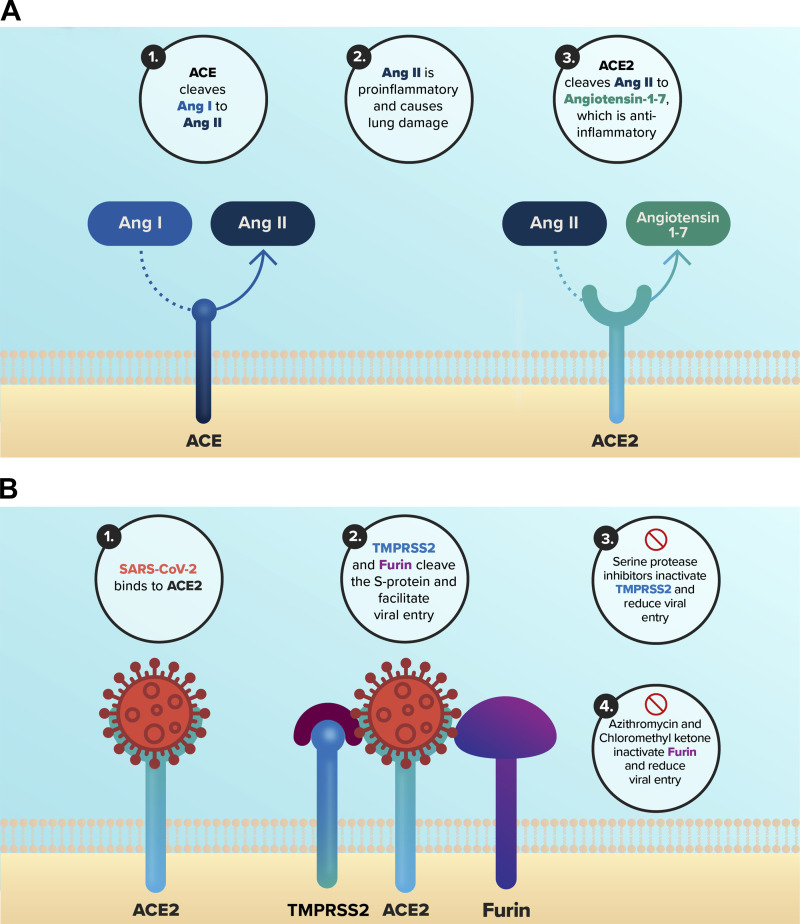
Fig. 1.
Role of angiotensin-converting enzyme (ACE), ACE2, and TMPRSS2 in SARS-CoV-2 lung infection. A: ACE cleaves angiotensin I (ANG I) to angiotensin II (ANG II), which is proinflammatory and causes lung damage. ACE2 processes ANG II to angiotensin-1–7, which is anti-inflammatory. Thus, a decrease in ACE and/or an increase in ACE2 would reduce inflammation and lung damage due to SARS-CoV-2. B: 1) SARS-CoV-2 binds to ACE2. 2) The S protein is cleaved by TMPRSS2 and furin, which facilitates viral entry into cells. 3) Several serine protease inhibitors in the cystic fibrosis (CF) lung, including ecotin and SERP1NR1, and the drugs camostat mesylate and nelfinavir mesylate inhibit TMPRSS2. 4). Azithromycin and chloromethyl ketone inhibit furin. The inhibitors of TMPRSS2 and furin are predicted to reduce SARS-CoV-2 entry into airway epithelial cells.
Deletions in the ACE gene have also been described that effect the severity of chronic obstructive pulmonary disease (COPD) and CF. A deletion of a 287-bp DNA fragment in intron 16 of the ACE gene increases the level of ACE activity and subsequent ANG II levels and has been associated with increased severity of COPD (68). In addition, CF individuals homozygous for the deletion (D/D genotype) in the ACE gene have early onset of respiratory disease, increased inflammation, enhanced colonization by Burkholderia cepacia, and reduced lung function (44). By contrast, those who lack the deletion (I/I genotype), an effect predicted to decrease ACE activity, have better lung function (44). The genotype frequencies in a CF cohort of 180 patients were 40% with D/D, 47% with D/I, and 13% with I/I. The increased ACE activity with the D/D genotype shifts the ACE/ACE2 balance toward ACE and elevated ANG II, leading to increased proinflammatory cytokine release, and lends credence to the hypothesis that decreased ACE2 activity increases the severity of lung inflammation (79)(Fig. 1A). Clearly, additional studies on the role of ACE2 and ACE variants and the effect of ACE, ACE2, and ANG II levels on SARS-CoV-2 infection in CF are required.
Because changes in ACE2 levels may affect the severity of SARS-CoV-2 infection (2, 32), we analyzed publicly available gene microarray data to determine whether ACE2 and TMPRSS2 gene expression is altered in CF, an effect that could explain why SARS-CoV-2 may not lead to worse outcomes in CF patients than in the general population (18, 20, 59). Our analysis revealed that ACE2 mRNA is elevated and TMPRSS2 mRNA is decreased in CF airway epithelial cells compared with non-CF cells (26, 76) (Fig. 2). Increased ACE2 is predicted to enhance SARS-CoV-2 binding to epithelial cells but would increase conversion of ANG II, which is proinflammatory, to angiotensin-1–7, which is anti-inflammatory (Fig. 1A). Thus, increased ACE2 would reduce inflammation and lung damage due to SARS-CoV-2 (2, 32). Moreover, decreased TMPRSS2 would reduce SARS-CoV-2 entry into airway epithelial cells. Taken together, this analysis suggests that mutations in the CFTR gene may alter the protein abundance of ACE2 and TMPRSS2 in such a way as to mitigate the effects of SARS-CoV-2 infection on lung damage (Fig. 2). In our review of studies examining the proteome of CF and non-CF airway epithelial cells, none identified ACE2 or TMPRSS2, perhaps because of detection limits of proteomic analysis (55, 62). Thus, an analysis of ACE2 and TMPRSS2 protein abundance in CF and non-CF airway epithelial cells is warranted to determine whether mutations in the CFTR gene affect ACE2 and TMPRSS2 protein abundance, and therefore may affect the severity of lung damage in CF patients infected with SARS-CoV-2.
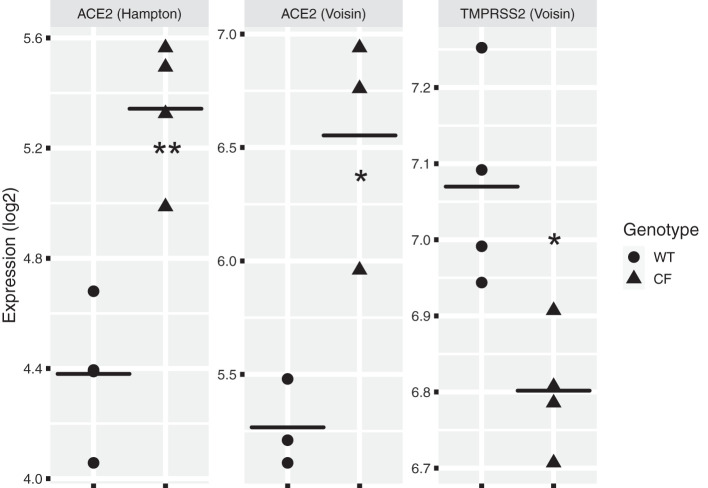
Fig. 2.
Analysis of published gene array data using ScanGeo (39) to examine the effect of mutations in the CFTR gene on angiotensin-converting enzyme (ACE), ACE2, and TMPRSS2 mRNA. We used ScanGEO (http://scangeo.dartmouth.edu/ScanGEO/) to conduct a meta-analysis of ACE2 and TMPRSS2 genes in human airway epithelial cell microarray data (39). Twenty-three data sets were identified in ScanGEO by selecting genus (Homo) from a pulldown list and entering “airway epithelia” into a text box. Next, ACE, ACE2, and TMPRSS2 were uploaded from a comma-separated value file. Two studies comparing cystic fibrosis (CF) and non-CF [wild type (WT)] airway epithelia were identified by ScanGEO, and statistical differences in gene expression between the CF and non-CF data sets in each study were analyzed by ANOVA using a threshold of P < 0.05, the default setting. Data from these studies were downloaded from ScanGEO, as well as data from an additional RNA-seq data set (89) and 1 other microarray data set (76) and further analyzed in R. ACE2 in the studies by Hampton et al. (26) and Voisin et al. (76) was increased in CF compared with non-CF airway epithelial cells. TMPRSS2 mRNA was decreased in CF vs. non-CF airway epithelial cells (76). However, 1 RNA-seq study on CF and non-CF cells did not identify a difference in ACE2 and TMPRSS2 mRNA levels (89). Our analysis also revealed that ACE mRNA was not differentially expressed in CF cells vs. non-CF airway epithelial cells. There are many possible reasons why mutations in CFTR do not elicit a reproducible effect on ACE2 and TMPRSS2 mRNA levels, including differences in the methods used to assess mRNA levels, differences in the cells examined, and differences in cell culture techniques. Thus, additional studies are needed to examine ACE2 and TMPRSS2 mRNA and protein levels, preferably on freshly isolated CF and non-CF nasal and airway epithelial cells. Each data point in this figure represents a sample. *P < 0.05, **P < 0.01 (Welch’s t test).
DOES AZITHROMYCIN REDUCE THE SEVERITY OF SARS-CoV-2 IN CF BY REDUCING INFLAMMATION AND INHIBITING VIRAL REPLICATION?
It has been suggested that the antibiotic azithromycin in combination with chloroquine may be useful to treat SARS-CoV; however, studies have identified adverse cardiovascular effects with chloroquine including risk of corrected QT prolongation and cardiac arrhythmias (11, 48). However, many CF patients are routinely prescribed azithromycin, without chloroquine, and the clinical efficacy of azithromycin in CF has been attributed to its anti-inflammatory effect (60). Azithromycin has also been demonstrated to have antiviral effects in a model of COPD (47). Moreover, azithromycin reduces ENaC-mediated sodium reabsorption by airway epithelial cells, an effect predicted to increase mucociliary clearance of lung pathogens (25). Recent studies have also suggested that azithromycin and the antibiotic ciprofloxacin, which are weak bases, may reduce SARS-CoV-2 entry into airway epithelial cells by increasing the pH of endosomes and lysosomes (60), since it has been shown that an increase in organelle pH reduces SARS-CoV entry (81). Thus, because severe cases of SARS-CoV-2 are associated with a cytokine storm and excessive inflammation, clinical studies with azithromycin and ciprofloxacin alone in SARS-CoV-2 infections may be warranted.
SERINE PROTEASE INHIBITORS AND FURIN IN THE CF LUNG: EFFECTS ON SARS-CoV-2 DISEASE SEVERITY
Several studies have shown that serine protease inhibitors reduce SARS-CoV infection, most likely by inactivating the serine protease TMPRSS2 (30, 87). The serine protease inhibitor camostat mesylate, a drug approved in Japan for several diseases including chronic pancreatitis, some cancers, and viral infections, reduced mortality after SARS-CoV infection in mice from 100% to 30–35% (87). In addition, camostat mesylate also reduced SARS-CoV-2 infection in Calu-3 cells, a lung cell line (30). The anti-HIV drug nelfinavir mesylate (Viracept), also an inhibitor of serine proteases, reduces cell fusion induced by the SARS-CoV-2 S protein (52). Camostat mesylate is now in clinical trials for SARS-CoV-2 (ClinicalTrials.gov Identifier: NCT04321096).
Elevated levels of serine protease inhibitors including ecotin and Serpin Family B Member 1 (SERPINB1) have been identified in the CF lung (9, 19, 75). Ecotin, a serine protease inhibitor secreted by P. aeruginosa, which chronically infects the lungs in a majority of adults with CF (20a, 42), has been shown to protect P. aeruginosa from neutrophil elastase-mediated killing (75). SERPINB1, a serine protease inhibitor expressed and released by neutrophils, which are abundant in the CF lungs, is found at increased levels in CF bronchoalveolar lavage fluid and inactivates three neutrophil elastases that mediate lung damage in P. aeruginosa infection (9, 19). Taken together, these studies suggest that elevated levels of ecotin and SERPINB1 in the CF lung may be protective by inhibiting the protease activity of TMPRSS2, which plays a key role in SARS-CoV-2 infection (Fig. 1B). Additional studies are required to determine whether serine protease inhibitors, including camostat mesylate, nelfinavir mesylate, ecotin, and SERPINB1, reduce SARS-CoV-2 lung infection in CF.
By contrast, the levels of furin, a serine protease, are elevated in the CF lung, an effect predicted to enhance SARS-CoV-2 infection in CF, since the SARS-CoV-2 S-glycoprotein has a furin cleavage site (Fig. 1B) (1, 33, 54). Furin-mediated cleavage at the S1/S2 site in infected cells might enhance SARS-CoV-2 infection, as reported for Middle East respiratory syndrome-related coronavirus (MERS-CoV) (38). Other serine proteases in the lungs, including trypsin and plasmin, may enhance SARS-CoV-2 entry into epithelial cells by activating the S protein (21, 34). It is interesting to note that azithromycin reduces furin levels in CF airway epithelial cells, an observation that lends additional support to the hypothesis that azithromycin may be a useful treatment for SARS-CoV-2 infection (Fig. 1B) (60). Additional studies are required to determine whether elevated furin in the CF lungs exacerbates SARS-CoV-2 infection and whether a furin convertase inhibitor (chloromethyl ketone), which reduces MERS-CoV entry in cells in vitro, reduces SARS-CoV-2 infection in CF (Fig. 1B) (49, 73).
DO MUTATIONS IN CFTR HAVE ADVERSE EFFECTS ON THE PROCESSING AND FUNCTION OF ACE, ACE2, AND TMPRSS2 AND THEREBY REDUCE SARS-CoV-2 ENTRY AND VIRAL REPLICATION IN CF?
CFTR interacts with a large number of proteins in airway epithelial cells including scaffolding proteins, other ion channels, electroneutral ion transporters, and membrane receptors—the so-called CFTR interactome—and mutations in CFTR have adverse effects on the function of CFTR interacting proteins (3–5, 84). For example, mutations in CFTR lead to an increase in sodium reabsorption by the ENaC sodium channel in airway epithelial cells, which decreases airway surface fluid volume and reduces mucociliary clearance. In addition, mutations in CFTR also increase the amount of lysophosphatidic acid receptor 2 in the plasma membrane of airway epithelial cells, which increases the secretion of IL-8, an effect that stimulates the migration of neutrophils into the lungs and enhances phagocytosis of bacteria by macrophages (84). Moreover, mutations in CFTR, by altering the pH of organelles in the protein secretory pathway, may alter the glycosylation of ACE, ACE2, and TMPRSS2 as well as the SARS-CoV-2 spike protein (60). Thus, we speculate that mutations in CFTR may have adverse effects on the processing and/or function of ACE, ACE2, TMPRSS2, and the S protein and thereby reduce SARS-CoV-2 entry and viral replication in CF. Because, to our knowledge, nothing is known about the effect of CFTR mutations on ACE, ACE2, TMPRSS2, and SARS-CoV-2 spike protein levels or activity, studies on this topic may provide novel insight into why CF may not be associated with increased morbidity in SARS-CoV-2 infection.
CONCLUSIONS
Taken together, the studies cited in this review suggest that a variety of factors may mitigate the severity of SARS-CoV-2 in CF patients and identify several potential targets for additional studies to reduce the severity of SARS-CoV-2 in individuals with CF and in the general population. These include ecotin, SERPINB1, camostat mesylate, nelfinavir mesylate, chloromethyl ketone, azithromycin, and ciprofloxacin, several of which are already in clinical trials.
GRANTS
The research described by the authors in this article was supported by the National Institutes of Health (R01 HL074175, R01 HL122372, R01 HL151385, and P30 DK117469), a Cystic Fibrosis Foundation Research Development Program grant (STANTO19R0), and Cystic Fibrosis Foundation research grants (STANTO16GO, STANTO20PO).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.A.S. prepared figures; B.A.S. and A.A. drafted manuscript; B.A.S., T.H.H., and A.A. edited and revised manuscript; B.A.S., T.H.H., and A.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank our colleagues who have contributed to the development of our research on CF, and Small Island Design for Figure 1.
REFERENCES
- 1.Abassi ZA, Skorecki K, Heyman SN, Kinaneh S, Armaly Z. Covid-19 infection and mortality: a physiologist’s perspective enlightening clinical features and plausible interventional strategies. Am J Physiol Lung Cell Mol Physiol 318: L1020–L1022, 2020. doi: 10.1152/ajplung.00097.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.AlGhatrif M, Cingolani O, Lakatta EG. The dilemma of coronavirus disease 2019, aging, and cardiovascular disease: insights from cardiovascular aging science. JAMA Cardiol 5: 747–748, 2020. doi: 10.1001/jamacardio.2020.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amaral MD, Balch WE. Hallmarks of therapeutic management of the cystic fibrosis functional landscape. J Cyst Fibros 14: 687–699, 2015. doi: 10.1016/j.jcf.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amaral MD, Hutt DM, Tomati V, Botelho HM, Pedemonte N. CFTR processing, trafficking and interactions. J Cyst Fibros 19, Suppl 1: S33–S36, 2020. doi: 10.1016/j.jcf.2019.10.017. [DOI] [PubMed] [Google Scholar]
- 5.Balch WE, Roth DM, Hutt DM. Emergent properties of proteostasis in managing cystic fibrosis. Cold Spring Harb Perspect Biol 3: a004499, 2011. doi: 10.1101/cshperspect.a004499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ballok AE, O’Toole GA. Pouring salt on a wound: Pseudomonas aeruginosa virulence factors alter Na+ and Cl− flux in the lung. J Bacteriol 195: 4013–4019, 2013. doi: 10.1128/JB.00339-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnaby R, Koeppen K, Nymon A, Hampton TH, Berwin B, Ashare A, Stanton BA. Lumacaftor (VX-809) restores the ability of CF macrophages to phagocytose and kill Pseudomonas aeruginosa. Am J Physiol Lung Cell Mol Physiol 314: L432–L438, 2018. doi: 10.1152/ajplung.00461.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartoszewski R, Matalon S, Collawn JF. Ion channels of the lung and their role in disease pathogenesis. Am J Physiol Lung Cell Mol Physiol 313: L859–L872, 2017. doi: 10.1152/ajplung.00285.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benarafa C, Priebe GP, Remold-O’Donnell E. The neutrophil serine protease inhibitor serpinb1 preserves lung defense functions in Pseudomonas aeruginosa infection. J Exp Med 204: 1901–1909, 2007. doi: 10.1084/jem.20070494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benhaim MA, Lee KK. New biophysical approaches reveal the dynamics and mechanics of type I viral fusion machinery and their interplay with membranes. Viruses 12: 413, 2020. doi: 10.3390/v12040413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bessière F, Roccia H, Delinière A, Charrière R, Chevalier P, Argaud L, Cour M. Assessment of QT intervals in a case series of patients with coronavirus disease 2019 (COVID-19) infection treated with hydroxychloroquine alone or in combination with azithromycin in an intensive care unit. JAMA Cardiol, 2020. doi: 10.1001/jamacardio.2020.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blanco-Melo D, Nilsson-Payant BE, Liu WC, Uhl S, Hoagland D, Møller R, Jordan TX, Oishi K, Panis M, Sachs D, Wang TT, Schwartz RE, Lim JK, Albrecht RA, tenOever BR. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 181: 1036–1045.e9, 2020. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bose SJ, Krainer G, Ng DR, Schenkel M, Shishido H, Yoon JS, Haggie PM, Schlierf M, Sheppard DN, Skach WR. Towards next generation therapies for cystic fibrosis: folding, function and pharmacology of CFTR. J Cyst Fibros 19, Suppl 1: S25–S32, 2020. doi: 10.1016/j.jcf.2019.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruscia EM, Bonfield TL. Cystic fibrosis lung immunity: the role of the macrophage. J Innate Immun 8: 550–563, 2016. doi: 10.1159/000446825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bucher J, Boelle PY, Hubert D, Lebourgeois M, Stremler N, Durieu I, Bremont F, Deneuville E, Delaisi B, Corvol H, Bassinet L, Grenet D, Remus N, Vodoff MV, Boussaud V, Troussier F, Leruez-Ville M, Treluyer JM, Launay O, Sermet-Gaudelus I. Lessons from a French collaborative case-control study in cystic fibrosis patients during the 2009 A/H1N1 influenza pandemy. BMC Infect Dis 16: 55, 2016. doi: 10.1186/s12879-016-1352-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chattoraj SS, Ganesan S, Faris A, Comstock A, Lee WM, Sajjan US. Pseudomonas aeruginosa suppresses interferon response to rhinovirus infection in cystic fibrosis but not in normal bronchial epithelial cells. Infect Immun 79: 4131–4145, 2011. doi: 10.1128/IAI.05120-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colasurdo GN, Fullmer JJ, Elidemir O, Atkins C, Khan AM, Stark JM. Respiratory syncytial virus infection in a murine model of cystic fibrosis. J Med Virol 78: 651–658, 2006. doi: 10.1002/jmv.20589. [DOI] [PubMed] [Google Scholar]
- 18.Colombo C, Burgel PR, Gartner S, van Koningsbruggen-Rietschel S, Naehrlich L, Sermet-Gaudelus I, Southern KW. Impact of COVID-19 on people with cystic fibrosis. Lancet Respir Med 8: e35–e36, 2020. doi: 10.1016/S2213-2600(20)30177-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooley J, Sontag MK, Accurso FJ, Remold-O’Donnell E. SerpinB1 in cystic fibrosis airway fluids: quantity, molecular form and mechanism of elastase inhibition. Eur Respir J 37: 1083–1090, 2011. doi: 10.1183/09031936.00073710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cosgriff R, Ahern S, Bell SC, Brownlee K, Burgel PR, Byrnes C, Corvol H, Cheng SY, Elbert A, Faro A, Goss CH, Gulmans V, Marshall BC, McKone E, Middleton PG, Ruseckaite R, Stephenson AL, Carr SB. A multinational report to characterise SARS-CoV-2 infection in people with cystic fibrosis. J Cyst Fibros 19: 355–358, 2020. doi: 10.1016/j.jcf.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20a.Cystic Fibrosis Foundation Cystic Fibrosis Foundation Patient Registry 2018 Annual Data Report. Bethesda, MD: Cystic Fibrosis Foundation, 2019. [Google Scholar]
- 21.Donaldson SH, Hirsh A, Li DC, Holloway G, Chao J, Boucher RC, Gabriel SE. Regulation of the epithelial sodium channel by serine proteases in human airways. J Biol Chem 277: 8338–8345, 2002. doi: 10.1074/jbc.M105044200. [DOI] [PubMed] [Google Scholar]
- 22.Elborn JS. Cystic fibrosis. Lancet 388: 2519–2531, 2016. doi: 10.1016/S0140-6736(16)00576-6. [DOI] [PubMed] [Google Scholar]
- 23.Esther CR Jr, Lin FC, Kerr A, Miller MB, Gilligan PH. Respiratory viruses are associated with common respiratory pathogens in cystic fibrosis. Pediatr Pulmonol 49: 926–931, 2014. doi: 10.1002/ppul.22917. [DOI] [PubMed] [Google Scholar]
- 25.Fujikawa H, Kawakami T, Nakashima R, Nasu A, Kamei S, Nohara H, Eto Y, Ueno-Shuto K, Takeo T, Nakagata N, Suico MA, Kai H, Shuto T. Azithromycin inhibits constitutive airway epithelial sodium channel activation in vitro and modulates downstream pathogenesis in vivo. Biol Pharm Bull 43: 725–730, 2020. doi: 10.1248/bpb.b19-01091. [DOI] [PubMed] [Google Scholar]
- 26.Hampton TH, Ballok AE, Bomberger JM, Rutkowski MR, Barnaby R, Coutermarsh B, Conejo-Garcia JR, O’Toole GA, Stanton BA. Does the ΔF508-CFTR mutation induce a proinflammatory response in human airway epithelial cells? Am J Physiol Lung Cell Mol Physiol 303: L509–L518, 2012. doi: 10.1152/ajplung.00226.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harutyunyan M, Huang Y, Mun KS, Yang F, Arora K, Naren AP. Personalized medicine in CF: from modulator development to therapy for cystic fibrosis patients with rare CFTR mutations. Am J Physiol Lung Cell Mol Physiol 314: L529–L543, 2018. doi: 10.1152/ajplung.00465.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hendricks MR, Bomberger JM. Digging through the obstruction: insight into the epithelial cell response to respiratory virus infection in patients with cystic fibrosis. J Virol 90: 4258–4261, 2016. doi: 10.1128/JVI.01864-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hendricks MR, Lashua LP, Fischer DK, Flitter BA, Eichinger KM, Durbin JE, Sarkar SN, Coyne CB, Empey KM, Bomberger JM. Respiratory syncytial virus infection enhances Pseudomonas aeruginosa biofilm growth through dysregulation of nutritional immunity. Proc Natl Acad Sci USA 113: 1642–1647, 2016. doi: 10.1073/pnas.1516979113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181: 271–280.e8, 2020. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu M, Bogoyevitch MA, Jans DA. Impact of respiratory syncytial virus infection on host functions: implications for antiviral strategies. Physiol Rev 100: 1527–1594, 2020. doi: 10.1152/physrev.00030.2019. [DOI] [PubMed] [Google Scholar]
- 32.Ingraham NE, Barakat AG, Reilkoff R, Bezdicek T, Schacker T, Chipman JG, Tignanelli CJ, Puskarich MA. Understanding the renin-angiotensin-aldosterone-SARS-CoV axis: a comprehensive review. Eur Respir J 56: 2000912, 2020. doi: 10.1183/13993003.00912-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ji HL, Zhao R, Matalon S, Matthay MA. Elevated plasmin(ogen) as a common risk factor for COVID-19 susceptibility. Physiol Rev 100: 1065–1075, 2020. doi: 10.1152/physrev.00013.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kam YW, Okumura Y, Kido H, Ng LF, Bruzzone R, Altmeyer R. Cleavage of the SARS coronavirus spike glycoprotein by airway proteases enhances virus entry into human bronchial epithelial cells in vitro. PLoS One 4: e7870, 2009. doi: 10.1371/journal.pone.0007870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khoury M, Cuenca J, Cruz FF, Figueroa FE, Rocco PR, Weiss DJ. Current status of cell-based therapies for respiratory virus infections: applicability to COVID-19. Eur Respir J 55: 2000858, 2020. doi: 10.1183/13993003.00858-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kiedrowski MR, Bomberger JM. Viral-bacterial co-infections in the cystic fibrosis respiratory tract. Front Immunol 9: 3067, 2018. doi: 10.3389/fimmu.2018.03067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kieninger E, Singer F, Tapparel C, Alves MP, Latzin P, Tan HL, Bossley C, Casaulta C, Bush A, Davies JC, Kaiser L, Regamey N. High rhinovirus burden in lower airways of children with cystic fibrosis. Chest 143: 782–790, 2013. doi: 10.1378/chest.12-0954. [DOI] [PubMed] [Google Scholar]
- 38.Kleine-Weber H, Elzayat MT, Hoffmann M, Pöhlmann S. Functional analysis of potential cleavage sites in the MERS-coronavirus spike protein. Sci Rep 8: 16597, 2018. doi: 10.1038/s41598-018-34859-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koeppen K, Stanton BA, Hampton TH. ScanGEO: parallel mining of high-throughput gene expression data. Bioinformatics 33: 3500–3501, 2017. doi: 10.1093/bioinformatics/btx452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kong M, Maeng P, Hong J, Szczesniak R, Sorscher E, Sullender W, Clancy JP. Respiratory syncytial virus infection disrupts monolayer integrity and function in cystic fibrosis airway cells. Viruses 5: 2260–2271, 2013. doi: 10.3390/v5092260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, Slutsky AS, Liu D, Qin C, Jiang C, Penninger JM. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med 11: 875–879, 2005. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malhotra S, Hayes D Jr, Wozniak DJ. Cystic fibrosis and Pseudomonas aeruginosa: the host-microbe interface. Clin Microbiol Rev 32: e00138-18, 2019. doi: 10.1128/CMR.00138-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mall MA, Mayer-Hamblett N, Rowe SM. Cystic fibrosis: emergence of highly effective targeted therapeutics and potential clinical implications. Am J Respir Crit Care Med 201: 1193–1208, 2020. doi: 10.1164/rccm.201910-1943SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marson FA, Bertuzzo CS, Hortencio TD, Ribeiro JD, Bonadia LC, Ribeiro AF. The ACE gene D/I polymorphism as a modulator of severity of cystic fibrosis. BMC Pulm Med 12: 41, 2012. doi: 10.1186/1471-2466-12-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matalon S, Bartoszewski R, Collawn JF. Role of epithelial sodium channels in the regulation of lung fluid homeostasis. Am J Physiol Lung Cell Mol Physiol 309: L1229–L1238, 2015. doi: 10.1152/ajplung.00319.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McClure ML, Barnes S, Brodsky JL, Sorscher EJ. Trafficking and function of the cystic fibrosis transmembrane conductance regulator: a complex network of posttranslational modifications. Am J Physiol Lung Cell Mol Physiol 311: L719–L733, 2016. doi: 10.1152/ajplung.00431.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Menzel M, Akbarshahi H, Bjermer L, Uller L. Azithromycin induces anti-viral effects in cultured bronchial epithelial cells from COPD patients. Sci Rep 6: 28698, 2016. doi: 10.1038/srep28698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mercuro NJ, Yen CF, Shim DJ, Maher TR, McCoy CM, Zimetbaum PJ, Gold HS. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19). JAMA Cardiol, 2020. doi: 10.1001/jamacardio.2020.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Millet JK, Whittaker GR. Host cell entry of Middle East respiratory syndrome coronavirus after two-step, furin-mediated activation of the spike protein. Proc Natl Acad Sci USA 111: 15214–15219, 2014. doi: 10.1073/pnas.1407087111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miró-Cañís S, Capilla-Rubio S, Marzo-Checa L, Fontanals-Aymerich D, Sanfeliu-Sala I, Espasa-Soley M, Asensio-de-la-Cruz O. Multiplex PCR reveals that viruses are more frequent than bacteria in children with cystic fibrosis. J Clin Virol 86: 1–4, 2017. doi: 10.1016/j.jcv.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morty RE, Ziebuhr J. Call for Papers: The pathophysiology of COVID-19 and SARS-CoV-2 infection. Am J Physiol Lung Cell Mol Physiol 318: L1016–L1019, 2020. doi: 10.1152/ajplung.00136.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Musarrat F, Chouljenko V, Dahal A, Nabi R, Chouljenko T, Jois SD, Kousoulas KG. The anti-HIV drug nelfinavir mesylate (Viracept) is a potent inhibitor of cell fusion caused by the SARSCoV-2 spike (S) glycoprotein warranting further evaluation as an antiviral against COVID-19 infections. J Med Virol jmv.25985, 2020. doi: 10.1002/jmv.25985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nixon GM, Armstrong DS, Carzino R, Carlin JB, Olinsky A, Robertson CF, Grimwood K. Clinical outcome after early Pseudomonas aeruginosa infection in cystic fibrosis. J Pediatr 138: 699–704, 2001. doi: 10.1067/mpd.2001.112897. [DOI] [PubMed] [Google Scholar]
- 54.Ornatowski W, Poschet JF, Perkett E, Taylor-Cousar JL, Deretic V. Elevated furin levels in human cystic fibrosis cells result in hypersusceptibility to exotoxin A-induced cytotoxicity. J Clin Invest 117: 3489–3497, 2007. doi: 10.1172/JCI31499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pankow S, Bamberger C, Calzolari D, Martínez-Bartolomé S, Lavallée-Adam M, Balch WE, Yates JR 3rd. ∆F508 CFTR interactome remodelling promotes rescue of cystic fibrosis. Nature 528: 510–516, 2015. doi: 10.1038/nature15729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parker D, Cohen TS, Alhede M, Harfenist BS, Martin FJ, Prince A. Induction of type I interferon signaling by Pseudomonas aeruginosa is diminished in cystic fibrosis epithelial cells. Am J Respir Cell Mol Biol 46: 6–13, 2012. doi: 10.1165/rcmb.2011-0080OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parker D, Prince A. Type I interferon response to extracellular bacteria in the airway epithelium. Trends Immunol 32: 582–588, 2011. doi: 10.1016/j.it.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Petersen NT, Høiby N, Mordhorst CH, Lind K, Flensborg EW, Bruun B. Respiratory infections in cystic fibrosis patients caused by virus, chlamydia and mycoplasma—possible synergism with Pseudomonas aeruginosa. Acta Paediatr Scand 70: 623–628, 1981. doi: 10.1111/j.1651-2227.1981.tb05757.x. [DOI] [PubMed] [Google Scholar]
- 59.Poli P, Timpano S, Goffredo M, Padoan R, Badolato R. Asymptomatic case of Covid-19 in an infant with cystic fibrosis. J Cyst Fibros 19: e18, 2020. doi: 10.1016/j.jcf.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Poschet JF, Perkett EA, Timmins GS, Deretic V. Azithromycin and ciprofloxacin have a chloroquine-like effect on respiratory epithelial cells (Preprint). bioRxiv, 2020. doi: 10.1101/2020.03.29.008631. [DOI] [PMC free article] [PubMed]
- 61.Ratjen F, Bell SC, Rowe SM, Goss CH, Quittner AL, Bush A. Cystic fibrosis. Nat Rev Dis Primers 1: 15010, 2015. doi: 10.1038/nrdp.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rauniyar N, Gupta V, Balch WE, Yates JR 3rd. Quantitative proteomic profiling reveals differentially regulated proteins in cystic fibrosis cells. J Proteome Res 13: 4668–4675, 2014. doi: 10.1021/pr500370g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Saint-Criq V, Gray MA. Role of CFTR in epithelial physiology. Cell Mol Life Sci 74: 93–115, 2017. doi: 10.1007/s00018-016-2391-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Santos RA, Oudit GY, Verano-Braga T, Canta G, Steckelings UM, Bader M. The renin-angiotensin system: going beyond the classical paradigms. Am J Physiol Heart Circ Physiol 316: H958–H970, 2019. doi: 10.1152/ajpheart.00723.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schögler A, Kopf BS, Edwards MR, Johnston SL, Casaulta C, Kieninger E, Jung A, Moeller A, Geiser T, Regamey N, Alves MP. Novel antiviral properties of azithromycin in cystic fibrosis airway epithelial cells. Eur Respir J 45: 428–439, 2015. doi: 10.1183/09031936.00102014. [DOI] [PubMed] [Google Scholar]
- 66.Sharma G, Volgman AS, Michos ED. Sex differences in mortality from COVID-19 pandemic: are men vulnerable and women protected? JACC Case Rep 2: 1407–1410, 2020. doi: 10.1016/j.jaccas.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shteinberg M, Taylor-Cousar JL. Impact of CFTR modulator use on outcomes in people with severe cystic fibrosis lung disease. Eur Respir Rev 29: 190112, 2020. doi: 10.1183/16000617.0112-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Simsek S, Tekes S, Oral D, Turkyilmaz A, Isik B, Isik MR, Akkoc H. The insertion/deletion polymorphism in the ACE gene and chronic obstructive pulmonary disease. Genet Mol Res 12: 1392–1398, 2013. doi: 10.4238/2013.April.25.10. [DOI] [PubMed] [Google Scholar]
- 69.Skopelja S, Hamilton BJ, Jones JD, Yang ML, Mamula M, Ashare A, Gifford AH, Rigby WF. The role for neutrophil extracellular traps in cystic fibrosis autoimmunity. JCI Insight 1: e88912, 2016. doi: 10.1172/jci.insight.88912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Somayaji R, Goss CH, Khan U, Neradilek M, Neuzil KM, Ortiz JR. Cystic fibrosis pulmonary exacerbations attributable to respiratory syncytial virus and influenza: a population-based study. Clin Infect Dis 64: 1760–1767, 2017. doi: 10.1093/cid/cix203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stanton BA. Effects of Pseudomonas aeruginosa on CFTR chloride secretion and the host immune response. Am J Physiol Cell Physiol 312: C357–C366, 2017. doi: 10.1152/ajpcell.00373.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stawiski EW, Diwanji D, Suryamohan K, Gupta R, Fellouse FA, Sathirapongsasuti JF, Liu J, Jiang YP, Ratan A, Mis M, Santhosh D, Somasekar S, Mohan S, Phalke S, Kuriakose B, Antony A, Junutula JR, Schuster SC, Jura N, Seshagiri S. Human ACE2 receptor polymorphisms predict SARS-CoV-2 susceptibility (Preprint). bioRxiv 2020. doi: 10.1101/2020.04.07.024752. [DOI] [PMC free article] [PubMed]
- 73.Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LF. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol 20: 363–374, 2020. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Theprungsirikul J, Skopelja-Gardner S, Meagher RE, Clancy JP, Zemanick ET, Ashare A, Rigby WF. Dissociation of systemic and mucosal autoimmunity in cystic fibrosis. J Cyst Fibros 19: 196–202, 2020. doi: 10.1016/j.jcf.2019.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tseng BS, Reichhardt C, Merrihew GE, Araujo-Hernandez SA, Harrison JJ, MacCoss MJ, Parsek MR. A biofilm matrix-associated protease inhibitor protects Pseudomonas aeruginosa from proteolytic attack. MBio 9: e00543-e18, 2018. doi: 10.1128/mBio.00543-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Voisin G, Bouvet GF, Legendre P, Dagenais A, Massé C, Berthiaume Y. Oxidative stress modulates the expression of genes involved in cell survival in ΔF508 cystic fibrosis airway epithelial cells. Physiol Genomics 46: 634–646, 2014. doi: 10.1152/physiolgenomics.00003.2014. [DOI] [PubMed] [Google Scholar]
- 77.Wat D. Impact of respiratory viral infections on cystic fibrosis. Postgrad Med J 79: 201–203, 2003. doi: 10.1136/pmj.79.930.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wat D, Gelder C, Hibbitts S, Cafferty F, Bowler I, Pierrepoint M, Evans R, Doull I. The role of respiratory viruses in cystic fibrosis. J Cyst Fibros 7: 320–328, 2008. doi: 10.1016/j.jcf.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wösten-van Asperen RM, Lutter R, Specht PA, Moll GN, van Woensel JB, van der Loos CM, van Goor H, Kamilic J, Florquin S, Bos AP. Acute respiratory distress syndrome leads to reduced ratio of ACE/ACE2 activities and is prevented by angiotensin-(1-7) or an angiotensin II receptor antagonist. J Pathol 225: 618–627, 2011. doi: 10.1002/path.2987. [DOI] [PubMed] [Google Scholar]
- 80.Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, Graham BS, McLellan JS. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 367: 1260–1263, 2020. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang N, Shen HM. Targeting the endocytic pathway and autophagy process as a novel therapeutic strategy in COVID-19. Int J Biol Sci 16: 1724–1731, 2020. doi: 10.7150/ijbs.45498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang S, Shrestha CL, Kopp BT. Cystic fibrosis transmembrane conductance regulator (CFTR) modulators have differential effects on cystic fibrosis macrophage function. Sci Rep 8: 17066, 2018. doi: 10.1038/s41598-018-35151-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang W, Zhang Z, Zhang Y, Naren AP. CFTR-NHERF2-LPA2 complex in the airway and gut epithelia. Int J Mol Sci 18: 1896, 2017. doi: 10.3390/ijms18091896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zheng S, De BP, Choudhary S, Comhair SA, Goggans T, Slee R, Williams BR, Pilewski J, Haque SJ, Erzurum SC. Impaired innate host defense causes susceptibility to respiratory virus infections in cystic fibrosis. Immunity 18: 619–630, 2003. doi: 10.1016/S1074-7613(03)00114-6. [DOI] [PubMed] [Google Scholar]
- 86.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395: 1054–1062, 2020. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhou Y, Vedantham P, Lu K, Agudelo J, Carrion R Jr, Nunneley JW, Barnard D, Pöhlmann S, McKerrow JH, Renslo AR, Simmons G. Protease inhibitors targeting coronavirus and filovirus entry. Antiviral Res 116: 76–84, 2015. doi: 10.1016/j.antiviral.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ziegler CG, et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell 181: 1016–1035.e19, 2020. doi: 10.1016/j.cell.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zoso A, Sofoluwe A, Bacchetta M, Chanson M. Transcriptomic profile of cystic fibrosis airway epithelial cells undergoing repair. Sci Data 6: 240, 2019. doi: 10.1038/s41597-019-0256-6. [DOI] [PMC free article] [PubMed] [Google Scholar]