Abstract
The family of resistin-like molecules (RELMs) consists of four members in rodents (RELMα/FIZZ1/HIMF, RELMβ/FIZZ2, Resistin/FIZZ3, and RELMγ/FIZZ4) and two members in humans (Resistin and RELMβ), all of which exhibit inflammation-regulating, chemokine, and growth factor properties. The importance of these cytokines in many aspects of physiology and pathophysiology, especially in cardiothoracic diseases, is rapidly evolving in the literature. In this review article, we attempt to summarize the contribution of RELM signaling to the initiation and progression of lung diseases, such as pulmonary hypertension, asthma/allergic airway inflammation, chronic obstructive pulmonary disease, fibrosis, cancers, infection, and other acute lung injuries. The potential of RELMs to be used as biomarkers or risk predictors of these diseases also will be discussed. Better understanding of RELM signaling in the pathogenesis of pulmonary diseases may offer novel targets or approaches for the development of therapeutics to treat or prevent a variety of inflammation, tissue remodeling, and fibrosis-related disorders in respiratory, cardiovascular, and other systems.
Keywords: FIZZ1, HIMF, inflammation, lung, RELMs
INTRODUCTION TO RELM SIGNALING
Pulmonary diseases, including pulmonary hypertension (PH), asthma/allergic airway inflammation, chronic obstructive pulmonary disease, fibrosis, cancers, infection, and other lung injuries, are multifactorial diseases that cause morbidity and mortality worldwide. The pathogenesis of these lung disorders is tremendously complex, with many questions left unanswered. One potentially unifying factor is the resistin-like molecule (RELM) family of proteins. This family comprises pleiotropic cytokines and growth factors that have been linked to obesity, type 2 diabetes, and alternate (such as Th2) inflammation (30, 98). The role of these cytokines in many aspects of physiology and pathophysiology, especially in cardiothoracic diseases, is rapidly evolving in the literature. In this review, we summarize the involvement of RELM signaling in pulmonary diseases. We focus on the immunoregulatory activities of RELMs, the immunotherapies that target RELM signaling in a variety of lung inflammation-related pathologies, and the potential to use RELMs as biomarkers of these diseases.
Nomenclature of the Resistin-Family Proteins
Resistin was first discovered in rodents as an adipokine with insulin resistance properties (116). Proteins that comprise the RELM family are characterized by a unique cysteine-rich motif at the COOH terminus (9, 18, 58, 117). Four rodent and two human isoforms of the resistin family have been discovered to date (36, 44, 117, 122). Members of this protein family have been identified as “found in inflammatory zone” (FIZZ) in an airway reactivity model (44), as “hypoxia-induced mitogenic factor” (HIMF) in a model of PH (122), as adipokines (resistin) (116, 117), as insulin resistance proteins (ADSF) (58), and as chemokines (XCP) (18). The diverse nomenclature of RELMs is shown in Table 1. In this review, we will predominantly refer to this family of proteins as RELMα, RELMβ, resistin, and RELMγ. Given the species variants (30), human (h) and rodent/murine (m) RELMs also will be specifically indicated.
Table 1.
Nomenclature for the RELM/FIZZ/XCP family of proteins
| RELM Family Members | Cellular Source (RELM-Producing Cells) |
|---|---|
| Murine/rodent (m) classification | |
| FIZZ1 = RELMα = HIMF = XCP2 | Macrophages (107), dendritic cells (20), and other immune cells (bone marrow cells and splenocytes) (30); bronchial epithelial cells, and type II pneumocytes |
| FIZZ2 = RELMβ = XCP3 | Goblet cells (7), lung epithelial cells (78) |
| FIZZ3 = Resistin = ADSF = XCP4 | Adipocytes (59) |
| FIZZ4 = RELMγ = XCP1 | Immune cells (bone marrow and white blood cells, splenocytes and thymocytes) (36, 115), adipocytes, and pneumonocytes (36) |
| Human (h) classification | |
| Resistin = RELMα = FIZZ1 = XCP1 | Macrophages, monocytes, and neutrophils (50) |
| RELMβ = FIZZ2 = XCP2 | SMCs, macrophages, and T cells (6), ECs and fibroblasts (33) |
ADSF, adipose tissue-specific secretory factor; ECs, endothelial cells; FIZZ, found in inflammatory zone; HIMF, hypoxia-induced mitogenic factor; RELM, resistin-like molecule; SMCs, smooth muscle cells; XCP, 10-cysteine protein.
RELM Structure and Biochemistry
Proteins in the RELM family have a secretory signal peptide at the NH2 terminus and a unique structure with no known homology to any other protein (44). Both human and rodent variants contain between 105 and 117 amino acids, with notable interspecies (33–59%) and intraspecies (21–51%) homology (36, 44, 117, 132), especially at the cysteine-rich COOH terminus. The common feature of these RELMs is the presence of five disulfide bonds formed by a group of 10 equally spaced cysteine residues in the COOH terminus (44). In mice, the genes for RELMα, β, and γ are aligned sequentially on chromosome 16B5 as Retnlb, Retnla, and Retnlg; the resistin gene is located separately on chromosome 8A1. In humans, resistin is located on chromosome 19p13.2, and RELMβ is located on chromosome 3q13.1 (30).
The Immunoregulatory Roles of RELM Signaling
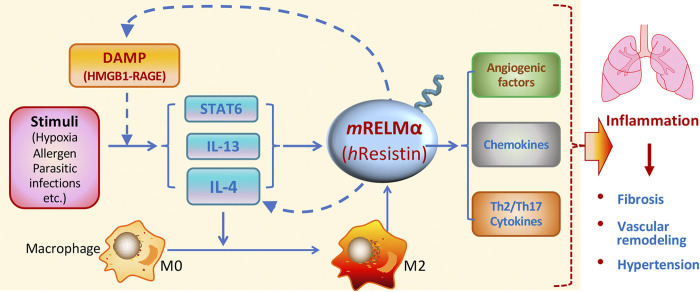
RELM signaling is an important component of the Th2-activated macrophage inflammatory response to tissue injury in the lung and other organs (54). Rodent (m) RELMα has been shown to play a critical role in the development of pulmonary arterial hypertension in hypoxia models and Th2 inflammatory models induced by ovalbumin (OVA) sensitization, schistosomiasis, and HIV-related stimuli (4, 6, 22, 31, 54, 120, 122). Our laboratory has shown that mRELMα promotes lung inflammation by activating the vascular endothelial growth factor (VEGF) and canonical Th2 cytokine interleukin (IL)-4 pathways (129, 130). In the OVA-induced Th2 immune response, mRELMα upregulates inflammatory cytokines such as IL-1β, IL-1ra, IL-16, and IL-17 and chemokines CXCL-1, CXCL-2, CXCL-9, CXCL-10, and CXCL-13, MCP-1, M-CSF, TIMP-1, and TREM-1 (31). The mRELMα ortholog could not be identified in humans (132), and human (h) resistin shows a greater similarity in expression pattern and inflammation-modulatory functions to mRELMα than to mResistin (92, 95, 132). Using mRELMα knockout mice and humanized mice expressing hResistin, we and others have identified additional similarities in the immunoregulatory properties of hResistin and mRELMα (52, 76). Recently, researchers have proposed the idea that alarmins might initiate Th2 inflammation (11). Indeed, high mobility group box (HMGB) 1, a key damage-associated molecular pattern (DAMP) protein, serves as an alarmin to drive inflammation (41). HMGB1 polarizes and activates M2 macrophages through the receptor for advanced glycation end products (RAGE) around hypoxic tumors (47) and mediates Th2 airway inflammation in acute allergic asthma (82). We have identified mRELMα and its human homolog hResistin as the upstream activators of DAMPs HMGB1 and S100A11 in the development of pulmonary vascular remodeling (29, 76, 77). Hence, RELMs might initiate Th2 inflammation in the lungs though the DAMP-RAGE pathway. Th2 stimuli also can induce mRELMα expression through IL-4, IL-13, and STAT-6 pathways (93, 102, 118). Thus, RELM signaling may act as a crucial hub of a positive feedback loop to trigger, amplify, and sustain inflammation through its immunoregulatory activities in the pathogenesis of lung diseases (Fig. 1).
Fig. 1.
Schematic illustration of inflammatory signals regulated by resistin-like molecules (RELMs). Through immunoregulatory properties, RELMα or human resistin may create a positive feedback loop that mediates the amplification of lung inflammation via Th2 (IL-4) and damage-associated molecular pattern (DAMP) (high mobility group box 1, HMGB1) pathways.
Putative Receptors and Binding Partners of RELMs
Receptors for RELMs have not yet been identified. Reports in the literature have suggested an isoform of decorin (23), mouse receptor tyrosine kinase-like orphan receptor (ROR) 1 (113), and Toll-like receptor (TLR) 4 (121) as putative receptors for Resistin. However, neither decorin nor ROR1 have been shown to mediate the inflammatory effects of hResistin (71). Additionally, biochemical binding assays have not been conducted to show interaction between TLR4 and hResistin (71), and TLR4 knockdown did not significantly affect hResistin-mediated monocyte migration and inflammatory cytokine production (71). The adenylyl cyclase-associated protein 1 (CAP1) has been implicated as a functional receptor that mediates the inflammatory actions of hResistin in human monocytes (71). However, as CAP1 lacks a transmembrane domain, the biological mechanism underlying its membrane location is still unclear (71). m/hRELMβs were also identified as bactericidal proteins that bind to negatively charged bacterial lipids and form a membrane-permeabilizing pore to lyse the targeted cells (105). We previously identified Bruton’s tyrosine kinase (BTK) as the first known functional binding partner of mRELMα to mediate chemokine actions in myeloid cells (119). More recently, the extracellular calcium-sensing receptor (CaSR) was suggested as a binding partner for mRELMα in pulmonary artery smooth muscle cells (SMCs) (134). A limitation of that study was that the PH model was induced by intermittent, not continuous, hypoxia (134). Therefore, the results need validation.
Tissue Distribution of RELMs
In healthy humans, hResistin is expressed predominantly in bone marrow, monocytes, and leukocytes, whereas hRELMβ is found primarily in colon and small intestine and to a lesser extent in testis and spleen (18, 96). In normal mice, mRELMα is expressed predominantly in lung, bone marrow, and spleen (44, 80, 117); mRELMβ is expressed in intestinal epithelial cells (7, 42, 117); mResistin is expressed in white adipose tissue (44, 116); and mRELMγ is expressed in hematopoietic tissues and lung (18, 36). Under pathophysiologic conditions, hResistin and hRELMβ have been observed in the lungs of humans with asthma, scleroderma (SSc), idiopathic PH, and other Th2 inflammatory diseases (6, 45, 77, 89, 102). The main cellular source of hResistin is the myeloid cells, especially macrophages, and its expression pattern shows a greater similarity to that of mRELMα than to that of mResistin (92, 95, 132). We also have reported that mRELMα, mRELMβ, and mRELMγ are expressed in rodent models of allergic (asthmatic) inflammation and chronic hypoxia-induced PH (5, 30, 31, 122).
On the basis of the above-described RELM tissue distribution, and because lung is the primary location of most RELM isoforms (30, 36, 44, 122), research into the association between RELMs and the pathogenesis of cardiothoracic and respiratory diseases is now beginning to expand rapidly. We believe that summarizing the involvement and clinical implications of RELM signaling in pulmonary disease will be helpful to the development of novel therapeutic approaches for a variety of related thoracic diseases.
ASSOCIATION OF RELM SIGNALING WITH PULMONARY DISEASE
Pulmonary Hypertension
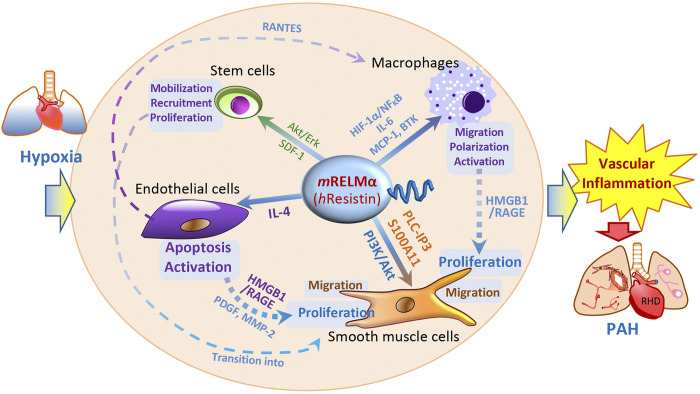
The pathogenesis of PH features pulmonary vascular remodeling, increases in pulmonary artery pressure, and right heart failure (133). In humans, severe PH is characterized by plexiform lesions that contain phenotypically altered pulmonary SMCs and endothelial cells (ECs) (125). Using gene array technology, we have shown that mRELMα is dramatically upregulated in hypoxic lungs and produces potent mitogenic effects (122). The expression of mRELMα is regulated by hypoxia, likely via the C/EBP pathway (18), as the mRELMα gene was shown to possess six C/EBP binding motifs (122). In rodent models, transtracheal delivery of mRELMα gene by adeno-associated virus causes vascular remodeling and hemodynamic changes like those of PH (4). Conversely, in vivo knockdown of mRELMα markedly reduces PH development caused by chronic hypoxia or Th2 inflammatory stimuli (4, 22, 89), indicating an etiologic role for mRELMα in PH. In vitro and in vivo gain- and loss-of-function studies carried out in PH-related human primary cells and rodent models with mRELMα knockout, knockdown, or overexpression have suggested mRELMα to be a proinflammatory, angiogenic, vasoconstrictive, fibrotic, and chemokine-like cytokine in hypoxic lungs (Fig. 2) (3, 4, 22, 29, 32, 55, 62, 76, 77, 119, 122, 129–131). These cytokine-like pro-PH properties of mRELMα are shared to a great extent with hResistin in humans (76, 77, 92, 95, 131, 132). It has been found that mRELMα promotes the proliferation of pulmonary vascular SMCs, microvascular (PMV) ECs, and fibroblasts in vitro via the Akt or ERK1/2 signal transduction pathway (19, 122, 123). mRELMα induces lung vascular inflammation via IL-4 and VEGF/VEGFR2 (129, 130). Systemic administration of mRELMα causes EC apoptosis and subsequent IL-4-dependent PH in mice (131). Interestingly in embryonic mouse lung, RELMα has antiapoptotic properties that may regulate lung alveolarization and maturation (127). HIF-1α has been identified as a downstream actor of RELMα based on its ability to mediate mRELMα-induced myeloid cell migration and vascular tube formation (55). In addition to its molecular mechanisms, we have uncovered the mRELMα-regulated stem cell biology. mRELMα activation may promote stem cell recruitment from bone marrow to lungs in hypoxic mice in vivo (3). In vitro, mRELMα maintains the multipotency of cultured mesenchymal stem cells by preserving their differentiation potential and phenotypic changes (62).
Fig. 2.
Schematic illustration of resistin-like molecule-α (RELMα)/resistin-governed cellular pathology and interaction in pulmonary arterial hypertension (PAH). Initiated by hypoxia, RELMα/resistin signaling regulates proinflammatory, proangiogenic, and promigratory pathways in PAH-related cells, including endothelial cells, smooth muscle cells, macrophages, and stem cells. By mediating vascular inflammation, RELMα/resistin drives pulmonary vascular remodeling and PAH development. BTK, Bruton’s tyrosine kinase; h, human; HIF, hypoxia-inducible factor; HMGB1, high mobility group box 1; m, mouse; MCP-1, monocyte chemoattractant protein-1; MMP-2, matrix metalloproteinase-2; PDGF, platelet-derived growth factor; RAGE, receptor for advanced glycation end products; RANTES, regulated on activation, normal T cell expressed and secreted, also known as chemokine (C-C motif) ligand 5 (CCL5); RHD, right heart dysfunction; SDF-1, stromal cell-derived factor-1.
The roles of mRELMα also have been interrogated in non-hypoxia-induced PH models. mRELMα was shown to be elevated in bronchoalveolar lavage fluid (BALF) of a mouse AIDS (acquired immune deficiency syndrome)-related PH model created by pneumocystis infection plus CD4 T-cell depletion (120). mRELMα expression also was increased in a schistosomiasis-induced PH mouse model, and the protein interacted with IL-13 signaling to exacerbate vascular remodeling in the infected lungs (40). In mice expressing a hypomorphic BMPR2 mutation, OVA-triggered PH-upregulated expression of mRELMα, but not mRELMγ, in the right ventricle (100). mRELMα was also upregulated in a mouse PH model induced by coexposure to OVA and urban ambient particulate matter (mimicking air pollution) (99), but mice lacking mRELMα also fully exhibited a severe remodeling response. These findings suggest that the role of mRELMα in arterial thickening and remodeling could vary between different types of PH inducers such as hypoxia and antigens (5).
In patients with SSc-associated PH, hRELMβ expression is upregulated in vascular cells and leukocytes, as well as in myofibroblast-like cells (6). Elevated serum hResistin levels in SSc patients also correlate with high right ventricular systolic pressure (RVSP), indicating that hResistin contributes to the pathogenesis of SSc-associated PH and might be useful as a biomarker (85). An in vitro study revealed that mRELMα stimulates intracellular Ca2+ release in human pulmonary artery (PA) SMCs through the phospholipase C signaling pathway involving Gαq/11 protein-coupled receptors and ryanodine receptors (32). mRELMα also induces PASMC migration by regulating the EF-hand calcium-binding DAMP protein S100A11 (29). hResistin promotes the EC-mediated alteration of PASMCs to a proliferative phenotype (131). hRELMβ induces proliferation and activation of ERK1/2 in human PASMCs and PMVECs in vitro (6). Besides being upregulated in pulmonary vascular cells, hRELMβ was shown to be upregulated in hypoxia-exposed human lung A549 cells and primary adventitial fibroblasts, and its overexpression enhanced mitogenesis via PI3K activation (110). hRELMβ also increases HIF-1α and IL-6 expression through an NF-κB-mediated mechanism in human primary lung fibroblasts (55). Thus, PH-related vascular, inflammatory, and resident cells, as well as stem/progenitor cells, all might be regulated by RELM signaling under inflammatory conditions.
Recently, our mechanistic studies showed that signaling of mRELMα, as well as its human homolog hResistin (92, 132), triggered immune-vascular cell crosstalk by activating DAMP signaling during hypoxic inflammation (76, 77). Our human studies support the biomarker potential of hResistin based on the unpublished data that serum hResistin levels in patients with idiopathic pulmonary arterial hypertension and SSc-PH correlate directly with disease severity and survival. In addition, ongoing studies indicate that RELMs drive mitochondrial and metabolic dysfunction of PASMCs and cardiomyocytes, and modulate M1/M2 macrophage polarization in PH development. These findings provide deeper mechanistic insight into RELM-induced PH.
Asthma and Allergic Lung Diseases
Th2 allergic airway inflammation, especially asthma, shares some key pathological features with PH, including increased RVSP and induction of vascular inflammation and remodeling (31, 112). However, animal models of severe Th2-associated allergic inflammation still fail to develop PH (5). One mechanism that might underlie this distinction is a difference in mRELMα expression patterns: mRELMα, which is expressed in the lung vasculature of animals with PH, is missing in allergic models (5). In rodent models, mRELMα is still one of the most upregulated gene products in allergen-associated Th2 responses (91). Similar to the way that we discovered HIMF in hypoxia-exposed animals and PH-related cells (122), FIZZ1 was first described in the OVA-induced asthmatic mouse model (44). Our genomic analysis confirmed mRELMα to be the most consistently upregulated gene in OVA-challenged mouse lungs (31). FIZZ2 (mRELMβ) and FIZZ4 (mRELMγ), but not FIZZ3 (mResistin), also were upregulated by OVA challenge in the mouse acute pulmonary inflammation model (31, 118). Activation of IL-4/IL-13, STAT-6, and C/EBP mediates mRELMα induction by Th2 allergic inflammation (91, 93, 118). In contrast, OVA-induced mRELMβ production is entirely dependent on the IL-13 receptor-α1 chain (91). In OVA-treated rats, upregulated mRELMα expression and mRELMα-enhanced myofibroblast differentiation are characteristic of the early stages of asthmatic airway remodeling (26). Other studies of OVA-challenged rodents have identified mRELMα as a potential oxidative stress marker (136). In a prolonged allergic inflammation model induced by repeated OVA inhalation, lung mRELMα expression was implicated in the pulmonary arterial remodeling driven by the IL-25-natural killer T-cell pathway (57). Genetic ablation of mRELMα prevented OVA-induced inflammation, vascular remodeling, and cardiac hypertrophy, further indicating the proallergic properties of mRELMα (31).
RELM activation also displays pathogenicity in fungus-associated asthma. Mice challenged with the aeroallergen Aspergillus fumigatus (Asp) exhibit significantly augmented mRELMα expression in areas surrounding remodeled vessels (5). Similar to OVA-induced mRELMβ, Asp-triggered mRELMα production and secretion by epithelial cells is regulated by IL-13Rα1 (91). Mice sensitized to a combination of dust mite, ragweed, and Asp extracts exhibited mRELMα induction in lungs that was dependent on the transcription factor PU.1, a regulator of macrophage differentiation and maturation (106). In mice exposed to alternaria, but not Asp or candida, gene microarray analysis of airway epithelial cell brushings demonstrated that fungal allergen sensitization caused a STAT6-dependent increase in mRELMα expression of more than 20-fold (25). mRELMβ was shown to promote lung remodeling in mice via proinflammatory and fibroblast mitogenic activity after intratracheal delivery and after Asp challenge (89). In response to Asp, lung epithelial cells, macrophages, fibroblasts, and vascular endothelial cells produce mRELMβ, which precipitates airway remodeling by extracellular matrix protein deposition and mucosal hypertrophy, suggesting a target potential of hRELMβ in human asthma (33).
Clinically, plasma hResistin levels are elevated in patients with asthma and correlate with disease severity (68). Indeed, hResistin is elevated in patients with acute asthma exacerbation as well as in those with stable asthma (1), suggesting that it could be used as a disease activity marker and predictor of asthma risk. Another study found that the hResistin:adiponectin ratio is a negative predictor of lung function in patients with asthma (8). The ratio is elevated in patients with asthma, much higher in those with more severe disease, and highest in asthmatic patients who are obese and male (8), supporting the premise that hResistin may contribute to increased severity in the obese population. These findings bolster the idea that this ratio might be a potential therapeutic target for management of the obese asthma phenotype.
Nevertheless, data regarding the association between RELM signaling and asthma are conflicting. The mRELMα-overexpressing mice generated by Lee et al. (70) showed attenuated Th2 airway inflammation after OVA-sensitization. However, in another mouse study, mRELMα gene mutation did not significantly suppress Th2 immune responses induced by OVA or Asp (91), mirroring a redundant functional role for mRELMα in these acute allergic models. Clinically, high plasma hResistin levels in steroid-naïve women with asthma predicted a favorable anti-inflammatory effect of inhaled glucocorticoids (73). Another study of childhood asthma showed that serum hResistin levels negatively correlated with atopy parameters, including eosinophil count and total serum IgE (60), suggesting a protective role of hResistin with disease-modifying effects against asthma in children. In an atopic adult study, sensitization to allergen Dermatophagoides pteronyssinus transiently increased serum hResistin levels (63). Unexpectedly, patients with normal airway responsiveness had higher bronchoalveolar lavage and serum hResistin concentrations than did hyperresponsive individuals (63). These results are directly opposed to reports in the literature of elevated serum hResistin in those with moderate or severe asthma (68). Hence, additional studies are required to dissect the immune-modifying mechanism that underlies RELM-related allergic inflammation and resolve these conflicting findings.
Pulmonary Fibrosis
Lung fibrosis is often complicated by PH and promoted by Th2 and Th17 inflammation. The pro-PH (Fig. 2) (3, 4, 22, 29, 32, 55, 62, 76, 77, 119, 122, 129–131) and Th2/Th17 regulatory (Fig. 1) (31) activities of mRELMα, or its human homolog hResistin (92, 132), suggest that it might contribute to fibrogenesis. Indeed, profibrotic effects of mRELMα were first identified in the rat model of bleomycin-induced pulmonary fibrosis (79). cDNA microarray screening showed that of the 628 genes upregulated or downregulated in that model, mRELMα exhibited the most dramatic increase (79). Additional in vivo and in vitro analyses revealed that mRELMα derived from alveolar epithelial cells stimulates α-SMA and collagen I production in fibroblasts (79). IL-4 and IL-13 strongly induce epithelial mRELMα production via STAT6 in bleomycin-treated lungs (80). In turn, mRELMα reduces myofibroblast apoptosis by suppressing the ERK pathway (19). mRELMα also activates collagen-1-producing fibroblasts to promote fungal-induced airway fibrosis (25) and links M2/wound-healing macrophage development with lung fibrogenesis induced by herpesvirus (35). In a mouse Pneumocystis-infected model, BALF-derived macrophages showed consistent mRELMα upregulation concomitant with fibrosis development (120). CC10-driven mRELMα overexpression in transgenic mice was sufficient to recruit bone marrow-derived dendritic cells to lungs, but was not sufficient to cause or alter lung fibrosis induced by bleomycin or silica (84). In support of these findings, experiments using mRELMα knockout mice and adenoviral mRELMα-overexpressing rats showed that mRELMα has profibrogenic properties essential for bleomycin-induced pulmonary fibrosis in vivo (81).
Other RELMs also have been implicated in the pathogenesis of fibrotic lung diseases. mRELMβ is highly induced in bleomycin-treated fibrotic rodent lungs (78), and research with gene-deficient mice showed that mRELMβ is essential to pulmonary fibrosis development (33, 78). hRELMβ expression is upregulated in primary cultured human pulmonary artery adventitial fibroblasts (110) and in patients with idiopathic pulmonary fibrosis (78). The pulmonary fibrosis that develops after both asbestos and silica exposure is also associated with high plasma hResistin levels (72, 114). The data suggest that hResistin mediates immune responses in fiber- or particle-induced parenchymal fibrotic disorders (72, 114) and might be useful as a biomarker of silicosis (114). Recently, hResistin levels were found to be elevated in the plasma and sputum of patients with cystic fibrosis lung disease and to correlate with lung function impairment (34). As discussed above, it also has been suggested that hRELMβ may contribute to the inflammation-induced fibrosis and vascular remodeling in SSc-associated PH (6). In comparison to hResistin, hRELMβ may play a more direct role in matrix protein deposition during tissue and vascular remodeling by activating local fibroblasts (55). Additional mechanistic studies are needed to uncover the hRELMβ-modulated endothelium-mesenchymal transition in human primary pulmonary artery ECs (53).
Lung Cancer
PH bears some resemblance to cancer, as pulmonary vascular cells acquire cancer-like traits, including excessive proliferation and resistance to apoptosis (13). The pro-PH role of mRELMα thus suggests that it has protumor effects. Interestingly, mRELMα gene expression was highly induced in response to tobacco carcinogen nitrosamine in lungs of tumor-susceptible A/J mice but not in those of resistant C3H mice (39). The upregulated mRELMα exhibited immunomodulating action to create a protumorigenic environment in lungs (39).
hResistin has a closer relationship with pulmonary carcinoma in humans than mRELMα has in mice. Serum hResistin levels are significantly elevated in patients with non-small-cell lung carcinoma (NSCLC) (14) and are associated with weight loss (56). In the study by Karapanagiotou et al. (56), although baseline serum hResistin level did not correlate significantly with overall survival of patients with NSCLC, the data showed a trend toward an association between hResistin levels and time to disease relapse in the responders at the end of chemotherapy. A recent study in another cohort showed that serum hResistin has inflammation-modulating effects on cancer cachexia in patients with advanced-stage NSCLC (24), indicating that it has potential as an adjuvant diagnostic or prognostic biomarker for lung cancer (14, 24). Moreover, hResistin gene (RETN) polymorphisms have been correlated with lung cancer progression (46), and hResistin activation was shown to promote human lung adenocarcinoma metastasis (38). Kuo et al. (67) reported that hResistin was elevated in tumor-infiltrating dendritic cells and in human lung cancer samples. Interestingly, they also showed that local expression of mResistin was increased in mice transplanted with lung cancer cells (67). Mechanistically, they found that hResistin-expressing dendritic cells promote lung cancer progression by increasing the Wolf–Hirschhorn syndrome candidate 1/Twist pathway (67). Thus, targeting hResistin may constitute a novel therapeutic approach to manipulate immune cells in the cancer microenvironment. Moreover, Tsai et al. (124) showed that hResistin-enhanced lung cancer metastasis is dependent on miR-519d downregulation in human chondrosarcoma cells. Hence, hResistin-governed micro-RNAs may also represent a novel focus for the development of strategies to treat lung cancer.
Chronic Obstructive Pulmonary Disease
Chronic obstructive pulmonary disease (COPD), including chronic bronchitis and emphysema, is the most common chronic lung condition in the world (66) and the most common cause of secondary PH (37). Chronic cigarette smoking-induced airway hyperresponsiveness is the main cause of COPD. The underlying mechanism has been suggested to be the upregulation of mRELMα, as demonstrated in experimental rat lungs (83). Indeed, serum hResistin levels are elevated in COPD patients and are associated with smoking, inflammatory marker complement C3 and C4 (1), and hyperinflation (111). These findings are supported by the observation that proper exercise training prevents cigarette smoking-induced augmentation of mRELMα production and thereby reduces the risk of COPD development (83). Consistently, patients with COPD who benefit from high-intensity exercise training exhibit lower serum levels of hResistin (12). Thus, hResistin has the potential as a biomarker for inflammation, risk assessment, severity, and prognosis of COPD disease progression (66). In a study by Mineo et al. (88), lung volume reduction surgery in patients with moderate-to-severe emphysema restored respiratory dynamics and ameliorated cachexia. The postoperative reduction in peripheral blood hResistin levels in these patients was associated with favorable clinical change, further highlighting the biomarker potential of hResistin and suggesting its contribution to COPD pathogenesis (88). Additionally, because hResistin is proinflammatory, its levels also correlate with tumor necrosis factor receptors in COPD patients who develop osteoporosis (126).
Interestingly, in COPD patients with chronic Chlamydia pneumoniae infection who had frequent exacerbations, upregulated circulating hResistin was shown to positively correlate with the level of anti-C. pneumoniae IgA (97). In the group with serological features of chronic C. pneumoniae infection, serum IL-6 correlated with hResistin (97). Thus, hResistin is also a critical mediator of the immune response to lung infection, as discussed in the following section.
Lung Infection
We discussed above how RELMs mediate allergic lung inflammatory responses to viral or fungal infection and in infection-induced PH. mRELMα is one of the most upregulated gene products in parasite-associated Th2 responses (91). Pulmonary expression of mRELMα induced by Nippostrongylus brasiliensi (Nb) correlates with the extent of long-term lung damage (43, 109). Interestingly, malaria (Plasmodium chabaudi) coinfection suppresses this nematode-induced increase in mRELMα to alter pulmonary repair processes (43). The accumulation of mRELMα-positive cells in mouse lungs is also induced by Schistosoma mansoni (Sm) eggs and is associated with augmented granulomatous inflammation (101). Moreover, in combination with granuloma obstruction of local capillary beds, mRELMα is related to the development of plexiform-like lesions in a murine schistosomiasis model of lung vascular remodeling (21, 54).
Other studies have shown that RELM activation produces anti-inflammatory effects. In Sm egg-challenged mice, mRELMα deficiency led to exacerbated lung inflammation and fibrosis (94). In a study by Pesce et al. (102) that used three mouse parasite models, including acute and chronic infection with Sm, Sm eggs, and the nematode Nb, the authors reported that mRELMα mediated IL-4/IL-13-dependent Th2-suppressing actions in the inflamed lungs. The mRELMα-suppressed Th2 adaptive immunity in the parasite-infected lungs was believed to protect the host from excessive and potentially fatal lung inflammation (10, 17, 51, 64). Interestingly, M2 macrophages are the main cellular source of this mRELMα (64), suggesting a feedback mechanism to control the Th2 inflammation in anti-parasite responses. Moreover, the suppression of Th2 immunity by mRELMα was offset by an mRELMβ-enhanced immune response in a study that used mRELMβ single- and mRELMβ/α double-deficient mice infected with Nb (17). However, a side effect of increased mRELMα would be its secondary stimulation of vascular remodeling in these infected lungs, as mentioned above (54). Thus, the perceived benefits of enhancing mRELMα signaling must be balanced against the risks associated with the provasculogenic properties of this PH inducer.
As the human homolog of mRELMα, hResistin is also essential for the immune response to helminths. In humanized mice with hResistin gene knock-in, hResistin overexpression exacerbated Nb-induced lung inflammation (50). In individuals infected with soil-transmitted helminths or filarial nematode Wuchereria bancrofti, increased serum hResistin was associated with higher parasite load and augmented proinflammatory responses, identifying hResistin as detrimental in lungs with multiple helminth infections (50). Plasma hResistin is elevated in patients with pulmonary tuberculosis (TB) (15, 90) and can be used for early assessment of drug and vaccine efficacy (15, 28, 90). It correlates directly with TB disease stage and has been suggested to be a key player in TB-associated energy dysregulation (15). Moreover, in patients with type 2 diabetes who also have TB infection, the increased hResistin may suppress the mycobacterium-induced inflammasome activation by leukocytes (16). Thus, hResistin has not only biomarker potential, but also immune-regulatory activities in pulmonary TB.
Other Lung Diseases
Because of their pluripotent effects, RELMs have been implicated in other lung injury conditions. Researchers identified mRELMα as the most promising target in radiation-induced acute lung injury (ALI) by comparing differences in radiation-induced gene expression in radio-sensitive and radio-tolerant mouse strains (48). mRELMα also is a causal factor for acute pancreatitis-associated lung injury (APALI) (128). In a rat model of APALI, mRELMα lung expression was significantly upregulated and associated with pulmonary injury severity. Overexpression and knockdown experiments in rats revealed that mRELMα mediates inflammation in APALI pathogenesis by activating the PI-3K/Akt-NF-κB pathway (128).
Resistin has been implicated in severe pulmonary system dysfunction. hResistin levels are elevated in patients with end-stage lung disease (ESLD) and correlate with the pathogenesis of osteopenic syndrome in ESLD (61). In ALI and acute respiratory distress syndrome (ARDS), the presence of severe PH is associated with increased morbidity and mortality (104). In a model of lipopolysaccharide-induced ALI in humanized mice that exclusively express hResistin (hRTN+/−/−) but not mResistin, hResistin overexpression promoted neutrophil-mediated inflammation involving DAMP molecules HMGB1 and TLR4 (52). In a rat model of ALI induced by blunt chest trauma, mResistin expression increased significantly in the injured lungs beginning in the initial posttraumatic inflammatory phase and remained upregulated throughout the experiment (27). These findings indicate that hResistin may be a potential target candidate for the development of therapeutic strategies in patients with life-threatening organ system failure such as ALI, ARDS, or sepsis. Lung mResistin levels were also increased in BALF of mice with acute exposure to ozone (O3) (108). Data from gene knockout mice indicated that mResistin suppressed chemokine keratinocyte chemoattractant expression in the lung but did not promote O3-induced lung pathology (108).
Baseline resistin expression in normal lungs has also been examined. The ozone challenge study described above was the first to demonstrate the presence of mResistin in mouse lung epithelial lining fluid in the absence of any inciting stimulus (108). Moreover, one human study has detected hResistin in the alveolar lining fluid of healthy adults (87). The authors of that study reported that the concentration of hResistin is significantly higher in alveolar lining fluid than in serum and that protein net charge may influence trafficking and compartmentalization to the alveolar airspace more than molecular weight or hydrophobicity (87). Data on baseline lung hResistin expression and localization in healthy subjects will be critical for understanding its homeostasis and for developing aerosolized drug delivery to or through the lungs.
CONCLUSIONS AND PERSPECTIVES
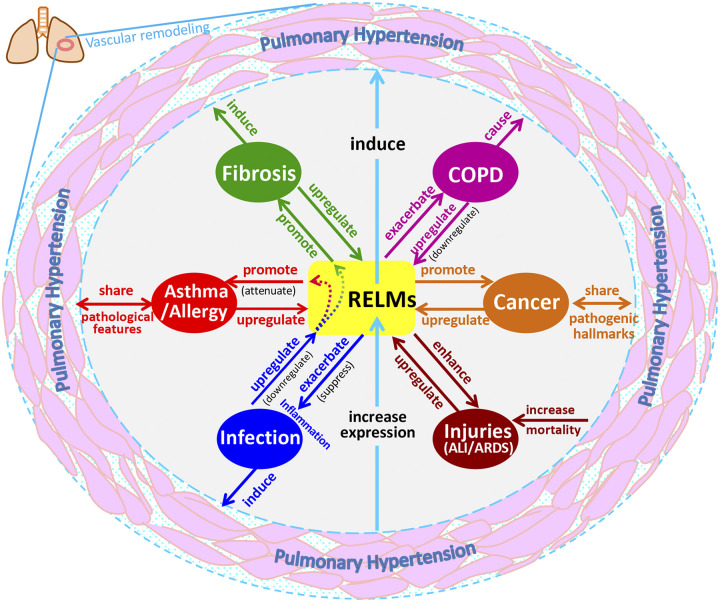
In this review, we have summarized the involvement of RELM signaling in pulmonary diseases (Table 2), with a focus on the immunoregulatory activities of m/hRELMs and the immunotherapies targeting RELM signaling in a variety of lung inflammation-related pathologies. Asthma, fibrosis, COPD, infection, and other lung injuries all are strongly related to each other and could result in or from immune dysfunction caused by RELM signaling (Fig. 3). Activation of RELMs may be a pivotal event that links these interconnected pulmonary disorders, contributing to or being implicated in vascular remodeling and PH development (Fig. 3).
Table 2.
Involvement of RELM family members in lung diseases
| Lung Pathologies | Rodent/Murine (m) RELMs |
Human (h) RELMs |
|||
|---|---|---|---|---|---|
| Increased expression/elevated level | Contribution to pathogenesis* | Elevated level/increased expression | Therapeutic target potential | Biomarker potential | |
| Pulmonary hypertension | RELMα (5, 40, 120, 122) | RELMα (4, 55, 76, 77, 122, 129-131) | RELMβ (6); Resistin (85) | Resistin (76, 77, 131); RELMβ (6, 55) | Resistin (85) |
| Asthma/allergy | RELMα (5, 25, 31, 44, 91); RELMβ and RELMγ (31, 33, 118) | RELMα (25, 26, 31), (anti-inflammatory effect) (70) | RELMβ (33); Resistin (1, 68, 73) | Resistin (8), (anti-inflammatory effect) (73) | Resistin (1, 60, 68) |
| Fibrosis | RELMα (79, 80); RELMβ (78) | RELMα (79, 81); RELMβ (33, 78) | RELMβ (6, 78, 110); Resistin (34, 72, 114) | RELMβ (6) | Resistin (34, 114) |
| Cancer | RELMα (39, 67) | RELMα (39) | Resistin (14, 67) | Resistin (38, 124) | Resistin (14, 24, 46) |
| COPD | RELMα (83) | RELMα (83) | Resistin (1, 111) | Resistin (66, 88) | |
| Infection | RELMα (parasite infection) (43, 91, 109) | RELMα (anti- inflammatory effect in parasite infection) (10, 17, 64, 94, 102) | Resistin (parasite infection) (50), (in TB) (15, 90) | Resistin (proinflammatory effect in parasite infection) (50), (immunosuppressive effect in TB with DM) (16) | Resistin (TB) (28) |
| Other lung diseases/injuries | RELMα (irradiated lung) (128); resistin (ALI) (27), (O3-induced injury) | RELMα (APALI) (128) | Resistin (ESLD) (61), (ALI/ARDS) | Resistin (ALI) (52) | |
| Healthy lung | RELMα (detactable) (117), (highest in lung vs. other organs) (44); RELMγ (18, 36) and resistin (108) (detectable) | Resistin (detectable) (18, 87) | |||
Contribution to pathogenesis primarily indicates the promotion of lung disease development unless specifically noted.
ALI, acute lung injury; APALI, acute pancreatitis-associated lung injury; ARDS, acute respiratory distress syndrome; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; ESLD, end-stage lung disease; N/A, not applicable; O3, ozone; RELMs, resistin-like molecules; TB, (pulmonary) tuberculosis.
Fig. 3.
Overview of the involvement of the resistin-like molecule (RELM) family in the interconnected etiologies of pulmonary diseases. ALI, acute lung injury; ARDS, acute respiratory distress syndrome; COPD, chronic obstructive pulmonary disease.
To date, the tremendously complex pathogenesis of lung diseases such as PH remains unclear. The cytokines, chemokines, and other mediators, together with the cells that release and respond to them, form a complex network that involves multiple signaling pathways. Knowledge about the roles of RELMs is vital for sorting out these pathways and their regulation during lung inflammation, and for defining an effective therapy that addresses the mechanism of the disease rather than just the symptoms. RELMs also mediate the tumorigenicity of lung cancers. Inflammation in the tumor microenvironment affects every aspect of tumor development, including initiation, promotion, malignant conversion, invasion, and metastasis, as well as response to therapy. In turn, elucidating the signaling network of the inflammatory tumor microenvironment in lungs is crucial to developing novel therapies for complex multifactorial pulmonary inflammatory diseases, especially PH (75), given the striking pathogenic analogies between it and cancer (13). We have a long way to go before the etiologies of these pulmonary diseases become clear. Nevertheless, the advances in our understanding of RELM signaling recapped here are filling in the picture.
RELM-induced vascular lesions and inflammation might also lead to heart failure, atherosclerosis, thrombosis, angiogenesis, and other cardiovascular diseases (2, 49, 65, 135). Additionally, RELMs mediate a variety of pathological processes in fatty liver disease, autoimmune disease, diabetes, inflammatory bowel disease, and chronic kidney disease (49), as well as immune dysfunction in gut, spleen, pancreas (128), etc. (103). Most of these diseases involve the RELM-related lung pathologies discussed in this review (18, 35, 69, 86, 96, 102, 128). All of these inflammation-related diseases remain clinical challenges in current medical practice. Although the study of RELM proteins in physiology and disease has begun to expand rapidly and involvement has been identified in a wide range of inflammatory responses, little is yet known regarding the exact biological function, pleiotropic roles, tissue and cellular distribution, receptors, and downstream signaling of different RELM isoforms. Consequently, some of their reported contributions to lung disease progression and prediction remain controversial. These limitations urge us to define the detailed functions of these family members and further dissect their roles in human disease. The mechanistic findings have been applied to the development of anti-hResistin blocking antibodies for use in PH (74) (Q. Lin, J. T. Skinner, C. Fan, E. N. Hunter, J. J. Gray, N. Biswas, K. Yamaji-Kegan, R. A. Johns, unpublished observations) and other related disorders. These pharmacologic intervention studies are likely to help researchers acquire more critical insights into disease pathogenesis. Nevertheless, we believe that the information summarized here provides a deeper understanding of the mechanisms by which RELMs alter the inflammatory milieu, opening a door for the identification of precise therapeutic targets within the pulmonary microenvironment. Identification of such targets is vital to the development of novel treatment approaches for a variety of disorders in lung and other organs, such as diabetic retinopathy, rheumatoid arthritis, psoriasis, and cancers.
GRANTS
This work was supported by National Institutes of Health (NIH) Centers for Advanced Diagnostics and Experimental Therapeutics in Lung Diseases Stage II (CADET II) Grant 5UH2HL123827-02 and NIH National Heart, Lung, and Blood Institute Grant 1R01HL138497-01A1 (to R.A.J.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Q.L. and R.A.J. prepared figures; Q.L. and R.A.J. drafted manuscript; Q.L. and R.A.J. edited and revised manuscript; Q.L. and R.A.J. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Claire F. Levine for editing this article in manuscript form.
REFERENCES
- 1.Al Mutairi SS, Mojiminiyi OA, Shihab-Eldeen A, Al Rammah T, Abdella N. Putative roles of circulating resistin in patients with asthma, COPD and cigarette smokers. Dis Markers 31: 1–7, 2011. doi: 10.1155/2011/297591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Biltagi MA, Tolba OA, Mawlana W, Abd El Hamed A, Ghazy M. Resistin and right ventricular function in children with recently diagnosed type-1 diabetes mellitus: a case control study. J Pediatr Endocrinol Metab 28: 299–308, 2015. doi: 10.1515/jpem-2014-0264. [DOI] [PubMed] [Google Scholar]
- 3.Angelini DJ, Su Q, Kolosova IA, Fan C, Skinner JT, Yamaji-Kegan K, Collector M, Sharkis SJ, Johns RA. Hypoxia-induced mitogenic factor (HIMF/FIZZ1/RELM alpha) recruits bone marrow-derived cells to the murine pulmonary vasculature. PLoS One 5: e11251, 2010. doi: 10.1371/journal.pone.0011251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angelini DJ, Su Q, Yamaji-Kegan K, Fan C, Skinner JT, Champion HC, Crow MT, Johns RA. Hypoxia-induced mitogenic factor (HIMF/FIZZ1/RELMalpha) induces the vascular and hemodynamic changes of pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 296: L582–L593, 2009. doi: 10.1152/ajplung.90526.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angelini DJ, Su Q, Yamaji-Kegan K, Fan C, Skinner JT, Poloczek A, El-Haddad H, Cheadle C, Johns RA. Hypoxia-induced mitogenic factor (HIMF/FIZZ1/RELMα) in chronic hypoxia- and antigen-mediated pulmonary vascular remodeling. Respir Res 14: 1, 2013. doi: 10.1186/1465-9921-14-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Angelini DJ, Su Q, Yamaji-Kegan K, Fan C, Teng X, Hassoun PM, Yang SC, Champion HC, Tuder RM, Johns RA. Resistin-like molecule-β in scleroderma-associated pulmonary hypertension. Am J Respir Cell Mol Biol 41: 553–561, 2009. doi: 10.1165/rcmb.2008-0271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Artis D, Wang ML, Keilbaugh SA, He W, Brenes M, Swain GP, Knight PA, Donaldson DD, Lazar MA, Miller HR, Schad GA, Scott P, Wu GD. RELMβ/FIZZ2 is a goblet cell-specific immune-effector molecule in the gastrointestinal tract. Proc Natl Acad Sci USA 101: 13596–13600, 2004. doi: 10.1073/pnas.0404034101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ballantyne D, Scott H, MacDonald-Wicks L, Gibson PG, Wood LG. Resistin is a predictor of asthma risk and resistin:adiponectin ratio is a negative predictor of lung function in asthma. Clin Exp Allergy 46: 1056–1065, 2016. doi: 10.1111/cea.12742. [DOI] [PubMed] [Google Scholar]
- 9.Banerjee RR, Lazar MA. Dimerization of resistin and resistin-like molecules is determined by a single cysteine. J Biol Chem 276: 25970–25973, 2001. doi: 10.1074/jbc.M103109200. [DOI] [PubMed] [Google Scholar]
- 10.Batugedara HM, Li J, Chen G, Lu D, Patel JJ, Jang JC, Radecki KC, Burr AC, Lo DD, Dillman AR, Nair MG. Hematopoietic cell-derived RELMα regulates hookworm immunity through effects on macrophages. J Leukoc Biol 104: 855–869, 2018. doi: 10.1002/JLB.4A0917-369RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Becerra-Díaz M, Wills-Karp M, Heller NM. New perspectives on the regulation of type II inflammation in asthma. F1000Res 6: 1014, 2017. doi: 10.12688/f1000research.11198.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boeselt T, Nell C, Lütteken L, Kehr K, Koepke J, Apelt S, Veith M, Beutel B, Spielmanns M, Greulich T, Vogelmeier CF, Kenn K, Janciauskiene S, Alter P, Koczulla AR. Benefits of high-intensity exercise training to patients with chronic obstructive pulmonary disease: a controlled study. Respiration 93: 301–310, 2017. doi: 10.1159/000464139. [DOI] [PubMed] [Google Scholar]
- 13.Boucherat O, Vitry G, Trinh I, Paulin R, Provencher S, Bonnet S. The cancer theory of pulmonary arterial hypertension. Pulm Circ 7: 285–299, 2017. doi: 10.1177/2045893217701438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cai D, Xu Y, Ding R, Qiu K, Zhang R, Wang H, Huang L, Xie X, Yan H, Deng Y, Lin X, Shao J, Luo X, Duan C. Extensive serum biomarker analysis in patients with non-small-cell lung carcinoma. Cytokine 126: 154868, 2020. doi: 10.1016/j.cyto.2019.154868. [DOI] [PubMed] [Google Scholar]
- 15.Chang SW, Pan WS, Lozano Beltran D, Oleyda Baldelomar L, Solano MA, Tuero I, Friedland JS, Torrico F, Gilman RH. Gut hormones, appetite suppression and cachexia in patients with pulmonary TB. PLoS One 8: e54564, 2013. doi: 10.1371/journal.pone.0054564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chao WC, Yen CL, Wu YH, Chen SY, Hsieh CY, Chang TC, Ou HY, Shieh CC. Increased resistin may suppress reactive oxygen species production and inflammasome activation in type 2 diabetic patients with pulmonary tuberculosis infection. Microbes Infect 17: 195–204, 2015. doi: 10.1016/j.micinf.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Chen G, Wang SH, Jang JC, Odegaard JI, Nair MG. Comparison of RELMα and RELMβ single- and double-gene-deficient mice reveals that RELMα expression dictates inflammation and worm expulsion in hookworm infection. Infect Immun 84: 1100–1111, 2016. doi: 10.1128/IAI.01479-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chumakov AM, Kubota T, Walter S, Koeffler HP. Identification of murine and human XCP1 genes as C/EBP-epsilon-dependent members of FIZZ/Resistin gene family. Oncogene 23: 3414–3425, 2004. doi: 10.1038/sj.onc.1207126. [DOI] [PubMed] [Google Scholar]
- 19.Chung MJ, Liu T, Ullenbruch M, Phan SH. Antiapoptotic effect of found in inflammatory zone (FIZZ)1 on mouse lung fibroblasts. J Pathol 212: 180–187, 2007. doi: 10.1002/path.2161. [DOI] [PubMed] [Google Scholar]
- 20.Cook PC, Jones LH, Jenkins SJ, Wynn TA, Allen JE, MacDonald AS. Alternatively activated dendritic cells regulate CD4+ T-cell polarization in vitro and in vivo. Proc Natl Acad Sci USA 109: 9977–9982, 2012. doi: 10.1073/pnas.1121231109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crosby A, Jones FM, Southwood M, Stewart S, Schermuly R, Butrous G, Dunne DW, Morrell NW. Pulmonary vascular remodeling correlates with lung eggs and cytokines in murine schistosomiasis. Am J Respir Crit Care Med 181: 279–288, 2010. doi: 10.1164/rccm.200903-0355OC. [DOI] [PubMed] [Google Scholar]
- 22.Daley E, Emson C, Guignabert C, de Waal Malefyt R, Louten J, Kurup VP, Hogaboam C, Taraseviciene-Stewart L, Voelkel NF, Rabinovitch M, Grunig E, Grunig G. Pulmonary arterial remodeling induced by a Th2 immune response. J Exp Med 205: 361–372, 2008. doi: 10.1084/jem.20071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daquinag AC, Zhang Y, Amaya-Manzanares F, Simmons PJ, Kolonin MG. An isoform of decorin is a resistin receptor on the surface of adipose progenitor cells. Cell Stem Cell 9: 74–86, 2011. doi: 10.1016/j.stem.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 24.Demiray G, Değirmencioğlu S, Uğurlu E, Yaren A. Effects of serum leptin and resistin levels on cancer cachexia in patients with advanced-stage non-small cell lung cancer. Clin Med Insights Oncol 11: 1179554917690144, 2017. doi: 10.1177/1179554917690144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doherty TA, Khorram N, Sugimoto K, Sheppard D, Rosenthal P, Cho JY, Pham A, Miller M, Croft M, Broide DH. Alternaria induces STAT6-dependent acute airway eosinophilia and epithelial FIZZ1 expression that promotes airway fibrosis and epithelial thickness. J Immunol 188: 2622–2629, 2012. doi: 10.4049/jimmunol.1101632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong L, Wang SJ, Camoretti-Mercado B, Li HJ, Chen M, Bi WX. FIZZ1 plays a crucial role in early stage airway remodeling of OVA-induced asthma. J Asthma 45: 648–653, 2008. doi: 10.1080/02770900802126941. [DOI] [PubMed] [Google Scholar]
- 27.Ehrnthaller C, Flierl M, Perl M, Denk S, Unnewehr H, Ward PA, Radermacher P, Ignatius A, Gebhard F, Chinnaiyan A, Huber-Lang M. The molecular fingerprint of lung inflammation after blunt chest trauma. Eur J Med Res 20: 70, 2015. doi: 10.1186/s40001-015-0164-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ehtesham NZ, Nasiruddin M, Alvi A, Kumar BK, Ahmed N, Peri S, Murthy KJ, Hasnain SE. Treatment end point determinants for pulmonary tuberculosis: human resistin as a surrogate biomarker. Tuberculosis (Edinb) 91: 293–299, 2011. doi: 10.1016/j.tube.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 29.Fan C, Fu Z, Su Q, Angelini DJ, Van Eyk J, Johns RA. S100A11 mediates hypoxia-induced mitogenic factor (HIMF)-induced smooth muscle cell migration, vesicular exocytosis, and nuclear activation. Mol Cell Proteomics 10: M110.000901, 2011. doi: 10.1074/mcp.M110.000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fan C, Johns BA, Su Q, Kolosova IA, Johns RA. Choosing the right antibody for resistin-like molecule (RELM/FIZZ) family members. Histochem Cell Biol 139: 605–613, 2013. doi: 10.1007/s00418-012-1042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fan C, Meuchel LW, Su Q, Angelini DJ, Zhang A, Cheadle C, Kolosova I, Makarevich OD, Yamaji-Kegan K, Rothenberg ME, Johns RA. Resistin-like molecule α in allergen-induced pulmonary vascular remodeling. Am J Respir Cell Mol Biol 53: 303–313, 2015. doi: 10.1165/rcmb.2014-0322OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fan C, Su Q, Li Y, Liang L, Angelini DJ, Guggino WB, Johns RA. Hypoxia-induced mitogenic factor/FIZZ1 induces intracellular calcium release through the PLC-IP(3) pathway. Am J Physiol Lung Cell Mol Physiol 297: L263–L270, 2009. doi: 10.1152/ajplung.90416.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fang CL, Yin LJ, Sharma S, Kierstein S, Wu HF, Eid G, Haczku A, Corrigan CJ, Ying S. Resistin-like molecule-β (RELM-β) targets airways fibroblasts to effect remodelling in asthma: from mouse to man. Clin Exp Allergy 45: 940–952, 2015. doi: 10.1111/cea.12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Forrest OA, Chopyk DM, Gernez Y, Brown MR, Conrad CK, Moss RB, Tangpricha V, Peng L, Tirouvanziam R. Resistin is elevated in cystic fibrosis sputum and correlates negatively with lung function. J Cyst Fibros 18: 64–70, 2019. doi: 10.1016/j.jcf.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 35.Gangadharan B, Hoeve MA, Allen JE, Ebrahimi B, Rhind SM, Dutia BM, Nash AA. Murine gammaherpesvirus-induced fibrosis is associated with the development of alternatively activated macrophages. J Leukoc Biol 84: 50–58, 2008. doi: 10.1189/jlb.0507270. [DOI] [PubMed] [Google Scholar]
- 36.Gerstmayer B, Küsters D, Gebel S, Müller T, Van Miert E, Hofmann K, Bosio A. Identification of RELMγ, a novel resistin-like molecule with a distinct expression pattern. Genomics 81: 588–595, 2003. doi: 10.1016/S0888-7543(03)00070-3. [DOI] [PubMed] [Google Scholar]
- 37.Gologanu D, Stanescu C, Bogdan MA. Pulmonary hypertension secondary to chronic obstructive pulmonary disease. Rom J Intern Med 50: 259–268, 2012. [PubMed] [Google Scholar]
- 38.Gong WJ, Liu JY, Yin JY, Cui JJ, Xiao D, Zhuo W, Luo C, Liu RJ, Li X, Zhang W, Zhou HH, Liu ZQ. Resistin facilitates metastasis of lung adenocarcinoma through the TLR4/Src/EGFR/PI3K/NF-κB pathway. Cancer Sci 109: 2391–2400, 2018. doi: 10.1111/cas.13704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gordon W, Galitovskiy V, Edwards R, Andersen B, Grando SA. The tobacco carcinogen nitrosamine induces a differential gene expression response in tumour susceptible A/J and resistant C3H mouse lungs. Eur J Cancer 49: 725–733, 2013. doi: 10.1016/j.ejca.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Graham BB, Mentink-Kane MM, El-Haddad H, Purnell S, Zhang L, Zaiman A, Redente EF, Riches DW, Hassoun PM, Bandeira A, Champion HC, Butrous G, Wynn TA, Tuder RM. Schistosomiasis-induced experimental pulmonary hypertension: role of interleukin-13 signaling. Am J Pathol 177: 1549–1561, 2010. doi: 10.2353/ajpath.2010.100063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harris HE, Andersson U, Pisetsky DS. HMGB1: a multifunctional alarmin driving autoimmune and inflammatory disease. Nat Rev Rheumatol 8: 195–202, 2012. doi: 10.1038/nrrheum.2011.222. [DOI] [PubMed] [Google Scholar]
- 42.He W, Wang ML, Jiang HQ, Steppan CM, Shin ME, Thurnheer MC, Cebra JJ, Lazar MA, Wu GD. Bacterial colonization leads to the colonic secretion of RELMβ/FIZZ2, a novel goblet cell-specific protein. Gastroenterology 125: 1388–1397, 2003. doi: 10.1016/j.gastro.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 43.Hoeve MA, Mylonas KJ, Fairlie-Clarke KJ, Mahajan SM, Allen JE, Graham AL. Plasmodium chabaudi limits early Nippostrongylus brasiliensis-induced pulmonary immune activation and Th2 polarization in co-infected mice. BMC Immunol 10: 60, 2009. doi: 10.1186/1471-2172-10-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holcomb IN, Kabakoff RC, Chan B, Baker TW, Gurney A, Henzel W, Nelson C, Lowman HB, Wright BD, Skelton NJ, Frantz GD, Tumas DB, Peale FV Jr, Shelton DL, Hébert CC. FIZZ1, a novel cysteine-rich secreted protein associated with pulmonary inflammation, defines a new gene family. EMBO J 19: 4046–4055, 2000. doi: 10.1093/emboj/19.15.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Homer RJ. Airway remodeling and RELM-β. Am J Physiol Lung Cell Mol Physiol 293: L303–L304, 2007. doi: 10.1152/ajplung.00226.2007. [DOI] [PubMed] [Google Scholar]
- 46.Hu WW, Tang CH, Sun Y, Lu TT, Jiang P, Wu YM, Wang CQ, Yang SF, Su CM. Correlation between resistin gene polymorphism and clinical aspects of lung cancer. Medicine (Baltimore) 96: e9485, 2017. doi: 10.1097/MD.0000000000009485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huber R, Meier B, Otsuka A, Fenini G, Satoh T, Gehrke S, Widmer D, Levesque MP, Mangana J, Kerl K, Gebhardt C, Fujii H, Nakashima C, Nonomura Y, Kabashima K, Dummer R, Contassot E, French LE. Tumour hypoxia promotes melanoma growth and metastasis via high mobility group box-1 and M2-like macrophages. Sci Rep 6: 29914, 2016. doi: 10.1038/srep29914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jackson IL, Zhang Y, Bentzen SM, Hu J, Zhang A, Vujaskovic Z. Pathophysiological mechanisms underlying phenotypic differences in pulmonary radioresponse. Sci Rep 6: 36579, 2016. [Erratum in Sci Rep 7: 46782, 2017]. doi: 10.1038/srep36579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jamaluddin MS, Weakley SM, Yao Q, Chen C. Resistin: functional roles and therapeutic considerations for cardiovascular disease. Br J Pharmacol 165: 622–632, 2012. doi: 10.1111/j.1476-5381.2011.01369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jang JC, Chen G, Wang SH, Barnes MA, Chung JI, Camberis M, Le Gros G, Cooper PJ, Steel C, Nutman TB, Lazar MA, Nair MG. Macrophage-derived human resistin is induced in multiple helminth infections and promotes inflammatory monocytes and increased parasite burden. PLoS Pathog 11: e1004579, 2015. doi: 10.1371/journal.ppat.1004579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jang JC, Li J, Gambini L, Batugedara HM, Sati S, Lazar MA, Fan L, Pellecchia M, Nair MG. Human resistin protects against endotoxic shock by blocking LPS-TLR4 interaction. Proc Natl Acad Sci USA 114: E10399–E10408, 2017. doi: 10.1073/pnas.1716015114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiang S, Park DW, Tadie JM, Gregoire M, Deshane J, Pittet JF, Abraham E, Zmijewski JW. Human resistin promotes neutrophil proinflammatory activation and neutrophil extracellular trap formation and increases severity of acute lung injury. J Immunol 192: 4795–4803, 2014. doi: 10.4049/jimmunol.1302764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiang Y, Zhou X, Hu R, Dai A. TGF-β1-induced SMAD2/3/4 activation promotes RELM-β transcription to modulate the endothelium-mesenchymal transition in human endothelial cells. Int J Biochem Cell Biol 105: 52–60, 2018. doi: 10.1016/j.biocel.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 54.Johns RA. Th2 inflammation, hypoxia-induced mitogenic factor/FIZZ1, and pulmonary hypertension and vascular remodeling in schistosomiasis. Am J Respir Crit Care Med 181: 203–205, 2010. doi: 10.1164/rccm.200912-1827ED. [DOI] [PubMed] [Google Scholar]
- 55.Johns RA, Takimoto E, Meuchel LW, Elsaigh E, Zhang A, Heller NM, Semenza GL, Yamaji-Kegan K. Hypoxia-inducible factor 1α is a critical downstream mediator for hypoxia-induced mitogenic factor (FIZZ1/RELMα)-induced pulmonary hypertension. Arterioscler Thromb Vasc Biol 36: 134–144, 2016. doi: 10.1161/ATVBAHA.115.306710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karapanagiotou EM, Tsochatzis EA, Dilana KD, Tourkantonis I, Gratsias I, Syrigos KN. The significance of leptin, adiponectin, and resistin serum levels in non-small cell lung cancer (NSCLC). Lung Cancer 61: 391–397, 2008. doi: 10.1016/j.lungcan.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 57.Kawashima S, Hirose K, Takahashi K, Tamachi T, Ikeda K, Tokoyoda K, Nakayama T, Nakajima H. Interleukin-25 induces pulmonary arterial remodeling via natural killer T cell-dependent mechanisms. Int Arch Allergy Immunol 161, Suppl 2: 118–124, 2013. doi: 10.1159/000350379. [DOI] [PubMed] [Google Scholar]
- 58.Kim KH, Lee K, Moon YS, Sul HS. A cysteine-rich adipose tissue-specific secretory factor inhibits adipocyte differentiation. J Biol Chem 276: 11252–11256, 2001. doi: 10.1074/jbc.C100028200. [DOI] [PubMed] [Google Scholar]
- 59.Kim KH, Zhao L, Moon Y, Kang C, Sul HS. Dominant inhibitory adipocyte-specific secretory factor (ADSF)/resistin enhances adipogenesis and improves insulin sensitivity. Proc Natl Acad Sci USA 101: 6780–6785, 2004. doi: 10.1073/pnas.0305905101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim KW, Shin YH, Lee KE, Kim ES, Sohn MH, Kim KE. Relationship between adipokines and manifestations of childhood asthma. Pediatr Allergy Immunol 19: 535–540, 2008. doi: 10.1111/j.1399-3038.2007.00690.x. [DOI] [PubMed] [Google Scholar]
- 61.Kochetkova EA, Ugaĭ LG, Buria KA, Nevzorova VA, Massard J. [Association between adipokines and the development of osteopenic syndrome in end-stage lung disease]. Ter Arkh 84: 40–44, 2012. [PubMed] [Google Scholar]
- 62.Kolosova IA, Angelini D, Fan C, Skinner J, Cheadle C, Johns RA. Resistin-like molecule α stimulates proliferation of mesenchymal stem cells while maintaining their multipotency. Stem Cells Dev 22: 239–247, 2013. doi: 10.1089/scd.2012.0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kramer MM, Hirota JA, Sood A, Teschke K, Carlsten C. Airway and serum adipokines after allergen and diesel exposure in a controlled human crossover study of atopic adults. Transl Res 182: 49–60, 2017. doi: 10.1016/j.trsl.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 64.Krljanac B, Schubart C, Naumann R, Wirtz S, Culemann S, Krönke G, Voehringer D. RELMα-expressing macrophages protect against fatal lung damage and reduce parasite burden during helminth infection. Sci Immunol 4: eaau3814, 2019. doi: 10.1126/sciimmunol.aau3814. [DOI] [PubMed] [Google Scholar]
- 65.Kumar S, Wang G, Liu W, Ding W, Dong M, Zheng N, Ye H, Liu J. Hypoxia-induced mitogenic factor promotes cardiac hypertrophy via calcium-dependent and hypoxia-inducible factor-1α mechanisms. Hypertension 72: 331–342, 2018. doi: 10.1161/HYPERTENSIONAHA.118.10845. [DOI] [PubMed] [Google Scholar]
- 66.Kumor-Kisielewska A, Kierszniewska-Stępień D, Pietras T, Kroczyńska-Bednarek J, Kurmanowska Z, Antczak A, Górski P. Assessment of leptin and resistin levels in patients with chronic obstructive pulmonary disease. Pol Arch Med Wewn 123: 215–220, 2013. doi: 10.20452/pamw.1724. [DOI] [PubMed] [Google Scholar]
- 67.Kuo CH, Chen KF, Chou SH, Huang YF, Wu CY, Cheng DE, Chen YW, Yang CJ, Hung JY, Huang MS. Lung tumor-associated dendritic cell-derived resistin promoted cancer progression by increasing Wolf-Hirschhorn syndrome candidate 1/Twist pathway. Carcinogenesis 34: 2600–2609, 2013. doi: 10.1093/carcin/bgt281. [DOI] [PubMed] [Google Scholar]
- 68.LaRochelle J, Freiler J, Dice J, Hagan L. Plasma resistin levels in asthmatics as a marker of disease state. J Asthma 44: 509–513, 2007. doi: 10.1080/02770900701495785. [DOI] [PubMed] [Google Scholar]
- 69.Lederer DJ, Kawut SM, Wickersham N, Winterbottom C, Bhorade S, Palmer SM, Lee J, Diamond JM, Wille KM, Weinacker A, Lama VN, Crespo M, Orens JB, Sonett JR, Arcasoy SM, Ware LB, Christie JD; Lung Transplant Outcomes Group . Obesity and primary graft dysfunction after lung transplantation: the Lung Transplant Outcomes Group Obesity Study. Am J Respir Crit Care Med 184: 1055–1061, 2011. doi: 10.1164/rccm.201104-0728OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee MR, Shim D, Yoon J, Jang HS, Oh SW, Suh SH, Choi JH, Oh GT. Retnla overexpression attenuates allergic inflammation of the airway. PLoS One 9: e112666, 2014. doi: 10.1371/journal.pone.0112666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee S, Lee HC, Kwon YW, Lee SE, Cho Y, Kim J, Lee S, Kim JY, Lee J, Yang HM, Mook-Jung I, Nam KY, Chung J, Lazar MA, Kim HS. Adenylyl cyclase-associated protein 1 is a receptor for human resistin and mediates inflammatory actions of human monocytes. Cell Metab 19: 484–497, 2014. doi: 10.1016/j.cmet.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Leivo-Korpela S, Lehtimäki L, Nieminen R, Oksa P, Vierikko T, Järvenpää R, Uitti J, Moilanen E. Adipokine adipsin is associated with the degree of lung fibrosis in asbestos-exposed workers. Respir Med 106: 1435–1440, 2012. doi: 10.1016/j.rmed.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 73.Leivo-Korpela S, Lehtimäki L, Vuolteenaho K, Nieminen R, Kankaanranta H, Saarelainen S, Moilanen E. Adipokine resistin predicts anti-inflammatory effect of glucocorticoids in asthma. J Inflamm (Lond) 8: 12, 2011. doi: 10.1186/1476-9255-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lin Q, Fan C, Skinner JT, Bejia D, Van Raemdonck K, Nakahara M, Yamaji-Kegan K, Johns RA. Therapeutic effects of the generated antibodies targeting human resistin in pulmonary hypertension (Abstract). Am J Respir Crit Care Med 201: A7398, 2018. [Google Scholar]
- 75.Lin Q, Jin S, Han M, Zheng W, Liu J, Wei X. Inflammation in the tumor microenvironment. J Immunol Res 2018: 1965847, 2018. doi: 10.1155/2018/1965847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lin Q, Fan C, Gomez-Arroyo J, Van Raemdonck K, Meuchel LW, Skinner JT, Everett AD, Fang X, Macdonald AA, Yamaji-Kegan K, Johns RA. HIMF (hypoxia-induced mitogenic factor) signaling mediates the HMGB1 (high mobility group Box 1)-dependent endothelial and smooth muscle cell crosstalk in pulmonary hypertension. Arterioscler Thromb Vasc Biol 39: 2505–2519, 2019. doi: 10.1161/ATVBAHA.119.312907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lin Q, Fan C, Skinner JT, Hunter EN, Macdonald AA, Illei PB, Yamaji-Kegan K, Johns RA. RELMα licenses macrophages for damage-associated molecular pattern activation to instigate pulmonary vascular remodeling. J Immunol 203: 2862–2871, 2019. doi: 10.4049/jimmunol.1900535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu T, Baek HA, Yu H, Lee HJ, Park BH, Ullenbruch M, Liu J, Nakashima T, Choi YY, Wu GD, Chung MJ, Phan SH. FIZZ2/RELM-β induction and role in pulmonary fibrosis. J Immunol 187: 450–461, 2011. doi: 10.4049/jimmunol.1000964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu T, Dhanasekaran SM, Jin H, Hu B, Tomlins SA, Chinnaiyan AM, Phan SH. FIZZ1 stimulation of myofibroblast differentiation. Am J Pathol 164: 1315–1326, 2004. doi: 10.1016/S0002-9440(10)63218-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu T, Jin H, Ullenbruch M, Hu B, Hashimoto N, Moore B, McKenzie A, Lukacs NW, Phan SH. Regulation of found in inflammatory zone 1 expression in bleomycin-induced lung fibrosis: role of IL-4/IL-13 and mediation via STAT-6. J Immunol 173: 3425–3431, 2004. doi: 10.4049/jimmunol.173.5.3425. [DOI] [PubMed] [Google Scholar]
- 81.Liu T, Yu H, Ullenbruch M, Jin H, Ito T, Wu Z, Liu J, Phan SH. The in vivo fibrotic role of FIZZ1 in pulmonary fibrosis. PLoS One 9: e88362, 2014. doi: 10.1371/journal.pone.0088362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ma L, Zeng J, Mo B, Wang C, Huang J, Sun Y, Yu Y, Liu S. High mobility group box 1: a novel mediator of Th2-type response-induced airway inflammation of acute allergic asthma. J Thorac Dis 7: 1732–1741, 2015. doi: 10.3978/j.issn.2072-1439.2015.10.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ma WL, Cai PC, Xiong XZ, Ye H. Exercise training attenuated chronic cigarette smoking-induced up-regulation of FIZZ1/RELMα in lung of rats. J Huazhong Univ Sci Technolog Med Sci 33: 22–26, 2013. doi: 10.1007/s11596-013-1065-3. [DOI] [PubMed] [Google Scholar]
- 84.Madala SK, Edukulla R, Davis KR, Schmidt S, Davidson C, Kitzmiller JA, Hardie WD, Korfhagen TR. Resistin-like molecule α1 (Fizz1) recruits lung dendritic cells without causing pulmonary fibrosis. Respir Res 13: 51, 2012. doi: 10.1186/1465-9921-13-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Masui Y, Asano Y, Akamata K, Aozasa N, Noda S, Taniguchi T, Takahashi T, Ichimura Y, Toyama T, Sumida H, Kuwano Y, Yanaba K, Tada Y, Sugaya M, Sato S, Kadono T. Serum resistin levels: a possible correlation with pulmonary vascular involvement in patients with systemic sclerosis. Rheumatol Int 34: 1165–1170, 2014. doi: 10.1007/s00296-013-2880-3. [DOI] [PubMed] [Google Scholar]
- 86.Mavi P, Niranjan R, Dutt P, Zaidi A, Shukla JS, Korfhagen T, Mishra A. Allergen-induced resistin-like molecule-α promotes esophageal epithelial cell hyperplasia in eosinophilic esophagitis. Am J Physiol Gastrointest Liver Physiol 307: G499–G507, 2014. doi: 10.1152/ajpgi.00141.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mendivil CO, Koziel H, Brain JD. Metabolic hormones, apolipoproteins, adipokines, and cytokines in the alveolar lining fluid of healthy adults: compartmentalization and physiological correlates. PLoS One 10: e0123344, 2015. doi: 10.1371/journal.pone.0123344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mineo D, Ambrogi V, Frasca L, Cufari ME, Pompeo E, Mineo TC. Effects of lung volume reduction surgery for emphysema on glycolipidic hormones. Chest 134: 30–37, 2008. doi: 10.1378/chest.07-3042. [DOI] [PubMed] [Google Scholar]
- 89.Mishra A, Wang M, Schlotman J, Nikolaidis NM, DeBrosse CW, Karow ML, Rothenberg ME. Resistin-like molecule-β is an allergen-induced cytokine with inflammatory and remodeling activity in the murine lung. Am J Physiol Lung Cell Mol Physiol 293: L305–L313, 2007. doi: 10.1152/ajplung.00147.2007. [DOI] [PubMed] [Google Scholar]
- 90.Moideen K, Kumar NP, Nair D, Banurekha VV, Babu S. Altered systemic adipokine levels in pulmonary tuberculosis and changes following treatment. Am J Trop Med Hyg 99: 875–880, 2018. doi: 10.4269/ajtmh.18-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Munitz A, Cole ET, Karo-Atar D, Finkelman FD, Rothenberg ME. Resistin-like molecule-α regulates IL-13-induced chemokine production but not allergen-induced airway responses. Am J Respir Cell Mol Biol 46: 703–713, 2012. doi: 10.1165/rcmb.2011-0391OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nagaev I, Smith U. Insulin resistance and type 2 diabetes are not related to resistin expression in human fat cells or skeletal muscle. Biochem Biophys Res Commun 285: 561–564, 2001. doi: 10.1006/bbrc.2001.5173. [DOI] [PubMed] [Google Scholar]
- 93.Nair MG, Cochrane DW, Allen JE. Macrophages in chronic type 2 inflammation have a novel phenotype characterized by the abundant expression of Ym1 and Fizz1 that can be partly replicated in vitro. Immunol Lett 85: 173–180, 2003. doi: 10.1016/S0165-2478(02)00225-0. [DOI] [PubMed] [Google Scholar]
- 94.Nair MG, Du Y, Perrigoue JG, Zaph C, Taylor JJ, Goldschmidt M, Swain GP, Yancopoulos GD, Valenzuela DM, Murphy A, Karow M, Stevens S, Pearce EJ, Artis D. Alternatively activated macrophage-derived RELM-alpha is a negative regulator of type 2 inflammation in the lung. J Exp Med 206: 937–952, 2009. doi: 10.1084/jem.20082048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nair MG, Guild KJ, Artis D. Novel effector molecules in type 2 inflammation: lessons drawn from helminth infection and allergy. J Immunol 177: 1393–1399, 2006. doi: 10.4049/jimmunol.177.3.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nohira T, Nagao K, Kameyama K, Nakai H, Fukumine N, Okabe K, Kitano S, Hisatomi H. Identification of an alternative splicing transcript for the resistin gene and distribution of its mRNA in human tissue. Eur J Endocrinol 151: 151–154, 2004. doi: 10.1530/eje.0.1510151. [DOI] [PubMed] [Google Scholar]
- 97.Paplińska-Goryca M, Rubinsztajn R, Nejman-Gryz P, Przybyłowski T, Krenke R, Chazan R. The association between serological features of chronic Chlamydia pneumoniae infection and markers of systemic inflammation and nutrition in COPD patients. Scand J Clin Lab Invest 77: 644–650, 2017. doi: 10.1080/00365513.2017.1393694. [DOI] [PubMed] [Google Scholar]
- 98.Park HK, Kwak MK, Kim HJ, Ahima RS. Linking resistin, inflammation, and cardiometabolic diseases. Korean J Intern Med (Korean Assoc Intern Med) 32: 239–247, 2017. doi: 10.3904/kjim.2016.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Park SH, Chen WC, Esmaeil N, Lucas B, Marsh LM, Reibman J, Grunig G. Interleukin 13- and interleukin 17A-induced pulmonary hypertension phenotype due to inhalation of antigen and fine particles from air pollution. Pulm Circ 4: 654–668, 2014. doi: 10.1086/678511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Park SH, Chen WC, Hoffman C, Marsh LM, West J, Grunig G. Modification of hemodynamic and immune responses to exposure with a weak antigen by the expression of a hypomorphic BMPR2 gene. PLoS One 8: e55180, 2013. doi: 10.1371/journal.pone.0055180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Perrigoue JG, Li J, Zaph C, Goldschmidt M, Scott P, de Sauvage FJ, Pearce EJ, Ghilardi N, Artis D. IL-31-IL-31R interactions negatively regulate type 2 inflammation in the lung. J Exp Med 204: 481–487, 2007. doi: 10.1084/jem.20061791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pesce JT, Ramalingam TR, Wilson MS, Mentink-Kane MM, Thompson RW, Cheever AW, Urban JF Jr, Wynn TA. Retnla (Relmα/Fizz1) suppresses helminth-induced Th2-type immunity. PLoS Pathog 5: e1000393, 2009. doi: 10.1371/journal.ppat.1000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pine GM, Batugedara HM, Nair MG. Here, there and everywhere: resistin-like molecules in infection, inflammation, and metabolic disorders. Cytokine 110: 442–451, 2018. doi: 10.1016/j.cyto.2018.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Price LC, McAuley DF, Marino PS, Finney SJ, Griffiths MJ, Wort SJ. Pathophysiology of pulmonary hypertension in acute lung injury. Am J Physiol Lung Cell Mol Physiol 302: L803–L815, 2012. doi: 10.1152/ajplung.00355.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Propheter DC, Chara AL, Harris TA, Ruhn KA, Hooper LV. Resistin-like molecule β is a bactericidal protein that promotes spatial segregation of the microbiota and the colonic epithelium. Proc Natl Acad Sci USA 114: 11027–11033, 2017. doi: 10.1073/pnas.1711395114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Qian F, Deng J, Lee YG, Zhu J, Karpurapu M, Chung S, Zheng JN, Xiao L, Park GY, Christman JW. The transcription factor PU.1 promotes alternative macrophage polarization and asthmatic airway inflammation. J Mol Cell Biol 7: 557–567, 2015. doi: 10.1093/jmcb/mjv042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Raes G, De Baetselier P, Noël W, Beschin A, Brombacher F, Hassanzadeh Gh G. Differential expression of FIZZ1 and Ym1 in alternatively versus classically activated macrophages. J Leukoc Biol 71: 597–602, 2002. [PubMed] [Google Scholar]
- 108.Razvi SS, Richards JB, Malik F, Cromar KR, Price RE, Bell CS, Weng T, Atkins CL, Spencer CY, Cockerill KJ, Alexander AL, Blackburn MR, Alcorn JL, Haque IU, Johnston RA. Resistin deficiency in mice has no effect on pulmonary responses induced by acute ozone exposure. Am J Physiol Lung Cell Mol Physiol 309: L1174–L1185, 2015. doi: 10.1152/ajplung.00270.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Reece JJ, Siracusa MC, Southard TL, Brayton CF, Urban JF Jr, Scott AL. Hookworm-induced persistent changes to the immunological environment of the lung. Infect Immun 76: 3511–3524, 2008. doi: 10.1128/IAI.00192-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Renigunta A, Hild C, Rose F, Klepetko W, Grimminger F, Seeger W, Hänze J. Human RELMβ is a mitogenic factor in lung cells and induced in hypoxia. FEBS Lett 580: 900–903, 2006. doi: 10.1016/j.febslet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 111.Rubinsztajn R, Przybyłowski T, Maskey-Warzęchowska M, Paplińska-Goryca M, Karwat K, Nejman-Gryz P, Chazan R. Correlation between hyperinflation defined as an elevated RV/TLC ratio and body composition and cytokine profile in patients with chronic obstructive pulmonary disease. Pneumonol Alergol Pol 83: 120–125, 2015. doi: 10.5603/PiAP.2015.0019. [DOI] [PubMed] [Google Scholar]
- 112.Said SI, Hamidi SA, Gonzalez Bosc L. Asthma and pulmonary arterial hypertension: do they share a key mechanism of pathogenesis? Eur Respir J 35: 730–734, 2010. doi: 10.1183/09031936.00097109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sánchez-Solana B, Laborda J, Baladrón V. Mouse resistin modulates adipogenesis and glucose uptake in 3T3-L1 preadipocytes through the ROR1 receptor. Mol Endocrinol 26: 110–127, 2012. doi: 10.1210/me.2011-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sauni R, Oksa P, Lehtimäki L, Toivio P, Palmroos P, Nieminen R, Moilanen E, Uitti J. Increased alveolar nitric oxide and systemic inflammation markers in silica-exposed workers. Occup Environ Med 69: 256–260, 2012. doi: 10.1136/oemed-2011-100347. [DOI] [PubMed] [Google Scholar]
- 115.Schinke T, Haberland M, Jamshidi A, Nollau P, Rueger JM, Amling M. Cloning and functional characterization of resistin-like molecule γ. Biochem Biophys Res Commun 314: 356–362, 2004. doi: 10.1016/j.bbrc.2003.12.100. [DOI] [PubMed] [Google Scholar]
- 116.Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature 409: 307–312, 2001. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 117.Steppan CM, Brown EJ, Wright CM, Bhat S, Banerjee RR, Dai CY, Enders GH, Silberg DG, Wen X, Wu GD, Lazar MA. A family of tissue-specific resistin-like molecules. Proc Natl Acad Sci USA 98: 502–506, 2001. doi: 10.1073/pnas.98.2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Stütz AM, Pickart LA, Trifilieff A, Baumruker T, Prieschl-Strassmayr E, Woisetschläger M. The Th2 cell cytokines IL-4 and IL-13 regulate found in inflammatory zone 1/resistin-like molecule alpha gene expression by a STAT6 and CCAAT/enhancer-binding protein-dependent mechanism. J Immunol 170: 1789–1796, 2003. doi: 10.4049/jimmunol.170.4.1789. [DOI] [PubMed] [Google Scholar]
- 119.Su Q, Zhou Y, Johns RA. Bruton’s tyrosine kinase (BTK) is a binding partner for hypoxia induced mitogenic factor (HIMF/FIZZ1) and mediates myeloid cell chemotaxis. FASEB J 21: 1376–1382, 2007. doi: 10.1096/fj.06-6527com. [DOI] [PubMed] [Google Scholar]
- 120.Swain SD, Han S, Harmsen A, Shampeny K, Harmsen AG. Pulmonary hypertension can be a sequela of prior Pneumocystis pneumonia. Am J Pathol 171: 790–799, 2007. doi: 10.2353/ajpath.2007.070178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tarkowski A, Bjersing J, Shestakov A, Bokarewa MI. Resistin competes with lipopolysaccharide for binding to toll-like receptor 4. J Cell Mol Med 14: 1419–1431, 2010. doi: 10.1111/j.1582-4934.2009.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Teng X, Li D, Champion HC, Johns RA. FIZZ1/RELMα, a novel hypoxia-induced mitogenic factor in lung with vasoconstrictive and angiogenic properties. Circ Res 92: 1065–1067, 2003. doi: 10.1161/01.RES.0000073999.07698.33. [DOI] [PubMed] [Google Scholar]
- 123.Tong Q, Zheng L, Lin L, Li B, Wang D, Huang C, Matuschak GM, Li D. Participation of the PI-3K/Akt-NF-κB signaling pathways in hypoxia-induced mitogenic factor-stimulated Flk-1 expression in endothelial cells. Respir Res 7: 101, 2006. doi: 10.1186/1465-9921-7-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tsai CH, Tsai HC, Huang HN, Hung CH, Hsu CJ, Fong YC, Hsu HC, Huang YL, Tang CH. Resistin promotes tumor metastasis by down-regulation of miR-519d through the AMPK/p38 signaling pathway in human chondrosarcoma cells. Oncotarget 6: 258–270, 2015. doi: 10.18632/oncotarget.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tuder RM, Chacon M, Alger L, Wang J, Taraseviciene-Stewart L, Kasahara Y, Cool CD, Bishop AE, Geraci M, Semenza GL, Yacoub M, Polak JM, Voelkel NF. Expression of angiogenesis-related molecules in plexiform lesions in severe pulmonary hypertension: evidence for a process of disordered angiogenesis. J Pathol 195: 367–374, 2001. doi: 10.1002/path.953. [DOI] [PubMed] [Google Scholar]
- 126.Vondracek SF, Voelkel NF, McDermott MT, Valdez C. The relationship between adipokines, body composition, and bone density in men with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 4: 267–277, 2009. doi: 10.2147/COPD.S2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wagner KF, Hellberg AK, Balenger S, Depping R, Dodd-O J, Johns RA, Li D. Hypoxia-induced mitogenic factor has antiapoptotic action and is upregulated in the developing lung: coexpression with hypoxia-inducible factor-2alpha. Am J Respir Cell Mol Biol 31: 276–282, 2004. doi: 10.1165/rcmb.2003-0319OC. [DOI] [PubMed] [Google Scholar]
- 128.Wang WY, Chen Y, Su X, Tang D, Ben QW, Yao WY, Chen P, Yuan YZ. Resistin-like molecule-α causes lung injury in rats with acute pancreatitis by activating the PI-3K/Akt-NF-κB pathway and promoting inflammatory cytokine release. Curr Mol Med 16: 677–687, 2016. doi: 10.2174/1566524016666160802145700. [DOI] [PubMed] [Google Scholar]
- 129.Yamaji-Kegan K, Su Q, Angelini DJ, Champion HC, Johns RA. Hypoxia-induced mitogenic factor has proangiogenic and proinflammatory effects in the lung via VEGF and VEGF receptor-2. Am J Physiol Lung Cell Mol Physiol 291: L1159–L1168, 2006. doi: 10.1152/ajplung.00168.2006. [DOI] [PubMed] [Google Scholar]
- 130.Yamaji-Kegan K, Su Q, Angelini DJ, Myers AC, Cheadle C, Johns RA. Hypoxia-induced mitogenic factor (HIMF/FIZZ1/RELMα) increases lung inflammation and activates pulmonary microvascular endothelial cells via an IL-4-dependent mechanism. J Immunol 185: 5539–5548, 2010. doi: 10.4049/jimmunol.0904021. [DOI] [PubMed] [Google Scholar]
- 131.Yamaji-Kegan K, Takimoto E, Zhang A, Weiner NC, Meuchel LW, Berger AE, Cheadle C, Johns RA. Hypoxia-induced mitogenic factor (FIZZ1/RELMα) induces endothelial cell apoptosis and subsequent interleukin-4-dependent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 306: L1090–L1103, 2014. doi: 10.1152/ajplung.00279.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yang RZ, Huang Q, Xu A, McLenithan JC, Eisen JA, Shuldiner AR, Alkan S, Gong DW. Comparative studies of resistin expression and phylogenomics in human and mouse. Biochem Biophys Res Commun 310: 927–935, 2003. doi: 10.1016/j.bbrc.2003.09.093. [DOI] [PubMed] [Google Scholar]
- 133.Zaiman A, Fijalkowska I, Hassoun PM, Tuder RM. One hundred years of research in the pathogenesis of pulmonary hypertension. Am J Respir Cell Mol Biol 33: 425–431, 2005. doi: 10.1165/rcmb.F307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zeng X, Zhu L, Xiao R, Liu B, Sun M, Liu F, Hao Q, Lu Y, Zhang J, Li J, Wang T, Wei X, Hu Q. Hypoxia-induced mitogenic factor acts as a nonclassical ligand of calcium-sensing receptor, therapeutically exploitable for intermittent hypoxia-induced pulmonary hypertension. Hypertension 69: 844–854, 2017. doi: 10.1161/HYPERTENSIONAHA.116.08743. [DOI] [PubMed] [Google Scholar]
- 135.Zhang HM, Li XY, He ZY, Xu LZ, Jin Q, Tan H. Resistin-like molecule alpha enhances the proliferation and migration of aortic vascular smooth muscle cells. Cardiology 126: 91–95, 2013. doi: 10.1159/000351599. [DOI] [PubMed] [Google Scholar]
- 136.Zhang L, Wang M, Kang X, Boontheung P, Li N, Nel AE, Loo JA. Oxidative stress and asthma: proteome analysis of chitinase-like proteins and FIZZ1 in lung tissue and bronchoalveolar lavage fluid. J Proteome Res 8: 1631–1638, 2009. doi: 10.1021/pr800685h. [DOI] [PMC free article] [PubMed] [Google Scholar]