Abstract
Objective
Numerous findings have demonstrated a strong association between parental health during pregnancy and cardiovascular disease in adult offspring. This study investigated whether sensitivity to angiotensin II (Ang II) is enhanced in offspring of renovascular hypertensive animals and whether hydrogen sulfide (H2S) can attenuate the increased response to Ang II in offspring.
Method
The systolic blood pressure (SBP) was measured by non-invasive tail-cuff plethysmograpy every two weeks in all offspring from 8 to 16 weeks. After intracerebroventricular microinjection of Ang II in the offspring, blood pressure, heart rate (HR), and renal sympathetic nerve activity (RSNA) were recorded to test the response to Ang II in the offspring. Western blot analysis was used to examine the protein expression of AT1R, AT1R-associated protein (ATRAP), Nox2, p67phox, and nitrotyrosine in the nucleus tractus solitarii (NTS).
Results
The SBP in the offspring of hypertensive rats were significantly higher than that in control group, and the above effects were significantly improved by prenatal or postnatal administration of H2S. Intralateroventricular microinjection of Ang II induced greater sympathetic responses in offspring of hypertensive rats than control group. The expression of AT1R and oxidative stress-related protein was increased, whereas that of ATRAP was decreased in the NTS in offspring of hypertensive rats. Exogenous administration of H2S prenatally or postnatally improved the above effects.
Conclusion
Prenatal or postnatal administration of H2S attenuated AngII-induced sympathetic excitation in offspring of hypertensive rats, which may occur by modulating the balance between AT1R and ATRAP and downregulating oxidative stress-related protein expression in the NTS.
Keywords: sympathetic excitation, hypertension, nucleus tractus solitarii, angiotensin II, fetal programming
Introduction
Increasing evidence has shown that an adverse intrauterine environment, such as in mothers with hypertension and diabetes, increases the risk of cardiovascular diseases in adult offspring (Barker and Osmond, 1986; Kobori et al., 2007; Samuelsson et al., 2013). For example, in women with preeclampsia in pregnancy, their children tend to have cardiovascular disease later in life (Bokslag et al., 2016). A similar study reported that children of women with preeclampsia showed increased risk of stroke in adulthood (Kajantie et al., 2009). In pregnant women with diabetes, the probability of cardiovascular diseases was increased in their adult offspring (Ma et al., 2015). Moreover, nicotine exposure during pregnancy leads to increased risk of hypertension and metabolic disorders in adult offspring (Xiao et al., 2008; Somm et al., 2009). Animal experiments have shown that uteroplacental insufficiency, protein restriction, chronic secondary hypertension, or glucocorticoid treatment during pregnancy leads to hypertension in offspring (Liang et al., 2013). However, early intervention can prevent the development of adult hypertension (Koeners et al., 2010; Koeners et al., 2011).
Renovascular hypertension (RVH) induced by partial renal artery stenosis could lead to persistent high blood pressure (BP) and increased angiotensin II (AngII) level (Higashi et al., 2002; Takahashi and Smithies, 2004). AngII, the major effector peptide of the renin-angiotensin-aldosterone system (RAAS), increased the production of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, especially superoxide anion (O2 -), in a rat model of renovascular hypertension (Oliveira-Sales et al., 2009) via activating AngII type I receptor (AT1R). AT1R-associated protein (ATRAP) specifically combines with the carboxyl-terminal domain of AT1R (Lopez-Ilasaca et al., 2003). In vitro study showed that ATRAP suppressed AngII-mediated pathological responses in cardiovascular cells by promoting AT1R internalization (Tamura et al., 2013), and ATRAP also suppressed the role of AT1R in the pathological process of hypertension (Dejima et al., 2011).
The nucleus tractus solitarius (NTS) is the primary site of peripheral afferent impulses to the central nervous system, receiving cardiovascular afferent signals after preliminary integration and transfer to nuclei that regulate the circulatory variables. Previous studies have shown that AT1R are widely distributed in the central nervous system and play an important role in the development of hypertension (Xu et al., 1998). Also, AT1R is abundantly expressed in the NTS (Hirooka, 2011). Abnormalities of the RAAS in the NTS is involved in the pathophysiological process of hypertension (Matsumura et al., 1998; Diz et al., 2011). Therefore, RAAS expressed in the NTS could play a key role in regulating BP stability, sympathetic outflow, and baroreceptor reflex control of cardiovascular function (Hirooka et al., 2003; Zanutto et al., 2010; Zanutto et al., 2011).
Hydrogen sulfide (H2S) is considered the third gas signal molecule after NO and CO and is involved in many physiological and pathophysiological processes in the cardiovascular system (Wang, 2002). Physiological concentrations of H2S can relax blood vessels, inhibit oxidative stress, decrease myocardial injury, attenuate inflammation (Polhemus and Lefer, 2014), and reduce BP both in normotensive and hypertensive rats (Ford et al., 2013; Ahmad et al., 2014). We found that the plasma level of H2S was significantly decreased in the model of RVH (Xiao et al., 2018), which leads to oxidative stress. However, exogenous administration of NaHS could rapidly increase the H2S level in renovascular hypertensive rats (Xiao et al., 2016). By inhibiting AT1 receptor and oxidative stress-related proteins, H2S could lower BP in RVH rats (Xue et al., 2015; Guo et al., 2017).
Maternal environment plays an important role in maintaining normal physiological function after birth. Therefore, preventive measures are needed to prevent and cure cardiovascular disease in the perinatal period (Marco et al., 2012). In this study, we examined whether prenatal and postnatal treatment with H2S could attenuate AngII-induced sympathetic excitation in hypertensive offspring by decreasing the level of AT1 receptor and increasing that of ATRAP.
Methods
Preparation of Parental Hypertensive Model
Seven-week male and female Sprague-Dawley rats (180–220 g) were obtained from the Animal Research Center of Hebei Medical University and kept in ordinary cages at 22 ± 2°C with 12-h dark/light cycles (lights on 6:00) with food and water adlibitum. All animal procedures complied with the Animal Management Rule of the Ministry of Health, People’s Republic of China (documentation no. 55, 2001) and the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication no. 85-23, revised in 1996) and were approved by the Animal Care Committee of Hebei Medical University. Seven-week-old male and female Sprague-Dawley (SD) rats, weighting 160–180 g, were anesthetized with pentobarbital sodium (50 mg/kg, i.p.). The absence of a withdrawal response to a paw pinch was used to confirm adequate anesthesia. The left kidney was exposed via laparotomy, and the left renal artery was carefully separated from the left renal vein and connective tissue. Then, the left renal artery was clipped with a rigid U-shaped solid clip. The stainless-steel clip with an opening of 0.25 mm (Aowang Medical Instrument Company, Shanghai) was used to clamp the left renal artery to induce stenosis, thus resulting in hypertension. Thereafter, animals were given a subcutaneous injection of penicillin G (30,000 U/100 g) and meloxicam (1 mg/kg).
Mating and Groups
The parental hypertensive rats were trained to become accustomed to the procedures for systolic blood pressure (SBP) measurement. Each rat was placed on a heating pad for 15–20 min to promote vasodilatation of the tail artery before measurement of SBP. SBP was measured in conscious rats each week after surgery for 5 weeks by tail-cuff plethysmography (Chengdu Instrument Factory, Sichuan, China). While SBP in RVH rats was up to 150 mmHg in 5 weeks after surgery, we selected 12 pairs of male and female RVH rats to mate and produce offspring. There were four groups in the experiment: The control group was offspring of SD rats, F1NF2N rats were offspring of RVH rats, F1HF2N rats were maternal RVH rats treated with NaHS (H2S donor, 56 μmol/kg per day, i.p.) during pregnancy and lactation and offspring without NaHS, and F1NF2H rats were maternal RVH rats treated without NaHS during pregnancy and lactation and offspring with NaHS after weaning. On the first day of pregnancy, each mother received NaHS treatment or not. Each female rat was separately mated overnight. The day of pregnancy was defined as the day when spermatozoa were found in a vaginal smear. In the offspring in the above groups, SBP was measured from 8 to 16 weeks.
Blood Pressure Measurement in Conscious Offspring
SBP was measured non-invasively by tail-cuff plethysmography in conscious offspring from 8 to 16 weeks, every 2 weeks. The rats were trained to become accustomed to the procedures for blood pressure measurement before the experiments. Each rat was placed on a heating pad for 15–20 min to promote vasodilatation of the tail artery prior to measurement of SBP. BP measurement was always carried out between 9:00 and 12:00, and the data were obtained with the average of three to four times.
Implanting Cannula Into the Lateral Ventricle of Offspring
After anesthetization with pentobarbital sodium (50 mg/kg, i.p.), 15 weeks offspring were placed in a prone position, and the head of the animal was fixed on a stereotaxic frame. The skin of the skull bone was removed via a midline incision to expose the bregma. The stereotaxic coordinates used were the following (in mm): caudal to bregma, 0.8; lateral to midline, 1.5; and ventral to dura, 3.5 to 3.8. Offspring were mounted on a stereotaxic apparatus and implanted with a lateral cerebroventricular cannula. At 1 week after surgery, rats were anesthetized with pentobarbital sodium, and then Ang II (10 and 30 ng, 5 μL) was injected in the lateral ventricle via the cannula connected to a microsyringe with a polyethylene tube. BP, HR, and RSNA were recorded simultaneously by using PowerLab software (AD Instruments, Australia).
Recording of BP, HR, and RSNA
Pentobarbital sodium (50 mg/kg, i.p.) was used to anesthetize rats, and then the PE tube was cannulated into their trachea for ventilation. A thermostatic bed was used to maintain body temperature in the range of 37–38℃. The left femoral artery was cannulated and connected with a pressure transducer to record arterial BP (ABP). The HR was measured along with BP. The left kidney was exposed via a retroperitoneal approach. One branch of the left renal sympathetic nerve around the renal vessels was isolated from the surrounding tissue carefully. To eliminate the nerve’s afferent activity, it was cutted distally. The nerve was placed on a bipolar platinum electrode for potential recording and immersed in warm (37°C) mineral oil. The nerve signal was amplified (20,000–30,000) and band-pass–filtered (100–3,000 Hz) by an alternating current amplifier (model P511; Grass Instruments, West Warwick, RI, USA). The pressure and nerve discharge signal were amplifier-fed into a PowerLab 15T data acquisition system (AD Instruments, QUAD, Bridge, Australia) to record ABP and RSNA simultaneously. A customized computer program was used to integrate the RSNA; the integrating time was 0.16 s before, and 5 min after, rats were euthanized by an overdose of sodium pentobarbital (200 mg/kg, i.v.) to suppress RSNA completely to obtain background electrical noise. The electrical noise level measured with these two methods was similar and was subtracted from the integrated RSNA values, and the percentage change in RSNA from baseline was calculated.
Collecting NTS Tissue
At the end of the experiment, brains were dissected, and fresh brainstem tissue was transected coronally at the level of the NTS, which was identified as a distinct translucent structure in the dorsomedial medulla. Histology verification referred to Paxinos and Watson′s coordinates. At the level of the area postrema, a 250-μm thick piece of NTS was punched out bilaterally by using fine-tip forceps under a binocular microscope. The tissues were immersed in artificial cerebrospinal fluid at 0°C in the whole process. The NTS tissues from each rat were rapidly removed and placed in liquid nitrogen, then stored at −80°C.
Western Blot Detection of Protein
NTS tissues were added with pre-cooled lysate and then cracked by ultrasonography. The specimens were statically placed on ice for 30 min to be fully cracked and then centrifuged at 12,000 rpm for 15 min at 4°CC. Supernatant was collected for protein assay. The Bradford assay (Generay Biotechnology, Shanghai) was used to determine the concentration of protein in tissue. An amount of 5 μg of protein was loaded in each lane. The protein was separated by electrophoresis, transferred onto polyvinylidene fluoride membranes, which were blocked with 0.1% Tween-20 tris-buffered saline containing 10% nonfat milk for 1 h at room temperature, and then incubated overnight at 4°C with the rabbit polyclonal antibodies anti-AT1R (1:800; ab18801, Abcam, USA), anti-ATRAP (1:500; sc-134652, Santa Cruz Biotechnology), anti-Nox2 (1:1,000; Abcam), anti-p67phox (1:1,000; EPITOMICS, USA), and anti-nitrotyrosine (1:1,000; Millipore), followed by the peroxidase-conjugated goat anti-rabbit secondary antibody (1:2,000; SA00001-2, Proteintech Biotechnology). Western blotting reagents (Millipore Corp., Billerica, MA) were used to detect the signal and blots were exposed to X-ray film for densitometric analysis.
Statistical Analysis
All data are expressed as means ± SE. Repeated measures analysis of variance was tested to compare differences between groups at several times. One-way ANOVA was applied to compare differences between groups. Student-Newman-Keuls test and Bonferroni correction were used for analysis at one time. Differences were considered statistically significant at P < 0.05.
Results
SBP in Conscious Offspring
We measured SBP every 2 weeks from 8 to 16 weeks. Firstly for SBP, the interaction between times and groups had significant effect on subjects (P < 0.05). Times had no significant effect (P > 0.05) on subjects while groups had (P < 0.05). The SBP in male offspring of renovascular hypertensive dams significantly higher compared with that in control group from 8 to 16 weeks. And the above effects were significantly improved by exogenous administration with H2S. When we treated the hypertensive mother with H2S during pregnancy and lactation, the offspring exhibited a persistence decrease in blood pressure compared with hypertensive offspring. The blood pressure was decreased gradually in the offspring which treated with H2S after weaning. In female offspring of renovascular hypertensive dams, the SBP significantly elevated when compared with control from 8 to 16 weeks. In addition, the SBP in male offspring of renovascular hypertensive dams was higher than that in female offspring, while there was no sex different in normal rat (Table 1).
Table 1.
Time courses of changes in systolic blood pressure (SBP) in four different groups of male and female offspring.
| Groups | 8W | 10W | 12W | 14W | 16W | |
|---|---|---|---|---|---|---|
| Male | F1NF2N F1HF2N F1NF2H Control |
131.4 ± 2.6* 120.8 ± 2.7# 126.6 ± 2.1 111.6 ± 1.8 |
133 ± 1.9* 120.2 ± 1.2# 124.3 ± 1.8# 112.3 ± 1.5 |
132.4 ± 1.9* 119.1 ± 0.9# 124.6 ± 1.2# 111 ± 1.8 |
133.2 ± 1.9* 120.2 ± 1.6# 118.8 ± 1.2# 112.2 ± 1.4 |
135 ± 2.8* 117.5 ± 2.3# 114.8 ± 1.2# 113.1 ± 1.6 |
| Female | F1NF2N F1HF2N F1NF2H Control |
123.4 ± 1.3*♦ 115 ± 2# 124.9 ± 1.8 107.6 ± 2 |
122.7 ± 1.4*♦ 119.9 ± 1.5 122.5 ± 2.7 108.2 ± 1.3 |
124.2 ± 1.7*♦ 117.2 ± 2.4 120.7 ± 2.2 109.2 ± 2.1 |
119.9 ± 2.6*♦ 117.8 ± 1 115.9 ± 2.8 108.8 ± 2.2 |
115.9 ± 0.6*♦ 115.4 ± 1.3 108 ± 2.8# 108.7 ± 2.3 |
Date are showed as mean ± SE (n = 40). *P < 0.05 compared with control, #P < 0.05 compared with F1NF2N, and ♦P < 0.05 compared with male F1NF2N.
Sympathetic Response to AngⅡ in Rat Offspring
Sympathetic Response to Low-Dose Ang II in Rat Offspring
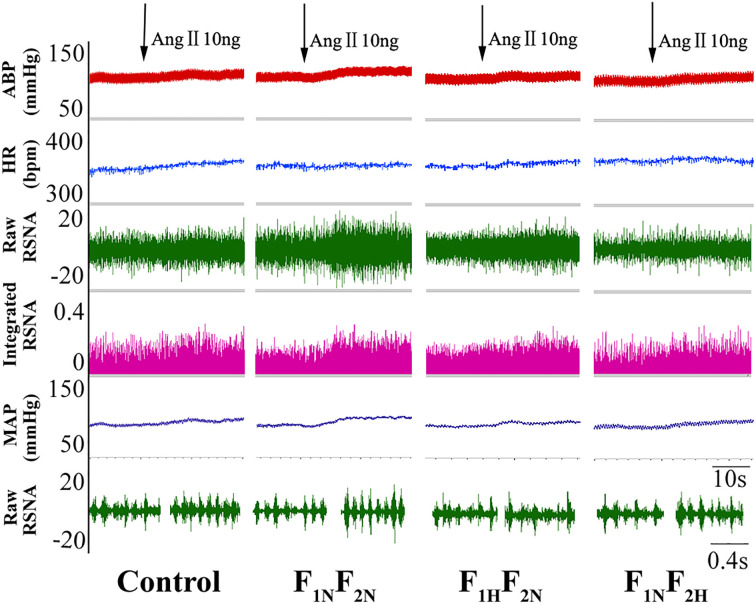
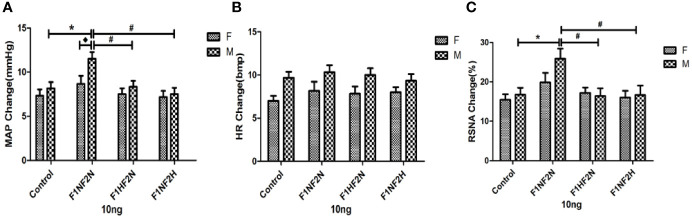
Intracerebroventricular infusion of 10 ng of Ang II increased MAP, HR, and RSNA in both male and female offspring. Figure 1 shows an original trace of the sympathetic response to the lower dose of Ang II (10 ng, 5 μL) with intracerebroventricular injection in male offspring. Sympathoexcitatory responses to intracerebroventricular injection of 10 ng of Ang II began within 1 min, reached a plateau within 3 to 5 min, and returned to the normal steady state within 10 min. Figures 2A–C show summary data in male and female offspring. Changes in MAP and RSNA were higher in the F1NF2N than control group among male offspring (ΔMAP: 11.5 ± 0.76 vs. 8.17 ± 0.70 mmHg; ΔRSNA: 25.90 ± 2.53% vs. 16.78 ± 1.70%; P<0.05). As compared with the F1NF2N group, in the F1HF2N and F1NF2H groups, the above effects were significantly attenuated (F1HF2N:ΔMAP, 8.33 ± 0.67 mmHg; ΔRSNA, 16.42 ± 1.98%; F1NF2H:ΔMAP, 7.50 ± 0.72 mmHg; ΔRSNA, 16.68 ± 2.41%; P<0.05). MAP, HR, and RSNA did not differ between female groups. However, the sympathetic responses change to lower dose of Ang II (10 ng, 5 μL) was higher in male than female RVH offspring (ΔMAP, 11.5 ± 0.76 vs. 8.67 ± 0.92 mmHg; P<0.05), with no sex differences in control offspring. Table 2 showed the basal blood pressure and HR in offspring responsed to intracerebroventricular injection of 10 ng of Ang II. Sympathoexcitatory effects were similar with Figures 2A–C.
Figure 1.
A raw trace recordings showing microinjection of Ang II (10 ng, 5 μL) into the lateral ventricles (LVs) of rats increased mean aterial pressure (MAP), HR, and renal sympathetic nerve activity (RSNA) in male offspring from the control, F1NF2N (offspring of renovascular hypertension [RVH] rats), F1HF2N (RVH rats treated with NaHS, offspring without NaHS), and F1NF2H (maternal RVH rats treated without NaHS and offspring with NaHS after weaning) groups (n = 8).
Figure 2.
Microinjection of Ang II 10 ng into the LVs in both male and female offspring. Summary data showing the peak effect of Ang II (10 ng, 5 μL) microinjected into LVs on MAP (A), HR (B), and RSNA (C). Data are mean ± SE (n = 8). *P < 0.05 compared with control, #P < 0.05 compared with F1NF2N, and ◆P <0.05 male F1NF2N compared with female F1NF2N.
Table 2.
Basal MAP and HR were measured under anaesthesia with a pressure transducer through a catheter placed in the left femoral artery.
| Parameter | Control | F1NF2N | F1HF2N | F1NF2H | |||
|---|---|---|---|---|---|---|---|
| MAP(mmHg) | Ang II 10 ng | M | Baseline | 98.3 ± 2.6 | 109.8 ± 2.7 | 99.5 ± 3.1 | 101.8 ± 2.8 |
| Ang II10 ng | 106.5 ± 2.5 | 121.3 ± 2.9* | 107.8 ± 2.8# | 109.3 ± 2.5# | |||
| F | Baseline | 96.7 ± 2.8 | 106 ± 2.3 | 101.8 ± 1.6 | 98.8 ± 2.5 | ||
| Ang II10 ng | 104 ± 3.2 | 114.7 ± 3 | 109.3 ± 1.5 | 106 ± 2.2 | |||
| Ang II 30 ng | M | Baseline | 98 ± 3.2 | 110 ± 3 | 102.7 ± 2.6 | 101.5 ± 2.8 | |
| Ang II30 ng | 108.2 ± 3.3 | 123.3 ± 2.9* | 111.5 ± 2.1# | 110.2 ± 3# | |||
| F | Baseline | 98.3 ± 2.9 | 108.7 ± 2.5 | 99.2 ± 2.4 | 97.8 ± 2.8 | ||
| Ang II30 ng | 106.3 ± 2.7 | 117.8 ± 2.7* | 106.8 ± 2.4# | 105 ± 2.7# | |||
| HR(bpm) | Ang II 10 ng | M | Baseline | 341.7 ± 6.3 | 352 ± 8.4 | 346 ± 7.8 | 343.2 ± 6.2 |
| Ang II10 ng | 351.3 ± 7 | 362.3 ± 8.8 | 356 ± 8 | 352.5 ± 5.8 | |||
| F | Baseline | 342.8 ± 7.2 | 354.5 ± 5.6 | 349 ± 6.7 | 338.3 ± 8.9 | ||
| Ang II10 ng | 349.8 ± 6.8 | 362.7 ± 5.2 | 356.8 ± 6.7 | 346.3 ± 9 | |||
| Ang II 30 ng | M | Baseline | 344.7 ± 6.2 | 358.5 ± 7.2 | 345.5 ± 7.7 | 348.2 ± 6.1 | |
| Ang II30 ng | 354.8 ± 6.4 | 370.8 ± 6.7 | 355.7 ± 7.4 | 357.8 ± 6.4 | |||
| F | Baseline | 349 ± 7.7 | 259.2 ± 7.2 | 353.2 ± 4.2 | 351.3 ± 5.3 | ||
| Ang II30 ng | 353 ± 5.9 | 365.3 ± 6.9 | 361.3 ± 5.1 | 359.5 ± 5.2 | |||
Date are showed as mean ± SE (n = 8).*P < 0.05 compared with control group treatment with AngⅡ and #P < 0.05 compared with F1NF2N group treatment with AngⅡ.
Sympathetic Response to High-Dose Ang II in Offspring
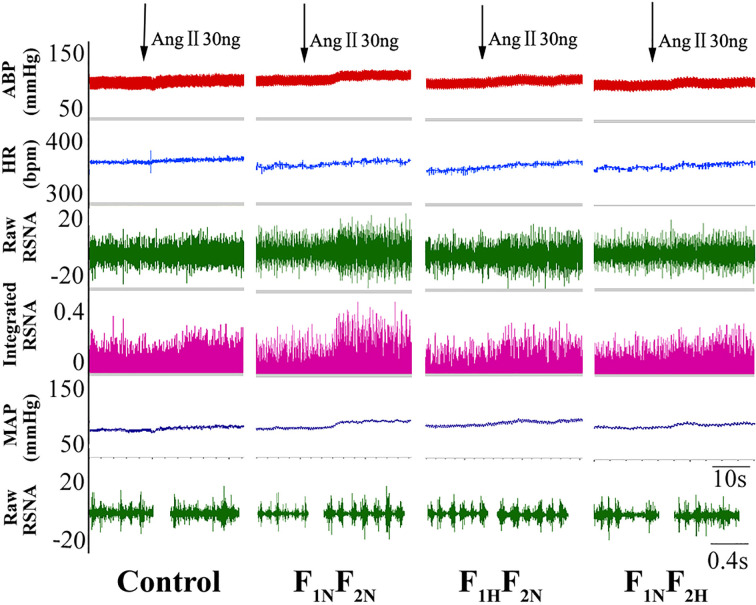
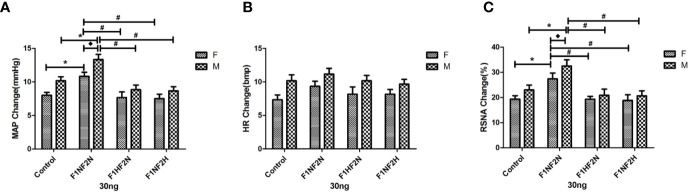
Figure 3 shows the original tracing of the larger dose of Ang II (30 ng) inducing increased sympathetic outflow in female offspring. Figures 4A–C show summary data for sympathetic outflow change and that MAP and RSNA were higher in F1NF2N than control female and male offspring (female offspring:ΔMAP, 10.83 ± 0.60 vs. 8.00 ± 0.45 mmHg; ΔRSNA, 27.38 ± 2.34% vs. 19.34 ± 1.39%, P<0.05; male offspring: ΔMAP, 13.33 ± 0.76 vs. 10.17 ± 0.60 mmHg;ΔRSNA, 32.50 ± 2.47% vs. 23.00 ± 1.93%; P < 0.05). As compared with the F1NF2N group, F1HF2N and F1NF2H groups showed significantly decreased hypertension. HR showed no change at any dose of Ang II (Figure 4). Effects were similar with intracerebroventricular infusion of Ang II (3 and 10 ng) and were not dose-dependent. As compared with control offspring, RVH offspring showed sex differences. The sympathetic response to higher dose of Ang II (30 ng, 5 μL) was higher in male than female RVH offspring (ΔMAP, 13.33 ± 0.76 vs. 10.83 ± 0.60 mmHg; P<0.05; ΔRSNA:27.38 ± 2.34% vs. 32.5 ± 2.47%, P < 0.05). Table 2 showed the basal blood pressure and HR in offspring responsed to intracerebroventricular injection of Ang II (30 ng).
Figure 3.
Original trace recordings showing microinjection of Ang II (30 ng, 5 μL) into LVs increased MAP, HR, and RSNA in female offspring from the control, F1NF2N (offspring of RVH rats), F1HF2N (RVH rats treated with NaHS, offspring without NaHS), and F1NF2H (maternal RVH rats treated without NaHS and offspring with NaHS after weaning) groups (n = 8).
Figure 4.
Microinjection of Ang II 30 ng into the LVs in male and female offspring. Summary data show the peak change of Ang II (30 ng, 5 μL) microinjected into LVs on MAP (A), HR (B), and RSNA (C). Data are mean ± SE (n = 8). *P < 0.05 compared with control, #P < 0.05 compared with F1NF2N, and ◆P < 0.05 male F1NF2N compared with female F1NF2N.
Protein Expression in NTS of Offspring
Protein Expression in NTS of Male Offspring
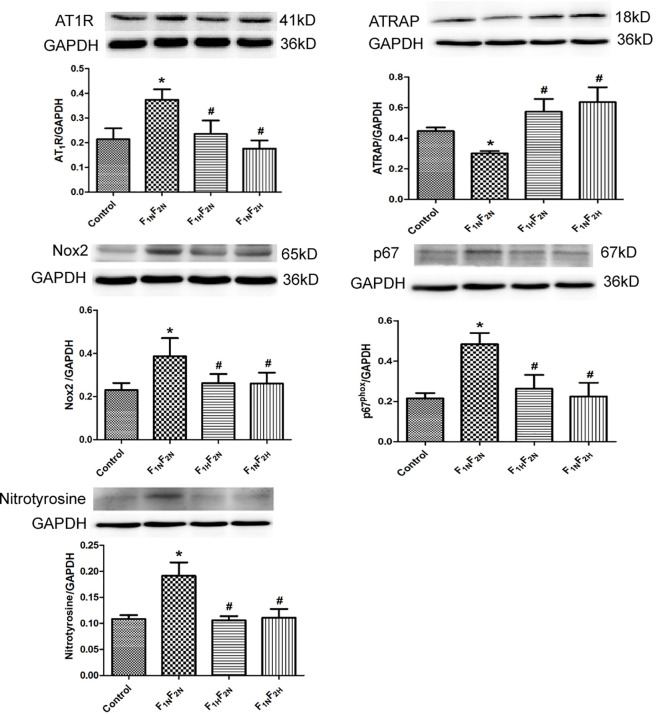
The protein expression of AT1R, Nox2, p67phox, and nitrotyrosine was significantly increased and that of ATRAP was decreased in the NTS in the F1NF2N group; exogenous administration of hydrogen sulfide during prenatal or postnatal significantly reversed the above changes (Figure 5).
Figure 5.
Western blot analysis of protein expression of AT1R, ATRAP, Nox2, p67phox, and nitrotyrosine in nucleus tractus solitarii (NTS) in male offspring from the control, F1NF2N (offspring of RVH rats), F1HF2N (RVH rats treated with NaHS, offspring without NaHS), and F1NF2H (maternal RVH rats treated without NaHS and offspring with NaHS after weaning) groups. Data are mean ± SE (n = 12). *P < 0.05 compared with control and #P < 0.05 compared with F1NF2N. GAPDH was used to normalize.
Protein Expression in NTS of Female Offspring
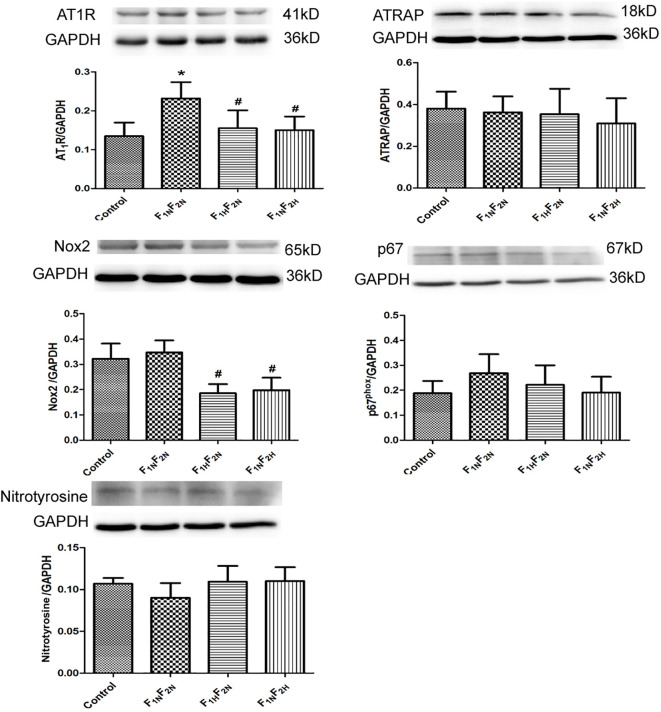
As compared with controls, AT1R protein expression was significantly increased in F1NF2N group, whereas the protein expression of ATRAP, Nox2, p67phox, and nitrotyrosine did not differ between groups. Prenatal or postnatal treatment with hydrogen sulfide significantly decreased the protein expression of AT1R in group (Figure 6).
Figure 6.
Western blot analysis of protein expression of AT1R, ATRAP, Nox2, p67phox, and nitrotyrosine in NTS in female offspring from the control, F1NF2N (offspring of RVH rats), F1HF2N (RVH rats treated with NaHS, offspring without NaHS), and F1NF2H (maternal RVH rats treated without NaHS and offspring with NaHS after weaning) groups. Data are mean ± SE (n = 12). *P < 0.05 compared with control and #P < 0.05 compared with F1NF2N. GAPDH was used to normalize.
Discussion
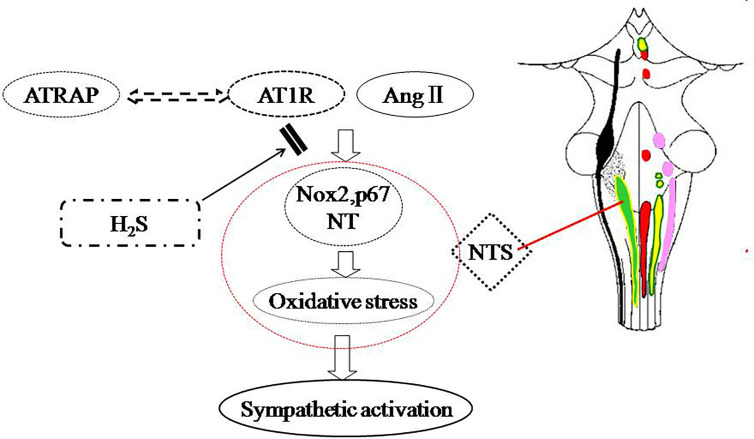
In this study, we have found that SBP in offspring of renovascular hypertensive significantly increased from 8 to 16 weeks; with intracerebroventricular infusion of Ang II, the parameters MAP, HR and RSNA were greater in offspring of parents with than without RVH. Offspring of normal rats showed no sex differences in BP or sympathetic outflow, but for RVH dams, BP and the sympathetic response to Ang II were both higher in male than female offspring; The expression of AT1 was increased and that of ATRAP decreased in NTS in offspring of RVH animals; prenatal or postnatal treatment with H2S could prevent the sympathetic activation in offspring (Figure 7).
Figure 7.
Schematic representation of the ameliorative effect of H2S on sympathetic activation in offspring of parental hypertension.
Epidemiologic studies have shown that the maternal intrauterine environment is positively associated with BP in children (Longo et al., 2016). Maternal exposure to the nitric oxide (NO) synthase (NOS) inhibitor NG-nitro-l-arginine-methyl ester (L-NAME) during pregnancy induced hypertension in male offspring (Tain et al., 2016). Another research demonstrated that basal blood pressure in adult offspring of mothers with secondary renovascular hypertension was not increased. However, in our study, the baseline blood pressure in offspring of parents RVH animals was increased, which meant paternal or maternal status both have contribution to the offspring phenotype. Meanwhile, there is a study have demonstrated that sex difference in programmed hypertension is in male but not female adult offspring (Chiossi et al., 2016; Gray et al., 2016). To the contrary, our study have found that both male and female adult offspring of renovascular hypertensive rats have higher blood pressure, and the blood pressure was also higher in male offspring of renovascular hypertensive rats than that in female offspring. However, there was no sex difference in the offspring of normotensive rats.
Sex-specific differences in programmed disease have been well described in the literature. Denton et al. found that maternal secondary hypertension can “program” hypertension in female adult offspring. These results suggest sex-specific differences in sensitivity to the altered uteral environment (Denton et al., 2003). Consistent with these studies, our study demonstrated that parental secondary hypertension conferred sympathetic activation in adult offspring of both sexes. Furthermore, the sympathetic response to Ang II was greater in hypertensive male than female offspring. We found sex differences in the development of hypertension in offspring of RVH dams. Parental renovascular hypertension induced sympathetic activity in offspring sex-specifically. Our findings in conjunction with the above studies suggest that the offspring of hypertensive animals are prone to hypertension themselves.
Several mechanisms are considered involved in programmed hypertension, including NO deficiency, oxidative stress, epigenetic regulation, altered renin–angiotensin system and sodium transporters. The RAS plays a fundamental role in regulating sympathetic activity and BP control. Altered RAS in the brain and kidney may be an important mechanism for the fetal programming of hypertension (Guo et al., 2017; Xue et al., 2017). In our previous study, we found the AT1 receptor was epigenetically regulated in the kidney of maternal secondary hypertensive offspring (Guo et al., 2017). In the present study, intracerebroventricular infusion of Ang II (10 ng/5 µl) increased BP, HR, and RSNA in male hypertensive offspring and Ang II (30 ng/5 µl) in female hypertensive offspring.
ATRAP, a molecule that directly binds to the carboxyl-terminal domain of AT1R, could suppress the Ang II-AT1R–mediated pathological process (Lopez-Ilasaca et al., 2003). Also, increasing ATRAP level could ameliorate Ang II-induced hypertrophy in mice, with no effect on baseline cardiovascular function, including BP. Systemic ATRAP deficiency provoked vasoconstriction and increased sodium retention, exacerbating hypertension induced by chronic Ang II infusion. Increased ATRAP level inhibited salt-sensitive BP elevation (Wakui et al., 2015). All the above evidence indicate that activation of ATRAP plays an important role in inhibiting Ang II-dependent hypertension. Similarity, in our study, ATRAP was significantly decreased in the NTS of male offspring of hypertensive animals, and the sympathetic outflow was activated. However, ATRAP expression did not differ in female offspring, which suggests that ATRAP was not only involved in the development of hypertension in both male and female offspring but also was responsible for the sex differences in BP in these pups.
Oxidative stress is a key mediator in the pathogenesis of programmed hypertension. NADPH oxidases (Nox), a family of reactive oxygen species-producing enzymes, are a source of oxidative stress in diseases. Nox2 is the main ROS-producing enzyme producing superoxide and reducing NO bioavailability (Judkins et al., 2010). Preventing both the maternal and fetal adverse effects by inhibiting Nox2 may be an effective therapy for preeclampsia. Also, nicotine exposure during pregnancy leads to programming vascular dysfunction via a Nox2-dependent mechanism and increases the risk of hypertension in adult offspring (Xiao et al., 2011; Lim et al., 2015). One of the NADPH oxidase subunits, p67phox, also participates in fetal programming. In mothers with a low-protein diet, vascular remodeling was induced in offspring, which was attributed to increased p67phox level (Chisaka et al., 2016). Nitrotyrosine is considered a biomarker for endogenous level of peroxynitrite, and its level is correlated with elevated levels of other indices of oxidative stress. In line with previous studies, our study showed that Nox2, p67phox, and nitrotyrosine levels were increased in the NTS of male offspring of RVH animals. However, different from male offspring, female offspring showed no significant differences in Nox2, p67phox, and nitrotyrosine levels. Estrogen may play a protective role. Indeed, estrogen can combine with estrogen receptor and then inhibit the increase in reactive oxygen products and exert an antioxidant effect (Lagranha et al., 2010; Grundt et al., 2010; Siqueira et al., 2011).
H2S acts as a vasodilator and is widely expressed in mammals. Our previous study found that H2S could decrease BP, reduce the product of reactive oxygen species, and increase the methylation of AT1b receptor in kidney (Xue et al., 2015). Several studies revealed that H2S is involved in fetal programming. More importantly, reduced H2S levels in preeclampsia can lead to fetal growth restriction, whereas H2S treatment prevented pre-eclampsia and fetal growth restriction (Wang et al., 2013; Wang et al., 2014; Tai et al., 2016). Our data showed that prenatal or postnatal treatment with H2S could decrease sympathetic activation and basal blood pressure in hypertensive offspring, so H2S could modulate the balance between AT1R and ATRAP, reduce oxidative stress, and then improve the embryonic environment for offspring.
Our study has some limitations. We did not examine birth weight in offspring or the epigenetic mechanism of the AT1 receptor in the brain. Nevertheless, the data from the present study suggest the importance of sex differences in programmed hypertension, which is worthy of further investigation. Also, H2S may be a potential drug to prevent fetal programmed hypertension and can be a new method to treat hypertension.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by Animal Care Committee of Hebei Medical University.
Author Contributions
Conception and design: QG, XF, and YW. Conducted the research: XF, QG, and HX. Acquisition, analysis, and interpretation of data: XD and SJ. Manuscript preparation: QG, XF, and YW.
Funding
This work is supported by Program for National Natural Science Foundation of China (NSFC) (31671185, 31701008, 31871154, and 91849120), Youth Top Talent Program Foundation, Hebei Province of China, the Province Natural Science Foundation of Hebei (No. H2020206307), and China Scholarship Foundation (CSC.201808130056).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Ahmad F. U., Sattar M. A., Rathore H. A., Tan Y. C., Akhtar S., Jin O. H., et al. (2014). Hydrogen sulphide and tempol treatments improve the blood pressure and renal excretory responses in spontaneously hypertensive rats. Ren. Fail 36 (4), 598–605. 10.3109/0886022X.2014.882218 [DOI] [PubMed] [Google Scholar]
- Barker D. J., Osmond C. (1986). Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. LANCET 1 (8489), 1077–1081. 10.1016/s0140-6736(86)91340-1 [DOI] [PubMed] [Google Scholar]
- Bokslag A., van Weissenbruch M., Mol B. W., de Groot C. J. (2016). Preeclampsia; short and long-term consequences for mother and neonate. Early Hum. Dev. 102, 47–50. 10.1016/j.earlhumdev.2016.09.007 [DOI] [PubMed] [Google Scholar]
- Chiossi G., Costantine M. M., Tamayo E., Hankins G. D., Saade G. R., Longo M. (2016). Fetal programming of blood pressure in a transgenic mouse model of altered intrauterine environment. J. Physiol. 594 (23), 7015–7025. 10.1113/JP272602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisaka T., Mogi M., Nakaoka H., Kan-No H., Tsukuda K., Wang X. L., et al. (2016). Low-Protein Diet-Induced Fetal Growth Restriction Leads to Exaggerated Proliferative Response to Vascular Injury in Postnatal Life. Am. J. Hypertens. 29 (1), 54–62. 10.1093/ajh/hpv072 [DOI] [PubMed] [Google Scholar]
- Dejima T., Tamura K., Wakui H., Maeda A., Ohsawa M., Kanaoka T., et al. (2011). Prepubertal angiotensin blockade exerts long-term therapeutic effect through sustained ATRAP activation in salt-sensitive hypertensive rats. J. Hypertens. 29 (10), 1919–1929. 10.1097/HJH.0b013e32834a5a46 [DOI] [PubMed] [Google Scholar]
- Denton K. M., Flower R. L., Stevenson K. M., Anderson W. P. (2003). Adult rabbit offspring of mothers with secondary hypertension have increased blood pressure. HYPERTENSION 41 (3 Pt 2), 634–639. 10.1161/01.HYP.0000052949.85257.8E [DOI] [PubMed] [Google Scholar]
- Diz D. I., Arnold A. C., Nautiyal M., Isa K., Shaltout H. A., Tallant E. A. (2011). Angiotensin peptides and central autonomic regulation. Curr. Opin. Pharmacol. 11 (2), 131–137. 10.1016/j.coph.2011.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford A., Al-Magableh M., Gaspari T. A., Hart J. L. (2013). Chronic NaHS Treatment Is Vasoprotective in High-Fat-Fed ApoE(-/-) Mice. Int. J. Vasc. Med. 2013, 915983. 10.1155/2013/915983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray C., Gardiner S. M., Elmes M., Gardner D. S. (2016). Excess maternal salt or fructose intake programmes sex-specific, stress- and fructose-sensitive hypertension in the offspring. Br. J. Nutr. 115 (4), 594–604. 10.1017/S0007114515004936 [DOI] [PubMed] [Google Scholar]
- Grundt A., Grundt C., Knoth K., Lemmer B. (2010). Rhythmic supplementation of 17beta-estradiol according to the physiological estrous cycle: effect on blood pressure in female ovariectomized rats. Horm. Metab. Res. 42 (2), 130–136. 10.1055/s-0029-1241807 [DOI] [PubMed] [Google Scholar]
- Guo Q., Feng X., Xue H., Teng X., Jin S., Duan X., et al. (2017). Maternal Renovascular Hypertensive Rats Treatment With Hydrogen Sulfide Increased the Methylation of AT1b Gene in Offspring. Am. J. Hypertens. 30 (12), 1220–1227. 10.1093/ajh/hpx124 [DOI] [PubMed] [Google Scholar]
- Higashi Y., Sasaki S., Nakagawa K., Matsuura H., Oshima T., Chayama K. (2002). Endothelial function and oxidative stress in renovascular hypertension. N Engl. J. Med. 346 (25), 1954–1962. 10.1056/NEJMoa013591 [DOI] [PubMed] [Google Scholar]
- Hirooka Y., Sakai K., Kishi T., Ito K., Shimokawa H., Takeshita A. (2003). Enhanced depressor response to endothelial nitric oxide synthase gene transfer into the nucleus tractus solitarii of spontaneously hypertensive rats. Hypertens. Res. 26 (4), 325–331. 10.1291/hypres.26.325 [DOI] [PubMed] [Google Scholar]
- Hirooka Y. (2011). Oxidative stress in the cardiovascular center has a pivotal role in the sympathetic activation in hypertension. Hypertens. Res. 34 (4), 407–412. 10.1038/hr.2011.14 [DOI] [PubMed] [Google Scholar]
- Judkins C. P., Diep H., Broughton B. R., Mast A. E., Hooker E. U., Miller A. A., et al. (2010). Direct evidence of a role for Nox2 in superoxide production, reduced nitric oxide bioavailability, and early atherosclerotic plaque formation in ApoE-/- mice. Am. J. Physiol. Heart Circ. Physiol. 298 (1), H24–H32. 10.1152/ajpheart.00799.2009 [DOI] [PubMed] [Google Scholar]
- Kajantie E., Eriksson J. G., Osmond C., Thornburg K., Barker D. J. (2009). Pre-eclampsia is associated with increased risk of stroke in the adult offspring: the Helsinki birth cohort study. STROKE 40 (4), 1176–1180. 10.1161/STROKEAHA.108.538025 [DOI] [PubMed] [Google Scholar]
- Kobori H., Nangaku M., Navar L. G., Nishiyama A. (2007). The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol. Rev. 59 (3), 251–287. 10.1124/pr.59.3.3 [DOI] [PubMed] [Google Scholar]
- Koeners M. P., Braam B., van der Giezen D. M., Goldschmeding R., Joles J. A. (2010). Perinatal micronutrient supplements ameliorate hypertension and proteinuria in adult fawn-hooded hypertensive rats. Am. J. Hypertens. 23 (7), 802–808. 10.1038/ajh.2010.48 [DOI] [PubMed] [Google Scholar]
- Koeners M. P., Braam B., Joles J. A. (2011). Perinatal inhibition of NF-kappaB has long-term antihypertensive effects in spontaneously hypertensive rats. J. Hypertens. 29 (6), 1160–1166. 10.1097/HJH.0b013e3283468344 [DOI] [PubMed] [Google Scholar]
- Lagranha C. J., Deschamps A., Aponte A., Steenbergen C., Murphy E. (2010). Sex differences in the phosphorylation of mitochondrial proteins result in reduced production of reactive oxygen species and cardioprotection in females. Circ. Res. 106 (11), 1681–1691. 10.1161/CIRCRESAHA.109.213645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang M., Cowley A. J., Mattson D. L., Kotchen T. A., Liu Y. (2013). Epigenomics of hypertension. Semin. Nephrol. 33 (4), 392–399. 10.1016/j.semnephrol.2013.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim R., Acharya R., Delpachitra P., Hobson S., Sobey C. G., Drummond G. R., et al. (2015). Activin and NADPH-oxidase in preeclampsia: insights from in vitro and murine studies. Am. J. Obstet. Gynecol. 212 (1), 81–86. 10.1016/j.ajog.2014.07.021 [DOI] [PubMed] [Google Scholar]
- Longo M., Refuerzo J. S., Mann L., Leon M., Moussa H. N., Sibai B. M., et al. (2016). Adverse Effect of High-Fat Diet on Metabolic Programming in Offspring Born to a Murine Model of Maternal Hypertension. Am. J. Hypertens. 29 (12), 1366–1373. 10.1093/ajh/hpw088 [DOI] [PubMed] [Google Scholar]
- Lopez-Ilasaca M., Liu X., Tamura K., Dzau V. J. (2003). The angiotensin II type I receptor-associated protein, ATRAP, is a transmembrane protein and a modulator of angiotensin II signaling. Mol. Biol. Cell 14 (12), 5038–5050. 10.1091/mbc.e03-06-0383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma R. C., Tutino G. E., Lillycrop K. A., Hanson M. A., Tam W. H. (2015). Maternal diabetes, gestational diabetes and the role of epigenetics in their long term effects on offspring. Prog. Biophys. Mol. Biol. 118 (1-2), 55–68. 10.1016/j.pbiomolbio.2015.02.010 [DOI] [PubMed] [Google Scholar]
- Marco L. J., McCloskey K., Vuillermin P. J., Burgner D., Said J., Ponsonby A. L. (2012). Cardiovascular disease risk in the offspring of diabetic women: the impact of the intrauterine environment. Exp. Diabetes Res. 2012, 565160. 10.1155/2012/565160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura K., Averill D. B., Ferrario C. M. (1998). Angiotensin II acts at AT1 receptors in the nucleus of the solitary tract to attenuate the baroreceptor reflex. Am. J. Physiol. 275 (5 Pt 2), R1611–R1619. 10.1152/ajpregu.1998.275.5.R1611 [DOI] [PubMed] [Google Scholar]
- Oliveira-Sales E. B., Nishi E. E., Carillo B. A., Boim M. A., Dolnikoff M. S., Bergamaschi C. T., et al. (2009). Oxidative stress in the sympathetic premotor neurons contributes to sympathetic activation in renovascular hypertension. Am. J. Hypertens. 22 (5), 484–492. 10.1038/ajh.2009.17 [DOI] [PubMed] [Google Scholar]
- Polhemus D. J., Lefer D. J. (2014). Emergence of hydrogen sulfide as an endogenous gaseous signaling molecule in cardiovascular disease. Circ. Res. 114 (4), 730–737. 10.1161/CIRCRESAHA.114.300505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson A. M., Matthews P. A., Jansen E., Taylor P. D., Poston L. (2013). Sucrose feeding in mouse pregnancy leads to hypertension, and sex-linked obesity and insulin resistance in female offspring. Front. Physiol. 4, 14. 10.3389/fphys.2013.00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siqueira R., Campos C., Colombo R., Becker C. U., Fernandes T. R., Araujo A. S., et al. (2011). Influence of estrogen on pulmonary arterial hypertension: role of oxidative stress. Cell Biochem. Funct. 29 (7), 543–548. 10.1002/cbf.1784 [DOI] [PubMed] [Google Scholar]
- Somm E., Schwitzgebel V. M., Vauthay D. M., Aubert M. L., Huppi P. S. (2009). Prenatal nicotine exposure and the programming of metabolic and cardiovascular disorders. Mol. Cell Endocrinol. 304 (1-2), 69–77. 10.1016/j.mce.2009.02.026 [DOI] [PubMed] [Google Scholar]
- Tai I. H., Sheen J. M., Lin Y. J., Yu H. R., Tiao M. M., Chen C. C., et al. (2016). Maternal N-acetylcysteine therapy regulates hydrogen sulfide-generating pathway and prevents programmed hypertension in male offspring exposed to prenatal dexamethasone and postnatal high-fat diet. Nitric. Oxide 53, 6–12. 10.1016/j.niox.2015.12.006 [DOI] [PubMed] [Google Scholar]
- Tain Y. L., Lee C. T., Chan J. Y., Hsu C. N. (2016). Maternal melatonin or N-acetylcysteine therapy regulates hydrogen sulfide-generating pathway and renal transcriptome to prevent prenatal N(G)-Nitro-L-arginine-methyl ester (L-NAME)-induced fetal programming of hypertension in adult male offspring. Am. J. Obstet. Gynecol. 215 (5), 631–636. 10.1016/j.ajog.2016.07.036 [DOI] [PubMed] [Google Scholar]
- Takahashi N., Smithies O. (2004). Human genetics, animal models and computer simulations for studying hypertension. Trends Genet. 20 (3), 136–145. 10.1016/j.tig.2004.01.004 [DOI] [PubMed] [Google Scholar]
- Tamura K., Wakui H., Maeda A., Dejima T., Ohsawa M., Azushima K., et al. (2013). The physiology and pathophysiology of a novel angiotensin receptor-binding protein ATRAP/Agtrap. Curr. Pharm. Des. 19 (17), 3043–3048. 10.2174/1381612811319170010 [DOI] [PubMed] [Google Scholar]
- Wakui H., Uneda K., Tamura K., Ohsawa M., Azushima K., Kobayashi R., et al. (2015). Renal tubule angiotensin II type 1 receptor-associated protein promotes natriuresis and inhibits salt-sensitive blood pressure elevation. J. Am. Heart Assoc. 4 (3), e1594. 10.1161/JAHA.114.001594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Ahmad S., Cai M., Rennie J., Fujisawa T., Crispi F., et al. (2013). Dysregulation of hydrogen sulfide producing enzyme cystathionine gamma-lyase contributes to maternal hypertension and placental abnormalities in preeclampsia. CIRCULATION 127 (25), 2514–2522. 10.1161/CIRCULATIONAHA.113.001631 [DOI] [PubMed] [Google Scholar]
- Wang K., Ahmad S., Cai M., Rennie J., Fujisawa T., Crispi F., et al. (2014). Response to letter regarding article, “dysregulation of hydrogen sulfide (H2S) producing enzyme cystathionine gamma-lyase (CSE) contributes to maternal hypertension and placental abnormalities in preeclampsia”. CIRCULATION 129 (22), e517–e518. 10.1161/CIRCULATIONAHA.114.010273 [DOI] [PubMed] [Google Scholar]
- Wang R. (2002). Two’s company, three’s a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J. 16 (13), 1792–1798. 10.1096/fj.02-0211hyp [DOI] [PubMed] [Google Scholar]
- Xiao D., Xu Z., Huang X., Longo L. D., Yang S., Zhang L. (2008). Prenatal gender-related nicotine exposure increases blood pressure response to angiotensin II in adult offspring. HYPERTENSION 51 (4), 1239–1247. 10.1161/HYPERTENSIONAHA.107.106203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao D., Huang X., Yang S., Zhang L. (2011). Antenatal nicotine induces heightened oxidative stress and vascular dysfunction in rat offspring. Br. J. Pharmacol. 164 (5), 1400–1409. 10.1111/j.1476-5381.2011.01437.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L., Dong J. H., Jin S., Xue H. M., Guo Q., Teng X., et al. (2016). Hydrogen Sulfide Improves Endothelial Dysfunction via Downregulating BMP4/COX-2 Pathway in Rats with Hypertension. Oxid. Med. Cell Longev. 2016, 8128957. 10.1155/2016/8128957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L., Dong J. H., Teng X., Jin S., Xue H. M., Liu S. Y., et al. (2018). Hydrogen sulfide improves endothelial dysfunction in hypertension by activating peroxisome proliferator-activated receptor delta/endothelial nitric oxide synthase signaling. J. Hypertens. 36 (3), 651–665. 10.1097/HJH.0000000000001605 [DOI] [PubMed] [Google Scholar]
- Xu L., Collister J. P., Osborn J. W., Brooks V. L. (1998). Endogenous ANG II supports lumbar sympathetic activity in conscious sodium-deprived rats: role of area postrema. Am. J. Physiol. 275 (1 Pt 2), R46–R55. 10.1152/ajpregu.1998.275.1.R46 [DOI] [PubMed] [Google Scholar]
- Xue H., Zhou S., Xiao L., Guo Q., Liu S., Wu Y. (2015). Hydrogen sulfide improves the endothelial dysfunction in renovascular hypertensive rats. Physiol. Res. 64 (5), 663–672. 10.33549/physiolres.932848 [DOI] [PubMed] [Google Scholar]
- Xue B., Yin H., Guo F., Beltz T. G., Thunhorst R. L., Johnson A. K. (2017). Maternal Gestational Hypertension-Induced Sensitization of Angiotensin II Hypertension Is Reversed by Renal Denervation or Angiotensin-Converting Enzyme Inhibition in Rat Offspring. HYPERTENSION 69 (4), 669–677. 10.1161/HYPERTENSIONAHA.116.08597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanutto B. S., Valentinuzzi M. E., Segura E. T. (2010). Neural set point for the control of arterial pressure: role of the nucleus tractus solitarius. BioMed. Eng. Online 9, 4. 10.1186/1475-925X-9-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanutto B. S., Frias B. C., Valentinuzzi M. E. (2011). Blood pressure long term regulation: a neural network model of the set point development. BioMed. Eng. Online 10, 54. 10.1186/1475-925X-10-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.