Abstract
Much more serious than the previous severe acute respiratory syndrome (SARS) coronavirus (CoV) outbreaks, the novel SARS-CoV-2 infection has spread speedily, affecting 213 countries and causing ∼17,300,000 cases and ∼672,000 (∼+1,500/day) deaths globally (as of July 31, 2020). The potentially fatal coronavirus disease (COVID-19), caused by air droplets and airborne as the main transmission modes, clearly induces a spectrum of respiratory clinical manifestations, but it also affects the immune, gastrointestinal, hematological, nervous, and renal systems. The dramatic scale of disorders and complications arises from the inadequacy of current treatments and absence of a vaccine and specific anti-COVID-19 drugs to suppress viral replication, inflammation, and additional pathogenic conditions. This highlights the importance of understanding the SARS-CoV-2 mechanisms of actions and the urgent need of prospecting for new or alternative treatment options. The main objective of the present review is to discuss the challenging issue relative to the clinical utility of plants-derived polyphenols in fighting viral infections. Not only is the strong capacity of polyphenols highlighted in magnifying health benefits, but the underlying mechanisms are also stressed. Finally, emphasis is placed on the potential ability of polyphenols to combat SARS-CoV-2 infection via the regulation of its molecular targets of human cellular binding and replication, as well as through the resulting host inflammation, oxidative stress, and signaling pathways.
Keywords: complications, microbiota, oxidative stress, polyphenols, viral infection
INTRODUCTION
Coronavirus disease-19 (COVID-19) is a novel coronavirus originating from the city of Wuhan in China. With more than 213 countries and territories affected, the pandemic spread across the globe. So far, nearly 17,300,000 people have been infected worldwide and 672,000 have died (as of July 31, 2020). Although the main part of the pandemic passed in China, the viral crisis is now hitting many countries by infecting and killing thousands: 4,582,000/154,200 in the United States; 301,455/45,961 in the United Kingdom; 329,721/28,441 in Spain; 246,776/35,129 in Italy; 2,556,210/90,215 in Brazil; 185,196/30,238 in France; and 115,617/8,957 in Canada, to name a few. Everywhere, the healthcare system is facing a looming shortage of medical equipment, the residents are confined to their homes, the economy and financial markets are profoundly shaken, the usual consumption pattern is distorted, and the world panics (142, 144, 201).
Although governments have launched unprecedented public-health and economic responses, thousands of new cases are reported every day (201). The majority of people with mild symptoms recover on their own. Notably, it has been shown that high levels of the virus are present in respiratory secretions in asymptomatic subjects or during the “presymptomatic” period prior the development of the fever and cough, which are characteristic of COVID-19 (144). Moreover, many patients with COVID-19 display major complications and are hospitalized in intensive care units (77, 89, 206). Although there is no novel or proven therapy against COVID-19, patients are treated with antiviral drugs such as oseltamivir, ganciclovir, and remdesivir in the hopes of inhibiting SARS-CoV-2 replication (130). In view of the inflammatory cytokine storm highly present in patients with severe COVID-19 causing acute lung injury, anti-inflammatory medications are proposed. Although several governments and international health authorities have promoted and encouraged the treatment with chloroquine/hydroxychloroquine (71, 76, 128, 157), it must nonetheless be noted that these compounds present critical risk and benefit balance issues (44, 185, 216). In this context, recent clinical investigations have assessed promising alternatives, including chloroquine and its hydroxyl analog hydroxychloroquine, to evaluate whether they could prevent the virus from entering host cells, preclude viral replication, and dampen exacerbation of the immune response (44, 182, 185, 216). The findings did not reveal high-quality evidence to support these proposed aminoquinoline therapies (35, 74). Outcomes from large cohort trials are needed to definitely inform the international medical community about the proven clinical effectiveness.
Polyphenols are plant-derived phenolic compounds (Fig. 1) endowed with antioxidants and anti-inflammatory, immune, antitumor, and prebiotic properties. They show benefits for the prevention and treatment of a wide array of chronic diseases, such as insulin resistance, metabolic syndrome, type 2 diabetes (T2D), nonalcoholic fatty liver disease, and atherosclerosis (3–6, 51, 152, 155, 163, 175).
Fig. 1.
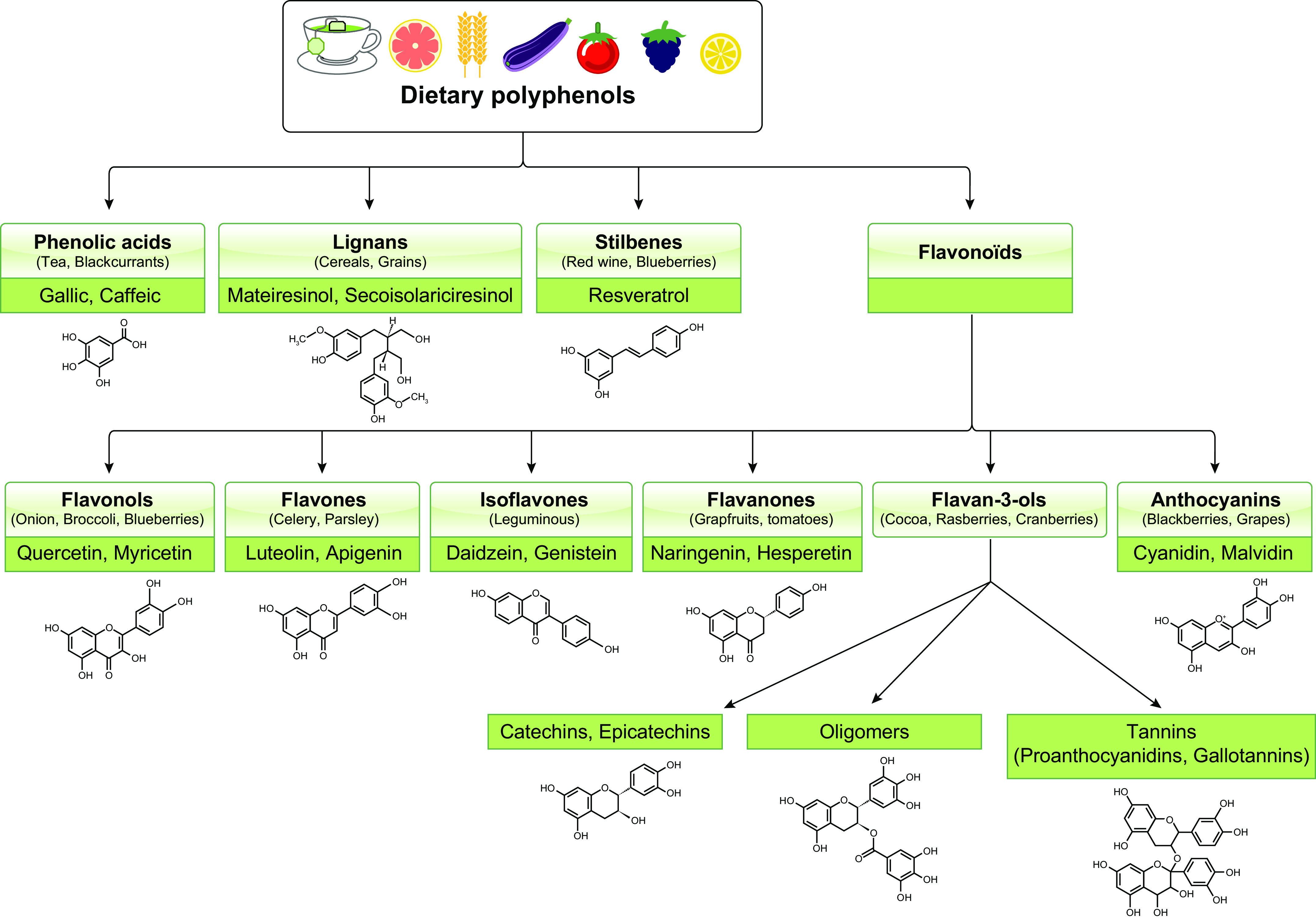
Main classes of polyphenols. Polyphenols are classified into flavonoids and nonflavonoids that contain a diverse group of compounds with a phenolic acid.
Healthful pharmacological properties were also observed in inflammatory pulmonary diseases in response to naturally occurring polyphenols (199, 215). Additionally, polyphenols disclose inhibitory activity against many viral components and actions (110, 165). Currently, there is no specific antiviral treatment or effective vaccine to control COVID-19; hence, there is an unmet need to determine whether alternative treatments offer improved efficacy. The objective of the present article is precisely to exhaustively analyze existing literature to evaluate the promising hypothesis regarding the potential of powerful polyphenols to fight COVID-19 infection while discussing related mechanisms through comparisons of their effects in other acute infectious diseases.
COVID-19 GENOME, PROTEIN STRUCTURE, AND LIFE CYCLE
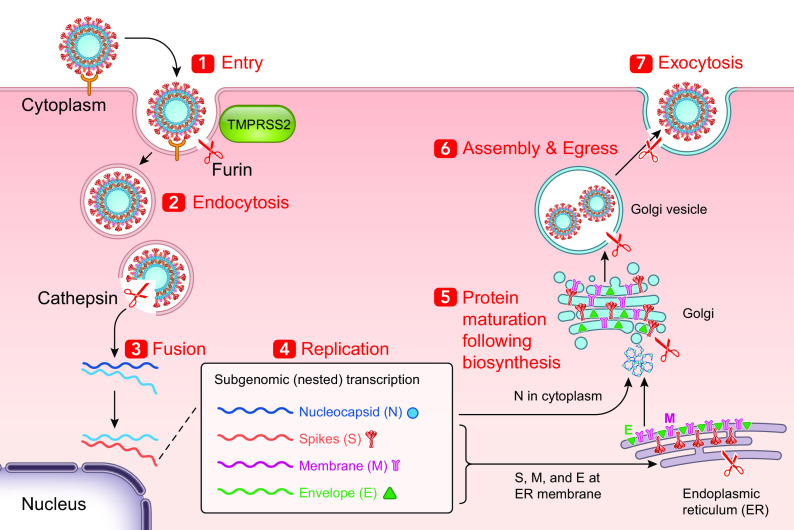
SARS-CoV-2 is the seventh identified coronavirus (CoV) capable of infecting humans (232). As in the case of other CoVs, SARS-CoV-2 is pleomorphic or spherical with a particle size of 150–160 nm, containing positive single-stranded RNA, nucleoprotein, capsid, matrix, and S-protein (Fig. 2). More precisely, the characteristic viral proteins comprise envelope protein (E), nucleocapsid proteins (N), membrane glycoproteins (M), and spike glycoprotein (S) (167). Separately from other CoVs, SARS-CoV-2 displays an additional glycoprotein that has acetyl esterase and hemagglutination properties (117, 232). As per the typical genome organization of the -COVs, SARS-CoV-2 exhibits the 5′ untranslated region (UTR), S gene, E gene, M gene, N gene, 3′ UTR, and other open reading frames (ORFs). Recently, on the basis of various computational tools, ORF 1a and 1b of SARS-CoV-2 are fitted with ∼2/3 of the whole genome and code for 16 nonstructural proteins (nsp1‐16) (117). The −1 frameshift located between ORF1a and ORF1b regions imparts to the formation of pp1a and pp1ab polypeptides (164), which are further processed by viral-encoded proteases into 16 nsp. A section within the nsp2 and the nsp3, with no homology with other CoVs, may provide COVID-19 with the potency of infectiousness. The spike glycoprotein S is composed of two subunits S1 and S2, which mediate binding of COVID-19 (driven by S1) to its receptor and host cell membrane fusion (driven by S2) allowing virus entry (248). Host cell proteins contribute to viral S protein cleavage at the S1/S2 site [transmembrane protease serine 2 (TMPRSS2)] and cell attachment (ACE2 for angiotensin-converting enzyme 2), thereby leading to the merging of the viral capsid and cellular membrane. If presently the spike protein S is considered as the outermost component, which determines the host specificity and infectivity of the virion, the N protein is instrumental for the capsid development and the full viral structure (198). Additionally, the N terminal domain of the N protein binds to the viral (+) sense RNA, thus resulting in the CoV ribonucleoprotein complex, which is essential for the virus replication (181). With regard to the other proteins, the E moiety serves for the viral assembly and comprises ion channel actions (186), and the M protein also participates in the assembly of new virus particles (159). To initiate its life cycle and proficiently replicate for the production of progeny viruses, several processes (i.e., attachment, entry, induction of replicase proteins, replication, transcription, assembly, and discharge of mature viral particles) have to take place (Fig. 3). Following attachment of the virus to host cells via binding of the S1 region (C-terminus) to the receptor (ACE2, DPP4), the S protein is cleaved by host proteases (endosomal cathepsins, TMPRSS, furin, or trypsin) to be functional and activate fusogenicity, with an impact on tropism and pathogenicity (150). Thereafter, there is a fusion of the viral envelope with a host plasma membrane and acidified endosomes resulting in the release of the viral genome into the cytoplasm of the infected cell. The latter process is facilitated by the low pH of the endosomes and the S2 functional subunit of the S spike protein (17). It is at this stage that the SARS-CoV-2 takes advantage of the host endoplasmic reticulum (ER) to form numerous double membrane vesicles to shield viral genome and allow the replication to occur through the replication−transcription complex (85). Making use of the host cell protein translation machinery, the viral genome is translated into viral polyproteins, which are split into structural and nonstructural viral proteins by the viral proteases Mpro and PLpro (137). The assembly of viral particles (virions) is performed in the ER/Golgi compartment (48). Assembled virions are then carried to the cell surface, discharged from the cells through exocytosis, and proceed to infect other cells (Fig. 3).
Fig. 2.
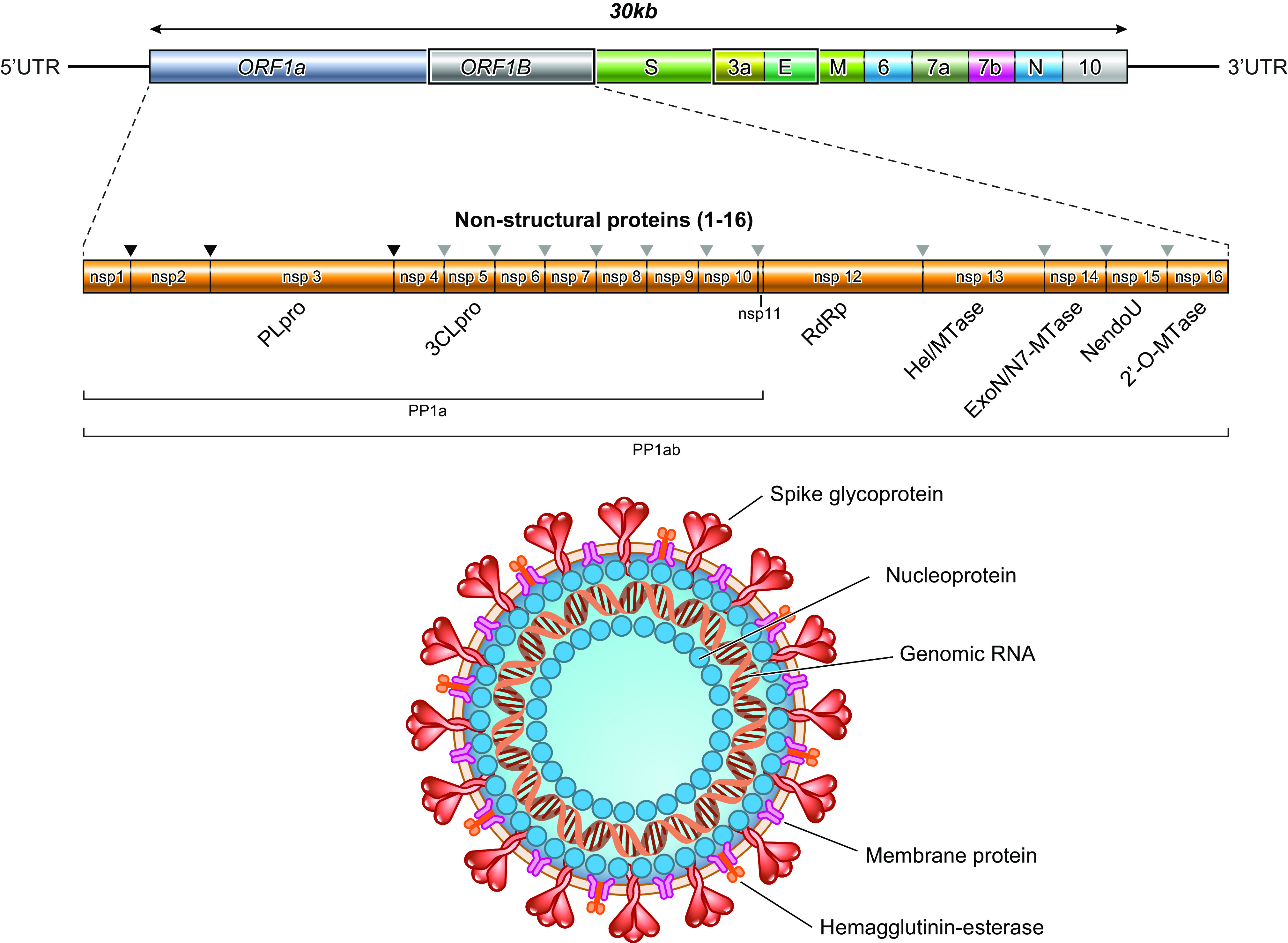
Genomic organization and structure of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [created with BioRender.com]. The genome sequencing includes two large genes, open reading frame (ORF)1A and ORF1B, which encode 1–16 nonstructural proteins constituting a replication–transcription complex, closely involved in the genome transcription and replication. The structural genes encode the following structural proteins: spike (S), envelope (E), membrane (M), and nucleocapsid (N). The additional accessory proteins are specific for SARS-CoV-2 in terms of number, genomic organization, sequence, and function. 3CLpro, 3-chymotrypsin-like protease; UTR, untranslated region.
Fig. 3.
Replication cycle of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the target cell [created with BioRender.com]. The illustration details the replication cycle of the virus starting from binding of the S Spike to the receptor angiotensin-converting enzyme 2 (ACE2) and other potential receptors. Through endosomal membrane fusion, the virus enters the cell and releases its RNA genome. Following transcription and translation, the products (viral structural and nonstructural proteins and genomic RNA) are assembled into virions, transported by vesicles, and released out of the target cell.
COVID-19 SYMPTOMS
Following a 5-day incubation, the patients may present with at least one of the symptoms such as fever, cough, breath shortness, sore throat, and fatigue, which represent the dominant symptoms of COVID-19 illness. However, many subjects also complain of headaches, hemoptysis, muscle soreness, diarrhea, and dyspnea. Subsequently, the patients may develop pneumonia, acute respiratory distress syndrome, acute cardiac problems, and multiorgan failure (184). The loss of sense of smell and taste has often been reported. Even if the coronavirus primarily targets the respiratory system, it has the potential to spread from the respiratory tract (through the olfactory bulb) to the central nervous system, causing inflammation and demyelination, with related complications (from confusion and headache to febrile seizures or encephalitis) (8, 53). Additionally, numerous hospitalized patients had underlying conditions, including obesity, hypertension, diabetes mellitus, and cardiovascular diseases. Clearly, the SARS-CoV-2 infection represents a challenge for physicians because no specific and effective drug is currently available for COVID-19, thereby posing a serious threat to human health.
ANTI-CORONAVIRUS THERAPEUTICS AND PROPHYLACTICS
From the outset, the absence of specific vaccine and antiviral agents makes patient management difficult. The current pharmacotherapy refers to a broad-spectrum antiviral arsenal, which has been used to treat previous viral infections. Therefore, collecting hospitalization data and tracking clinical features and outcomes are mandatory to establish the clinical spectrum of COVID-19, which is instrumental to containing and managing this new pandemic.
Drugs formerly employed for SARS-CoV and Middle East respiratory syndrome-coronavirus (MERS-CoV) have been among the management options for COVID-19 pneumonia. In China, the antiretroviral drugs used for the human immunodeficiency virus (HIV) have also been considered, including lopinavir/ritonavir in combination with the anti-flu drug oseltamivir (45). Even remdesivir, known as a powerful agent to treat patients with Ebola virus, has been administered in the United States against COVID-19. Moreover, the antiviral and anti-inflammatory effects of antimalarial chloroquine and its derivatives (e.g., hydroxychloroquine) have been tested without success, and more studies are needed to prove the dose-response effectiveness (74, 182).
In view of the paucity of therapeutic findings and scantiness of related mechanisms, it is surprising that polyphenolic compounds have not been tested to combat viral activities. However, it is worth noting that, in addition to their antioxidative and anti-inflammatory characteristics, polyphenols exhibit anti-infective properties (220). Indeed, in the last two decades, numerous benefits of polyphenols against diverse families of viruses were suggested, as these safe and reliable nutrients displayed the capacity to interrupt the life cycle of viruses and halt viral replication, boost immune responses, and protect against inflammation in infected patients. In the following sections, polyphenol properties, potential impact on various harmful viruses, and underlying molecular mechanisms will be discussed before their application in managing COVID-19 infection is proposed (Fig. 4).
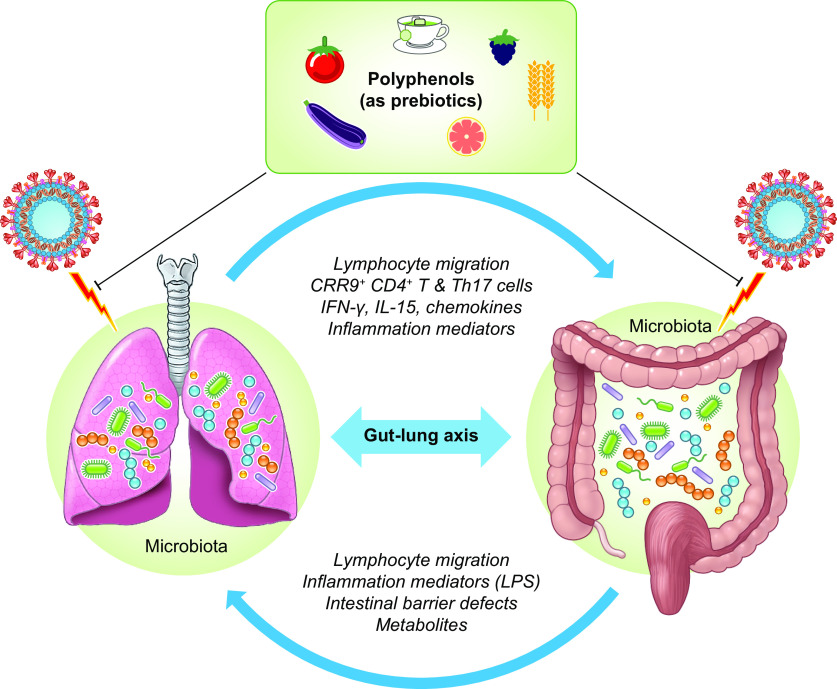
Fig. 4.
Potential cross-talk between gut microbiota and lungs during severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection [created with Servier Medical Art]. The gut–lung axis is bidirectional and able to induce changes in the blood and respective microbiota, illustrating the association of gut microbiota dysbiosis with local and distal pulmonary system. For example, the inflammation occurring in the pulmonary system leads to modifications in gut microbiota, and vice versa. LPS: Lipopolysaccharide.
ANTIVIRAL ACTIVITIES OF POLYPHENOLS
Herpes Simplex Virus Type 1
Propolis is produced by bees and contains polyphenols, including flavonoids and phenylcarboxylic as major constituents (190). Preincubation of propolis extracts with African green monkey kidney cells (Vero cells) before infection with herpes simplex virus type 1 (HSV-1) strain KOS resulted in inhibition of >98% viral plaque formation (infectivity) and herpetic activity at maximum noncytotoxic concentrations in mice (196). Administration of propolis before or at the time of infection yielded the most significant inhibitory effect. Propolis extracts were proposed to interfere with HSV-1 attachment to cells via its surface structures and proteins, thereby blocking its adsorption and penetration into host cells (238). Contrary to acyclovir, propolis extracts do not inhibit viral DNA polymerase responsible for intracellular replication of HSV-1 in cell lines (238). In men and women, the administration of propolis was effective in respiratory tract infections (42) and genital herpes, respectively, hasting the healing process in the presence of acyclovir (223). The aforementioned observations highlight the promising actions of propolis polyphenol content, but additional efforts are still necessary to indicate whether propolis extracts directly cause virion damage. Validation of the propolis findings was obtained with almond skin polyphenols. These compounds hampered HSV-1 adsorption to Vero cells and restricted the virion inside the cells (20). Later, the same group demonstrated a radical drop of the HSV‐1 viral infectivity along with a significant decrease in the accumulation of viral proteins (e.g., ICP0 or UL42) and DNA in the standard American Type Culture Collection (ATCC) and clinical strains of Staphylococcus aureus (158), which prompts the use of polyphenol extracts for the development of novel virucide agents.
Resveratrol impact was assessed in HeLa, Vero, and HEK-293 T cells with respect to HSV-2 infection. Resveratrol was capable of stimulating histone acetylation and activation of NF-κB transcription factor, thereby releasing the chromatin structure from depressed condition and triggering HSV transcription (56). These data are not in accordance with previous investigations, which reported inhibition of HSV-1 and HSV-2 replication in human-lung cell lines (MRC-5) by resveratrol in a dose- and time-dependent manner (59). Resveratrol also exhibited a comparable efficient blockade of HSV replication like acyclovir in the vagina of mice (39). Differences in cell types and doses may explain the opposite data observed, which argues for clarification with additional studies.
Influenza Virus
Many types of berries display antiviral activities. Some of them such as elderberry and blueberry are able to impede the replication in cell lines (104), which may relieve the symptoms of influenza virus. Several blueberry varieties exert anti‐influenza viral activities, especially viral adsorption, which positively correlated with the total polyphenol content in Madin-Darby canine kidney cells (192).
Resveratrol exhibited potent inhibitory effects against the influenza in MDCK cells via the blockade of nuclear-cytoplasmic translocation of viral ribonucleoprotein complexes, the decrease in the expression of late viral proteins, and the inhibition of cellular protein kinase C activity and its dependent pathways (162). In view of these observations, the authors proposed that resveratrol acts on posttranscriptional phases of the viral life cycle. Other studies were able to show the attenuation of replication of influenza A and H1N1 in A549 lung cancer cells, not only through stimulation of INF-through Toll-like receptor (TLR)9, but also via the downregulation of hemagglutinin (facilitating the binding of the virus to target cells) and neuraminidase (responsible for the cleavage of the host cellular receptors and release of the progeny virus) (135). On that basis, resveratrol may serve for the development of novel antiviral therapies against influenza provided that the data are validated in clinical trials.
Zika Virus
Resveratrol (3, 4′, 5-trihydroxystilbene), a natural polyphenolic compound found in the skins of red fruits, shows broad therapeutic benefits. Its effects were examined on Zika virus (ZIKV) belonging to the Flaviviridae family of the genus Flavivirus. At low concentrations (80 µM), resveratrol administration led to >90% inhibition without cytotoxicity to human hepatocellular carcinoma (Huh7) and Vero cells (153). Not only does resveratrol inhibit binding of ZIKV particles to cells, but it also reduces circulating ZIKV particles, which underlines its potential of limiting disease severity during the viraemic phase. Furthermore, the decline of ZIKV mRNA copy numbers in response to the resveratrol treatment suggests inhibitory effects against intracellular ZIKV replication.
Dengue Virus
Resveratrol exerts an inhibitory effect on dengue infection, which is transmitted to humans by Aedes mosquitoes and threatens more than one-third of the world’s population, as 96 million individuals (particularly in children between 2 and 16 yr) seem to be affected annually (19). Despite the recently developed vaccine against dengue virus (DENV), the observations in two phase 3 trials with 30,000 children provide only partial efficacy (222), with ∼60% overall depending on the four serotypes (DENV1–4) (84). Therefore, various groups focused on resveratrol and its analogs (e.g., PNR-4-44 and PNR-5-02) to reveal anti-DENV activities, an initiative that has proven to be true (88). Subsequently, it turned out that resveratrol mediates the inhibitory effect on DENV by retaining the high-mobility group box 1 (HMGB1) in the nucleus of human hepatocellular carcinoma cells, thus preventing its migration to cytoplasm and binding to viral proteins to enhance viral replication (240). As a significant complement of information for this mechanism of action, resveratrol induces sirtuin (Sirt)1 synthesis to initiate HMGB1 deacetylation and retention of HMGB1 in the nucleus of various cell lines (102). Altogether, resveratrol may likely target factors or pathways inside host cells, contributing to anti-DENV activities, which will alleviate dengue symptoms ranging from mild fever to hemorrhagic fever and dengue shock syndrome.
Respiratory Syncytial Virus
Resveratrol was also tested in respiratory syncytial virus (RSV), characterized by persistent airway inflammation, airway hyperresponsiveness, and childhood recurrent wheezing and asthma (70). Resveratrol was able to lower the expression of Toll-like receptors-Toll/IL-1-receptor domain containing adaptor protein inducing IFN-β pathway in 9HTEo cells derived from human tracheal epithelium to block the formation of RSV-induced IFN-γ and IL-6, thereby ameliorating acute airway inflammation and airway hyperresponsiveness (138) via the suppression of nerve growth factor production in respiratory syncytial virus-infected mice (241). Similarly, resveratrol depleted mouse natural killer cells, thereby reducing the type 2 cytokines and ameliorating the airway disease (139).
Hepatitis C Virus
Resveratrol multimers (oligostilbenoid compounds) were identified as potent replication inhibitors of hepatitis C virus (HCV), which contains a single-stranded positive RNA genome and has infected 170 million people worldwide (195). Among the oligostilbenoids [e.g., ampelopsin A, (+)-ε-viniferin, wilsonol C, vitisin A, and vitisin B], the resveratrol tetramer vitisin B is the most powerful to affect anti-HCV activity [EC50 = 6 nM and 50% cytotoxic concentration (CC50) > 10 μM] via neutralization of its NS3 helicase in several cell lines (154). Similarly, the more stable (+)-ε-viniferin dimer markedly lowered viral protein expression and HCV replication with minimal cytotoxicity in vitro and in vivo (127). In view of their promising antiviral and pharmacokinetic properties, oligostilbenoids warrant further anti-HCV therapeutics investigation, which will help reduce chronic liver diseases (e.g., liver cirrhosis and hepatocellular carcinoma) in HCV-positive carriers.
Epstein-Barr Virus
In preclinical studies, resveratrol disclosed prevention capacity malignant transformation of human B cells in response to Epstein-Barr virus (EBV) along with potent antitumor activities against EBV-transformed B cells (66). Therefore, resveratrol is a promising avenue, unlike the classical immunosuppression approaches that lead to severe EBV reactivation with possible lymphoproliferative disorders (168). The mechanisms proposed for resveratrol include reduced miR-155 and miR-34a expression in EBV-infected B cells along with significantly declined anti-apoptotic viral gene BHRF1 expression, thereby interfering with crucial processes implicated in EBV transformation and the survival of human EBV-transformed cells (66). Recently, another mechanism was described in extranodal natural killer (NK)/T-cell lymphoma cell lines (NT-8, SNK-10, and SNT-16), resting on the curtailment of AKT and Stat3 phosphorylation, as well as activated DNA damage response and Zta upregulation of EBV, knowing that Zta induces EBV into lytic phase and viral replication (207). However, clinical trials are required to determine whether resveratrol alone or combined with available treatments could constitute a definite advantage to combat EBV-related cancers (i.e., Hodgkin’s lymphoma, Burkitt’s lymphoma, and diffuse large B-cell lymphoma, nasopharyngeal carcinoma).
Human Immunodeficiency Virus Type 1
Although resveratrol could not stop HIV-1 and related mutant replication in activated T cells or in transformed T-cell lines (41, 93), it does potentiate inhibition of reverse transcription by nucleoside analog reverse transcriptase inhibitors, including tenofovir, didanosine, zidovudine, and emtricitabine (47). Additional reports emphasized anti-HIV activities by demonstrating cellular ribonucleotide reductase inhibition, causing a marked decrease in 2-deoxynucleotide 5′-triphosphate (dNTP) levels, which boosts the competition of nucleoside analog drugs for cellular dNTPs in U373-MAGI cells (178). Recently, resveratrol drastically arrested HIV-1 infection through the obstruction of generation of reverse transcripts in CD4 T cells (33). This is a promising set of findings, although reinforcement is necessary to support the potential use of resveratrol as an adjuvant in anti-HIV prophylaxis formulation.
Varicella-Zoster Virus
Resveratrol administration to human diploid MRC-5 cells resulted in the inhibition of varicella-zoster virus (VZV) replication in a dose-dependent manner (60). In fact, resveratrol affects the first stage of VZV replication by suppression of the synthesis of IE62, an essential immediate early viral protein (58).
Catechins and Various Types of Viral Infections
Catechins are polyphenolic flavonoids contained in green tea leaves (Camellia sinensis) and they include: (−)-epigallocatechin-3-gallate (EGCG), (−)-epicatechin-3-gallate, (−)-epigallocatechin, and (−)-epicatechin (57, 235). Catechins provide diverse health benefits such as antioxidants (197), anti-inflammatory (236), antitumor (18), antiobesity, antidiabetic, hypotensive, and anti-allergic effects in humans (147). Studies have shown that catechins, particularly EGCG, have antimicrobial (Gram-positive and Gram-negative bacteria) (87) and antiviral activities with different modes of action (202). The negative effects of catechins were reported, mostly in cultured cells, against a wide range of DNA viruses, including HSV (43, 172), human papillomavirus (90), and hepatitis B virus (91); against (+)-RNA viruses such as HCV (23), ZIKV (29, 220), DENV (173), West Nile viruses (220), Chikungunya virus (CHIKV; Togaviridae) (141), and porcine reproductive and respiratory virus (245); and against (−)-RNA viruses such as HIV (131), Ebola virus (180), and influenza virus (188).
The different mechanisms of action triggered by catechins are largely reported in the scientific literature (113, 234). Overall, natural catechins may inhibit viral activity of enveloped DNA, (+)-RNA, and (−)-RNA viruses, but EGCG appears to be the most promising in achieving this function. Through its interactions with viral membrane proteins or/and cellular proteins, EGCG exhibits high ability to block the early stages of infections, including attachment, postadsorption entry, and genome replication by inhibiting reverse transcriptase in vitro and in vivo (113). Importantly, polyphenol 3-galloyl and 5′-OH groups possess a superior inhibitory power.
To date, many companies and academic research groups around the world have focused on searching and developing the specific vaccine or antiviral drug to prevent or control emerging infection of SARS-CoV-2 (e.g., vaccines, monoclonal antibodies, and small-molecule drugs). However, these options need several months to years for the developing process. For the urgent requirement to alleviate the COVID-19 pandemic, the use of polyphenols and repurposed existing antiviral drugs approved for treatment of other viral infections, such as HIV, hepatitis B virus, HCV, and influenza, is somewhat promising based on previous success of the therapeutic treatment with two relevant human coronaviruses, including SARS-CoV and MERS-CoV.
POLYPHENOLS AND SARS-COV-2 CELL ENTRY
3-Chymotrypsin-Like Protease
3-Chymotrypsin-like protease (3CLpro) is a nonstructural protein of coronaviruses, which has been identified as the most attractive target to combat sarcoviruses. Its function consists in cleaving polyproteins into viral replication-related proteins, an essential process for the replication and maturation of sarcoviruses. SARS-CoV-2 also has the 3CLpro with 96.1% sequence identity compared with the SARS-CoV family, such as SARS-CoV and MERS-CoV. Another crucial function of the 3CLpro is the cleavage of host proteins related to innate immune response, among them the powerful signal transducer and activator of transcription 2 and NF-κB transcription factor as an essential modulator-signaling protein (167). Thus, neutralizing 3CLpro can serve a twofold objective regarding SARS-CoV-2 infection, e.g., averting viral maturation and restoring the natural immune response. A series of inhibitors was reported to act against 3CLpro from CoVs to prevent viral replication since the SARS outbreak in 2003 (112, 237).
As mentioned previously, the water-soluble flavonoid quercetin displays the capacity to inhibit a wide range of viruses, such as the human T-lymphotropic virus 1, the Japanese encephalitis virus, the DENV-2, and HCV (38, 108). Its main mode of action is the suppression of 3CLpro (12). Molecular docking studies and enzymatic inhibition assays clearly documented 3CLpro binding and inhibition by quercetin-3-β-galactoside (36). Based on the similarity between the 3CLpro physical structure and function, it can be plausibly deduced that quercetin may bind to the 3CLpro of SARS-CoV-2 and impede its catalytic activity similar to its effects on the various aforementioned viruses. The advantage of the quercetin small molecule is that it is abundant in nature and can easily be extracted from fruits, vegetables,grains, and leaves on a large scale, and in different formulations as well, including quercetin-3-O-β-d-glucuronide, quercetin-enriched lecithin formulations, and quercetin 7-rhamnoside, which exhibit high efficacy against different viruses (231). Among other benefits, quercetin as a natural compound has low cell toxicity activities and has been found to accumulate in various tissues, including the lungs (132), which will facilitate the interaction with SARS-CoV-2. Importantly, not only does quercetin exert inhibitory activity and replication by interactions occurring in the 3CLpro’s active site, but it might also fight concomitant comorbidities (T2D, obesity, chronic respiratory disease) characterizing old patients (95, 100). Notably, quercetin reduces pulmonary arterial pressure through the abolition of pulmonary artery smooth muscle cell proliferation and inhibits the plate-derived growth factor receptor β signaling pathways, thereby exhibiting potential cardiovascular protective effects probably by equilibrating ACE-AngII-AT1R and ACE2-Ang1-7-Mas (244). Finally, pulmonary SARS-CoV-2 infection may be complicated by secondary bacterial infection, and again quercetin may provide protection against clinical pneumococcal infections, even in resistant Streptococcus pneumoniae pathogenesis (143).
Transmembrane Serine Protease TMPRSS2
Virus cell entry also depends on S protein priming by host cell proteases to promote infection. TMPRSS2 (also known as epitheliasin) activity is key for SARS-CoV-2 spread and pathogenesis of infected host tissues (Fig. 3) (97). It also has the capacity to cleave ACE2 to promote viral entry (94). However, little is known about its biological function and regulation. Protease inhibitor drugs may strategically be designed to block the active site of these proteolytic enzymes, which may produce a significant reduction in SARS-CoV-2 activation in humans. For example, mimetic peptides could block the activities of TMPRSS2 and TMPRSS11D, which stimulate influenza infection by A/Memphis/14/96 (H1N1), A/Mallard/Alberta/205/98 (H2N9), and A/Texas/6/96 (H3N2) in lung epithelia (13). Although polyphenols have been the focus on the development of various antiviral proteases (e.g., SARS-CoV 3-chymotrypsin-like protease and papain-like protease, MERS-CoV cysteine proteases) against CoV-induced diseases (165), no specific study has been devoted to TMPRSS2. However, it is important to remember that polyphenols are endowed with the properties of potentially useful broad-spectrum antiviral agents based on their activity on a variety of enveloped viruses (229). Furthermore, polyphenols have α-glucosidase inhibitory activity responsible for the removal of terminal glucose residues from N-glycan chains attached to nascent glycoproteins, extremely essential for viral envelope (34). Preventing enzymatic activity by polyphenols thwarts folding and function of many glycoproteins, which are central for nucleocapsid formation (34). Concentration efforts on the polyphenol knowledge development would help design effective therapeutic and prophylactic SARS-CoV-2 inhibitors.
Angiotensin-Converting Enzyme 2
ACE2 receptors function as a gate for the SARS-CoV-2 (97, 224). This transmembrane protein is found on the cell surface of various tissues (e.g., nasal mucosa, lung parenchyma, gastrointestinal and renal tract, vascular endothelium, lymphoid tissues, reproductive system, and cerebral neurons) and may theoretically give the virus access to several organs (133, 242), which explains respiratory problems (pneumonia leading to acute respiratory distress syndrome) and disorders of heart, kidneys, and digestive tract. The S spike protein of SARS-CoV-2 displays a strong binding affinity for ACE2, probably due to the four-residue motif from 482 to 485 in the human ACE2 ridge (78, 224). Following endocytosis and membrane fusion, viral RNA is transcribed by the host cell ribosomes in the ER to produce viral component proteins, allowing the assembly of full viruses and discharge from the cell. Downregulation of ACE2 is known to occur following viral infection and may lead to angiotensin II effects, such as hypertension, enhanced inflammation, and thrombosis (2, 68, 204). Patients with structural variations of ACE2 receptors have an enhanced protection given the low binding affinity of the S protein (81).
The strategy of supercomputer-based in silico drug-docking to the COVID-19 viral spike protein allowed the identification of quercetin as a likely ligand of ACE2, which may interfere with virus-host interactions (32).
Resveratrol has the ability to upregulate ACE2 protein expression in various cardiometabolic conditions and displays protective actions against oxidative stress (OxS), inflammation, platelet oxidation, thrombus formation, and aortic aneurysm (69, 221). In the cardiovascular context, resveratrol lessened reactive oxygen species (ROS) concentration and proinflammatory cytokine production while preventing arterial aging in association with reduced activity of the PRR-ACE-Ang II axis and stimulation of the ACE2-Ang-(1–7)-ATR2-MasR axis (118). Similarly, resveratrol exerts protective effects on aging kidneys by decreasing OxS, inflammation, and fibrosis through Ang II suppression and MasR activation (109).
Because resveratrol is endowed with the ability to upregulate ACE2 protein expression, we might then think that the polyphenol would promote viral infection and subsequent symptoms as ACE2 protein expression is required for SARS-CoV-2 host cell entry. Validation is obtained in ACE2 knockout mice, which become resistant to SARS-CoV-2 infections (105, 123). Despite this, ACE2 activity plays a protective role because acute lung injury and respiratory distress syndrome are more severe in mice with ACE2 inactivation following SARS-CoV-2 infection (106). Obviously, after being infected, animals with total ACE2 deficiency exhibited worsened vascular permeability, lung edema, neutrophil accumulation, and lung dysfunction. Amelioration was recorded with the administration of catalytically active recombinant ACE2 protein (15, 106, 151). Therefore, resveratrol may help mitigate the detrimental viral impact.
Dipeptidyl Peptidase 4
Dipeptidyl peptidase 4 (DPP4), also known as cluster of differentiation 26 and as an antidiabetic drug, is another host receptor for COVID-19 (46). This serine exopeptidase has capacity in hydrolyzing several substrates with proline or alanine residue at position 2 and is expressed ubiquitously in several tissues such as lung, kidney, liver, gut, and immune cells, but it can also be found in a soluble form in the circulation (99). Given its ability to cleave numerous substrates, including growth factors, chemokines, neuropeptides, and vasoactive peptides, DPP4 is considered as a regulator in multiple physiological 11processes (62, 119, 160). Using a docked complex model of the SARS‐CoV‐2 spike glycoprotein and DPP4, a large interface was observed, predicting DPP4 as a functional receptor (62). Furthermore, human DPP4 has recently been noted to interact with the S1 domain of the viral spike glycoprotein (219). Even if validation is eagerly awaited concerning the question of whether DPP4 is directly involved in SARS‐CoV‐2 cell adhesion/virulence, some investigators think that DPP4 inhibition may represent a therapeutic strategy to slow the progression of COVID-19 infection or at least to modulate inflammation and fibrotic activity. To substantiate this claim, scientists mention the aptitude of DPP4 to reduce the production of proinflammatory cytokines, severity of symptoms in autoimmune encephalomyelitis myelin basic protein-specific CD4+ T-cell clones, the risk of incident autoimmune diseases, pulmonary fibrosis, and especially lung injury via lowering inflammatory cytokines in an experimental model of acute respiratory distress syndrome, which represents the main death cause of SARS-CoV-2 infected patients (193, 200). On the other hand, human DPP4 was identified as a functional receptor for the spike protein of the MERS-CoV (176) using antibodies against it (176). A preventive role against HIV1 was also evidenced with the inhibition and downregulation of DPP4 (79). Additionally, mice were made susceptible to MERS-CoV by expressing human DPP4 (124). Finally, upon inoculation with MERS-CoV, human DPP4−/− mice supported virus replication in the lungs (212).
Whether DPP4 inhibition represents a plausible approach to mitigate COVID-19 requires much work to be done, as other studies reported divergent findings. The largely used inhibitors (e.g., sitagliptin, alogliptin, vildagliptin, saxagliptin, linagliptin) bind to the catalytic site of DPP4, whereas SARS-CoV-2 is directed to another DPP4 pocket site (219) and without interference with DPP4 inhibitors (65).
Experimental evidence showed that polyphenols inhibit DPP4 and stimulate intestinal L cells to secrete GLP1, a DPP substrate (61). Similarly, resveratrol, cocoa-contained flavonols and polyphenol-rich grape seed-contained catechin, epicatechin, procyanidin B2, and gallic acid were able to inhibit DPP4 activity (61, 208). The administration of the resveratrol phytochemical in presence of DPP4 inhibitors (alogliptin, sitagliptin, saxagliptin) slowed down the metabolism rate of these drugs while extending their pharmacological effects (208). Given its potential, resveratrol may achieve synergistic actions with pharmacological agents particularly in diabetic patients who contract COVID-19 and have worse prognosis (83, 225). Of note, diabetic ketoacidosis, hyperosmolar hyperglycemia state, and acute metabolic complications are precipitated by COVID-19 infection, resulting in catastrophic outcomes (21). Because COVID-19 has diverse disproportionately worse outcome, especially in patients with preexisting T2D, hypertension, and cardiovascular disease, administration of polyphenols known to alleviate these disorders can be a major source of benefit. For example, persons that consume polyphenol-rich foods can reduce the risk of T2D (189). This section will be developed below.
POLYPHENOLS AND VIRAL SIRTUIN REGULATION
SIRTs (1–7) represent a large family of NAD+-deacetylase enzymes with a broad range of physiological and biological functions, such as the regulation of gene expression, cell survival, apoptosis, energy metabolism, circadian clocks, mitochondrial biogenesis inflammation, DNA repair, development, neuroprotection, and longevity (86). For example, the most studied SIRT1 splits the nicotinamide ribosyl bond of NAD+ and transfers the acetyl group from proteins to their cosubstrate through an ADP-ribose-peptidyl imidate intermediate. Recently, mounting attention has been paid to the anti- and proviral role of SIRTs in viral infection control, as acetylation constitutes a significant regulatory process in infection.
Oligonol, a polyphenolic antioxidant compound, shows anti-influenza activity (75). Its administration in human lung epithelial cells, after influenza virus infection, simultaneously upregulated SIRT1 expression and downregulated viral hemagglutinin expression (166). Accordingly, SIRT knockout or addition of SIRT antagonists in human MRC-5 embryonic lung fibroblasts or canine MDCK kidney epithelial cells stimulated the growth of diverse human viruses (human pathogen human cytomegalovirus, influenza A H1N1 virus) (122).
Similarly, SIRT1 overexpression resulted in HIV transcription, whereas SIRT1 lessening by siRNAs or inactivation by nicotinamide increased viral gene expression via multifunctional Tat protein‐induced HIV‐1 transactivation (243). An opposing outcome (probably due to differences in their experimental conditions) was reported by another study, which documented that SIRT1 directly binds to Tat to deacetylate it and synergistically activate the HIV promoter transcription (161).
Stimulation of SIRT1 by resveratrol repressed human T-cell leukemia virus type 1 (HTLV-1) replication (210). Mechanistically, activation of SIRT1 by resveratrol was seen to inhibit HTLV-1 transcription by blunting and counteracting the activity of Tax oncoprotein, the viral transcriptional regulator. This is the first proof of concept for the effectiveness of resveratrol as a SIRT1 modulator and as an antiviral defense molecule for poorly treatable HTLV-1, causing aggressive adult T-cell leukemia in ∼20 million people worldwide.
As well documented above, polyphenols display antiviral effects against a variety of viruses via different cellular pathways by actuating SIRT1, a central actor-providing defense against viral pathogens. Like a certain number of virus types, SARS‐CoV‐2 may act to deviate sirtuins from their regular physiological processes. Unfortunately, the surprising outbreak of COVID-19 did not leave scientists with enough time to scrutinize its molecular mechanisms by which disease-causing SARS‐CoV‐2 dramatically infects and replicates in human cells and concomitantly probe the status of SIRTs and histone/nonhistone targets. As SIRT1 holds promise to have a broad impact in the field of infectious diseases, it is possible that its activation by polyphenols may exert protective effects against SARS‐CoV‐2. It has already been established that resveratrol is able to activate SIRT1, which is able to modulate ANG type 1a receptor and ACE2 (146). It is therefore mandatory to assess whether the modulation of SIRT1 expression by polyphenols in COVID-19 presents a new insight in the discovery of a number of therapeutics.
POLYPHENOLS AND MODIFICATION OF RESPIRATORY AND INTESTINAL MICROBIOTA
Viral infections disturb synergistic and competitive/antagonistic interactions of microbial communities in respiratory and intestinal systems. The resulting dysbiosis, which reflects abnormal microbiota diversity and composition, affects local and general health. If the microbiota-host interplay in normal conditions promotes immune homeostasis (26, 156), viral infection in turn instigates microbiota alterations along with immune pathogenesis while upsurging infectious diseases and leading to the disruption of physical barriers, dysregulation of immune responses, and delays in a return to homeostasis (26, 209).
Upper Respiratory Tract
Commensal and pathogenic bacteria, including Streptococcus pneumoniae, Hemophilus influenzae, Moraxella catarrahlis, Staphylococcus aureus, and Neisseria meningitidis constitute the microbiota in the nasopharynx of healthy humans (92, 203). However, viral infections of the upper respiratory tract modify bacterial adherence (10), phyla, and diversity (126), as well as host immune response (149). There is a common understanding that viral infection leads to microbiota shift and dysfunction in the upper respiratory tract, in association with secondary bacterial infections and disease, as exemplified by infective human rhinovirus and respiratory syncytial virus (103). Clusters of H. influenzae and Streptococcus were positively linked to respiratory syncytial virus infection and hospitalization (187), but commensal Corynebacterium pseudodiphtheriticum alleviates respiratory syncytial virus and S. pneumoniae superinfection (114).
Lower Respiratory Tract
If viral infections are usually transitory and restricted to the upper respiratory tract, their severity can make them progress to the lower respiratory tract along with marked morbidity and mortality. In this context, alterations of microbiota have been described in the lower respiratory tract after influenza viral infection (82). During the acute period, this site microbiota was characterized by an abundance of the genera Halomonas, Shewanella, and Clostridiales, whereas Bacillales and Actinobacteria were the preponderant genera in the recovery period. Furthermore, the enrichment with Gammaproteobacteria and pulmonary gram-negative pathogens like Acinetobacter, Pseudomonas, and Stenotrophomonas and facultative anaerobes such as Staphylococcus Streptococcus and the anaerobe Prevotella in Bacteroidetes can cause pneumonia. Similarly, the lower airway microbiota of Ugandan HIV-infected acute pneumonia patients contained dominant bacterial family (Prevotellaceae, Streptococcaceae, or Pseudomonadaceae) (194). Furthermore, dysbiosis and dominance of Enterobacteriaceae were noted in influenza A virus-infected lower respiratory tract (179).
Gastrointestinal Tract and Gut–Lung Axis
Although the shift and function of gut microbiota in response to viral pathogens remains poorly studied, the literature available for the moment indicates marked changes in gut microbiota composition in association with immune activation and chronic inflammation, as is the case for HIV (9), hepatitis B virus (228), HCV (107), and rotavirus (73). Consequently, the viral replication is accompanied by defects in epithelial barrier integrity and immune homeostasis, leading to microbial translocation that, in turn, contributes to immune activation, inflammation, and disease progression. Therefore, changes of gut microbiota communities and functions in response to viral infection may lead to adverse clinical outcomes.
Similarly, dysbiosis and subsequent dysregulation of gut microbiota-related immunological processes are observed in respiratory diseases following respiratory infection by viruses such as influenza A virus (239), respiratory syncytial virus (226), and recombinant pneumonia virus (64) exhibiting tropism for the gastrointestinal tract and additional systems. Importantly, accumulating data underline the mutual interactions between intestinal microbiota and the lung with effects of respiratory infections (Fig. 4) (148). Disturbances of gut microbiota raise the risk of respiratory syncytial virus infections in infants (22). Otherwise, the gut microbiota in physiological conditions mediates the differentiation and expansion of regulatory T cells (Treg) (211), whereas mononuclear phagocytes’ intestinal and respiratory tract sample antigens in the lumen possibly act through metabolites (e.g., short-chain fatty acids), thereby fine-tuning the threshold of antiviral immune responses and activating adaptive immunity to promote the clearance of viruses and other pathogens (22). Although the gut–lung interactions, notably gut microbiota-lung immunity (referred to as the “gut–lung axis”) (Fig. 4) appear more and more important for respiratory diseases (63), the cross-talk between their respective microbial communities is ill defined, particularly during viral infections. Factors disturbing gut microbiota (e.g., smoking, antibiotics, diets, pre- and probiotics) cause inflammation and predispose to microbial infection at distal organs such as the lung (22). Just to name a few examples: fecal transplantation induces changes in the lung microbiota (145); intestinal germ-free animals are protected from lung injury (205); and the modification of gut microbiome is closely implicated in the pathogenesis of the acute respiratory distress syndrome (54). Reciprocally, lung microbiota may bear upon gut microbiota composition as reflected by influenza infection, which enhanced Enterobacteriaceae and lowered Lactobacilli and Lactococci in the intestine (140).
Impact of Polyphenols on the Gut-Lung Axis in the Context of Viral Infection
Although the respiratory tract is almost exclusively invaded by human influenza viruses, infected individuals very often suffer from gastrointestinal symptoms (55). Investigations, designed to elucidate the mechanisms, found that this infection unbalanced gut microbiota through the induction of type I INFs (52). Conversely, probiotics and prebiotics, which reinforce gut microbiota (14, 27, 31, 50, 96, 183, 191, 217), ameliorate the immune system and influenza virus-related damage (25, 177, 218, 230), suggesting that gut microbiota manipulation represents a tool to treat lung infections and related diseases.
In addition to the hydrolysis of dietary fibers and polysaccharides by intestinal microbiota, leading to the production of short-chain fatty acid metabolites with anti-inflammatory and immunomodulatory capabilities, polyphenols emerge as powerful prebiotics (116). They can be catabolized, deglycosylated, and biotransformed in strong metabolites by gut microbiota, with evident health host benefits (28). By exerting specific effects on bacterial strains (e.g., reducing pathogens and enhancing favorable communities), polyphenols can sustain gut–lung axis integrity and provide protection against viral infections (Fig. 4). As its name suggests, SARS-CoV-2 is linked to an acute respiratory syndrome, but also to gastrointestinal (GI) symptoms (diarrhea, vomiting, nausea, abdominal pain) (247). The GI manifestations not only coexist but may also precede the respiratory events (98, 170). As normal gut microbiota restoration may influence the immune response to viral diseases and improve respiratory symptoms, polyphenols known as powerful prebiotics may represent an efficient therapeutic arsenal to fight the fatal COVID-19. This is all the more important because the gut microbiome of COVID-19 patients has been reported to be affected and may predict the severity of the disease.
POLYPHENOLS AND VIRAL INFLAMMATION
Following viral infection, it is possible to observe dysregulated host immune response and production of inflammatory cytokines. COVID-19 patients exhibit elevated concentrations of plasma IL2, IL6, IL7, IL10, granulocyte colony-stimulating factor (GCSF), IFN-γ-induced protein 10 (IP-10), monocyte chemoattractant protein 1 (MCP1), macrophage inflammatory protein 1-α (MIP1A), and TNFα (37, 101), indicating an increased output of inflammatory components. With increasing state of severity, NK and T cells run through, and the decline of their number leads to lymphopenia. In compensation and for continuing the fight against SARS-CoV-2 infection, the immune system is hyper-stimulated to produce a profusion of cytokines tapping highs, named “cytokine storm,” leading immune cells to affect healthy tissues and cause organ failure (1). Remarkably, polyphenols are known for their ability to prevent and counteract inflammation (Fig. 5) by mitigating inflammatory response in adipocytes, macrophages, and additional immune cells (129). As a polyphenolic phytochemical, curcumin can modulate dendritic cells (DCs) and transform them into tolerogenic DCs with anti-inflammatory and immunomodulatory activities in health and disease (174). Accordingly, curcumin impedes the maturation and migration of cytokines and chemokines while disturbing the antigen-presenting machinery of DCs, rendering them non- or hyporesponsive to immunostimulants (80). These alterations may be due to the downregulation of transcription factors, including NF-κB, AP-1, MAPKs (p38, JNK, ERK), and other intracellular signaling molecules such as JAK/STAT/SOCS (171). Similarly, polyphenols from tea such as the bioactive EGCG could repress the biogenesis of blood angiotensin II-associated C-reactive protein, which plays a vital role in the progression of inflammation characterizing metabolic syndrome, atherosclerosis, and hepatic diseases through the angiotensin II type 1 receptor-ROS-ERK1/2 signaling pathway (246), the modulation of Notch pathway in human macrophages (227), and the upregulation of E3 ubiquitin ligase RNF 216, followed by downregulation of toll-like receptor 4 (125). As we can therefore infer from these brief examples and a multitude of others available in the scientific literature, polyphenols may not only be applied as antiviral tools, but also as powerful anti-inflammatory agents capable of boosting antiviral response.
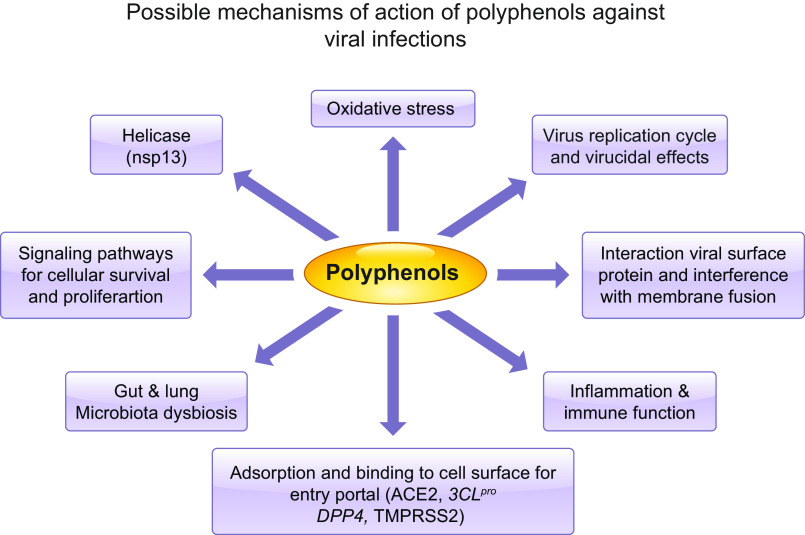
Fig. 5.
Possible mechanisms of polyphenols against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. The multiple biological properties may be actuated to counteract viral infection and related complications. 3CLpro, 3-chymotrypsin-like protease; ACE2, angiotensin-converting enzyme 2; DPP4, dipeptidyl peptidase 4; TMPRSS2, transmembrane protease serine 2.
POLYPHENOLS AND VIRAL OXIDATIVE STRESS
A molecular link is apparent between viral infection and OxS pathways (16). For instance, OxS has been induced by the respiratory syncytial virus as reflected by raised NADPH oxidase-1, an enzyme implicated in the formation of ROS, and declined endogenous antioxidant defense (e.g., catalase, glutathione S-transferase A2), likely resulting from downregulation of the nuclear factor erythroid 2-related factor 2 (Nrf2), a strong transcription factor undergoing histone deacetylation and proteasomal degradation (120). The administration of antioxidants reduced ROS generation and simultaneously lessened pulmonary inflammation and lung pathologies associated with respiratory syncytial virus infection, pointing out OxS as a causal agent (7). Accordingly, lowering NADPH oxidase 2 expression prevents NF-κB activation in respiratory syncytial virus in infected cells and drastically counteracts the induction proinflammatory cytokines and chemokines (72). Other respiratory viral infections (influenza, human metapneumovirus, parainfluenza, adenovirus, CoV, and human rhinovirus) are also associated with redox imbalance/OxS and antioxidant mechanism slackness, highly necessary for viral infection and complications (49). For their part, SARS-CoVs trigger OxS mechanisms, which contribute to the pathogenesis and progression of respiratory diseases (121). The abundant ROS production, as a result of increased inflammatory cell recruitment at the site of viral infection, is coupled with innate immunity and SARS-CoV 3CLpro-mediated NF-κB activation, leading to an intensified proinflammatory host response (136). Clearly, it was shown that OxS-NF-κB-toll-like receptor (mainly TLR4) signaling pathways are elicited by SARS-CoV to magnify host inflammatory response, thus contributing to acute lung injury. Such abnormalities can be prevented by the intake polyphenols, known as influential antioxidants capable of halting virus-driven ROS production and protecting host cells from OxS and the vicious inflammation cycle (Fig. 5). By strengthening the antioxidant capacity and scavenging ROS, polyphenols may therefore represent a promising tool to stop the negative effects of SARS-CoV-2 while ameliorating the clinical course of COVID-19.
POLYPHENOLS AND CARDIOMETABOLIC DISORDERS
Preexisting cardiometabolic ailments display poorer prognosis for the COVID-19 course. Available epidemiological information from the United States indicates that at least 25% of patients who perished following COVID-19 had obesity (233). Elevated body mass index was predictive of the need for hospitalization (169) and related to admission to the intensive care unit (134). The mechanistic link is probably the induction of inflammation by the immune system in the adipose tissue, increasing the vulnerability to develop infections (115). Notably, adipose tissue is composed of adipocytes, macrophages, endothelial cells, and lymphocytes (30), which secrete, among others, adipokines, cytokines, and chemokines with a substantial influence on surrounding tissues, allowing a potential cross-talk with the respiratory system (40). Not only may the adipose tissue serve as a pathogen reservoir for SARS-CoV-2 given its high ACE2 expression (146), but its obesity-associated inflammation likely plays a role in cardiometabolic pathogenesis and, of course, in the aggravation of viral outcomes.
In a comprehensive meta-analysis totaling 4,659 patients, additional metabolic complications (e.g., T2D, hypertension, coronary heart disease) have been noted to be closely associated with an enhanced COVID-19 death risk (213) and adverse outcomes (67). In these conditions, investigators propose that compounding SARS-CoV-2 infection and T2D provokes dysregulated immune response, which leads to aberrant immune response with exacerbated and extended lung pathology (124).
Consequently, use of polyphenols in controlling the cardiometabolic disorders (Fig. 6) appears as a biocontrol strategy to reduce the chances of viral infection and/or exaggerated adverse outcomes of SARS-CoV-2. These bioactive molecules yield beneficial effects via regulation of multiple metabolic pathways, which promote antioxidants and anti-inflammatory, antithrombotic, anti-atherogenic, and antiviral effects (Fig. 5) (147). What is more, growing evidence reveals that polyphenols reduce body weight, modify body composition, and subvert systemic and adipose tissue inflammation while ameliorating metabolic diseases (129). The constellation of risk factors of the metabolic syndrome, including dyslipidemia, insulin resistance, hypertension, and obesity, responds advantageously to polyphenols (Fig. 6) (24). Consistent antidiabetic and cardioprotective effects are observed following administration of polyphenolic flavonoid experimental models and human studies (11, 111, 214). Therefore, polyphenols may represent a promise to mitigate SARS-CoV-2-associated metabolic disorders and atherosclerosis risks, especially diabetes mellitus and cardiovascular diseases as principal comorbidities. However, it should be pointed out that despite their health-promoting effects, polyphenols have limited bioavailability, as a substantial proportion is lost during the digestive process and only a small fraction is directly absorbed by the small intestine. In fact, the bulk of polyphenols reaches the colon, where they interact with the gut microbiota and regulate microbial growth/proliferation. For their part, intracolonic microorganisms are crucial for polyphenol metabolism to produce bioactive metabolites that exert evident health benefits.
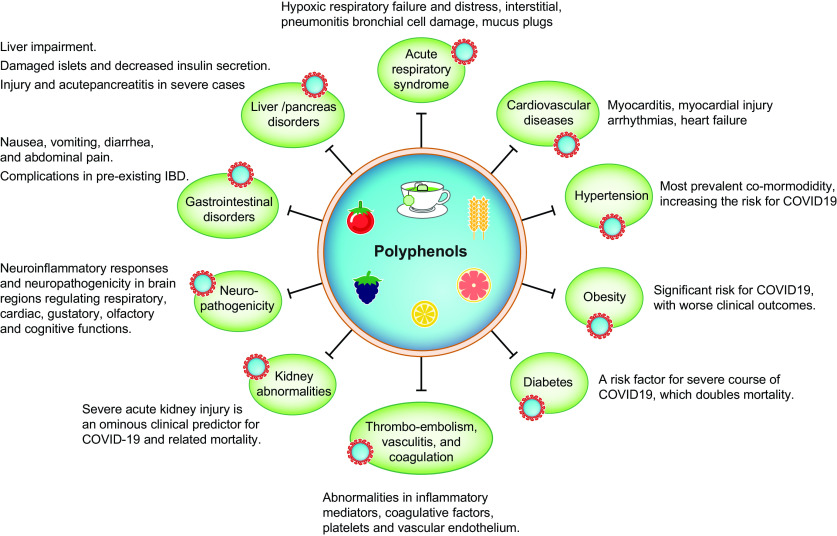
Fig. 6.
Beneficial effects of polyphenols against diverse disorders mediated by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This illustration summarizes the main health benefits of polyphenols in pathological conditions, which can be built upon and produce beneficial results in the threatening coronavirus disease (COVID-19).
SUMMARY POINTS
-
1.
The potential mechanisms for SARS-CoV-2 adhesion, entry, and replication into host cells, along with multiple related complications, have been emphasized to prospect and design treatment options.
-
2.
Given the lack of vaccine and effective antiviral agents for COVID-19, evidence is provided to propose that natural polyphenolic compounds may serve as a useful arsenal to mitigate coronaviral infection in view of their established beneficial properties and functions.
-
3.
The effectiveness of polyphenols has been first substantiated by their powerful antiviral activities, as reflected by the modulation of viral envelope proteins (namely Spike S glycoprotein instrumental for the specificity and infectivity of the virion) and host cell receptors (e.g., ACE2 functioning as a gate for SARS-CoV-2) and transmembrane proteases (e.g., TMPRSS2 mediating SARS-CoV-2 cell invasion).
-
4.
As amply supported by the scientific literature, polyphenols are endowed with the redoubtable antioxidant and anti-inflammatory actions capable of counteracting redox imbalance and inflammatory processes elicited by SARS-CoV-2 infection and accounting for enhanced patients’ susceptibility to severe organ insults.
-
5.
A plethora of evidence underlines the favorable regulation of microbiota by polyphenols, which may both amend dysbiosis triggered by invading SARS-CoV-2 and boost antiviral response, thereby turning down viral infectivity.
-
6.
Bioactive polyphenols are known to improve cardiometabolic health and alleviate the constellation of the risk factors associated with the metabolic syndrome (e.g., dyslipidemia, insulin resistance, hypertension, and obesity), which would be most helpful for preexisting cardiometabolic ailments displaying poorer prognosis for the COVID-19 course.
CONCLUSIONS
The speed and severity of the present, deadly COVID-19 pandemic took the whole world by surprise. It has caused widespread woefulness, fear, anxiety, loneliness, and economic uncertainty. This crisis is challenging health systems worldwide, forcing countries to make difficult choices, mainly because of the lack of a vaccine and effective standard treatment. In recognition of the seriousness of the SARS-CoV-2 strike and the urgency to limit the virus spread, polyphenols represent a valuable therapeutic strategy for the management of SARS-CoV-2-infected patients. Overall, from available evidence documented by the present review, polyphenols are endowed with multifaceted beneficial properties. Their antiviral, antioxidant, anti-inflammatory, antiobesogenic, antidiabetic, antithrombotic, and prebiotic effects can be put in use to combat COVID-19. At least, their potency must be tested given their ability to modulate the different molecular, metabolic, and clinical targets of SARS-CoV-2. Thorough investigation will allow for the definition of whether they may act synergistically with existing drugs against the viral infection and related complications.
FUTURE ISSUES
-
1.
The direct impact of polyphenols on SARS-CoV-2 infectivity and mechanisms of action deserves an in-depth investigation.
-
2.
Emerging animal models for coronavirus should be exploited to progress and gain insights into the protective role of polyphenols against SARS-CoV-2 entry and reproduction in host cells.
-
3.
Intensive efforts are highly needed to carry out clinical trials to test the effectiveness of polyphenols in suppressing SARS-CoV-2 replication and inflammation while reversing organ failure.
-
4.
Polyphenols will have to be comprehensively evaluated on preexisting inflammatory and cardiometabolic conditions to get COVID-19 prognosis and outcomes.
-
5.
Information is required as to the safety, stability, and bioavailability of polyphenols along with the interactions with antiviral drugs.
GRANTS
This study was supported by the Canadian Institutes of Health Research Grants (PJT 153113), NSERC (RGPIN-2016-04761), Leahy Orchards Inc., and the J. A. DeSève Research Chair in Nutrition.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.L. and S.S. prepared figures; E.L. and S.S. drafted manuscript; E.L., E.D., V.M., and S.S. edited and revised manuscript; E.L., E.D., V.M., and S.S. approved final version of the manuscript.
REFERENCES
- 1.An PJ, Zhu YZ, Yang LP. Biochemical indicators of coronavirus disease 2019 exacerbation and the clinical implications. Pharmacol Res 159: 104946, 2020. doi: 10.1016/j.phrs.2020.104946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anguiano L, Riera M, Pascual J, Soler MJ. Circulating ACE2 in cardiovascular and kidney diseases. Curr Med Chem 24: 3231–3241, 2017. doi: 10.2174/0929867324666170414162841. [DOI] [PubMed] [Google Scholar]
- 3.Anhê FF, Nachbar RT, Varin TV, Vilela V, Dudonné S, Pilon G, Fournier M, Lecours MA, Desjardins Y, Roy D, Levy E, Marette A. A polyphenol-rich cranberry extract reverses insulin resistance and hepatic steatosis independently of body weight loss. Mol Metab 6: 1563–1573, 2017. doi: 10.1016/j.molmet.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anhê FF, Pilon G, Roy D, Desjardins Y, Levy E, Marette A. Triggering Akkermansia with dietary polyphenols: a new weapon to combat the metabolic syndrome? Gut Microbes 7: 146–153, 2016. doi: 10.1080/19490976.2016.1142036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anhê FF, Roy D, Pilon G, Dudonné S, Matamoros S, Varin TV, Garofalo C, Moine Q, Desjardins Y, Levy E, Marette A. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 64: 872–883, 2015. doi: 10.1136/gutjnl-2014-307142. [DOI] [PubMed] [Google Scholar]
- 6.Anhê FF, Varin TV, Le Barz M, Desjardins Y, Levy E, Roy D, Marette A. Gut microbiota dysbiosis in obesity-linked metabolic diseases and prebiotic potential of polyphenol-rich extracts. Curr Obes Rep 4: 389–400, 2015. doi: 10.1007/s13679-015-0172-9. [DOI] [PubMed] [Google Scholar]
- 7.Antoniu SA. Infliximab for the therapy of chronic sarcoidosis. Expert Opin Investig Drugs 16: 753–756, 2007. doi: 10.1517/13543784.16.5.753. [DOI] [PubMed] [Google Scholar]
- 8.Asadi-Pooya AA, Simani L. Central nervous system manifestations of COVID-19: a systematic review. J Neurol Sci 413: 116832, 2020. doi: 10.1016/j.jns.2020.116832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ashuro AA, Lobie TA, Ye DQ, Leng RX, Li BZ, Pan HF, Fan YG. Review on the alteration of gut microbiota: the role of HIV infection and old age. AIDS Res Hum Retroviruses 36: 556–565, 2020. doi: 10.1089/aid.2019.0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avadhanula V, Rodriguez CA, Devincenzo JP, Wang Y, Webby RJ, Ulett GC, Adderson EE. Respiratory viruses augment the adhesion of bacterial pathogens to respiratory epithelium in a viral species- and cell type-dependent manner. J Virol 80: 1629–1636, 2006. doi: 10.1128/JVI.80.4.1629-1636.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Babu PV, Liu D, Gilbert ER. Recent advances in understanding the anti-diabetic actions of dietary flavonoids. J Nutr Biochem 24: 1777–1789, 2013. doi: 10.1016/j.jnutbio.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bachmetov L, Gal-Tanamy M, Shapira A, Vorobeychik M, Giterman-Galam T, Sathiyamoorthy P, Golan-Goldhirsh A, Benhar I, Tur-Kaspa R, Zemel R. Suppression of hepatitis C virus by the flavonoid quercetin is mediated by inhibition of NS3 protease activity. J Viral Hepat 19: e81–e88, 2012. doi: 10.1111/j.1365-2893.2011.01507.x. [DOI] [PubMed] [Google Scholar]
- 13.Bahgat MM, Błazejewska P, Schughart K. Inhibition of lung serine proteases in mice: a potentially new approach to control influenza infection. Virol J 8: 27, 2011. doi: 10.1186/1743-422X-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ballan R, Battistini C, Xavier-Santos D, Saad SMI. Interactions of probiotics and prebiotics with the gut microbiota. Prog Mol Biol Transl Sci 171: 265–300, 2020. doi: 10.1016/bs.pmbts.2020.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Bao H, Gao F, Xie G, Liu Z. Angiotensin-converting enzyme 2 inhibits apoptosis of pulmonary endothelial cells during acute lung injury through suppressing MiR-4262. Cell Physiol Biochem 37: 759–767, 2015. doi: 10.1159/000430393. [DOI] [PubMed] [Google Scholar]
- 16.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245–313, 2007. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 17.Belouzard S, Chu VC, Whittaker GR. Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites. Proc Natl Acad Sci USA 106: 5871–5876, 2009. doi: 10.1073/pnas.0809524106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beltz LA, Bayer DK, Moss AL, Simet IM. Mechanisms of cancer prevention by green and black tea polyphenols. Anticancer Agents Med Chem 6: 389–406, 2006. doi: 10.2174/187152006778226468. [DOI] [PubMed] [Google Scholar]
- 19.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI. The global distribution and burden of dengue. Nature 496: 504–507, 2013. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bisignano C, Mandalari G, Smeriglio A, Trombetta D, Pizzo MM, Pennisi R, Sciortino MT. Almond skin extracts abrogate HSV-1 replication by blocking virus binding to the cell. Viruses 9: 178, 2017. doi: 10.3390/v9070178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bornstein SR, Rubino F, Khunti K, Mingrone G, Hopkins D, Birkenfeld AL, Boehm B, Amiel S, Holt RI, Skyler JS, DeVries JH, Renard E, Eckel RH, Zimmet P, Alberti KG, Vidal J, Geloneze B, Chan JC, Ji L, Ludwig B. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol 8: 546–550, 2020. doi: 10.1016/S2213-8587(20)30152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Budden KF, Gellatly SL, Wood DL, Cooper MA, Morrison M, Hugenholtz P, Hansbro PM. Emerging pathogenic links between microbiota and the gut-lung axis. Nat Rev Microbiol 15: 55–63, 2017. doi: 10.1038/nrmicro.2016.142. [DOI] [PubMed] [Google Scholar]
- 23.Calland N, Sahuc ME, Belouzard S, Pène V, Bonnafous P, Mesalam AA, Deloison G, Descamps V, Sahpaz S, Wychowski C, Lambert O, Brodin P, Duverlie G, Meuleman P, Rosenberg AR, Dubuisson J, Rouillé Y, Séron K. Polyphenols inhibit hepatitis C virus entry by a new mechanism of action. J Virol 89: 10053–10063, 2015. doi: 10.1128/JVI.01473-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calvano A, Izuora K, Oh EC, Ebersole JL, Lyons TJ, Basu A. Dietary berries, insulin resistance and type 2 diabetes: an overview of human feeding trials. Food Funct 10: 6227–6243, 2019. doi: 10.1039/C9FO01426H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cani PD. Microbiota and metabolites in metabolic diseases. Nat Rev Endocrinol 15: 69–70, 2019. doi: 10.1038/s41574-018-0143-9. [DOI] [PubMed] [Google Scholar]
- 26.Cani PD, Everard A. Talking microbes: when gut bacteria interact with diet and host organs. Mol Nutr Food Res 60: 58–66, 2016. doi: 10.1002/mnfr.201500406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cardarelli HR, Martinez RC, Albrecht S, Schols H, Franco BD, Saad SM, Smidt H. In vitro fermentation of prebiotic carbohydrates by intestinal microbiota in the presence of Lactobacillus amylovorus DSM 16998. Benef Microbes 7: 119–133, 2016. doi: 10.3920/BM2014.0151. [DOI] [PubMed] [Google Scholar]
- 28.Cardona F, Andrés-Lacueva C, Tulipani S, Tinahones FJ, Queipo-Ortuño MI. Benefits of polyphenols on gut microbiota and implications in human health. J Nutr Biochem 24: 1415–1422, 2013. doi: 10.1016/j.jnutbio.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 29.Carneiro BM, Batista MN, Braga ACS, Nogueira ML, Rahal P. The green tea molecule EGCG inhibits Zika virus entry. Virology 496: 215–218, 2016. doi: 10.1016/j.virol.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 30.Caspar-Bauguil S, Cousin B, Galinier A, Segafredo C, Nibbelink M, André M, Casteilla L, Pénicaud L. Adipose tissues as an ancestral immune organ: site-specific change in obesity. FEBS Lett 579: 3487–3492, 2005. doi: 10.1016/j.febslet.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 31.Castanet M, Costalos C, Haiden N, Hascoet JM, Berger B, Sprenger N, Grathwohl D, Brüssow H, De Groot N, Steenhout P, Pecquet S, Benyacoub J, Picaud JC. Early effect of supplemented infant formulae on intestinal biomarkers and microbiota: a randomized clinical trial. Nutrients 12: 1481, 2020. doi: 10.3390/nu12051481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cavalcante MB, Saccon TD, Nunes AD, Kirkland JL, Tchkonia T, Schneider A, Masternak MM. Dasatinib plus quercetin prevents uterine age-related dysfunction and fibrosis in mice. Aging (Albany NY) 12: 2711–2722, 2020. doi: 10.18632/aging.102772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan CN, Trinité B, Levy DN. Potent inhibition of HIV-1 replication in resting CD4 T cells by resveratrol and pterostilbene. Antimicrob Agents Chemother 61: e00408-17, 2017. doi: 10.1128/AAC.00408-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang J, Warren TK, Zhao X, Gill T, Guo F, Wang L, Comunale MA, Du Y, Alonzi DS, Yu W, Ye H, Liu F, Guo JT, Mehta A, Cuconati A, Butters TD, Bavari S, Xu X, Block TM. Small molecule inhibitors of ER α-glucosidases are active against multiple hemorrhagic fever viruses. Antiviral Res 98: 432–440, 2013. doi: 10.1016/j.antiviral.2013.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen J, Liu D, Liu L, Liu P, Xu Q, Xia L, Ling Y, Huang D, Song S, Zhang D, Qian Z, Li T, Shen Y, Lu H. A pilot study of hydroxychloroquine in treatment of patients with moderate COVID-19 [in Chinese]. Zhejiang Da Xue Xue Bao Yi Xue Ban 49: 215–219, 2020. doi: 10.3785/j.issn.1008-9292.2020.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen L, Li J, Luo C, Liu H, Xu W, Chen G, Liew OW, Zhu W, Puah CM, Shen X, Jiang H. Binding interaction of quercetin-3-β-galactoside and its synthetic derivatives with SARS-CoV 3CLpro: structure-activity relationship studies reveal salient pharmacophore features. Bioorg Med Chem 14: 8295–8306, 2006. doi: 10.1016/j.bmc.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395: 507–513, 2020. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiow KH, Phoon MC, Putti T, Tan BK, Chow VT. Evaluation of antiviral activities of Houttuynia cordata Thunb. extract, quercetin, quercetrin and cinanserin on murine coronavirus and dengue virus infection. Asian Pac J Trop Med 9: 1–7, 2016. doi: 10.1016/j.apjtm.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chuanasa T, Phromjai J, Lipipun V, Likhitwitayawuid K, Suzuki M, Pramyothin P, Hattori M, Shiraki K. Anti-herpes simplex virus (HSV-1) activity of oxyresveratrol derived from Thai medicinal plant: mechanism of action and therapeutic efficacy on cutaneous HSV-1 infection in mice. Antiviral Res 80: 62–70, 2008. doi: 10.1016/j.antiviral.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 40.Chwalba A, Machura E, Ziora K, Ziora D. The role of adipokines in the pathogenesis and course of selected respiratory diseases. Endokrynol Pol 70: 504–510, 2019. doi: 10.5603/EP.a2019.0051. [DOI] [PubMed] [Google Scholar]
- 41.Clouser CL, Chauhan J, Bess MA, van Oploo JL, Zhou D, Dimick-Gray S, Mansky LM, Patterson SE. Anti-HIV-1 activity of resveratrol derivatives and synergistic inhibition of HIV-1 by the combination of resveratrol and decitabine. Bioorg Med Chem Lett 22: 6642–6646, 2012. doi: 10.1016/j.bmcl.2012.08.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohen HA, Varsano I, Kahan E, Sarrell EM, Uziel Y. Effectiveness of an herbal preparation containing echinacea, propolis, and vitamin C in preventing respiratory tract infections in children: a randomized, double-blind, placebo-controlled, multicenter study. Arch Pediatr Adolesc Med 158: 217–221, 2004. doi: 10.1001/archpedi.158.3.217. [DOI] [PubMed] [Google Scholar]
- 43.Colpitts CC, Schang LM. A small molecule inhibits virion attachment to heparan sulfate- or sialic acid-containing glycans. J Virol 88: 7806–7817, 2014. doi: 10.1128/JVI.00896-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cortegiani A, Ippolito M, Ingoglia G, Iozzo P, Giarratano A, Einav S. Update I. A systematic review on the efficacy and safety of chloroquine/hydroxychloroquine for COVID-19. J Crit Care 59: 176–190, 2020. doi: 10.1016/j.jcrc.2020.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Costanzo M, De Giglio MAR, Roviello GN. SARS-CoV-2: recent reports on antiviral therapies based on lopinavir/ritonavir, darunavir/umifenovir, hydroxychloroquine, remdesivir, favipiravir and other drugs for the treatment of the new coronavirus. Curr Med Chem 27: 4536–4541, 2020. doi: 10.2174/0929867327666200416131117. [DOI] [PubMed] [Google Scholar]
- 46.Dalan R. Is DPP4 inhibition a comrade or adversary in COVID-19 infection. Diabetes Res Clin Pract 164: 108216, 2020. doi: 10.1016/j.diabres.2020.108216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Date AA, Destache CJ. Natural polyphenols: potential in the prevention of sexually transmitted viral infections. Drug Discov Today 21: 333–341, 2016. doi: 10.1016/j.drudis.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 48.de Haan CA, Kuo L, Masters PS, Vennema H, Rottier PJ. Coronavirus particle assembly: primary structure requirements of the membrane protein. J Virol 72: 6838–6850, 1998. doi: 10.1128/JVI.72.8.6838-6850.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Delgado-Roche L, Mesta F. Oxidative stress as key player in severe acute respiratory syndrome coronavirus (SARS-CoV) infection. Arch Med Res 51: 384–387, 2020. doi: 10.1016/j.arcmed.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Delzenne NM, Bindels LB. Food for thought about manipulating gut bacteria. Nature 577: 32–34, 2020. doi: 10.1038/d41586-019-03704-z. [DOI] [PubMed] [Google Scholar]
- 51.Denis MC, Desjardins Y, Furtos A, Marcil V, Dudonné S, Montoudis A, Garofalo C, Delvin E, Marette A, Levy E. Prevention of oxidative stress, inflammation and mitochondrial dysfunction in the intestine by different cranberry phenolic fractions. Clin Sci (Lond) 128: 197–212, 2015. doi: 10.1042/CS20140210. [DOI] [PubMed] [Google Scholar]
- 52.Deriu E, Boxx GM, He X, Pan C, Benavidez SD, Cen L, Rozengurt N, Shi W, Cheng G. Influenza virus affects intestinal microbiota and secondary Salmonella infection in the gut through type I Interferons. PLoS Pathog 12: e1005572, 2016. doi: 10.1371/journal.ppat.1005572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Desforges M, Le Coupanec A, Dubeau P, Bourgouin A, Lajoie L, Dubé M, Talbot PJ. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses 12: 14, 2019. doi: 10.3390/v12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dickson RP. The microbiome and critical illness. Lancet Respir Med 4: 59–72, 2016. doi: 10.1016/S2213-2600(15)00427-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dilantika C, Sedyaningsih ER, Kasper MR, Agtini M, Listiyaningsih E, Uyeki TM, Burgess TH, Blair PJ, Putnam SD. Influenza virus infection among pediatric patients reporting diarrhea and influenza-like illness. BMC Infect Dis 10: 3, 2010. doi: 10.1186/1471-2334-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ding L, Jiang P, Xu X, Lu W, Yang C, Zhou P, Liu S. Resveratrol promotes HSV-2 replication by increasing histone acetylation and activating NF-κB. Biochem Pharmacol 171: 113691, 2020. doi: 10.1016/j.bcp.2019.113691. [DOI] [PubMed] [Google Scholar]
- 57.Ding S, Jiang H, Fang J. Regulation of immune function by polyphenols. J Immunol Res 2018: 1264074, 2018. doi: 10.1155/2018/1264074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Disney GH, Everett RD. A herpes simplex virus type 1 recombinant with both copies of the Vmw175 coding sequences replaced by the homologous varicella-zoster virus open reading frame. J Gen Virol 71: 2681–2689, 1990. doi: 10.1099/0022-1317-71-11-2681. [DOI] [PubMed] [Google Scholar]
- 59.Docherty JJ, Fu MM, Stiffler BS, Limperos RJ, Pokabla CM, DeLucia AL. Resveratrol inhibition of herpes simplex virus replication. Antiviral Res 43: 145–155, 1999. doi: 10.1016/S0166-3542(99)00042-X. [DOI] [PubMed] [Google Scholar]
- 60.Docherty JJ, Sweet TJ, Bailey E, Faith SA, Booth T. Resveratrol inhibition of varicella-zoster virus replication in vitro. Antiviral Res 72: 171–177, 2006. doi: 10.1016/j.antiviral.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 61.Domínguez Avila JA, Rodrigo García J, González Aguilar GA, de la Rosa LA. The antidiabetic mechanisms of polyphenols related to increased glucagon-like peptide-1 (GLP1) and insulin signaling. Molecules 22: 903, 2017. doi: 10.3390/molecules22060903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Drucker DJ. Coronavirus infections and type 2 diabetes-shared pathways with therapeutic implications. Endocr Rev 41: 457–470, 2020. doi: 10.1210/endrev/bnaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dumas A, Bernard L, Poquet Y, Lugo-Villarino G, Neyrolles O. The role of the lung microbiota and the gut-lung axis in respiratory infectious diseases. Cell Microbiol 20: e12966, 2018. doi: 10.1111/cmi.12966. [DOI] [PubMed] [Google Scholar]
- 64.Dyer KD, Drummond RA, Rice TA, Percopo CM, Brenner TA, Barisas DA, Karpe KA, Moore ML, Rosenberg HF. Priming of the respiratory tract with immunobiotic Lactobacillus plantarum limits infection of alveolar macrophages with recombinant pneumonia virus of mice (rK2-PVM). J Virol 90: 979–991, 2015. doi: 10.1128/JVI.02279-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Engel M, Hoffmann T, Wagner L, Wermann M, Heiser U, Kiefersauer R, Huber R, Bode W, Demuth HU, Brandstetter H. The crystal structure of dipeptidyl peptidase IV (CD26) reveals its functional regulation and enzymatic mechanism. Proc Natl Acad Sci USA 100: 5063–5068, 2003. doi: 10.1073/pnas.0230620100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Espinoza JL, Takami A, Trung LQ, Kato S, Nakao S. Resveratrol prevents EBV transformation and inhibits the outgrowth of EBV-immortalized human B cells. PLoS One 7: e51306, 2012. doi: 10.1371/journal.pone.0051306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fadini GP, Morieri ML, Longato E, Avogaro A. Prevalence and impact of diabetes among people infected with SARS-CoV-2. J Endocrinol Invest 43: 867–869, 2020. doi: 10.1007/s40618-020-01236-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fan Z, Wu G, Yue M, Ye J, Chen Y, Xu B, Shu Z, Zhu J, Lu N, Tan X. Hypertension and hypertensive left ventricular hypertrophy are associated with ACE2 genetic polymorphism. Life Sci 225: 39–45, 2019. doi: 10.1016/j.lfs.2019.03.059. [DOI] [PubMed] [Google Scholar]
- 69.Fang Y, Gao F, Liu Z. Angiotensin-converting enzyme 2 attenuates inflammatory response and oxidative stress in hyperoxic lung injury by regulating NF-κB and Nrf2 pathways. QJM 112: 914–924, 2019. doi: 10.1093/qjmed/hcz206. [DOI] [PubMed] [Google Scholar]
- 70.Feldman AS, He Y, Moore ML, Hershenson MB, Hartert TV. Toward primary prevention of asthma. Reviewing the evidence for early-life respiratory viral infections as modifiable risk factors to prevent childhood asthma. Am J Respir Crit Care Med 191: 34–44, 2015. doi: 10.1164/rccm.201405-0901PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ferner RE, Aronson JK. Chloroquine and hydroxychloroquine in covid-19. BMJ 369: m1432, 2020. doi: 10.1136/bmj.m1432. [DOI] [PubMed] [Google Scholar]
- 72.Fink K, Duval A, Martel A, Soucy-Faulkner A, Grandvaux N. Dual role of NOX2 in respiratory syncytial virus- and Sendai virus-induced activation of NF-κB in airway epithelial cells. J Immunol 180: 6911–6922, 2008. doi: 10.4049/jimmunol.180.10.6911. [DOI] [PubMed] [Google Scholar]
- 73.Fix J, Chandrashekhar K, Perez J, Bucardo F, Hudgens MG, Yuan L, Twitchell E, Azcarate-Peril MA, Vilchez S, Becker-Dreps S. Association between gut microbiome composition and rotavirus vaccine response among Nicaraguan infants. Am J Trop Med Hyg 102: 213–219, 2020. doi: 10.4269/ajtmh.19-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Galiev VZ. Fight of revolutionist physicians against hunger and cholera in Kazakhstan (1891-1892) [in Russian]. Sov Zdravookhr 4: 62–65, 1976. [PubMed] [Google Scholar]
- 75.Gangehei L, Ali M, Zhang W, Chen Z, Wakame K, Haidari M. Oligonol a low molecular weight polyphenol of lychee fruit extract inhibits proliferation of influenza virus by blocking reactive oxygen species-dependent ERK phosphorylation. Phytomedicine 17: 1047–1056, 2010. doi: 10.1016/j.phymed.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 76.Gao J, Tian Z, Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends 14: 72–73, 2020. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 77.George I, Salna M, Kobsa S, Deroo S, Kriegel J, Blitzer D, Shea NJ, D’Angelo A, Raza T, Kurlansky P, Takeda K, Takayama H, Bapat V, Naka Y, Smith CR, Bacha E, Argenziano M. The rapid transformation of cardiac surgery practice in the coronavirus disease 2019 (COVID-19) pandemic: Insights and clinical strategies from a center at the epicenter. J Thorac Cardiovasc Surg.;S0022-5223(20)31012-6, 2020 (Epub Ahead of Print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong JC, Turner AJ, Raizada MK, Grant MB, Oudit GY. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res 126: 1456–1474, 2020. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Godfrey DI, Kennedy J, Suda T, Zlotnik A. A developmental pathway involving four phenotypically and functionally distinct subsets of CD3-CD4-CD8- triple-negative adult mouse thymocytes defined by CD44 and CD25 expression. J Immunol 189: 4203–4211, 2012. [PubMed] [Google Scholar]
- 80.Gontijo VS, Viegas FP, Ortiz CJ, de Freitas Silva M, Damasio CM, Rosa MC, Campos TG, Couto DS, Tranches Dias KS, Viegas C. Molecular hybridization as a tool in the design of multi-target directed drug candidates for neurodegenerative diseases. Curr Neuropharmacol 18: 348–407, 2020. doi: 10.2174/1385272823666191021124443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gu H, Xie Z, Li T, Zhang S, Lai C, Zhu P, Wang K, Han L, Duan Y, Zhao Z, Yang X, Xing L, Zhang P, Wang Z, Li R, Yu JJ, Wang X, Yang P. Angiotensin-converting enzyme 2 inhibits lung injury induced by respiratory syncytial virus. Sci Rep 6: 19840, 2016. doi: 10.1038/srep19840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gu L, Deng H, Ren Z, Zhao Y, Yu S, Guo Y, Dai J, Chen X, Li K, Li R, Wang G. Dynamic changes in the microbiome and mucosal immune microenvironment of the lower respiratory tract by influenza virus infection. Front Microbiol 10: 2491, 2019. doi: 10.3389/fmicb.2019.02491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DS, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19 . Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382: 1708–1720, 2020. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hadinegoro SR, Arredondo-García JL, Capeding MR, Deseda C, Chotpitayasunondh T, Dietze R, Muhammad Ismail HI, Reynales H, Limkittikul K, Rivera-Medina DM, Tran HN, Bouckenooghe A, Chansinghakul D, Cortés M, Fanouillere K, Forrat R, Frago C, Gailhardou S, Jackson N, Noriega F, Plennevaux E, Wartel TA, Zambrano B, Saville M; CYD-TDV Dengue Vaccine Working Group . Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N Engl J Med 373: 1195–1206, 2015. doi: 10.1056/NEJMoa1506223. [DOI] [PubMed] [Google Scholar]
- 85.Hagemeijer MC, Verheije MH, Ulasli M, Shaltiël IA, de Vries LA, Reggiori F, Rottier PJ, de Haan CA. Dynamics of coronavirus replication-transcription complexes. J Virol 84: 2134–2149, 2010. doi: 10.1128/JVI.01716-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Haigis MC, Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol 5: 253–295, 2010. doi: 10.1146/annurev.pathol.4.110807.092250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hamilton-Miller JM. Anti-cariogenic properties of tea (Camellia sinensis). J Med Microbiol 50: 299–302, 2001. doi: 10.1099/0022-1317-50-4-299. [DOI] [PubMed] [Google Scholar]
- 88.Han YS, Penthala NR, Oliveira M, Mesplède T, Xu H, Quan Y, Crooks PA, Wainberg MA. Identification of resveratrol analogs as potent anti-dengue agents using a cell-based assay. J Med Virol 89: 397–407, 2017. doi: 10.1002/jmv.24660. [DOI] [PubMed] [Google Scholar]
- 89.Hartman WR, Hess AS, Connor JP. Hospitalized COVID-19 patients treated with convalescent plasma in a mid-size city in the Midwest (Preprint). bioRxiv 2020.06.19.20135830, 2020. doi: 10.1101/2020.06.19.20135830. [DOI] [PMC free article] [PubMed]
- 90.He L, Zhang E, Shi J, Li X, Zhou K, Zhang Q, Le AD, Tang X. (–)-Epigallocatechin-3-gallate inhibits human papillomavirus (HPV)-16 oncoprotein-induced angiogenesis in non-small cell lung cancer cells by targeting HIF-1α. Cancer Chemother Pharmacol 71: 713–725, 2013. doi: 10.1007/s00280-012-2063-z. [DOI] [PubMed] [Google Scholar]
- 91.He W, Li LX, Liao QJ, Liu CL, Chen XL. Epigallocatechin gallate inhibits HBV DNA synthesis in a viral replication - inducible cell line. World J Gastroenterol 17: 1507–1514, 2011. doi: 10.3748/wjg.v17.i11.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hendley JO, Hayden FG, Winther B. Weekly point prevalence of Streptococcus pneumoniae, Hemophilus influenzae and Moraxella catarrhalis in the upper airways of normal young children: effect of respiratory illness and season. APMIS 113: 213–220, 2005. doi: 10.1111/j.1600-0463.2005.apm1130310.x. [DOI] [PubMed] [Google Scholar]
- 93.Heredia A, Davis C, Amin MN, Le NM, Wainberg MA, Oliveira M, Deeks SG, Wang LX, Redfield RR. Targeting host nucleotide biosynthesis with resveratrol inhibits emtricitabine-resistant HIV-1. AIDS 28: 317–323, 2014. doi: 10.1097/QAD.0000000000000168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Heurich A, Hofmann-Winkler H, Gierer S, Liepold T, Jahn O, Pöhlmann S. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J Virol 88: 1293–1307, 2014. doi: 10.1128/JVI.02202-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hickson LJ, Langhi Prata LGP, Bobart SA, Evans TK, Giorgadze N, Hashmi SK, Herrmann SM, Jensen MD, Jia Q, Jordan KL, Kellogg TA, Khosla S, Koerber DM, Lagnado AB, Lawson DK, LeBrasseur NK, Lerman LO, McDonald KM, McKenzie TJ, Passos JF, Pignolo RJ, Pirtskhalava T, Saadiq IM, Schaefer KK, Textor SC, Victorelli SG, Volkman TL, Xue A, Wentworth MA, Wissler Gerdes EO, Zhu Y, Tchkonia T, Kirkland JL. Senolytics decrease senescent cells in humans: preliminary report from a clinical trial of dasatinib plus quercetin in individuals with diabetic kidney disease. EBioMedicine 47: 446–456, 2019. [Erratum in EBio Medicine 52: 102595, 2020]. doi: 10.1016/j.ebiom.2019.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hiel S, Gianfrancesco MA, Rodriguez J, Portheault D, Leyrolle Q, Bindels LB, Gomes da Silveira Cauduro C, Mulders MD, Zamariola G, Azzi AS, Kalala G, Pachikian BD, Amadieu C, Neyrinck AM, Loumaye A, Cani PD, Lanthier N, Trefois P, Klein O, Luminet O, Bindelle J, Paquot N, Cnop M, Thissen JP, Delzenne NM. Link between gut microbiota and health outcomes in inulin -treated obese patients: lessons from the Food4Gut multicenter randomized placebo-controlled trial. Clin Nutr.; S0261-5614(20)30160-6. In press. doi: 10.1016/j.clnu.2020.04.005. [DOI] [PubMed] [Google Scholar]
- 97.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181: 271–280.e8, 2020. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, Spitters C, Ericson K, Wilkerson S, Tural A, Diaz G, Cohn A, Fox L, Patel A, Gerber SI, Kim L, Tong S, Lu X, Lindstrom S, Pallansch MA, Weldon WC, Biggs HM, Uyeki TM, Pillai SK; Washington State 2019-nCoV Case Investigation Team . First Case of 2019 Novel Coronavirus in the United States. N Engl J Med 382: 929–936, 2020. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hopsu-Havu VK, Glenner GG. A new dipeptide naphthylamidase hydrolyzing glycyl-prolyl-β-naphthylamide. Histochemie 7: 197–201, 1966. doi: 10.1007/BF00577838. [DOI] [PubMed] [Google Scholar]
- 100.Hu HL, Forsey RJ, Blades TJ, Barratt ME, Parmar P, Powell JR. Antioxidants may contribute in the fight against ageing: an in vitro model. Mech Ageing Dev 121: 217–230, 2001. doi: 10.1016/S0047-6374(00)00212-8. [DOI] [PubMed] [Google Scholar]
- 101.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395: 497–506, 2020. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hwang JS, Choi HS, Ham SA, Yoo T, Lee WJ, Paek KS, Seo HG. Deacetylation-mediated interaction of SIRT1-HMGB1 improves survival in a mouse model of endotoxemia. Sci Rep 5: 15971, 2015. doi: 10.1038/srep15971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hyde ER, Petrosino JF, Piedra PA, Camargo CA Jr, Espinola JA, Mansbach JM. Nasopharyngeal Proteobacteria are associated with viral etiology and acute wheezing in children with severe bronchiolitis. J Allergy Clin Immunol 133: 1220–1222, 2014. doi: 10.1016/j.jaci.2013.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ikuta K, Hashimoto K, Kaneko H, Mori S, Ohashi K, Suzutani T. Anti-viral and anti-bacterial activities of an extract of blackcurrants (Ribes nigrum L.). Microbiol Immunol 56: 805–809, 2012. doi: 10.1111/j.1348-0421.2012.00510.x. [DOI] [PubMed] [Google Scholar]
- 105.Imai Y, Kuba K, Ohto-Nakanishi T, Penninger JM. Angiotensin-converting enzyme 2 (ACE2) in disease pathogenesis. Circ J 74: 405–410, 2010. doi: 10.1253/circj.CJ-10-0045. [DOI] [PubMed] [Google Scholar]
- 106.Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, Yang P, Sarao R, Wada T, Leong-Poi H, Crackower MA, Fukamizu A, Hui CC, Hein L, Uhlig S, Slutsky AS, Jiang C, Penninger JM. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 436: 112–116, 2005. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Inoue T, Nakayama J, Moriya K, Kawaratani H, Momoda R, Ito K, Iio E, Nojiri S, Fujiwara K, Yoneda M, Yoshiji H, Tanaka Y. Gut dysbiosis associated with hepatitis C virus infection. Clin Infect Dis 67: 869–877, 2018. doi: 10.1093/cid/ciy205. [DOI] [PubMed] [Google Scholar]
- 108.Ismail NA, Jusoh SA. Molecular docking and molecular dynamics simulation studies to predict flavonoid binding on the surface of DENV2 E protein. Interdiscip Sci 9: 499–511, 2017. doi: 10.1007/s12539-016-0157-8. [DOI] [PubMed] [Google Scholar]
- 109.Jang IA, Kim EN, Lim JH, Kim MY, Ban TH, Yoon HE, Park CW, Chang YS, Choi BS. Effects of resveratrol on the renin-angiotensin system in the aging kidney. Nutrients 10: 1741, 2018. doi: 10.3390/nu10111741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jasso-Miranda C, Herrera-Camacho I, Flores-Mendoza LK, Dominguez F, Vallejo-Ruiz V, Sanchez-Burgos GG, Pando-Robles V, Santos-Lopez G, Reyes-Leyva J. Antiviral and immunomodulatory effects of polyphenols on macrophages infected with dengue virus serotypes 2 and 3 enhanced or not with antibodies. Infect Drug Resist 12: 1833–1852, 2019. doi: 10.2147/IDR.S210890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jennings A, Welch AA, Spector T, Macgregor A, Cassidy A. Intakes of anthocyanins and flavones are associated with biomarkers of insulin resistance and inflammation in women. J Nutr 144: 202–208, 2014. doi: 10.3945/jn.113.184358. [DOI] [PubMed] [Google Scholar]
- 112.Jo S, Kim S, Shin DH, Kim MS. Inhibition of SARS-CoV 3CL protease by flavonoids. J Enzyme Inhib Med Chem 35: 145–151, 2020. doi: 10.1080/14756366.2019.1690480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kaihatsu K, Yamabe M, Ebara Y. Antiviral mechanism of action of epigallocatechin-3-O-gallate and its fatty acid esters. Molecules 23: 2475, 2018. doi: 10.3390/molecules23102475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kanmani P, Clua P, Vizoso-Pinto MG, Rodriguez C, Alvarez S, Melnikov V, Takahashi H, Kitazawa H, Villena J. Respiratory commensal bacteria Corynebacterium pseudodiphtheriticum improves resistance of infant mice to respiratory syncytial virus and Streptococcus pneumoniae superinfection. Front Microbiol 8: 1613, 2017. doi: 10.3389/fmicb.2017.01613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kassir R. Risk of COVID-19 for patients with obesity. Obes Rev 21: e13034, 2020. doi: 10.1111/obr.13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kawabata K, Yoshioka Y, Terao J. Role of intestinal microbiota in the bioavailability and physiological functions of dietary polyphenols. Molecules 24: 370, 2019. doi: 10.3390/molecules24020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Khailany RA, Safdar M, Ozaslan M. Genomic characterization of a novel SARS-CoV-2. Gene Rep 19: 100682, 2020. doi: 10.1016/j.genrep.2020.100682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kim EN, Kim MY, Lim JH, Kim Y, Shin SJ, Park CW, Kim YS, Chang YS, Yoon HE, Choi BS. The protective effect of resveratrol on vascular aging by modulation of the renin-angiotensin system. Atherosclerosis 270: 123–131, 2018. doi: 10.1016/j.atherosclerosis.2018.01.043. [DOI] [PubMed] [Google Scholar]
- 119.Kim TH, Kim MK, Cheong YH, Chae YN, Lee Y, Ka SO, Jung IH, Shin CY, Bae EJ, Son MH. Hepatic role in an early glucose-lowering effect by a novel dipeptidyl peptidase 4 inhibitor, evogliptin, in a rodent model of type 2 diabetes. Eur J Pharmacol 771: 65–76, 2016. doi: 10.1016/j.ejphar.2015.11.029. [DOI] [PubMed] [Google Scholar]
- 120.Komaravelli N, Tian B, Ivanciuc T, Mautemps N, Brasier AR, Garofalo RP, Casola A. Respiratory syncytial virus infection down-regulates antioxidant enzyme expression by triggering deacetylation-proteasomal degradation of Nrf2. Free Radic Biol Med 88: 391–403, 2015. doi: 10.1016/j.freeradbiomed.2015.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kovács T, Mikó E, Ujlaki G, Sári Z, Bai P. The microbiome as a component of the tumor microenvironment. Adv Exp Med Biol 1225: 137–153, 2020. doi: 10.1007/978-3-030-35727-6_10. [DOI] [PubMed] [Google Scholar]
- 122.Koyuncu E, Budayeva HG, Miteva YV, Ricci DP, Silhavy TJ, Shenk T, Cristea IM. Sirtuins are evolutionarily conserved viral restriction factors. MBio 5: e02249-14, 2014. doi: 10.1128/mBio.02249-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, Slutsky AS, Liu D, Qin C, Jiang C, Penninger JM. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med 11: 875–879, 2005. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kulcsar KA, Coleman CM, Beck SE, Frieman MB. Comorbid diabetes results in immune dysregulation and enhanced disease severity following MERS-CoV infection. JCI Insight 4: e13177, 2019. doi: 10.1172/jci.insight.131774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kumazoe M, Nakamura Y, Yamashita M, Suzuki T, Takamatsu K, Huang Y, Bae J, Yamashita S, Murata M, Yamada S, Shinoda Y, Yamaguchi W, Toyoda Y, Tachibana H. Green tea polyphenol epigallocatechin-3-gallate suppresses Toll-like receptor 4 expression via up-regulation of E3 ubiquitin-protein ligase RNF216. J Biol Chem 292: 4077–4088, 2017. doi: 10.1074/jbc.M116.755959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Langille MG, Meehan CJ, Koenig JE, Dhanani AS, Rose RA, Howlett SE, Beiko RG. Microbial shifts in the aging mouse gut. Microbiome 2: 50, 2014. doi: 10.1186/s40168-014-0050-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lee S, Mailar K, Kim MI, Park M, Kim J, Min DH, Heo TH, Bae SK, Choi W, Lee C. Plant-derived purification, chemical synthesis, and in vitro/in vivo evaluation of a resveratrol dimer, viniferin, as an HCV replication inhibitor. Viruses 11: 890, 2019. doi: 10.3390/v11100890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lenzer J. Covid-19: US gives emergency approval to hydroxychloroquine despite lack of evidence. BMJ 369: m1335, 2020. doi: 10.1136/bmj.m1335. [DOI] [PubMed] [Google Scholar]
- 129.Li D, Zhang T, Lu J, Peng C, Lin L. Natural constituents from food sources as therapeutic agents for obesity and metabolic diseases targeting adipose tissue inflammation. Crit Rev Food Sci Nutr 28: 1–19, 2020. doi: 10.1080/10408398.2020.1768044. [DOI] [PubMed] [Google Scholar]
- 130.Li H, Wang YM, Xu JY, Cao B. Potential antiviral therapeutics for 2019 Novel Coronavirus [in Chinese]. Zhonghua Jie He He Hu Xi Za Zhi 43: 170–172, 2020. doi: 10.3760/cma.j.issn.1001-0939.2020.03.004. [DOI] [PubMed] [Google Scholar]
- 131.Li S, Hattori T, Kodama EN. Epigallocatechin gallate inhibits the HIV reverse transcription step. Antivir Chem Chemother 21: 239–243, 2011. doi: 10.3851/IMP1774. [DOI] [PubMed] [Google Scholar]
- 132.Li Y, Yao J, Han C, Yang J, Chaudhry MT, Wang S, Liu H, Yin Y. Quercetin, Inflammation and Immunity. Nutrients 8: 167, 2016. doi: 10.3390/nu8030167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol 92: 552–555, 2020. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lighter J, Phillips M, Hochman S, Sterling S, Johnson D, Francois F, Stachel A. Obesity in patients younger than 60 years is a risk factor for Covid-19 hospital admission. Clin Infect Dis 71: 896–897, 2020. doi: 10.1093/cid/ciaa415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lin CJ, Lin HJ, Chen TH, Hsu YA, Liu CS, Hwang GY, Wan L. Polygonum cuspidatum and its active components inhibit replication of the influenza virus through toll-like receptor 9-induced interferon beta expression. PLoS One 10: e0117602, 2015. [Erratum in PLoS One 10: e0125288, 2015]. doi: 10.1371/journal.pone.0117602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lin CW, Lin KH, Hsieh TH, Shiu SY, Li JY. Severe acute respiratory syndrome coronavirus 3C-like protease-induced apoptosis. FEMS Immunol Med Microbiol 46: 375–380, 2006. doi: 10.1111/j.1574-695X.2006.00045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Liu C, Zhou Q, Li Y, Garner LV, Watkins SP, Carter LJ, Smoot J, Gregg AC, Daniels AD, Jervey S, Albaiu D. Research and development on therapeutic agents and vaccines for COVID-19 and related human coronavirus diseases. ACS Cent Sci 6: 315–331, 2020. doi: 10.1021/acscentsci.0c00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Liu T, Zang N, Zhou N, Li W, Xie X, Deng Y, Ren L, Long X, Li S, Zhou L, Zhao X, Tu W, Wang L, Tan B, Liu E. Resveratrol inhibits the TRIF-dependent pathway by upregulating sterile alpha and armadillo motif protein, contributing to anti-inflammatory effects after respiratory syncytial virus infection. J Virol 88: 4229–4236, 2014. doi: 10.1128/JVI.03637-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Long X, Xie J, Zhao K, Li W, Tang W, Chen S, Zang N, Ren L, Deng Y, Xie X, Wang L, Fu Z, Liu E. NK cells contribute to persistent airway inflammation and AHR during the later stage of RSV infection in mice. Med Microbiol Immunol (Berl) 205: 459–470, 2016. doi: 10.1007/s00430-016-0459-9. [DOI] [PubMed] [Google Scholar]
- 140.Looft T, Allen HK. Collateral effects of antibiotics on mammalian gut microbiomes. Gut Microbes 3: 463–467, 2012. doi: 10.4161/gmic.21288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lu JW, Hsieh PS, Lin CC, Hu MK, Huang SM, Wang YM, Liang CY, Gong Z, Ho YJ. Synergistic effects of combination treatment using EGCG and suramin against the chikungunya virus. Biochem Biophys Res Commun 491: 595–602, 2017. doi: 10.1016/j.bbrc.2017.07.157. [DOI] [PubMed] [Google Scholar]
- 142.Lu Y, Li Y, Deng W, Liu M, He Y, Huang L, Lv M, Li J, Du H. Symptomatic Infection is associated with prolonged duration of viral shedding in mild coronavirus disease 2019: a retrospective study of 110 children in Wuhan. Pediatr Infect Dis J 39: e95–e99, 2020. doi: 10.1097/INF.0000000000002729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lv Q, Zhang P, Quan P, Cui M, Liu T, Yin Y, Chi G. Quercetin, a pneumolysin inhibitor, protects mice against Streptococcus pneumoniae infection. Microb Pathog 140: 103934, 2020. doi: 10.1016/j.micpath.2019.103934. [DOI] [PubMed] [Google Scholar]
- 144.Machhi J, Herskovitz J, Senan AM, Dutta D, Nath B, Oleynikov MD, Blomberg WR, Meigs DD, Hasan M, Patel M, Kline P, Chang RC, Chang L, Gendelman HE, Kevadiya BD. The natural history, pathobiology, and clinical manifestations of SARS-CoV-2 Infections. J Neuroimmune Pharmacol 22: 1–28, 2020. doi: 10.1007/s11481-020-09944-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Madan JC, Koestler DC, Stanton BA, Davidson L, Moulton LA, Housman ML, Moore JH, Guill MF, Morrison HG, Sogin ML, Hampton TH, Karagas MR, Palumbo PE, Foster JA, Hibberd PL, O’Toole GA. Serial analysis of the gut and respiratory microbiome in cystic fibrosis in infancy: interaction between intestinal and respiratory tracts and impact of nutritional exposures. MBio 3: e00251-12, 2012. doi: 10.1128/mBio.00251-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Magrone T, Magrone M, Jirillo E. Focus on receptors for coronaviruses with special reference to angiotensin-converting enzyme 2 as a potential drug target—a perspective. Endocr Metab Immune Disord Drug Targets 20: 807–811, 2020. doi: 10.2174/1871530320666200427112902. [DOI] [PubMed] [Google Scholar]
- 147.Mancini E, Beglinger C, Drewe J, Zanchi D, Lang UE, Borgwardt S. Green tea effects on cognition, mood and human brain function: a systematic review. Phytomedicine 34: 26–37, 2017. doi: 10.1016/j.phymed.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 148.Marsland BJ, Trompette A, Gollwitzer ES. The gut-lung axis in respiratory disease. Ann Am Thorac Soc 12, Suppl 2: S150–S156, 2015. doi: 10.1513/AnnalsATS.201503-133AW. [DOI] [PubMed] [Google Scholar]
- 149.McNamee LA, Harmsen AG. Both influenza-induced neutrophil dysfunction and neutrophil-independent mechanisms contribute to increased susceptibility to a secondary Streptococcus pneumoniae infection. Infect Immun 74: 6707–6721, 2006. doi: 10.1128/IAI.00789-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Millet JK, Whittaker GR. Host cell proteases: critical determinants of coronavirus tropism and pathogenesis. Virus Res 202: 120–134, 2015. doi: 10.1016/j.virusres.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Min F, Gao F, Li Q, Liu Z. Therapeutic effect of human umbilical cord mesenchymal stem cells modified by angiotensin-converting enzyme 2 gene on bleomycin-induced lung fibrosis injury. Mol Med Rep 11: 2387–2396, 2015. doi: 10.3892/mmr.2014.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mirabelli M, Chiefari E, Arcidiacono B, Corigliano DM, Brunetti FS, Maggisano V, Russo D, Foti DP, Brunetti A. Mediterranean diet nutrients to turn the tide against insulin resistance and related diseases. Nutrients 12: 1066, 2020. doi: 10.3390/nu12041066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mohd A, Zainal N, Tan KK, AbuBakar S. Resveratrol affects Zika virus replication in vitro. Sci Rep 9: 14336, 2019. doi: 10.1038/s41598-019-50674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Moradpour D, Penin F, Rice CM. Replication of hepatitis C virus. Nat Rev Microbiol 5: 453–463, 2007. doi: 10.1038/nrmicro1645. [DOI] [PubMed] [Google Scholar]
- 155.Morissette A, Kropp C, Songpadith JP, Junges Moreira R, Costa J, Mariné-Casadó R, Pilon G, Varin TV, Dudonné S, Boutekrabt L, St-Pierre P, Levy E, Roy D, Desjardins Y, Raymond F, Houde VP, Marette A. Blueberry proanthocyanidins and anthocyanins improve metabolic health through a gut microbiota-dependent mechanism in diet-induced obese mice. Am J Physiol Endocrinol Metab 318: E965–E980, 2020. doi: 10.1152/ajpendo.00560.2019. [DOI] [PubMed] [Google Scholar]
- 156.Mu C, Yang Y, Zhu W. Crosstalk between the immune receptors and gut microbiota. Curr Protein Pept Sci 16: 622–631, 2015. doi: 10.2174/1389203716666150630134356. [DOI] [PubMed] [Google Scholar]
- 157.Multicenter collaboration group of Department of S, Technology of Guangdong P, and Health Commission of Guangdong Province for chloroquine in the treatment of novel coronavirus pneumonia Expert consensus on chloroquine phosphate for the treatment of novel coronavirus pneumonia [in Chinese] Zhonghua Jie He He Hu Xi Za Zhi 43: 185–188 , 2020. doi: 10.3760/cma.j.issn.1001-0939.2020.03.009. [DOI] [PubMed] [Google Scholar]
- 158.Musarra-Pizzo M, Ginestra G, Smeriglio A, Pennisi R, Sciortino MT, Mandalari G. The antimicrobial and antiviral activity of polyphenols from almond (Prunus dulcis L.) skin. Nutrients 11: 2355, 2019. doi: 10.3390/nu11102355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Neuman BW, Kiss G, Kunding AH, Bhella D, Baksh MF, Connelly S, Droese B, Klaus JP, Makino S, Sawicki SG, Siddell SG, Stamou DG, Wilson IA, Kuhn P, Buchmeier MJ. A structural analysis of M protein in coronavirus assembly and morphology. J Struct Biol 174: 11–22, 2011. doi: 10.1016/j.jsb.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ou X, O’Leary HA, Broxmeyer HE. Implications of DPP4 modification of proteins that regulate stem/progenitor and more mature cell types. Blood 122: 161–169, 2013. doi: 10.1182/blood-2013-02-487470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pagans S, Pedal A, North BJ, Kaehlcke K, Marshall BL, Dorr A, Hetzer-Egger C, Henklein P, Frye R, McBurney MW, Hruby H, Jung M, Verdin E, Ott M. SIRT1 regulates HIV transcription via Tat deacetylation. PLoS Biol 3: e41, 2005. doi: 10.1371/journal.pbio.0030041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Palamara AT, Nencioni L, Aquilano K, De Chiara G, Hernandez L, Cozzolino F, Ciriolo MR, Garaci E. Inhibition of influenza A virus replication by resveratrol. J Infect Dis 191: 1719–1729, 2005. doi: 10.1086/429694. [DOI] [PubMed] [Google Scholar]
- 163.Paquette M, Medina Larqué AS, Weisnagel SJ, Desjardins Y, Marois J, Pilon G, Dudonné S, Marette A, Jacques H. Strawberry and cranberry polyphenols improve insulin sensitivity in insulin-resistant, non-diabetic adults: a parallel, double-blind, controlled and randomised clinical trial. Br J Nutr 117: 519–531, 2017. doi: 10.1017/S0007114517000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Paraskevis D, Kostaki EG, Magiorkinis G, Panayiotakopoulos G, Sourvinos G, Tsiodras S. Full-genome evolutionary analysis of the novel corona virus (2019-nCoV) rejects the hypothesis of emergence as a result of a recent recombination event. Infect Genet Evol 79: 104212, 2020. doi: 10.1016/j.meegid.2020.104212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Park JY, Yuk HJ, Ryu HW, Lim SH, Kim KS, Park KH, Ryu YB, Lee WS. Evaluation of polyphenols from Broussonetia papyrifera as coronavirus protease inhibitors. J Enzyme Inhib Med Chem 32: 504–515, 2017. doi: 10.1080/14756366.2016.1265519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Park SK, Seong RK, Kim JA, Son SJ, Kim Y, Yokozawa T, Shin OS. Oligonol promotes anti-aging pathways via modulation of SIRT1-AMPK-autophagy Pathway. Nutr Res Pract 10: 3–10, 2016. doi: 10.4162/nrp.2016.10.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Perlman S. Another decade, another coronavirus. N Engl J Med 382: 760–762, 2020. doi: 10.1056/NEJMe2001126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Petrara MR, Giunco S, Serraino D, Dolcetti R, De Rossi A. Post-transplant lymphoproliferative disorders: from epidemiology to pathogenesis-driven treatment. Cancer Lett 369: 37–44, 2015. doi: 10.1016/j.canlet.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 169.Petrilli CM, Jones SA, Yang J, Rajagopalan H, O’Donnell L, Chernyak Y, Tobin KA, Cerfolio RJ, Francois F, Horwitz LI. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ 369: m1966, 2020. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Phan LT, Nguyen TV, Luong QC, Nguyen TV, Nguyen HT, Le HQ, Nguyen TT, Cao TM, Pham QD. Importation and human-to-human transmission of a novel coronavirus in Vietnam. N Engl J Med 382: 872–874, 2020. doi: 10.1056/NEJMc2001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Porro C, Cianciulli A, Trotta T, Lofrumento DD, Panaro MA. Curcumin regulates anti-inflammatory responses by JAK/STAT/SOCS signaling pathway in BV-2 microglial cells. Biology (Basel) 8: 51, 2019. doi: 10.3390/biology8030051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Pradhan P, Nguyen ML. Herpes simplex virus virucidal activity of MST-312 and epigallocatechin gallate. Virus Res 249: 93–98, 2018. doi: 10.1016/j.virusres.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 173.Raekiansyah M, Buerano CC, Luz MAD, Morita K. Inhibitory effect of the green tea molecule EGCG against dengue virus infection. Arch Virol 163: 1649–1655, 2018. doi: 10.1007/s00705-018-3769-y. [DOI] [PubMed] [Google Scholar]
- 174.Rahimi K, Hassanzadeh K, Khanbabaei H, Haftcheshmeh SM, Ahmadi A, Izadpanah E, Mohammadi A, Sahebkar A. Curcumin: a dietary phytochemical for targeting the phenotype and function of dendritic cells. Curr Med Chem. 2020. Online ahead of print. doi: 10.2174/0929867327666200515101228. [DOI] [PubMed] [Google Scholar]
- 175.Raimundo AF, Félix F, Andrade R, García-Conesa MT, González-Sarrías A, Gilsa-Lopes J, do Ó D, Raimundo A, Ribeiro R, Rodriguez-Mateos A, Santos CN, Schär M, Silva A, Cruz I, Wang B, Pinto P, Menezes R. Combined effect of interventions with pure or enriched mixtures of (poly)phenols and anti-diabetic medication in type 2 diabetes management: a meta-analysis of randomized controlled human trials. Eur J Nutr 59: 1329–1343, 2020. doi: 10.1007/s00394-020-02189-1. [DOI] [PubMed] [Google Scholar]
- 176.Raj VS, Mou H, Smits SL, Dekkers DH, Müller MA, Dijkman R, Muth D, Demmers JA, Zaki A, Fouchier RA, Thiel V, Drosten C, Rottier PJ, Osterhaus AD, Bosch BJ, Haagmans BL. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature 495: 251–254, 2013. doi: 10.1038/nature12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Rastelli M, Cani PD, Knauf C. The gut microbiome influences host endocrine functions. Endocr Rev 40: 1271–1284, 2019. doi: 10.1210/er.2018-00280. [DOI] [PubMed] [Google Scholar]
- 178.Rawson JM, Roth ME, Xie J, Daly MB, Clouser CL, Landman SR, Reilly CS, Bonnac L, Kim B, Patterson SE, Mansky LM. Synergistic reduction of HIV-1 infectivity by 5-azacytidine and inhibitors of ribonucleotide reductase. Bioorg Med Chem 24: 2410–2422, 2016. doi: 10.1016/j.bmc.2016.03.052. [DOI] [PubMed] [Google Scholar]
- 179.Reddinger RM, Luke-Marshall NR, Hakansson AP, Campagnari AA. Host physiologic changes induced by influenza a virus lead to staphylococcus aureus biofilm dispersion and transition from asymptomatic colonization to invasive disease. MBio 7: e01235-16, 2016. doi: 10.1128/mBio.01235-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Reid SP, Shurtleff AC, Costantino JA, Tritsch SR, Retterer C, Spurgers KB, Bavari S. HSPA5 is an essential host factor for Ebola virus infection. Antiviral Res 109: 171–174, 2014. doi: 10.1016/j.antiviral.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 181.Risco C, Antón IM, Enjuanes L, Carrascosa JL. The transmissible gastroenteritis coronavirus contains a spherical core shell consisting of M and N proteins. J Virol 70: 4773–4777, 1996. doi: 10.1128/JVI.70.7.4773-4777.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Rizk JG, Kalantar-Zadeh K, Mehra MR, Lavie CJ, Rizk Y, Forthal DN. Pharmaco-immunomodulatory therapy in COVID-19. Drugs. In press. doi: 10.1007/s40265-020-01367-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Rodriguez J, Hiel S, Neyrinck AM, Le Roy T, Pötgens SA, Leyrolle Q, Pachikian BD, Gianfrancesco MA, Cani PD, Paquot N, Cnop M, Lanthier N, Thissen JP, Bindels LB, Delzenne NM. Discovery of the gut microbial signature driving the efficacy of prebiotic intervention in obese patients. Gut In press. doi: 10.1136/gutjnl-2019-319726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun 109: 102433, 2020. doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Rowland Yeo K, Zhang M, Pan X, Ban Ke A, Jones HM, Wesche D, Almond LM. Impact of disease on plasma and lung exposure of chloroquine, hydroxychloroquine and azithromycin: application of PBPK modeling. Clin Pharmacol Ther In press. doi: 10.1002/cpt.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ruch TR, Machamer CE. The coronavirus E protein: assembly and beyond. Viruses 4: 363–382, 2012. doi: 10.3390/v4030363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sande CJ, Njunge JM, Mwongeli Ngoi J, Mutunga MN, Chege T, Gicheru ET, Gardiner EM, Gwela A, Green CA, Drysdale SB, Berkley JA, Nokes DJ, Pollard AJ. Airway response to respiratory syncytial virus has incidental antibacterial effects. Nat Commun 10: 2218, 2019. [Erratum in Nat Commun 10: 3291, 2019]. doi: 10.1038/s41467-019-10222-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Santambrogio L, Rammensee HG. Contribution of the plasma and lymph degradome and peptidome to the MHC ligandome. Immunogenetics 71: 203–216, 2019. doi: 10.1007/s00251-018-1093-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Satija A, Bhupathiraju SN, Rimm EB, Spiegelman D, Chiuve SE, Borgi L, Willett WC, Manson JE, Sun Q, Hu FB. Plant-based dietary patterns and incidence of type 2 diabetes in US men and women: results from three prospective cohort studies. PLoS Med 13: e1002039, 2016. doi: 10.1371/journal.pmed.1002039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Schnitzler P, Neuner A, Nolkemper S, Zundel C, Nowack H, Sensch KH, Reichling J. Antiviral activity and mode of action of propolis extracts and selected compounds. Phytother Res 24, Suppl 1: S20–S28, 2010. doi: 10.1002/ptr.2868. [DOI] [PubMed] [Google Scholar]
- 191.Scorletti E, Afolabi PR, Miles EA, Smith DE, Almehmadi A, Alshathry A, Childs CE, Del Fabbro S, Bilson J, Moyses HE, Clough GF, Sethi JK, Patel J, Wright M, Breen DJ, Peebles C, Darekar A, Aspinall R, Fowell AJ, Dowman JK, Nobili V, Targher G, Delzenne NM, Bindels LB, Calder PC, Byrne CD. Synbiotics alter fecal microbiomes, but not liver fat or fibrosis, in a randomized trial of patients with nonalcoholic fatty liver disease. Gastroenterology 158: 1597–1610.e7, 2020. doi: 10.1053/j.gastro.2020.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sekizawa H, Ikuta K, Ohnishi-Kameyama M, Nishiyama K, Suzutani T. Identification of the components in a Vaccinium oldhamii Extract showing inhibitory activity against influenza virus adsorption. Foods 8: 172, 2019. doi: 10.3390/foods8050172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Seong JM, Yee J, Gwak HS. Dipeptidyl peptidase-4 inhibitors lower the risk of autoimmune disease in patients with type 2 diabetes mellitus: a nationwide population-based cohort study. Br J Clin Pharmacol 85: 1719–1727, 2019. doi: 10.1111/bcp.13955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Shenoy MK, Iwai S, Lin DL, Worodria W, Ayakaka I, Byanyima P, Kaswabuli S, Fong S, Stone S, Chang E, Davis JL, Faruqi AA, Segal MR, Huang L, Lynch SV. Immune response and mortality risk relate to distinct lung microbiomes in patients with HIV and pneumonia. Am J Respir Crit Care Med 195: 104–114, 2017. doi: 10.1164/rccm.201603-0523OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis 5: 558–567, 2005. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- 196.Shimizu T, Takeshita Y, Takamori Y, Kai H, Sawamura R, Yoshida H, Watanabe W, Tsutsumi A, Park YK, Yasukawa K, Matsuno K, Shiraki K, Kurokawa M. Efficacy of Brazilian propolis against herpes simplex virus type 1 infection in mice and their modes of antiherpetic efficacies. Evid Based Complement Alternat Med 2011: 976196, 2011. doi: 10.1155/2011/976196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Singh BN, Shankar S, Srivastava RK. Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications. Biochem Pharmacol 82: 1807–1821, 2011. doi: 10.1016/j.bcp.2011.07.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Siu YL, Teoh KT, Lo J, Chan CM, Kien F, Escriou N, Tsao SW, Nicholls JM, Altmeyer R, Peiris JS, Bruzzone R, Nal B. The M, E, and N structural proteins of the severe acute respiratory syndrome coronavirus are required for efficient assembly, trafficking, and release of virus-like particles. J Virol 82: 11318–11330, 2008. doi: 10.1128/JVI.01052-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sivanantham A, Pattarayan D, Rajasekar N, Kannan A, Loganathan L, Bethunaickan R, Mahapatra SK, Palanichamy R, Muthusamy K, Rajasekaran S. Tannic acid prevents macrophage-induced pro-fibrotic response in lung epithelial cells via suppressing TLR4-mediated macrophage polarization. Inflamm Res 68: 1011–1024, 2019. doi: 10.1007/s00011-019-01282-4. [DOI] [PubMed] [Google Scholar]
- 200.Soare A, Györfi HA, Matei AE, Dees C, Rauber S, Wohlfahrt T, Chen CW, Ludolph I, Horch RE, Bäuerle T, von Hörsten S, Mihai C, Distler O, Ramming A, Schett G, Distler JHW. Dipeptidylpeptidase 4 as a marker of activated fibroblasts and a potential target for the treatment of fibrosis in systemic sclerosis. Arthritis Rheumatol 72: 137–149, 2020. doi: 10.1002/art.41058. [DOI] [PubMed] [Google Scholar]
- 201.Sohrabi C, Alsafi Z, O’Neill N, Khan M, Kerwan A, Al-Jabir A, Iosifidis C, Agha R. World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19). Int J Surg 76: 71–76, 2020. [Erratum in Int J Surg 77: 217, 2020]. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Song JM. Anti-infective potential of catechins and their derivatives against viral hepatitis. Clin Exp Vaccine Res 7: 37–42, 2018. doi: 10.7774/cevr.2018.7.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sormani MP. Modeling the distribution of new MRI cortical lesions in multiple sclerosis longitudinal studies by Sormani MP, Calabrese M, Signori A, Giorgio A, Gallo P, De Stefano N [PLoS One 2011;6(10):e26712. Epub 2011 October 20] Mult Scler Relat Disord 1: 108, 2012. doi: 10.1016/j.msard.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 204.South AM, Diz DI, Chappell MC. COVID-19, ACE2, and the cardiovascular consequences. Am J Physiol Heart Circ Physiol 318: H1084–H1090, 2020. doi: 10.1152/ajpheart.00217.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Souza DG, Vieira AT, Soares AC, Pinho V, Nicoli JR, Vieira LQ, Teixeira MM. The essential role of the intestinal microbiota in facilitating acute inflammatory responses. J Immunol 173: 4137–4146, 2004. doi: 10.4049/jimmunol.173.6.4137. [DOI] [PubMed] [Google Scholar]
- 206.Suen CM, Hui DSC, Memtsoudis SG, Chung F. Obstructive sleep apnea, obesity, and noninvasive ventilation: considerations during the COVID-19 pandemic. Anesth Analg 131: 318–322, 2020. doi: 10.1213/ANE.0000000000004928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Sui X, Zhang C, Zhou J, Cao S, Xu C, Tang F, Zhi X, Chen B, Wang S, Yin L. Resveratrol inhibits extranodal NK/T cell lymphoma through activation of DNA damage response pathway. J Exp Clin Cancer Res 36: 133, 2017. doi: 10.1186/s13046-017-0601-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Surendran S, Sapkal R, Paul D, Nanjappan S. Effect of resveratrol on dipeptidyl peptidase-4 inhibitors pharmacokinetics: an in vitro and in vivo approach. Chem Biol Interact 315: 108909, 2020. doi: 10.1016/j.cbi.2019.108909. [DOI] [PubMed] [Google Scholar]
- 209.Takiishi T, Fenero CIM, Câmara NOS. Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers 5: e1373208, 2017. doi: 10.1080/21688370.2017.1373208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Tang HM, Gao WW, Chan CP, Cheng Y, Deng JJ, Yuen KS, Iha H, Jin DY. SIRT1 suppresses human T-cell leukemia virus type 1 transcription. J Virol 89: 8623–8631, 2015. doi: 10.1128/JVI.01229-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Thorburn AN, McKenzie CI, Shen S, Stanley D, Macia L, Mason LJ, Roberts LK, Wong CH, Shim R, Robert R, Chevalier N, Tan JK, Mariño E, Moore RJ, Wong L, McConville MJ, Tull DL, Wood LG, Murphy VE, Mattes J, Gibson PG, Mackay CR. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat Commun 6: 7320, 2015. doi: 10.1038/ncomms8320. [DOI] [PubMed] [Google Scholar]
- 212.Tian A, Wang B, Jiang J. Injury-stimulated and self-restrained BMP signaling dynamically regulates stem cell pool size during Drosophila midgut regeneration. Proc Natl Acad Sci USA 114: E2699–E2708, 2017. doi: 10.1073/pnas.1617790114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Tian W, Jiang W, Yao J, Nicholson CJ, Li RH, Sigurslid HH, Wooster L, Rotter JI, Guo X, Malhotra R. Predictors of mortality in hospitalized COVID-19 patients: a systematic review and meta-analysis. J Med Virol In press. doi: 10.1002/jmv.26050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Tresserra-Rimbau A, Rimm EB, Medina-Remón A, Martínez-González MA, de la Torre R, Corella D, Salas-Salvadó J, Gómez-Gracia E, Lapetra J, Arós F, Fiol M, Ros E, Serra-Majem L, Pintó X, Saez GT, Basora J, Sorlí JV, Martínez JA, Vinyoles E, Ruiz-Gutiérrez V, Estruch R, Lamuela-Raventós RM; PREDIMED Study Investigators . Inverse association between habitual polyphenol intake and incidence of cardiovascular events in the PREDIMED study. Nutr Metab Cardiovasc Dis 24: 639–647, 2014. doi: 10.1016/j.numecd.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 215.Tsai MJ, Chang WA, Liao SH, Chang KF, Sheu CC, Kuo PL. The Effects of epigallocatechin gallate (EGCG) on pulmonary fibroblasts of idiopathic pulmonary fibrosis (IPF)—a next-generation sequencing and bioinformatic approach. Int J Mol Sci 20: 1958, 2019. doi: 10.3390/ijms20081958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Uzelac I, Iravanian S, Ashikaga H, Bhatia NK, Herndon C, Kaboudian A, Gumbart JC, Cherry EM, Fenton FH. Fatal arrhythmias: another reason why doctors remain cautious about chloroquine/hydroxychloroquine for treating COVID-19. Heart Rhythm 17(9): 1445–1451, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Valero-Cases E, Cerdá-Bernad D, Pastor JJ, Frutos MJ. Non-dairy fermented beverages as potential carriers to ensure probiotics, prebiotics, and bioactive compounds arrival to the gut and their health benefits. Nutrients 12: 1666, 2020. doi: 10.3390/nu12061666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Van Hul M, Cani PD. Targeting carbohydrates and polyphenols for a healthy microbiome and healthy weight. Curr Nutr Rep 8: 307–316, 2019. doi: 10.1007/s13668-019-00281-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Vankadari N, Wilce JA. Emerging (COVID-19) coronavirus: glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerg Microbes Infect 9: 601–604, 2020. doi: 10.1080/22221751.2020.1739565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Vázquez-Calvo Á, Jiménez de Oya N, Martín-Acebes MA, Garcia-Moruno E, Saiz JC. Antiviral properties of the natural polyphenols delphinidin and epigallocatechin gallate against the flaviviruses West Nile virus, Zika virus, and Dengue virus. Front Microbiol 8: 1314, 2017. doi: 10.3389/fmicb.2017.01314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Verma A, Xu K, Du T, Zhu P, Liang Z, Liao S, Zhang J, Raizada MK, Grant MB, Li Q. Expression of human ACE2 in Lactobacillus and beneficial effects in diabetic retinopathy in mice. Mol Ther Methods Clin Dev 14: 161–170, 2019. [Erratum in Mol Ther Methods Clin Dev 17: P400, 2020]. doi: 10.1016/j.omtm.2019.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Villar L, Dayan GH, Arredondo-García JL, Rivera DM, Cunha R, Deseda C, Reynales H, Costa MS, Morales-Ramírez JO, Carrasquilla G, Rey LC, Dietze R, Luz K, Rivas E, Miranda Montoya MC, Cortés Supelano M, Zambrano B, Langevin E, Boaz M, Tornieporth N, Saville M, Noriega F; CYD15 Study Group . Efficacy of a tetravalent dengue vaccine in children in Latin America. N Engl J Med 372: 113–123, 2015. doi: 10.1056/NEJMoa1411037. [DOI] [PubMed] [Google Scholar]
- 223.Vynograd N, Vynograd I, Sosnowski Z. A comparative multi-centre study of the efficacy of propolis, acyclovir and placebo in the treatment of genital herpes (HSV). Phytomedicine 7: 1–6, 2000. doi: 10.1016/S0944-7113(00)80014-8. [DOI] [PubMed] [Google Scholar]
- 224.Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 181: 281–292.e6, 2020. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA: 323: 1061–1069, 2020. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Wang J, Li F, Wei H, Lian ZX, Sun R, Tian Z. Respiratory influenza virus infection induces intestinal immune injury via microbiota-mediated Th17 cell-dependent inflammation. J Exp Med 211: 2397–2410, 2014. [Erratum in J Exp Med 211: 2683, 2014]. doi: 10.1084/jem.20140625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Wang T, Xiang Z, Wang Y, Li X, Fang C, Song S, Li C, Yu H, Wang H, Yan L, Hao S, Wang X, Sheng J. (–)-Epigallocatechin gallate targets notch to attenuate the inflammatory response in the immediate early stage in human macrophages. Front Immunol 8: 433, 2017. doi: 10.3389/fimmu.2017.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Wang Y, Pan CQ, Xing H. Advances in gut microbiota of viral hepatitis cirrhosis. BioMed Res Int 2019: 9726786, 2019. doi: 10.1155/2019/9726786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Whitby K, Pierson TC, Geiss B, Lane K, Engle M, Zhou Y, Doms RW, Diamond MS. Castanospermine, a potent inhibitor of dengue virus infection in vitro and in vivo. J Virol 79: 8698–8706, 2005. doi: 10.1128/JVI.79.14.8698-8706.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Wieërs G, Belkhir L, Enaud R, Leclercq S, Philippart de Foy JM, Dequenne I, de Timary P, Cani PD. How probiotics affect the microbiota. Front Cell Infect Microbiol 9: 454, 2020. doi: 10.3389/fcimb.2019.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Wong G, He S, Siragam V, Bi Y, Mbikay M, Chretien M, Qiu X. Antiviral activity of quercetin-3-β-O-D-glucoside against Zika virus infection. Virol Sin 32: 545–547, 2017. doi: 10.1007/s12250-017-4057-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, Hu Y, Tao ZW, Tian JH, Pei YY, Yuan ML, Zhang YL, Dai FH, Liu Y, Wang QM, Zheng JJ, Xu L, Holmes EC, Zhang YZ. A new coronavirus associated with human respiratory disease in China. Nature 579: 265–269, 2020. [Erratum in Nature 580: E7, 2020]. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Xie J, Tong Z, Guan X, Du B, Qiu H. Clinical characteristics of patients who died of coronavirus disease 2019 in China. JAMA Netw Open 3: e205619, 2020. doi: 10.1001/jamanetworkopen.2020.5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Xu J, Gu W, Li C, Li X, Xing G, Li Y, Song Y, Zheng W. Epigallocatechin gallate inhibits hepatitis B virus via farnesoid X receptor alpha. J Nat Med 70: 584–591, 2016. doi: 10.1007/s11418-016-0980-6. [DOI] [PubMed] [Google Scholar]
- 235.Xu J, Xu Z, Zheng W. A review of the antiviral role of green tea catechins. Molecules 22: 1337, 2017. doi: 10.3390/molecules22081337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Yang CS, Lambert JD, Sang S. Antioxidative and anti-carcinogenic activities of tea polyphenols. Arch Toxicol 83: 11–21, 2009. doi: 10.1007/s00204-008-0372-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Ye G, Wang X, Tong X, Shi Y, Fu ZF, Peng G. Structural basis for inhibiting porcine epidemic diarrhea virus replication with the 3C-like protease inhibitor GC376. Viruses 12: 240, 2020. doi: 10.3390/v12020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Yildirim A, Duran GG, Duran N, Jenedi K, Bolgul BS, Miraloglu M, Muz M. Antiviral activity of hatay propolis against replication of herpes simplex virus type 1 and type 2. Med Sci Monit 22: 422–430, 2016. doi: 10.12659/MSM.897282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Yildiz S, Mazel-Sanchez B, Kandasamy M, Manicassamy B, Schmolke M. Influenza A virus infection impacts systemic microbiota dynamics and causes quantitative enteric dysbiosis. Microbiome 6: 9, 2018. doi: 10.1186/s40168-017-0386-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Zainal N, Chang CP, Cheng YL, Wu YW, Anderson R, Wan SW, Chen CL, Ho TS, AbuBakar S, Lin YS. Resveratrol treatment reveals a novel role for HMGB1 in regulation of the type 1 interferon response in dengue virus infection. Sci Rep 7: 42998, 2017. doi: 10.1038/srep42998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Zang N, Li S, Li W, Xie X, Ren L, Long X, Xie J, Deng Y, Fu Z, Xu F, Liu E. Resveratrol suppresses persistent airway inflammation and hyperresponsivess might partially via nerve growth factor in respiratory syncytial virus-infected mice. Int Immunopharmacol 28: 121–128, 2015. doi: 10.1016/j.intimp.2015.05.031. [DOI] [PubMed] [Google Scholar]
- 242.Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med 46: 586–590, 2020. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Zhang HS, Wu MR. SIRT1 regulates Tat-induced HIV-1 transactivation through activating AMP-activated protein kinase. Virus Res 146: 51–57, 2009. doi: 10.1016/j.virusres.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 244.Zhang Y, Cui Y, Deng W, Wang H, Qin W, Huang C, Li C, Zhang J, Guo Y, Wu D, Guo H. Isoquercitrin protects against pulmonary hypertension via inhibiting PASMCs proliferation. Clin Exp Pharmacol Physiol 44: 362–370, 2017. doi: 10.1111/1440-1681.12705. [DOI] [PubMed] [Google Scholar]
- 245.Zhao C, Liu S, Li C, Yang L, Zu Y. In vitro evaluation of the antiviral activity of the synthetic epigallocatechin gallate analog-epigallocatechin gallate (EGCG) palmitate against porcine reproductive and respiratory syndrome virus. Viruses 6: 938–950, 2014. doi: 10.3390/v6020938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Zhao J, Liu J, Pang X, Zhang X, Wang S, Wu D. Epigallocatechin-3-gallate inhibits angiotensin II-induced C-reactive protein generation through interfering with the AT1-ROS-ERK1/2 signaling pathway in hepatocytes. Naunyn Schmiedebergs Arch Pharmacol 389: 1225–1234, 2016. doi: 10.1007/s00210-016-1279-6. [DOI] [PubMed] [Google Scholar]
- 247.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395: 1054–1062, 2020. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579: 270–273, 2020. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]