Abstract
Background/Objective
To evaluate the impact of the COVID-19 pandemic on hospital admissions and outcomes in patients admitted with acute ischemic stroke.
Methods
Single-center retrospective analysis of patients admitted to the hospital with acute ischemic stroke, between December 1st, 2019 and June 30th, 2020. Outcomes were classified as none-to-minimal disability, moderate-to-severe disability, and death based on discharge disposition, and compared between two time periods: pre-COVID-19 era (December 1st, 2019 to March 11th, 2020) and COVID-19 era (March 12th to June 30th, 2020). We also performed a comparative trend analysis for the equivalent period between 2019 and 2020.
Results
Five hundred and seventy-five patients with a mean age (years±SD) of 68±16 were admitted from December 1st, 2019 to June 30th, 2020, with a clinical diagnosis of acute ischemic stroke. Of these, 255 (44.3%) patients were admitted during the COVID-19 era. We observed a 22.1% and 39.5% decline in admission for acute ischemic stroke in April and May 2020, respectively. A significantly higher percentage of patients with acute ischemic stroke received intravenous thrombolysis during the COVID-19 era (p = 0.020). In patients with confirmed COVID-19, we found a higher percentage of older men with preexisting comorbidities such as hyperlipidemia, coronary artery disease, and diabetes mellitus but a lower rate of atrial fibrillation. In addition, we found a treatment delay in both intravenous thrombolysis (median 94.5 min versus 38 min) and mechanical thrombectomy (median 244 min versus 86 min) in patients with confirmed COVID-19 infection. There were no differences in patients’ disposition including home, short-term, and long-term facility (p = 0.60).
Conclusions
We observed a reduction of hospital admissions in acute ischemic strokes and some delay in reperfusion therapy during the COVID-19 pandemic. Prospective studies and a larger dataset analysis are warranted.
Key Words: COVID-19, Pandemic, Acute Ischemic Stroke, IV thrombolysis, Mechanical Thrombectomy, Hospital Admission, Mortality
Introduction
The Coronavirus disease 2019 (COVID-19) pandemic has placed an unprecedented strain on healthcare systems and resources.1 , 2 , 3 Despite the modifications in acute ischemic stroke (AIS) care due to the COVID-19 pandemic,4 there is limited data to provide quantitative estimates of such changes. One of the research priorities identified by an international panel of stroke experts 4 was to better characterize the impact of COVID-19 pandemic on AIS epidemiology and workflow and guide resource allocation. A few recent publications reported reduced hospital admission for acute coronary syndrome, myocardial infarction, and AIS during the COVID-19 pandemic in several countries.5 , 6 , 7 , 8 , 9 , 10 , 11 In addition, recent publications have reported decreased volume and delay in initiation of reperfusion therapy for AIS during the COVID-19 pandemic.6 , 8 , 12
This study aims to assess the impact of the COVID-19 pandemic on AIS care at a large regional tertiary care center, focusing on admission volumes, time to reperfusion therapy, and patient outcomes.
Methods
Patients and Study Design
This study was approved by the Institutional Review Board of Inova Fairfax Medical Center.
We identified all patients admitted to Inova Fairfax Medical Campus with a confirmed diagnosis of AIS or transient ischemic attack (TIA) from December 1st, 2019 to June 30th, 2020 using a prospectively maintained stroke registry. We analyzed data from two distinct periods. First, we compared data from the 2.5 months prior to the first hospitalized COVID-19 patient at our facility (December 1st, 2019 to March 11th, 2020; pre-COVID-19 era) to the subsequent 2.5 month period (March 12th to June 30th, 2020; COVID era). Second, we performed a trend analysis which was compared to historic data in 2019 to assess potential seasonal variations.
Baseline Characteristics and Outcomes
Patient demographics including the primary diagnosis on admission, past medical history, the National Institutes of Health Stroke Scale (NIHSS), treatment modalities (intravenous thrombolytic and mechanical thrombectomy), and discharge disposition were collected. Outcomes were classified based on discharge disposition: none-to-minimal disability when patients were discharged to home or a short term facility; and moderate-to-severe disability when patients were discharged to a long term facility including acute rehabilitation, skilled nursing facility, and hospice. In-hospital mortality data were also collected. Previous studies have verified discharge destination as a surrogate for functional outcomes.13
Statistical Analysis
Categorical variables are reported as absolute numbers and percentages. Continuous variables are presented as mean and standard deviation (SD) or median ± interquartile range (IQR). The Two-sample and paired t-tests were used to compare the numeric variables. Statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC). All analyses were two-tailed, and the significance level was determined by p < 0.05.
Results
From December 1st, 2019 to June 30th, 2020, a total of 575 patients were admitted to Inova Fairfax Medical Campus with a clinical diagnosis of AIS or TIA, of whom 255 patients (44.3%) were admitted during the COVID-19 era. Baseline patient characteristics are summarized in Table 1 . Patients admitted during the COVID-19 pandemic were more likely to undergo intravenous thrombolysis (p = 0.020) and mechanical thrombectomy (p = 0.060). There were no differences in patient disposition between pre-COVID and COVID era (p = 0.600).
Table 1.
Baseline characteristics in patients admitted with acute ischemic stroke during the pre-COVID-19 and COVID-19 eras
| Pre-COVID era | COVID era | p-value | |
|---|---|---|---|
| (12/1/19–03/11/20) | (03/12/20–06/30/20) | ||
| Admission for AIS and TIA, n | 320 | 255 | |
| Age, median (IQR) | 70 (62–81) | 73 (62–83) | 0.513 |
| Male, n (%) | 166 (51.9%) | 136 (53.3%) | 0.728 |
| Initial NIHSS, median (IQR) | 4 (1–11) | 5 (1–12) | 0.906 |
| IV tPA, n (%) | 20 (6.3%) | 30 (11.8%) | 0.020 |
| MET, n (%) | 43 (13.4%) | 49 (19.2%) | 0.060 |
| DNT, min, median (IQR) | 52.5 (33.5–89) | 41.5 (31–51) | 0.100 |
| DGPT, min,median (IQR) | 72 (41–109) | 85.5 (46–116) | 0.812 |
| LOS, median (IQR) | 4 (2–8) | 4 (2–8) | 0.801 |
| Death at discharge, n (%) | 25 (7.8%) | 12 (4.7%) | 0.131 |
| Home/Short term Facility, n (%) | 167 (52.2%) | 133 (52.2%) | 0.994 |
| Long term Facility, n (%) | 128 (40.0%) | 110 (43.1%) | 0.448 |
AIS, Acute Ischemic Stroke; DGPT, door-to-groin puncture time; DNT, door-to-needle time; IQR, interquartile range; IV tPA, intravenous alteplase; LOS, length of stay; MET, mechanical thrombectomy; NIHSS, National Institutes of Health Stroke Scale; TIA, transient ischemic attack. Values in bold font indicate statistical significance.
Out of the 255 patients admitted with AIS, 130 patients (54.3%) were tested for COVID-19 infection using real-time reverse transcription polymerase chain reaction (rRT-PCR), of whom 9 patients (7%) tested positive. These 9 patients were more likely to be male, of older age, and with preexisting vascular comorbidities such as hyperlipidemia, coronary artery disease, and diabetes; however, they had a lower rate of atrial fibrillation. Only two patients received intravenous alteplase with a longer median door-to-needle time (94.5 versus 38.5 min), and one patient underwent mechanical thrombectomy with a longer median door-to-groin time (244 versus 86 min) compared to pre-COVID-19 era (Table 2).
Table 2.
Baseline characteristics of patients admitted with acute ischemic stroke during the COVID-19 era.
| COVID testing not performed | COVID (-) | COVID (+) | |
|---|---|---|---|
| Hospital admission for AIS and TIA, n | 125 | 121 | 9 |
| Age, median (IQR) | 73 (63–81) | 72 (60–84) | 73 (61–80) |
| Male, n (%) | 56.80% | 47.90% | 77.80% |
| BMI | 26 (24–30) | 27 (24–31) | 26 (23–28) |
| Coronary Artery Disease | 10.40% | 19.80% | 44.40% |
| Diabetes | 34.40% | 25.60% | 44.40% |
| Hypertension | 79.20% | 71.90% | 66.70% |
| Atrial Fibrillation | 17.60% | 28.90% | 0.00% |
| Dyslipidemia | 53.60% | 48.80% | 66.70% |
| AIS and TIA, n | 125 | 121 | 9 |
| NIHSS on arrival | 3 (1–7) | 8 (2–16) | 6 (1–20) |
| IV tPA, n (%) | 10 | 18 | 2 |
| MET, n (%) | 14 | 34 | 1 |
| DNT, min, median (IQR) | 48 (29–52) | 38.5 (34–48) | 94.5 (24–165) |
| DGPT, min, median (IQR) | 78.5 (44–96) | 86 (48–116) | 244 (244–244) |
| LOS, median (IQR) | 3 (2–5) | 5 (3–9) | 17 (9–46) |
AIS, Acute Ischemic Stroke; IQR, interquartile range; IV tPA, intravenous alteplase; MET, mechanical thrombectomy; DNT, door-to-needle time; DGPT, door-to-groin puncture time; NIHSS, National Institutes of Health Stroke Scale; LOS, length of stay; TIA, transient ischemic attack.
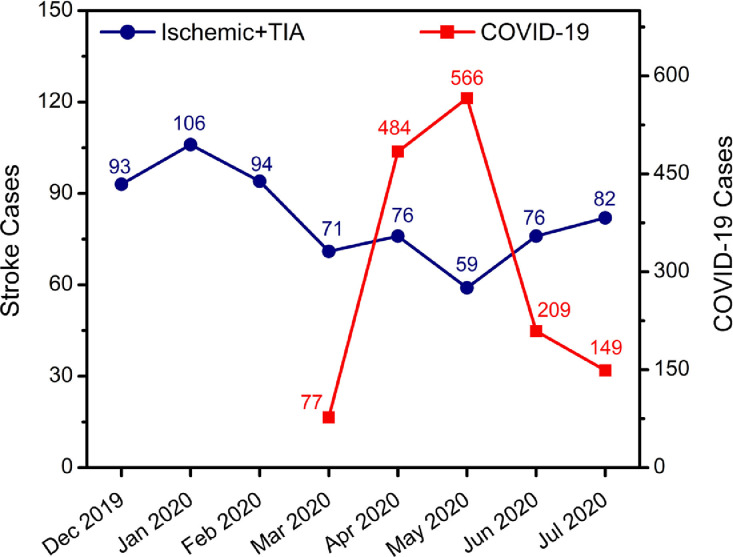
Since the outbreak of the COVID-19 pandemic, we observed a significant reduction of hospital admissions for AIS using the Cochran-Armitage trend test (p = 0.002). The sharp decrease in volume started in March and persisted throughout May 2020, with 22.1% and 39.5% decline in April and May 2020, respectively. The number of AIS cases inversely correlated to the number of hospital admissions of COVID-19 cases (Fig. 1 ). Acute ischemic stroke admission, when compared to the same period in 2019, remained relatively unchanged (Supplementary Figure 1a).
Fig. 1.
The trend of monthly admissions for acute ischemic stroke during the pre-COVID-19 and the COVID-19 pandemic from December 1st, 2019 to July 31th, 2020, and comparison of stroke hospital admissions to those with confirmed COVID-19 infection from March 12th since the outbreak of COVID-19 to June 30th, 2020. The blue line represents the monthly stroke admissions, and the red line represents the number of monthly admissions for COVID-19 infection.
Discussion
The impact of the COVID-19 pandemic on hospital admissions for vascular diseases, including acute coronary syndrome14, 15 and acute ischemic stroke,6 , 15, 16, 17, 18, 19, 20 has been reported. A 36.4% reduction in AIS admissions was seen in Brazil after COVID-19 compared to the same period in 2019.16 In addition, data from four German comprehensive stroke centers showed reduced hospital admissions for AIS and TIA.17 Our data, in line with the findings above, showed a reduction in AIS admissions associated with the COVID-19 pandemic in 2020. We found a relatively stable number of acute stroke admissions throughout 2019, devoid of seasonal variability (Supplementary data).
Interestingly, despite a reduction in the number of hospital admissions for AIS, a higher proportion of patients received reperfusion therapies compared to both pre-COVID-19 era in 2020 and from the same period in 2019. However, previous reports have shown a decreased number21, 22, 23 but a relatively unchanged proportion6 , 18 of patients who received reperfusion therapy during COVID-19 pandemic. The first confirmed case in Virginia was reported on March 7, 2020, and the first confirmed case was admitted to our facility on March 12, 2020. Stay-at-home orders were issued on March 30th, 2020, in the Commonwealth of Virginia and Maryland and April 1st, 2020, in the Washington D.C. We did not find a significant difference in initial NIHSS between pre-COVID and COVID eras in AIS patients who received IV thrombolysis, mechanical thrombectomy, or both although a trend towards higher initial NIHSS was observed during the COVID-19 era (Supplementary Table 1a). This finding suggests that the increased rate of reperfusion therapies during the COVID-19 era is more likely due to a decline in minor and non-disabling stroke admissions, rather than an increase in stroke severity, leading to the increased ratio of patients presenting with disabling and large vessel occlusion. In patients with confirmed COVID-19 infection, we observed a higher percentage of older males with preexisting comorbidities, such as hyperlipidemia, coronary artery disease, and diabetes, similar to a previous report from Wuhan, China,24 although we observed a lower rate of atrial fibrillation.
Acute Ischemic stroke may be the initial and the only presenting symptom in patients with COVID-19 infection.25 , 26 Early recognition and prompt reperfusion therapy are pivotal in reducing morbidity and mortality in AIS. We observed a significant delay in mechanical thrombectomy. Thorough assessment of one patient with a door-to-groin-puncture time more than 4 hours, highlighted several contributing factors, many attributable to the COVID-19 pandemic, including added time required for donning personal protection equipment leading to delay in symptoms recognition, neurological assessment, communication, transport, imaging, and procedures. Nevertheless, an unchanged median door-to-needle time for intravenous thrombolysis represents a consistent hospital-level approach to AIS treatment.
We did observe an increased length of stay in patients with COVID-19 infection. Factors that may be attributed to the delay are multifactorial. All patients required a negative COVID-19 test before discharge to rehabilitation facilities; two consecutive negative tests were required for those with COVID-19 infection. Completion of stroke workup including echocardiography and follow-up imaging, were delayed; and some studies such as brain MRI were postponed in COVID-19+ patients. We observed an increased percentage of patients with severe AIS and a decline in TIA and mild stroke admission that can also contribute to the increased length of stay.
Our study has a few limitations. This is an observational single-center retrospective study, and some prehospital data, including baseline functional status, symptom-onset to presentation time as well as discharge functional status and long-term functional outcomes, are not currently available for all patients. Only half of the patients with AIS were tested for COVID-19 infection. Also, stroke occurrence could have remained undetected in COVID-19 patients who were not clinically or radiographically evaluated for stroke.
In conclusion, we observed a reduction in the number of admission for AIS during the COVID-19 pandemic. A larger proportion of patients with AIS received reperfusion therapy. The long-term impact of COVID-19 pandemic on functional outcomes of patients with AIS, including those with TIA and minor strokes who did not seek medical care, warrants further investigation.
Authors Contribution
Study concept and design: JW, SAC
Acquisition, analysis, and interpretation of data: JW, ZB, SAC, Statistical analysis: YF, SAC
Drafting of the manuscript: JW, PTF, SAC, LA, SB
Declaration of Competing Interest
The authors have no conflicts to report.
Source of Funding
This study was not funded.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.jstrokecerebrovasdis.2020.105344.
Appendix. Supplementary materials
References
- 1.Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020 19;91(1):157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fauci AS, Clifford Lane H, Redfield RR. Covid-19 — Navigating the Uncharted. N Engl J Med. 2020 26;382(13):1268–1269. doi: 10.1056/NEJMe2002387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahase E, Covid-19 Kmietowicz Z. Doctors are told not to perform CPR on patients in cardiac arrest. BMJ. 2020 29;368:m1282. doi: 10.1136/bmj.m1282. [DOI] [PubMed] [Google Scholar]
- 4.Qureshi AI, Abd-Allah F, Al-Senani F. Management of acute ischemic stroke in patients with COVID-19 infection: Report of an international panel. Int J Stroke. 2020;15(5):540–554. doi: 10.1177/1747493020923234. [DOI] [PubMed] [Google Scholar]
- 5.Solomon MD, McNulty EJ, Rana JS. The Covid-19 pandemic and the incidence of acute myocardial infarction. N Engl J Med. 2020 13;383(7):691–693. doi: 10.1056/NEJMc2015630. [DOI] [PubMed] [Google Scholar]
- 6.Zhao J, Li H, Kung D. Impact of the COVID-19 epidemic on stroke care and potential solutions. Stroke. 2020;51(7):1996–2001. doi: 10.1161/STROKEAHA.120.030225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morelli N, Rota E, Terracciano C. The baffling case of ischemic stroke disappearance from the casualty department in the COVID-19 era. Eur Neurol. 2020;83(2):213–215. doi: 10.1159/000507666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Rosa S, Spaccarotella C, Basso C. Reduction of hospitalizations for myocardial infarction in Italy in the COVID-19 era. Eur Heart J. 2020 7;41(22):2083–2088. doi: 10.1093/eurheartj/ehaa409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Filippo O, D'Ascenzo F, Angelini F. Reduced rate of hospital admissions for ACS during Covid-19 outbreak in Northern Italy. N Engl J Med. 2020 2;383(1):88–89. doi: 10.1056/NEJMc2009166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Metzler B, Siostrzonek P, Binder RK. Decline of acute coronary syndrome admissions in Austria since the outbreak of COVID-19: the pandemic response causes cardiac collateral damage. Eur Heart J. 2020 14;41(19):1852–1853. doi: 10.1093/eurheartj/ehaa314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia S, Albaghdadi MS, Meraj PM. Reduction in ST-segment elevation cardiac catheterization laboratory activations in the United States during COVID-19 pandemic. J Am Coll Cardiol. 2020 9;75(22):2871–2872. doi: 10.1016/j.jacc.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kerleroux B, Fabacher T, Bricout N. Mechanical thrombectomy for acute ischemic stroke amid the COVID-19 Outbreak. Stroke. 2020;51(7):2012–2017. doi: 10.1161/STROKEAHA.120.030373. [DOI] [PubMed] [Google Scholar]
- 13.Qureshi AI, Chaudhry SA, Sapkota BL. Discharge destination as a surrogate for modified rankin scale defined outcomes at 3- and 12-months poststroke among stroke survivors. Arch Phys Med Rehabil. 2012;93(8):1408–1413. doi: 10.1016/j.apmr.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pessoa-Amorim G, Camm CF, Gajendragadkar P. Admission of patients with STEMI since the outbreak of the COVID-19 pandemic. a survey by the European society of cardiology. Eur Heart J Qual Care Clin Outcomes. 2020 1;6(3):210–216. doi: 10.1093/ehjqcco/qcaa046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niccoli G, Luescher TF, Crea F. Decreased myocardial infarction admissions during COVID times: what can we learn? Cardiovasc Res. 2020 1;116(10):e126–e128. doi: 10.1093/cvr/cvaa146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diegoli H, Magalhães PSC, Martins SCO. Decrease in hospital admissions for transient ischemic attack, mild, and moderate stroke during the COVID-19 era. Stroke. 2020;51(8):2315–2321. doi: 10.1161/STROKEAHA.120.030481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoyer C, Ebert A, Huttner HB. Acute stroke in times of the COVID-19 pandemic: a multicenter study. Stroke. 2020;51(7):2224–2227. doi: 10.1161/STROKEAHA.120.030395. [DOI] [PubMed] [Google Scholar]
- 18.Meza HT, Á Lambea Gil, Saldaña AS. Impact of COVID-19 outbreak on ischemic stroke admissions and in-hospital mortality in North-West Spain. Int J Stroke. 2020 26 doi: 10.1177/1747493020938301. 1747493020938301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bullrich MB, Fridman S, Mandzia JL. COVID-19: stroke admissions, emergency department visits, and prevention clinic referrals. Can J Neurol Sci. 2020 26:1–4. doi: 10.1017/cjn.2020.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siegler JE, Heslin ME, Thau L. Falling stroke rates during COVID-19 pandemic at a comprehensive stroke center. J Stroke Cerebrovasc Dis. 2020 14 doi: 10.1016/j.jstrokecerebrovasdis.2020.104953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pop R, Quenardelle V, Hasiu A. Impact of the COVID‐19 outbreak on acute stroke pathways – insights from the Alsace region in France. Eur J Neurol. 2020 12 doi: 10.1111/ene.14316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meza HT, Tejada Meza H, Lambea Gil Á. Ischaemic stroke in the time of coronavirus disease. Eur J Neurol. 2019;16 doi: 10.1111/ene.14327. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montaner J, Barragán-Prieto A, Pérez-Sánchez S. Break in the Stroke Chain of survival due to COVID-19. Stroke. 2020;51(8):2307–2314. doi: 10.1161/STROKEAHA.120.030106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou F, Yu T, Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 28;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oxley TJ, Mocco J, Majidi S. Large-vessel stroke as a presenting feature of Covid-19 in the Young. N Engl J Med. 2020 14;382(20):e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mao L, Jin H, Wang M. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020 10;77(6):1–9. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.