Highlights
-
•
The immune status of COVID-19 patients is different in each stage.
-
•
DN and DP cells are negatively correlated with IL-10 and IL-6, respectively.
-
•
Immune indexes help to distinguish COVID-19 and its severity early.
-
•
Dynamic immune monitoring can provide a reference for clinical drug selection.
Keywords: COVID-19, Immune monitoring, Lymphocyte subsets, Cytokines
Abstract
Background
COVID-19 is threating human health worldwide. We aim to investigate the dynamic changes of immune status in COVID-19 patients with clinical evolution.
Methods
Sixty-one COVID-19 patients (42 mild cases and 19 severe cases, 51 cases without secondary infection as non-infection group and 10 cases with secondary bacterial/fungal infection as infection group) and 52 healthy controls (HCs) were enrolled from our hospital. Leucocyte classification, lymphocyte subsets and cytokines were detected by full-automatic blood cell analyzer and flow cytometer, respectively.
Results
Upon admission, eosinophils and lymphocyte subsets decreased significantly, while neutrophils, monocytes, basophils, IL-2, IL-6, IL-10 and IFN-γ increased significantly in COVID-19 patients compared to HCs. CD3+ T and DN (CD3+CD4−CD8−) cells appeared sustained decline, leucocytes, neutrophils and IL-10 showed sustained increase in severe group compared to mild group. Compared with the non-infection group, we observed a depletion of eosinophils, CD3+ T and CD4+ T cells, but leucocytes, neutrophils, IL-6 and IL-10 on the contrary in the infection group. Besides, in severe group of COVID-19 patients, DN cells were negatively correlated with IL-10, and DP (CD3+CD4+CD8+) cells were negatively correlated with IL-6. Lymphocytes, eosinophils, CD3+ T cells, CD4+ T cells, IL-6 and IL-10 all had great diagnostic efficacy (AUC, 0.905-0.975) for COVID-19. The laboratory indicators of COVID-19 patients with improved condition also showed a recovery trend with time.
Conclusions
The immune status of COVID-19 patients is different in each stage, and dynamic monitoring of related indicators can help predict the disease and may avoid cytokine storms.
1. Introduction
In the past six months, coronavirus disease 2019 (COVID-19) has become a global epidemic that seriously threatens human health due to its high infectivity, high mortality, and lack of directed antiviral drugs and vaccines. As of July 10, 2020, over 12 million people worldwide have been diagnosed with COVID-19 and 550384 people have died from it, the mortality has increased from 3.7% reported in March to about 4.6% [1]. He W et al. reported that the basic reproduction number of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) which induced COVID-19 was 3.15, and the asymptomatic infection rate was as high as 46% [2]. In the fight against COVID-19, the scientific community generally focuses on understanding its pathophysiology mechanism and clinical evolution to develop targeted drugs and vaccines, of which immunotherapy has become one of the most promising research directions, such as angiotensin converting enzyme 2 (ACE2) inhibitors, convalescent plasma, natural killer (NK) cells, monoclonal antibodies, etc [3], [4]. Intriguingly, the therapeutic effect of tocilizumab, a cytokine interleukin-6 (IL-6) antagonist for COVID-19 patients, is being conducted clinical controlled trials worldwide. Following used in cytokine release syndrome (CRS) patients receiving chimeric antigen receptor (CAR) T cell therapy, tocilizumab is expected to be used in COVID-19 to promote the recovery of patients' antiviral immunity [5], [6].
Lymphocytes reduction and their correlation with the disease severity as one of the key features of SARS-CoV-2 infection have recently attracted attention. SARS-CoV-2 can infect monocytes, macrophages and dendritic cells to promote the secretion of cytokines, IL-6 can further activate the classic cis signal, trans signal and trans presentation, resulting in multiple effects of acquired immune system (most lymphocytes) and innate immune system (neutrophils, monocytes, NK cells, etc.) [5]. However, due to the variability of SARS-CoV-2 and the complexity of human immune regulatory network, the dynamic changes of immune status in mild and severe COVID-19 patients are still controversial. In some COVID-19 patients, leucocytes and neutrophils could even appear increased or declined, lymphocytes appeared normal, and IL-10 was normal or elevated [7]. What's more, there are few reports on double positive (CD3+CD4+CD8+) T cells (DP), double negative (CD3+CD4−CD8−) T cells (DN) and (CD3+CD16+/CD56+) NKT cells in COVID-19 patients.
We aim to analyze data derived from a designated hospital for referral severe COVID-19 patients in Wenzhou, China, to investigate the immune changes after SARS-CoV-2 infection through dynamic monitoring of laboratory indicators in COVID-19 patients, including leucocyte classification, lymphocyte subsets and cytokines. Simultaneously, we would also focus on the longitudinal changes of DP, DN and NKT cells with disease recovering.
2. Materials and methods
2.1. Patients
We recruited 61 COVID-19 patients who were confirmed by real-time reversetranscriptase polymerase-chain reaction (RT-PCR) assay of nasopharyngeal swab samples. The enrolled patients were divided into mild group (42 cases, age 53.02 ± 13.78, male/female 25/17) and severe group (19 cases, age 65.16 ± 14.94, male/female 13/6) according to the Seventh Version New Coronavirus Pneumonia Diagnosis and Treatment Guidelines of China [8]. Patients in the mild group showed mild clinical symptoms, without imaging manifestations of pneumonia or had fever, respiratory symptoms and imaging manifestations of pneumonia; patients who met any of the following conditions could be included in the severe group: shortness of breath with respiratory rate > 30 times/min, oxygen saturation ≤ 93% in resting state, partial pressure of arterial oxygen (PaO2)/oxygen concentration (FiO2) ≤ 300 mmHg (1 mmHg = 0.133 kPa), progression of pulmonary imaging lesions > 50% within 24–48 h. In addition, we divided enrolled patients into non-infection group (51 cases) and infection group (10 cases) according to whether they had secondary bacterial or fungal infections during hospitalization. Except for SARS-CoV-2, patients with other respiratory virus, hematological diseases, specimens contaminated, or incomplete clinical information were excluded. The common clinical symptoms of the enrolled patients mainly included fever, dry cough, shortness of breath, fatigue and myalgia, a few patients presented with expectoration or hemoptysis, upper respiratory tract symptoms such as sore throat, gastrointestinal symptoms such as diarrhea, which were basically consistent with the previous literature summary [9]. All COVID-19 patients received standardized treatment in accordance with the above guidelines, including antiviral drugs such as α-interferon (α-IFN), lopinavir/ritonavir, ribavirin, chloroquine, etc., oxygen therapy, fluid management, antibiotics treatment if occasion required, symptomatic relief and supportive treatment. Only one COVID-19 patient who was classified as a member of the severe group died, others were relieved and followed up until the patients were discharged. Besides, 52 age- and sex-matched healthy individuals (HCs) with SARS-CoV-2 nucleic acid and antibody negative, other respiratory viruses and bacteria negative, were recruited as control group. The demographic and clinical baseline characteristics of the enrolled COVID-19 patients were shown in Table 1 .
Table 1.
The demographic and clinical baseline characteristics of the enrolled COVID-19 patients.
| Characteristics | COVID-19 | Mild | Severe |
|---|---|---|---|
| Patient count[N] | 61 | 42 | 19 |
| Age[mean ± SD] | 56.80 ± 15.13 | 53.02 ± 13.78 | 65.16 ± 14.94 |
| Male[N (%)] | 38(62.30) | 25(59.52) | 13(68.42) |
| ICU patients[N] | 6 | 0 | 6 |
| ECMO[N] | 1 | 0 | 1 |
| Secondary infection[N] | 10 | 0 | 10 |
| Bacterial infections | 5 | ||
| Gram-negative bacteria | 2 | ||
| Gram-negative and Gram-positive bacteria | 3 | ||
| Fungal infections | 2 | ||
| Mixed bacterial and fungal infections | 3 | ||
| Average hospitalization time[Days] | 25 | 20 | 35 |
| Death[N] | 1 | 0 | 1 |
N (%), sample size (percentage); SD, standard deviation; ICU, intensive care unit; ECMO, extracorporeal membrane oxygenation.
2.2. Laboratory examination
We tested the COVID-19 patients' leucocyte classification, absolute value of lymphocyte subsets and cytokines in 1–3 days (as soon as possible) after admission, and based on the double-blind principle, we randomly selected 17–34 (40–81%) mild patients and 10-17 (56–94%) severe patients (except the dead case) at the following 5 different period to detect the above indicators: 4–7 days, 8–10 days, 11–15 days, 16–20 days, 21–30 days (1–3 days before patients discharged from hospital) after patients admission. At the same time, we tested cytokines of 52 enrolled HCs, then randomly selected 18 cases for leucocyte classification detection, and 20 cases for lymphocyte subsets detection.
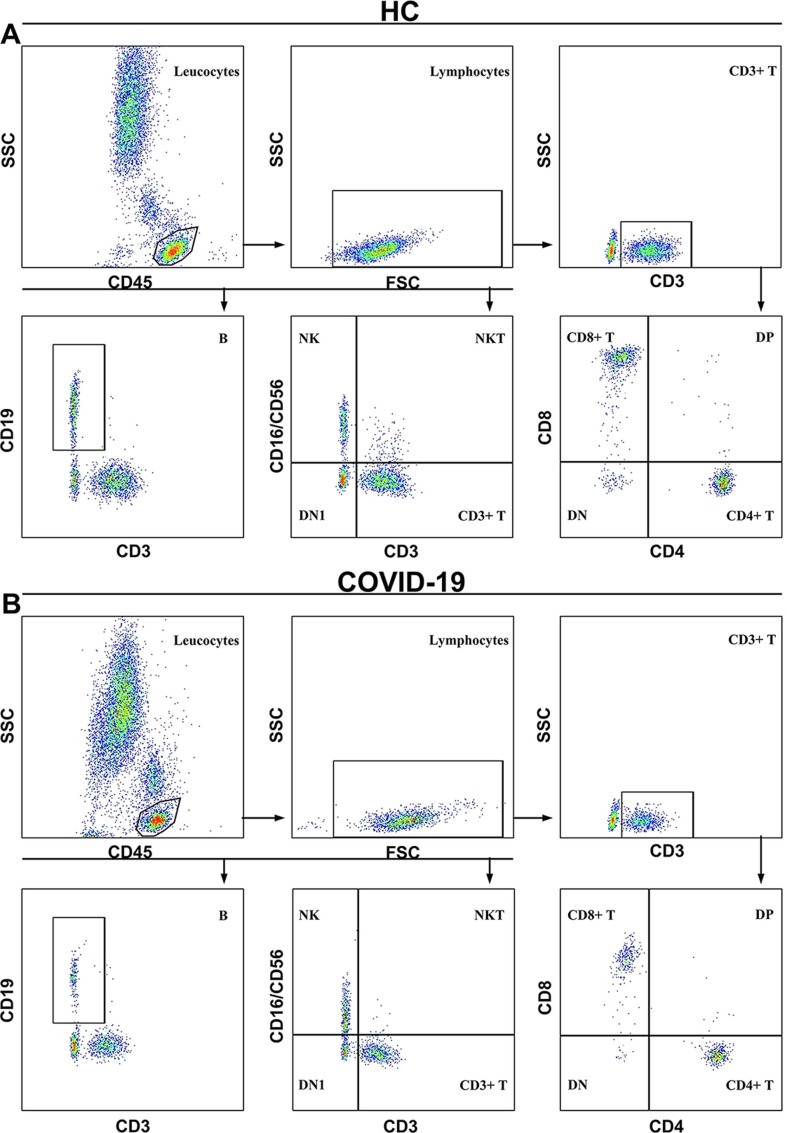
Two ml of whole blood was collected with ethylenediaminetetraacetic acid anticoagulation tube for leucocyte classification (leucocytes, neutrophils, lymphocytes, monocytes, eosinophils and basophils) detection by full-automatic blood cell analyzer (Sysmex XE2100, Japan). Four ml of blood without anticoagulant was took to coagulate naturally and separated serum for lymphocyte subsets (CD3+ T, CD4+ T, CD8+ T, B, NK, DP, DN and NKT cells) and cytokines (IL-2, IL-4, IL-6, IL-10, TNF-α and IFN-γ) detection by FACSCanto II flow cytometer (BD, USA), the reagents were BD Multitest IMK kit (BD Multitest™ CD3/CD8/CD45/CD4 reagent and BD Multitest™ CD3/CD16+CD56/CD45/CD19 reagent) and Th1/2 cytokine kit II, respectively, which purchased from BD Biosciences (San Jose, CA, USA). The antibody protocol used for cell staining of lymphocyte subsets was as follows: PerCP-anti-CD45, FITC-anti-CD3, APC-anti-CD4, PE-anti-CD8, APC-anti-CD19, PE-antiCD16, and PE-anti-CD56. The gating strategy (Fig. 1 ) of CD4+ T, CD8+ T, B, NK, DP, DN and NKT cells was executed as CD3+CD4+, CD3+CD8+, CD3−CD19+, CD3−CD16+/CD56+, CD3+CD4+CD8+, CD3+CD4−CD8− and CD3+CD16+/CD56+, respectively.
Fig. 1.
The representative gating strategy of lymphocyte subsets data. (A) and (B) showed the representative gating strategy for a healthy control (HC) and a COVID-19 patient selected randomly in the study, respectively; DN1, CD3−CD16−/CD56− cells.
2.3. Statistical analysis
GraphPad Prism software (Version 8.0.2, La Jolla, CA) was used for processing data. Continuous variables with normal distribution were expressed as mean ± standard deviation, those with non-normal distribution were expressed as median and quartile intervals. The comparisons between two groups were performed by t-test or Mann-Whitney test. One-way ANOVA test or Kruskal-Wallis rank sum test was used to analyze the variables for three groups. Qualitative variables were expressed as frequency or percentage, tested by Chi-square test or Fisher's exact test. Correlation between variables was analyzed by Spearman correlation test. Diagnostic value of each parameters was assessed by the receiver operation characteristic (ROC) curves and the area under the ROC curve (AUC), the cut-off value was selected when the Jordan index was at it's maximum. For all the tests, definition of statistical significance: the two-tailed P value < 0.05, represented as follows: ****P < 0.0001, ***P < 0.001, **P < 0.01 and *P < 0.05.
3. Results
3.1. The immune status of COVID-19 patients upon admission
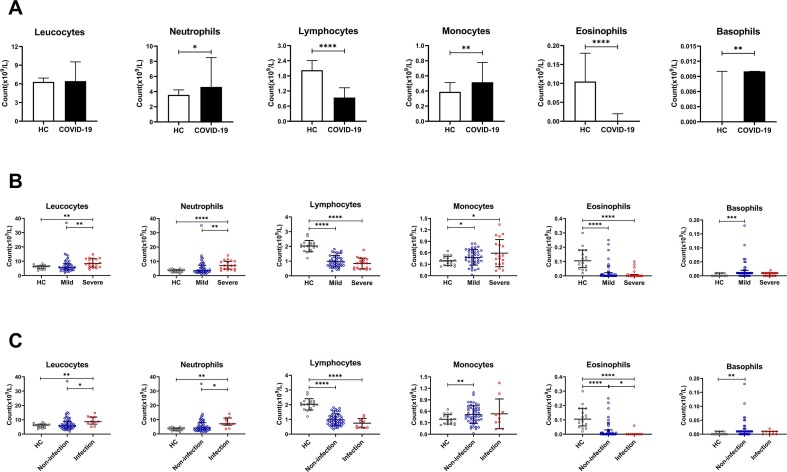
First, we conducted experimental analyses of the earliest collected specimens after the enrolled patients admitted to our hospital. For leucocyte classification, we found that compared with HCs, neutrophils, monocytes, and basophils were significantly increased, and lymphocytes, eosinophils were significantly decreased, while leucocytes had no statistical significance in COVID-19 patients (Fig. 2 A). The leucocytes and neutrophils of the severe group were higher than the mild group, but lymphocytes, monocytes, eosinophils and basophils did not differ between the two groups in COVID-19 patients (Fig. 2B). Interestingly, we found that in COVID-19 patients, leucocytes and neutrophils in the infection group were also higher than those in the non-infection group, while eosinophils were declined; lymphocytes, monocytes and basophils were not markedly different between the two groups (Fig. 2C).
Fig. 2.
The leucocyte classification in COVID-19 patients upon admission. Including the healthy control (HC) and COVID-19 groups (A), mild and severe groups (B), non-infection and infection groups (C); ****P < 0.0001, ***P < 0.001, **P < 0.01 and *P < 0.05.
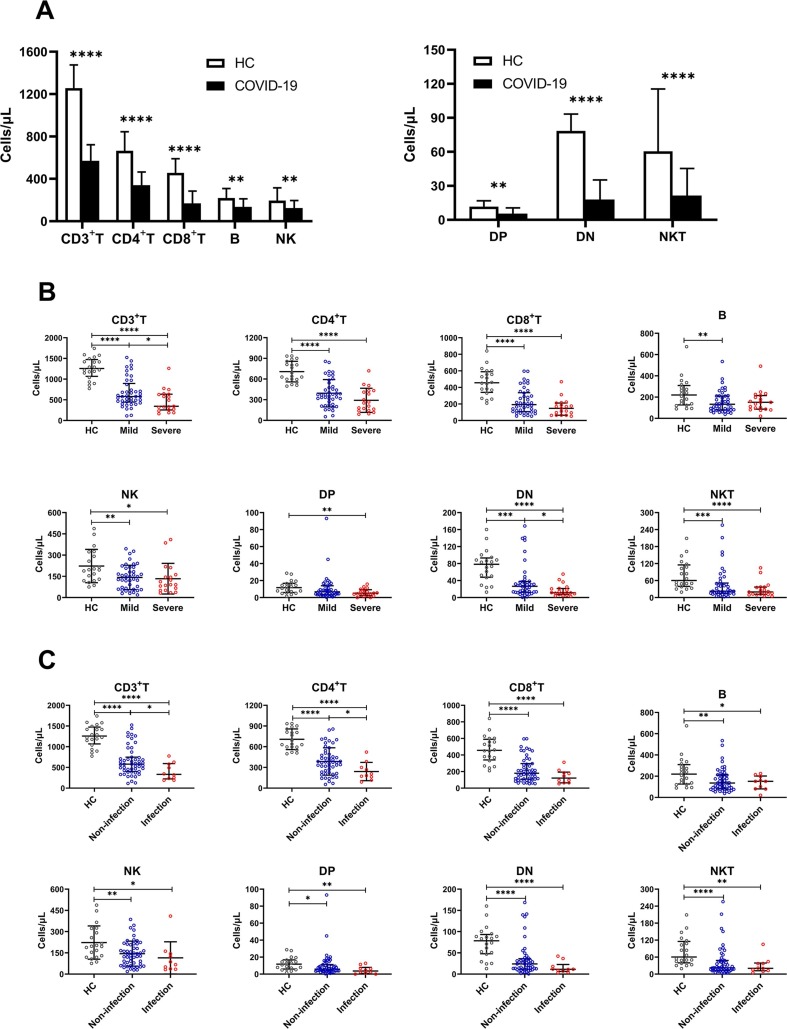
Next, in the analysis of lymphocyte subsets, we found that compared with HCs, all indicators of COVID-19 patients showed a downward trend, especially CD3+ T, CD4+ T, CD8+ T, DN and NKT cells (P < 0.0001) (Fig. 3 A). Among severe cases, only CD3+ T and DN cells continued to decline compared with mild cases, although CD4+ T, CD8+ T and NKT cells also showed a downward trend, there was no statistical difference; B, NK and DP cells did not change significantly (Fig. 3B). In COVID-19 patients with secondary infection, CD3+ T and CD4+ T cells were significantly lower than those without infection, and other lymphocyte subsets were not statistically different (Fig. 3C).
Fig. 3.
The absolute value of lymphocyte subsets in COVID-19 patients upon admission. Including the healthy control (HC) and COVID-19 groups (A), mild and severe groups (B), non-infection and infection groups (C); ****P < 0.0001, ***P < 0.001, **P < 0.01 and *P < 0.05.
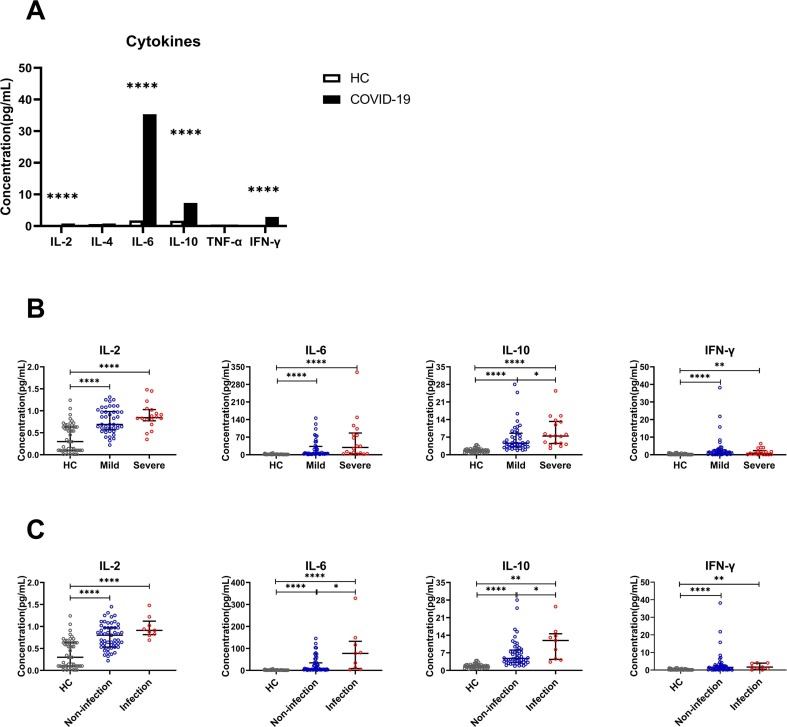
Another interesting finding, the expression of IL-2, IL-6, IL-10 and IFN-γ in COVID-19 patients was significantly higher than that of HCs, IL-4 and TNF-α didn't seem to change significantly (Fig. 4 A). Then, we analyzed IL-2, IL-6, IL-10 and IFN-γ between mild and severe COVID-19 patients, only the expression of IL-10 increased with the severity of the disease, no significant increase in IL-2, IL-6 and IFN-γ (Fig. 4B). However, compared with the non-infection group, we found that the expression of IL-6 and IL-10 in the infection group was both significantly increased, there was no significant difference in IL-2 and IFN-γ expression (Fig. 4C).
Fig. 4.
The cytokines in COVID-19 patients upon admission. Including the healthy control (HC) and COVID-19 groups (A), mild and severe groups (B); non-infection and infection groups (C). ****P < 0.0001, ***P < 0.001, **P < 0.01 and *P < 0.05.
3.2. Comparison of correlation between lymphocyte subsets and cytokines
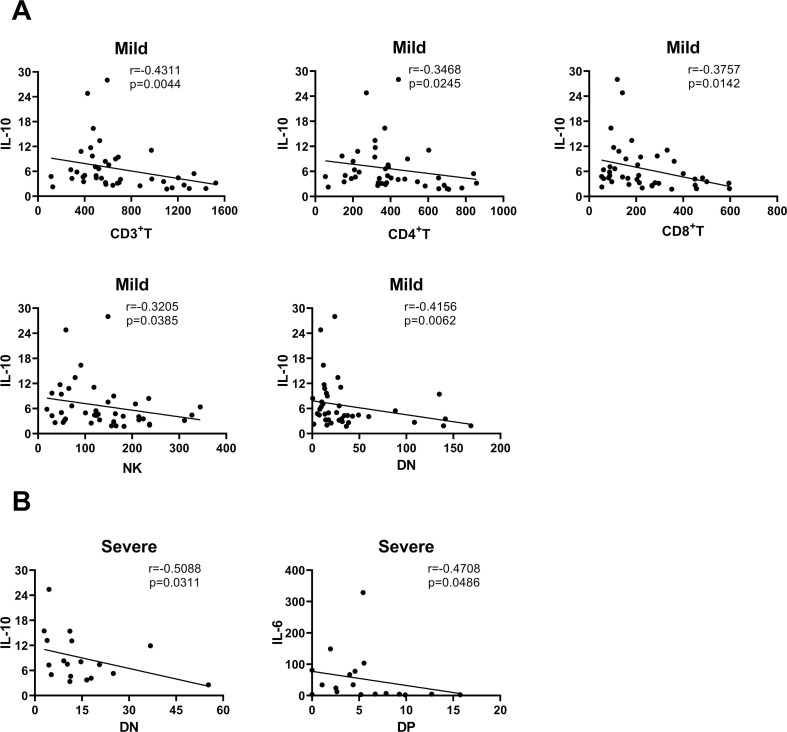
To comprehend the connection among laboratory parameters which assess the immune status of COVID-19 patients, we performed Spearman correlation test for lymphocyte subsets and cytokines of mild and severe COVID-19 patients upon admission. Absorbingly, we found that lymphocyte subsets CD3+ T, CD4+ T, CD8+ T, NK and DN cells had a certain correlation with IL-10 in mild cases, the correlation coefficient of CD3+ T (r, -0.4311; p < 0.01) and DN (r, -0.4156; p < 0.01) cells was relatively higher than other lymphocyte subsets (Fig. 5 A). Besides, DN cells (r, -0.5088; p < 0.05) were associated with IL-10, DP cells (r, -0.4708; p < 0.05) were associated with IL-6 in severe cases (Fig. 5B). There was no obvious correlation (p > 0.05) among other lymphocyte subsets and cytokines (data not shown).
Fig. 5.
The correlation analysis of lymphocyte subsets absolute value and cytokines. Including the analysis of mild (A) and severe (B) groups; r, correlation coefficient; the figure only showed those correlation coefficients with significant differences (P < 0.05).
3.3. Diagnostic efficacy of laboratory parameters for COVID-19 patients
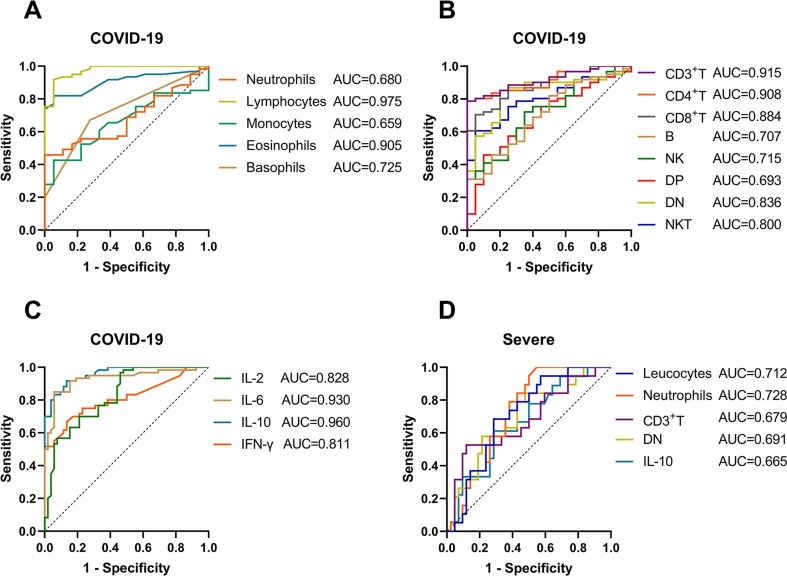
We used the above indicators to perform ROC curve analysis on the COVID-19 and HC groups, severe and mild groups, respectively. In terms of leucocyte classification (Fig. 6 A, Table 2 ), lymphocytes (AUC, 0.975) had the best value in the diagnosis of COVID-19 with sensitivity of 91.8% and specificity of 94.4%; the sensitivity of eosinophils (AUC, 0.905), basophils (AUC, 0.725), neutrophils (AUC, 0.680) and monocytes (AUC, 0.659) were 82.0%, 67.2%, 45.9%, 42.6%, the specificity of those were 94.4%, 72.2%, 100.0%, 94.4%, respectively; the ROC curve of leucocytes was not statistically significant. The lymphocyte subset parameters all showed a certain discrimination ability for COVID-19 patients (Fig. 6B, Table 2), the AUC of CD3+ T, CD4+ T, CD8+ T, DN, NKT, NK, B and DP cells were 0.915, 0.908, 0.884, 0.836, 0.800, 0.715, 0.707, 0.693, respectively; DN cells had the highest sensitivity (85.3%), followed by B (82.0%), CD3+ T (78.7%) and CD4+ T (78.7%) cells; CD3+ T and CD4+ T cells had the highest specificity (100.0%), followed by CD8+ T (95.0%) and NKT (95.0%) cells. Also, in the ROC curves for cytokines to distinguish COVID-19 patients from HCs (Fig. 6C, Table 2), we found IL-10 (0.960) and IL-6 (0.930) had greater AUC than IL-2 (0.828) and IFN-γ (0.811) with sensitivity of 91.7%, 85.0%, 96.7%, 66.7% and specificity of 86.5%, 94.2%, 53.9%, 86.5%, respectively; IL-4 and TNF-α had no statistical difference.
Fig. 6.
The ROC curves of immune indexes to discriminate COVID-19 and its severity. Figures 6A-C showed the ROC curves of leucocyte classification, lymphocyte subsets absolute value and cytokines to distinguish COVID-19 patients from healthy controls, respectively; figure 6D used mild COVID-19 patients as control, ROC curve showed the diagnostic efficacy of the above indicators for severe cases; this figure only showed the parameters with p < 0.05.
Table 2.
Summary of ROC curves parameters for predicting COVID-19 and its severity.
| Indexes | AUC | P | 95% CI | Cut-off value | Jordan index | Sensitivity | Specificity |
|---|---|---|---|---|---|---|---|
| Neutrophils1 | 0.680 | 0.0207 | 0.562–0.799 | >5.08 | 0.459 | 45.9% | 100.0% |
| Lymphocyte1 | 0.975 | <0.0001 | 0.943–1.000 | <1.59 | 0.862 | 91.8% | 94.4% |
| Monocyte1 | 0.659 | 0.0414 | 0.537–0.781 | >0.55 | 0.371 | 42.6% | 94.4% |
| Eosinophils1 | 0.905 | <0.0001 | 0.840–0.970 | <0.04 | 0.764 | 82.0% | 94.4% |
| Basophils1 | 0.725 | 0.0040 | 0.602–0.847 | >0.01 | 0.394 | 67.2% | 72.2% |
| CD3+T1 | 0.915 | <0.0001 | 0.856–0.974 | <760.20 | 0.787 | 78.7% | 100.0% |
| CD4+T1 | 0.908 | <0.0001 | 0.847–0.970 | <494.10 | 0.787 | 78.7% | 100.0% |
| CD8+T1 | 0.884 | <0.0001 | 0.812–0.957 | <233.00 | 0.655 | 70.5% | 95.0% |
| B1 | 0.707 | 0.0056 | 0.583–0.832 | <228.60 | 0.320 | 82.0% | 50.0% |
| NK1 | 0.715 | 0.0041 | 0.593–0.837 | <166.50 | 0.371 | 72.1% | 65.0% |
| DP1 | 0.693 | 0.0097 | 0.567–0.820 | <4.73 | 0.359 | 45.9% | 90.0% |
| DN1 | 0.836 | <0.0001 | 0.741–0.931 | <44.97 | 0.653 | 85.3% | 80.0% |
| NKT1 | 0.800 | <0.0001 | 0.704–0.896 | <28.66 | 0.557 | 60.7% | 95.0% |
| IL-21 | 0.828 | <0.0001 | 0.752–0.903 | >0.35 | 0.505 | 96.7% | 53.9% |
| IL-61 | 0.930 | <0.0001 | 0.881–0.979 | >2.92 | 0.792 | 85.0% | 94.2% |
| IL-101 | 0.960 | <0.0001 | 0.930–0.990 | >2.47 | 0.782 | 91.7% | 86.5% |
| IFN-γ1 | 0.811 | <0.0001 | 0.731–0.891 | >0.70 | 0.532 | 66.7% | 86.5% |
| Leucocytes2 | 0.712 | 0.0089 | 0.581–0.840 | >7.17 | 0.399 | 68.4% | 71.4% |
| Neutrophils2 | 0.728 | 0.0046 | 0.605–0.851 | >3.42 | 0.452 | 100.0% | 45.2% |
| CD3+T2 | 0.679 | 0.0259 | 0.528–0.830 | <356.60 | 0.407 | 52.6% | 88.1% |
| DN2 | 0.691 | 0.0179 | 0.549–0.832 | <25.47 | 0.366 | 84.2% | 52.4% |
| IL-102 | 0.665 | 0.0446 | 0.520–0.810 | >7.21 | 0.325 | 61.1% | 71.4% |
The ROC curves parameters of distinguishing COVID-19 patients from healthy controls.
The ROC curves parameters of predicting severe patients from mild patients; AUC, area under the curve; 95% CI, 95% confidence interval.
What made us more curious was whether the above indicators also had clinical value in distinguishing severe cases from mild COVID-19 cases. Through statistical analysis we found that neutrophils (AUC, 0.728), leucocytes (AUC, 0.712), DN cells (AUC, 0.691), CD3+ T cells (AUC, 0.679) and IL-10 (AUC, 0.665) had the ability to identify with sensitivity of 100.0%, 68.4%, 84.2%, 52.6%, 61.1% and specificity of 45.2%, 71.4%, 52.4%, 88.1%, 71.4%, respectively (Fig. 6D, Table 2). There was no statistical difference in other parameters (data not shown).
3.4. The immune status changes dynamically with clinical evolution in COVID-19 patients
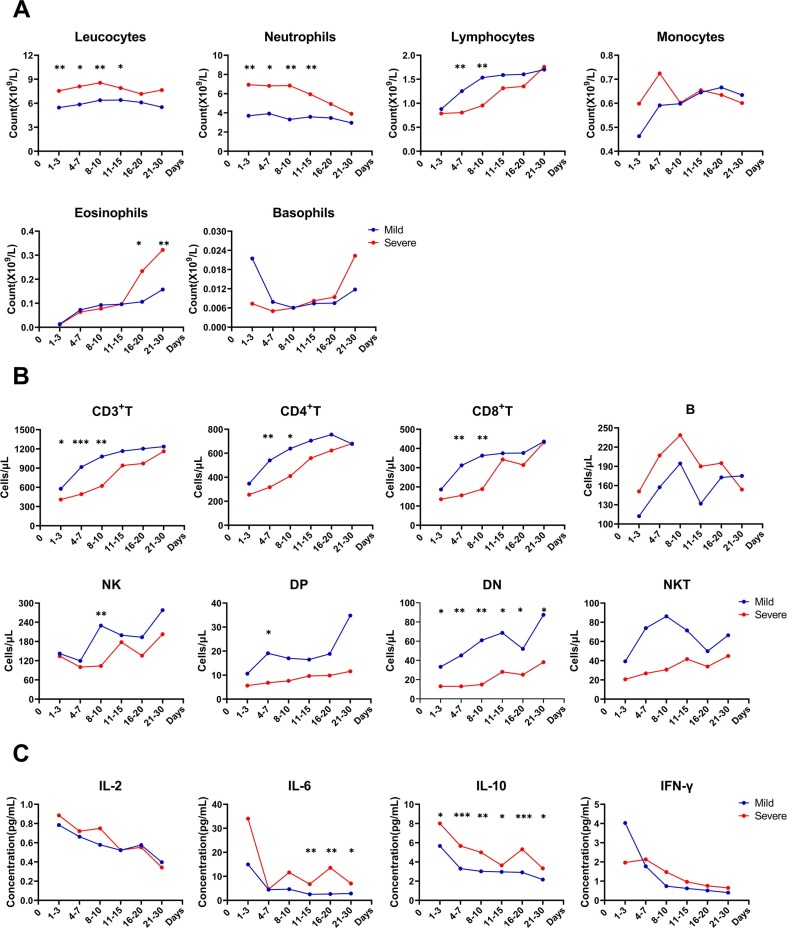
In order to further clarify the dynamic changes of immune status in mild and severe cases, we continuously monitored the above indicators during patients' hospitalization. With regard to leucocyte classification (Fig. 7 A), we found leucocyte and neutrophil counts in the severe group were always greater than those in the mild group, and could reduce to the same level (no statistical difference) in 16–20 days; in contrast, the lymphocytes in the severe group maintained at a lower level during 4–7 days compared to the mild group and began to recovery in 11–15 days, which was completely inconsistent with eosinophils that began to increase significantly during 16–20 days, and until patients' SARS-CoV-2 nucleic acid turned negative, the disease was controlled, and patients were discharged, there was no downward trend for eosinophils; in severe patients, monocytes had an early increase trend and basophils had a late increase trend, but there was no statistical difference between the two groups.
Fig. 7.
The continuous monitoring of patients' immune indexes during hospitalization (except the one dead case). Including the leucocyte classification (A), lymphocyte subsets absolute value (B) and cytokines (C); the detection value of each period in the graph were expressed as mean. ****P < 0.0001, ***P < 0.001, **P < 0.01 and *P < 0.05.
Except for B cells, the lymphocyte subset counts in severe patients were lower than those in mild patients, and all had an upward trend with time (Fig. 7B). Although compared with the mild group, CD3+ T cells in the severe group began to be lower in 1–3 days, and CD4+ T, CD8+ T cells began to be lower in 4–7 days, all of which increased to the same level in both groups in 11–15 days; NK and DP cells decreased significantly with the severity of the disease in 8–10 days and 4–7 days, respectively; interestingly, DN cells of severe cases were significantly lower than those of mild cases during hospitalization; there was no statistical difference for B and NKT cells between the two groups in each period.
Combining with the previous results, here we only continuously monitored the expression of cytokines IL-2, IL-6, IL-10 and IFN-γ. By comparison, it was not difficult to find that the expression of the above four cytokines was the highest in 1–3 days, and then showed a downward trend with time, the severe group is generally higher than the mild group (Fig. 7C). From 11 to 15 days, until the patients were discharged, severe cases expressed IL-6 higher than mild cases, the expression of IL-10 in severe patients throughout the hospitalization was significantly higher than the mild patients, the difference was statistically significant; there was no statistical difference in the change of IL-2 and IFN-γ between the two groups in each period.
4. Discussion
Facing the challenge brought by COVID-19 to human health, we still have no way to explain the huge differences in the symptoms and immune response of COVID-19 patients. Comprehensive understanding of the contribution of COVID-19 patients' various immune indicators to inflammation changes from onset to remission is the key. Wenzhou was one of the cities with the largest number of COVID-19 cases except Wuhan, in China, and the First Affiliated Hospital of Wenzhou Medical University served as the designated hospital for severe and critical COVID-19 patients in Wenzhou city. We hope to provide new ideas and perspectives to overcome COVID-19 through dynamic analysis of leucocyte classification, lymphocyte subsets and cytokines.
Suyu Sun et al. reported that the counts of leucocytes, lymphocytes, and eosinophils in COVID-19 patients were decline, there was no statistical difference for neutrophils and monocytes compared to controls; while in severe COVID-19 cases, the counts of leucocytes and neutrophils increased, lymphocytes and eosinophils continued to decline, monocytes were still not statistically different [10], which is partially consistent with our results. We consider that compared to HCs, the increase of monocytes in COVID-19 patients may be caused by inflammation-mediated monocyte infiltration and activation after SARS-CoV-2 infection [11]. The progressive increase of basophils was believed to be related to non-survivors of COVID-19 patients [12]. In this study, basophils increased slightly in the mild group and the non-infected group compared to HCs. It was reported that basophil histamine release can be enhanced in influenza A virus infection [13], however the effect in COVID-19 is not yet clear. Leukocytes and neutrophils were mainly increased in severe patients and patients with secondary infections, the following mechanism may explain the reason for the change reasonably, the down regulation of ACE2 during SARS-CoV-2 infection triggers the infiltration of neutrophils into the tissue, which can strengthen tissue damage, activate complement, and promote venous thrombosis formation [14]. In the infection group, we also observed a significant decrease in eosinophils compared to the non-infection group, which may be related to involvement of eosinophils in immune regulation and antiviral roles [15]. It is confusing that although the lymphocytes showed a continuous downward trend in the severe group and the infection group, there was no statistical difference compared to the mild group and the non-infection group, this seems to be a bit different from previous reports [16]. We suspect that the critical patients in our hospital are mainly referrals from other hospitals rather than first diagnosed in our hospital, so our data may be more likely to reflect the changes in laboratory parameters during the patients' remission period.
In this study, compared with the mild patients, the decrease of CD3+ T cells in the severe group mainly due to the decrease of DN cells, as for why it were not CD4+/CD8+ T cells, we deemed the same reason as the lymphocytes mentioned earlier. Nevertheless, the decrease in CD3+ T cells was mainly accompanied by the decrease of CD4+ T cells, which was more statistically significant between infection group and non-infection group. This is consistent with the conclusion of Xiaonan Zhang et al. They believed that CD3+ T cells were the principal cell type suppressed in infected organisms, but B and NK cells were relatively less suppressed [17]. Science also reported on May 22 that the severity of COVID-19 could depend on the intensity of helper T cell and killer T cell responses [18]. It is undeniable that the downward trend of CD4+/CD8+T cells in severe patients and patients with secondary infections is still very prominent, and more interestingly, this study observed that the expression of DN cells seems to have significant difference between different severity groups. Although the proportion of DN cells is small and rarely attracts everyone's attention, it can't be used as a reason to ignore its functional performance in immune regulation, such as DN cells can down-regulate the immune response, be resistant to activation-induced cell death, and highly expresses perforin to regulate DN cell-mediated suppression, etc [19].
Meanwhile, we found that IL-2, IL-6, IL-10 and IFN-γ in COVID-19 patients increased significantly, which were partly similar to the study of Jing Liu and her colleagues [20]. IL-10 increased significantly with the severity of the disease; compared with the non-infection group, IL-6 and IL-10 in the infection group also increased in our research. It is not surprising that IL-6 as a pro-inflammatory cytokine is elevated in infectious diseases, often accompanied by an increase of anti-inflammatory cytokine IL-10. We further analyzed the correlation between lymphocyte subsets and cytokines, and found that DN cells were negatively correlated with IL-10 in COVID-19 patients, DP cells and IL-6 also showed a negative correlation in severe patients. The specific mechanism between DN cells and IL-10 is unclear, and it is suspected that this may be related to the previously mentioned DN cells' role in suppressing immune response. In addition, research showed that lymphocyte counts were negatively correlated with IL-6 [17], which also verified our view from the side. Misme-Aucouturier B etc. pointed out in a study of Candida albicans infection that DP cells were involved in adaptive immune response and could secrete IL-4 and IL-10 [21]. However, the mechanism of DP cells action in SARS-CoV-2 infection and its regulatory role with IL-6 are still unclear.
We next used ROC curve analysis to verify the difference of the above indicators in COVID-19 patients. Lymphocytes, eosinophils, CD3+ T cells, CD4+ T cells, CD8+ T cells, DN cells, IL-2, IL-6, IL-10 and IFN-γ all had great diagnostic efficacy (AUC > 0.800) for COVID-19 patients. Distinguishing severe cases from the mild cases, only the following indicators have statistical value: leucocytes, neutrophils, CD3+ T cells, DN cells and IL-10 with AUC of 0.712, 0.728, 0.679, 0.691, 0.665. Many studies have tried to predict the severity of COVID-19, among which the more conspicuous indicators included the neutrophil-to-lymphocyte ratio (NLR) (AUC, 0.888-0.93), neutrophil-to-CD8+T cell ratio (N8R) (AUC, 0.94) and monocyte-to-lymphocytes ratio (MLR) (AUC, 0.862) [10], [20]. We also confirmed that in severe patients, neutrophils and monocytes appeared an upward tendency, lymphocytes and CD8+ T cells showed a downward trend, so further increase in NLR, N8R and MLR should be inevitable. Given the uncontrollability of the patients' immune changes and the difficulty of unifying tests' accuracy in various laboratories, we recommend that distinguishing the severity of COVID-19 patients by referring to the above indicators, and combining the patients' clinical manifestations and other imaging tests are also indispensable.
Besides, in the dynamic detection of the patients' immune status during hospitalization, we observed in the early stages of SARS-CoV-2 infection, leucocytes and neutrophils increased significantly, lymphocytes, CD3+ T cells, CD4+ T cells and CD8+ T cells decreased significantly in severe patients. According to previous discussion, this may be a sign of increased tissue damage and declined immunity in COVID-19 patients, so it would be more likely to develop into severe disease. The time of leucocytes and neutrophils in all severe patients who were relieved by standardized treatments began to match the level of mild patients (16–20 days) later than lymphocytes, CD3+ T cells, CD4+ T cells and CD8+ T cells (11–15 days). Ruyuan He et al. held that T lymphocyte value in patients with improved conditions began to increase after about 15 days of treatment, until they returned to normal levels [22]. The CD3+ T, CD4+ T, and CD8+ T cells we observed started to rise slowly after being hospitalized. It may be because the starting point of observation for the enrolled severe patients was that COVID-19 had been diagnosed in external hospitals, and we could implement effective treatment measures as early as possible. However, the most significant increase for T lymphocytes was about 10 days after treatment. Supplementary note, in the later stages (about 16–20 days after treatment) of the disease and until the patients were discharged, eosinophils of severe patients were significantly higher than those in mild patients, it should be that eosinophils, as a part of antiviral immunity, increasing irritably in the later stages of the disease due to the patients' immunity had been restored to a certain extent after treatment. It was puzzling that DN cells in severe cases were always lower than those in mild group until the patients were discharged, the reason for which has been unclear. Maybe we need to extend the follow-up time of patients to clarify the changes of DN cells in the later period. The expression of IL-6 gradually decreased with time, but after 11–15 days of treatment, the severe group began to appear higher than the mild group, this may be related to secondary bacterial/fungal infections in 53% (Table 1) of severe patients in the late-stage. IL-6 expression helped the host resist infection and tissue damage, but overexpression may induce cytokine storm [23]. The expression of IL-10 in severe group had been decreased at a higher level than the mild group with time, this indicates that the body's anti-inflammatory ability persistently exist during infection, but it needs to be balanced with the level of pro-inflammatory to achieve a normal physiological function of the body.
In this research, we noticed another interesting and possibly controversial issue, that is, under the COVID-19 pandemic, what kind of impact might the current therapeutic interventions have on the body's immunity? Whether this would interfere with our longitudinal detection for COVID-19 patients' immune status? Firstly, for COVID-19 patients, the antiviral interventions what we commonly implemented included virus-targeted drugs lopinavir/ritonavir and ribavirin, host-targeted drugs α-IFN and chloroquine as mentioned in the part of materials and methods. Lopinavir/ritonavir was initially used as human immunodeficiency virus (HIV) protease inhibitor to play an antiviral role; ribavirin, a nucleoside analogue, was approved for use in hepatitis C virus (HCV) and respiratory syncytial virus (RSV) infections, both of which have been evaluated in severe acute respiratory syndrome (SARA) and Middle East respiratory syndrome (MERS) patients; IFN was one of the conventional drugs used to treat hepatitis B virus (HBV) and HCV infections, it was reported that IFN could promote innate antiviral effects; chloroquine had an outstanding position in the fight against malaria and meanwhile acted as immune modulator to suppress SARS-CoV-2 [24]. The anti-SARS-CoV-2 effects of such drugs still need a certain period of clinical trial to confirm. Dhiraj Acharya etc. proposed that although IFN can regulate monocyte-derived macrophages, NK cells and T cells in coronavirus infection, whether these immune cell changes are caused by IFN for SARS-CoV-2 infection remain unclear [25]. Early studies have shown that chloroquine could promote the function of CD8+ T cells during human HCV, HBV and HIV infections [26]. However, recently chloroquine seems to be on the cusp of public opinion regarding the treatment of COVID-19, its Emergency Use Authorization was withdrawn by the US Food and Drug Administration (FDA), the efficacy of chloroquine and the effect on the body's immunity should be in the active exploration stage [27], [28], [29]. So far, at least one thing is clear to us, we can evaluate the dynamic changes between host and SARS-CoV-2 infection under current treatment through immune monitoring and guide the choice of drugs and optimal medication time in clinic. Secondly, the selection of different therapeutic interventions would be strictly implemented in accordance with the Seventh Version New Coronavirus Pneumonia Diagnosis and Treatment Guidelines of China, videlicet, both the mild patients and the severe patients received reasonable treatment based on the same treatment principles and evaluation criteria, therefore, in the continuous monitoring of patients' immune indexes, we have reason to believe the effects of different treatments on the research results between different COVID-19 severity groups could be matched. Moreover, the biomarkers measured in actual clinical management could more veritably reflect the clinical significance of COVID-19 patients' immune status.
In conclusion, we conducted a systematic analysis of the dynamic changes in the immune status of COVID-19 patients through the continuous detection of laboratory indicators. Our research showed the changes of leucocytes, neutrophils, lymphocytes, eosinophils, CD3+ T, CD4+ T, CD8+ T, DN cells, IL-6 and IL-10 were more outstanding with the severity of the disease. Moreover, DN cells were negatively correlated with IL-10, and DP cells in severe group were negatively correlated with IL-6. Leucocytes, neutrophils, CD3+ T cells, DN cells and IL-10 had the ability to identify severe patients to a certain extent, but combined with other clinical characteristics of patients for comprehensive analysis to be needed.
CRediT authorship contribution statement
Jingjing Guan: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Data curation, Writing - original draft, Writing - review & editing. Xin Wei: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Data curation, Writing - original draft, Writing - review & editing. Shuang Qin: Validation, Formal analysis, Investigation, Data curation, Visualization. Xiaoyuan Liu: Validation, Formal analysis, Investigation, Data curation, Visualization. Yujie Jiang: Validation, Formal analysis, Investigation, Data curation, Visualization. Yingxiao Chen: Conceptualization, Resources, Methodology, Validation. Yanfan Chen: Conceptualization, Resources, Methodology, Validation. Hong Lu: Methodology, Resources, Data curation. Jingjing Qian: Methodology, Resources, Data curation. Zhongyong Wang: Funding acquisition. Xiangyang Lin: Conceptualization, Methodology, Investigation, Data curation, Writing - review & editing, Visualization, Supervision, Project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Funding
This work was supported by the Natural Science Foundation of Zhejiang Province of China [grant number LY16H200004]. The funding bodies had no role in the study design; data collection, analysis and interpretation; the writing of the report; and the decision to submit the article for publication.
Ethical approval
The research protocol was approved by the Institutional Review Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University.
Consent to participate
Written informed consent had been obtained from studied patients or their authorized family members.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.intimp.2020.107034.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.World Health Organization (WHO). Coronavirus disease (COVID-19) outbreak situation reports. Available at: https://www.who.int/emergencies/diseases/novel- coronavirus-2019. Accessed 10 July 2020.
- 2.He W., Yi G.Y., Zhu Y. Estimation of the basic reproduction number, average incubation time, asymptomatic infection rate, and case fatality rate for COVID-19: Meta-analysis and sensitivity analysis. J. Med. Virol. 2020 doi: 10.1002/jmv.26041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen J. Vaccine designers take first shots at COVID-19. Science (New York, N.Y.) 2020;368:14–16. doi: 10.1126/science.368.6486.14. [DOI] [PubMed] [Google Scholar]
- 4.Pooladanda V., Thatikonda S., Godugu C. The current understanding and potential therapeutic options to combat COVID-19. Life Sci. 2020;254:117765. doi: 10.1016/j.lfs.2020.117765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science (New York, N.Y.) 2020;368:473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 6.Mazzoni A., Salvati L., Maggi L. Impaired immune cell cytotoxicity in severe COVID-19 is IL-6 dependent. J. Clin. Invest. 2020 doi: 10.1172/jci138554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Velavan T.P., Meyer C.G. Mild versus severe COVID-19: Laboratory markers. Int J Infect Dis.: IJID: Off. Publ. Int. Soc. Infect. Dis. 2020;95:304–307. doi: 10.1016/j.ijid.2020.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Health Commission of China. New coronavirus pneumonia prevention and control program. Available at: http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml?spm=C73544894212.P59511941341.0.0. Accessed 5 July 2020.
- 9.Harapan H., Itoh N., Yufika A. Coronavirus disease 2019 (COVID-19): a literature review. Journal of Infection and Public Health. 2020;13:667–673. doi: 10.1016/j.jiph.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun S., Cai X., Wang H. Abnormalities of peripheral blood system in patients with COVID-19 in Wenzhou, China. Clin. Chim. Acta. 2020;507:174–180. doi: 10.1016/j.cca.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat. Rev. Immunol. 2020;20:355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen R., Sang L., Jiang M. Longitudinal hematologic and immunologic variations associated with the progression of COVID-19 patients in China. J. Allergy Clin. Immunol. 2020 doi: 10.1016/j.jaci.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clementsen P., Norn S., Kristensen K.S., Hannoun C. Influenza A virus enhances basophil histamine release and the enhancement is abolished by carbohydrates. Allergy. 1990;45:471–476. doi: 10.1111/j.1398-9995.1990.tb01099.x. [DOI] [PubMed] [Google Scholar]
- 14.Tomar B., Anders H.-J., Desai J., Mulay S.R. Neutrophils and neutrophil extracellular traps drive necroinflammation in COVID-19. Cells. 2020;9 doi: 10.3390/cells9061383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindsley A.W., Schwartz J.T., Rothenberg M.E. Eosinophil responses during COVID-19 infections and coronavirus vaccination. J. Allergy Clin. Immunol. 2020 doi: 10.1016/j.jaci.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guan W.J., Ni Z.Y., Hu Y. Clinical Characteristics of coronavirus disease 2019 in China. New England J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X., Tan Y., Ling Y. Viral and host factors related to the clinical outcome of COVID-19. Nature. 2020 doi: 10.1038/s41586-020-2355-0. [DOI] [PubMed] [Google Scholar]
- 18.Leslie M. T cells found in coronavirus patients ‘bode well’ for long-term immunity. Science (New York, N.Y.) 2020;368:809–810. doi: 10.1126/science.368.6493.809. [DOI] [PubMed] [Google Scholar]
- 19.Zhang D., Yang W., Degauque N. New differentiation pathway for double-negative regulatory T cells that regulates the magnitude of immune responses. Blood. 2007;109:4071–4079. doi: 10.1182/blood-2006-10-050625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J., Li S., Liu J. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55:102763. doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Misme-Aucouturier B., Touahri A., Albassier M. Double positive CD4+CD8+ T cells are part of the adaptive immune response against Candida albicans. Hum. Immunol. 2019;80(12):999–1005. doi: 10.1016/j.humimm.2019.09.008. [DOI] [PubMed] [Google Scholar]
- 22.He R., Lu Z., Zhang L., Fan T., Xiong R., Shen X., Feng H., Meng H., Lin W., Jiang W., Geng Q. The clinical course and its correlated immune status in COVID-19 pneumonia. J. Clin. Virol. 2020;127:104361. doi: 10.1016/j.jcv.2020.104361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang S., Tanaka T., Narazaki M., Kishimoto T. Targeting interleukin-6 signaling in clinic. Immunity. 2019;50:1007–1023. doi: 10.1016/j.immuni.2019.03.026. [DOI] [PubMed] [Google Scholar]
- 24.Li G., De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat. Rev. Drug Discov. 2020;19(3):149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- 25.Acharya D., Liu GuanQun, Gack M.U. Dysregulation of type I interferon responses in COVID-19. Nat. Rev. Immunol. 2020;20(7):397–398. doi: 10.1038/s41577-020-0346-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Accapezzato D., Visco V., Francavilla V. Chloroquine enhances human CD8+ T cell responses against soluble antigens in vivo. J. Exp. Med. 2005;202:817–828. doi: 10.1084/jem.20051106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saitz R., Schwitzer G. Communicating science in the time of a pandemic. JAMA. 2020;324:443. doi: 10.1001/jama.2020.12535. [DOI] [PubMed] [Google Scholar]
- 28.Kupferschmidt K. Big studies dim hopes for hydroxychloroquine. Science. 2020;368:1166–1167. doi: 10.1126/science.368.6496.1166. [DOI] [PubMed] [Google Scholar]
- 29.Hernandez A.V., Roman Y.M., Pasupuleti V. Update alert: hydroxychloroquine or chloroquine for the treatment or prophylaxis of COVID-19. Ann. Intern. Med. 2020;173(4):78–79. doi: 10.7326/L20-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.