Abstract
Purpose
To describe the clinical characteristics and outcomes of coronavirus disease-2019 (COVID-19)-associated pulmonary thromboembolism (PTE).
Materials and methods
A case series of five patients, representing the clinical spectrum of COVID-19 associated PTE. Patients were admitted to four hospitals in Germany, Italy, and France. Infection with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) was confirmed using a real-time reverse transcription polymerase chain reaction test.
Results
The onset of PTE varied from 2 to 4 weeks after the occurrence of the initial symptoms of SARS-CoV-2 infection and led to deterioration of the clinical picture in all cases. PTE was the primary reason for hospital admission after a 2-week period of self-isolation at home (1 patient) and hospital readmission after initial uncomplicated hospital discharge (2 patients). Three of the patients had no past history of clinically relevant risk factors for venous thromboembolism (VTE). Severe disease progression was associated with concomitant increases in IL-6, ferritin, and D-Dimer levels. The outcome from PTE was related to the extent of vascular involvement, and associated complications.
Conclusion
PTE is a potential life-threatening complication, which occurs frequently in patients with COVID-19. Intermediate therapeutic dose of anticoagulants and extend thromboprophylaxis are necessary after meticulous risk-benefit assessment.
Keywords: SARS-CoV-2, COVID-19, Pulmonary embolism, Thromboprophylaxis, Venous thromboembolism
Highlights
-
•
Pulnonary thromboembolism (PTE) is a frequent life-threatening complication in patients with COVID-19.
-
•
The onset of PTE varies from 2 to 4 weeks after the occurrence of the initial symptoms.
-
•
PTE may occur in patients without past history of risk factors for venous thromboembolism and in those receiving standard prophylactic anticoagulation.
-
•
Intermediate therapeutic dose of anticoagulants and extend thromboprophylaxis are necessary in these patients after meticulous risk-benefit assessment.
List of abbreviations
| aPTT | Activated partial thromboplastin time |
| BE | Base excess |
| ECMO | Extra corporeal membrane oxygenator |
| GGO | Ground glass opacities |
| CTA | Computer Tomographic Angiography |
| COVID-19 | Corona virus disease-2019 |
| DVT | Deep vein thrombosis |
| ICU | Intensive care unit |
| IL | Interleukin |
| IMC | Intermediate Care Unit |
| INR | International normalized ratio |
| I.U. | International unit |
| IQ | interquartile range |
| LMWH | Low molecular weight heparin |
| MAP | Mean arterial pressure |
| PT | Prothrombin time |
| PAP | Pulmonary artery pressure |
| PTE | Pulmonary thromboembolism |
| RT-PCR | Real-time reverse transcription polymerase chain reaction |
| r-tPA | Recombinant tissue-type plasminogen activator |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| TT | Thrombin time |
| VTE | Venous thromboembolism |
1. Introduction
Pulmonary thromboembolism (PTE) is a potentially fatal complication that has been frequently reported in patients with coronavirus disease-2019 (COVID-19) [[1], [2], [3], [4]]. Infection with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) is associated with coagulation abnormalities [[4], [5], [6], [7]], which predispose to considerable procoagulant effects [4,5,8].
In this report, we describe the clinical characteristics and outcomes of five patients admitted to four hospitals in Germany, Italy, and France with COVID-19-associated PTE and discuss the diagnostic and therapeutic implications of PTE in COVID-19.
2. Main text
2.1. Case series
2.1.1. Case #1
A 66-year-old male patient was admitted to the interdisciplinary intensive care unit (ICU) of Jena University Hospital with rapidly progressive dyspnea and swelling of the right thigh. Two weeks prior to ICU admission, the patient had complained of cough, malaise, and fever up to 38 °C. He self-isolated at home after being tested positive for SARS-CoV-2 using a real-time reverse transcription polymerase chain reaction (RT-PCR) test. The patient was known to have systemic hypertension, treated with an angiotensin converting enzyme inhibitor.
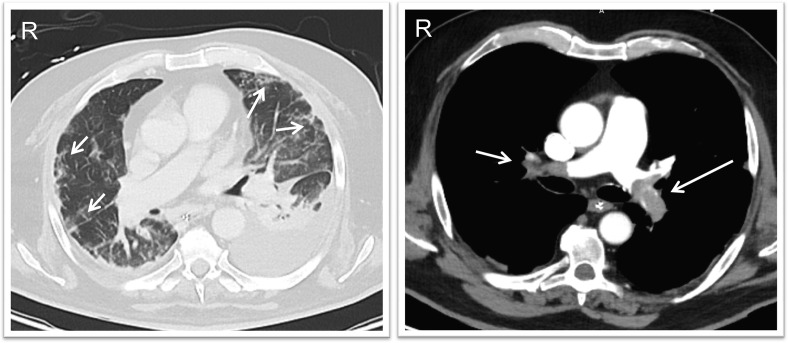
Initial clinical examination on admission to the ICU revealed marked dyspnea and arterial oxygen saturation (SO2) of 92% (with a 10 L/min oxygen mask). Transthoracic echocardiography showed a markedly dilated right ventricle and paradoxical septal motion. Bilateral peripheral ground glass opacities (GGO) were observed on the computed tomography angiography (CTA) scan, together with bilateral central PTE with subtotal occlusion of the left lower lobe and right upper lobe arteries, and multiple segmental occlusions of the right upper and lower lung lobes (Fig. 1 ). A right femoral vein thrombosis was identified on compression ultrasonography of the lower limb. Angiographic ultrasound-enhanced lysis was performed with infusion of recombinant tissue-type plasminogen activator (r-tPA) and heparin (800–1200 IU/h).
Fig. 1.
Computed tomography scan images (case #1), showing bilateral ground glass opacities (arrows, left panel) and central thrombi occluding the main pulmonary arteries bilaterally (arrows, right panel).
Twenty-four hours following ICU admission, the patient's dyspnea and preexisting hypoxemia worsened despite oxygen therapy. Non-invasive ventilation was started but when there was no marked improvement in the respiratory condition after 2 h, tracheal intubation and controlled invasive mechanical ventilation were necessary and prone positioning was started. The hemodynamic situation deteriorated rapidly after intubation and a CTA scan revealed progression of the PTE into the segmental arteries of the right upper lobe and both lower lung lobes, in addition to progression of the GGO bilaterally. Systemic lysis was performed using intravenous r-tPA. Over subsequent days, the patient developed progressive multi-organ failure (Table 1 ). Despite maximal supportive therapy, the patient died on the 6th day in the ICU due to therapeutic refractory shock and multi-organ failure.
Table 1.
Case #1: Laboratory parameters during the ICU stay.
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | |
|---|---|---|---|---|---|---|
| C-reactive protein, mg/dL | 85 | 82 | 101 | 137 | 135 | 89 |
| Procalcitonin, ng/ml | 0.18 | 0.21 | 4.34 | 16 | 17 | 7.2 |
| Interleukin-6, pg/ml | 47 | 76 | 776 | 168 | 401 | 2464 |
| Leukocyte count, x103/μL | 7.1 | 7.2 | 18.1 | 10.1 | 14.2 | 13.6 |
| Lymphocyte count, x103/μL | 0.87 | . | 0.68 | |||
| Creatinine, μmol/l | 83 | 68 | 194 | 303 | 364 | 293 |
| Urea, mmol/l | 5.6 | 5.8 | 6.8 | 7.2 | 8.5 | 6.3 |
| Bilirubin, μmol/l | 15 | 15 | 38 | 43 | 74 | 116 |
| Thrombocytes, x103/μL | 176 | 154 | 155 | 193 | 120 | 54 |
| aPTT, sec | 25–39 | 24–71 | 48–150 | 74–61 | 70–80 | 65 |
| Arterial blood gases | ||||||
| pH, range | 7.47 | 7.18–7.49 | 7.18–7.29 | 7.17–7.29 | 7.18–7.38 | 7.24–7.33 |
| PCO2, kPa, range | 4.23–5.41 | 4.76–11.1 | 6.66–10.7 | 5.65–7.67 | 5.57–7.67 | 4.88–6.56 |
| PO2, kPa, range | 11.4–14.6 | 6.56–15.5 | 10.8–17.8 | 8.1–21.1 | 10–19.9 | 9.6–9.8 |
| SO2%, range | 97 | 85–98 | 94–98 | 91–99 | 94–99 | 91–92 |
| Serum lactate, mmol/L, range | 2.5 | 1.5–6.2 | 6.2–11.6 | 5.7–13.3 | 6.9–20 | 20–21 |
| Ferritin, μg/l | 1267 | 1029 | 30,116 | 170,694 | 43,615 | 24,688 |
| Troponin I, pg/ml | 96.5 | 91.6 | 959 | 483 | 4227 | 4113 |
| BNP, pg/ml | 289 | – | 16,389 | 1212 | 695 | – |
| LDH, μmol/l | 6.4 | 8.11 | 41.2 | 132 | 58 | 41 |
| ASAT, μmol/l | 0.69 | 0.9 | 23.2 | 170 | 94.9 | 85.3 |
| ALAT, μmol/l | 0.52 | 0.54 | 11.5 | 46.4 | 32 | 55.6 |
| Gamma GT, μmol/l | 1.38 | 1.26 | 3.46 | 2.78 | 2.39 | 1.46 |
| GLDH, μmol/l | 54 | 69 | 1049 | 34,800 | 30,398 | 13,932 |
| AT III, % | 70 | 54 | 37 | 33 | 10–60 | 32 |
| D-Dimer, μg/l | 857 | 31,608 | 65,448 | 48,588 | 17,605 | 8682 |
| Fibrinogen, g/l | 3.6 | 3 | 2.2 | 2.3 | 0.8 | 1 |
| Blood sugar, mmol/l, range | 16.7–9.4 | 8.9–15.6 | 6.2–10.2 | 5.3–7.3 | 3.9–6.4 | 5.1–5.4 |
aPTT: activated partial thromboplastin time, ALT: Alanin-Aminotransferase, AST: Aspartat-Aminotransferase, AT: antithrombin, BNP: B-Typ Natriuretisches Peptid, LDH: lactate dehydrogenase.
2.1.2. Case #2
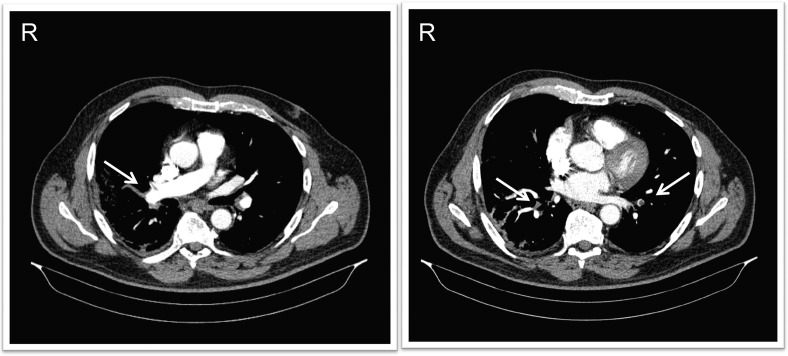
A 65-year-old male patient presented to the emergency room of Ospedale Guglielmo da Saliceto (Piacenza, Italy) with thoracic pain and dyspnea. He had no history of comorbid conditions. Two weeks prior to his emergency room presentation, he had been discharged home from the same hospital, where he had been admitted for 7 days because of a 10-day history of cough and fever. A CT scan of the lungs at that time had shown bilateral peripheral GGO (Fig. 1 of the electronic supplement material) and a nasopharyngeal swab confirmed SARS-CoV-2 infection using RT-PCR. During this presentation to the emergency room, his SO2 was 82% while breathing ambient air. The initial blood gas analysis showed mild hypoxemia (arterial partial pressure of oxygen (PaO2)73 mmHg) on 4 L/min oxygen via nasal prongs. Transthoracic echocardiography showed mild dilatation of the right ventricle, with preserved left ventricular function (Fig. 2 of the electronic supplement material). Compression ultrasound of the lower extremities excluded deep vein thrombosis. A CTA scan showed evidence of subtotal occlusion of the right pulmonary artery at the level of the middle lung lobe and subsegmental obstruction at the level of the inferior lobes (Fig. 2). The patient was admitted to the intermediate care unit (IMC) and treated with 8000 IU b.i.d. of low molecular weight heparin (LMWH, enoxaparin sodium) and standard oxygen therapy. The clinical picture improved over the subsequent 5 days and the patient was discharged home after resolution of the respiratory dysfunction to continue therapeutic anticoagulation using LMWH.
Fig. 2.
Computed tomographic angiography images showing subtotal occlusion of the right pulmonary artery at the level of the middle lung lobe (arrows, left panel) and subsegmental obstruction at the level of the inferior lobes (arrows, right panel).
2.1.3. Case #3
A 56-year-old male patient was admitted to the emergency room of Ospedale Guglielmo da Saliceto (Piacenza, Italy) because of sudden-onset, progressive dyspnea. He had no past history of comorbid conditions. Two days before presentation, he had been discharged from the same hospital, where he had been admitted for 13 days because of confirmed SARS-CoV-2 infection with dyspnea. A CT scan of the lungs had shown extended bilateral GGO and inferior lobar pneumonia in the right lung (Fig. 3 of the electronic supplement material).
At his subsequent presentation to the emergency room, his oxygen saturation was 88% while breathing ambient air. Arterial blood gas analysis showed moderate hypoxemia (pH 7.45, arterial partial pressure of carbon dioxide (PaCO2) 37 mmHg, PaO2 86 mmHg on 12 L/min). Lung CTA confirmed thromboembolic obstruction of lobar and segmental vessels of the pulmonary artery of both superior and inferior lobes and of the middle lobe (Fig. 4 of the electronic supplement material). The patient was admitted to the IMC and anticoagulation initiated with a bolus of 6000 IU of LMWH (enoxaparin sodium) followed by 8000 IU daily. A three-point compression ultrasound of the lower extremities was negative for deep vein thrombosis. During the IMC stay, the patient remained hemodynamically stable and afebrile. Dyspnea resolved within 3 days. He was discharged home after resolution of the respiratory dysfunction to continue therapeutic LMWH anticoagulation.
2.1.4. Case #4
A 41-year-old male was admitted to the emergency room of Hȏpital Nord Marseille (Marseille, France) because of progressive dyspnea. The patient had diabetes mellitus type II and hypothyroidism in his past medical history. Flu-like symptoms had started 7 days prior to admission and the patient was treated on an ambulatory basis. Initial clinical examination revealed marked dyspnea and a SaO2 94% (under 10 L/min oxygen). A chest CT scan revealed multifocal GGO and nasopharyngeal RT-PCR swab was positive for SARS-CoV-2. The patient was transferred to the ICU, where tracheal intubation and invasive mechanical ventilation were necessary because of severe dyspnea, metabolic acidosis, and hypoxemia despite oxygen therapy (15 L/min via oxygen mask). Prophylactic anticoagulation was given with LMWH (enoxaparin at 0.4 IU/d).
At day 3, the patient developed ventilator-associated pneumonia due to Enterobacter cloacae and was treated with cefepime for 7 days. His clinical condition improved over the subsequent days, and he was successfully weaned from mechanical ventilation; his trachea was extubated on day 7 in the ICU. However, the patient suddenly developed severe hypoxemia (PaO2:FiO2 ratio at 90 mmHg) on day 8, so that invasive mechanical ventilation and prone positioning were again necessary. Hemodynamics deteriorated rapidly after intubation and high dose norepinephrine was needed. Tranesophageal echocardiography showed acute right heart failure with paradoxical septal motion (Fig. 5 of the electronic supplement material). Compression ultrasonography of the lower extremities showed extended thrombosis of the left femoral vein (Fig. 6 of the electronic supplement material). Transesophageal echocardiography revealed a large thrombus in the right pulmonary artery (Fig. 5 of the electronic supplement material). Thrombolysis with r-tPA was unsuccessful so that veno-arterial extracorporeal membrane oxygenation (ECMO) was initiated because of marked hemodynamic instability and ultrasound-enhanced thrombolysis was performed with subsequent successful dissolution of the clot.
Although evolution was favorable and ECMO was weaned on day 27, thrombi were identified in the internal right jugular vein and the left femoral vein. Because of persistent thrombosis the patients was treated with tinzaparine (18,000 IU/d). After gradual weaning from mechanical ventilation, the patient's trachea was successfully extubated on day 35. He was discharged to a medical ward in the same institution on day 40 on therapeutic LMWH.
2.1.5. Case #5
A 49-year-old male patient was admitted to the emergency department of S.Orsola-Malpighi Hospital (Bologna, Italy) because of acute respiratory failure. Fever and cough had started 15 days prior to admission and SARS-CoV-2 infection had been confirmed 10 days earlier with a RT-PCR positive swab. The patient had been self-isolating at home. Known comorbidities were obesity and diabetes mellitus type II.
On presentation, the patient was febrile (39 °C) and his respiratory rate was >30 breaths/min. Blood gas analysis showed hypoxemia despite oxygen therapy (non-rebreather facemask 15 L/min) and respiratory acidosis, so that tracheal intubation and invasive mechanical ventilation were necessary and the patient was referred to the ICU. CT scan showed diffuse GGOs with multiple zones of consolidation (Fig. 7 of the electronic supplement material). Prophylactic LMWH was started (enoxaparin at 4000 IU/d). As the patient showed no signs of clinical improvement, a CTA of the lung was performed on day 9, and revealed a thrombus in the right interlobar artery extending into segmental arteries of the right lower lobe. Worsening of the right lower lobe consolidation was also observed (Fig. 8 of the electronic supplement material). Upper and lower limb Doppler ultrasonography excluded deep vein thrombosis. LMWH dosage was increased to 8000 IU B.I.D. On day 14, chest CTA was repeated and showed necrotizing pneumonia of the right lower lobe with persistence of diffuse GGOs without further extension of the pulmonary thrombosis. Despite full clinical and therapeutic support, the patient died of refractory septic shock on day 16.
3. Discussion
The clinical cases we describe represent the broad spectrum of PTE in patients with COVID-19. The onset of PTE varied from 2 to 4 weeks after the occurrence of the initial symptoms of SARS-CoV-2 infection and led to deterioration of the clinical picture in all cases. In 100 hospitalized patients with COVID-19, Grillet et al. [2] found radiologic evidence of PTE in 23% of cases in CTA, performed in the average of 12 days after the onset of symptoms. This underscores the importance of adequate follow-up of these patients and the relatively high degree of clinical suspicion to early detect and treat PTE. Indeed, 3 patients in our series developed acute severe respiratory failure after a rather stable early clinical course of the disease, necessitating mechanical ventilation and/or ECMO therapy.
Interestingly, 3 of the patients had no past history of clinically relevant risk factors for venous thromboembolism (VTE) and one patient (Case #4) developed PE despite prophylactic anticoagulation using LMWH. The occurrence of PTE despite of standard prophylactic thromboprophylaxis underscores the potential procoagulant effect of COVID-19 and the likely inadequacy of standard prophylactic anticoagulation in these patients. Although embolic events are possible in patients with COVID-19, pulmonary microthrombosis (PMT) may be another relevant phenotype in these patients [9]. The pronounced inflammation of the lung tissue due to viral infection triggers activates hemostasis and may induce the formation of thrombi in the microvasculature as a physiological effort to limit the viral load. This may be explained by the interaction between platelets and endothelium, inducing endothelial dysfunction, which may play a major role in COVID-19 associated microthrombosis [10,11]. Subsequent platelet aggregation and leukocytes' recruitment may extend PMT to sufficiently occlude large pulmonary vessels [10]. These aggregates continue to grow until they become sufficiently large to induce extended PMT.
Few studies [[12], [13], [14], [15], [16], [17], [18]], mostly retrospective [[13], [14], [15], [16], [17], [18]], have reported the epidemiology and clinical characteristics of patients with COVID-19 requiring ICU admission. The incidence of PTE was 13.6–35.3% in this population despite of prophylactic anticoagulation. The hypercoagulable state observed in these patients is probably multifactorial and can be explained by several mechanisms, including cytokine release [19], complement activation [20], endothelial dysfunction [21,22], and interactions between hemostatic and immune systems [21]. Taken together, intermediate dose of LMWH (e.g., enoxaparin 1 mg/kg/day in patients with normal renal function) is a reasonable option in patients with risk factors for VTE [23], especially those who require ICU admission [1,2,[12], [13], [14], [15], [16], [17], [18],[24], [25], [26], [27]].
We may also speculate that COVID-19 may predispose to a resistance to heparin therapy as evident from the relatively high dose of heparin needed to achieve the therapeutic target in Case #1. Similarly, Beun et al. [16] reported that high dose unfractionated heparin (UFH) of more than 35,000 IU/day was required in 4 patients with PTE to achieve therapeutic targets. Monitoring and dose-adjustment of anticoagulation therapy in these patients is, therefore, of utmost importance to avoid life-threatening thromboembolic events. In particular, obese patients may require higher weight-based doses [28,29].
Another important observation in patient #1 was that the severe disease progression was associated with concomitant increases in IL-6, ferritin, and D-Dimer levels, which highlights the potential role of these markers for identifying severe cases of COVID-19 who may warrant a more meticulous diagnostic assessment to exclude the presence of PTE. These abnormalities were shown to be associated with poor prognosis these patients [5] and have been reported to predict VTE in COVID-19 patients and monitor the effectiveness of anticoagulant therapy [30]. They should be interpreted, however, within the clinical context and should not be used, per se, to establish the diagnosis of VTE or indicate the need for diagnostic procedures [30,31]. Clinical deterioration of the respiratory function or the lack of improvement despite of supportive therapy, together with laboratory evidence of severe inflammation and hypercoagulable state should prompt rapid diagnostic procedures to identify patients with PTE. Indeed, the International Society of Thrombosis and Haemostasis (ISTH) did not recommend the routine screening for DVT in COVID-19 patients.Nonetheless, high index of clinical suspicion of PTE should be adopted in these patients, especially those at increased risk of VTE [31].
In case #1, PTE was the primary reason for hospital and ICU admission after a 2-week period of self-isolation at home, raising concerns about the possible influence of self-isolation on potentiating the procoagulant effects of COVID-19 because of relative restrictions in physical activity. The magnitude of this problem is probably underestimated because of the lack of reliable data on the epidemiology of thromboembolic events in this population. It may reasonable, therefore, to consider prophylactic anticoagulation for 1–2 weeks in the pre-hospital phase during home self isolation, especially in patients with VTE risk factors, such as reduced mobility, obesity, and previous VTE [23].
Two patients (Cases #2 and #3) were readmitted after being discharged from the same hospital, suggesting that the assumed procoagulant effect of COVID-19 may extend some weeks after hospital discharge of apparently stable, asymptomatic patients. It would be prudent, therefore, to have a high degree of clinical suspicion of PE in COVID-19 patients readmitted to the hospital after surviving an initial hospitalization. Extended post-discharge prophylactic anticoagulation for 1–2 weeks may also be useful in some patients after meticulous assessment of the risk-benefit ratio of this treatment [7,8,23]. Further studies are needed to assess these aspects.
The outcome from PTE seems to be related to the extent of the thromboembolism, associated complications (e.g., superinfections) and associated organ dysfunction/failure, especially cardiovascular failure. Highly invasive treatment options, such as ECMO, may be useful in selected cases but require long durations of therapy and adequate supportive treatment (Case #4). Indeed, therapeutic anticoagulation is the cornerstone in the management of these patients and should be adapted according to the preexisting comorbidities, such as renal dysfunction [7]. LMWH has been suggested as the first-line treatment, unless closer dose adjustment is required due to expected invasive procedures or in patients with severe renal impairment [32]. In the later cases intravenous UFH followed by the subcutaneous route is recommended to allow regular monitoring and dose adjustment. Urgent thrombolytic therapy should be considered in case of hemodynamic instability, evidence of new onset increased right-ventricular load, or pulmonary arterial hypertension [7,32].
4. Conclusion
PTE is a potential life-threatening complication, which occurs frequently in patients with COVID-19. Intermediate therapeutic dose of anticoagulants can be considered in patients with COVID-19 with risk factors for VTE, especially those requiring ICU admission. Extending thromboprophylaxis after hospital discharge or during home self-isolation may be reasonable after meticulous risk-benefit assessment, especially in patients with high risk of VTE.
Ethics approval and consent to participate
The report was approved by the institutional review board of Jena university hospital (Bachstrasse 8, 07743 Jena, Germany; Reference # 2020-585,174-Daten) and the corresponding institutional review board of the respective centers. Informed consents were waived due to the retrospective and anonymous nature of data collection.
Consent for publication
Informed consents were waived by the corresponding institutional review boards due to the retrospective and anonymous nature of data collection.
Availability of data and material
The datasets used and analysed during the current report are available from the corresponding author on reasonable request.
Authors' contributions
YS, IA, VMR, AK, MB, and ML, designed the scientific work. YS, SB, EA, and MG reviewed the literature. YS, EA, MG, ML, GP, TT, GD, and LZ wrote the first draft of the manuscript. All the authors reviewed, revised, and approved the submitted manuscript. YS, EA, ML, VMR, AK had complete access to the clinical and radiologic data of the reported cases and hold responsibility for integrity and correctness of data.
Declarations of Competing Interest
The authors declare that they do not have conflict of interests or financial relationship in relation to this manuscript.
Funding
No funding has been obtained for this report.
Acknowledgements
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jcrc.2020.09.021.
Appendix A. Supplementary data
Supplementary material.
References
- 1.Lodigiani C., Iapichino G., Carenzo L., Cecconi M., Ferrazzi P., Sebastian T. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grillet F., Behr J., Calame P., Aubry S., Delabrousse E. Acute pulmonary embolism associated with COVID-19 pneumonia detected by pulmonary CT angiography. Radiology. 2020;201544 doi: 10.1148/radiol.2020201544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galeano-Valle F., Oblitas C.M., Ferreiro-Mazon M.M., Alonso-Munoz J., Del Toro-Cervera J., di Natale M. Antiphospholipid antibodies are not elevated in patients with severe COVID-19 pneumonia and venous thromboembolism. Thromb Res. 2020;192:113–115. doi: 10.1016/j.thromres.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stoneham S.M., Milne K.M., Nuttall E., Frew G.H., Sturrock B.R., Sivaloganathan H. Thrombotic risk in COVID-19: A case series and case-control study. Clin Med (Lond) 2020;20(4):e76–e81. doi: 10.7861/clinmed.2020-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rouhezamin M.R., Haseli S. Diagnosing pulmonary thromboembolism in COVID-19: A stepwise clinical and imaging approach. Acad Radiol. 2020;27(6):896–897. doi: 10.1016/j.acra.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bikdeli B., Madhavan M.V., Jimenez D., Chuich T., Dreyfus I., Driggin E. COVID-19 and thrombotic or thromboembolic disease: Implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kollias A., Kyriakoulis K.G., Dimakakos E., Poulakou G., Stergiou G.S., Syrigos K. Thromboembolic risk and anticoagulant therapy in COVID-19 patients: Emerging evidence and call for action. Br J Haematol. 2020;189(5):846–847. doi: 10.1111/bjh.16727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thachil J., Srivastava A. SARS-2 coronavirus-associated hemostatic lung abnormality in COVID-19: Is it pulmonary thrombosis or pulmonary embolism? Semin Thromb Hemost. 2020 doi: 10.1055/s-0040-1712155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varatharajah N., Rajah S. Microthrombotic complications of COVID-19 are likely due to embolism of circulating endothelial derived ultralarge Von Willebrand factor (eULVWF) decorated-platelet strings. Fed Pract. 2020;37(6):e1–e2. [PMC free article] [PubMed] [Google Scholar]
- 11.Mosleh W., Chen K., Pfau S.E., Vashist A. Endotheliitis and endothelial dysfunction in patients with COVID-19: Its role in thrombosis and adverse outcomes. J Clin Med. 2020:9(6). doi: 10.3390/jcm9061862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X. High risk of thrombosis in patients with severe SARS-CoV-2 infection: A multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klok F.A., Kruip M., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Llitjos J.F., Leclerc M., Chochois C., Monsallier J.M., Ramakers M., Auvray M. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost. 2020;18(7):1743–1746. doi: 10.1111/jth.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poissy J., Goutay J., Caplan M., Parmentier E., Duburcq T., Lassalle F. Pulmonary embolism in patients with COVID-19: Awareness of an increased prevalence. Circulation. 2020;142(2):184–186. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 16.Beun R., Kusadasi N., Sikma M., Westerink J., Huisman A. Thromboembolic events and apparent heparin resistance in patients infected with SARS-CoV-2. Int J Lab Hematol. 2020;42(Suppl. 1):19–20. doi: 10.1111/ijlh.13230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Middeldorp S., Coppens M., van Haaps T.F., Foppen M., Vlaar A.P., Muller M.C.A. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18(8):1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klok F.A., Kruip M., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oudkerk M., Buller H.R., Kuijpers D., van Es N., Oudkerk S.F., McLoud T.C. Diagnosis, prevention, and treatment of thromboembolic complications in COVID-19: Report of the National Institute for Public Health of the Netherlands. Radiology. 2020;201629 doi: 10.1148/radiol.2020201629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magro C., Mulvey J.J., Berlin D., Nuovo G., Salvatore S., Harp J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Transl Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giannis D., Ziogas I.A., Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol. 2020;127:104362. doi: 10.1016/j.jcv.2020.104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Lorenzo A., Escobar S., Tibirica E. Systemic endothelial dysfunction: A common pathway for COVID-19, cardiovascular and metabolic diseases. Nutr Metab Cardiovasc Dis. 2020;30(8):1401–1402. doi: 10.1016/j.numecd.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marietta M., Ageno W., Artoni A., De Candia E., Gresele P., Marchetti M. COVID-19 and haemostasis: A position paper from Italian Society on Thrombosis and Haemostasis (SISET) Blood Transfus. 2020;18(3):167–169. doi: 10.2450/2020.0083-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leonard-Lorant I., Delabranche X., Severac F., Helms J., Pauzet C., Collange O. Acute pulmonary embolism in COVID-19 patients on CT angiography and relationship to D-dimer levels. Radiology. 2020;201561 doi: 10.1148/radiol.2020201561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wichmann D., Sperhake J.P., Lutgehetmann M., Steurer S., Edler C., Heinemann A. Autopsy findings and venous thromboembolism in patients with COVID-19: A prospective cohort study. Ann Intern Med. 2020;173(4):268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bompard F., Monnier H., Saab I., Tordjman M., Abdoul H., Fournier L. Pulmonary embolism in patients with COVID-19 pneumonia. Eur Respir J. 2020:56(1). doi: 10.1183/13993003.01365-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomas W., Varley J., Johnston A., Symington E., Robinson M., Sheares K. Thrombotic complications of patients admitted to intensive care with COVID-19 at a teaching hospital in the United Kingdom. Thromb Res. 2020;191:76–77. doi: 10.1016/j.thromres.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pannucci C.J., Fleming K.I., Holoyda K., Moulton L., Prazak A.M., Varghese T.K., Jr. Enoxaparin 40 mg per day is inadequate for venous thromboembolism prophylaxis after thoracic surgical procedure. Ann Thorac Surg. 2018;106(2):404–411. doi: 10.1016/j.athoracsur.2018.02.085. [DOI] [PubMed] [Google Scholar]
- 29.Wang TF, Milligan PE, Wong CA, Deal EN, Thoelke MS, Gage BF. Efficacy and safety of high-dose thromboprophylaxis in morbidly obese inpatients. Thromb Haemost 2014;111(1):88–93. [DOI] [PMC free article] [PubMed]
- 30.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18(6):1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thachil J., Tang N., Gando S., Falanga A., Cattaneo M., Levi M. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18(5):1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhai Z., Li C., Chen Y., Gerotziafas G., Zhang Z., Wan J. Prevention and treatment of venous thromboembolism associated with coronavirus disease 2019 infection: A consensus statement before guidelines. Thromb Haemost. 2020;120(6):937–948. doi: 10.1055/s-0040-1710019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.
Data Availability Statement
The datasets used and analysed during the current report are available from the corresponding author on reasonable request.