Abstract
Patients with severe coronavirus disease 2019 from infection with severe acute respiratory syndrome coronavirus 2 mount a profound inflammatory response and are predisposed to thrombotic complications. Pulmonary vein thrombosis is a rare disease process resulting in pulmonary congestion, infarction, and potential mortality. This report describes a patient with coronavirus disease 2019 requiring venovenous extracorporeal membrane oxygenation for hypoxic respiratory failure who developed hemorrhagic infarction of the right lower lobe. During emergency exploration the patient was found to have a right inferior vein thrombosis and marked lobar hemorrhage mandating lobectomy.
Dr Kon discloses a financial relationship with Medtronic and Breethe.
Although most patients with coronavirus disease 2019 (COVID-19) develop moderate symptoms, patients hospitalized with severe disease may require mechanical ventilation for hypoxic respiratory failure.1 Venovenous extracorporeal membrane oxygenation (ECMO) has been used as rescue therapy in select patients failing ventilator support.2 Patients with severe COVID-19 also mount a profound inflammatory response and are predisposed to thrombotic complications.3 In this report we describe a patient with severe COVID-19 who developed hemorrhagic infarction of the right lower lobe while on venovenous ECMO support. During emergency exploration the patient was found to have a right inferior vein thrombosis and marked lobar hemorrhage mandating lobectomy. This report describes a patient requiring lobectomy for COVID-19 disease.
A 33-year-old man with no past medical history presented to the emergency department with a week-long history of fever, progressive dyspnea, and nonproductive cough despite antibiotic therapy prescribed 4 days before for suspected pneumonia. The patient was found to be positive for COVID-19 and hypoxic with diffuse bilateral pulmonary infiltrates suggestive of acute respiratory distress syndrome. The patient was admitted to the intensive care unit and intubated within 8 hours for progressive hypoxia and hypercarbia. Over the following 48 hours the patient was sedated and paralyzed for ventilator dyssynchrony and persistent hypoxia despite maximal ventilator settings and required vasopressors for hemodynamic support. Given the patient’s young age, baseline normal functional and medical status, and lack of end-organ injury, he was evaluated for venovenous ECMO and cannulated.
Approximately 1 month after cannulation in the setting of therapeutic heparinization the patient had acute bleeding from a right pleural chest tube, which had been placed a week before for a secondary spontaneous pneumothorax. The patient was taken to the operating room emergently for exploration through a right thoracotomy. A significant volume of hemothorax was evacuated, with significant congestion of the right lower lobe and multiple visceral pleural tears along the posterior basilar segment. The remainder of the chest was evaluated, and no other source of bleeding was identified. A wedge resection of the hemorrhagic parenchyma significantly reduced bleeding. Because of some diffuse coagulopathic bleeding while on ECMO the chest was packed, and the patient returned to the intensive care unit.
Over the subsequent hours the patient had increasing bloody output from the chest tubes with continued pressor requirement and limited EMCO flows. The thoracotomy incision was opened in the intensive care unit, and bleeding from the right lower lobe was controlled manually to allow resuscitation. On return to the operating room the right lower lobe was noted to have worsening parenchymal congestion and bleeding and the inferior pulmonary vein was dark and firm, consistent with venous thrombosis. The decision was made to perform a right lower lobectomy for definitive control of bleeding. The fissure was opened and the pulmonary artery dissected. The pulmonary artery branches of the right lower lobe were ligated with 2-0 silk sutures and divided sharply. The inferior pulmonary vein was carefully encircled, attempting to not dislodge the clot. A vascular load stapler was used to divide the vein at the level of the pericardium. The bronchus and parenchyma were subsequently divided, completing the right lower lobectomy, with good hemostasis noted thereafter. The chest was irrigated and drained widely and the thoracotomy incision closed in layers. Postoperatively the patient was decannulated from ECMO and was on room air. Additionally he had no neurologic deficits and was ambulatory with physical therapy.
Pathologic evaluation of the right lower lobe revealed hemorrhagic infarction with cavities resulting from ischemic necrosis of the lung parenchyma and alveolar hemorrhage (Figure 1 ). The lung distant to the infarction showed focal residual hyaline membranes and interstitial inflammation consisting primarily of T cells and macrophages with extensive peribronchiolar metaplasia. Numerous megakaryocytes and platelets were present (Figure 2 ). The inferior pulmonary vein was verified as thrombosed.
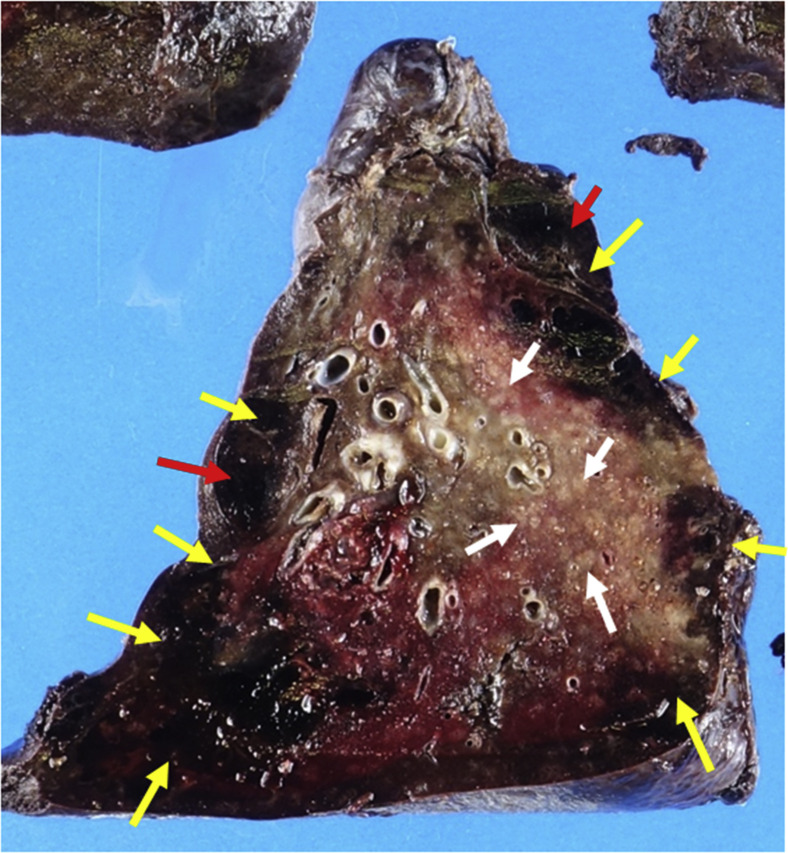
Figure 1.
Cut surface of the right lower lobe shows extensive pleural based hemorrhagic infarction (yellow arrows) with cavitation (red arrows) resulting from ischemic necrosis of the lung parenchyma. The rest of the cut surface shows patchy pale tan areas (white arrows) that correspond to the inflammation and peribronchiolar metaplasia.
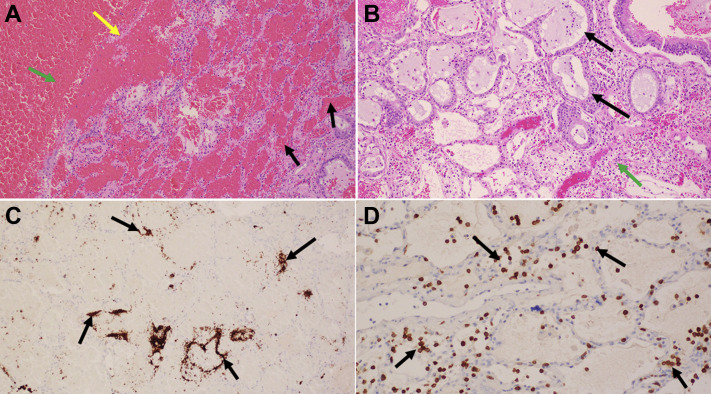
Figure 2.
Histologic sections of right lower lobe parenchyma, (A) patchy areas of intraalveolar hemorrhage (black arrows), and hemorrhagic infarct with cavity formation. The yellow arrow points to the residual necrotic alveolar septa and the green arrow to the cavity with blood. Alveolar septa are not seen. (B) Moderate inflammation (green arrow) with peribronchiolar metaplasia (black arrows) consisting of bronchiolar epithelium lining alveoli. (A and B are stained with hematoxylin and eosin, magnification ×100.) (C) Numerous megakaryocytes and platelets are present (black arrows). (Immunohistochemical stain for megakaryocytes, magnification ×100.) (D) Most inflammatory cells are T cells (black arrows). (Immunohistochemical stain for platelets [CD61] and T [CD3] cells, magnification ×200.)
Comment
Pulmonary vein thrombosis is a rare clinical entity, typically occurring in the setting of malignancy or lung transplantation.4 Patients with severe COVID-19 are predisposed to both venous and arterial thrombosis, with pulmonary embolism emerging as the most common thrombotic complication.1 , 3 In autopsy examinations of COVID-19 patients, nearly all patients had capillary congestion and platelet–fibrin thrombi in small arterial vessels (<1 mm).5
This is a case of a patient requiring pulmonary lobectomy due to COVID-19. Although a parenchymal-sparing strategy was initially attempted considering the patient’s ongoing respiratory failure, lobectomy was eventually required for definitive hemorrhagic control in the setting of inferior venous thrombosis. Although there is no consensus regarding the management of inferior vein thrombosis, most patients with complicated disease undergo pulmonary resection.4
References
- 1.Richarson S., Hirsch J.S., Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kowalewski M., Fina D., Slomka A. COVID-19 and ECMO: the interplay between coagulation and inflammation—a narrative review. Crit Care. 2020;24:205. doi: 10.1186/s13054-020-02925-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flok F.A., Kruip M.J.H.A., van der Meer N.J.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaaya G., Vishnubhotla P. Pulmonary vein thrombosis: a recent systematic review. Cureus. 2017;9:e993. doi: 10.7759/cureus.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carsana L., Sonzogni A., Nasr A. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis. 2020;20:1135–1140. doi: 10.1016/S1473-3099(20)30434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]