Abstract
Personal protective equipment (PPE) is critical to protect healthcare workers (HCWs) from highly infectious diseases such as COVID-19. However, hospitals have been at risk of running out of the safe and effective PPE including personal protective clothing needed to treat patients with COVID-19, due to unprecedented global demand. In addition, there are only limited manufacturing facilities of such clothing available worldwide, due to a lack of available knowledge about relevant technologies, ineffective supply chains, and stringent regulatory requirements. Therefore, there remains a clear unmet need for coordinating the actions and efforts from scientists, engineers, manufacturers, suppliers, and regulatory bodies to develop and produce safe and effective protective clothing using the technologies that are locally available around the world. In this review, we discuss currently used PPE, their quality, and the associated regulatory standards. We survey the current state-of-the-art antimicrobial functional finishes on fabrics to protect the wearer against viruses and bacteria and provide an overview of protective medical fabric manufacturing techniques, their supply chains, and the environmental impacts of current single-use synthetic fiber-based protective clothing. Finally, we discuss future research directions, which include increasing efficiency, safety, and availability of personal protective clothing worldwide without conferring environmental problems.
Keywords: protective clothing, sustainability, personal protective equipment, PPE, antimicrobial, COVID-19, antiviral, medical textiles, single-use PPE, environmental impact
In pandemics of highly infectious diseases such as COVID-19,1 the risk of healthcare workers (HCWs) being infected is much greater than the general population, as they are in direct contact with patients. Personal protective equipment (PPE) is considered to be a critical component that can be used to protect HCWs from droplets from coughs, sneezes, and aerosol-generating procedures, in addition to other contaminated body fluids and surfaces from infected patients.2 PPE may include aprons, gowns, or coveralls, masks or respirators, and goggles.3 The supplies of safe and effective protective clothing needed to treat COVID-19 patients have been severely depleted due to the unprecedented global demand. In addition, in some cases, the standard of PPE has not been of the required quality for medical uses, thus adding to delay and waste. A recent survey by Nursing Times showed that ∼73% of National Health Services (NHS) nurses were without long-sleeved disposable gowns, eye protection, and FFP3 respirators. In addition, ∼63% did not have fluid-repellent face masks due to the current crisis.4 Furthermore, PPE needs to be “donned” and “doffed” correctly, and it may be uncomfortable to wear. Although there have been many advisory publications from various organizations and regulatory bodies such as the World Health Organization (WHO), the NHS in the U.K., and the Centre for Disease Control and Prevention (CDC) in the USA about the specification and use of PPE, there remains the unmet need for safer and more effective PPE for HCWs around the world and a clear understanding and knowledge about the regulatory standards for such equipment.
Medical textiles are used in the manufacturing of personal protective clothing for healthcare or medical applications, specifically to mitigate the risks from exposure to hazardous substances including body fluids and to minimize the risk of cross-infections.5 There are several different types of medical clothing products, including coveralls, footwear covers, full body suits, gloves, independent sleeves, scrubs, surgical gowns, surgical masks, and scrub hats. Medical textiles are also used in the manufacture of drapes and bedding textiles for healthcare settings as well as wound dressings, bandages, and other products. Medical protective clothing, usually made of synthetic fibers due to better liquid barrier properties, could be manufactured using nonwoven, weaving, or knitting technologies. Among them, nonwoven fabrics are the most popular for such clothing as they facilitate relatively fast and cheap manufacturing, high levels of sterility, and infection control. As such, they are commonly used in the manufacture of disposable medical textiles including surgical caps, surgical gowns, and surgical masks.6 Such nonwoven fabrics are typically made from polypropylene and usually have a spun-bond–melt-blown–spun-bond (SMS) construction.7 In contrast, woven fabrics typically made from cotton or polyester/cotton blends are commonly used in the manufacture of scrubs. Providing HCWs with protection from contaminated body fluids and other hazardous substances from infected patients is important, and specialist finishes can be applied to disposable or reusable medical textiles in order to impart protective effects. Fluid repellent finishes, for example, can be used to create a barrier which prevents adsorbed fluids from penetrating contact fabrics.8 A further challenge for HCWs is exposure to biological fluids that can transmit diseases caused by a variety of deadly pathogens including coronavirus (such as the coronavirus which causes COVID-19), Hepatitis B Virus (HBV), Hepatitis C Virus (HCV), Ebola Virus, and Human Immunodeficiency Virus (HIV). Antimicrobial finishes can be highly effective against such pathogens in preventing infections either by killing or by inhibiting viruses and bacteria and could be applied onto protective medical clothing via various highly scalable and cost-effective fabrication techniques.7,9
While there have been many advances in high-performance and functional protective clothing thus far, there remains a lack of comprehensive reviews that provide guidelines on the use of PPE, specifications and regulatory standards for PPE, a summary of fabrication techniques for medical protective clothing, and antimicrobial finishes of such clothing, and their environmental impacts and economic landscape. In this comprehensive review, we describe the mechanisms of viral infection, followed by a summary of the types of PPE used within a healthcare environment. We then discuss natural and synthetic antimicrobial agents and their mechanisms to kill or inhibit pathogens. The review also discusses the various manufacturing techniques and antimicrobial coating techniques used, followed by the regulatory standards and required properties for producing protective clothing for healthcare applications. We then review the global market size and supply chain for such clothing and discuss the environmental impacts of single-use and reusable protective medical clothing. Finally, we present our views on current trends, future research directions, and recommendations for potential solutions with current or future crises with PPE, due to epidemics and pandemics such as COVID-19.
Mechanism and Transmission of Viruses
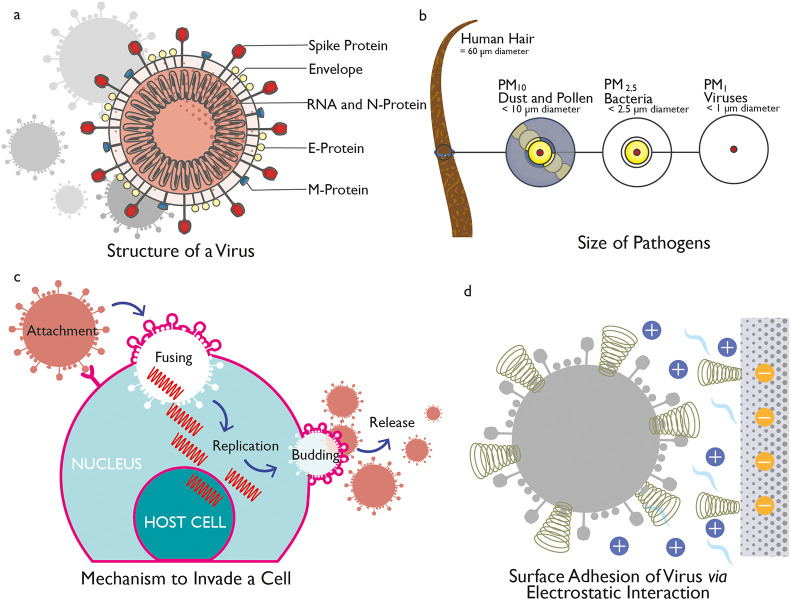
Viruses are small (∼10–200 nm) infectious agents (Figure 1a), which are typically 100 times smaller than the average bacterium, and can be most easily observed using an electron microscope (Figure 1b). Viruses are dependent on the “host cells” of other living organisms to survive, thrive, and reproduce and cannot function or replicate on their own outside of a host cell. A virus particle occurs as “packets” of DNA or RNA genetic material encompassed in a protein coating and is termed as a virion. The virion is usually composed of DNA or RNA genomic material that contains the genetic instructions for reproducing the virus and a protein coating called a capsid, which surrounds and protects DNA or RNA (Figure 1a).10 Some viruses also have an outer lipid-based envelope and are known as enveloped viruses (H1N1, coronaviruses, etc.).11 The coronavirus which causes COVID-19 has such a fatty envelope, which can be destroyed by the application of soap-like materials. Other viruses without such an envelope are called naked viruses (e.g., Rotavirus). The diameter of the coronavirus is typically between 60 and 140 nm and has spike-like surface projections creating a “crown-like appearance” under the electron microscope (Figure 1a).12,13
Figure 1.
Structure of virus and mechanistic action. (a) Structure of a coronavirus. (b) Relative size of various pathogens. (c) Mechanism to invade a cell via a virus. (d) Surface addition of viruses via electrostatic interaction.
A virus can spread via aerosols generated by coughing and sneezing in air, by vectors such as insects like mosquitoes, or by the transmission of body fluids such as saliva, blood, or semen.14 Once a virus infects a cell, it starts to replicate and reproduce virions rapidly (Figure 1c). As a result, the host cell produces more viral material than it does its own genetic material, and the virus could kill the host cell if left unchecked.15 The human body has some natural defenses against viruses and uses its immune system to produce antibodies that bind to the viruses and render them incapable of replicating. The immune system also releases T-cells, which work to kill viruses. In addition, several vaccines have been developed to produce an artificial immune system to the specific viral infections. However, some viruses, including those that cause Acquired Immune Deficiency Syndrome (AIDS), Human Papillomavirus (HPV) infection, and viral hepatitis, are less susceptible to natural immune responses and result in chronic infections. COVID-19 is another highly infectious viral disease caused by the recently discovered coronavirus. Coronaviruses are a large family of viruses which may cause illness in animals or humans. In humans, several coronaviruses are known to cause respiratory infections. The severity of these infections can range from mild, as in the case of the common cold, but some are more severe as in the cases of Middle East Respiratory Syndrome (MERS) and Severe Acute Respiratory Syndrome (SARS). The most recently discovered Coronavirus is Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), which was identified in Wuhan, China, in December 2019 and is responsible for the COVID-19 disease.1
The main mechanisms for viral adsorption on surfaces are physical adsorption and electrostatic interactions (Figure 1d). The amount of virus adsorbed on a surface is the linear functional of the square root of time. Therefore, the more time virus stays on a surface the more opportunity to be strongly adsorbed onto a surface and become available to attack the population. One strategy to reduce viral infection is to decrease the amount of time the virus interacts with a material, whereas another strategy is to impart materials with surface properties which are unfavorable for viruses. There are several factors which influence the movement of viruses or pathogens through fabrics, including the shape and dimensions of the microbe, the properties of carriers, and physicochemical nature of the fabric.2,16 There are several pathogens that can be found in healthcare environments including fungi, bacteria, and viruses. Such pathogens vary in cell dimensions and morphology, mobility, and sensitivity to environmental extremes. In general, fungal microbes are larger than bacterial microbes (∼1–5 μm), which, in turn, are larger than viral microbes (e.g., the size of the HIV virus is ∼13 nm). Pathogens can be carried by any persons present in healthcare settings, and they can be transported by a variety of carriers including respiratory droplets expelled by coughing or sneezing, body fluids, shed skin cells, lint, and dust.17,18 Overall, pathogens are transmitted most easily through liquids such as respiratory droplets and body fluids, but they can also be transmitted without the presence of liquids.16
The transmission of liquid through textile materials could be described by two interchangeable but fundamentally different terminologies: penetration and permeation. Penetration involves the flow of gas, vapor, or liquid through a porous material, whereas permeation involves the diffusion of gas or vapor through a porous material. Penetration and permeation usually take place due to a pressure gradient and concentration gradient across the barrier, respectively. Pathogens are larger in size than gas and vapor molecules and are considered to penetrate and not permeate through materials.2 The coronavirus which causes COVID-19 has been found to be transmitted via aerosols.19
Personal Protective Equipment for HCWs
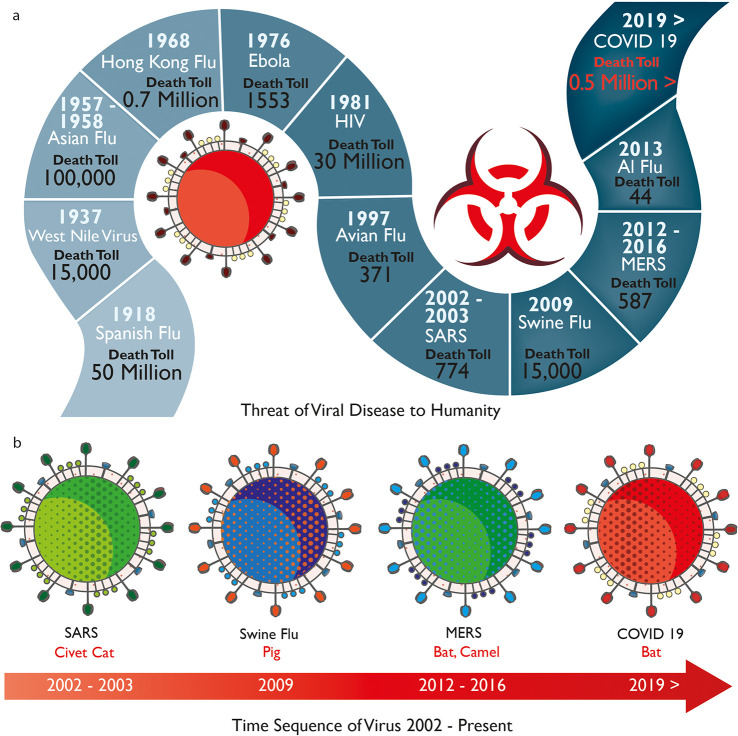
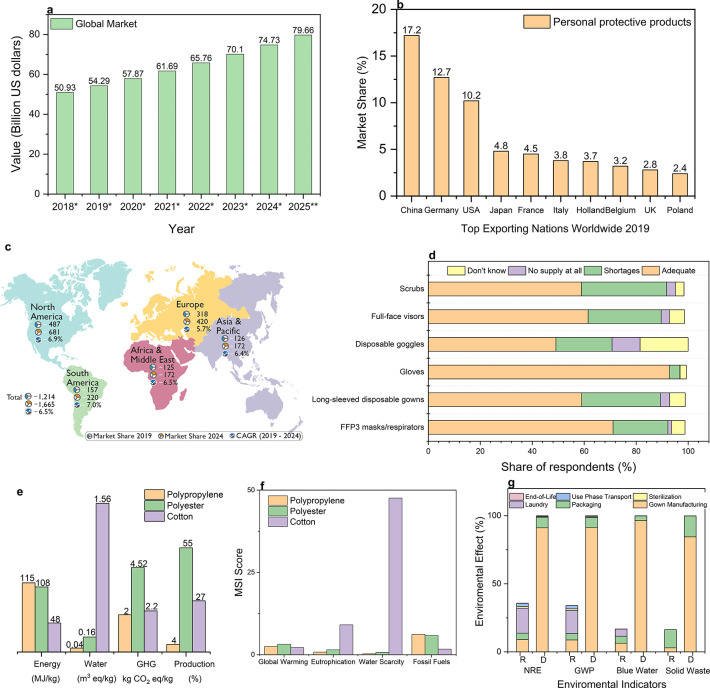
About 80 million people will be working in the healthcare industry worldwide by 2030.20 HCWs are at constant risk of exposure to pathogens and acquiring infections while treating patients with infectious diseases (Figure 2).2 In the last century, millions of people died from these highly infectious viruses (Figure 2a). This century has already seen breakouts of several deadly viruses including SARS, Swine Flu, MERS, and the recent COVID-19 pandemic (Figure 2b). The CDC states that such pathogens could be transmitted to the human body using three primary routes: direct or indirect contact with an infected person which is the most usual, airborne transmission, and respiratory droplet transmission though coughing, sneezing, or talking.21 PPE for HCWs could prevent or reduce such contact and droplet exposures by creating a barrier between the human body and the pathogens. The Occupational Safety & Health Administration (OSHA) in the USA defines PPE as “specialized clothing or equipment worn by an employee for protection against infectious materials”. OSHA issued regulations that require the use of PPE in healthcare environments to protect healthcare personnel from any exposure to potential infectious diseases. As per the regulations, employers are required to supply PPE of the correct specifications to their staff. Furthermore, in the case of reusable PPE, employers must arrange the appropriate cleaning, repair, and storage of products. Additionally, employers must ensure that any “end of life” PPE is disposed of correctly.22
Figure 2.
History of viruses. (a) Threat of viral diseases to humanity at various years with number of human deaths. (b) Timeline of recent highly infectious viruses such as SARS, Swine Flu, MERS, and COVID-19.
Factors Affecting the Selection of PPE
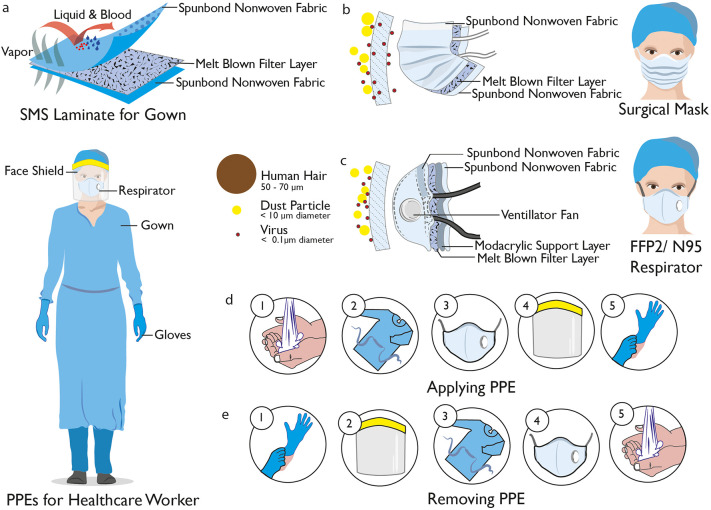
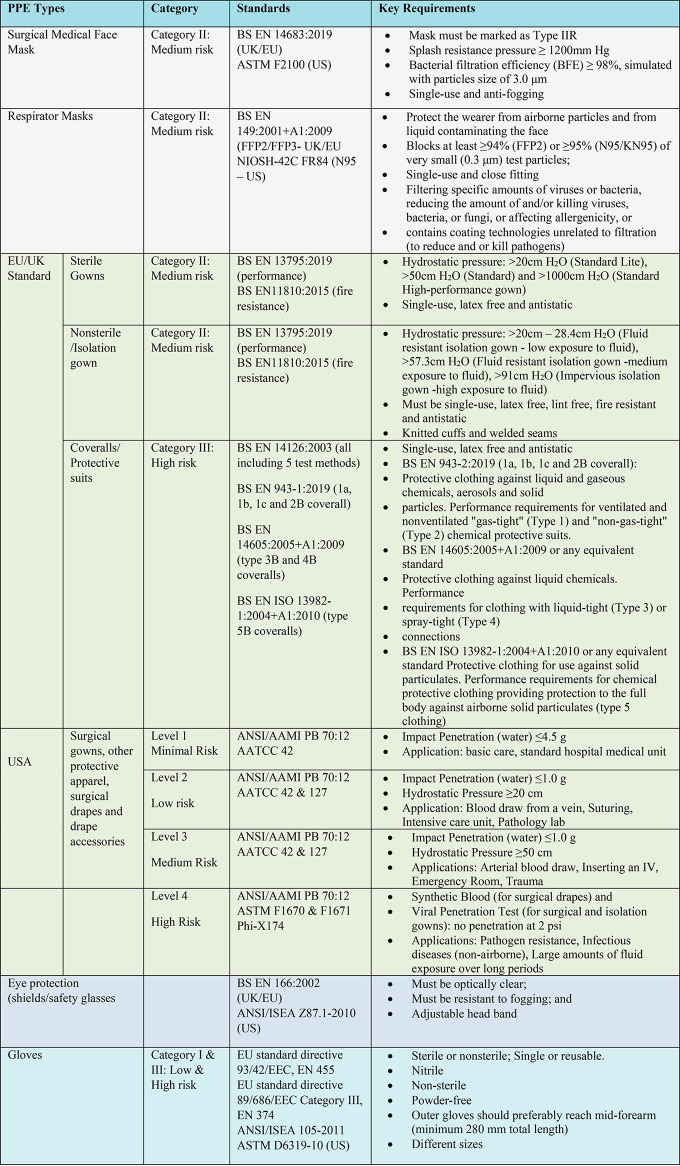
The most common types of PPE within a healthcare environment are gloves, gowns or aprons, masks or respirators, goggles and face shields (Figure 3a–e). In order to select PPE, three important points need to be considered: the types and amounts of body fluids to which the wearer might be exposed to and the ways in which these fluids might be transported, the durability and appropriateness of the PPE for the task, and the fit of the PPE for individual users.3 Gloves are the most commonly used PPE to protect hands and are manufactured from natural and synthetic rubbers for sterile and nonsterile usage, and it is essential that they are comfortable, fit, and do not tear or damage easily. Gowns are also widely used for PPE in order to protect skin and other clothing and should fully cover the torso, fit comfortably over the body, and have long sleeves that fit snuggly at the wrist. All or parts of the face (nose, mouth, and eyes) are protected using a combination of PPE types such as masks or respirators, goggles, and face shields. Masks should fully cover the nose and mouth and prevent fluid penetration, whereas goggles should fit softly over and around the eyes or personal prescription lenses. In some cases, face shields are used to substitute masks or goggles, where skin protection is required in addition to mouth, nose, and eye protection. The face shield should cover the forehead, extend below the chin, and wrap around the sides of the face. HCWs are protected from hazardous or infectious aerosols, such as the coronavirus which causes COVID-19, and Mycobacterium tuberculosis, using respirators that filter the air before it is inhaled. The most widely used healthcare respirators are the N95, N99, or N100 particulate respirators, which have a submicron filter capable of excluding particles that are less than 5 μm in diameter (Table 1). However, a higher level of respiratory protection, such as that provided by a powered air-purifying respirator (PAPR), is required when high-risk aerosol-generating procedures such as bronchoscopies are being performed.3
Figure 3.
Personal protective equipment for HCWs. (a) Healthcare worker with safe PPEs such as gown, visor respirator, visor, and gloves. Spun-bond–melt-blown–spun-bond (SMS) laminate fabric used for a disposable medical gown. It provides protection from liquid and blood at the same time maintaining comfort. (b) Surgical mask with SMS structure, which only provides protection against larger particles but is not effective against airborne viruses. (c) FFP2/N95 respirator, which provides efficient protection against airborne viruses by stopping >95% of particles. (d) Stages to put on PPEs for healthcare setting and (e) Steps to remove PPEs safely without any contamination.
Table 1. Comparison of FFP2, KN95, and N95 and Other Filtering Facepiece Respirator Classes28.
| certification/class (standard) | N95 (NIOSH-42C FR84) | FFP2 (EN 149-2001) | KN95 (GB2626-20 06) | P2 (AS/NZ 1716:2012) | Korea 1st class (KMOEL-2017-64) | DS2 (Japan JMHLW-notification 214, 2018) |
|---|---|---|---|---|---|---|
| filter performance (must be ≥X% efficient) | ≥95% | ≥94% | ≥95% | ≥94% | ≥94% | ≥95% |
| test agent | NaCl | NaCl and paraffin oil | NaCl | NaCl | NaCl and paraffin oil | NaCl |
| flow rate | 85 L/min | 95 L/min | 85 L/min | 95 L/min | 95 L/min | 85 L/min |
| total inward leakage (TIL), tested on human subjects each performing exercises | N/A | ≤8% leakage (arithmetic mean) | ≤8% leakage (arithmetic mean) | ≤8% leakage (individual and arithmetic mean) | ≤8% leakage (arithmetic mean) | inward leakage measured and included in user instructions |
| inhalation resistance, max pressure drop | ≤343 Pa | ≤70 Pa (at 30 L/min), ≤240 Pa (at 95 L/min), ≤500 Pa (clogging) | ≤350 Pa | ≤70 Pa (at 30 L/min), ≤240 Pa (at 95 L/min) | ≤70 Pa (at 30 L/min), ≤240 Pa (at 95 L/min) | ≤70 Pa (w/valve), ≤50 Pa (no valve) |
| flow rate | 85 L/min | varied; see above | 85 L/min | varied; see above | varied; see above | 40 L/min |
| exhalation resistance, max pressure drop | ≤245 Pa | ≤300 Pa | ≤250 Pa | ≤120 Pa | ≤300 Pa | ≤70 Pa (w/valve), ≤50 Pa (no valve) |
| flow rate | 85 L/min | 160 L/min | 85 L/min | 85 L/min | 160 L/min | 40 L/min |
| exhalation valve leakage requirement | leak rate ≤30 mL/min | N/A | depressurization to 0 Pa ≥ 20 s | leak rate ≤30 mL/min | visual inspection after 300 L/min for 30 s | depressurization to 0 Pa ≥ 15 s |
| force applied | –245 Pa | N/A | –1180 Pa | –250 Pa | N/A | –1470 Pa |
| CO2 clearance requirement | N/A | ≤1% | ≤1% | ≤1% | ≤1% | ≤1% |
How to Put on (“Don”), Use, and Remove (“Doff”) PPE Safely
The type of PPE used will vary based on the level of protection required and will consider factors such as the level of patient contact, exposure to droplets or airborne infections, and isolation precautions.23,24 The process for donning, using, and doffing PPE should respond and adjust to the specific type of PPE being worn and be carried out as per the guidance from CDC (Figure 3d,e).3,25 Proper hygiene should also be performed per international recommendation before putting on any PPE.26 The first item of PPE to be donned is the gown, followed by the mask or respirator. The mask or respirator should be properly adjusted to fit the face of the user. After the mask or respirator, it is recommended that the goggles or face shield is put on, followed by gloves as the last step. While using the PPE, it is important for users to follow safe working practice and avoid contamination by keeping hands away from the face and not touching or adjusting PPE. Gloves should be removed if they are torn, and recommended hand hygiene should be performed before putting on a new pair of gloves. Touching surfaces and other items with contaminated gloves should be avoided to prevent the possible spread of pathogens.3 During the removal of PPE, self-contamination should be avoided by removing the most contaminated gloves first. The face shield or goggles are then removed, followed by the gown and then the mask or respirator.3,25
Medical Gowns and Drapes
Gowns are items of protective apparel designed to ensure the protection of the wearer from the spread of infection should they come into contact with potentially infectious liquids and solid materials (Figure 3a). Gowns can also prevent the transfer of pathogens to vulnerable patients with weakened immune systems.27 The American National Standards Institute/Association of the Advancement of Medical Instrumentation (ANSI/AAMI) introduced standard PB70:2003, “liquid barrier performance and classification of protective apparel and drapes intended for use in health care facilities” for gowns and other protective apparel intended for use in health care facilities in 2004. The standard describes the barrier protection levels of such apparel and specifies test methods and performance levels necessary to verify and validate that the gown provides the necessary defined levels of protection (section 6). Many descriptions have been used to characterize medical gowns; however, the most commonly used types are surgical gowns, surgical isolation gowns, and nonsurgical gowns. As regulated by the U.S. Food and Drug Administration (FDA), both surgical and surgical isolation gowns are categorized as a Class II medical device that requires a 510(k) premarket notification, whereas nonsurgical gowns are Class I devices and do not require a 510(k) premarket review. Surgical isolation gowns and nonsurgical gowns have much larger zones of protection than surgical gowns. Nonsurgical gowns are used in low or minimal risk patient isolation situations and should never be used during surgical and invasive procedures or when there is a medium to high risk of contamination. Unlike nonsurgical gowns, surgical isolation gowns could be used in medium to high risk contamination environments, whereas surgical gowns are suitable for any risk level (Levels 1–4).
Mask or Respirators
Surgical masks or respirators are used to prevent airborne particles and liquids from contaminating the face of the wearer. Medical masks are composed of a three-layer nonwoven SMS (spun-bond–melt-blown–spun-bond) fabric laminate (Figure 2b). The inner spun-bond nonwoven fabric layer absorbs moisture released by the wearer, and the outer layer is a waterproof nonwoven fabric, which is mainly used to create a barrier between the external liquids and the users. The middle melt-blown nonwoven fabric of polypropylene is the filter layer which provides protection from airborne particles. The filtering mechanism of medical masks is dominated by Brownian diffusion entrapment, inertial collision, gravity sedimentation, and electrostatic adsorption. The first four physical processes are delivered by the melt-blown nonwoven polypropylene fabric and achieves ∼35% filtration. However, further electrostatic treatment on this layer can significantly improve the capture of aerosols or airborne particles through ionic interaction.
Although a surgical mask may be effective in blocking splashes and large-particle droplets, it does not provide protection against very small airborne particles, airborne viruses, or other nanoscale contaminants. However, an N95/FFP2 respirator provides barrier protection against at least 95% of very small (0.3 μm) test particles, while still allowing respiration through the microscopically porous shell (Table 1). N95 respirators do not usually require 510(k) premarket notification in the U.S. market and are categorized as Class II medical devices. Unlike the loose fit surgical mask, N95 respirators have extra filtration layers (Figure 3c) and are designed to achieve a very close facial fit and very efficient filtration of airborne particles. However, the risks of illness, being infected by viruses, or death are still not eliminated even with a properly fitted N95 respirator. It is worth noting, however, that a weakness of most fabric masks is that they seldom form a perfect seal against the face. As such, a mask which allows air to be drawn in through gaps caused by a poor facial seal will be ineffective, regardless of how efficient fabric filtration is. Nevertheless, in the light of the recent COVID-19 pandemic, several governments and world organizations. including WHO and CDC recommended the use cloth face covering, especially in areas of significant community transmissions.29,30 In addition, combinations of various commonly available fabrics used in fabric masks can potentially provide significant protection against the transmission of aerosol particles.31,32
Antimicrobial Agents and Finishes
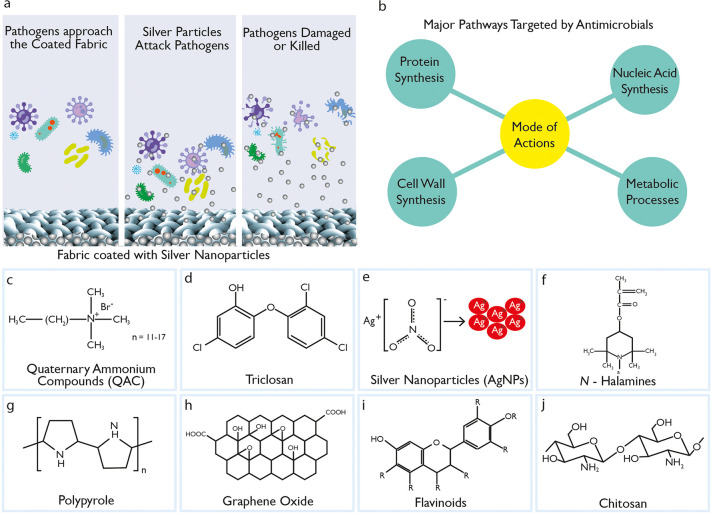
HCWs treating patients with highly infectious diseases require high level (Level 4) protection against pathogens. The recent COVID-19 pandemic has highlighted the urgent need for effective antiviral fabrics for both medical and day-to-day apparel applications that could protect the wearer from potentially infectious pathogens. As per the Association for the Advancement of Medical Instrumentation (AAMI) PB70:2012 standard,9 protective medical clothing must be able to prevent viruses and fluid penetration for up to an hour and pass three tests: water impact, pressurizing the materials, and barrier against simulated blood containing a virus. Water repellent or barrier finishes based on fluorocarbons have been a popular choice for hospital gowns to provide resistance against water and liquid.33 However, once wet, they no longer provide an effective barrier against pathogen ingress. Additionally, some pathogens may even penetrate when no visible liquid penetration is present. In addition to repellent finishes, antimicrobial finishes have recently been widely used in medical gowns to control, destroy, or suppress the growth of pathogens (Figure 4a) and their negative effects of odor, staining, and deterioration.34 There are four major pathways targeted by antimicrobial agents to inhibit or destroy pathogens, which are cell wall synthesis, protein synthesis, nucleic acid synthesis, and metabolic process (Figure 4b).
Figure 4.
Antimicrobial agents and their mechanism. (a) Antimicrobial action via silver-nanoparticle-coated fabrics. (b) Major pathways targeted by antimicrobial agents to inhibit or destroy pathogens. The chemical structure of some commonly used antimicrobial agents: (c) quaternary ammonium compounds, (d) triclosan, (e) N-halamines, (f) graphene oxide, (g) silver nanoparticles, (h) polypyrrole, (i) chitosan, and (j) flavonoids.
Natural fibers such as cotton or wool suffer degradation, unpleasant odors, and potential health risks from microbial growth due to their high surface area and moisture regain capability.35,36 In addition, various textile materials such as those made from cotton, poly/cotton, and polypropylene provide favorable environments for bacteria or fungal growth, allowing such pathogens to survive for 1–90 days on textile materials in a hospital environment.37 Furthermore, the polio and vaccinia viruses were found to survive on wool fabrics for up to 20 and 14 weeks, respectively, and for shorter duration on cotton fabrics.38 Therefore, the use of antimicrobial textiles in healthcare facilities could diminish microbial infections compared with the use of textiles without antimicrobial finishes.39 A polyurethane-based antimicrobial material, N,N-dodecyl,methyl polyurethane (Quat-12-PU) when coated on surfaces or electrospun into fiber, was able to kill airborne Gram-positive Staphylococcus aureus and Gram-negative Escherichia coli bacteria, as well as inactivate the influenza virus.40 In another study N-halamine-coated nonwoven fabrics completely inactivated Avian Influenza (AI) viruses and disrupted their RNA and were found to be very effective in reducing airborne pathogens in the poultry production environment.41 In addition, several other studies have examined the use of antimicrobial finishes on textiles to inhibit the growth of viruses and were found to be highly effective against bacteria and viruses such as influenza viruses,42,43 cytomegalovirus (CMV),44 and adenovirus type 5 and poliovirus type 1.45
An antimicrobial agent is defined as any substance of natural, semisynthetic, or synthetic origin that kills (biocidal) or inhibits (biostatic) the growth of pathogens but causes little or no damage to the host. The term “antimicrobials” include all agents that act against all types of pathogens including bacteria (antibacterial), viruses (antiviral), fungi (antifungal), and protozoa (antiprotozoal). In textile applications, such as clothing, antimicrobial agents need to be effective in providing protection from a wide variety of pathogens.46 In addition, such agents have to be durable to washing, dry cleaning, and ironing, simple and easy to apply on textiles, and should not compromise appearance and hand quality of textiles.47 Most importantly, the antimicrobial agent should be safe to wear next to the skin and should not interfere with the skin’s natural flora. Table 2 summarizes most commonly used antimicrobial agents for textiles applications.
Table 2. Summary of Antimicrobial Agents.
| agents | possible mechanism | type of pathogens | application techniques on textiles | fiber types | references/limitations |
|---|---|---|---|---|---|
| QAC | damage cell membranes | Gram-positive and Gram-negative bacteria, fungi and certain viruses64,69 | electrospinning,68,69 exhaust, padding | cotton, polyester, nylon and wool | (50,68,69,135,136) |
| denature proteins | poor durability due to the fast leaching from textiles. | ||||
| inhibit DNA production, avoiding multiplication | |||||
| triclosan | blocks lipid biosynthesis, affecting the integrity of cell membranes67,70 | Gram-negative and Gram-positive bacteria, some antifungal and antiviral properties67,70−72 | exhaust,70 melt-mixing (spinning),73padding64 | polyester, nylon, polypropylene, cellulose acetate and acrylic | (67,70−72) |
| photochemical conversion of triclosan to 2,8-dichlorodibenzo-p-dioxin in aqueous solutions is another great concern, due to its toxicity | |||||
| metals and metallic salts | generate reactive oxygen species, damaging cellular proteins, lipids, and DNA75,76 | broad spectrum of action against bacteria | exhaust, padding, melt-mixing47 | cotton, wool, polyester, nylon | cost, technical and environmental challenges, and requirement of additional plasma, UV, or acidic pretreatment80 |
| PHMB | interacts with membrane phospholipids to disrupt and cause the lethal leakage of cytoplasmic materials67,81,82 | exhaust and padding50 | cotton, polyester, nylon | ||
| N-halamines | precludes the cell enzymatic and metabolic processes, causing the consequent pathogen destruction64,84 | broad spectrum of bacteria, fungi, and viruses | polymerization, electrogeneration, or chemical grafting86 | cotton, polyester, nylon, wool | unpleasant odor or even discoloration of fabrics84 |
| conjugated polymers (PPy) | attack on the cell by charged N and CL ions of PPy91 | Gram-negative and Gram-positive bacteria | in situ polymerization, coating | cotton, polyester | insoluble in water |
| graphene derivatives | bacterial membrane perturbation caused by sharp edges and oxidative stress induction | bacteria and viruses | coating137 | cotton, polyester, poly-cotton, nylon | no consensus in terms of the intrinsic antibacterial properties of bare graphene oxide |
| chitosan | electrostatic interactions or the binding with microbial DNA or the excellent metal-binding capacity of chitosan due to the amine groups47,138 | wide spectrum of pathogens, including fungi, algae, and some bacteria | dyeing/printing139 | cotton, silk, wool, viscose, synthetic fabrics | as temperature and pH activity dependence and poor handling |
| pad–dry–cure140 |
Mechanism of Antimicrobial Activity
The antimicrobial effect on textiles is achieved via either cell growth inhibition (called “biostatic”) or killing of the pathogens (called “biocidal”). Most of the antimicrobial agents used in commercial textiles provide a biocidal effect via damage or inhibition of cell wall synthesis, inhibition of cell membrane function, inhibition of protein synthesis, inhibition of nucleic acid synthesis (DNA and RNA), and inhibition of other metabolic processes such as the disruption of the folic acid pathway (Figure 4b).48−50 In addition, the vast majority of antimicrobial products work by leaching when in contact with moisture, as they migrate from a textile surface to the external environments to attack the pathogen.46,47 However, the challenge with such an antimicrobial effect is that it may also kill the “good” natural bacterial flora associated with the skin and may be less durable due to exposure to the external environment. Another set of antimicrobial agents are those that covalently bond with the textile and provide greater durability but still function by rupturing the cell wall membrane of pathogens to which they come into contact.
An antiviral effect on a surface could be achieved via either destroying and inactivating viruses (called “virucidal”) or inhibiting the cell entry and/or virus replication (called “virustatic”). Therefore, virucidal agents (such as chlorine-based bleach and Lysol) attack and inactivate viruses outside of host cells by damaging their protein shell capsid, destroying the genetic materials such as RNA and DNA, or damaging the virion structure.51 For example, metal nanoparticles are proven to exhibit virucidal activity against a wide variety of viruses by interacting with the viral surface glycoproteins directly, as well as gaining entry into an infected cell to destroy its genome (DNA or RNA) and stop its replication process. Moreover, metal particles are active against the “naked” viruses, as such particles can interact with virus particles in a well-defined spatial arrangement.52 However, it is easier to attack and inactivate enveloped viruses in comparison to naked viruses, as they can be neutralized via various chemical and physical methods. Several studies report binding and/or destroying anionic viruses via cationic surfaces or materials (polymers, metals),53 heterocoagulation with positively charged aerosols,54 photocatalytic effect,55 methylene blue photochemical treatment,56 nanoparticles,52 aqueous and gaseous ozone,57 antifouling surfaces,58 and self-cleaning surfaces.59
Self-cleaning and antifouling are important properties for antimicrobial textiles. Contamination of fibers by destroying and inactivating viruses and bacteria leads to a decrease in antimicrobial activity. Stimuli-responsive polyelectrolyte multilayers attached to fibers provide cationic and anionic sites to neutral fibers60 and control bacterial adhesion.61 When compared to uncoated fabric, a high degree of surface charge density leads to a reduction in adhesion of Staphylococcus aureus by 50%. Self-cleaning and antifouling mechanisms are based on both electrostatic repulsion of contaminants and the change in mechanical properties of polymer nanocoatings in response to biological contaminants and products of their living cycle62 and degradation.63 Uncharged polymer chains are in the collapsed inactive state, but in the presence of biological contaminants, functional groups of the polymers become charged and an active gel-like nanocoating. Such reversible phase transitions in polycationic/polyanionic multilayers provide textiles with both antimicrobial and self-cleaning properties. For instance, Gram-positive Lactococcus lactis produce lactic acid, which decreases the local pH to 4. However, when the pH reaches 4, the polymer becomes charged, the length of the charged chains increases, and the adsorbed bacteria are mechanically detached from the surface.62
Synthetic Antimicrobial Agents
Quaternary Ammonium Compounds (QACs)
A class of cationic surface-active agents (Figure 4c)64 are commonly used in textile manufacturing as biocides. They are also used as detergents, softening agents, or antistatic agents at different stages of textile processing such as pretreatment, dyeing, and finishing.65 Conventionally, QACs (R4N+X–) refer to the subgroup of linear alkyl ammonium compounds, which are composed of a hydrophobic alkyl chain (C12–C18) and a hydrophilic counterpart.66 Such positively charged cationic agents are usually attached to anionic fibers (cotton, polyester, nylon, and wool) via ionic interaction.64,67 The antimicrobial effect on textiles with QACs is obtained by the interaction between positively charged surfaces and negatively charged cell membranes of the microbes, resulting in damage of the cell membranes, denaturation of proteins, and inhibition of DNA replication.67,68 QACs are effective against a broad spectrum of pathogens such as Gram-positive and Gram-negative bacteria, fungi, and certain viruses.64,69 However, they suffer from poor durability due to the fast leaching from textiles.50
Triclosan
(2,4,4′-trichloro-2′-hydroxydiphenyl ether) (C12H7Cl3O2) is an odorless synthetic chlorinated bisphenol (Figure 4d), which does not ionize in solutions unlike other cationic biocides and thus improves its resistance to laundering. Triclosan is also effective against Gram-positive and Gram-negative bacteria and to some virus and fungi67,70−72 by blocking lipid biosynthesis of phospholipids, lipopolysaccharides, or lipoproteins and affecting the integrity of cell membranes.67,70 Triclosan has been used in hospitals and personal care products such as antimicrobial soap, toothpaste, and deodorants for decades.73 It is suitable to be applied to polyester, nylon, polypropylene, cellulose acetate, and acrylic fibers.50 However, a number of leading retailers and governments in Europe have banned triclosan because it could potentially cause skin irritation, as well as being non-biodegradable and toxic to aquatic and other organisms.74
Metal oxide or salt compounds
, mostly based on silver, but also on copper, zinc, and cobalt have commonly been used as antimicrobial agents, due to their ability to bind to O, N, or S donor ligands present in the pathogen cells, inducing oxidative stress and damaging cellular proteins, lipids, and DNA.75,76 Among them, silver nanoparticles (Figure 4e) have been widely exploited in textiles, mainly in the form of salts, due to their broad spectrum of actions on pathogens.77 Recently, metal nanoparticles have received significant interest because of their relatively higher surface area, higher solubility, and faster release of the metal ions, resulting in a strong antimicrobial effect.78,79 The size of ZnO nanoparticles was found to be inversely proportional to its antibacterial activity.78 The limitation of metal nanoparticles is their cost, technical and environmental challenges, and requirement of additional plasma, UV or acidic pretreatment.80
Polyhexamethylene biguanide (PHMB)
((C8H17N5)n) is a polycationic amine, which causes the disruption of cell membranes and lethal leakage of cytoplasmic materials by interacting with microbial cell membranes via electrostatic and hydrophobic interactions.67,81,82 The antimicrobial activity increases with the increased level of polymerization.83
Regenerable N-halamines
are heterocyclic organic compounds, which contain one or more nitrogen and halogen (N–X) covalent bonds, where X is usually chlorine (N–Cl) but could also be bromine or iodine (Figure 4f). Such covalent bonds could be formed via the chlorination of an amine (RR′–NX), amide (−C(O)–NX–R), or imide (−C(O)–NX–C(O)−) group in dilute sodium hypochlorite. The order of stability is as follows: imide < amide < amine and, in terms of biocidal action effectiveness, imide > amide > amine.64,67,84N-halamines provide biocidal actions against a wide variety of bacteria, fungi, and viruses, which is achieved via the electrophilic substitution of Cl with H in the presence of water. The free Cl anions then bind with the acceptor regions of the pathogen to prevent the enzymatic and metabolic processes of their cells, thus causing the destruction of the pathogen.85 The imide N-halamines work better for the rapid destruction of pathogens; however, amine N-halamines are the better choice for durable and sustainable antimicrobial properties on textiles.84,86 The advantage of N-halamines are lower cost and long-term stability and action against a broad spectrum of pathogens. In addition, their antimicrobial effect could be recharged using a bleaching solution during laundering. Such bleaching solutions usually contain sodium hypochlorite, sodium hypobromite, trichloroisocyanuric acid, or sodium dichlorocyanurate, which donates Cl or Br to improve antimicrobial effect with N-halamines.87 However, the disadvantage of N-halamines antimicrobial finish on fabric is the presence of a substantial amount of adsorbed Cl or even other halogens on the fiber surface that can discolor fabric and produce an unpleasant odor.
Conjugated polymers such as polypyrrole (PPy)
are conductive polymers (Figure 4g) that are produced via chemical oxidative polymerization from water solutions of the monomer and applied in situ onto textile fibers, yarns, and fabrics in the oxidation bath during the polymerization process. Monomers and oligomers of conjugated polymers are very toxic.88 The presence of such low-molecular-weight compounds in fibers after polymerization can significantly restrict the application of conjugated polymers for textiles that can be in contact with skin. Compared to other conjugated polymers such as polyaniline, PPy is more biocompatible and less cytotoxic.89 The antimicrobial properties of PPy are due to the presence of positive charge distributed along the backbone chains and are very effective against both Gram-negative and Gram-positive bacteria.81 In addition, PPy’s nonleaching behavior provides better safety for both outside environments and the wearer of the garment. The report on the antimicrobial activity of PPy suggests that the addition of the antimicrobial agent CuCl2 to PPy increases the biocidal efficiency by up to 93, 98, and 100% against S. aureus, E. coli, and Candida albicans, respectively.91 In addition, PPy has also been investigated for antimicrobial applications in combination with silver,92,93 using silver nitrate as an oxidant92 or silver-coated fabric.94 The presence of silver in PPy/silver composites increases the antimicrobial activity of coated fabrics by increasing the inhibition zone. The major obstacles to using of PPy and other conductive polymers are the presence of toxic monomers and the low processability of polymers. Both problems can be solved by incorporation of conjugated polymers as nanoparticles.95
Graphene materials (GMs)
such as graphene, graphene oxide (GO), reduced GO (rGO), and graphene quantum dots (GQDs) have shown promise as a new class of broad spectrum antimicrobial agents.96 Additionally, graphene-based materials have successfully been applied on textiles,97−100 and their scalable production methods have been reported.101−103 GO, a derivative of graphene, is a two-dimensional one-atom-thick sheet composed of sp2-hybridized carbon atoms (Figure 4h).104 In 2010, a study on the antibacterial activity of graphene materials (GO and rGO) against E. coli bacterial growth was reported. Since then, several studies104−107 have reported antibacterial activity of such materials, which is mainly due to the combined mechanisms of bacterial membrane perturbation caused by sharp edges and oxidative stress induction. In addition, the presence of abundant oxygen-containing functional groups such as hydroxyl, epoxy, and carboxyl groups on the graphene oxide surface enhance its hydrophilicity and biocompatibility and facilitate its surface modification with other molecules or polymers significantly.104−106 However, there is currently no consensus in terms of the intrinsic antibacterial properties of “bare” GO.108,109 Previous studies report that GO possesses strong,105,110 very weak,111 or no112 antimicrobial activity and even facilitates bacterial proliferation.113 Nevertheless, the antimicrobial activity of graphene materials has been investigated as nanocomposites with other antimicrobial agents such as metal nanoparticles (mainly silver),114,115 metal oxides (e.g., Cu2O),116 photocatalysts (e.g., TiO2),116,117 quaternary ammonium salts (QAS),118−121 and polymers (e.g., polypyrrole).122 In such nanocomposites, graphene-based materials are claimed to enhance the antibacterial performance mainly due to their large surface and sharp edges. Only a few studies reported the broad spectrum antiviral activity of GO and GO-AgNP composites against viruses such as pseudorabies virus (PRV, a DNA virus), porcine epidemic diarrhea virus (PEDV, an RNA virus),123 respiratory syncytial virus (RSV),124,125 and Novel duck reovirus (NDRV).126
Natural Antimicrobial Agents
Recently, many eco-friendly natural antimicrobial agents such as peroxy acids, chitosan and its derivatives, or specific dyes have drawn significant interests for textile applications, due to growing environmental concerns with synthetic antimicrobial agents, as well as increased awareness about consumer safety. For example, materials extracted from different parts of plants such as bark, leaves, roots, and flowers containing tannin, flavonoids (Figure 4i), and quinonoids but also alkaloids, saponins, terpenoids, and phenolic compounds, with strong antimicrobial properties, have been studied and were found to be very effective antimicrobial agents.127−131 In addition, essential oils have been investigated as efficient antimicrobial agents.130,132 Moreover, natural dyes extracted from bark, leaves, roots, fruits, seeds, and flowers or from pathogens such as fungi, algae, and bacteria could offer low-cost and environmentally friendly colors with inherent antimicrobial properties from different coloring materials such as tannin, flavonoids, and quinonoids.133 Furthermore, natural antimicrobial peptides that are present in every living organism could be ideal candidates for antimicrobial textile applications.134 The use of natural high-molecular-weight antimicrobial compounds can overcome the issues of loss of antimicrobial activity after washing and chemical treatment, leaching out from the fabrics, and contamination of the environment and users’ skin as well as high costs.81
Chitosan
(2-amino-2-deoxy-(1→4)-β-d-glucopyranan), a biodegradable, biocompatible, nontoxic, noncarcinogenic, and environmentally friendly antimicrobial agent, is derived from the deacetylation of chitin. It is the second most abundant biopolymer in the world after cellulose, which consists of 20–30% of the exoskeleton of crustaceans (Figure 4j).141 It offers a strong antimicrobial activity against a wide variety of pathogens, including fungi, algae, and some bacteria. The interaction between the positively charged chitosan side groups and the negatively charged microbial cell membranes occurs through either electrostatic interaction, binding with the microbial DNA, or through the excellent metal-binding capacity of chitosan due to its amine groups.47,138 Chitosan could be incorporated into textiles with dyes and pigments due to the presence of reactive amine groups139 and also with binder,140 which enables flexible application methods and a durable antimicrobial effect. However, chitosan suffers from some disadvantages for textile applications such as sensitivity to temperature, pH activity dependence, and imparting a poor handle to the fabric. In addition, thermal curing following padding or exhaustion is the most common application of chitosan on textiles and involves a high temperature with associated energy consumption costs and possible fabric degradation. To mitigate such problems, UV curing has been proposed as a fast and eco-friendly process, which is carried out at room temperature, with a cost lower than that of the traditional thermal process.142−145 The stimuli-responsive properties of chitosan are used for the design of self-cleaning, anti-fouling, and self-healing146 nanocoatings as well as the formation of polymer nanocarriers for release of antimicrobial compounds on demand for biomedical textiles.147
Personal Protective Textiles
Medical textiles are typically soft goods used for healthcare and hygiene applications and have been critical components in the protective healthcare sector.148 Such textiles are broadly categorized into implantable (sutures, vascular grafts, artificial ligaments, etc.), nonimplantable (wound dressings, bandages, and pressure garments), extracorporeal devices (artificial kidneys, artificial lungs, liver, etc.), and protective, hygienic, and healthcare products (surgeons’ and operating theater wear, operating drapes, and medical staff uniforms).5,149 Surgical textiles, which fall within the class of healthcare and hygiene products, have been in unprecedented demand in recent months due to the COVID-19 pandemic. Since the first COVID-19 case was reported in China, surgical textiles such as gowns, gloves, and masks have been used extensively in healthcare environments for the protection of HCWs and to stop the spreading of the highly infectious coronavirus COVID-19. Therefore, in this section, we discuss the nature of current surgical textiles, the associated performance standards, fiber and polymer types, and the fabric structures, manufacturing techniques, and functional finishes used for such textiles.
Fiber and Fabric Types
The properties of an item of protective clothing is defined by the physical and chemical properties of its smallest component, the fibers. The fibers, with their high surface area and relatively shorter length, prevent transmission of particles. Microfibers, in particular, are generally preferred for manufacturing barrier materials which provide higher levels of protection. However, less absorbent or hygroscopic fibers wick liquid along the fiber surface, enhancing the capillary movement of liquid which contains pathogens. Thus, less absorbent synthetic fibers (such as polypropylene and polyester), which neither absorb liquid nor admit bacteria to be trapped inside their structure, provide better liquid barrier properties than those of natural origin (such as cotton, wool, silk, etc.) and are commonly used for protective clothing. Furthermore, the capillary absorption of fibrous assembly is governed by the following factors:150 the characteristics of the fluid (surface tension, viscosity, and density); fiber surface energy and surface morphology; fluid’s interaction with the fiber surface (interfacial tension and contact angle); and pore characteristics (size, volume, geometry, and orientation). In addition, the level of twist in textile yarns can also influence the barrier properties of fabrics.
Medical gowns can either be “disposable/single-use” or “reusable/multi-use”. Reusable gowns, washed after each use, are usually tightly woven fabrics with a plain weave structure that are chemically finished via a pad–dry–cure process to improve liquid barrier properties. They are washed after each use and used typically for more than 50 washing and drying cycles, which is monitored via a suitable tracking system.2 Such gowns are typically made of 100% cotton, 100% polyester, or a polyester/cotton blend. Historically, loosely woven cotton muslin fabrics with high air permeability and breathability were very popular as medical textiles; however, they were eliminated from the market due to their poor resistance to liquid penetration.7 Cotton/polyester blend fabrics also faced the same problem, even with a 180 thread count, where the blend fabrics met wearer comfort requirements but failed to resist microbial penetration.75 Woven polyester (T280) fabrics provided better water-repellency and increased protection against strike-through liquids and pathogens, but the thermal comfort could be a problem.151 A recent study of the North America market152 reported that the majority of modern reusable surgical gowns are composed of woven polyethylene terephthalate (PET) fabric in the noncritical zones and knitted PET fabric in the critical zones. A barrier fabric is used to reinforce the knitted PET in the critical zones, where 70% of the barrier fabric is based on expanded polytetrafluorethylene (PTFE) and the remaining 30% is based on breathable polyurethane (PU) barrier membranes.
Single-use or disposable nonwoven medical gowns and drapes are becoming the primary choice for healthcare professionals,153 due to their ability to provide excellent protection against fluids and pathogens154 as well as maintaining breathability and comfort. Single-use or disposable gowns are usually composed of nonwoven fabrics and polyethylene films with a weight range of ∼30–45 g/m2.153 Disposable gowns are typically based on synthetic fiber (such as polypropylene, polyester, and polyethylene) nonwoven fabrics, which could be engineered to achieve desired properties using particular fiber types, bonding processes, and fabric finishes (chemical or physical). There have also been recent approaches, such as electrospinning, which is a well-established technique to manufacture nonwovens made from polymer fibers with diameters in the range of 100–600 nm, to produce protective clothing with improved comfort without compromising the protective performance. We discuss various manufacturing and finishing (functional) processes in detail for medical protective clothing in the following section.
There is a considerable variation between reusable and disposable protective medical textiles in terms of design and performance. They both have pros and cons in terms of protection, maintenance, comfort, cost, and environmental impact.155 Several studies7,152,155,156 have evaluated and compared the performance of both reusable and disposable medical gowns and, in most cases, report that the impermeable materials are effective in reducing transfer of pathogens; however, the thermal comfort of the wearer is adversely affected.154 Moreover, disposable surgical gowns made of SMS polypropylene laminate offer higher fluid resistance than the gowns made of a polyester–cellulosic blend and only allow passage of methicillin-resistant S. aureus at pressures >1 psi.157 Reusable items of protective clothing are subjected to abrasion and damage over time and also undergo further mechanical stress during laundering processes. Indeed, several studies7,154,156,158 have highlighted that laundering processes cause fabric to break down and, in turn, reduce a fabric’s ability to prevent the penetration of pathogens through its surface. Nevertheless, gowns with reinforced layers demonstrate better durability to laundering. Furthermore, both disposable and reusable protective clothing have an environmental impact, which is discussed later in this review.
Manufacturing Process
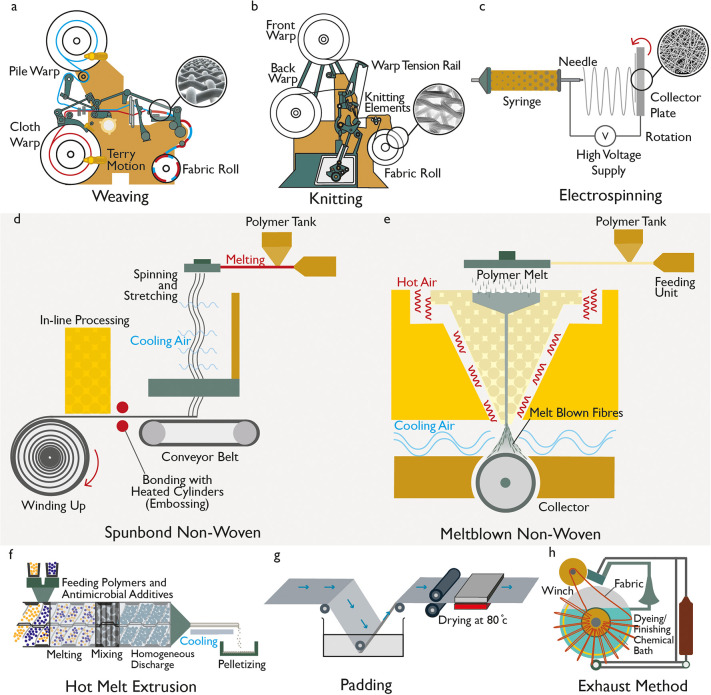
The fabrics used for protective medical clothing are usually of woven, knitted, or nonwoven structure. Woven fabrics are manufactured via a weaving process, where fabrics are formed by interlacing or interweaving of warp (lengthwise or vertical) and weft (widthwise or horizontal) yarns (Figure 5a).159 Woven fabrics can be customized to have specific strength, thickness, extensibility, porosity, and durability by varying their weave construction, the thread spacing or the raw materials (fiber) structure. Such fabrics are usually strong and durable; however, they are less extensible than knitted fabrics, are porous, and have poor barrier properties. After weaving, knitting is the second most popular technique for manufacturing fabrics, and it involves interlooping one yarn or a group of yarns (Figure 5b). Compared with weaving, knitting is a faster and more economical method of converting yarn into fabrics. Additionally, knitted fabrics are more stretchable and have potential for very high extensibility, up to 100%, and offer improved comfort and a better fit in most types of apparel.160 However, knitted fabrics also provide poor barrier properties due to their highly porous structure. Nevertheless, the barrier properties of both woven and knitted fabrics can be improved by engineering a dense fabric structure and using hygroscopic synthetic fibers.
Figure 5.
Manufacturing processes for personal protective fabric. (a) Weaving mechanism and woven fabric structure (inset). (b) Knitting mechanism and knitted fabric structure (inset). (c) Electrospinning process and resulting fabric with random orientation (inset). (d) Spun-bond nonwoven fabric manufacturing technique. (e) Melt-blown nonwoven fabric manufacturing technique. Application of antimicrobial finish into/on textiles: (f) hot melt extrusion process for melt-mixing antimicrobial additives to fiber polymers, (g) pad–dry–cure technique to apply antimicrobial finish on fabric, and (h) exhaustion method to apply antimicrobial finish on fabric.
The most popular fabrics for medical applications are nonwoven fabrics (Figure 5c–e), which are defined by ISO 9092:1988 as “a manufactured sheet, web or batt of directionally or randomly orientated fibers, bonded by friction, and/or cohesion and/or adhesion, excluding paper and products which are woven, knitted, tufted, stitch-bonded incorporating binding yarns or filaments, or felted by wet-milling, and may be additionally needled.” Single-use disposable medical textiles are usually made of nonwoven fabrics alone or in combination with other materials (for example, plastic) in order to increase the fluid repellent property. Nonwoven fabrics are manufactured via bonding or interlocking fibers or filaments of various size and shape by mechanical, thermal, chemical, or solvent treatment to provide integrity and strength to the fabrics rather than the interlocking geometries associated with woven and knitted materials. In the manufacture of nonwoven fabrics, both staple and filament fibers can be used separately or in blends of different sizes and types, which are selected on the basis of the desired properties and performance of end products.160,161 Fibers are arranged randomly in a nonwoven fabric structure, which successfully reduces liquid transmission by providing a filtering media and reducing the capillary formation.162 The most commonly used nonwoven fabrics for surgical gowns and drapes are spun-lace, spun-bond–melt-blown–spun-bond (SMS), and wet-laid.7 Synthetic fibers (such as polypropylene, polyester, and polyethylene) are typically used for single-use items. To improve the barrier resistance, absorbency and nonslippage performance of both single-use and reusable products, additional materials in the forms of coatings, reinforcements, laminates, or plastic films are often added to obtain a composite material.7
Nonwoven fabrics are manufactured in two stages: web formation and bonding. First, the fibers or filaments are laid on a forming or conveying surface via dry-laid, wet-laid, or spun-laid techniques. Such web-forming techniques originate from traditional carding, suspending fibers in liquid and polymer extrusion techniques used traditionally in the textiles, paper, and plastic industries, respectively. The laid fibers are then arranged in the desired orientation using mechanical or fluid means. Second, the webs are then bonded together by mechanical, chemical, and thermal methods to form nonwoven fabrics. In the dry-laid process, staple fibers are converted into web or batt structure with uniform weight per unit area via carding, garneting, and air-laying. Meanwhile, wet-laid nonwovens are obtained by the swelling and dispersion of fibers in water, web formation, and drying and bonding of the web. The spun-laid process, which is most commonly used for protective medical textiles, involves the extrusion of the filaments from the raw polymer material, drawing the filaments, and laying them into a batt. It is a continuous polymer-to-fabric operation. There are several methods that can be used to produce spun-laid nonwoven fabrics including spun-bond, melt-blown, aperture films, and the many-layered combinations.160,163 The development of spun-bond technology was a major manufacturing breakthrough, which was followed by the development of melt-blown technology which enabled the production of finer microfibers.164 The melt-blown process provides advantages of better filament distribution, better filtration via smaller pores between the fibers, softer feel, and also the possibility of manufacturing lighter weight fabrics. Generally, high and broad molecular weight thermoplastic polymers such as polypropylene, polyester, and polyamide are processed by a melt-extrusion process in all commercially available spun-laid machines.160
In the spun-bond process
, melt fiber spinning is combined with web formation by placing the bonding device in line with spinning (Figure 5d). Briefly, thermoplastic polymer pellets or powder granules are fed from a hopper into an extrusion chamber where the polymer is heated to a molten state with other additives. The molten polymer is then passed through the heated screw and extruded from the extruder through a gear pump, which precisely controls the flow rate of polymer mixture into a die block or spin-pack. The spin-pack, which contains a spinneret with thousands of microdiameter holes, maintains uniform temperature and polymer distribution. The molten polymer flows through spinneret holes, jets into a quenching chamber, and is converted into fibers which are solidified by cooling air. In the attenuator, fibers are stretched by high-speed air flux to reduce their diameter while traveling from the spinneret to a collecting belt. The flight velocity of the fibers slows down as they are collected onto a conveyer belt via a vacuum system which sucks the air flux and facilitates a nonwoven web formation on the collector belt. The untreated nonwoven fabric is then progressed to the bonding line by a conveyer belt, where its is bonded via a mechanical, chemical, or thermal process before wound onto a take-up roller.165,166 Spun-bond fabrics provide good thermal properties, high tear strength, and good permeability. Therefore, they are widely used in hygiene-related, medical, construction, and agricultural applications, as well as in other end-uses in daily life.167
The melt-blown process
is similar to the spun-bond process in that the thermoplastic polymers are extruded through a spinning die to form filament fibers (Figure 5e). The main difference between the spun-bond and melt-blown processes is the way in which the air is introduced to cool and/or attenuate the fibers as they come out of the spinneret. In the spun-bond process, the quenching airflow is horizontal to the vertical fibers emerging from the spinneret, whereas in the melt-blown process, the heated air with high velocity is injected near the die tips, which will which will converge with the filaments to attenuate them to very fine diameters. The attenuated filaments are quenched with cool air and collected on a moving collector screen to form a fine fibrous and self-bonding web.168 As the air action is more dynamic in melt-blown manufacture, much finer fibers are obtained, which results in softer and weaker nonwoven fabrics. The attenuated filaments are generally 1–4 μm in diameter and form a very uniform web at low grammage. The melt-blown process is unique, as it is used almost exclusively to produce microfibers rather than traditional coarser textiles fibers. The fine fiber network and large fiber surface area of such fabrics result in enhanced filtration efficiency, excellent barrier properties, and good wicking action. Melt-blown fabrics have been widely used for applications in filtration (e.g., surgical mask and respiratory filtration), insulation, and liquid and oil absorption.163
Composite nonwoven fabrics
are also very attractive for medical applications. They offer the opportunity to combine strong and durable spun-bond materials with the relatively weaker melt-blown materials with better wicking and barrier properties and finer and higher surface area fibers. Therefore, by combining the two fabric types, a spun-bond–melt-blown composite can create a single product with enhanced performance. The most commonly used nonwoven composite laminates are spun-bond–melt-blown–spun-bond (SMS), spun-bond–melt-blown–melt-blown–spun-bond (SMMS), or spun-bond–spun-bond–melt-blown–melt-blown-spun–bond (SSMMS) in weights ranging from 10 to 25 g/m2 comprising 1–5 g/m2 melt-blown (MB) microfibers. The use of polypropylene (PP)/polyethylene (PE) biocomponent materials in the preparation of MB webs enhances the production and properties of the composite fabrics. Excellent levels of protection with softness and comfort have been achieved in SMS products with weight ranging from 10 to 70 g/m2.153 Trilayer antiviral and antibacterial nonwoven composite fabrics with an additional antimicrobial finish on the outer layer have shown Level 4 protection for surgical application, according to the barrier protection classification of AAMI.169,170 To improve the barrier resistance, absorbency, and nonslippage performance of both single-use and reusable products, additional materials in the form of coatings, reinforcements, laminates, or plastic film are often added.7 However, such membranes or reinforcements may impair comfort in applications such as medical gowns due to less heat transfer and more sweating.
Newer techniques such as electrospinning have been evaluated for use in the manufacture of gown materials to improve comfort without sacrificing the protective performance. Electrospinning is one of the most simple and effective methods of fabricating ultra-fine fibers with diameters ranging from a few nanometers to several micrometers.171 Electrospinning uses an electrostatic field and extrusion technology (Figure 5c) to generate ultrafine fibers in a very short period of time with minimum initial investment, training, and supervision. Initially, electrospinning received only a small amount of attention due to issues associated with low productivity and fiber nonuniformity. However, such issues have been resolved with the advancement of needleless electrospinning, near-field electrospinning, and electrospinning with rotating strings of electrodes.172Table 3 summarizes recent progress on filtration performance using an electrospun membrane. As emerging nonwoven filters, electrospun fibers have successfully been applied for PM1.0 and PM2.5 level filtration and now used for personal proection against COVID-19.
Table 3. Comparison of Filtration Performance.
| filtration type | material | test agent | flow rate | collection efficiency (%) | pressure drop (Pa) | quality factor (Pa–1) | refs |
|---|---|---|---|---|---|---|---|
| fibrous filtration | PSa/PANb/PAc-6 | NaCl | 32 L/min | 99.992 | 118 | 0.0799 | (173) |
| fibrous filtration | PVDFd-NIPes | NaCl | 16.6 cm/s | 98.33 | 97 | 0.042 | (174) |
| fibrous filtration | PAN/PVDF | NaCl | 0.3–0.5 m/s | 99.99 | 86 | 0.1071 | (175) |
| fibrous filtration | PAN | KCl | 5 cm/s | 96.6 | 172 | 0.0196 | (176) |
| fibrous filtration | nylon-6 | incense smoke | 1 m/s | 99.6 | 349 | 0.0158 | (177) |
| fibrous filtration | PAN | incense smoke | 0.21 m/s | 96.12 | 133 | 0.024 | (178) |
| electrostatic filtration | Al-coated polyester | KCl | 10 cm/s | 99.99 | 4.9 | 2.2 | (179) |
| fibrous filtration | PVDF/SDBSf | NaCl | 32 L/min | 99.985 | 66.7 | 0.132 | (180) |
| fibrous filtration | ZIFg-8/PAN | cigarette smoke | 0.05 L/min | 88.33 | 20 | 0.1074 | (181) |
| fibrous filtration | cellulose-PVPh | NaCl | 5.3 cm/s | 86.4 | 17 | 0.117 | (182) |
Polysulfone.
Polyacrylonitrile.
Polyamide.
Polyvinylidene fluoride.
Negative ion powder.
Sodium dodecyl benzenesulfonate.
Zeolitic imidazolate framework.
Polyvinylpyrrolidone.
Antimicrobial Finish Techniques
Antimicrobial finishes can be applied to textiles by embedding an antimicrobial reagent into the polymer bulk during the fiber processing (Figure 5f) or by applying a surface coating or modification as a chemical or physical finishing treatment (Figure 5g,h). Among these methods, the application at the textile finishing stage is a more common and popular choice via traditional pad–dry–cure (Figure 5g) or exhaust (Figure 5h) techniques. The “pad–dry–cure” technique is the most commonly used method for applying functional or soft finishes onto textiles such as water repellent, antimicrobial, wrinkle-free, moisture management, etc. onto textiles. In this technique, the fabric is passed through a padding bath containing the finishing agent (such as antimicrobial agent), and the mangle nip rollers squeeze any excess solution from the fabric surface, thus producing a uniform treatment. The fabric is subsequently dried and cured using a stenter at recommended temperatures (Figure 5g). Such a technique provides very high production speed (∼150 m/min)101,103 and is applicable to a wide range of fabrics of different structures (woven, knit, and nonwoven) and fiber compositions (cotton, polyester, polypropylene, and nylon). The “pad–dry–cure” technique is readily scalable and could be used for large-scale industrial production of protective clothing with antimicrobial and fluid-repellent properties. Another popular method for the application of functional finishes onto textiles is an “exhaustion” technique, which is most commonly used to dye yarn, knit, and woven fabrics and garments. The principle of such processing is the migration of dyes or finishing agent from the solution into the fiber or fabric until the dye or finishing agent has fully exhausted onto the fibrous materials (Figure 5h). Like the “pad–dry–cure” technique, the “exhaust” finishing technique is also highly scalable102 and could be used finish tonnes (∼1000 kg) of textiles in a short period, providing an even distribution and good wash fastness of finishing agents. To improve the durability of antimicrobial finishes to the fabrics, cross-linkers183,184 are used to introduce intermolecular covalent bridges between the polymer chains and the antibacterial molecule by chemical,185 radiation,186 or physical methods.187 In addition, surface modification methods, such as oxygen plasma treatment, ultrasound technology, UV radiation, surface bridging, and enzyme treatment, have all been investigated for improving the durability of antimicrobial finishes on natural fiber-based products.188
The other popular approach is to incorporate the antimicrobial agents into the polymer matrix of the textile fibers before or during spinning or during the web formation process. Such agents can be mixed (commonly by melt-mixing) with polymer granules prior to production or added to the reservoir chamber during the extrusion or electrospinning process (Figure 5f).67,189,190 Several studies191−193 report electrospinning of nanofibers based on polymers and nanoparticles with antimicrobial properties that provide several advantages such as higher surface area to volume ratio, adjustable porosity, and the ability to customize nanofiber composition. Additionally, the approach of mixing antimicrobial agents into thermoplastic polymers for fiber spinning or web formation processes has received substantial interests due to the durability and scalability it offers. Furthermore, such a method has no negative effect on the mechanical properties of the fibrous end product. However, it requires a higher extrusion temperature, and the antimicrobial agents are usually trapped in the polymer matrix, which restricts their ability to diffuse through the matrix to perform their biocidal or biostatic function. Thus, such an approach would potentially provide a lower antimicrobial effect and be limited to antimicrobial agents that are stable at higher temperature such as metallic particles.189,194,195 There are also natural fibers with intrinsic antimicrobial properties based on chitosan and cellulose fibers; however, their antimicrobial effect is generally less effective.50
Standards and Requirements for Protective Medical Clothing
The protective clothing products used in healthcare environments are considered as medical devices and are therefore subject to stringent regulations. Such clothing is required to meet or be equivalent to certain standards originated under the auspices of the International Standards Organization (ISO), European Committee for Standardization (CEN), or under various U.S. standards organizations (e.g., ANSI/AAMI, ASTM), Table 4. The selection and use of personal protective clothing depend on the hazards and the risks that a wearer is exposed to. Therefore, a first critical step is to identify and assess the physical and health hazards (i.e., risk and hazard assessment) in the workplace. The standards will then enable the selection of the appropriate protective clothing or PPE based on the risk and hazard assessment. There are mainly three types of standards: test methods, product or performance specifications, and technical reports or guidance documents.196 Test methods usually describe a testing method simulating the real-life exposure of protective clothing and what would be observed. The product or performance specification standards set the pass or fail criteria for protective clothing by defining the levels of performance that must be met for different properties related to hazards or risks. The guidance documents provide useful information about the selection, correct use, and maintenance of protective clothing.
Table 4. Typical Standards for PPE.
Regulatory Standards
For EU and U.K. markets, PPE must conform to European Commission (EC) regulations 2016/425, which covers the design, manufacture, and marketing of personal protective equipment. CE marking must be clearly evident on the product and/or packaging. CE marking is defined as a certification mark, which indicates that a product conforms with health, safety, and environmental protection standards for products sold within the European Economic Area. In addition, any products containing phthalates should be packaged in such a way that this information is clearly indicated in accordance with medical devices regulation 2017/745. Furthermore, the products and packaging should be latex free with a minimum 3 year shelf life from the date of manufacture and instructions for use and disposal/recycling instructions for use and disposal/recycling. As per EU 2016/425, PPE is generally categorized into three types on the basis of the risks and hazards a wearer is subject to Category I (minimal risk), Category II (PPE not covered within category I or III), and Category III (high risks that may cause very serious consequences such as death or irreversible damage to health).197 In the U.S., all PPE is regulated by the FDA and should meet applicable voluntary consensus standards for protection. Premarket Notification or 510(k) clearance is required for some PPE before it can be legally sold in the U.S. In such cases, PPE is reviewed by the FDA to make sure it meets specific criteria for performance, labeling, and intended use to demonstrate substantial equivalence by conforming to consensus standards for barrier performance and resistance to tears and snags. In addition, voluntary consensus standards may also be used to demonstrate sterility (when applicable), biocompatibility, fluid resistance, and flammability.
Functional/Barrier Properties Against Pathogens
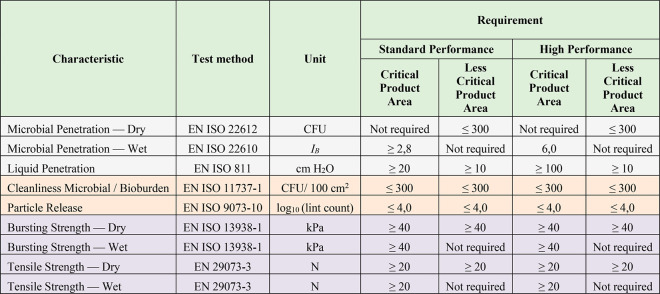
Pathogens are transported by carriers such as body fluids, sloughed skin cells, lint, dust, and respiratory droplets. Therefore, protective clothing with functional properties such as protection against liquids and pathogens are extremely important for healthcare applications.198 In order to determine the antiviral activity of textile materials, ISO 18184:2019 has been developed and provides a quantitative test method to assess the antiviral performance of such products against specific viruses including SARS-CoV-2 (NL63).199 Recently, several commercial products claimed that their products were effective against coronavirus (kills such viruses) after testing their antiviral performance against human coronavirus (NL63) as per ISO 18184:2019. In addition, several standard test methods have been developed to investigate the functional performance of protective clothing against liquids and pathogens by simulating real-life conditions.200 Such test methods include the water impact penetration test, the hydrostatic pressure test, resistance to liquid penetration,201 and protection against pathogen.202 The EN 13795 European Standard for surgical gowns and drapes specifies the performance requirements, manufacturing standards, and testing methods for both reusable and single-use surgical drapes, gowns, and clean-air products. In this standard, the barrier performance of the protective clothing against liquid and pathogens is tested by a liquid penetration resistance test (EN IS0 811:2018), a wet microbial penetration resistance test (EN ISO 22610:2006), and a dry microbial penetration resistance test (EN ISO 22612:2015).203 In the U.S., American National Standards Institute (ANSI) and the Association of the Advancement of Medical Instrumentation (AAMI). ANSI/AAMI PB70:2003. identifies four levels (Levels 1–4: minimal to high risk) of protection based on barrier performance against liquids and pathogen. Such levels of protection are assessed by the spray impact penetration test, the hydrostatic head test, and synthetic blood (for surgical drapes) and viral penetration test (for surgical and isolation gowns). Table 5 shows the characteristics to be evaluated and performance requirements for surgical gowns as per BS EN 13795-1:2019. As per the standard if the manufacturer does not categorize product areas, all areas of the protective clothing should meet the requirements for critical product areas.
Table 5. Characteristics to be Evaluated and Performance Requirements for Surgical Gowns (BS EN 13795-1:2019)203.
Physical Properties
The protective garment should be durable enough to last the intended life cycle. The critical physical and mechanical properties need to be considered while selecting protective clothing, which include tensile and burst strength, dimensional stability, and lint generation. Such properties are assessed by a tensile test (EN 29073-3 or ASTM D5034, ASTM D1682), burst test (EN ISO 13938-1), and lint generation test (EN ISO 9073-10). In addition, the seam strength (ASTM D751) and the barrier properties of seams/closures are critical to provide the overall barrier protection by fluid-resistant or impermeable garments. Several seaming techniques with barrier properties are used in the construction of protective clothing. Examples of durable and effective seams include serged or sewn, bound, taped, double taped, and ultrasonic welded seams. Furthermore, HCW should also know the appropriate size of their protective garments as it may catch or snag on objects if they are too large. PPE that fits well and is comfortable to wear will encourage employee use of PPE. The American national standard ANSI/ISEA 101-2014 provides a sizing chart and a set of exercises in which a user can validate whether that garment is the “proper” size.198 All reusable protective clothing and linen should be laundered at 71 °C for a minimum of 3 min or 65 °C for at least 10 min per the BS EN 14065:2016 standard and still provide the specified protection after washings.204
Comfort
Surgeons are less likely to tolerate poor thermal comfort and fit while wearing personal protective clothing. A balance between the heat loss and gain of a human body is needed to enable thermal comfort. Such thermal comfort with a medical gown depends on the surgical environment, length of procedure, and amount of exposure.148 The temperature and relative humidity of an operating room are usually ∼15.6–25.6 °C and 30–60%, respectively. However, the operating room temperature could increase during the procedure due to the radiant heat from the overhead lighting. In addition, doctors and nurses’ own body heat released in a high-stress environment under strong lighting add to the feeling of discomfort,205 which may contribute to increased mistakes, impaired performance, and less efficient work.7 Therefore, protective medical clothing should provide an isothermal environment for HCWs during use. To improve barrier resistance, absorbency, and nonslippage performance of both single-use and reusable protective clothing, additional materials in the form of coatings, reinforcements, laminates, or plastic film are often added.7 However, such membranes or reinforcements may impair the wearing comfort of the reinforced gowns due to less heat transfer and more sweating in areas where the gown is covered by another layer of fabric or a plastic. To provide enhanced protective medical garments without compromising the comfort/breathability, several advanced technology-based approaches have been introduced to increase the permeability and flexibility of the fabric. For example, phase change materials have been used to improve the regulation of the temperature of treated fabrics within the normal comfort range.206 Another approach is to use layered, breathable materials with an active cooling mechanism. Because of the environments in which they work and the nature of the tasks which they must perform, surgeons tend to express a preference toward comfortable products which have moisture management properties while also providing a high level of protection. To achieve such clothing, an impervious “breathable” plastic film is sandwiched between two layers of spun-bonded nonwoven, allowing moisture vapor to pass through the fabric from the inside of the garment but preventing the passage of fluids or pathogen from outside.207
Biocompatibility
A biocompatibility test of protective medical clothing has been developed to determine its compatibility with a biological system and fitness for human use. The International Organization for Standardization (ISO) established ISO 10993-1, “Biological evaluation of medical devices - Part 1: Evaluation and testing within a risk management process”, for evaluating the biocompatibility of a medical device prior to a clinical study. The biocompatibility test involves the application of a high temperature to a medical device to extract leachable materials and then investigating potentially harmful chemicals or cytotoxicity in the leachable extracts. There are a range of biocompatibility tests available, from a skin irritation test to hemocompatibility and implantation tests, which could be in vitro or in vivo or both depending on the intended use. The FDA recommends that cytotoxicity (ISO 10993-5), sensitization (ISO 10993-10), and irritation or intracutaneous reactivity (ISO 10993-10) is evaluated for a medical device that is contact with the skin.27,208
Global Market and Supply Chain for Personal Protective Clothing
The global market size of personal protective products was valued at ∼$50.93 billion in 2018 and is expected to grow to ∼$79.66 billion at a compound annual growth rate (CAGR) of ∼6.6% from 2018 to 2024 (Figure 6a).209 In 2019, China was the largest exporter of personal protective products with a ∼17.2% market share followed by Germany and USA (Figure 6b).210 The protective clothing market for the healthcare industry was valued at ∼$1.2 billion and forecast to reach ∼$1.7 billion by 2024 at a CAGR of ∼6.5%.211 As expected, the demand for medical protective clothing in North America was the highest (∼$0.49 billion) followed by Europe (∼$0.32 billion) and was forecast to continue to grow until 2024 (Figure 6c) at a CAGR of ∼6.9% (North America) and ∼5.7% (Europe), due to the presence of technologically advanced countries in these regions and stringent regulations regarding the use of protective clothing in every industry. South America and Asia-Pacific (APAC) are also projected to experience high growth in the global protective clothing market during the forecasted period. The markets in these regions are estimated to register CAGRs of 7.0 and 6.4%, respectively, between 2019 and 2024. Such growth is mainly driven by the manufacturing industry and rapid urbanization in these regions. China is the largest market for protective clothing in APAC.211
Figure 6.
Global personal protective equipment and clothing market and their environmental impacts. (a) Value of the personal protective equipment market worldwide from 2018 to 2025 in billion U.S. dollars (source: Statista). (b) Share of the leading exporters of personal protective products worldwide in 2019 (source: Statista). (c) Protective clothing market in healthcare/medical industry, by region, 2019–2024 in millions U.S. dollars (source: Market and Market Research). (d) PPE supply levels for doctors working in high risk areas in the U.K. during COVID-19 pandemic as of April 2020. (e) Energy consumptions, water consumptions, greenhouse gas emission (GHG), and fiber production for polypropylene, polyester, and cotton fibers. (f) Materials sustainability index (MSI) score for polypropylene, polyester, and cotton fibers. (g) Comparison of environmental impact of reusable (R) and disposable (D) surgical gowns.152 NRE = natural resource energy, GWP = global warming potential.
Since the outbreak of COVID-19 in December 2019, there has been a surge in demand for PPE products, notably surgical masks and gowns which are made from polypropylene nonwoven fabrics. In 2018, nonwoven fabrics accounted for a 64.3% share of the global medical textiles market in volume terms. Prior to the COVID-19 pandemic, global demand for nonwoven fabrics for the medical sector was projected to grow by an average of 5.0% per annum between 2018 and 2025.212 However, during the COVID-19 pandemic, several hospitals around the world reported that supplies of PPE were running low. Indeed, a recent survey showed that over 30 and 21% of doctors working in high-risk areas during the pandemic reported shortages of long-sleeved disposable gowns and FFP3 masks or respirators, respectively (Figure 6d).213 Therefore, it can be assumed that this growth figure will have accelerated in light of the global COVID-19 outbreak.
Overall, producers of nonwoven fabrics account for some 70% of global polypropylene fiber demand. As a result of the surge in demand for nonwoven fabrics following the COVID-19 pandemic, prices for polypropylene fiber in Asia increased during April 2020, particularly in China. China is the largest producer of many of the raw materials required in the manufacture of PPE, and it is also the largest supplier of finished PPE products. Other PPE supplying countries include Taiwan, India, Japan, South Korea, Malaysia, Mexico, Thailand, USA, and several countries in Europe.214 The supply of PPE and associated raw materials has been disrupted during the COVID-19 pandemic, not least because government-imposed lockdowns in China disrupted manufacturing operations in the country during February 2020. Since March 2020, PPE manufacturing operations in China have largely returned to normal, although there is still some concern regarding the supply of raw materials.214 However, increasing numbers of companies in China are turning to the manufacture of polypropylene fiber in light of the increase in demand.215
Some countries have imposed export bans on PPE products following the COVID-19 outbreak. The Indian government, for example, has imposed an export ban on all PPE products and associated raw materials. In addition, the EU has enforced a regulation to prevent the export of PPE without authorization from the manufacturing member state. Overall, demand for PPE during the COVID-19 pandemic appears to be outstripping supply,216 and this is a cause for concern. Furthermore, ensuring adequate supply of PPE is particularly challenging for low-income countries and middle-income countries, whose governments cannot afford to compete against high-income countries in bidding wars for PPE. To help meet increasing demand for PPE and other medical apparel such as scrubs, several companies which usually manufacture consumer products have converted their production lines to manufacture PPE or medical scrubs. Several such companies are concentrating on boosting domestic supplies in the light of global shortages of PPE and other medical apparel.214
Environmental Impact
The environmental impacts of the textile industry are well-documented, and the textile industry is reported to be the second largest polluter of the environment after the oil industry, by contributing to ∼8–10% global CO2 emission and ∼20% of the global waste.217 About 95% of textiles are fully recyclable; however, 85% of all textiles are still sent to landfills or incinerated.218 Synthetic fibers such as polyester, which dominate the textiles market due to their low cost and performance characteristics, are the biggest source of carbon emission. As synthetic fibers provide better protection against pathogens, protective clothing products for healthcare applications—particularly single-use surgical products—are predominantly made of polypropylene or polyester. At the end of their useful lives, such products are either incinerated, thereby generating further carbon emissions, or sent to a landfill, where they can persist for years (∼450 years) due to their non-biodegradability. Thus, the environmental impacts of such clothing are significant. Indeed, it is estimated that, during the COVID-19 pandemic, frontline workers worldwide are using some 44 million nonwoven PPE items on a daily basis, the majority of which are made from polypropylene. This, in turn, is resulting in the generation of some 15,000 tons of waste every 24 h.219 However, it is challenging to accurately assess these environmental impacts due to their complex supply chains and lack of extensive studies in this area. Nevertheless, in this section, we discuss the current understanding of the environmental impacts of the protective medical clothing on energy consumption, carbon footprint, blue water consumption, and solid waste generation. We also compare the environmental impacts of synthetic fiber-based clothing with the most commonly used natural fibers such as cotton.
Polyester dominates worldwide fiber consumption, with ∼55% of the total textile fiber by volume, followed by cotton (∼27%). By contrast, the consumption of polypropylene fibers is far less as they are used for very specialized applications such as surgical masks and medical gowns. However, the consumption of polypropylene fibers has seen a significant rise in recent months due to the COVID-19 pandemic. Synthetic fibers consume the highest amount of energy during the fiber manufacturing, as they are usually manufactured from fossil fuels. For example, the production of polyester and polypropylene consumes almost double the amount of energy compared with that of cotton fibers. Additionally, greenhouse gas emission (GHG) is also influenced by the energy consumption rate and the sources of the energy consumption during textile processing including spinning, weaving, dyeing, and finishing. For example, coal-based energy sources used in China have a higher carbon footprint than that of other “greener” sources used in Europe. The textile industry also uses large amounts of water, ∼79 billion m3 in 2015 alone, and about 90% of this consumption is associated with the cultivation and wet processing of cotton-based textile materials. Cotton fibers therefore have the highest water footprint among textile fibers. In reviewing the Higgs Materials Sustainability Index (MSI) score from the life cycle assessment (LCA) to understand and quantify the sustainability impacts of polyester, polypropylene, and cotton textiles, it is apparent that the fossil fuel consumption associated with polyester and polypropylene is higher, which, in turn, results in higher global warming (Figure 6e,f). However, the cotton fiber contribution to water usage/scarcity is substantial and to eutrophication is higher than polyester and polypropylene (Figure 6e,f). Nevertheless, natural fibers such as cotton are biodegradable, unlike synthetic fibers which can remain in the environment for hundreds of years.
Medical textiles including surgical gowns are available as reusable and disposable products. The recent COVID-19 pandemic has driven the market toward single-use disposable products due to hygiene concerns and the need to avoid spreading infection or diseases. After use, all single-use PPE are discarded using standard infection control measures. NHS England usually labels waste as either infectious (contaminated with bodily fluids), offensive (contaminated but not infectious), or municipal (similar to household disposals). All infectious PPE waste is incinerated at high temperature. However, such processing is expensive and emits unwanted toxic gases, which contributes toward the overall pollution and carbon emission. Nevertheless, modern gas cleaning or “scrubbing” technologies can remove these potentially harmful gases, and additionally, the heat generated from waste incineration could be used as a source of energy. In the U.K., waste incineration contributes ∼2% of the total energy generation. Other medical waste, which is not incinerated, is usually discarded in a landfill, while a very small proportion is recycled. It is worth noting that, in the case of non-PPE clothing, by doubling a garments’ lifetime, it is possible to reduce GHG emission by ∼44% and save $460 billion each year. To become environmentally sustainable, it is therefore important to reuse and recycle textile materials. Similarly, there is a growing interest in reusable medical textiles due to better environmental sustainability. A recent study shows that the selection of reusable medical apparel could reduce natural resource energy consumption (∼64%), greenhouse gas emissions (∼66%), blue water consumption (∼83%), and solid waste generation (84%) (Figure 6f).152
The environmental impact of antimicrobial textiles is also a growing global concern, which is mainly associated with antimicrobial chemical production, their application on textile materials, and the subsequent use and disposal of such products.66 The ideal antimicrobial agents would have high efficacy at low dosing, be durable to washing, and their effectiveness should outweigh the potential environmental consequences and the cost of their usage.50 In addition, the removal of such agents from textile wastewater is important in order to avoid discharge to the wider aquatic environment. For example, silver is one of the most popular antimicrobial agents and is non-biodegradable. Silver is immobilized by the formation of stable sulfide complexes, which are insoluble and much less toxic and bioavailable than dissolved silver. Thus, the potential risk with silver in the aquatic environment is reduced by removing 85–99% of silver during the textile wastewater treatment.66,220 Additionally, the health impact of antimicrobial agents needs to be considered in order to evaluate the safety of antimicrobial compounds for animals and humans. The extent to which humans could be exposed to antimicrobial textiles is usually influenced by the type of action of the antimicrobial agent (diffusion or contact), its concentration in the textile, the exposure routes, and the frequency of use. The risk associated with antimicrobial textiles is usually evaluated via acute and chronic toxicity, skin sensitization and irritation, and the disturbance of skin ecology. For instance, silver may interact with so-called “good” bacteria such as skin flora to weaken the skin defense barrier221,222 and also lead to the deposition of Ag metal/Ag sulfide particles in skin, causing discoloration (argyria) or even ocular discoloration (argyrosis), which although not life threatening is cosmetically undesirable.221
Future Directions and Conclusions
Toward Smart and Sustainable Protective Clothing
Smart wearable electronic textiles (e-textiles) are becoming increasingly popular due to their ability to make human life safer, healthier, and more comfortable.97,99 Such smart e-textiles could potentially interface with the human body and continuously monitor, collect, and communicate various physiological data and the vital signs of the wearer, including temperature, heart rate, and oxygen saturation level, and communicate these data wirelessly to a processing device. Such data relating to vital signs can alert health care professionals of any deterioration in a patient’s health at a very early stage and enable them to intervene more quickly. This could be considered to be particularly important in health care settings such as care homes where staffing shortages are a major issue. Smart textiles could also allow patients to monitor their health at home, thereby freeing up hospital beds. Self-monitoring of medical conditions with connected wearable devices could potentially reduce NHS costs by ∼60%. In addition, the health and well-being of HCWs is immensely important in order to ensure that they are available to treat patients with infectious diseases, such as COVID-19. As such, e-textiles could be a useful tool in monitoring the health of HCWs, who regularly experience stress during long shifts or in operation theaters and are exposed to several risks in their work environment.
Smart wearable e-textile technologies could be integrated with protective clothing to produce truly “Smart” wearable medical clothing, which can then continuously monitor the physiological conditions of both HCWs and patients (Figure 7a). Furthermore, recent studies have also shown promise in tracking COVID-19 symptoms continuously via wearable sensors, which could potentially monitor coughs, fever, and respiratory activity. Researchers have developed such a wearable device and are creating a set of data algorithms specifically tailored to detect early signs and symptoms associated with COVID-19 and to monitor patients as the illness progresses.223 Recently, we also reported washable, durable, and flexible graphene-based wearable e-textiles, which are highly scalable, cost-effective, and potentially more environmentally friendly than existing metals-based technologies.98,101−103 In addition, graphene and other 2D materials have drawn significant interest in flexible and wearable electronics applications, due to their outstanding electrical, mechanical, and other performance properties. Such properties could also be exploited in heterostructures,224 where different 2D materials are inkjet printed on top of each other via rapid, precise, and reproducible deposition of controlled quantities of 2D materials in a nonimpact, additive patterning, and mask-less approach. Therefore, integrated graphene-based wearable e-textiles for protective clothing could potentiality address current challenges associated with the early detection of highly infectious diseases, ensuring good health and well-being of frontline workers.
Figure 7.
Future research directions and recommendations. (a) Smart wearable protective clothing that can monitor a wearer’s physiological conditions such as temperature, heart rate, and oxygen saturation level. (b) Sustainable protective clothing which are reusable, washable, and recyclable. (c) Use of green, natural, and novel materials for functional finishes on textiles. (d) Use of digital technologies for processing protective clothing. (e) Local manufacturing of personal protective clothing for healthcare applications. (f) Industry 4.0 for manufacturing of protective clothing. (g) Government legislation for using sustainable PPE. (h) Public and private funding in R&D to develop new and innovative technologies.
The outbreak of the COVID-19 pandemic has resulted in significant increases in the consumption of disposable face masks and gloves. Such plastic-based disposable items, used by the general public, are often not disposed of properly, thereby adding to the mass of plastic pollution which poses a significant threat to oceans and marine life.225 Recent news has highlighted concerns from conservationists about such “new” pollution, which is set to become ubiquitous after millions of people around the world have turned to single-use plastic-based protective products (e.g., surgical mask made of polypropylene plastics) to help prevent the spread of COVID-19.226 Such plastic-based products are not biodegradable and could stay in the environment for many years. In addition, used PPE is incinerated because it is classified as a biohazard. Increased volumes of PPE consumption as a result of the COVID-19 pandemic and the subsequent incineration of these products will, in turn, contribute to increased carbon emissions, which is also a problem. Therefore, the textile industry now has an opportunity to design new types of environmentally sustainable protective clothing that would be washable and reusable, which could potentially reduce the amount of medical waste contributing toward environmental pollution. In addition, new technologies that sterilize waste and separate/reduce the mixing of infectious waste with general waste need to be investigated urgently.227 Furthermore, the use of biodegradable polymers (e.g., polylactic acid, PLA)228 and recycled polymers (e.g., rPET or rPP) for manufacturing protective clothing and recycling of current disposable plastic-based protective clothing products would help to reduce the environmental impact and support a shift toward more sustainable protective medical clothing and a circular economy.
Toward Safe and Green Functional Materials and Techniques
Although the benefits of antimicrobial textiles that protect the wearer from pathogens are evident, there are some concerns associated with the use of antimicrobial agents on textiles, including their potential to kill good flora bacteria from the skin, their toxic breakdown products, and the consequent risks to human health and environment.50 It is important that the overall advantages and effectiveness of antimicrobial agents should outperform any potential environmental concern and the cost of the product. Therefore, future antimicrobial agents should be highly effective at very low dosages while also being extremely durable to ensure the development of products with longer useful lives. This, in turn, will help to reduce the quantities of textiles waste disposed of and improve the overall carbon footprint associated with protective clothing. In addition, antimicrobial agents should not release unwanted toxic chemicals in aquatic environment and should efficiently be removed via green and less energy-intensive wastewater treatments. Furthermore, there must be efforts from researchers and manufacturers to develop safer and environment-friendly “green” agents, namely, natural antimicrobial agents, which may present an efficient antimicrobial effect, with safety, easy availability, and nontoxicity to skin.229,230 Natural biopolymers extracted from animals or plants, including chitosan, cyclodextrin, sericin, and alginate, are renewable and could be used as key resources for sustainable bioactive textiles (Figure 7c).231 Polyelemental nanoparticles (PE NPs) containing four or more elements in a single NP could also be explored to enable a multifunctional antimicrobial agent with a new physical and chemical phenomena.232
In addition to the growing interest in natural green antimicrobial agents, there has also been a drive toward the introduction of green processing techniques in textile manufacturing due to rigorous ecological legislation and growing environmental concerns (Figure 7d). For example, a “solvent-free” dry plasma treatment could be employed to apply antimicrobial finishes onto textiles. Unlike conventional treatments, the dry plasma treatment does not produce contaminated water or create mechanical hazards for treated fabrics.233 In addition, the uses of enzymes, UV radiation, and ultrasound energy could be considered to be more environmentally friendly processes which promote the adhesion of natural dyes with antimicrobial properties.234,235 Furthermore, a broader perspective and knowledge of antimicrobials, with considerations into risks and benefits of their use compared to alternative antimicrobial substances, is needed for the regulatory assessment of the single antimicrobial substance.66 For example, it has been reported that the textile industry uses the highest amount of triclosan (∼210 metric tonnes), which could be replaced by either <2 metric tonnes of silver nanoparticles or by ∼180 metric tonnes of Si-QAC, achieving similar antimicrobial results but with a lower environmental impact.50 Microencapsulation of antimicrobial additives into textile polymer matrix could also be explored to avoid the human and environment safety level. New fiber or fabric manufacturing techniques such as electrospinning and additive manufacturing could also be explored for making protective clothing with improved comfort and effective protections from pathogens.171,236,237
Toward Sustainable Supply Chain
The recent COVID-19 pandemic has resulted in unprecedented demand for PPE needed to protect the health of HCWs and prevent the spread of the disease. Hospitals around the world have experienced significant shortages in supplies of PPE, and the global supply chain was severely challenged216 due to rising demand, panic buying, hoarding, and misuse. The shortage of safe and effective PPE has been one of the major causes for the deaths of frontline workers treating COVID-19 patients around the world. PPE prices have also increased significantly with long delay in supplies, widespread market manipulation, and stocks frequently sold to the highest bidder. Per WHO’s modeling, an estimated 89 million medical masks, 76 million gloves, and 1.6 million goggles are required for the COVID-19 response each month. The WHO has also recommended that the industry should increase the PPE production by ∼40%.238 The WHO has additionally provided recommendations for the rational use of PPE in healthcare and community settings and strategies to optimize the availability of PPE including minimizing the use of PPE, recommending the appropriate use of PPE, and coordinating with the PPE supply chains.23 However, these are temporary measures to optimize the PPE supply chain in order to tackle the current COVID-19 pandemic. Sustainable and commercial approaches are needed to avoid similar shortages into the future and could involve the following.
First, there needs to be the creation of government-backed local or regional (e.g., EU, America, South Asia, Middle East, etc.) manufacturing, sourcing, and distribution facilities for PPE (Figure 7e). Second, digital technologies should be introduced in manufacturing and processing (e.g., water-less colorations, inkjet printing, digital finishing techniques, and 3D printing) of protective clothing. The traditional manufacturing and processing techniques of protective clothing may be challenging for some countries or regions such as the EU or U.S. due to higher labor costs and the associated environmental legislation. Nevertheless, the introduction of new technologies including robotics, additive manufacturing, and artificial intelligence in manufacturing facilities (Industry 4.0) would enable cost-effective manufacturing of such devices at scale (Figure 7f). Third, governments should encourage and introduce legislation to increase the use of sustainable and reusable protective equipment to lessen any environmental impacts (Figure 7g). In addition, more effort and resources should be invested into the design and development of environmentally sustainable recycling processes for single-use plastic-based PPE. Finally, governments should increase annual expenditure on research and development for developing innovative technologies to produce smart, highly effective, and extremely durable PPE with longer useful life, as well monitoring the wearer’s physiological conditions (Figure 7h).
Acknowledgments
The authors respectfully acknowledge all frontline workers (“Heroes”) around the world for the wonderful care and work they are providing on a daily basis during the current COVID-19 pandemic. This research was supported by Research England The Expanding Excellence in England (E3) funding (U.K.) and KAIST Institute for the NanoCentury and Global Singularity Research Project (N11200030).
Glossary
Vocabulary
- antimicrobial
is defined as any substance of natural, semisynthetic, or synthetic in origin that kills (biocidal) or inhibits (biostatic) the growth of pathogens but causes little or no damage to the host
- COVID-19
is short form of coronavirus disease 2019, and it belongs to a large family of viruses called coronaviruses (CoV)
- virucidal agents
attack and inactivate viruses outside of host cells by damaging their protein shell capsid, destroying the genetic materials such as RNA and DNA or damaging the virion structure
- virustatic agents
are used on surfaces to stop the growth of viruses
- personal protective equipment (PPE)
is equipment (such as gloves, eye protection, face masks, etc.) to protect the user against health or safety risks at work
Author Contributions
N.K. planned the manuscript, researched the literature, and wrote the article. All authors discussed, reviewed, edited, and approved the manuscript.
The authors declare no competing financial interest.
References
- Zhu N.; Zhang D.; Wang W.; Li X.; Yang B.; Song J.; Zhao X.; Huang B.; Shi W.; Lu R.; Niu P.; Zhan F.; Ma X.; Wang D.; Xu W.; Wu G.; Gao G. F.; Tan W. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilinc F. S. A Review of Isolation Gowns in Healthcare: Fabric and Gown Properties. J. Eng. Fibers Fabr. 2015, 10, 180–190. 10.1177/155892501501000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC . Using Personal Protective Equipment (PPE), https://www.cdc.gov/coronavirus/2019-ncov/hcp/using-ppe.html (accessed 2020-04-25).
- Ford S.Nurse Poll Hints at Scale of Shortage of Key Masks, Gowns and Respirators. Nursing Times, https://www.nursingtimes.net/author/steve-ford/page/15/?cmd=gotopage&=402 (access 2020-09-03). [Google Scholar]
- Rajendran S.; Anand S. Developments in Medical Textiles. Text. Prog. 2002, 32, 1–42. 10.1080/00405160208688956. [DOI] [Google Scholar]
- Virk R. K.; Ramaswamy G. N.; Bourham M.; Bures B. L. Plasma and Antimicrobial Treatment of Nonwoven Fabrics for Surgical Gowns. Text. Res. J. 2004, 74, 1073–1079. 10.1177/004051750407401208. [DOI] [Google Scholar]
- Rutala W. A.; Weber D. J. A Review of Single-Use and Reusable Gowns and Drapes in Health Care. Infect. Cont. Hosp. Ep. 2001, 22, 248–257. 10.1086/501895. [DOI] [PubMed] [Google Scholar]
- Telford G. L.; Quebbeman E. J. Assessing the Risk of Blood Exposure in the Operating Room. Am. J. Infect. Control 1993, 21, 351–356. 10.1016/0196-6553(93)90401-O. [DOI] [PubMed] [Google Scholar]
- ANSI/AAMI, PB70:2012 Liquid Barrier Performance and Classification of Protective Apparel and Drapes Intended for Use in Health Care Facilities. Association for the Advancement of Medical Instrumentation, https://my.aami.org/aamiresources/previewfiles/pb70_1206_preview.pdf (accessed 2020-08-05).
- Domingo E.Introduction to Virus Origins and Their Role in Biological Evolution. Virus as Populations, 2nd ed.; Academic Press: London, U.K., 2019; pp 1–33. [Google Scholar]
- Cui J.; Li F.; Shi Z. L. Origin and Evolution of Pathogenic Coronaviruses. Nat. Rev. Microbiol. 2019, 17, 181–192. 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrapp D.; Wang N.; Corbett K. S.; Goldsmith J. A.; Hsieh C. L.; Abiona O.; Graham B. S.; McLellan J. S. Cryo-EM Structure of the 2019-nCoV Spike in the Prefusion Conformation. Science 2020, 367, 1260–1263. 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal T. A Review of Coronavirus Disease-2019 (COVID-19). Indian J. Pediatr. 2020, 87, 281–286. 10.1007/s12098-020-03263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajouri L.What Is a Virus? How Do They Spread? How Do They Make Us Sick? The Conversation, https://theconversation.com/what-is-a-virus-how-do-they-spread-how-do-they-make-us-sick-133437 (accessed 2020-07-04). [Google Scholar]
- Baron S.; Fons M.; Albrecht T.. Viral Pathogenesis. Medical Microbiology, 4th ed.; University of Texas Medical Branch at Galveston, 1996. [PubMed] [Google Scholar]
- Leonas K. K.; Jinkins R. S. The Relationship of Selected Fabric Characteristics and the Barrier Effectiveness of Surgical Gown Fabrics. Am. J. Infect. Control 1997, 25, 16–23. 10.1016/S0196-6553(97)90048-1. [DOI] [PubMed] [Google Scholar]
- Blom A.; Gozzard C.; Heal J.; Bowker K.; Estela C. Bacterial Strike-Through of Re-Usable Surgical Drapes: The Effect of Different Wetting Agents. J. Hosp. Infect. 2002, 52, 52–55. 10.1053/jhin.2002.1262. [DOI] [PubMed] [Google Scholar]
- Flaherty A. L.; Wick T. M. Prolonged Contact Wild Blood Alters Surgical Gown Permeability. Am. J. Infect. Control 1993, 21, 249–256. 10.1016/0196-6553(93)90417-3. [DOI] [PubMed] [Google Scholar]
- Guo Z.-D.; Wang Z.-Y.; Zhang S.-F.; Li X.; Li L.; Li C.; Cui Y.; Fu R.-B.; Dong Y.-Z.; Chi X.-Y.; Zhang M.-Y.; Liu K.; Cao C.; Liu B.; Zhang K.; Gao Y.-W.; Lu B.; Chen W. Aerosol and Surface Distribution of Severe Acute Respiratory Syndrome Coronavirus 2 in Hospital Wards, Wuhan, China, 2020. Emerging Infect. Dis. 2020, 26, 1583–1591. 10.3201/eid2607.200885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. X.; Goryakin Y.; Maeda A.; Bruckner T.; Scheffler R. Global Health Workforce Labor Market Projections for 2030. Hum Resour Health 2017, 15, 11. 10.1186/s12960-017-0187-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel J. D.; Rhinehart E.; Jackson M.; Chiarello L. 2007 Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Health Care Settings. Am. J. Infect. Control 2007, 35, S65. 10.1016/j.ajic.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Labor . Occupational Exposure to Blood Borne Pathogens: Final Rule on Occupational Exposure to Bloodborne Pathogens. Federal Register #56.64004, 1991.
- WHO . Rational Use of Personal Protective Equipment for Coronavirus Disease (COVID-19): Interim Guidance, https://www.who.int/publications/i/item/rational-use-of-personal-protective-equipment-for-coronavirus-disease-(covid-19)-and-considerations-during-severe-shortages (accessed 2020-02-27).
- Ortega R.; Bhadelia N.; Obanor O.; Cyr K.; Yu P.; McMahon M.; Gotzmann D. Putting On and Removing Personal Protective Equipment. N. Engl. J. Med. 2015, 372, e16 10.1056/NEJMvcm1412105. [DOI] [PubMed] [Google Scholar]
- ECDC . Guidance for Wearing and Removing Personal Protective Equipment in Healthcare Settings for the Care of Patients with Suspected or Confirmed COVID-19. European Centre for Disease Prevention and Control: Stockholm, February 2020, https://www.ecdc.europa.eu/en/publications-data/guidance-wearing-and-removing-personal-protective-equipment-healthcare-settings (accessed 2020-04-25).
- Boyce J.; Chartier Y.; Chraiti M.; Cookson B.; Damani N.; Dharan S.. WHO Guidelines on Hand Hygiene in Health Care; WHO: Geneva, 2009. [Google Scholar]
- FDA . Personal Protective Equipment for Infection Control, https://www.fda.gov/medical-devices/general-hospital-devices-and-supplies/personal-protective-equipment-infection-control (accessed 2020-04-26).
- 3M . Comparison of FFP2, KN95, and N95 and Other Filtering Facepiece Respirator Classes; 3M: Minneapolis, MN, 2020; pp 1–3. [Google Scholar]
- CDC . Considerations for Wearing Cloth Face Coverings. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/cloth-face-cover-guidance.html (accessed 2020-06-28).
- WHO . Coronavirus Disease (COVID-19) Advice for the Public: When and How to Use Masks. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public/when-and-how-to-use-masks (accessed 2020-07-04).
- Konda A.; Prakash A.; Moss G. A.; Schmoldt M.; Grant G. D.; Guha S. Aerosol Filtration Efficiency of Common Fabrics Used in Respiratory Cloth Masks. ACS Nano 2020, 14, 6339–6347. 10.1021/acsnano.0c03252. [DOI] [PubMed] [Google Scholar]
- Aydin O.; Emon M. A. B.; Saif M. T. A.. Performance of Fabrics for Home-Made Masks against Spread of Respiratory Infection through Droplets: A Quantitative Mechanistic Study. medRxiv 2020, https://www.medrxiv.org/content/10.1101/2020.04.19.20071779v2.full.pdf+html (accessed 2020-07-04). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midha V. K.; Vashisht R.; Midha V. Durability of Fluoropolymer and Antibacterial Finishes on Woven Surgical Gown Fabrics. Fash. Text. 2014, 1, 12. 10.1186/s40691-014-0012-7. [DOI] [Google Scholar]
- Bonaldi R. R.Functional Finishes for High-Performance Apparel. In High-Performance Apparel; McLoughlin J., Sabir T., Eds.; Woodhead Publishing: Cambridge, U.K., 2018; pp 129–156. [Google Scholar]
- Lim S. H.; Hudson S. M. Application of a Fiber-Reactive Chitosan Derivative to Cotton Fabric as an Antimicrobial Textile Finish. Carbohydr. Polym. 2004, 56, 227–234. 10.1016/j.carbpol.2004.02.005. [DOI] [Google Scholar]
- Yazhini K. B.; Prabu H. G. Antibacterial Activity of Cotton Coated with ZnO and ZnO-CNT Composites. Appl. Biochem. Biotechnol. 2015, 175, 85–92. 10.1007/s12010-014-1257-8. [DOI] [PubMed] [Google Scholar]
- Neely A. N.; Maley M. P. Survival of Enterococci and Staphylococci on Hospital Fabrics and Plastic. J. Clin. Microbiol. 2000, 38, 724–726. 10.1128/JCM.38.2.724-726.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon G. J.; Sidwell R. W.; McNeil E. Quantitative Studies on Fabrics as Disseminators of Viruses. II. Persistence of Poliomyelitis Virus on Cotton and Wool Fabrics. Appl. Microbiol. 1966, 14, 183–188. 10.1128/AEM.14.2.183-188.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkova P.; Francesko A.; Perelshtein I.; Gedanken A.; Tzanov T. Simultaneous Sonochemical-Enzymatic Coating of Medical Textiles with Antibacterial ZnO Nanoparticles. Ultrason. Sonochem. 2016, 29, 244–250. 10.1016/j.ultsonch.2015.09.021. [DOI] [PubMed] [Google Scholar]
- Park D.; Larson A. M.; Klibanov A. M.; Wang Y. Antiviral and Antibacterial Polyurethanes of Various Modalities. Appl. Biochem. Biotechnol. 2013, 169, 1134–1146. 10.1007/s12010-012-9999-7. [DOI] [PubMed] [Google Scholar]
- Ren T.; Dormitorio T. V.; Qiao M.; Huang T. S.; Weese J. N-Halamine Incorporated Anti-Microbial Nonwoven Fabrics for Use against Avian Influenza Virus. Vet. Microbiol. 2018, 218, 78–83. 10.1016/j.vetmic.2018.03.032. [DOI] [PubMed] [Google Scholar]
- Monmaturapoj N.; Sri-on A.; Klinsukhon W.; Boonnak K.; Prahsarn C. Antiviral Activity of Multifunctional Composite Based on TiO2-Modified Hydroxyapatite. Mater. Sci. Eng., C 2018, 92, 96–102. 10.1016/j.msec.2018.06.045. [DOI] [PubMed] [Google Scholar]
- Imai K.; Ogawa H.; Bui V. N.; Inoue H.; Fukuda J.; Ohba M.; Yamamoto Y.; Nakamura K. Inactivation of High and Low Pathogenic Avian Influenza Virus H5 Subtypes by Copper Ions Incorporated in Zeolite-Textile Materials. Antiviral Res. 2012, 93, 225–233. 10.1016/j.antiviral.2011.11.017. [DOI] [PubMed] [Google Scholar]
- Neyts J.; Snoeck R.; Schols D.; Balzarini J.; Esko J. D.; Van Schepdael A.; De Clercq E. Sulfated Polymers Inhibit the Interaction of Human Cytomegalovirus with Cell Surface Heparan Sulfate. Virology 1992, 189, 48–58. 10.1016/0042-6822(92)90680-N. [DOI] [PubMed] [Google Scholar]
- Iyigundogdu Z. U.; Demir O.; Asutay A. B.; Sahin F. Developing Novel Antimicrobial and Antiviral Textile Products. Appl. Biochem. Biotechnol. 2017, 181, 1155–1166. 10.1007/s12010-016-2275-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta D. Antimicrobial Treatments for Textiles. Indian J. Fibre Text. 2007, 32, 254–263. [Google Scholar]
- Periolatto M.; Ferrero F.; Vineis C.; Varesano A.; Gozzelino G.. Novel Antimicrobial Agents and Processes for Textile Applications. In Antibacterial Agents; Kumavath R., Ed.; IntechOpen: London, 2017; p 17. [Google Scholar]
- Glazer A. N.; Nikaido H.. Microbial Biotechnology: Fundamentals of Applied Microbiology, 2nd ed.; Cambridge University Press: New York, 2007. [Google Scholar]
- Rahman M. A.; Ahsan T.; Islam S. Antibacterial and Antifungal Properties of the Methanol Extract from the Stem of Argyreia Argentea. Bangladesh J. Pharmacol. 2010, 5, 41–44. 10.3329/bjp.v5i1.4700. [DOI] [Google Scholar]
- Morais D. S.; Guedes R. M.; Lopes M. A. Antimicrobial Approaches for Textiles: From Research to Market. Materials 2016, 9, 498. 10.3390/ma9060498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galabov A. S. Virucidal Agents in the Eve of Manorapid Synergy. GMS Krankenh. Hyg. Interdiszip. 2007, 2, 18. [PMC free article] [PubMed] [Google Scholar]
- Galdiero S.; Falanga A.; Vitiello M.; Cantisani M.; Marra V.; Galdiero M. Silver Nanoparticles as Potential Antiviral Agents. Molecules 2011, 16, 8894–8918. 10.3390/molecules16108894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona-Ribeiro A. M.; de Melo Carrasco L. D. Cationic Antimicrobial Polymers and Their Assemblies. Int. J. Mol. Sci. 2013, 14, 9906–9946. 10.3390/ijms14059906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghernaout D.; Touahmia M.; Aichouni M. Disinfecting Water: Electrocoagulation as an Efficient Process. Appl. Eng. 2019, 3, 1–12. [Google Scholar]
- Hajkova P.; Spatenka P.; Horsky J.; Horska I.; Kolouch A. Photocatalytic Effect of TiO2 Films on Viruses and Bacteria. Plasma Processes Polym. 2007, 4, S397–S401. 10.1002/ppap.200731007. [DOI] [Google Scholar]
- Jin C.; Yu B.; Zhang J.; Wu H.; Zhou X.; Liu F.; Lu X.; Cheng L.; Jiang M.; Wu N.. Methylene Blue Photochemical Treatment as a Reliable SARS-COV-2 Plasma Virus Inactivation Method for Blood Safety and Convalescent Plasma Therapy for the COVID-19 Outbreak. Research Square, https://europepmc.org/article/ppr/ppr121848 (accessed 2020-07-04). [Google Scholar]
- Megahed A.; Aldridge B.; Lowe J. The Microbial Killing Capacity of Aqueous and Gaseous Ozone on Different Surfaces Contaminated with Dairy Cattle Manure. PLoS One 2018, 13, e0196555–e0196555. 10.1371/journal.pone.0196555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francolini I.; Vuotto C.; Piozzi A.; Donelli G. Antifouling and Antimicrobial Biomaterials: An Overview. APMIS 2017, 125, 392–417. 10.1111/apm.12675. [DOI] [PubMed] [Google Scholar]
- Pappas H. C.; Phan S.; Yoon S.; Edens L. E.; Meng X.; Schanze K. S.; Whitten D. G.; Keller D. J. Self-Sterilizing, Self-Cleaning Mixed Polymeric Multifunctional Antimicrobial Surfaces. ACS Appl. Mater. Interfaces 2015, 7, 27632–27638. 10.1021/acsami.5b06852. [DOI] [PubMed] [Google Scholar]
- Aubert-Viard F.; Mogrovejo-Valdivia A.; Tabary N.; Maton M.; Chai F.; Neut C.; Martel B.; Blanchemain N. Evaluation of Antibacterial Textile Covered by Layer-by-Layer Coating and Loaded with Chlorhexidine for Wound Dressing Application. Mater. Sci. Eng., C 2019, 100, 554–563. 10.1016/j.msec.2019.03.044. [DOI] [PubMed] [Google Scholar]
- Smith R. J.; Moule M. G.; Sule P.; Smith T.; Cirillo J. D.; Grunlan J. C. Polyelectrolyte Multilayer Nanocoating Dramatically Reduces Bacterial Adhesion to Polyester Fabric. ACS Biomater. Sci. Eng. 2017, 3, 1845–1852. 10.1021/acsbiomaterials.7b00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensel J.; Borke T.; Pazos Perez N.; Fery A.; Andreeva D. V.; Betthausen E.; Muller A. H. E.; Mohwald H.; Skorb E. V. Cavitation Engineered 3D Sponge Networks and Their Application in Active Surface Construction. Adv. Mater. 2012, 24, 985–989. 10.1002/adma.201103786. [DOI] [PubMed] [Google Scholar]
- Ulasevich S. A.; Brezesinski G.; Mohwald H.; Fratzl P.; Schacher F. H.; Poznyak S. K.; Andreeva D. V.; Skorb E. V. Light-Induced Water Splitting Causes High-Amplitude Oscillation of pH-Sensitive Layer-by-Layer Assemblies on TiO2. Angew. Chem., Int. Ed. 2016, 55, 13001–13004. 10.1002/anie.201604359. [DOI] [PubMed] [Google Scholar]
- Simoncic B.; Tomsic B. Structures of Novel Antimicrobial Agents for Textiles-A Review. Text. Res. J. 2010, 80, 1721–1737. 10.1177/0040517510363193. [DOI] [Google Scholar]
- Lacasse K.; Baumann W.. Textile Chemicals: Environmental Data and Facts, 1st ed.; Springer-Verlag: Switzerland, 2004. [Google Scholar]
- Windler L.; Height M.; Nowack B. Comparative Evaluation of Antimicrobials for Textile Applications. Environ. Int. 2013, 53, 62–73. 10.1016/j.envint.2012.12.010. [DOI] [PubMed] [Google Scholar]
- Yuan Gao; Cranston R. Recent Advances in Antimicrobial Treatments of Textiles. Text. Res. J. 2008, 78, 60–72. 10.1177/0040517507082332. [DOI] [Google Scholar]
- Yao C.; Li X. S.; Neoh K.; Shi Z. l.; Kang E. Antibacterial Poly (D, L-Lactide) (PDLLA) Fibrous Membranes Modified with Quaternary Ammonium Moieties. Chin. J. Polym. Sci. 2010, 28, 581–588. 10.1007/s10118-010-9094-x. [DOI] [Google Scholar]
- Yao C.; Li X.; Neoh K.; Shi Z.; Kang E. Surface Modification and Antibacterial Activity of Electrospun Polyurethane Fibrous Membranes with Quaternary Ammonium Moieties. J. Membr. Sci. 2008, 320, 259–267. 10.1016/j.memsci.2008.04.012. [DOI] [Google Scholar]
- Orhan M.; Kut D.; Gunesoglu C. Use of Triclosan as Antibacterial Agent in Textiles. Indian J. Fibre Text. Res. 2007, 32, 114–118. [Google Scholar]
- Yazdankhah S. P.; Scheie A. A.; Høiby E. A.; Lunestad B.-T.; Heir E.; Fotland T. Ø.; Naterstad K.; Kruse H. Triclosan and Antimicrobial Resistance in Bacteria: An Overview. Microb. Drug Resist. 2006, 12, 83–90. 10.1089/mdr.2006.12.83. [DOI] [PubMed] [Google Scholar]
- Jones R. D.; Jampani H. B.; Newman J. L.; Lee A. S. Triclosan: A Review of Effectiveness and Safety in Health Care Settings. Am. J. Infect. Control 2000, 28, 184–196. 10.1067/mic.2000.102378. [DOI] [PubMed] [Google Scholar]
- Mansfield R. G. Keeping It Fresh. Textile World 2002, 152, 42. [Google Scholar]
- Halden R. U.; Lindeman A. E.; Aiello A. E.; Andrews D.; Arnold W. A.; Fair P.; Fuoco R. E.; Geer L. A.; Johnson P. I.; Lohmann R.; McNeill K.; Sacks V. P.; Schettler T.; Weber R.; Zoeller R. T.; Blum A. The Florence Statement on Triclosan and Triclocarban. Environ. Health Perspect. 2017, 125, 064501 10.1289/EHP1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abreu M.; Silva M. E.; Schacher L.; Adolphe D. Designing Surgical Clothing and Drapes According to the New Technical Standards. Int. J. Cloth. Sci. Technol. 2003, 15, 69–74. 10.1108/09556220310461178. [DOI] [Google Scholar]
- Palza H. Antimicrobial Polymers with Metal Nanoparticles. Int. J. Mol. Sci. 2015, 16, 2099–2116. 10.3390/ijms16012099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahltig B.; Zhang J.; Wu L.; Darko D.; Wendt M.; Lempa E.; Rabe M.; Haase H. Effect Pigments for Textile Coating: A Review of The Broad Range of Advantageous Functionalization. J. Coat. Technol. Res. 2017, 14, 35–55. 10.1007/s11998-016-9854-9. [DOI] [Google Scholar]
- Raghupathi K. R.; Koodali R. T.; Manna A. C. Size-Dependent Bacterial Growth Inhibition and Mechanism of Antibacterial Activity of Zinc Oxide Nanoparticles. Langmuir 2011, 27, 4020–4028. 10.1021/la104825u. [DOI] [PubMed] [Google Scholar]
- Aruna S.; Vasugi Raaja N.; Sathiesh Kumar S. Fabrication of Antimicrobial Textiles Using Hydrothermally Synthesized Copper Oxide Nanoparticles. Int. J. Innov. Res. Sci. Eng. 2016, 5, 2112–2119. 10.15680/IJIRSET.2016.0502061. [DOI] [Google Scholar]
- Nikiforov A.; Deng X.; Xiong Q.; Cvelbar U.; DeGeyter N.; Morent R.; Leys C. Non-Thermal Plasma Technology for the Development of Antimicrobial Surfaces: A Review. J. Phys. D: Appl. Phys. 2016, 49, 204002. 10.1088/0022-3727/49/20/204002. [DOI] [Google Scholar]
- Varesano A.; Vineis C.; Aluigi A.; Rombaldoni F.. Antimicrobial Polymers for Textile Products. In Science Against Microbial Pathogens: Communicating Current Research and Technological Advances; Méndez-Vilas A., Ed.; Formatex Research Center: Spain, 2011; Vol. 3, pp 99–110. [Google Scholar]
- Chadeau E.; Brunon C.; Degraeve P.; Léonard D.; Grossiord C.; Bessueille F.; Cottaz A.; Renaud F.; Ferreira I.; Darroux C.; Simon F.; Rimbault F.; Oulahal N. Evaluation of Antimicrobial Activity of a Polyhexamethylene Biguanide-Coated Textile by Monitoring Both Bacterial Growth (ISO 20743/2005 Standard) and Viability (Live/Dead Baclight Kit). J. Food Saf. 2012, 32, 141–151. 10.1111/j.1745-4565.2011.00361.x. [DOI] [Google Scholar]
- Siadat S. A.; Mokhtari J. Fabrication of Novel Antimicrobial Bio-Fibers Using Silk Wastage, Study of Poly (hexamethylene) Biguanide, and Silver Nanoparticles Interaction. J. Nat. Fibers 2017, 14, 707–717. 10.1080/15440478.2016.1277818. [DOI] [Google Scholar]
- Zanoaga M.; Tanasa F. Antimicrobial Reagents as Functional Finishing for Textiles Intended for Biomedical Applications. I. Synthetic Organic Compounds. Chem. J. Mold. 2014, 9, 14–32. 10.19261/cjm.2014.09(1).02. [DOI] [Google Scholar]
- Ren X.; Jiang Z.; Liu Y.; Li L.; Fan X.. N-Halamines as Antimicrobial Textile Finishes. In Antimicrobial Textiles; Sun G., Ed.; Woodhead Publishing: Cambridge, U.K., 2016; pp 125–140. [Google Scholar]
- Hui F.; Debiemme-Chouvy C. Antimicrobial N-Halamine Polymers and Coatings: A Review of Their Synthesis, Characterization, and Applications. Biomacromolecules 2013, 14, 585–601. 10.1021/bm301980q. [DOI] [PubMed] [Google Scholar]
- Li L.; Ma K.; Liu Y.; Liu Y.; Li R.; Ren X.; Huang T. S. Regenerablity and Stability of Antibacterial Cellulose Containing Triazine N-Halamine. J. Eng. Fibers Fabr. 2016, 11, 23–30. 10.1177/155892501601100105. [DOI] [Google Scholar]
- Xu J.; Wang D.; Yuan Y.; Wei W.; Gu S.; Liu R.; Wang X.; Liu L.; Xu W. Polypyrrole-Coated Cotton Fabrics for Flexible Supercapacitor Electrodes Prepared Using Cuo Nanoparticles as Template. Cellulose 2015, 22, 1355–1363. 10.1007/s10570-015-0546-x. [DOI] [Google Scholar]
- Marakova N.; Humpolicek P.; Kasparkova V.; Capakova Z.; Martinkova L.; Bober P.; Trchova M.; Stejskal J. Antimicrobial Activity and Cytotoxicity of Cotton Fabric Coated with Conducting Polymers, Polyaniline or Polypyrrole, and with Deposited Silver Nanoparticles. Appl. Surf. Sci. 2017, 396, 169–176. 10.1016/j.apsusc.2016.11.024. [DOI] [Google Scholar]
- Seshadri D. T.; Bhat N. V. Synthesis and Properties of Cotton Fabrics Modified with Polypyrrole. Sen'i Gakkaishi 2005, 61, 103–108. 10.2115/fiber.61.103. [DOI] [Google Scholar]
- Firoz Babu K.; Dhandapani P.; Maruthamuthu S.; Anbu Kulandainathan M. One Pot Synthesis of Polypyrrole Silver Nanocomposite on Cotton Fabrics for Multifunctional Property. Carbohydr. Polym. 2012, 90, 1557–1563. 10.1016/j.carbpol.2012.07.030. [DOI] [PubMed] [Google Scholar]
- Shi Z.; Zhou H.; Qing X.; Dai T.; Lu Y. Facile Fabrication and Characterization of Poly (Tetrafluoroethylene)@ Polypyrrole/Nano-Silver Composite Membranes with Conducting and Antibacterial Property. Appl. Surf. Sci. 2012, 258, 6359–6365. 10.1016/j.apsusc.2012.03.040. [DOI] [Google Scholar]
- Varesano A.; Vineis C.; Tonetti C.; Mazzuchetti G.; Bobba V. Antibacterial Property on Gram-Positive Bacteria of Polypyrrole-Coated Fabrics. J. Appl. Polym. Sci. 2015, 132, 41670. 10.1002/app.41670. [DOI] [Google Scholar]
- Sanchez Ramirez D. O.; Varesano A.; Carletto R. A.; Vineis C.; Perelshtein I.; Natan M.; Perkas N.; Banin E.; Gedanken A. Antibacterial Properties of Polypyrrole-Treated Fabrics by Ultrasound Deposition. Mater. Sci. Eng., C 2019, 102, 164–170. 10.1016/j.msec.2019.04.016. [DOI] [PubMed] [Google Scholar]
- Karahan H. E.; Wiraja C.; Xu C.; Wei J.; Wang Y.; Wang L.; Liu F.; Chen Y. Graphene Materials in Antimicrobial Nanomedicine: Current Status and Future Perspectives. Adv. Healthcare Mater. 2018, 7, 1701406. 10.1002/adhm.201701406. [DOI] [PubMed] [Google Scholar]
- Karim N.; Afroj S.; Tan S.; Novoselov K. S.; Yeates S. G. All Inkjet-Printed Graphene-Silver Composite Ink on Textiles for Highly Conductive Wearable Electronics Applications. Sci. Rep. 2019, 9, 8035. 10.1038/s41598-019-44420-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim N.; Afroj S.; Malandraki A.; Butterworth S.; Beach C.; Rigout M.; Novoselov K. S.; Casson A. J.; Yeates S. G. All Inkjet-Printed Graphene-Based Conductive Patterns for Wearable E-Textile Applications. J. Mater. Chem. C 2017, 5, 11640–11648. 10.1039/C7TC03669H. [DOI] [Google Scholar]
- Abdelkader A. M.; Karim N.; Vallés C.; Afroj S.; Novoselov K. S.; Yeates S. G. Ultraflexible and Robust Graphene Supercapacitors Printed on Textiles for Wearable Electronics Applications. 2D Mater. 2017, 4, 035016 10.1088/2053-1583/aa7d71. [DOI] [Google Scholar]
- Bhattacharjee S.; Joshi R.; Chughtai A. A.; Macintyre C. R. Graphene Modified Multifunctional Personal Protective Clothing. Adv. Mater. Interfaces 2019, 6, 1900622. 10.1002/admi.201900622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim N.; Afroj S.; Tan S.; He P.; Fernando A.; Carr C.; Novoselov K. S. Scalable Production of Graphene-Based Wearable E-Textiles. ACS Nano 2017, 11, 12266–12275. 10.1021/acsnano.7b05921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afroj S.; Karim N.; Wang Z.; Tan S.; He P.; Holwill M.; Ghazaryan D.; Fernando A.; Novoselov K. S. Engineering Graphene Flakes for Wearable Textile Sensors via Highly Scalable and Ultrafast Yarn Dyeing Technique. ACS Nano 2019, 13, 3847–3857. 10.1021/acsnano.9b00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afroj S.; Tan S.; Abdelkader A. M.; Novoselov K. S.; Karim N. Highly Conductive, Scalable, and Machine Washable Graphene-Based E-Textiles for Multifunctional Wearable Electronic Applications. Adv. Funct. Mater. 2020, 30, 2000293. 10.1002/adfm.202000293. [DOI] [Google Scholar]
- Szunerits S.; Boukherroub R. Antibacterial Activity of Graphene-Based Materials. J. Mater. Chem. B 2016, 4, 6892–6912. 10.1039/C6TB01647B. [DOI] [PubMed] [Google Scholar]
- Liu S.; Zeng T. H.; Hofmann M.; Burcombe E.; Wei J.; Jiang R.; Kong J.; Chen Y. Antibacterial Activity of Graphite, Graphite Oxide, Graphene Oxide, and Reduced Graphene Oxide: Membrane and Oxidative Stress. ACS Nano 2011, 5, 6971–6980. 10.1021/nn202451x. [DOI] [PubMed] [Google Scholar]
- Ji H.; Sun H.; Qu X. Antibacterial Applications of Graphene-Based Nanomaterials: Recent Achievements and Challenges. Adv. Drug Delivery Rev. 2016, 105, 176–189. 10.1016/j.addr.2016.04.009. [DOI] [PubMed] [Google Scholar]
- Yousefi M.; Dadashpour M.; Hejazi M.; Hasanzadeh M.; Behnam B.; de la Guardia M.; Shadjou N.; Mokhtarzadeh A. Anti-Bacterial Activity of Graphene Oxide as a New Weapon Nanomaterial to Combat Multidrug-Resistance Bacteria. Mater. Sci. Eng., C 2017, 74, 568–581. 10.1016/j.msec.2016.12.125. [DOI] [PubMed] [Google Scholar]
- Palmieri V.; Carmela Lauriola M.; Ciasca G.; Conti C.; De Spirito M.; Papi M. The Graphene Oxide Contradictory Effects against Human Pathogens. Nanotechnology 2017, 28, 152001. 10.1088/1361-6528/aa6150. [DOI] [PubMed] [Google Scholar]
- Hegab H. M.; ElMekawy A.; Zou L.; Mulcahy D.; Saint C. P.; Ginic-Markovic M. The Controversial Antibacterial Activity of Graphene-Based Materials. Carbon 2016, 105, 362–376. 10.1016/j.carbon.2016.04.046. [DOI] [Google Scholar]
- Gurunathan S.; Woong Han J.; Abdal Daye A.; Eppakayala V.; Kim J.-h. Oxidative Stress-Mediated Antibacterial Activity of Graphene Oxide and Reduced Graphene Oxide in Pseudomonas Aeruginosa. Int. J. Nanomed. 2012, 7, 5901. 10.2147/IJN.S37397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L.; Liu J.; Wang Y.; Yan X.; Sun D. D. Facile Synthesis of Monodispersed Silver Nanoparticles on Graphene Oxide Sheets with Enhanced Antibacterial Activity. New J. Chem. 2011, 35, 1418–1423. 10.1039/c1nj20076c. [DOI] [Google Scholar]
- de Faria A. F.; de Moraes A. C. M.; Marcato P. D.; Martinez D. S. T.; Durán N.; Souza Filho A. G.; Brandelli A.; Alves O. L. Eco-Friendly Decoration of Graphene Oxide with Biogenic Silver Nanoparticles: Antibacterial and Antibiofilm Activity. J. Nanopart. Res. 2014, 16, 2110. 10.1007/s11051-013-2110-7. [DOI] [Google Scholar]
- Ruiz O. N.; Fernando K. S.; Wang B.; Brown N. A.; Luo P. G.; McNamara N. D.; Vangsness M.; Sun Y. P.; Bunker C. E. Graphene Oxide: A Nonspecific Enhancer of Cellular Growth. ACS Nano 2011, 5, 8100–8107. 10.1021/nn202699t. [DOI] [PubMed] [Google Scholar]
- Xu W. P.; Zhang L. C.; Li J. P.; Lu Y.; Li H. H.; Ma Y. N.; Wang W. D.; Yu S. H. Facile Synthesis of Silver@ Graphene Oxide Nanocomposites and Their Enhanced Antibacterial Properties. J. Mater. Chem. 2011, 21, 4593–4597. 10.1039/c0jm03376f. [DOI] [Google Scholar]
- de Faria A. F.; Martinez D. S. T.; Meira S. M. M.; de Moraes A. C. M.; Brandelli A.; Souza Filho A. G.; Alves O. L. Anti-Adhesion and Antibacterial Activity of Silver Nanoparticles Supported on Graphene Oxide Sheets. Colloids Surf., B 2014, 113, 115–124. 10.1016/j.colsurfb.2013.08.006. [DOI] [PubMed] [Google Scholar]
- Ditta I. B.; Steele A.; Liptrot C.; Tobin J.; Tyler H.; Yates H. M.; Sheel D. W.; Foster H. A. Photocatalytic Antimicrobial Activity of Thin Surface Films of TiO2, Cuo and TiO2/CuO Dual Layers on Escherichia Coli and Bacteriophage T4. Appl. Microbiol. Biotechnol. 2008, 79, 127–133. 10.1007/s00253-008-1411-8. [DOI] [PubMed] [Google Scholar]
- Ghosh S.; Das A. Modified Titanium Oxide (TiO2) Nanocomposites and Its Array of Applications: A Review. Toxicol. Environ. Chem. 2015, 97, 491–514. 10.1080/02772248.2015.1052204. [DOI] [Google Scholar]
- Liu T.; Liu Y.; Liu M.; Wang Y.; He W.; Shi G.; Hu X.; Zhan R.; Luo G.; Xing M.; Wu J. Synthesis of Graphene Oxide-Quaternary Ammonium Nanocomposite with Synergistic Antibacterial Activity to Promote Infected Wound Healing. Burns & Trauma 2018, 6, 16. 10.1186/s41038-018-0115-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X.; Feng J.; Zhang J.; Yang X.; Liao X.; Shi Q.; Tan S. Controlled Release and Long-Term Antibacterial Activity of Reduced Graphene Oxide/Quaternary Ammonium Salt Nanocomposites Prepared by Non-Covalent Modification. Colloids Surf., B 2017, 149, 322–329. 10.1016/j.colsurfb.2016.10.016. [DOI] [PubMed] [Google Scholar]
- Ye X.; Qin X.; Yan X.; Guo J.; Huang L.; Chen D.; Wu T.; Shi Q.; Tan S.; Cai X. Π–Π Conjugations Improve the Long-Term Antibacterial Properties of Graphene Oxide/Quaternary Ammonium Salt Nanocomposites. Chem. Eng. J. 2016, 304, 873–881. 10.1016/j.cej.2016.07.026. [DOI] [Google Scholar]
- Tu Q.; Tian C.; Ma T.; Pang L.; Wang J. Click Synthesis of Quaternized Poly (Dimethylaminoethyl Methacrylate) Functionalized Graphene Oxide with Improved Antibacterial and Antifouling Ability. Colloids Surf., B 2016, 141, 196–205. 10.1016/j.colsurfb.2016.01.046. [DOI] [PubMed] [Google Scholar]
- Berendjchi A.; Khajavi R.; Yousefi A. A.; Yazdanshenas M. E. Improved Continuity of Reduced Graphene Oxide on Polyester Fabric by Use of Polypyrrole to Achieve a Highly Electro-Conductive and Flexible Substrate. Appl. Surf. Sci. 2016, 363, 264–272. 10.1016/j.apsusc.2015.12.030. [DOI] [Google Scholar]
- Ye S.; Shao K.; Li Z.; Guo N.; Zuo Y.; Li Q.; Lu Z.; Chen L.; He Q.; Han H. Antiviral Activity of Graphene Oxide: How Sharp Edged Structure and Charge Matter. ACS Appl. Mater. Interfaces 2015, 7, 21571–21579. 10.1021/acsami.5b06876. [DOI] [PubMed] [Google Scholar]
- Yang X. X.; Li C. M.; Li Y. F.; Wang J.; Huang C. Z. Synergistic Antiviral Effect of Curcumin Functionalized Graphene Oxide against Respiratory Syncytial Virus Infection. Nanoscale 2017, 9, 16086–16092. 10.1039/C7NR06520E. [DOI] [PubMed] [Google Scholar]
- Du T.; Lu J.; Liu L.; Dong N.; Fang L.; Xiao S.; Han H. Antiviral Activity of Graphene Oxide–Silver Nanocomposites by Preventing Viral Entry and Activation of the Antiviral Innate Immune Response. ACS Appl. Bio Mater. 2018, 1, 1286–1293. 10.1021/acsabm.8b00154. [DOI] [PubMed] [Google Scholar]
- Du X.; Xiao R.; Fu H.; Yuan Z.; Zhang W.; Yin L.; He C.; Li C.; Zhou J.; Liu G.; Shu G.; Chen Z. Hypericin-Loaded Graphene Oxide Protects Ducks against a Novel Duck Reovirus. Mater. Sci. Eng., C 2019, 105, 110052. 10.1016/j.msec.2019.110052. [DOI] [PubMed] [Google Scholar]
- Katewaraphorn J.; Aldred A. K. A Study of Microcapsules Containing Psidium Guajava Leaf Extract for Antibacterial Agent on Cotton Fabric. Int. J. Chem. Eng. Appl. 2016, 7, 27. 10.7763/IJCEA.2016.V7.536. [DOI] [Google Scholar]
- Ravindra K.; Murugesh Babu K. Study of Antimicrobial Properties of Fabrics Treated with Ocimum Sanctum L (Tulsi) Extract as a Natural Active Agent. J. Nat. Fibers 2016, 13, 619–627. 10.1080/15440478.2015.1093577. [DOI] [Google Scholar]
- Hübsch Z.; Van Zyl R.; Cock I.; Van Vuuren S. Interactive Antimicrobial and Toxicity Profiles of Conventional Antimicrobials with Southern African Medicinal Plants. S. Afr. J. Bot. 2014, 93, 185–197. 10.1016/j.sajb.2014.04.005. [DOI] [Google Scholar]
- Chouhan S.; Sharma K.; Guleria S. Antimicrobial Activity of Some Essential Oils-Present Status and Future Perspectives. Medicines 2017, 4, 58. 10.3390/medicines4030058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y.; Yang Z. Y.; Tang R. C. Bioactive and UV Protective Silk Materials Containing Baicalin—The Multifunctional Plant Extract from Scutellaria Baicalensis Georgi. Mater. Sci. Eng., C 2016, 67, 336–344. 10.1016/j.msec.2016.05.063. [DOI] [PubMed] [Google Scholar]
- Pei R. S.; Zhou F.; Ji B. P.; Xu J. Evaluation of Combined Antibacterial Effects of Eugenol, Cinnamaldehyde, Thymol, and Carvacrol against E. Coli with an Improved Method. J. Food Sci. 2009, 74, M379–M383. 10.1111/j.1750-3841.2009.01287.x. [DOI] [PubMed] [Google Scholar]
- Pal A.; Tripathi Y.; Kumar R.; Upadhyay L. Antibacterial Efficacy of Natural Dye from Melia Composita Leaves and Its Application in Sanitized and Protective Textiles. J. Pharm. Res. 2016, 10, 154–159. [Google Scholar]
- Mouro C.; Gouveia I. C. Antimicrobial Functionalization of Wool: Assessment of the Effect of Cecropin-B And [Ala5]-Tritrp7 Antimicrobial Peptides. J. Text. Inst. 2016, 107, 1575–1583. 10.1080/00405000.2015.1130297. [DOI] [Google Scholar]
- Cai Z.; Sun G. Antimicrobial Finishing of Acrilan Fabrics with Cetylpyridinium Chloride: Affected Properties and Structures. J. Appl. Polym. Sci. 2005, 97, 1227–1236. 10.1002/app.21261. [DOI] [Google Scholar]
- Cai Z.; Sun G. Antimicrobial Finishing of Acrilan Fabrics with Cetylpyridinium Chloride. J. Appl. Polym. Sci. 2004, 94, 243–247. 10.1002/app.20876. [DOI] [Google Scholar]
- Karimi L.; Yazdanshenas M. E.; Khajavi R.; Rashidi A.; Mirjalili M. Optimizing the Photocatalytic Properties and the Synergistic Effects of Graphene and Nano Titanium Dioxide Immobilized on Cotton Fabric. Appl. Surf. Sci. 2015, 332, 665–673. 10.1016/j.apsusc.2015.01.184. [DOI] [Google Scholar]
- Hoque J.; Adhikary U.; Yadav V.; Samaddar S.; Konai M. M.; Prakash R. G.; Paramanandham K.; Shome B. R.; Sanyal K.; Haldar J. Chitosan Derivatives Active against Multidrug-Resistant Bacteria and Pathogenic Fungi: In Vivo Evaluation as Topical Antimicrobials. Mol. Pharmaceutics 2016, 13, 3578–3589. 10.1021/acs.molpharmaceut.6b00764. [DOI] [PubMed] [Google Scholar]
- Vakili M.; Rafatullah M.; Salamatinia B.; Abdullah A. Z.; Ibrahim M. H.; Tan K. B.; Gholami Z.; Amouzgar P. Application of Chitosan and Its Derivatives as Adsorbents for Dye Removal from Water and Wastewater: A Review. Carbohydr. Polym. 2014, 113, 115–130. 10.1016/j.carbpol.2014.07.007. [DOI] [PubMed] [Google Scholar]
- Nayak R.; Padhye R.. Antimicrobial Finishes for Textiles. In Functional Finishes for Textiles: Improving Comfort, Performance and Protection; Paul R., Ed.; Woodhead Publishing: Cambridge, U.K., 2014; pp 361–385. [Google Scholar]
- Vani R.; Stanley S. A. Studies on the Extraction of Chitin and Chitosan from Different Aquatic Organisms. Adv. Biotech. 2013, 12, 12–15. [Google Scholar]
- Ferrero F.; Periolatto M. Antimicrobial Finish of Textiles by Chitosan UV-Curing. J. Nanosci. Nanotechnol. 2012, 12, 4803–4810. 10.1166/jnn.2012.4902. [DOI] [PubMed] [Google Scholar]
- Periolatto M.; Ferrero F.; Vineis C. Antimicrobial Chitosan Finish of Cotton and Silk Fabrics by UV-Curing with 2-Hydroxy-2-Methylphenylpropane-1-One. Carbohydr. Polym. 2012, 88, 201–205. 10.1016/j.carbpol.2011.11.093. [DOI] [Google Scholar]
- Periolatto M.; Ferrero F.; Vineis C.; Rombaldoni F. Multifunctional Finishing of Wool Fabrics by Chitosan UV-Grafting: An Approach. Carbohydr. Polym. 2013, 98, 624–629. 10.1016/j.carbpol.2013.06.054. [DOI] [PubMed] [Google Scholar]
- Ferrero F.; Periolatto M.; Ferrario S. Sustainable Antimicrobial Finishing of Cotton Fabrics by Chitosan UV-Grafting: From Laboratory Experiments to Semi Industrial Scale-Up. J. Cleaner Prod. 2015, 96, 244–252. 10.1016/j.jclepro.2013.12.044. [DOI] [Google Scholar]
- Andreeva D. V.; Kollath A.; Brezhneva N.; Sviridov D. V.; Cafferty B. J.; Mohwald H.; Skorb E. V. Using A Chitosan Nanolayer as an Efficient pH Buffer to Protect pH-Sensitive Supramolecular Assemblies. Phys. Chem. Chem. Phys. 2017, 19, 23843–23848. 10.1039/C7CP02618H. [DOI] [PubMed] [Google Scholar]
- Kundu C. K.; Wang X.; Song L.; Hu Y. Chitosan-Based Flame Retardant Coatings for Polyamide 66 Textiles: One-Pot Deposition versus Layer-By-Layer Assembly. Int. J. Biol. Macromol. 2020, 143, 1–10. 10.1016/j.ijbiomac.2019.11.220. [DOI] [PubMed] [Google Scholar]
- Cao W.; Cloud R.. Balancing Comfort and Function in Textiles Worn by Medical Personnel. In Improving Comfort in Clothing; Song G., Ed.; Woodhead Publishing: Cambridge, U.K., 2011; pp 370–384. [Google Scholar]
- Rajendran S.; Anand S. C.; Rigby A. J.. Textiles for Healthcare and Medical Applications. In Handbook of Technical Textiles, 2nd ed.; Horrocks A. R., Anand S. C., Eds.; Woodhead Publishing: Cambridge, U.K., 2016; pp 135–168. [Google Scholar]
- Gupta B. The Effect of Structural Factors on the Absorbent Characteristics of Nonwovens. Tappi J. 1988, 71, 147–152. [Google Scholar]
- Song G.; Cao W.; Cloud R. M.. Medical Textiles and Thermal Comfort. In Handbook of Medical Textiles; Bartels V. T., Ed.; Woodhead Publishing: Cambridge, U.K., 2011; pp 198–218. [Google Scholar]
- Vozzola E.; Overcash M.; Griffing E. An Environmental Analysis of Reusable and Disposable Surgical Gowns. AORN J. 2020, 111, 315–325. 10.1002/aorn.12885. [DOI] [PubMed] [Google Scholar]
- Ajmeri J. R.; Joshi Ajmeri C.. Nonwoven Materials and Technologies for Medical Applications. In Handbook of Medical Textiles; Bartels V. T., Ed.; Woodhead Publishing: Cambridge, U.K., 2011; pp 106–131. [Google Scholar]
- Leonas K., Microorganism Protection. In Textiles for Protection, 1st ed.; Scott R. A., Ed.; Woodhead Publishing: Cambridge, U.K., 2005; pp 441–464. [Google Scholar]
- Overcash M. A Comparison of Reusable and Disposable Perioperative Textiles: Sustainability State-of-the-Art 2012. Anesth. Analg. 2012, 114, 1055–1066. 10.1213/ANE.0b013e31824d9cc3. [DOI] [PubMed] [Google Scholar]
- Leonas K. K. Effect of Laundering on the Barrier Properties of Reusable Surgical Gown Fabrics. Am. J. Infect. Control 1998, 26, 495–501. 10.1016/S0196-6553(98)70022-7. [DOI] [PubMed] [Google Scholar]
- Granzow J.; Smith J. W.; Nichols R. L.; Waterman R. S.; Muzik A. C. Evaluation of the Protective Value of Hospital Gowns Against Blood Strike-Through and Methicillin-Resistant Staphylococcus Aureus Penetration. Am. J. Infect. Control 1998, 26, 85–93. 10.1016/S0196-6553(98)80027-8. [DOI] [PubMed] [Google Scholar]
- Leonas K. K. Effect of Laundering on the Barrier Properties of Reusable Surgical Gown Fabrics. Am. J. Infect. Control 1998, 26, 495–501. 10.1016/S0196-6553(98)70022-7. [DOI] [PubMed] [Google Scholar]
- Hatch K. L.Textile Science; West Publishing Co.: Minneapolis, MN, 1993. [Google Scholar]
- Gong H.; Ozgen B.. Fabric Structures: Woven, Knitted, or Nonwoven. In Engineering of High-Performance Textiles; Miao M., Xin J. H., Eds.; Woodhead Publishing: Cambridge, U.K., 2018; pp 107–131. [Google Scholar]
- Qin Y.A Brief Description of the Manufacturing Processes for Medical Textile Materials. In Medical Textile Materials; Qin Y., Ed.; Woodhead Publishing: Cambridge, U.K., 2016; pp 43–54. [Google Scholar]
- Laufman H.; Eudy W. W.; Vandernoot A. M.; Harris C. A.; Liu D. Strike-Through of Moist Contamination by Woven and Nonwoven Surgical Materials. Ann. Surg. 1975, 181, 857–862. 10.1097/00000658-197506000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourmohammadi A.Nonwoven Materials and Joining Techniques. In Joining Textiles; Jones I., Stylios G. K., Eds.; Woodhead Publishing: Cambridge, U.K., 2013; pp 565–581. [Google Scholar]
- Wilson A.The Formation of Dry, Wet, Spunlaid and Other Types of Nonwovens. In Applications of Nonwovens in Technical Textiles; Chapman R. A., Ed.; Woodhead Publishing: Cambridge, U.K., 2010; pp 3–17. [Google Scholar]
- Chen K.; Ghosal A.; Yarin A. L.; Pourdeyhimi B. Modeling of Spunbond Formation Process of Polymer Nonwovens. Polymer 2020, 187, 121902. 10.1016/j.polymer.2019.121902. [DOI] [Google Scholar]
- Hosun L. A Review of Spun Bond Process. J. Text. Appar. Technol. Manag. 2010, 6, 1–13. [Google Scholar]
- Midha V.; Dakuri A. Spun Bonding Technology and Fabric Properties: A Review. J. Text. Eng. Fashion Technol. 2017, 1, 1–9. 10.15406/jteft.2017.01.00023. [DOI] [Google Scholar]
- Hutten I. M.Introduction to Nonwoven Filter Media. In Handbook of Nonwoven Filter Media, 2nd ed.; Hutten I. M., Ed.; Butterworth-Heinemann: Oxford, U.K., 2016; pp 1–52. [Google Scholar]
- Parthasarathi V.; Thilagavathi G. Development of Tri-Laminate Antiviral Surgical Gown for Liquid Barrier Protection. J. Text. Inst. 2015, 106, 1095–1105. 10.1080/00405000.2014.972711. [DOI] [Google Scholar]
- Parthasarathi V.; Thilagavathi G. Developing Antiviral Surgical Gown Using Nonwoven Fabrics for Health Care Sector. Afr. Health Sci. 2013, 13, 327–332. 10.4314/ahs.v13i2.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H.; Zhou Q.; Zhang Y.. Improving Fiber Alignment During Electrospinning. In Electrospun Nanofibers; Afshari M., Ed.; Woodhead Publishing: Cambridge, U.K., 2017; pp 125–147. [Google Scholar]
- Asmatulu R.; Khan W. S.. Historical Background of the Electrospinning Process. In Synthesis and Applications of Electrospun Nanofibers; Asmatulu R., Khan W. S., Eds.; Elsevier: Amsterdam, 2019; pp 17–39. [Google Scholar]
- Zhang S.; Tang N.; Cao L.; Yin X.; Yu J.; Ding B. Highly Integrated Polysulfone/Polyacrylonitrile/Polyamide-6 Air Filter for Multilevel Physical Sieving Airborne Particles. ACS Appl. Mater. Interfaces 2016, 8, 29062–29072. 10.1021/acsami.6b10094. [DOI] [PubMed] [Google Scholar]
- Zhao X.; Li Y.; Hua T.; Jiang P.; Yin X.; Yu J.; Ding B. Low-Resistance Dual-Purpose Air Filter Releasing Negative Ions and Effectively Capturing PM2.5. ACS Appl. Mater. Interfaces 2017, 9, 12054–12063. 10.1021/acsami.7b00351. [DOI] [PubMed] [Google Scholar]
- Zhao X.; Li Y.; Hua T.; Jiang P.; Yin X.; Yu J.; Ding B. Cleanable Air Filter Transferring Moisture and Effectively Capturing PM2.5. Small 2017, 13, 1603306. 10.1002/smll.201603306. [DOI] [PubMed] [Google Scholar]
- Al-Attabi R.; Dumée L. F.; Kong L.; Schütz J. A.; Morsi Y. High Efficiency Poly(acrylonitrile) Electrospun Nanofiber Membranes for Airborne Nanomaterials Filtration. Adv. Eng. Mater. 2018, 20, 1700572. 10.1002/adem.201700572. [DOI] [Google Scholar]
- Yang A.; Cai L.; Zhang R.; Wang J.; Hsu P. C.; Wang H.; Zhou G.; Xu J.; Cui Y. Thermal Management in Nanofiber-Based Face Mask. Nano Lett. 2017, 17, 3506–3510. 10.1021/acs.nanolett.7b00579. [DOI] [PubMed] [Google Scholar]
- Liu C.; Hsu P. C.; Lee H. W.; Ye M.; Zheng G.; Liu N.; Li W.; Cui Y. Transparent Air Filter for High-Efficiency PM2.5 Capture. Nat. Commun. 2015, 6, 6205. 10.1038/ncomms7205. [DOI] [PubMed] [Google Scholar]
- Choi D. Y.; Jung S. H.; Song D. K.; An E. J.; Park D.; Kim T. O.; Jung J. H.; Lee H. M. Al-Coated Conductive Fibrous Filter with Low Pressure Drop for Efficient Electrostatic Capture of Ultrafine Particulate Pollutants. ACS Appl. Mater. Interfaces 2017, 9, 16495–16504. 10.1021/acsami.7b03047. [DOI] [PubMed] [Google Scholar]
- Li X.; Wang C.; Huang X.; Zhang T.; Wang X.; Min M.; Wang L.; Huang H.; Hsiao B. S. Anionic Surfactant-Triggered Steiner Geometrical Poly(vinylidene Fluoride) Nanofiber/Nanonet Air Filter for Efficient Particulate Matter Removal. ACS Appl. Mater. Interfaces 2018, 10, 42891–42904. 10.1021/acsami.8b16564. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Yuan S.; Feng X.; Li H.; Zhou J.; Wang B. Preparation of Nanofibrous Metal–Organic Framework Filters for Efficient Air Pollution Control. J. Am. Chem. Soc. 2016, 138, 5785–5788. 10.1021/jacs.6b02553. [DOI] [PubMed] [Google Scholar]
- Balgis R.; Murata H.; Goi Y.; Ogi T.; Okuyama K.; Bao L. Synthesis of Dual-Size Cellulose–Polyvinylpyrrolidone Nanofiber Composites via One-Step Electrospinning Method for High-Performance Air Filter. Langmuir 2017, 33, 6127–6134. 10.1021/acs.langmuir.7b01193. [DOI] [PubMed] [Google Scholar]
- Li M.; Zhang G.; Xu S.; Zhao C.; Han M.; Zhang L.; Jiang H.; Liu Z.; Na H. Cross-Linked Polyelectrolyte for Direct Methanol Fuel Cells Applications Based on a Novel Sulfonated Cross-Linker. J. Power Sources 2014, 255, 101–107. 10.1016/j.jpowsour.2013.12.116. [DOI] [Google Scholar]
- Harifi T.; Montazer M. Past, Present and Future Prospects of Cotton Cross-Linking: New Insight into Nano Particles. Carbohydr. Polym. 2012, 88, 1125–1140. 10.1016/j.carbpol.2012.02.017. [DOI] [Google Scholar]
- Straccia M. C.; Romano I.; Oliva A.; Santagata G.; Laurienzo P. Crosslinker Effects on Functional Properties of Alginate/N-Succinylchitosan Based Hydrogels. Carbohydr. Polym. 2014, 108, 321–330. 10.1016/j.carbpol.2014.02.054. [DOI] [PubMed] [Google Scholar]
- Dragan E. S. Design and Applications of Interpenetrating Polymer Network Hydrogels. A Review. Chem. Eng. J. 2014, 243, 572–590. 10.1016/j.cej.2014.01.065. [DOI] [Google Scholar]
- Sathianarayanan M.; Bhat N.; Kokate S.; Walunj V. Antibacterial Finish for Cotton Fabric from Herbal Products. Indian J. Fibre Text. 2010, 35, 50–58. [Google Scholar]
- Tawiah B.; Tawiah B.; Badoe W.; Badoe W.; Fu S. Advances in the Development of Antimicrobial Agents for Textiles: The Quest for Natural Products. Review. Fibres Text. East. Eur. 2016, 24, 136–149. 10.5604/12303666.1196624. [DOI] [Google Scholar]
- Shahidi S.; Wiener J.. Antibacterial Agents in Textile Industry. In Antimicrobial Agents; Bobbarala V., Ed.; IntechOpen: London, 2012; pp 387–406. [Google Scholar]
- Bshena O.; Heunis T. D.; Dicks L. M.; Klumperman B. Antimicrobial Fibers: Therapeutic Possibilities and Recent Advances. Future Med. Chem. 2011, 3, 1821–1847. 10.4155/fmc.11.131. [DOI] [PubMed] [Google Scholar]
- Bhardwaj N.; Kundu S. C. Electrospinning: A Fascinating Fiber Fabrication Technique. Biotechnol. Adv. 2010, 28, 325–347. 10.1016/j.biotechadv.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Mirjalili M.; Zohoori S. Review for Application of Electrospinning and Electrospun Nanofibers Technology in Textile industry. J. Nanostruct. Chem. 2016, 6, 207–213. 10.1007/s40097-016-0189-y. [DOI] [Google Scholar]
- Torres-Giner S.Novel Antimicrobials Obtained by Electrospinning Methods. In Antimicrobial Polymers; Lagarón J. M., Ocio M. J., López-Rubio A., Eds.; John Wiley & Sons: Hoboken, NJ, 2011; pp 261–285. [Google Scholar]
- Palza H. Antimicrobial Polymers with Metal Nanoparticles. Int. J. Mol. Sci. 2015, 16, 2099–2116. 10.3390/ijms16012099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlarik V.Antimicrobial Modifications of Polymers. In Biodegradation - Life of Science; Chamy R., Ed.; IntechOpen: London, 2013. [Google Scholar]
- Van Wely E. Current Global Standards for Chemical Protective Clothing: How to Choose the Right Protection for the Right Job?. Ind. Health 2017, 55, 485–499. 10.2486/indhealth.2017-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Health and Social Care . Specification for Personal Protective Clothing (PPE), https://www.gov.uk/government/publications/technical-specifications-for-personal-protective-equipment-ppe (accessed 2020-05-04).
- NIOSH . Considerations for Selecting Protective Clothing Used in Healthcare for Protection against Microorganisms in Blood and Body Fluids, https://www.cdc.gov/niosh/npptl/topics/protectiveclothing/default.html (accessed 2020-05-04).
- ISO . 18184:2019 (en) Textiles — Determination of Antiviral Activity of Textile Products, https://www.iso.org/standard/71292.html (accessed 2020-05-04).
- Troynikov O.; Nawaz N.; Watson C.. Medical Protective Clothing. In Protective Clothing; Wang F., Gao C., Eds.; Woodhead Publishing: Cambridge, U.K., 2014; pp 192–224. [Google Scholar]
- ASTM . F16170/F1670M-17a: Standard Test Method for Resistance of Materials Used in Protective Clothing to Penetration by Synthetic Blood, https://www.astm.org/Standards/F1670.htm (accessed 2020-05-04).
- ASTM . F1671/F1671M-13: Standard Test Method for Resistance of Materials Used in Protective Clothing to Penetration by Blood-Borne Pathogens Using Phi-X174 Bacteriophage Penetration as a Test System, https://www.astm.org/Standards/F1671.htm (accessed 2020-05-04).
- BSI . BS EN 13795–1:2019: Surgical Clothing and Drapes. Requirements and Test Methods. Surgical Drapes and Gowns, 2019.
- Laird K.; Owen L.. The Role of Protective Clothing in Healthcare and Its Decontamination. In Decontamination in Hospitals and Healthcare, 2nd ed.; Walker J., Ed.; Woodhead Publishing: Cambridge, U.K., 2020; pp 209–226. [Google Scholar]
- Cao W.; Cloud R. Effects of Temperature on Liquid Penetration Performance of Surgical Gown Fabrics. Int. J. Cloth. Sci. Technol. 2010, 22, 319–332. 10.1108/09556221011071794. [DOI] [Google Scholar]
- Mondal S. Phase Change Materials for Smart Textiles–An Overview. Appl. Therm. Eng. 2008, 28, 1536–1550. 10.1016/j.applthermaleng.2007.08.009. [DOI] [Google Scholar]
- Akridge J.Raising the Bar on Surgical Gowns and Drapes. Healthcare Purchasing News 2004, https://cdn.hpnonline.com/inside/2004-09/0409gowns.html (accessed 2020-06-27). [Google Scholar]
- Qin Y.Biocompatibility Testing for Medical Textile Products. In Medical Textile Materials; Qin Y., Ed.; Woodhead Publishing: Cambridge, U.K., 2016; pp 191–201. [Google Scholar]
- Statista . Value of the Personal Protective Equipment Market Worldwide from 2018 to 2025, https://www.statista.com/statistics/711286/value-of-the-global-ppe-market/ (accessed 2020-06-27). [Google Scholar]
- Statista . Share of the Leading Exporters of Personal Protective Products Worldwide in 2019, https://www.statista.com/statistics/1121545/top-exporters-of-personal-protective-products-worldwide/ (accessed 2020-06-27).
- Markets and Markets . Protective Clothing Market Global Forecast to 2024, https://www.marketsandmarkets.com/Market-Reports/protective-clothing-market-1278.html (accessed 2020-06-27).
- Textiles Intelligence . Medical Textiles: Markets, Applications, Developments and Regulations, Textile Outlook International Issue 199. Wilmslow, U.K., 2019.
- Statista . Level of Supply of the Following Personal Protective Equipment (PPE) Items for Doctors Working in High-Risk Areas In The U.K. as of April 2020, https://www.statista.com/statistics/1111503/ppe-supply-in-high-risk-area-in-the-uk/ (accessed 2020-06-27).
- UNICEF . COVID-19 Impact Assessment and Outlook on Personal Protective Equipment, https://www.unicef.org/supply/stories/covid-19-impact-assessment-and-outlook-personal-protective-equipment (accessed 2020-06-27).
- Loo F.Asian Plastic Prices Rebound but Pandemic Limits Demand Support for Most Markets, https://www.icis.com/explore/resources/news/2020/04/16/10496614/asian-plastic-prices-rebound-but-pandemic-limits-demand-support-for-most-markets (accessed 2020-06-27).
- Queiroz M. M.; Ivanov D.; Dolgui A.; Fosso Wamba S. Impacts of Epidemic Outbreaks on Supply Chains: Mapping a Research Agenda Amid the COVID-19 Pandemic through a Structured Literature Review. Ann. Oper. Res. 2020, 10.1007/s10479-020-03685-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNCTAD . UN Launches Drive to Highlight Environmental Cost of Staying Fashionable, https://news.un.org/en/story/2019/03/1035161 (accessed 2020-07-09).
- UNFCC . UN Helps Fashion Industry Shift to Low Carbon, https://unfccc.int/news/un-helps-fashion-industry-shift-to-low-carbon (accessed 2020-05-09).
- Textiles Intelligence . Fast Track: Performance Apparel Supply Chains Have Mobalised in the Fight against COVID-19, Performance Apparel Market Issue 68; Wilmslow, U.K., May, 2020.
- Eckelman M. J.; Graedel T. Silver Emissions and Their Environmental Impacts: A Multilevel Assessment. Environ. Sci. Technol. 2007, 41, 6283–6289. 10.1021/es062970d. [DOI] [PubMed] [Google Scholar]
- Lansdown A. B.Silver In Health Care: Antimicrobial Effects and Safety in Use. Biofunctional Textiles and the Skin; Karger Publishers: Basel, 2006; Vol. 33, pp 17–34. [DOI] [PubMed] [Google Scholar]
- Wollina U.; Abdel-Naser M.; Verma S.. Skin Physiology and Textiles–Consideration of Basic Interactions. Biofunctional Textiles and the Skin; Karger Publishers: Basel, 2006; Vol. 33, pp 1–16. [DOI] [PubMed] [Google Scholar]
- Morris A.Monitoring COVID-19 from Hospital to Home: First Wearable Device Continuously Tracks Key Symptoms, https://news.northwestern.edu/stories/2020/04/monitoring-covid-19-from-hospital-to-home-first-wearable-device-continuously-tracks-key-symptoms/ (accessed 2020-07-09).
- Novoselov K. S.; Mishchenko A.; Carvalho A.; Castro Neto A. H. Science 2016, 353, aac9439. 10.1126/science.aac9439. [DOI] [PubMed] [Google Scholar]
- Boyle L.Discarded Coronavirus Face Masks and Gloves Rising Threat to Ocean Life, Conservationists Warn. The Independent, https://www.independent.co.uk/news/coronavirus-masks-gloves-oceans-pollution-waste-a9469471.html (accessed 2020-07-09). [Google Scholar]
- Kassam A.‘More Masks than Jellyfish’: Coronavirus Waste Ends Up in Ocean. The Guardian, https://www.theguardian.com/environment/2020/jun/08/more-masks-than-jellyfish-coronavirus-waste-ends-up-in-ocean (accessed 2020-07-09). [Google Scholar]
- Fletcher C.What Happens to Waste PPE During the Coronavirus Pandemic? The Conversation, https://theconversation.com/what-happens-to-waste-ppe-during-the-coronavirus-pandemic-137632 (accessed 2020-07-01). [Google Scholar]
- Karim M. N.; Afroj S.; Rigout M.; Yeates S. G.; Carr C. Towards UV-Curable Inkjet Printing of Biodegradable Poly (lactic Acid) Fabrics. J. Mater. Sci. 2015, 50, 4576–4585. 10.1007/s10853-015-9006-0. [DOI] [Google Scholar]
- Upadhyay A.; Upadhyaya I.; Kollanoor-Johny A.; Venkitanarayanan K. Combating Pathogenic Microorganisms Using Plant-Derived Antimicrobials: A Minireview of the Mechanistic Basis. BioMed Res. Int. 2014, 2014, 1–18. 10.1155/2014/761741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savoia D. Plant-Derived Antimicrobial Compounds: Alternatives to Antibiotics. Future Microbiol. 2012, 7, 979–990. 10.2217/fmb.12.68. [DOI] [PubMed] [Google Scholar]
- Shahid-ul-Islam; Shahid M.; Mohammad F. Green Chemistry Approaches to Develop Antimicrobial Textiles Based on Sustainable Biopolymers—A Review. Ind. Eng. Chem. Res. 2013, 52, 5245–5260. 10.1021/ie303627x. [DOI] [Google Scholar]
- Koo W. T.; Millstone J. E.; Weiss P. S.; Kim I. D. The Design and Science of Polyelemental Nanoparticles. ACS Nano 2020, 14, 6407–6413. 10.1021/acsnano.0c03993. [DOI] [PubMed] [Google Scholar]
- Kan C. W.A Novel Green Treatment for Textiles: Plasma Treatment as a Sustainable Technology; CRC Press: Boca Raton, FL, 2014. [Google Scholar]
- Kasiri M. B.; Safapour S. Natural Dyes and Antimicrobials for Green Treatment of Textiles. Environ. Chem. Lett. 2014, 12, 1–13. 10.1007/s10311-013-0426-2. [DOI] [Google Scholar]
- Kalia S.; Thakur K.; Celli A.; Kiechel M. A.; Schauer C. L. Surface Modification of Plant Fibers Using Environment Friendly Methods for Their Application in Polymer Composites, Textile Industry and Antimicrobial Activities: A Review. J. Environ. Chem. Eng. 2013, 1, 97–112. 10.1016/j.jece.2013.04.009. [DOI] [Google Scholar]
- Zuniga J. M.; Cortes A. The Role of Additive Manufacturing and Antimicrobial Polymers in the COVID-19 Pandemic. Expert Rev. Med. Devices 2020, 17, 477–481. 10.1080/17434440.2020.1756771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrañeta E.; Dominguez-Robles J.; Lamprou D. A., Additive Manufacturing Can Assist in the Fight against COVID-19 and Other Pandemics and Impact on the Global Supply Chain. 3D Print. Addit. Manuf. 2020, 7, 100–103. 10.1089/3dp.2020.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . Shortage of Personal Protective Equipment Endangering Health Workers Worldwide; World Health Organisation: Geneva, 2020.