After the first autochthonous case described on February 19, also in Italy the Severe Acute Respiratory Syndrome CoronaVirus 2 (SARS-CoV-2) infection rapidly circulated, mainly in the Northern regions of the country. The earliest reports on Coronavirus disease-19 (COVID-19) have described worldwide a high prevalence of severe respiratory illness [1]. A suggestive feature of COVID-19 has been a rapid progression of the respiratory impairment, leading to acute respiratory distress syndrome (ARDS) and often requiring ventilation support [2]. To date, whether clinical features at hospital presentation and outcome of COVID-19 have changed over the outbreak course is unknown. We explored this issue in a multicenter cohort of patients hospitalized for COVID-19 in Northern Italy.
We retrospectively revised the clinical records of all consecutive patients admitted for SARS-CoV-2 infection between March 1 and May 12, 2020 in three hospitals of the Piedmont region: “Maggiore della Carità Hospital” in Novara, “Sant'Andrea” Hospital, in Vercelli, “Santi Antonio e Biagio e Cesare Arrigo” Hospital, in Alessandria. These hospitals are the referral of a large homogenous territory in Eastern Piedmont, one of the most hit by the SARS-CoV-2 pandemic [3]. The study protocol was approved by the institutional ethical committee of Novara (IRB code CE 97/20). For the purpose of the analysis, we divided the whole study duration in two time spans: period 1 (from March 1 to April 13, i.e. during the maximal diffusion of SARS-CoV-2 infection) and period 2 (from April 14 to May 12, during the declining infection). The starting of period 2 was set on April 14 as this date represented the turning point when the increase in the daily number of individuals positive for SARS-CoV-2 infection in Piedmont has ceased [3]. Informed consent was waived due to the retrospective nature of the study and use of pseudonymized data.
The following endpoints were compared between patients admitted in the two abovementioned periods:
-
1.
Incidence of patients requiring mechanical ventilation on the day of admission.
-
2.
Incidence of patients with severe ARDS on the day of admission. Severe ARDS was defined according to the Berlin definition [4].
-
3.
A score indicative of critical COVID-19 on the day of admission. Patients were stratified according to the Shang's score, which represents a clinical classification of COVID-19 severity, as per recent description in a cohort of patients diagnosed with SARS-CoV-2 infection in Wuhan (China) [5]. Patients with score of critical disease were identified as those with respiratory failure requiring mechanical ventilation or with shock or other organ failure requiring Intensive Care Unit monitoring and treatment.
The daily percentage of patients with mechanical ventilation, severe ARDS and a score of critical disease on admission was obtained from the daily number of each outcome measure on admission and the total number of patients hospitalized in the same day. This was recorded for all 44 days of period 1 and 29 days of period 2. Incidence rate ratios (IRR) comparing period 1 and period 2 were calculated using Poisson regression. The weekly number of events per 100 patients was represented in a plot together with a Local polynomial regression smoothing curve. A span smoothing control of 0.75 with 2 degrees of the polynomials has been considered for the computation. The R2 model fitting statistics was also computed.
A total of 522 patients were overall included in this analysis, 416 admitted from March 1 to April 13 (period 1) and 106 from April 14 to May 12 (period 2). Main characteristics of the two groups are indicated in Table 1 . Patients hospitalized in the later phase of the SARS-CoV-2 outbreak were older and had a lower prevalence of male gender and diabetes mellitus. In period 2, cough, dyspnea and sputum production were less represented as clinical features upon Emergency Department presentation, body temperature was lower, and levels of C-reactive protein tended to be reduced. In the later phase patients were less frequently treated with hydroxychloroquine, lopinavir/ritonavir and tocilizumab.
Table 1.
Main characteristics of patients admitted in the two time periods of the SARS-CoV-2 infection.
| Period 1 (March 1 to April 13) N=416 | Period 2 (April 14 to May 12) N=106 | p value | |
|---|---|---|---|
| Age (years) | 67.5±15 | 73.1±16.7 | 0.001 |
| Male gender | 255 (61.3) | 51 (48.5) | 0.015 |
| Obesity* | 87 (31.4) | 16 (20.3) | 0.07 |
| Arterial hypertension | 241 (57.9%) | 56 (52.8) | 0.38 |
| Diabetes mellitus | 125 (30) | 16 (15) | 0.002 |
| Cardiomyopathy | 107 (25.7) | 23 (21.7) | 0.45 |
| Chronic obstructive pulmonary disease | 70 (16.8) | 14 (13.2) | 0.38 |
| Chronic renal failure | 66 (15.9) | 24 (22.6) | 0.10 |
| History of cancer | 110 (26.4) | 27 (25.4) | 0.90 |
| Chronic liver disease | 12 (2.9) | 6 (5.7) | 0.23 |
| Autoimmune diseases | 33 (7.9) | 6 (5.7) | 0.54 |
| Symptoms upon presentation | |||
| Cough or dyspnea | 232 (55.8) | 43 (40.6) | 0.006 |
| Sputum production | 49 (11.8) | 2 (1.9) | 0.002 |
| Chest pain | 53 (12.7) | 5 (4.7) | 0.023 |
| Syncope | 21 (5.1) | 4 (3.7) | 0.63 |
| Clinical signs upon presentation | |||
| Systolic blood pressure (mmHg) | 127±21 | 130±24 | 0.20 |
| Body temperature (°C) | 37.5±1.0 | 37.1±1.1 | 0.001 |
| Heart rate (bpm) | 86±17 | 87±22 | 0.68 |
| C-reactive protein (mg/dL) | 8.6±7.5 | 7.3±6.1 | 0.12 |
| In-hospital therapy | |||
| Hydroxychloroquine* | 325 (89.0) | 73 (80.2) | 0.034 |
| Lopinavir/ritonavir | 70 (16.8) | 5 (4.7) | 0.002 |
| Remdesivir | 8 (1.9) | 0 | 0.22 |
| Tocilizumab | 45 (10.8) | 1 (0.9) | 0.002 |
| Low molecular weight heparin* | 286 (78.4) | 84 (85.7) | 0.12 |
Data are expressed as n (%) or mean±standard deviation. * Data were missing for obesity in 166 patients (139 in period 1 and 27 in period 2), for hydroxychloroquine in 66 patients (51 in period 1 and 15 in period 2) and for low molecular weight heparin in 59 patients (51 in period 1 and 8 in period 2).
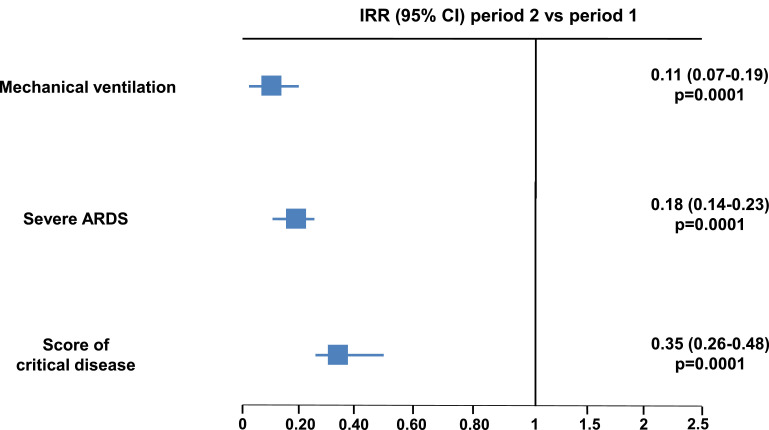
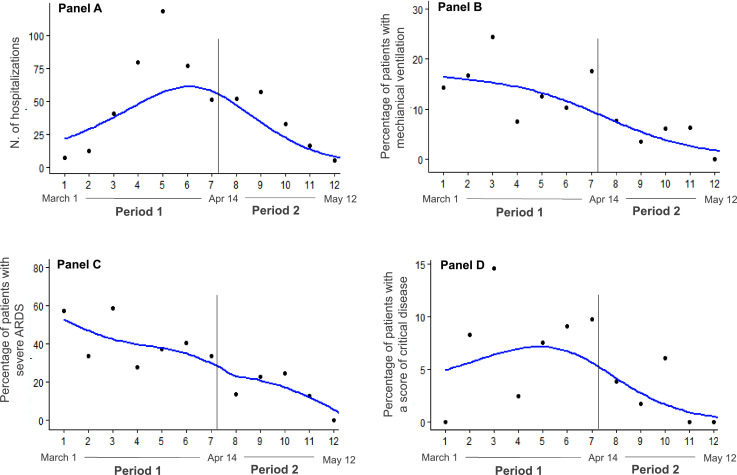
Mean IR of mechanical ventilation on admission was 5.0 per 100 patient-days in period 1 and 0.6 per 100 patient-days in period 2; IR of severe ARDS on admission was 12.9 per 100 patient-days and 2.3 per 100 patient-days, respectively. For both these outcome measures, the rates in period 2 were significantly lower compared to period 1 (IRR of mechanical ventilation 0.11, 95% CI 0.07-0.19; p=0.0001; IRR of severe ARDS 0.18, 95% CI 0.14-0.23; p=0.0001) (Fig. 1 ). The occurrence of a score on the day of admission indicating a critical COVID-19 was 11.1 per 100 patient-days in period 1 and 2.6 per 100 patient-days in period 2, with a significant 65% relative reduction in the latter time span (IRR 0.35, 95% CI 0.26-0.48; p=0.0001) (Fig. 1). As patients admitted in the later phase were older, we performed a sensitivity analysis in the subgroup with age ≥70 years, where consistent results on the event rates upon hospitalization were observed in period 2 vs 1: mechanical ventilation: OR 0.27, 95% CI 0.03-2.10; severe ARDS: OR 0.39, 95% CI 0.20-0.79; score of severe disease: OR 0.24, 95% CI 0.05-1.02. Fig. 2 depicts the weekly course of total number of hospitalizations, percentage of mechanical ventilations, percentage of severe ARDS and percentage of patients with a score of severe disease across the two periods.
Fig. 1.
Forrest plot comparing the IRR of mechanical ventilation, severe ARDS and a clinical score of critical disease on admission in period 2 (from April 14 to May 12, 2020) vs period 1 (from March 1 to April 13, 2020).
ARDS= Acute Respiratory Distress Syndrome; IRR= incidence rate ratio
Fig. 2.
Local Polynomial Regression smoothing (LOESS) plots.
Panel A: Total hospitalizations; a LOESS smoothing method has been considered for the analysis with a degree of non-linear approximation (span) of 0.75. R2=0.88. Panel B: Weekly number of patients with mechanical ventilation over number of hospitalizations; a LOESS smoothing method has been considered for the analysis with a degree of non-linear approximation (span) of 0.75. R2=0.75. Panel C: Weekly number of patients with severe ARDS over number of hospitalizations; a LOESS smoothing method has been considered for the analysis with a degree of non-linear approximation (span) of 0.75. R2=0.8. Panel D: Weekly number of patients with a clinical score of severe COVID-19 over number of hospitalizations; a LOESS smoothing method has been considered for the analysis with a degree of non-linear approximation (span) of 0.75. R2=0.6.
ARDS= Acute Respiratory Distress Syndrome
In this observational, retrospective, multicenter investigation on consecutive patients admitted for COVID-19 we found that with decreasing viral diffusion the severity of the respiratory tract involvement and the inflammatory status at hospital presentation were less pronounced, as demonstrated by a fewer prevalence of cough, dyspnea and sputum production, as well as by a lower body temperature and a trend towards reduced C-reactive protein levels. As a consequence, in the later phase we observed an 89% relative reduction in the incidence rate of mechanical ventilation on admission and an 82% relative reduction of early severe ARDS. A clinical score on admission indicating a critical COVID-19 was also less frequently observed in period 2, with a 65% relative reduction. During the SARS-CoV-2 outbreak we report a linear decrease over time of severe ARDS and mechanical ventilation use (Fig. 2). In fact, the incidence of both these parameters was highest in the first 3 weeks of period 1, i.e. when the total number of cases and hospitalizations was still low, and continued to decrease in the subsequent 3 weeks of period 1, while the total number of cases and hospitalizations was increasing. Nevertheless, the percentage of patients with a score of critical disease tended to increase in the first weeks of period 1, concomitantly with the increase in the number of hospitalizations; this apparent inconsistency may be due to the hospital overcrowding and limited bed capacity in the intensive care units that occurred at the beginning of the epidemic in Northern Italy, when severe cases with less life expectancy were not intubated. Notably, period 1 almost entirely refers to the time of national lock-down, decreed on March 8th, characterized by strict social containing measures and limitation of several economic activities.
The present study has strengths and limitations. It was performed comparing clinical features upon hospital presentation and outcome of COVID-19 over two subsequent periods in consecutive patients from the same area, receiving consistent in-hospital protocols of diagnosis or care and managed by the same care-workers. Our investigation has limitations inherent to observational and retrospective studies, mainly the risk of residual confounding. Individual data were accurately collected with a strict source verification for the event adjudication; however, the retrospective design and the conduction of the study during a National Emergency contributed to the lack of some, although limited, data, which were not available. Furthermore, data on COVID-19 patients who died before the Emergency Department presentation were not available. A selection bias may exist, as it is reasonable that the wider diffusion of the outbreak in the earlier period led to admit to the hospital only those patients with more critical clinical pictures. Finally, whether our results may also apply to regions with lower viral circulation or different health-care systems it is unknown. Actually, the exponential increase of COVID-19 cases at the beginning of the epidemic heavily affected the sustainability of hospital acceptance capacity, limiting hospitalization to more severe cases.
In conclusion, this investigation indicates a progressive decreasing severity of COVID-19 at hospital presentation over the pandemic period in Northern Italy. In particular, the percentage of patients with early severe ARDS or requiring early mechanical ventilation was significantly reduced. We believe these findings may be relevant, as large areas in the world are fully involved in the SARS-CoV-2 pandemic yet and may represent the basis for further evaluations by epidemiologists and virologists. It is reasonable that the wider diffusion of the outbreak in the earlier period led to admit to the hospital only those patients with critical clinical pictures. However, our findings might support that, during the intense virus circulation, expositions to a higher viral load or re-expositions have caused more severe disease presentations [6]; indeed, the relationship between viral load and COVID-19 severity is controversial, with recent data showing no significant difference in the viral load of symptomatic versus asymptomatic patients with SARS-CoV-2 infection [7]. Specific studies are needed to investigate this latter issue, as well as to explore whether our results can be also due to a decrease over time in the virulence of SARS-CoV-2, linked to intrinsic (i.e. virus mutations) and/or extrinsic (i.e. environmental) mechanisms.
Contributions
GP conceived the study; GP and MM designed the study; ES, EH, AR, LG, CC and VL performed data collection; GP, MM and DA performed statistical analysis; GP wrote the manuscript; all authors contributed to data interpretation; critical revision of the paper for important intellectual content was done by all authors.
Funding
None
Disclosures
All authors have no disclosure related to this paper.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank the following investigators for their contribution to this work: Emanuele Albano,1 Umberto Dianzani,1,2 Gianluca Gaidano,1,2 Alessandra Gennari,1,2 Carla Gramaglia,1,2 Martina Solli,2 Ailia Giubertoni,2 Alessia Veia,2 Carlo Cisari,1,2 4 Paolo Amedeo Tillio,1,3 Paolo Aluffi Valletti,1,2 Francesco Barone Adesi,1 Michela Barini,2 Daniela Ferrante,1 Simona De Vecchi,1,2 Matteo Santagostino,1,2 Antonio Acquaviva,1,2 Elisa Calzaducca,1,2 Francesco Giuseppe Casciaro,1,2 Federico Ceruti,1,2 Micol Giulia Cittone,1,2 Davide Di Benedetto,1,2 Ileana Gagliardi,1,2 Greta Maria Giacomini,1,2 Irene Cecilia Landi,1,2 Raffaella Landi,1,2 Giulia Francesca Manfredi,1,2 Anita Rebecca Pedrinelli,1,2 Cristina Rigamonti,1,2 Eleonora Rizzi,1,2 Carlo Smirne,1,2 Veronica Vassia,1,2 Roberto Arioli,1,2 Pietro Danna,1,2 Zeno Falaschi,1,2 Alessio Paschè,1,2 Ilaria Percivale,1,2 Domenico Zagaria,1,2 Michela Beltrame,1,2 Matteo Bertoli,1,2 Alessandra Galbiati 1,2 Clara Ada Gardino,1,2 Maria Luisa Gastaldello,1,2 Valentina Giai Via,1,2 Francesca Giolitti,1,2 Ilaria Inserra,1,2 Emanuela Labella,1,2 Ilaria Nerici,1,2 Laura Cristina Gironi,1,2 Edoardo Cammarata,1,2 Elia Esposto,1,2 Vanessa Tarantino,1,2 Elisa Zavattaro,1,2 Francesca Zottarelli,1,2 Tommaso Daffara,1,2 Alice Ferrero,1,2 Ilaria Leone,1,2 Alessandro Nuzzo,1,2 Giulia Baldon,1,2 Sofia Battistini,1,2 Emilio Chirico,1,2 Luca Lorenzini,1,2 Maria Martelli,1,2 Emanuela Barbero,1,2 Paolo Boffano,1,2 Matteo Brucoli,1,2 Massimiliano Garzaro,1,2 Alberto Pau,1,2 Stephanie Bertolin,1,2 Letizia Marzari,1,2 Gianluca Avino,1,2 Massimo Saraceno,1,2 Umberto Morosini,1,2 Alessio Baricich,1,2 Marco Invernizzi,1,2 Silvia Gallo,1,3 Claudia Montabone,1,3 Samuel Alberto Padelli,1,3 Lucio Boglione,1,3 Filippo Patrucco,1,3 Luigia Salamina,2 Francesca Baorda,1,3 Eleonora Croce,1,3 Irene Giacone.1,3, Marco Sciarra4, Andrea Schimmenti4, Andrea Pasini4, Roberto Angilletta4.
Footnotes
The following other authors from the COVID-UPO Clinical Team participated the study: Danila Azzolina,1 Eyal Hayden,1,2 Andrea Rognoni,2 Leonardo Grisafi,1,2 Crizia Colombo,1,2 Veronica Lio,1,2 Mario Pirisi,1,2 Rosanna Vaschetto,1,2 Gianluca Aimaretti,1,2 Marco Krengli,1,2 Gian Carlo Avanzi,1,2 Piero Emilio Balbo,2 Andrea Capponi,2 Luigi Mario Castello,1,2 Mattia Bellan,1,2 Mario Malerba,1,3 Pietro Luigi Garavelli,2 Patrizia Zeppegno,1,2 Paola Savoia,1,2 Guido Chichino,4 Carlo Olivieri,1,3 Roberta Re,1,3 Antonio Maconi,4 Cristoforo Comi,1,3 Annalisa Roveta4, Marinella Bertolotti,4 Alessandro Carriero,1,2 Marta Betti,4 Marco Mussa,4 Silvio Borrè,3 Vincenzo Cantaluppi,1,2 Roberto Cantello,1,2 Flavio Bobbio,2 Francesco Gavelli,1,2
References
- 1.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D., Hu B., Hu C. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Italian Civil Protection Department. Coronavirus Emergency. Accessed athttp://opendatadpc.maps.arcgis.com/apps/opsdashboard/index.html#/b0c68bce2cce478eaac82fe38d4138b1.
- 4.ARDS Definition Task Force Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 5.Shang W., Dong J., Ren Y. The value of clinical parameters in predicting the severity of COVID‐19. J Med Virol. 2020 doi: 10.1002/jmv.26031. May 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Y., Yan L.M., Wan L. Viral Dynamics in Mild and Severe Cases of COVID-19. Lancet Infect Dis. 2020;20:656–657. doi: 10.1016/S1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lavezzo E., Franchin E., Ciavarella C. Suppression of a SARS-CoV-2 outbreak in the Italian municipality of Vo'. Nature. 2020;584:425–429. doi: 10.1038/s41586-020-2488-1. [DOI] [PubMed] [Google Scholar]