ABSTRACT
Background: In the absence of confirmatory biopsy, the criteria for diagnosis of neuro-ophthalmic sarcoidosis are not well established. Diagnostic criteria for both intraocular sarcoidosis and neurosarcoidosis have been proposed, but the diagnosis of neuro-ophthalmic sarcoidosis remains challenging. It is our intention to augment what is currently known about the diagnosis of neuro-ophthalmic sarcoidosis by providing a series of biopsy-proven cases that contribute to the continued development of diagnostic criteria for this enigmatic condition.
Methods: Case series of four Caucasian women with biopsy-proven neuro-ophthalmic sarcoidosis.
Results: The first patient was initially diagnosed with traumatic optic neuropathy following a fall. Years later, the presence of pathologic submandibular lymphadenopathy was identified and biopsied, revealing non-caseating granulomas. The second and third cases involved sarcoidosis of the extraocular muscles without clear or common systemic features of sarcoidosis. In the fourth and final case, the patient presented with a Horner syndrome attributed to sarcoid infiltration of the ipsilateral sympathetic chain. Bronchoscopy with biopsy showed non-caseating granulomas consistent with sarcoidosis.
Conclusions: We describe four cases of neuro-ophthalmic sarcoidosis and propose possible neuro-orbital and neuro-ophthalmic criteria both with and without diagnostic biopsy.
KEYWORDS: Neuro-ophthalmic sarcoidosis, non-caseating granuloma, diagnostic criteria, sarcoidosis
Introduction
Sarcoidosis is a multi-systemic granulomatous disease that can present with neuro-ophthalmic findings.1,2 The typical presentation of sarcoidosis in the eye includes anterior or posterior uveitis, episcleritis, or keratitis in up to 25% of cases.3–5 Although typically a multisystemic disease, sarcoid may occur in the orbit, eye, or brain without any evidence of systemic involvement. Tissue biopsy demonstrating non-caseating granuloma remains the gold standard for diagnosis, but diagnostic criteria have been proposed both with and without a diagnostic biopsy.6
Zajicek et al. proposed a set of diagnostic criteria for neurosarcoidosis (Table 1).7 Likewise, in 2009, the first International Workshop on Ocular Sarcoidosis (IWOS) reported criteria for the diagnosis of intraocular sarcoidosis based on a combination of ophthalmic clinical signs and laboratory investigations (Table 2).8 However, the diagnosis of neuro-ophthalmic sarcoidosis – sarcoidosis of the central nervous system (CNS) with ophthalmic manifestations but without intraocular involvement – lacks nuance. CNS involvement is reported to occur with the frequency of 5% to 16% in clinical studies of patients with sarcoidosis,5 and neuro-ophthalmic symptoms may be the only presenting symptom in some patients,9 so neuro-ophthalmic sarcoidosis is a very uncommon condition that presents a significant diagnostic challenge. We describe four cases and propose a combined set of diagnostic criteria for neuro-ophthalmic sarcoidosis. To our knowledge, this is the first attempt to unify the various diagnostic criteria for neuro-ophthalmology in the English language ophthalmic literature.
Table 1.
Diagnostic criteria for neurosarcoidosis proposed by Zajicek, et al.7
| Definite | Clinical presentation suggestive of neurosarcoidosis with exclusion of other possible diagnoses and the presence of positive nervous system histology |
| Probable | Clinical syndrome suggestive of neurosarcoidosis with laboratory support for CNS inflammation (elevated levels of CSF protein and/or cells, the presence of oligoclonal bands and/or MRI evidence compatible with neurosarcoidosis) and exclusion of alternative diagnoses together with evidence for systemic sarcoidosis (either through positive histology, including Kveim test, and/or at least two indirect indicators from Gallium scan, chest imaging and serum ACE) |
| Possible | Clinical presentation suggestive of neurosarcoidosis with exclusion of alternative diagnoses where the above criteria are not met |
CNS central nervous system; MRI magnetic resonance imaging; ACE angiotensin-converting enzyme
Table 2.
Diagnostic criteria for intraocular sarcoidosis proposed by IWOS.8
|
Definite ocular sarcoidosis Biopsy supported diagnosis with a compatible uveitis |
|
Presumed ocular sarcoidosis Biopsy not done; presence of bilateral hilar lymphadenopathy (BHL) with a compatible uveitis |
|
Probable ocular sarcoidosis Biopsy not done and BHL negative; presence of three of the suggestive intraocular signs* and two positive investigational tests** |
|
Possible ocular sarcoidosis Biopsy negative, four of the suggestive intraocular signs* and two of the investigations are positive** |
*Suggestive intraocular signs
(1) mutton-fat keratic precipitates (KPs)/small granulomatous KPs and/or iris nodules
(2) trabecular meshwork (TM) nodules and/or tent-shaped peripheral anterior synechiae (PAS)
(3) vitreous opacities displaying snowballs/strings of pearls
(4) multiple chorioretinal peripheral lesions
(5) nodules and/or segmental peri-phlebitis and/or retinal microaneurysm in an inflamed eye
(6) optic disc nodule(s)/granuloma(s) and/or solitary choroidal nodule
(7) bilaterality
**Laboratory investigations
(1) negative tuberculin skin test in a BCG-vaccinated patient or one with prior positive test
(2) elevated serum angiotensin converting enzyme levels and/or elevated serum lysozyme
(3) chest x-ray revealing BHL
(4) abnormal liver enzyme tests
(5) chest computed tomography scan in patients with a negative chest x-ray result
Case series
Case 1
A 59-year-old Caucasian female presented with painless progressive vision loss in her left eye (OS) after an accidental fall. Magnetic resonance imaging (MRI) of the brain and orbits revealed enlargement and enhancement of the left intraorbital and intracanalicular optic nerve. A diagnosis of “traumatic optic neuropathy” was made, and oral corticosteroids were started at 80 mg per day for one month. Past medical history included hypothyroidism, hypertension, and hyperlipidemia, controlled with medication.
Six weeks later, her vision improved from 20/60 to 20/20 OS. She had a left relative afferent pupillary defect (RAPD) and an inferior nasal step OS. Her cup-to-disc ratio was 0.3 in the right eye (OD) and 0.6 OS without disc oedema or pallor.
She returned four months later complaining of worsening vision OS. Her visual acuity was 20/20 OD and count fingers OS. The prior left RAPD persisted. The left visual field examination revealed a worsening inferior nasal step with new central loss. Funduscopic examination revealed peripapillary atrophy and temporal pallor of the left optic nerve without oedema. Cranial and orbital MRI with contrast showed symmetric enlargement of the intracanalicular portion of the left optic nerve with a “tram-track” appearance. MRI confirmed an enhancing mass lesion encircling the left optic nerve (Figure 1a–b). Computed tomography (CT) of the chest with contrast showed no mediastinal or hilar lymphadenopathy. Positive laboratory findings included antinuclear antibody (ANA) titres of 1:160 in a speckled pattern. Other laboratory tests were unremarkable including angiotensin converting enzyme (ACE), lysozyme, liver function tests (LFT), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), complete metabolic panel (CMP), complete blood count (CBC), and coagulation panel. A diagnosis of possible “optic nerve sheath meningioma” (ONSM) was made. Optic nerve sheath biopsy, however was non-diagnostic, showing dense collagenous tissue consistent with dura, and did not confirm ONSM. She underwent 30 sessions of stereotactic external beam radiation therapy to treat presumed ONSM and concurrent intravenous (IV) corticosteroid treatment throughout the year. Her vision remained count fingers OS. A gallium scan revealed abnormal uptake in the right mid-neck region, with parajugular lymphadenopathy measuring 1.8 × 0.9 cm (Figure 1d) with normal uptake in the lacrimal glands. MRI of the cervical spine revealed an enhancing nodule at C3. Finally, positron emission tomography (PET) showed further bilateral lymphadenopathy in the neck. She declined biopsy, and interim follow-up was unremarkable. Three years following her initial presentation, she presented with new bilateral fullness below the jaw. Serial chest x-rays and ACE levels were negative, but CT with contrast of the neck revealed new pathologic submandibular lymphadenopathy measuring 2.2 × 2.3 cm on the left and 1.6 × 1.9 cm on the right. Biopsy of the left submandibular lymph node indicated non-caseating granulomatous inflammation (Figure 1g). A diagnosis of sarcoidosis was subsequently made. The patient declined steroids or immunosuppression. She has not had any new symptoms and her most recent vision was 20/20 OD and hand motion OS. Repeat imaging showed resolution of the previously noted sheath enhancement consistent with treated sarcoidosis of the optic nerve sheath rather than a neoplastic process such as ONSM.
Figure 1.
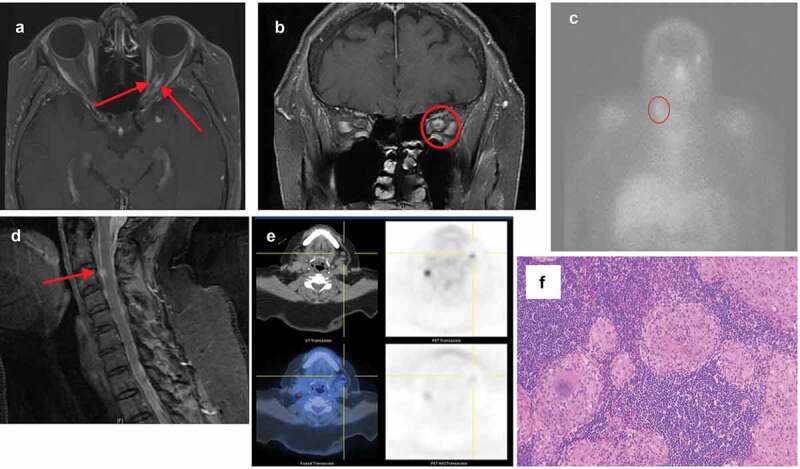
Case 1. (a) Axial T1 MRI showing mid-orbital thickening and enhancement of the left optic nerve sheath with a “tram-tracking” appearance. (b) Coronal T1 MRI at the posterior orbital apex showing soft-tissue enhancing mass surrounding the left optic nerve. (c) Gallium scan showing lymphadenopathy in the right cervical parajugular level III region. (d) Spinal sagittal T1 MRI showing an enhancing nodule at the level of C3. (e) PET scan showing bilateral neck lymphadenopathy. (f) Histological biopsy specimen with Hematoxylin-eosin staining of the left submandibular lymph node showing non-caseating granulomatous inflammation with focal necrosis and hyalinisation.
Case 2
A 50-year-old Caucasian woman developed left upper eyelid ptosis of one-month duration with increasing retrobulbar fullness, worsening proptosis and tearing OS. Her past ocular history included left high myopia and left presumed strabismic amblyopia OS (best corrected visual acuity 20/200 OS). She was otherwise healthy and took no medications. Extraocular motility exam of the left eye revealed normal adduction but all other ductions were impaired OS. The right eye moved normally. There was a 3 mm ptosis of the left upper eyelid (Figure 2a) and 3 mm of newly acquired left proptosis. There was a left RAPD and generalised left visual field depression. Slit lamp examination disclosed mild conjunctival chemosis OS. The fundus examination revealed chorioretinal striae nasal to the optic disc in the setting of a myopic fundus. The right eye was entirely normal on examination. MRI of the brain and orbits with contrast revealed marked thickening of extraocular muscles (EOM) and tendons with a lesser involvement of the lateral rectus muscle on the left side (Figure 2b). The lateral wall of the cavernous sinus was involved. Medial rectus muscle biopsy showed histologic findings of non-caseating granuloma consistent with orbital sarcoidosis (Figure 2c–d).
Figure 2.
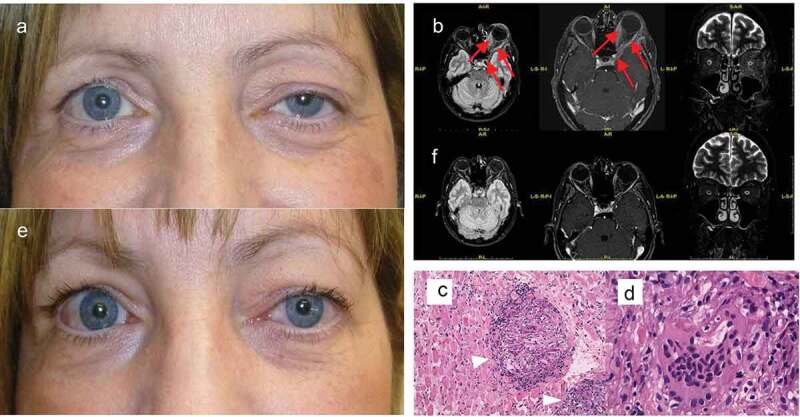
Case 2. (a) External appearance at presentation notable for left proptosis and upper eyelid ptosis. (b) After three months of steroid treatment the ptosis reduced. Brain MRI (Left: axial FLAIR; Middle: axial T1-weighted; Right: coronal long TE). (c) At presentation there is involvement of EOMs and tendons associated with a 3 mm proptosis of the left eye. (d) Three months later, after steroid treatment, there is improvement of proptosis and EOMs have returned to normal limits. Hematoxylin-eosin staining of the medial rectus muscle biopsy. (e) Multiple non-caseating sarcoid-type granulomas (arrowheads; magnification 20x) suggestive of sarcoidosis. (f) Higher magnification (63x) of a multinucleated giant cell.
Similar to case 1, systemic examination and testing including ACE, lysozyme, and high-resolution CT chest scan were unremarkable. The patient was treated with intravenous methylprednisolone followed by an oral prednisone taper with marked improvement of ptosis and proptosis (Figure 2e). Repeat MRI showed reduced EOM calibre (Figure 2f) and the patient improved clinically.
Case 3
A 49-year-old Caucasian female presented with an acutely painful right eye and progressive swelling accompanied by decreased vision OD. She was given daily oral prednisone 60 mg with some improvement subjectively. Five months later, she presented with chronic generalised abdominal pain, anorexia, and weight loss of sixty pounds.
Her past medical history was significant for hypertension and gastric ulcers, both managed with medication. Past family history was significant for glaucoma and amyotrophic lateral sclerosis. She smoked cigarettes for many years and drank alcohol but denied drug use.
Her visual acuity was 20/30 OD and 20/20 OS. Her right eye had impaired supraduction and abduction. Left eye motility was full. She had right upper and lower eyelid oedema, ptosis, fullness of the inferior fat pads, and decreased retropulsion of the globe. There was no lid lag, lid retraction, or obvious proptosis. Confrontation visual fields, pupillary examination and intraocular pressures bilaterally were normal. The right conjunctiva was chemotic inferiorly and showed diffuse injection that did not blanch with phenylephrine. The remainder of the slit lamp exam was normal.
The initial laboratory testing including CBC, CMP, LFTs, calcium, lactic acid, and lipase were unremarkable. Neuroimaging showed bilateral proptosis, enlarged EOM bellies, and prominent intraorbital fat with enhancement of intermuscular septum in the right orbit (Figure 3a). Abdominal imaging, performed to evaluate the lack of appetite and weight loss, showed abnormal soft tissue infiltration in the periaortic retroperitoneum concerning for fibrosis or malignancy that encased the coeliac trunk, superior mesenteric artery, right renal artery, and likely, the right ureter (Figure 3b).
Figure 3.
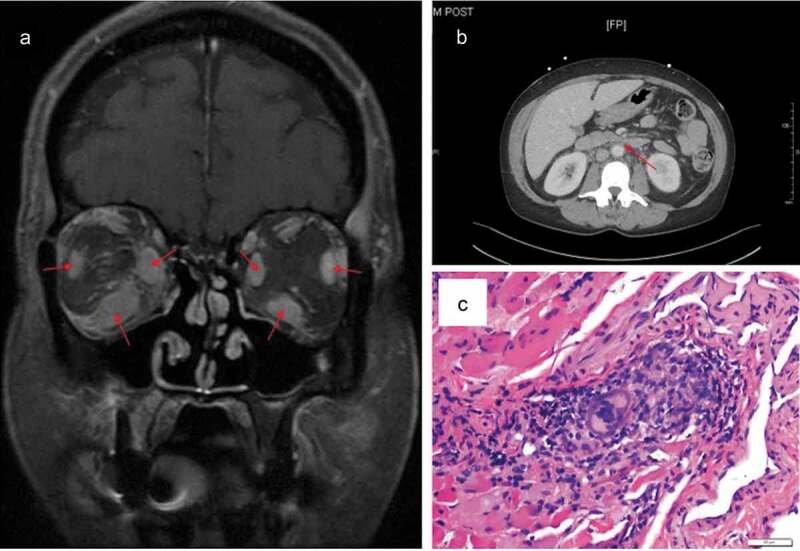
Case 3. (a) T1 fat-saturated coronal MRI of the orbit shows enlarged muscle bellies inferiorly, medially, and laterally in both eyes. The orbital fat and septum are enhancing. (b) CT of the abdomen reveals diffuse retroperitoneal fibrosis encasing the coeliac trunk, superior mesenteric artery, and right renal artery. (c) Hematoxylin-eosin stain of specimen obtained from the right lateral rectus showing infiltration of histiocytes, lymphocytes, and giant cells in skeletal muscle.
The patient was admitted to the hospital to rule out neoplastic, infectious, or autoimmune processes. Serum studies were negative for thyroid disease, sarcoidosis (ACE and lysozyme), tuberculosis, fungal disease, syphilis, hepatitis and HIV. Laboratory work up revealed elevated CRP of 17.05 mg/L and ESR of 52 mm/hr. Flow cytometry of the peripheral blood showed neither monoclonal B cells nor aberrant T cell antigen expression. She had normal levels of ANA, anti-neutrophil cytoplasmic antibodies, IgG subclasses, lactate dehydrogenase, SSA and SSB antibodies, C3, and C4. Additional neck and chest imaging revealed nonspecific bilateral hilar lymph nodes and no cervical lymphadenopathy.
A biopsy of the right lateral rectus muscle was performed, which revealed non-caseating granulomatous inflammatory infiltrates with lymphocytes, histiocytes, and giant cells (Figure 3c) consistent with orbital sarcoidosis. The patient was restarted on 60 mg of daily systemic steroids for biopsy-proven sarcoidosis for one month and noted marked improvement of all of her symptoms.
Case 4
A 65-year-old Caucasian female presented with chronic, recurrent, unilateral non-granulomatous anterior uveitis and recurrent macular oedema despite intravitreal and topical steroid treatment. Her past ocular history was significant for prior retinal tear treated with laser OS. The patient presented to the neuro-ophthalmology service with a new left-sided ptosis and miosis and a pharmacologically confirmed Horner syndrome OS. Head and neck imaging were unremarkable. CT scan of the chest showed mediastinal and bilateral hilar lymphadenopathy with calcifications. She underwent bronchoscopy with biopsy, which was non-diagnostic. Non-caseating granulomas were not seen. Interferon gamma release assay (QuantiFERON gold) was negative. Serum ACE was mildly elevated to 70 U/L (Ref: 9–67 U/L).
One year later, she complained of mild blurry vision in the left eye. On exam, a 2-mm left-sided ptosis with anhidrosis in a VI distribution was observed. The pupils measured 5 mm OD and 4 mm OS in the dark and 2.5 mm OD and 2 mm OS in the light. There was no RAPD. Her vision was 20/20 in both eyes. There was no anterior or posterior segment inflammation, macular oedema, vitreous cells, or peri-phlebitis. Her serum ACE was 56 U/L and calcium was 8.6 mg/dL.
At a follow-up appointment with her pulmonologist 6 months later, CT of the chest showed interval development of speculated nodules throughout bilateral lung fields and mediastinal lymphadenopathy. A second bronchoscopy with biopsy showed non-caseating granulomas consistent with sarcoidosis. Ipsilateral lymphadenopathy that directly infiltrated the sympathetic chain was suspected as the cause of Horner syndrome OS.
Discussion
Efforts to achieve diagnostic criteria for ocular sarcoidosis were previously made in Japan in 1991 by the Japanese Society for Sarcoidosis and Other Granulomatous Disorders (JSSOG)10 and were recently revised by the same group,11 but the universal applicability of their recommendations is unclear. Here we describe four cases of neuro-ophthalmic sarcoidosis confirmed by biopsy proven non-caseating granulomas.
Neurosarcoidosis is an uncommon aetiology of intracranial CNS lesions, and neurologic manifestations can include mass effect, cranial neuropathies, seizures and myelopathies.3,11 Ing, et al.12found less than twenty known cases of isolated neurosarcoidosis of the optic nerve. In the majority (14/18, 77.8%) of those cases, the leading presumptive diagnosis was ONSM, which is still the leading cause of progressive visual loss in a woman with unilateral optic nerve enlargement and optic disc pallor in the absence of other systemic symptoms.12–15 Without biopsy, differentiating neurosarcoidosis from ONSM is a challenge. With regard to our first case, there are reports of cases of ONSM that show reduced optic nerve swelling and decrease in tumour size following radiation therapy, but the enhancement of the optic nerve, proptosis, and ophthalmoplegia often persist.16,17 Given the lymph node biopsy findings of non-caseating granulomatous inflammation and the complete resolution of the patient’s symptoms and radiographic findings after administration of IV steroids, we feel sarcoidosis was the most appropriate diagnosis.
In a series of 20 patients with biopsy-proven neuro-ophthalmic sarcoidosis, only one (5%) had cavernous sinus involvement.18 Our patient in case 2 was treated with intravenous steroids, and resolution of symptoms was achieved without recurrence during follow-up. This particular case shows sarcoidosis presenting as an isolated unilateral orbital and cavernous sinus disease without systemic signs or symptoms. Serum ACE test, lysozyme, and high-resolution CT chest were unremarkable, making the diagnosis of sarcoidosis very challenging. The sensitivity and specificity of serum ACE and lysozyme are 0.55 & 0.88 and 0.82 & 0.50, respectively.19 The decision to perform a biopsy of the medial rectus was driven by the atypical presentation of EOM involvement causing proptosis. The most common cause of proptosis is thyroid eye disease, which typically involves the inferior or medial recti and presents with conjunctival injection, chemosis, and clinical or laboratory evidence of hyperthyroidism,20 which were not evident in our patient. Lateral rectus involvement is very uncommon in thyroid eye disease, so the finding of non-caseating granulomas on biopsy was critical in the diagnosis. Additionally, steroids may be of little to no benefit after the conclusion of the acute inflammatory phase of thyroid eye disease,20 but our patient responded exceptionally well to steroid therapy months after development of proptosis.
The patient in case 3 presented with an acute unilateral painful red eye with retroperitoneal fibrosis. Serum ACE level and chest x-ray were both negative in this patient. Extraocular muscle infiltration in sarcoidosis is a rare phenomenon. Hayashi, et al.21 reviewed eight separate cases of EOM sarcoidosis. In all cases, organ systems other than the eye were involved: the lung; lacrimal gland; and skeletal muscle. Despite the atypical presentation of this patient, she responded favourably to steroids. This case is the first published instance of neuro-ophthalmic sarcoidosis accompanied by retroperitoneal fibrosis. Though eye pain is not a particularly common finding in sarcoidosis, it can occur due to ciliary spasm or increased intraocular pressure.
While serum ACE levels have proven useful in monitoring the course of patients with sarcoidosis, the normal range of ACE is quite broad, and normal ACE levels could be seen in patients with the disease.22 It has also been suggested that the absolute serum ACE level is less important than a change in serum ACE level as a marker of disease activity.22 Liver function tests are also sometimes ordered because occult granulomatous inflammation can develop in the liver, but there is very little literature on the importance of this test in the workup of neuro-ophthalmic sarcoidosis.
The clinical spectrum of neuro-ophthalmic sarcoidosis was previously investigated in two large case series.9,23 In the first, more than half of the cases presented with a neuro-ophthalmic symptom as the sole manifestation of sarcoidosis.9 In the second, many of the classic fundal findings of periphlebitis and optic nerve granuloma were typically absent.23 Therefore, aggressive diagnostic evaluation may help establish the diagnosis early in its course. Between the two series, 39 patients with neuro-ophthalmic sarcoidosis were identified. Taken together, the most sensitive diagnostic tests included gallium scan (82%), brain MRI (76%), and CXR (70%). ACE, pulmonary function tests, lumbar puncture, and urine calcium were of variable sensitivity among their patients. Cerebrospinal fluid (CSF) analysis is often performed during a workup for neurosarcoidosis, but the findings are variable. In biopsy confirmed cases, elevated CSF cell count occurs in 54%, elevated CSF protein in 64%, elevated IgG in 43%, oligoclonal bands in 34%, and decreased glucose in 14%.24 On the contrary, most infections of the CSF will result in elevated opening pressure, very high cell counts regardless of the kind of causative organism, positive stains or cultures, and also elevated CSF protein.25 CSF findings in neoplastic disease are similar to infectious aetiologies but may have either mononuclear or mixed pleocytosis, and neoplastic cells may be seen on cytology.25
Ocular findings are more common in patients with neuro-ophthalmic sarcoidosis than neurosarcoidosis (31%) or systemic sarcoidosis (26%).4,26–30 One potential reason for this may be that neuro-ophthalmic sarcoidosis tends to be examined by people with ophthalmic training, whereas neurosarcoidosis does not. Neurologists without access to slit lamp biomicroscopy or dilated funduscopy may not detect signs of prior or mild active uveitis or other ocular signs such as conjunctival or iris nodules or retinal/choroidal findings, so the reported incidence rate may be falsely low. Nonetheless, what we have aimed to show with this case series is that clinicians should not forget sarcoidosis as a possible aetiology of neuro-ophthalmic disorders when other more common diagnoses are excluded. To this end, we have combined the findings of our case series with the findings in similar case series and the diagnostic criteria of intra-ocular sarcoidosis8 and neurosarcoidosis7 to propose a set of guidelines that may assist clinicians with identification of neuro-ophthalmic neurosarcoidosis (Table 3). If we consider all patients diagnosed with neuro-ophthalmic sarcoidosis from our case series, the series by Koczman, et al.,18 and the two series by Frohman, et al.,9,23 then our diagnostic criteria for “probable” neuro-ophthalmic sarcoidosis (Table 3) captures 67% of the 63 total cases. It should be noted, however, that not all 63 patients underwent all available testing, so this percentage is likely an underestimate. We present four cases of neuro-ophthalmic sarcoidosis. We proposed diagnostic criteria to bridge the gap between the ophthalmic criteria and the neurosarcoidosis criteria for use in patients with neuro-ophthalmic sarcoid. To our knowledge, this is the first such attempt in the English language ophthalmic literature.
Table 3.
Proposed diagnostic guidelines for neuro-ophthalmic sarcoidosis.
| Definite | Presence of non-caseating granulomatous inflammation on tissue biopsy and neuro-ophthalmic involvement with clinical features compatible with sarcoidosis | |
| Probable | Clinical syndrome suggestive of neuro-ophthalmic sarcoidosis in the absence of a confirmatory biopsy with each of the following:
|
|
EOM extraocular muscle; MRI magnetic resonance imaging; CXR chest x-ray; CT computed tomography; PFT pulmonary function test; ACE angiotensin-converting enzyme
Declaration of interest statement
The authors report no conflicts of interest.
References
- 1.Stern BJ, Aksamit A, Clifford D, Scott TF.. Neurologic presentations of neurosarcoidosis. Neurol Clin. 2010;28(1):185–198. doi: 10.1016/j.ncl.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 2.Allen RKA, Stellars RE, Sandstrom PA. A prospective study of 32 patients with neurosarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2003;20:118–215. [PubMed] [Google Scholar]
- 3.Lacomis D. Neurosarcoidosis. Curr Neuropharmacol. 2011;9(3):429–436. doi: 10.2174/157015911796557975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jabs DA, Johns CJ. Ocular involvement in chronic sarcoidosis. Am J Ophthalmol. 1986;102(3):297–301. doi: 10.1016/0002-9394(86)90001-2. [DOI] [PubMed] [Google Scholar]
- 5.Braswell RA, Kline LB. Neuro-ophthalmologic manifestations of sarcoidosis. Int Ophthalmol Clin. 2007;47:67–77. doi: 10.1097/IIO.0b013e3181571d65. [DOI] [PubMed] [Google Scholar]
- 6.Stewart P, Jumper MJ. Trans-scleral biopsy of choroidal lesions. In: Elliot D, Rao PK, eds. Surgical Management of Intraocular Inflammation and Infection. London: JP Medical Ltd; 2013:51–55. [Google Scholar]
- 7.Zajicek JP, Scolding NJ, Foster O, et al. Central nervous system sarcoidosis – diagnosis and management. Q J Med. 1999;92(2):103–117. doi: 10.1093/qjmed/92.2.103. [DOI] [PubMed] [Google Scholar]
- 8.Herbort CP, Rao NA, Mochizuki M. International criteria for the diagnosis of ocular sarcoidosis: results of the International Workshop on Ocular Sarcoidosis (IWOS). Ocular Immunol Inflamm. 2009;17(3):160–169. doi: 10.1080/09273940902818861. [DOI] [PubMed] [Google Scholar]
- 9.Frohman LP, Grigorian R, Bielory L. Neuro-ophthalmic manifestations of sarcoidosis: clinical spectrum, evaluation, and management. J Neuroophthalmol. 2001;21(1):132–137. doi: 10.1097/00041327-200106000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Goto H, Mochizuki M, Yamaki K, Kotake S, Usui M, Ohno S. Epidemiological survey of intraocular inflammation in Japan. Jpn J Ophthalmol. 2007;51(1):41–44. doi: 10.1007/s10384-006-0383-4. [DOI] [PubMed] [Google Scholar]
- 11.Research Committee of Diffuse Pulmonary Disorders of the Ministry of Health and Welfare of Japan . Diagnostic criteria of sarcoidosis. Jpn J Sarcoidosis Granulomatous Disord. 1991;10:159–162. [Google Scholar]
- 12.Ing EB, Garrity JA, Cross SA, Ebersold MJ. Sarcoid masquerading as optic nerve sheath meningioma. Mayo Clin Proc. 1997;72(1):38–43. doi: 10.1016/S0025-6196(11)64728-9. [DOI] [PubMed] [Google Scholar]
- 13.Elia M, Kombo N, Juang J. Neurosarcoidosis masquerading as a central nervous system tumor. Retin Cases Brief Rep. 2017;11:S166–S169. doi: 10.1097/ICB.0000000000000402. [DOI] [PubMed] [Google Scholar]
- 14.Jennings JW, Rojiani AM, Brem SS, Murtagh FR. Necrotizing neurosarcoidosis masquerading as a left optic nerve meningioma: a case report. Am J Neuroradiol. 2002;23:660–662. [PMC free article] [PubMed] [Google Scholar]
- 15.Tobias S, Prayson RA, Lee JH. Necrotizing neurosarcoidosis of the cranial base resembling an en plaque sphenoid wing meningioma: case report. Neurosurgery. 2002;51(5):1290–1294. doi: 10.1227/01.NEU.0000033461.20649.C2. [DOI] [PubMed] [Google Scholar]
- 16.Paridaens ADA, van Ruyven RLJ, Eijkenboom WMH, Mooy CM, van Den Bosch WA. Stereotactic radiation of biopsy proved optic nerve sheath meningioma. Br J Ophthalmol. 2003;87(2):246–247. doi: 10.1136/bjo.87.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moyer PD, Golnik KC, Breneman J. Treatment of optic nerve sheath meningioma with three-dimensional conformal radiation. Am J Ophthalmol. 2000;129(5):694–696. doi: 10.1016/S0002-9394(99)00477-8. [DOI] [PubMed] [Google Scholar]
- 18.Koczman JJ, Rouleau J, Gaunt M, Kardon RH, Wall M, Lee AG. Neuro-ophthalmic sarcoidosis: the University of Iowa experience. Semin Ophthalmol. 2008;23(3):157–168. doi: 10.1080/08820530802007382. [DOI] [PubMed] [Google Scholar]
- 19.Grajewski RS, Adler W, Frank KF, et al. Predictive value of serum markers for pulmonary involvement in ocular sarcoidosis. Acta Ophthalmol. 2014;92(3):e250–e251. doi: 10.1111/aos.12248. [DOI] [PubMed] [Google Scholar]
- 20.Li Z, Cestari DM, Fortin E. Thyroid eye disease: what is new to know? Curr Opin Ophthalmol. 2018;29:528–534. doi: 10.1097/ICU.0000000000000529. [DOI] [PubMed] [Google Scholar]
- 21.Hayashi Y, Ishii Y, Nagasawa J, et al. Subacute sarcoid myositis with ocular muscle involvement. A case report and review of the literature. Sarcoidosis Vasc Diffuse Lung Dis. 2016;33:297–301. [PubMed] [Google Scholar]
- 22.DeRemee RA, Rohrbach MS. Normal serum angiotensin converting enzyme activity in patients with newly diagnosed sarcoidosis. Chest. 1984;85(1):45–48. doi: 10.1378/chest.85.1.45. [DOI] [PubMed] [Google Scholar]
- 23.Frohman LP, Guirgis M, Turbin RE, Bielory L. Sarcoidosis of the anterior visual pathway: 24 new cases. J Neuroophthalmol. 2003;23(3):190–197. doi: 10.1097/00041327-200309000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Wengert O, Rothenfusser-Korber E, Vollrath B, et al. Neurosarcoidosis: correlation of cerebrospinal fluid findings with diffuse leptomeningeal gadolinium enhancement on MRI and clinical disease activity. J Neurol Sci. 2013;335(1–2):124–130. doi: 10.1016/j.jns.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Koski RR, Van Loo D. Etiology and management of chronic meningitis. US Pharm. 2010;35:HS-2-HS-8. [Google Scholar]
- 26.Stern BJ, Krumholz A, Johns C, Scott P, Nisim J. Sarcoidosis and its neurological manifestations. Arch Neurol. 1985;42(9):909–917. doi: 10.1001/archneur.1985.04060080095022. [DOI] [PubMed] [Google Scholar]
- 27.Chapelon C, Ziza JM, Piette JC, et al. Neurosarcoidosis: signs, course and treatment in 35 confirmed cases. Medicine (Baltimore). 1990;69:261–276. [PubMed] [Google Scholar]
- 28.Oksanen V, Gronhagen-Riska C, Fyhrquist F, Somer H. Systemic manifestations and enzyme studies in sarcoidosis with neurologic involvement. Acta Med Scand. 1985;218(1):123–127. doi: 10.1111/j.0954-6820.1985.tb08835.x. [DOI] [PubMed] [Google Scholar]
- 29.Sharma OP. Neurosarcoidosis: a personal perspective based on the study of 37 patients. Chest. 1997;112(1):220–228. doi: 10.1378/chest.112.1.220. [DOI] [PubMed] [Google Scholar]
- 30.Lower EE, Broderick JP, Brott TJ, Baughman RP. Diagnosis and management of neurological sarcoidosis. Arch Intern Med. 1997;157(16):1864–1868. doi: 10.1001/archinte.1997.00440370104011. [DOI] [PubMed] [Google Scholar]


