ABSTRACT
This study aimed to investigate pupillary involvement in patients with type 2 diabetes mellitus (DM) and to evaluate whether there is a relationship between severity of diabetic retinopathy (DR) and pupillary responses. The study included 133 individuals in four groups: proliferative DR, non-proliferative DR, DM group without retinal involvement and a control group. Static pupillometry measurements including scotopic pupil diameter (PD), mesopic PD, low photopic PD, high photopic PD, and dynamic pupillometry measurements, including resting diameter, amplitude, latency, velocity, duration of pupil contraction and latency, duration, and velocity of pupil dilatation were taken using an automated quantitative pupillometry system. The correlations between glycosylated haemoglobin values and duration of DM with these parameters were also investigated. The study showed that patients with DR may also have diabetic autonomic neuropathy and pupillometry can be a useful screening tool for detecting diabetic autonomic neuropathy.
KEYWORDS: Diabetes mellitus, diabetic retinopathy, dynamic pupillometry, pupil diameter, static pupillometry
Background
Diabetes mellitus (DM) is a very common metabolic disorder, and its prevalence is on the rise in developing countries as a result of dietary habits.1 Many cases of DM go undiagnosed until complications become clinically evident.2,3 DM can cause diabetic retinopathy (DR), diabetic macular oedema, vitreous haemorrhage, neovascular glaucoma, retinal detachment, and eventual blindness.4 Early detection of DR and timely and effective treatment are the key factors in the prevention of extensive vision loss.5
Diabetic autonomic neuropathy (DAN) is one of the least known and recognised complications of DM.6 DAN is generally subclinical and is an early consequence of DM. It negatively impacts quality of life and overall survival in DM patients.7,8 Hence, early clinical detection and precautions are necessary to help people with DM to live better.8 Both the parasympathetic and sympathetic divisions of the autonomic nervous system control pupillary responses. Therefore, pupillary responses to an external light stimulus may give us an indirect mean to assess the integrity of the neuronal pathways controlling pupil size.9 While reduced pupillary size and attenuated light responses are already recognised features of the autonomic nervous system damage that occurs in DM, pupillometry is a useful non-invasive method for screening autonomic dysfunction and has the potential to improve screening for DM and its complications without the need for a blood test.10,11
Recent developments in automated pupillometry devices have enabled objective, quantitative, non-invasive and repeatable measurements of static and dynamic pupillary responses.12 Patients with DM have been shown to have smaller resting pupil sizes and reflex amplitudes than those without this condition even before the disease is clinically apparent.10,13–15 While the static pupil diameter has been shown to be affected by DM, the dynamic pupil responses (latency, duration of contraction and dilatation, dilatation speed) have not been studied extensively. Although Park et al.14 and Jain et al.15 investigated the pupil responses in DM patients, they did not evaluate the static and dynamic pupil responses extensively in different illumination conditions. Additionally, the relationship between duration of DM and glycosylated haemoglobin (HbA1c) levels with pupil responses has not been determined elaborately previously.
From this perspective, we aimed to assess the relationship between static and dynamic pupillary responses and the severity of retinopathy and to determine whether a change in pupillary responses is useful for monitoring type 2 DM.
Methods
This cross-sectional prospective study was conducted at a tertiary eye hospital from May 2017 to December 2017. The study protocol was approved by the institutional board of the ethics committee (E-17-1542), and we carried out the study in accordance with the ethical standards of the Declaration of Helsinki. We obtained written informed consent from all participants prior to enrolment.
This study included four groups, as follows: Group 1 included newly diagnosed patients with treatment-naïve proliferative DR without diabetic macular oedema; Group 2 included patients with non-proliferative DR without diabetic macular oedema; Group 3 included patients with DM but without any retinal involvement and Group 4 included age- and sex-matched control subjects (control group). The presence and stages of the DR in the patients with DM were investigated using fundus photography, fundus fluorescein angiography, and optical coherence tomography, with all investigations being carried out by the same experienced retina specialist (MAS). The Early Treatment Diabetic Retinopathy Study criteria were used to describe the various stages of DR.16 All control subjects were healthy, without any systemic or ocular diseases, and were seen in the ophthalmology clinic for routine ophthalmic examination. In the DM cases, blood samples were taken for the measurement of HbA1c levels on the same day as the pupillometry measurements were taken. Detailed ophthalmic and systemic histories were collected, including the duration, or the time since the diagnosis of type 2 DM was made for each patient.
Patients were excluded from the study if they had any ocular disease other than DR or any systemic disorders other than type 2 DM. Moreover, we excluded patients who had used anticholinergic drugs for urinary symptoms or anti-prostate drugs such as alfuzosin, prazosin, or tamsulosin. Subjects with any of the following conditions that can affect pupillary motility were also excluded: iris or pupil anomalies including coloboma, synechia, rubeosis iridis, sphincter tear and anisocoria; pseudoexfoliation syndrome; glaucoma; a history of head or orbital trauma; ocular or orbital inflammation; a history of orbital and ocular surgery; intravitreal injection; laser treatment; use of topical medications which can affect iris mechanics, including tropicamide, pilocarpine, cyclopentolate and narcotic-derived medications and neurological or other diseases of the visual pathways. Subjects who were not cooperative enough to undergo pupillometry measurements were also excluded.
Each participant received a standard ophthalmologic examination, including the best-corrected visual acuity test using the Snellen chart, an intraocular pressure measurement using a pneumotonometer, a slit-lamp biomicroscopy examination, and a dilated fundus examination.
The same experienced clinician performed pupillometry measurements using the same automated quantitative pupillometry system (MonPack One, Vision Monitor System, Metrovision, France). Before the pupillometry examination, no contact ocular examination and pupil dilatation were performed. Pupillometry measurements were taken at least 3 days after pupil dilatation. The quantitative pupillometry system was equipped with near-infrared illumination (880 nm) and a high-resolution camera (720 x 480 pixels) which allowed measurements to be taken from binocular pupils and to provide precise control of stimulation parameters.12 The stimulus was white, obtained from a full-field backlight combining red (632 nm), green (523 nm), and blue (465 nm) light-emitting diode sources. This system allowed the clinician to take both static and dynamic pupillometry measurements and to make accurate measurements of pupil size (accuracy = 0.1 mm).12 Three consecutive measurements were taken for each participant and their average values were calculated for data analysis. The automatic-release mode of the device was used to minimise examiner-induced errors and only the images with high quality were included in the study. To minimise the effect of circadian variation on iris mechanics,17,18 the pupillometry measurements were performed at the same time of day (between 10 a.m. and 12 p.m.) and in the same environmental conditions. During the pupil recording, participants were required to fixate on a target in the centre of the test field in order to control fixation stability. Pupil contours of participants were outlined on the image to ensure the accuracy of measurements and the proprietary analysis. The proprietary analysis software of device was used to conduct automatic static and dynamic pupillometry. This software automatically outlined the pupillary contours on the images, allowing measurements to be accurate and taken under controlled lighting conditions. Afterwards, the software made an analysis of the temporal and average responses to successive visual stimuli with automated quantification of the following parameters: latency and duration of contraction and dilatation (ms); initial, minimum, maximum, and mean pupil diameter (mm); amplitude of contraction (mm) and contraction and dilatation speed (velocity) of the pupil (mm/s) (Figure 1).
Figure 1.
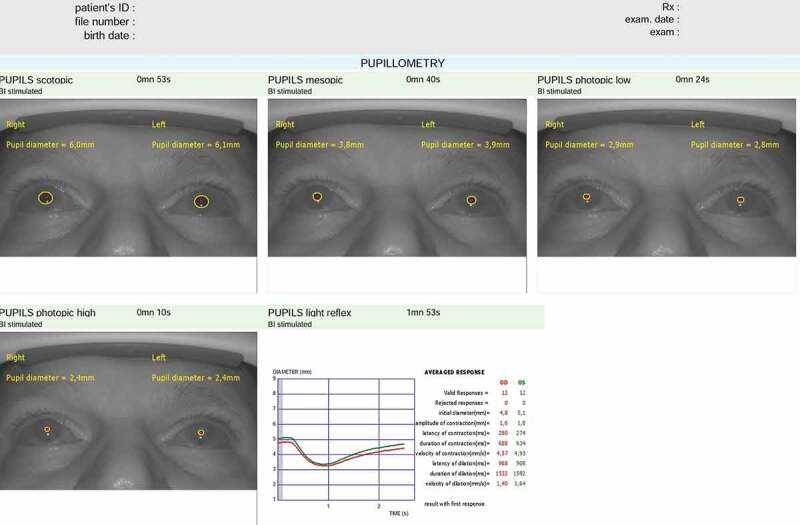
An output of static and dynamic pupillary characteristics via the automated quantitative pupillary measurement system (Vision Monitor System, Metrovision, France) is demonstrated.
Static pupillometry measurements were also performed under several illumination levels to measure pupil size in scotopic (0.1 cd/m2), mesopic (1 cd/m2), low photopic (10 cd/m2), and high photopic (100 cd/m2) vision conditions. Scotopic pupil diameter, mesopic pupil diameter, low photopic pupil diameter, and high photopic pupil diameter values were recorded. In darkness, after 5 min of darkness adaptation, dynamic pupillometry measurements were obtained for the duration of 90 s. Participants were examined using white light flashes (stimulation ON time: 200 ms; stimulation OFF time: 3300 ms). Because the stimulus on time is slower than pupil reflexes, waveforms contaminated by blinks were rejected. We acquired and processed images of each of the patients’ eyes in real time (30 images per second). A Minolta LS100 luminance metre was used to measure the luminance output and the average response to successive visual stimuli (light flashes) was measured using the following parameters: resting diameter; amplitude of pupil contraction; latency of pupil contraction; duration of pupil contraction; velocity of pupil contraction; latency of pupil dilatation; duration of pupil dilatation and velocity of pupil dilatation.
Statistical analysis
The data from the right eyes of the subjects were used for the statistical analysis using the Statistical Package for Social Sciences (SPSS) version 22.0 for Windows (SPSS Inc., Chicago, IL). Descriptive data are presented as mean values ± standard deviations, frequency distributions, and percentages. The chi-square test was used in the analysis of categorical variables. The normal distribution of the variables was tested by visual methods (histograms and probability graphs) and analytical methods (Kolmogorov–Smirnov/Shapiro–Wilk test). The equality of variances was checked by the Levene test. The one-way analysis of variance, Welch analysis of variance, and Kruskal–Wallis tests were used to determine if there were any significant differences among the four groups. Post-hoc tests for pairwise comparisons were also performed. Additionally, Pearson correlation tests were used to investigate the correlations of the pupillometry measurements with the HbA1c levels and the duration of the DM. A probability level of p < .05 was considered statistically significant.
Results
This study included 133 subjects: 21 were in the proliferative DR group; 35 were in the non-proliferative DR group; 31 were in the DM without any retinal involvement group and the remaining 46 were in the control group. Of the participants, 81 were women (60.9%), and 52 were men (39.1%). The mean ages of the cases in the proliferative DR group, non-proliferative DR group, DM without any retinal involvement group, and control group were 57.0 ± 7.0 (range: 47 to 71) years, 55.8 ± 7.8 (range: 37 to 70) years, 56.2 ± 11.0 (range: 36 to 76) years, and 57.0 ± 6.0 (range: 47 to 69) years, respectively. There were no statistically significant differences in age or gender among the groups (p > .05). The clinical and demographic characteristics and HbA1c values of the participants are displayed in Table 1.
Table 1.
Demographic characteristics and HbA1c values of all participants.
| Proliferative DR Group (n = 21) |
Non-proliferative DR Group (n = 35) |
DM non-DR Group (n = 31) |
Control Group (n = 46) |
P | |
|---|---|---|---|---|---|
| Age, years (mean±SD) | 57.0 ± 7.0 | 55.8 ± 7.8 | 56.2 ± 11.0 | 57.0 ± 6.0 | 0.246* |
| Female/Male (n/n) | 11/10 | 20/15 | 20/11 | 30/16 | 0.715** |
| Duration of DM, years | 11.2 ± 5.2 | 9.9 ± 6.0 | 6.7 ± 4.0 | _ | |
| HbA1C (%) | 8.8 ± 2.9 | 7.8 ± 2.0 | 7.1 ± 1.6 | _ |
Notes. HbA1C: Glycosylated haemoglobin; SD, Standard deviation.
*Significance in analysis of variance (comparison among four groups)
** Chi-square test
Although all static pupillometry measurements were lowest in the proliferative DR group and highest in the control group, only the low photopic pupil diameter and high photopic pupil diameter values were statistically significantly different among the groups (Table 2). The pairwise comparisons showed statistically significantly lower low photopic pupil diameter and high photopic pupil diameter values in the DM patients with proliferative DR and non-proliferative DR than in the controls. However, the DM without any retinal involvement group showed no statistically significant difference in the static pupillometry values than did the controls.
Table 2.
Static pupillometry measurements of the groups in all participants.
| Proliferative DR Group (n = 21) | Non-proliferative DR Group (n = 35) |
DM non-DR Group (n = 31) |
Control Group (n = 46) |
Pa | Pb | |
|---|---|---|---|---|---|---|
| Scotopic PD (mm) | 5.37 ± 0.94 | 5.49 ± 0.90 | 5.60 ± 0.71 | 5.79 ± 0.57 | 0.132 | |
| Mesopic PD (mm) | 4.49 ± 0.64 | 4.56 ± 0.66 | 4.67 ± 0.79 | 4.81 ± 0.88 | 0.525 | |
| Low photopic PD (mm) | 3.71 ± 0.39 | 3.80 ± 0.59 | 4.04 ± 0.63 | 4.31 ± 0.95 | <0.001 | PDR-Control: <0.001 NPDR-Control: 0.003 DM-nonDR-Control: 0.081 |
| High photopic PD (mm) | 3.17 ± 0.33 | 3.32 ± 0.50 | 3.48 ± 0.59 | 3.84 ± 0.99 | <0.001 | PDR-Control: <0.001 NPDR-Control: 0.004 DM-nonDR-Control: 0.367 |
Notes. NPDR: Non-proliferative diabetic retinopathy; PD: Pupil diameter; PDR: Proliferative diabetic retinopathy.
aSignificance in analysis of variance (comparison among four groups)
bBonferroni post hoc test
The dynamic pupillary measurements of the groups are shown in detail in Table 3. There were statistically significant differences among the groups in the mean values of the amplitude of pupil contraction, the velocity of pupil contraction, and the velocity of pupil dilatation (p < .001, p = .001, and p = .007, respectively). The pairwise comparisons showed statistically significantly lower amplitudes of pupil contraction, velocities of pupil contraction, and velocities of pupil dilatation in the DM patients with proliferative DR and non-proliferative DR than in the controls. However, the DM without any retinal involvement group showed no statistically significant difference in the dynamic pupillometry values than did the controls.
Table 3.
Dynamic pupillometry measurements of the groups in all participants.
| Proliferative DR Group (n = 21) |
Non-proliferative DR Group (n = 35) |
DM non-DR Group (n = 31) |
Control Group (n = 46) |
Pa | Pb | |
|---|---|---|---|---|---|---|
| Resting diameter (mm) | 5.06 ± 0.94 | 5.13 ± 0.74 | 5.15 ± 0.60 | 5.32 ± 0.53 | 0.391 | |
| Amplitude of pupil contraction (mm) | 0.99 ± 0.44 | 1.22 ± 0.43 | 1.46 ± 0.30 | 1.53 ± 0.39 | <0.001 | PDR-Control: <0.001 NPDR-Control: 0.001 DM-nonDR-Control: 0.370 |
| Latency of pupil contraction (ms) | 213.40 ± 63.04 | 233.14 ± 56.72 | 242.46 ± 52.31 | 253.11 ± 47.37 | 0.278 | |
| Duration of pupil contraction (ms) | 702.81 ± 207.91 | 679.34 ± 193.37 | 650.33 ± 152.15 | 645.53 ± 143.13 | 0.329 | |
| Velocity of pupil contraction (mm/s) | 3.87 ± 1.83 | 4.79 ± 1.88 | 5.18 ± 1.24 | 5.31 ± 1.29 | 0.001 | PDR-Control: <0.001 NPDR-Control: 0.005 DM-nonDR-Control: 0.655 |
| Latency of pupil dilatation (ms) | 975.91 ± 211.61 | 893.16 ± 153.92 | 882.74 ± 118.62 | 923.35 ± 115.72 | 0.293 | |
| Duration of pupil dilatation (ms) | 1532.14 ± 207.34 | 1483.38 ± 282.03 | 1421.24 ± 225.48 | 1349.74 ± 185.03 | 0.955 | |
| Velocity of pupil dilatation (mm/s) | 2.68 ± 1.59 | 2.78 ± 1.77 | 2.88 ± 1.88 | 2.98 ± 1.95 | 0.007 | PDR-Control: <0.001 NPDR-Control: 0.008 DM-nonDR-Control: 0.785 |
Notes. NPDR: Non-proliferative diabetic retinopathy; PDR: Proliferative diabetic retinopathy.
aSignificance in analysis of variance (comparison among four groups)
bBonferroni post hoc test
As shown in Figures 2 and 3 as well as in Table 4, both the duration of the DM and the HbA1c values were inversely and moderately correlated with the scotopic pupil diameter, high photopic pupil diameter, resting diameter, and velocity of pupil contraction values (p < .05 for each). Additionally, the duration of the DM was significantly correlated with the amplitude of pupil contraction (p < .001, r = −0.404) (Figure 2), and the HbA1c values were significantly correlated with low photopic pupil diameter (p = .006, r = −0.370) (Figure 3).
Figure 2.
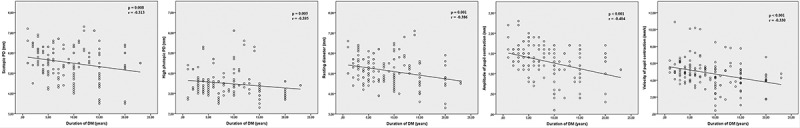
Statistically significant correlations between duration of DM with investigated static and dynamic pupillary parameters. The duration of DM shows statistically significant correlations with scotopic pupil diameter, high photopic pupil diameter, resting diameter, amplitude of pupil contraction and velocity of pupil contraction values.
Figure 3.
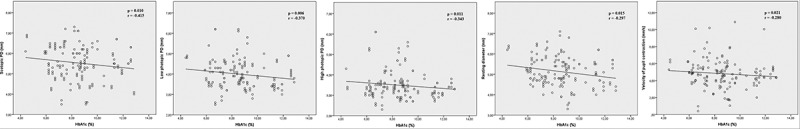
Statistically significant correlations between HbA1c levels with investigated static and dynamic pupillary parameters. The HbA1c levels show statistically significant correlations with scotopic pupil diameter, low photopic pupil diameter, high photopic pupil diameter, resting diameter and velocity of pupil contraction values.
Table 4.
Correlations between the duration of DM and HbA1c values with static and dynamic pupillometry measurements in DM patients.
| Duration of DM (years) | HbA1c values (%) | |
|---|---|---|
| Scotopic PD (mm) | r = −0.313, p = .008 | r = −0.415, p = .010 |
| Mesopic PD (mm) | r = −0.165, p = .152 | r = −0.155, p = .070 |
| Low photopic PD (mm) | r = −0.067, p = .437 | r = −0.370, p = .006 |
| High photopic PD (mm) | r = −0.395, p = .005 | r = −0.343, p = .011 |
| Resting diameter (mm) | r = −0.386, p = .001 | r = −0.297, p = .015 |
| Amplitude of pupil contraction (mm) | r = −0.404, p < .001 | r = −0.147, p = .085 |
| Latency of pupil contraction (ms) | r = 0.019, p = .825 | r = 0.057, p = .504 |
| Duration of pupil contraction (ms) | r = 0.085, p = .323 | r = −0.104, p = .225 |
| Velocity of pupil contraction (mm/s) | r = −0.330, p < .001 | r = −0.280, p = .021 |
| Latency of pupil dilation (ms) | r = 0.105, p = .222 | r = 0.220, p = .067 |
| Duration of pupil dilation (ms) | r = −0.111, p = .194 | r = −0.013, p = .878 |
| Velocity of pupil dilation (mm/s) | r = −0.076, p = .378 | r = −0.061, p = .475 |
Notes. PD: Pupil diameter; r: Pearson correlation coefficient. Bold values indicate statistically significant correlations.
Discussion
DR is a microangiopathy that results from damage to the small retinal vessels, arterioles, capillaries, and venules. Endothelial cell damage is the predisposing factor for these pathologies.19 These types of damage result in hypoxia in retinal cells. Vascular endothelial growth factor is synthesised from the retinal cells and neovascularisation, capillary leakage, and oedema can be seen. Increased levels of final products from the metabolism of sorbitol and glucose, hypercoagulability, thickening of the basal membrane and pericyte loss, reduced blood supply, and blood-retinal barrier breakdown are the other pathophysiological mechanisms of DM-related complications.19 All of these mechanisms play a role in DM-related complications such as DR, nephropathy, and DAN. In general, a five-year latent period between symptom onset and diagnosis has been defined; therefore, early detection of these complications may play a role in the prevention of morbidity and mortality.20
DAN is an early consequence of DM and is difficult to diagnose. It has a negative effect on patient quality of life and can decrease overall survival time.6 It can affect the cardiovascular, gastrointestinal, sudomotor, and ocular autonomic nerves and can result in morbidity and mortality.8,19 It is especially observed among patients with cardiac symptoms and subjects with undiagnosed cardiac autonomic neuropathy are at a much higher risk of mortality. An approach to screening DM patients for DAN is therefore necessary to minimise the social and economic burden of DAN. Pupillometry studies are performed to screen for DAN in DM patients.13,21–25 Lerner et al.22 reported that pupillometry is an inexpensive test for DAN but is not accurate enough for clinical use. Conversely, Ferrari et al.21 reported that pupillometry is useful for screening high-risk DM patients for DAN. Moreover, Yuan et al.23 compared pupillary involvement in DAN with that in non-DM causes of autonomic impairment and showed more frequent and more severe involvement in the DM cases. This study also indicated that pupillometry had significant potential as a screening tool for DAN.23 Similarly, Magure et al.24 found an association between the presence of microalbuminuria and retinopathy using baseline pupillometry tests and their study underlined the fact that pupillometry results may be an early indicator of future microvascular diseases. Pena et al.25 also found that pupillometry is a more sensitive test for DAN than the assessment of cardiovascular reflexes. We also believe that pupillometry can be useful for screening of DM-related complications and that it can provide beneficial information about the severity of DR.
Previous pupillometric studies on DM patients with and without DR indicated impaired pupil dynamics, such as reduced baseline pupil diameters and re-dilatation amplitudes due to dysfunctions in autonomic innervations.21,26,27 Ferrari et al.21 reported that DM subjects with or without cardiac autonomic neuropathy have diminished amplitude reflexes and smaller pupil radii than normals. Moreover, they indicated that pupillary autonomic dysfunction can occur before more generalised autonomic nervous system involvement. Similarly, Feigl et al.27 indicated that some pupillary changes can be detected in patients with DM even if it is not ophthalmoscopically or anatomically evident. Ortube et al.28 compared moderate to severe non-proliferative DR cases with a control group and found statistically significant differences in amplitude and constriction velocity. These values were highly correlated with the severity of the DR, but not with the duration of the DM.28 As in the study by Ortube et al.28, we detected significantly lower low photopic pupil diameter and high photopic pupil diameter values in DM patients with proliferative DR and non-proliferative DR than in controls. We also found statistically significantly lower amplitude of pupil contraction, velocity of pupil contraction, and velocity of pupil dilatation values in the DM patients with proliferative DR and non-proliferative DR than in the controls. A comparison of the DM patients who showed no DR findings with the control group also revealed lower values, but these differences were not statistically significant. The synergistic effects of retinopathy, loss of retinal ganglion cells and DAN are responsible for these abnormal pupil responses. Further investigations are needed to determine which of these factors lead to autonomic neuropathy: retinopathy, ganglion cell damage, or structural iris damage. Thus, we hypothesise that DR and DAN may be related to each other by the same pathophysiological mechanisms and that patients with DR should be carefully examined for DAN.
Similar to our study, different pupillometry systems were mostly used in studies that measured pupillary responses in DM patients. Park et al.14 investigated rod, cone, and melanopsin-mediated pupillary light reflexes in non-proliferative DR patients. They found significant alterations as the non-proliferative DR stage increased. They also indicated that chromatic pupillometry test could be used to assess neural dysfunction associated with DM.14 However, their study did not include DM patients without retinopathy, so they could not give any information about the period before the onset of retinopathy. Jain et al.15 classified the patients according to retinopathy severity and found that pupillary dynamics were abnormal in early stages of DR and progressed with increasing retinopathy severity. Similarly, we also grouped patients according to the severity of their DR and compared these groups with healthy subjects. The pupillometry system, which we used in this study, provides more comprehensive information about pupillary parameters compared with the pupillometry system used by Jain et al.15 This system allows the measurement of static pupil diameter in four different illumination conditions. It also allows the measurement of latency and duration of pupillary contraction and dilatation values. Therefore, we believe that our pupillometry system is better structured and provides more objective results. Additionally, the conclusions based on the findings of our study are more accurate. The main conclusion of our study is that DR (in parallel with the severity of DR) leads to significant changes in pupillary light responses and pupil diameter. We also found a relationship between pupillary measurements and both HbA1c levels and DM duration, which was not investigated by Jain et al.15
The possible relationship of HbA1c levels and the duration of DM with DAN has been previously studied.21,28,29 Karavanaki et al.29 used a portable pupillometer to study pupillary adaptation to darkness in children with DM as an index of sympathetic neuropathy. This study showed that mean pupillary diameter was negatively correlated with the duration of the disease and to HbA1c levels. This study also examined pupillary adaptation, grouping patients according to their HbA1c levels as having good, moderate, or poor control, and found that the group with the poorest control had the smallest mean pupillary diameter.29 However, Ferrari et al.21 did not find any statistically significant differences between cardiac autonomic neuropathy and non-cardiac autonomic neuropathy groups in the relationship of HbA1c levels and the duration of DM with pupillary measurements. Similarly, Ortube et al.27 did not find any correlation between pupillary involvements and the duration of DM. Consistent with Karavanaki et al.29, we found significant correlations, including inverse and moderate correlations of both HbA1c values and the duration of DM with the scotopic pupil diameter, high photopic pupil diameter, resting diameter, and velocity of pupil contraction values. Additionally, the duration of DM was statistically significantly correlated with the amplitude of pupil contraction and the HbA1c values were statistically significantly correlated with low photopic pupil diameter. This is unsurprising, as the HbA1c levels show effective glycaemic control and a high level of HbA1c is related to microvascular complications.
Previous studies have demonstrated that retinal laser treatments for proliferative DR can also affect pupillary anatomy and responses to light stimuli.29,30 Yilmaz et al.30 demonstrated that automated infrared pupillary measurements are significantly affected by pattern scan laser. Another study also demonstrated that pan-retinal laser photocoagulation may significantly influence pupil size but that focal/grid laser photocoagulation may not.31 In light of these findings, we selected our proliferative DR patients from among newly diagnosed, previously untreated patients so as to exclude any effects of laser treatment on pupil responses.
This study has several limitations. Because multiple factors, including systemic diseases and previous ocular treatments, may affect pupillary measurements in DM patients, the number of patients in the proliferative DR group was limited. We also did not investigate the patients’ uses of diabetic medication, including insulin doses or oral anti-diabetics. An important limitation of the study was the reporting of pupillometry measurements units in mm as opposed to reports on retinal and central disease which reported % from baseline metric.32,33 The usage of mm may also lead to spurious correlations between pupil metrics.34 The greatest strength of our study is that it is the first to report the relationship between the severity of DR and HbA1c levels, and a multitude of objective static and dynamic pupillary measurement parameters. In our study, it is an advantage that both the control group and the DM patients without retinopathy were also included.
To conclude, this study demonstrated via pupillometry that patients with DR may also have DAN. DAN is known to be associated with high morbidity and mortality rates, and all patients with DR should be referred for screening for cardiac autonomic neuropathy. We also suggest that pupillometry can be useful for detecting and screening for DAN.
Funding Statement
There is no funding for this study.
Declaration of interest statement
The authors report no conflicts of interest and have no proprietary interest in any of the materials mentioned in this article. The authors alone are responsible for the content and writing of the paper.
Ethic Committee approval
The study protocol was approved by the Institutional Board of the Ethics Committee (E-17-1542).
References
- 1.Gupta P, Verma N, Bhattacharya S, et al. Association of diabetic autonomic neuropathy with red blood cell aldose reductase activity. Can J Diabetes. 2014;38:22–25. doi: 10.1016/j.jcjd.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 2.Silva H, Hernandez-Hernandez R, Vinueza R, et al. CARMELA study investigators. Cardiovascular risk awareness, treatment, and control in urban Latin America. Am J Ther. 2010;17:159–166. doi: 10.1097/MJT.0b013e3181a84ec5. [DOI] [PubMed] [Google Scholar]
- 3.Lerner AG, Bernabe-Ortiz A, Gilman RH, Smeeth L, Miranda JJ.. The “rule of halves” does not apply in Peru: awareness, treatment, and control of hypertension and diabetes in rural, urban, and rural-to-urban migrants. Crit Pathw Cardiol. 2013;12:53–58. doi: 10.1097/HPC.0b013e318285ef60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang X, Saaddine JB, Chou CF, et al. Prevalence of diabetic retinopathy in the United States, 2005–2008. JAMA. 2010;304:649–656. doi: 10.1001/jama.2010.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heng LZ, Comyn O, Peto T, et al. Diabetic retinopathy: pathogenesis, clinical grading, management and future developments. Diabet Med. 2013;30:640–650. doi: 10.1111/dme.12089. [DOI] [PubMed] [Google Scholar]
- 6.Vinik AI, Erbas T. Recognizing and treating diabetic autonomic neuropathy. Cleve Clin J Med. 2001;68:928–944. doi: 10.3949/ccjm.68.11.928. [DOI] [PubMed] [Google Scholar]
- 7.Tesfaye S, Boulton AJ, Dyck PJ, et al. Update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33:2285–2293. doi: 10.2337/dc10-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vinik AI, Erbas T. Cardiovascular autonomic neuropathy: diagnosis and management. Curr Diab Rep. 2006;6:424–430. [DOI] [PubMed] [Google Scholar]
- 9.Fotiou F, Fountoulakis KN, Goulas A, Alexopoulos L, Palikaras A. Automated standardized pupillometry with optical method for purposes of clinical practice and research. Clin Physiol. 2000;20:336–347. [DOI] [PubMed] [Google Scholar]
- 10.Pittasch D, Lobmann R, Behrens-Baumann W, Lehnert H. Pupil signs of sympathetic autonomic neuropathy in patients with type 1 Diabetes. Diabetes Care. 2002;25:1545–1550. doi: 10.2337/diacare.25.9.1545. [DOI] [PubMed] [Google Scholar]
- 11.Meeker M, Du R, Bacchetti P, et al. Pupil examination: validity and clinical utility of an automated pupillometer. J Neurosci Nurs. 2005;37:34–40. [PubMed] [Google Scholar]
- 12.Tekin K, Sekeroglu MA, Kiziltoprak H, Doguizi S, Inanc M, Yilmazbas P. Static and dynamic pupillometry data of healthy individuals. Clin Exp Optom. 2018;101:659–665. doi: 10.1111/cxo.12659. [DOI] [PubMed] [Google Scholar]
- 13.Ferrari GL, Marques JL, Gandhi RA, et al. An approach to the assessment of diabetic neuropathy based on dynamic pupillometry. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:557–560. doi: 10.1109/IEMBS.2007.4352351. [DOI] [PubMed] [Google Scholar]
- 14.Park JC, Chen YF, Blair NP, et al. Pupillary responses in non-proliferative diabetic retinopathy. Sci Rep. 2017;7:44987. doi: 10.1038/srep44987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jain M, Devan S, Jaisankar D, Swaminathan G, Pardhan S, Raman R. Pupillary abnormalities with varying severity of diabetic retinopathy. Sci Rep. 2018;8:5636. doi: 10.1038/s41598-018-24015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Early Treatment Diabetic Retinopathy Study design and baseline characteristics . ETDRS report number 7. Ophthalmology. 1991;98:741–756. doi: 10.1016/s0161-6420(13)38009-9. [DOI] [PubMed] [Google Scholar]
- 17.Zele AJ, Feigl B, Smith SS, Markwell EL. The circadian response of intrinsically photosensitive retinal ganglion cells. PLoS One. 2011;6:17860. doi: 10.1371/journal.pone.0017860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Markwell EL, Feigl B, Zele AJ. Intrinsically photosensitive melanopsin retinal ganglion cell contributions to the pupillary light reflex and circadian rhythm. Clin Exp Optom. 2010;93:137–149. doi: 10.1111/j.1444-0938.2010.00479.x. [DOI] [PubMed] [Google Scholar]
- 19.Moreno A, Lozano M, Salinas P. Diabetic retinopathy. Nutr Hosp. 2013;28:53–56. doi: 10.3305/nh.2013.28.sup2.6714. [DOI] [PubMed] [Google Scholar]
- 20.Ewing DJ, Campbell IW, Clarke BF. The natural history of diabetic autonomic neuropathy. Q J Med. 1980;49:95–108. [PubMed] [Google Scholar]
- 21.Ferrari GL, Marques JL, Gandhi RA, et al. Using dynamic pupillometry as a simple screening tool to detect autonomic neuropathy in patients with diabetes: a pilot study. Biomed Eng Online. 2010;9:26. doi: 10.1186/1475-925X-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lerner AG, Bernabé-Ortiz A, Ticse R, et al. CRONICAS Cohort Study Group. Type 2 diabetes and cardiac autonomic neuropathy screening using dynamic pupillometry. Diabet Med. 2015;32:1470–1478. doi: 10.1111/dme.12752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuan D, Spaeth EB, Vernino S, Muppidi S. Disproportionate pupillary involvement in diabetic autonomic neuropathy. Clin Auton Res. 2014;24:305–309. doi: 10.1007/s10286-014-0258-6. [DOI] [PubMed] [Google Scholar]
- 24.Maguire AM, Craig ME, Craighead A, et al. Autonomic nerve testing predicts the development of complications: a 12-year follow-up study. Diabetes Care. 2007;30:77–82. doi: 10.2337/dc06-0793. [DOI] [PubMed] [Google Scholar]
- 25.Pena MM, Donaghue KC, Fung AT, et al. The prospective assessment of autonomic nerve function by pupillometry in adolescents with type 1 diabetes mellitus. Diabet Med. 1995;12:868–873. doi: 10.1111/j.1464-5491.1995.tb00388.x. [DOI] [PubMed] [Google Scholar]
- 26.Yang Y, Yu Y, Yao K. Pupillary dysfunction in type 2 diabetes mellitus to refine the early diagnosis of diabetic autonomic neuropathy. Neuro-ophthalmology. 2006;30:17–21. doi: 10.1080/01658100600599527. [DOI] [Google Scholar]
- 27.Feigl B, Zele AJ, Fader SM, et al. The post-illumination pupil response of melanopsin-expressing intrinsically photosensitive retinal ganglion cells in diabetes. Acta Ophthalmol. 2011;90:230–234. doi: 10.1111/j.1755-3768.2011.02226.x. [DOI] [PubMed] [Google Scholar]
- 28.Ortube MC, Kiderman A, Eydelman Y, et al. Comparative regional pupillography as a noninvasive biosensor screening method for diabetic retinopathy. Invest Ophthalmol Vis Sci. 2013;54:9–18. doi: 10.1167/iovs.12-10241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karavanaki K, Davies AG, Hunt LP, Morgan MH, Baum JD. Pupil size in diabetes. Arch Dis Child. 1994;71:511–515. doi: 10.1136/adc.71.6.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yilmaz I, Faiz M, Saracoglu B, Yazici AT. Changes in pupil size following panretinal and focal/grid retinal photocoagulation: automatic infrared pupillometry study. J Ocul Pharmacol Ther. 2016;32:172–177. doi: 10.1089/jop.2015.0080. [DOI] [PubMed] [Google Scholar]
- 31.Yilmaz I, Perente I, Saracoglu B, Yazici AT, Taskapili M. Changes in pupil size following panretinal retinal photocoagulation: conventional laser vs pattern scan laser (PASCAL). Eye. 2016;30:1359–1364. doi: 10.1038/eye.2016.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joyce DS, Feigl B, Kerr G, Roeder L, Zele AJ. Melanopsin-mediated pupil function is impaired in Parkinson’s disease. Sci Rep. 2018;8:7796. doi: 10.1038/s41598-018-26078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park JC, Moss HE, McAnany JJ. The pupillary light reflex in idiopathic intracranial hypertension. Invest Ophthalmol Vis Sci. 2016;57:23–29. doi: 10.1167/iovs.15-18181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joyce DS, Feigl B, Zele AJ. The effects of short-term light adaptation on the human post-illumination pupil response. Invest Ophthalmol Vis Sci. 2016;57:5672–5680. doi: 10.1167/iovs.16-19934. [DOI] [PubMed] [Google Scholar]


