ABSTRACT
The study aims to investigate the longitudinal changes in the circumpapillary retinal nerve fibre layer thickness (cpRNFLT) in progressive and non-progressive non-arteritic anterior ischaemic optic neuropathy (NAION). This retrospective observational case series study analysed 17 eyes with NAION. Patients sustaining any additional visual loss (additional decrease in visual acuity (VA) ≥0.2 logMAR) within two months after initial onset of symptoms were classified as having progressive NAION. Of the 17 eyes with NAION, 13 (76.5%) were diagnosed as non-progressive and 4 (23.5%) were diagnosed as progressive. Compared with control eyes, eyes with non-progressive NAION showed greater cpRNFLT in all four optic disc quadrants at the initial visit (temporal and superior: P < .001; nasal and inferior: P = .002). In contrast, compared with control eyes, eyes with progressive NAION showed greater cpRNFLT in the superior and nasal quadrants (P = .004 and 0.028, respectively), but not in the temporal and inferior quadrants. During progression, eyes with progressive NAION showed a significant increase in cpRNFLT in the inferior quadrants; furthermore, there was significant increase in cpRNFLT in the nasal sector before visual loss developed after the initial visit. Progressive NAION showed development of the disc swelling from the superior to inferior portion of optic disc via the nasal swelling, suggesting that swollen axons in one ischaemic part may lead to secondary vascular infarction in another part of the optic disc. This enlargement could constitute the earliest sign of progressive NAION.
KEYWORDS: Circumpapillary retinal nerve fibre layer thickness (cpRNFLT), disc swelling, non-arteritic anterior ischaemic optic neuropathy (NAION), optical coherence tomography (OCT), progressive and non-progressive NAION
Introduction
Non-arteritic anterior ischaemic optic neuropathy (NAION) is an optic nerve impairment characterised by sudden onset of visual loss with optic disc swelling.1–4 Although the exact pathogenic mechanism remains uncertain, NAION is considered to be caused by ischaemia of the optic nerve head, which is primarily supplied by the posterior ciliary artery circulation.5,6 In most patients with NAION, visual function remains unchanged or slightly improved after the sudden onset; however, some patients show significant deterioration in visual function.7
Progression and recurrence of NAION can cause visual deterioration. A second episode of visual deterioration within two months of onset is considered to be a progressive form of NAION, while a second episode occurring after two months is considered as a recurrence of NAION.2,8 According to current literature, progressive and recurrent NAION account for 15–40% 1,2,9-11 and 21–6.4%1,4,8,11-13 of eyes with NAION, respectively. Optic disc swelling seen during the acute phase of NAION usually resolves within two months.14 However, in progressive NAION, it remains unknown whether structural changes in the optic nerve may accompany an already swollen disc.
Optical coherence tomography (OCT) is a non-invasive imaging technique that has proven to be useful for monitoring circumpapillary retinal nerve fibre layer (RNFL) thickness (cpRNFLT) in various optic neuropathies.15–19 Measuring cpRNFLT enables us to detect subtle changes in the optic disc, such as mild optic disc swelling.15 Bellusci et al. studied cases of NAION and reported a decrease in cpRNFLT from a mean of 188.9 ± 56.0 µm (within two weeks of onset) to 63.1 ± 14.2 µm (at six months after onset).20 However, progressive NAION has never been investigated using OCT. Quantitatively comparing the changes in optic disc between progressive and non-progressive NAION may elucidate the structural changes in progressive NAION, and possibly establish OCT as a useful investigation to assess progression and prognosis of NAION. Here, we divided cases of NAION into progressive and non-progressive and compared the longitudinal changes in cpRNFLT between them.
Methods
A retrospective review was performed for all patients with NAION, who presented to the neuro-ophthalmology clinic of Kyoto University Hospital between November 2010 and February 2017 and fulfilled the inclusion criteria. At the initial visit, all patients underwent a comprehensive ophthalmic examination. The unaffected fellow eyes of the patients, without optic nerve diseases and retinal diseases, served as controls. All study participants provided consent and that the study design was approved by the Institutional Review Board at Kyoto University Graduate School of Medicine, and all study conduct adhered to the tenets of the Declaration of Helsinki.
Inclusion criteria and exclusion criteria
Inclusion criteria for diagnosis of NAION were:
history of sudden painless vision loss associated with a relative afferent pupillary defect (RAPD).
presence of optic disc swelling initially diagnosed by clinical examination at the time of presentation and confirmed through peripapillary RNFL thickness measurement using OCT.
optic disc-related visual field defects.
no evidence of any other neurologic or ocular disorder that could be responsible for optic disc swelling and visual impairment.
patients presented to the clinic within four weeks of onset of symptoms.
Exclusion criteria: Patients who were diagnosed with optic neuritis, compressive optic neuropathy, or invasive optic neuropathy using magnetic resonance imaging (MRI) with enhancement. Patients with elevated erythrocyte sedimentation (ESR) or C-reactive protein (CRP) were excluded. The upper limit of normal ESR for men was calculated as (age in years)/2 and for women (age in years+10)/2,21,22 and normal CRP was <0.1 mg/dl.23 Also, excluded were those with systemic symptoms suggestive of giant cell arteritis including: persistent, severe headache in the temple area; scalp tenderness; jaw claudication; polymyalgia rheumatica; unintended weight loss; and/or fever.
Patients experiencing any significant additional visual loss within two months of the initial VA assessment were classified as having progressive NAION.2,14 VA changes equivalent to 0.2 logMAR difference were considered to be statistically significant. We also chose for a ≥ 2 dB difference as the criterion for a significant mean deviation change in visual field score.2,24
Ophthalmological examination
30–2 Swedish Interactive Threshold Algorithm (SITA) standard visual field testing using the Humphrey Visual Field Analyser (HFA; model 750; Carl Zeiss Meditec), OCT with the Spectralis HRA + OCT (Heidelberg Engineering) using a standard peripapillary circular scan, and fundus photography (TRC NW8F plus Non-Mydriatic Retinal Camera; Topcon) were performed on all patients. To exclude all other optic nerve diseases, all patients underwent blood examinations (including ESR and CRP) and magnetic resonance imaging (MRI).
Tests to assess functional and structural outcomes, including best corrected visual acuity (BCVA), mean deviation of visual field test, and peripapillary RNFL thickness were analysed. Visual field tests in which results were reliable (i.e. with <33% fixation losses, false-positive responses, and false-negative responses) were analysed. Abnormal visual fields were classified as diffuse or localised loss. For localised deficits, the principal location of the visual field defect (VFD) was identified (i.e., inferior quadrant, inferior arcuate, inferior altitudinal, central or centrocaecal scotoma).25
Statistics
A paired t-test was used to compare eyes with progressive NAION at the first visit, at the second episode, prior to progression, and at the final visit. A Mann–Whitney U test was used to compare eyes with progressive NAION, eyes with non-progressive NAION, and control eyes. P values <.05 were considered to be statistically significant.
Results
In this study, patients with NAION who visited our clinic in the acute phase (≤4 weeks after onset) were analysed (11 men, five women – 17 eyes; median age: 67 years; range: 41–87 years). All of them showed normal MRI findings with no evidence of inflammatory diseases. At the initial visit, the mean logMAR VA was 0.31 ± 0.58 in NAION (17 eyes) and −0.09 ± 0.15 in the unaffected fellow eyes (P = .279). Of the 17 eyes with NAION, four eyes fulfilled the criteria for progressive NAION, i.e. additional deterioration in VA ≥0.2 logMAR within two months of the initial visit (Table 1). In eyes with non-progressive NAION, the mean logMAR VA did not differ between the initial and final visits (0.25 ± 0.61 and 0.25 ± 0.60, respectively). In contrast, eyes with progressive NAION showed a marginally significant decrease in mean logMAR VA at the final visit (from −0.11 ± 0.09 to 0.77 ± 0.96; P = .139). Of the four eyes with progressive NAION, two patients had hypertension (patients 1 and 4), one patient had sleep apnoea syndrome (patient 1), and one had insulin-independent diabetes mellitus (patient 4). One patient had familial hypercholesterolemia and had experienced a miscarriage (patient 3).
Table 1.
Visual acuity and visual field defects in eyes with progressive NAION at each time point.
| First attack |
Second attack |
PSL treatment |
Final test |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pt | Day | VA | MD(dB) | day | VA | MD(dB) | day | VA | MD(dB) | day | VA | MD(dB) |
| 1 | 9 | −0.176 | −6.15 | 31 | 0.398 | −8.81 | 50 | 0.301 | −5.86 | 184 | 0.222 | −7.31 |
| 2 | 3 | −0.079 | −17.1 | 37 | 0.222 | −19.22 | 79 | 1.155 | 173 | 1.046 | −22.76 | |
| 3 | 6 | −0.176 | −10.16 | 42 | −0.176 | −16.46 | 49 | −0.176 | −15.78 | 190 | −0.176 | −17.31 |
| 4 | 1 | 0 | −3.20 | 14 | 0.699 | −12.09 | 43 | 1 | −12.54 | 184 | 2 | −27.82 |
NAION = non-arteritic anterior ischaemic optic neuropathy; Pt = patient; PSL = prednisolone; VA = visual acuity (in logMAR);
MD = mean deviation; dB = decibels
Of the 13 eyes with non-progressive NAION, patterns of VFD were altitudinal or arcuate in eight (61.5%), diffuse in four (30.8%), and central scotoma in one eye (7.7%) at the initial visit. All eyes with non-progressive NAION showed stable VFDs during the follow-up period (data not shown). Eyes with progressive NAION showed arcuate scotoma in two eyes and diffuse pattern of VFD in two eyes at the initial visit. All eyes with progressive NAION showed VFD enlargement during the two months after the first episode, which remained unchanged after that.
The 13 eyes with non-progressive NAION showed a range of disc swelling at the initial visit: superior in seven (53.8%), inferior in one (7.7%), and diffuse in five (38.5%). When compared with the unaffected fellow eyes, affected eyes showed greater cpRNFLT in all four quadrants at the initial visit (temporal: P = .005; superior: P < .001; nasal: P = .023; and inferior: P = .012) (Table 2). At 1–2 months after onset (mean: 47.5 ± 15.5 days), affected eyes showed significantly decreased cpRNFLT in all four quadrants (temporal: P < .001; superior: P < .001; nasal: P = .002; and inferior: P = .002). On the other hand, when comparing progressive NAION with unaffected fellow eyes, affected eyes showed greater cpRNFLT in the superior (P = .004) and nasal (P = .028) quadrants, but not in the temporal and inferior quadrants (P = .173 and 0.283, respectively) at the initial visit (Table 2). However, cpRNFLT in the inferior sector significantly increased in the progression phase (P = .028) (Table 3). Notably, there was a significant increase in cpRNFLT in the nasal sector before the development of vision loss after the initial visit (from 128.5 ± 40.7 to 167.0 ± 32.7 μm; P = .011). All eyes with progressive NAION showed enlargement of the disc swelling from the upper to thelower portion in the progressive phase (Figure 1 and 2).
Table 2.
RNFL thickness of non-progressive and progressive NAION at the initial visit.
| Control (n = 8) | Non-progressive (n = 13) | Progressive (n = 4) | P* | P† | |
|---|---|---|---|---|---|
| Average (µm) | 97.2 (±9.4) | 174.1 (±62.6) | 163.9 (±47.7) | 0.159 × 10−4 | 0.003 |
| Temporal (µm) | 73.1 (±13.9) | 130.5 (±67.4) | 94.3 (±25.2) | 0.005 | 0.173 |
| Superior (µm) | 117.5 (±12.6) | 232.6 (±99.9) | 285.4 (±136.4) | 0.318 × 10−4 | 0.004 |
| Nasal (µm) | 72.9 (±20.5) | 113.1 (±44.7) | 128.5 (±40.7) | 0.023 | 0.028 |
| Inferior (µm) | 125.3 (±23.3) | 220 (±78.2) | 147.6 (±20.8) | 0.012 | 0.283 |
* = comparison between control and non-progressive NAION
† = comparison between control and progressive NAION
NAION = non-arteritic anterior ischaemic optic neuropathy; RNFL = retinal nerve fibre layer.
Table 3.
Development of RNFL thickness of progressive NAION.
| Progressive (n = 4) at onset | Progressive (n = 4) at progression | P | |
|---|---|---|---|
| Average (µm) | 163.9 (±47.7) | 179.4 (±22.0) | 0.612 |
| Temporal (µm) | 94.3 (±25.2) | 103.5 (±32.7) | 0.757 |
| Superior (µm) | 285.4 (±136.4) | 166.1 (±84.2) | 0.320 |
| Nasal (µm) | 128.5 (±40.7) | 134.8 (±9.5) | 0.781 |
| Inferior (µm) | 147.6 (±20.8) | 313.4 (±87.0) | 0.028 |
RNFL = retinal nerve fibre layer; NAION = non-arteritic anterior ischaemic optic neuropathy.
Figure 1.
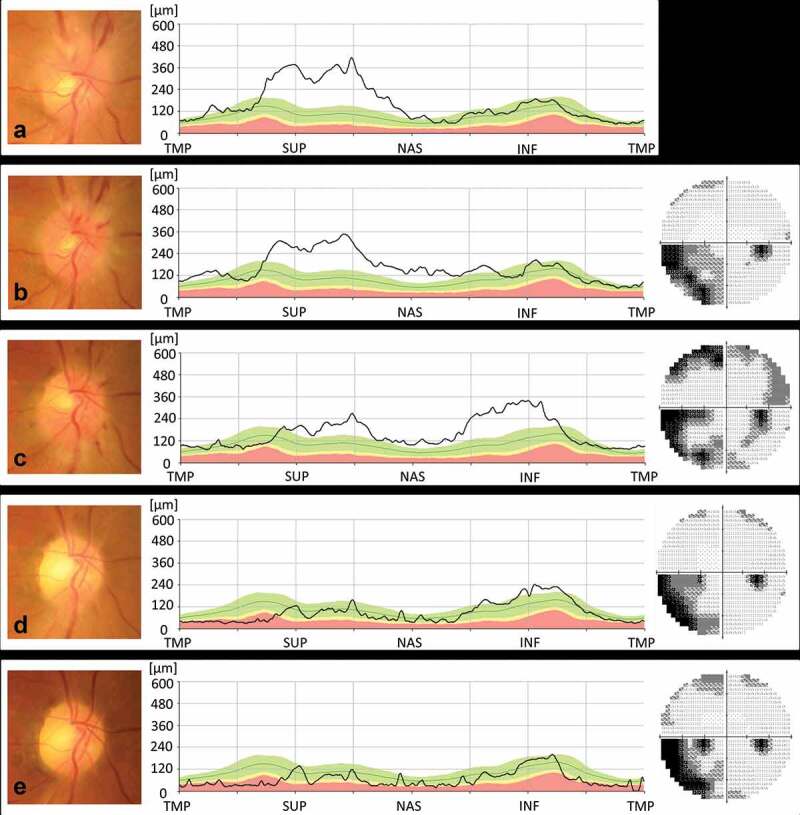
(a). At initial examination, swelling was noted in the superior portion of the right optic disc. BCVA was 30/20 in the right eye. (b). Over the next seven days, optic disc swelling, and nasal and peripapillary haemorrhage developed, but his BCVA did not change. Visual field testing showed an inferonasal visual field defect. (c). One month later, the BCVA decreased to 20/50, and the inferior portion of the right optic disc was swollen. Visual field testing detected a new upper visual field defect. (d). Following treatment with 40 mg of prednisone daily for a week followed by a reduction in dosage of 10 mg every week, optic disc swelling was no longer present. BCVA increased to 30/50, and the visual field improved. (e). The patient’s visual function remained unchanged for the next six months; however, RNFL thinning subsequently developed in the infero-temporal part of the right optic disc.
NAION = non-arteritic anterior ischaemic optic neuropathy; BCVA = best corrected visual acuity; TMP = temporal; SUP = superior; NAS = nasal; INF = inferior.
Figure 2.
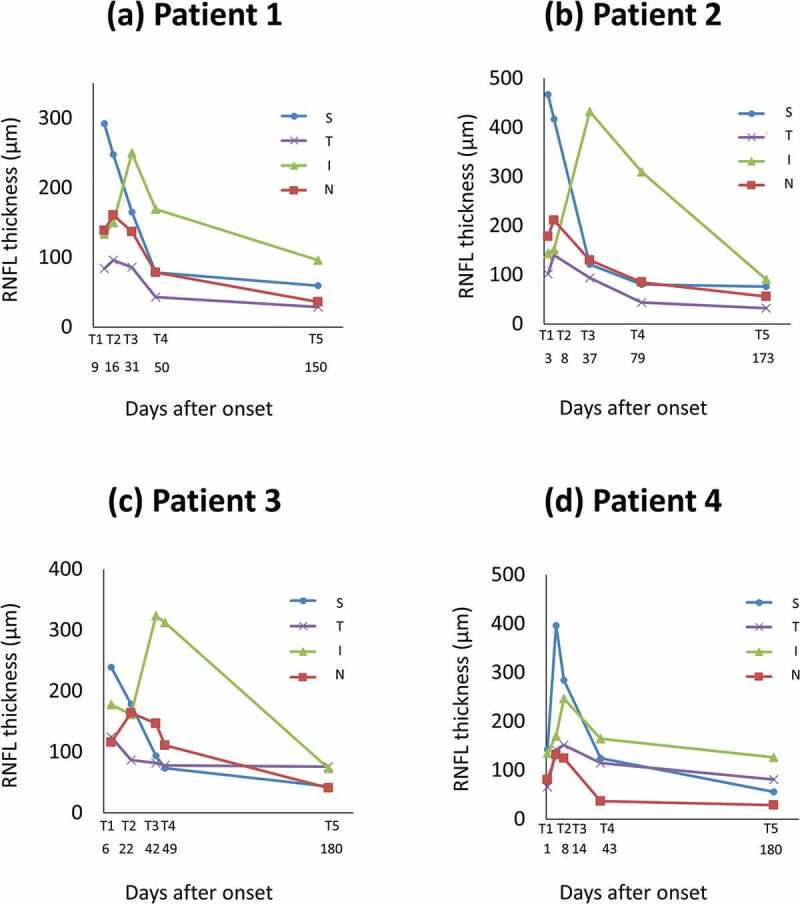
RNFL thickness in eyes with progressive NAION. Mean RNFL thickness values of four optic disc quadrants at each stage (T1–T5) of the follow-up period.
RNFL = retinal nerve fibre layer; T1 = onset; T2 = asymptomatic optic disc swelling; T3 = progressive phase; T4 = prednisone treatment; T5 = final testing.
Vertical cup-to-disc ratio of fellow eyes in patients with progressive NAION was 0.25 ± 0.11 on fundus images, while that in eyes with non-progressive NAION was 0.16 ± 0.15 (P = .334). The mean disc area of the eye affected with progressive NAION in the chronic phase was 2.07 ± 0.20 mm2, while that in non-progressive NAION was 1.87 ± 0.30 mm2 (P = .262).
All eyes with progressive NAION were treated with prednisone for at least four weeks (initial doses were 40 mg/day for patient 1, 60 mg/day for patients 2, 3, and 4). Steroid therapy was started immediately after diagnosis of progressive NAION. The visual function remained unchanged or improved after treatment with prednisone in three out of the four patients.
Discussion
This study analysed the longitudinal change in visual function and cpRNFLT in eyes with progressive NAION and compared them with eyes with non-progressive NAION and unaffected eyes. Of the 17 eyes with NAION, four eyes fulfilled the criteria for diagnosis of progressive NAION (i.e. an additional deterioration in VA ≥0.2 logMAR); all of them also showed VFD progression from inferior to superior portion. Consistently, all cases of progressive NAION showed development of the disc swelling from the upper portion to the lower portion. Notably, there was a significant increase in cpRNFLT in the nasal sector preceding vision loss after the initial visit.
Of all the reports on progressive NAION (Table 4), only a few have described the longitudinal changes in optic disc appearance in progressive or recurrent NAION.3,28,30 Borchert and Lessell reported that the disc swelling spread from the upper portion to thelower portion during the progressive phase of NAION in six out of eight patients.30 Beck et al. reported that three out of four patients with recurrent NAION showed disc swelling in the inferior portion during the second episode.28 Consistent with the previous reports, our study using OCT quantitatively demonstrated thickened RNFL in the superior portion during the first episode, and demonstrated the spread to the inferior portion during the second episode in all cases. In contrast, eyes with diffuse optic disc swelling during the first episode of NAION seldom progressed.
Table 4.
Review of literature on progressive of NAION in the same eye.
| Initial VA |
Final VA |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Authors | Year | Total eyes | Progressive form (%) | ≥ 20/30 20/30-20/70 ≥ 20/70 | ≥ 20/30 20/30-20/70 ≥ 20/70 | ||||
| Sanders MD26 | 1971 | 2 | 2 | 1 | 0 | 1 | 0 | 0 | 2 |
| Boghen and Glaser1 | 1975 | 34 | 11 (32%) | not given | not given | ||||
| Shults WT27 | 1977 | 1 | 1 | not given | not given | ||||
| Hayreh SS3 | 1981 | 4 | 1 (25%) | 1 | 0 | 0 | 1 | 0 | 0 |
| Beck RW et al.28 | 1983 | 4 | 3 | 2 | 1 | 0 | 1 | 0 | 2 |
| Lavin and Ellenberger29 | 1983 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 |
| Borchert and Lessell30 | 1988 | 11 | 11 | 8 | 3 | 0 | 1 | 4 | 6 |
| Kline LB31 | 1988 | 6 | 6 | 5 | 0 | 1 | 0 | 0 | 6 |
| Mutlukan and Cullen32 | 1990 | 1 | 1 | not given | not given | ||||
| Arnold and Hepler2 | 1994 | 27 | 6 (22%) | 4 | 0 | 2 | 1 | 2 | 3 |
| Purvin V et al.9 | 2004 | 24 | 7 (29%) | not given | not given | ||||
| Janaky M et al.33 | 2005 | 3 | 1 | not given | 0 | 0 | 1 | ||
| Contreras I et al.10 | 2007 | 27 | 4 (15%) | not given | not given | ||||
| Rebolleda and Munoz-Negrete34 | 2009 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 |
| Arnold AC et al.11 | 2013 | 294 | 119 (40%) | not given | not given | ||||
| Present study | 17 | 4 (24%) | 4 | 0 | 0 | 1 | 1 | 2 | |
VA = visual acuity;
The mechanisms of progression of NAION still remain unknown. Hayreh suggested that the pathogenesis of progressive or recurrent NAION is a vicious cycle: compression of capillaries in the optic nerve head by swollen axons causes more ischaemia and further swelling of axons.8,35 Borchert and Lessell also suggested that progressive NAION could progress until sufficient axons were lost and crowding was relieved.30 In the present study, all cases of progressive NAION showed thickened RNFL spreading from the superior to the inferior portion of optic disc via nasal swelling, suggesting that swollen axons in one ischaemic part may lead to secondary vascular infarction in another part of the optic disc.
The Zinn–Haller ring is an anastomotic circle between the lateral and medial short posterior ciliary arteries, which perfuses the optic nerve head. However, some cases have incomplete anastomoses or the Zinn–Haller ring is supplied only by the lateral or medial short posterior ciliary artery, and those eyes are vulnerable to anterior optic nerve ischaemia.36,37 In our cases, initially, hypoperfusion from the lateral short posterior ciliary artery may have occurred in the superior portion of optic disc, resulting in the superior disc swelling, followed by hypoperfusion from the medial short posterior ciliary artery.
The present study also revealed that thickening of the RNFL in the nasal sector preceded visual worsening in all cases. Several reports showed that asymptomatic optic disc swelling precedes the vision loss in NAION.3,30,34,35 Hayreh and Zimmerman reported that 25% of asymptomatic optic disc swelling progressed to symptomatic NAION.35 Therefore, monitoring cpRNFLT after the first episode of NAION using OCT may allow us to predict a second attack. Additionally, given that steroid therapy shortened the time taken for spontaneous resolution of disc swelling,14,24 steroid therapy may prevent the progression of VFDs by decreasing disc swelling. In fact, visual impairment did not progress after the commencement of steroid treatment in three out of the four patients.
The current study has several limitations including its small sample size and retrospective nature. However, despite the retrospective study design, we believe the diagnoses were accurate because ESR and CRP were normal in each patient during both the first and second episodes, and none had systemic symptoms or signs of giant cell arteritis. Additionally, MRI of the head and orbits with gadolinium contrast showed normal findings in all cases. Fluorescein angiography (FA) data is missing in two of the four patients because they did not consent to the procedure because of its invasive nature. The other two patients underwent FA, which revealed filling delays into the swollen parts of the optic discs in both cases (patients 3 and 4). In conclusion, we found that 23.5% of cases with NAION became progressive during the follow-up, and all cases with progressive NAION showed enlargement of optic disc swelling from the superior quadrant to the nasal quadrant; suggesting that this could constitute the earliest sign in the progressive phase of NAION. There is a possibility to predict the progression of NAION by following the evolution of optic disc swelling using OCT.
Funding Statement
This study was supported, in part, by the Japan Society for the Promotion of Science (JSPS, Tokyo, Japan, Grant-in-Aid for Scientific Research, no. 21592256) and the Japan National Society for the Prevention of Blindness (Tokyo, Japan). The funding sources had no involvement in study design, the collection, analysis and interpretation of data, the writing of the report, and in the decision to submit the article for publication.
Declaration of interest statement
There is no conflict to disclosure.
References
- 1.Boghen DR, Glaser JS.. Ischaemic optic neuropathy. The clinical profile and history. Brain. 1975;98(4):689–708. doi: 10.1093/brain/98.4.689. [DOI] [PubMed] [Google Scholar]
- 2.Arnold AC, Hepler RS. Natural history of nonarteritic anterior ischemic optic neuropathy. J Neuroophthalmol. 1994;14(2):66–69. doi: 10.1097/00041327-199406000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Hayreh SS. Anterior ischemic optic neuropathy. V. Optic disc edema an early sign. Arch Ophthalmol. 1981;99(6):1030–1040. doi: 10.1001/archopht.1981.03930011030010. [DOI] [PubMed] [Google Scholar]
- 4.Repka MX, Savino PJ, Schatz NJ, Sergott RC. Clinical profile and long-term implications of anterior ischemic optic neuropathy. Am J Ophthalmol. 1983;96(4):478–483. doi: 10.1016/S0002-9394(14)77911-5. [DOI] [PubMed] [Google Scholar]
- 5.Hayreh SS. Blood supply of the optic nerve head and its role in optic atrophy, glaucoma, and oedema of the optic disc. Br J Ophthalmol. 1969;53(11):721–748. doi: 10.1136/bjo.53.11.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hayreh SS. The blood supply of the optic nerve head and the evaluation of it - myth and reality. Prog Retin Eye Res. 2001;20(5):563–593. doi: 10.1016/S1350-9462(01)00004-0. [DOI] [PubMed] [Google Scholar]
- 7.Hayreh SS, Zimmerman MB. Nonarteritic anterior ischemic optic neuropathy: natural history of visual outcome. Ophthalmology. 2008;115(2):298–305.e2. doi: 10.1016/j.ophtha.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayreh SS, Podhajsky PA, Zimmerman B. Ipsilateral recurrence of nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol. 2001;132(5):734–742. doi: 10.1016/S0002-9394(01)01192-8. [DOI] [PubMed] [Google Scholar]
- 9.Purvin V, King R, Kawasaki A, Yee R. Anterior ischemic optic neuropathy in eyes with optic disc drusen. Arch Ophthalmol. 2004;122(1):48–53. doi: 10.1001/archopht.122.1.48. [DOI] [PubMed] [Google Scholar]
- 10.Contreras I, Noval S, Rebolleda G, Muñoz-Negrete FJ. Follow-up of nonarteritic anterior ischemic optic neuropathy with optical coherence tomography. Ophthalmology. 2007;114(12):2338–2344. doi: 10.1016/j.ophtha.2007.05.042. [DOI] [PubMed] [Google Scholar]
- 11.Arnold AC, Costa RM, Dumitrascu OM. The spectrum of optic disc ischemia in patients younger than 50 years (An American Ophthalmological Society thesis). Trans Am Ophthalmol Soc. 2013;111:93–118. [PMC free article] [PubMed] [Google Scholar]
- 12.Preechawat P, Bruce BB, Newman NJ, Biousse V. Anterior ischemic optic neuropathy in patients younger than 50 years. Am J Ophthalmol. 2007;144(6):953–960. doi: 10.1016/j.ajo.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 13.Ellenberger C Jr, Keltner JL, Burde RM. Acute optic neuropathy in older patients. Arch Neurol. 1973;28(3):182–185. doi: 10.1001/archneur.1973.00490210062008. [DOI] [PubMed] [Google Scholar]
- 14.Hayreh SS, Zimmerman MB. Optic disc edema in non-arteritic anterior ischemic optic neuropathy. Graefes Arch Clin Exp Ophthalmol. 2007;245(8):1107–1121. doi: 10.1007/s00417-006-0494-0. [DOI] [PubMed] [Google Scholar]
- 15.Savini G, Bellusci C, Carbonelli M, et al. Detection and quantification of retinal nerve fiber layer thickness in optic disc edema using stratus OCT. Arch Ophthalmol. 2006;124(8):1111–1117. doi: 10.1001/archopht.124.8.1111. [DOI] [PubMed] [Google Scholar]
- 16.Wollstein G, Schuman JS, Price LL, et al. Optical coherence tomography longitudinal evaluation of retinal nerve fiber layer thickness in glaucoma. Arch Ophthalmol. 2005;123(4):464–470. doi: 10.1001/archopht.123.4.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barboni P, Savini G, Valentino ML, et al. Retinal nerve fiber layer evaluation by optical coherence tomography in Leber’s hereditary optic neuropathy. Ophthalmology. 2005;112(1):120–126. doi: 10.1016/j.ophtha.2004.06.034. [DOI] [PubMed] [Google Scholar]
- 18.Kanamori A, Nakamura M, Matsui N, et al. Optical coherence tomography detects characteristic retinal nerve fiber layer thickness corresponding to band atrophy of the optic discs. Ophthalmology. 2004;111(12):2278–2283. doi: 10.1016/j.ophtha.2004.05.035. [DOI] [PubMed] [Google Scholar]
- 19.Medeiros FA, Moura FC, Vessani RM, Susanna R Jr. Axonal loss after traumatic optic neuropathy documented by optical coherence tomography. Am J Ophthalmol. 2003;135(3):406–408. doi: 10.1016/S0002-9394(02)02049-4. [DOI] [PubMed] [Google Scholar]
- 20.Bellusci C, Savini G, Carbonelli M, Carelli V, Sadun AA, Barboni P. Retinal nerve fiber layer thickness in nonarteritic anterior ischemic optic neuropathy: OCT characterization of the acute and resolving phases. Graefes Arch Clin Exp Ophthalmol. 2008;246(5):641–647. doi: 10.1007/s00417-008-0767-x. [DOI] [PubMed] [Google Scholar]
- 21.Griffiths RA, Good WR, Watson NP, O’Donnell HF, Fell PJ, Shakespeare JM. Normal erythrocyte sedimentation rate in the elderly. Br Med J (Clin Res Ed). 1984;289(6447):724–725. doi: 10.1136/bmj.289.6447.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller A, Green M, Robinson D. Simple rule for calculating normal erythrocyte sedimentation rate. Br Med J (Clin Res Ed). 1983;286(6361):266. doi: 10.1136/bmj.286.6361.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eshaghian J, Goeken JA. C-reactive protein in giant cell (cranial, temporal) arteritis. Ophthalmology. 1980;87(11):1160–1166. doi: 10.1016/S0161-6420(80)35110-5. [DOI] [PubMed] [Google Scholar]
- 24.Hayreh SS, Zimmerman MB. Non-arteritic anterior ischemic optic neuropathy: role of systemic corticosteroid therapy. Graefes Arch Clin Exp Ophthalmol. 2008;246(7):1029–1046. doi: 10.1007/s00417-008-0805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keltner JL, Johnson CA, Spurr JO, Beck RW. Baseline visual field profile of optic neuritis. The experience of the optic neuritis treatment trial. Optic Neuritis Study Group. Arch Ophthalmol. 1993;111(2):231–234. doi: 10.1001/archopht.1993.01090020085029. [DOI] [PubMed] [Google Scholar]
- 26.Sanders MD. Ischaemic papillopathy. Trans Ophthalmol Soc UK. 1971;91:369–386. [PubMed] [Google Scholar]
- 27.Shults WT. Ischemic optic neuropathy: some interesting features. Trans Pac Coast Otoophthalmol Soc Annu Meet. 1977;58:281–298. [PubMed] [Google Scholar]
- 28.Beck RW, Savino PJ, Schatz NJ, Smith CH, Sergott R. Anterior ischaemic optic neuropathy: recurrent episodes in the same eye. Br J Ophthalmol. 1983;67(10):705–709. doi: 10.1136/bjo.67.10.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lavin PJM, Ellenberger C. Recurrent ischemic optic neuropathy. Neuro-Ophthalmology. 1983;3(3):193–198. doi: 10.3109/01658108309009737. [DOI] [Google Scholar]
- 30.Borchert M, Lessell S. Progressive and recurrent nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol. 1988;106(4):443–449. doi: 10.1016/0002-9394(88)90881-1. [DOI] [PubMed] [Google Scholar]
- 31.Kline LB. Progressive of visual defects in ischemic optic neuropathy. Am J Ophthalmol. 1988;106(2):199–203. doi: 10.1016/0002-9394(88)90835-5. [DOI] [PubMed] [Google Scholar]
- 32.Mutlukan E, Cullen JF. Can empty sella syndrome be mistaken for a progressive form of nonarteritic ischemic optic neuropathy? Arch Ophthalmol. 1990;108(8):1066–1067. doi: 10.1001/archopht.1990.01070100022010. [DOI] [PubMed] [Google Scholar]
- 33.Janaky M, FÜlÖp Z, Palffy A, Benedek K, Benedek G. Non-arteritic ischaemic optic neuropathy(NAION) in patients under 50 years of age. Acta Ophthalmol Scand. 2005;83(4):499–503. doi: 10.1111/j.1600-0420.2005.00446.x. [DOI] [PubMed] [Google Scholar]
- 34.Rebolleda G, Muñoz-Negrete FJ. [Incipient or presymptomatic nonarteritic anterior ischemic optic neuropathy]. Arch Soc Esp Oftalmol. 2009;84(3):151–154. doi: 10.4321/s0365-66912009000300008. [DOI] [PubMed] [Google Scholar]
- 35.Hayreh SS, Zimmerman MB. Incipient nonarteritic anterior ischemic optic neuropathy. Ophthalmology. 2007;114(9):1763–1772. doi: 10.1016/j.ophtha.2006.11.035. [DOI] [PubMed] [Google Scholar]
- 36.Olver JM, Spalton DJ, McCartney AC. Quantitative morphology of human retrolaminar optic nerve vasculature. Invest Ophthalmol Vis Sci. 1994;35:3858–3866. [PubMed] [Google Scholar]
- 37.Ohno-Matsui K, Futagami S, Yamashita S, Tokoro T. Zinn-Haller arterial ring observed by ICG angiography in high myopia. Br J Ophthalmol. 1998;82(12):1357–1362. doi: 10.1136/bjo.82.12.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]


